Introduction
Diabetes and the complications arising from it have
become a global public health problem. The rate of lower limb
amputation is between 0.2-4.8% in diabetic patients worldwide,
which is 25 times higher compared with that among non-diabetic
individuals (1,2). A total of 85% of amputations occur
after foot ulcers among diabetic patients. Peripheral artery
disease (PAD) is not only the most important risk factor for
diabetic foot (DF) ulcers, but also an independent factor to
predict the outcome and recovery in patients with DF ulcers
(3,4).
To date, the treatment of diabetic peripheral
vascular lesions is limited to drug treatment; however, it is
usually long-term, with high-cost and limited effectiveness
(5). Vascular surgery or
interventional therapy are ideal for non-extensive vascular lesions
but unable to treat diffused vascular stenosis in lower extremity
diabetic complications (6).
Exercise can improve metabolism and ameliorate vascular lesions
(7). However, the ability to
exercise is impaired in most patients with DF. These limitations
have prompted further studies on the mechanism of DF to aid the
development of novel and effective treatments.
The dysfunction of vascular endothelial cells is the
major cause of PAD, which has been documented in numerous studies
in the past decades (8,9). Therefore, angiogenic factors, such as
VEGF, are beneficial for ulcer healing (10). VEGF is known to act directly on
numerous types of cells, including skeletal muscle cells (11). The skeletal muscle is the main organ
for sugar metabolism in humans, also acting as the exoskeleton
environment for vascular endothelial cell growth. Based on the
source of ATP production, skeletal muscle fibers are divided into
two types: Glycolytic and oxidative, and both can be converted to
each other. In patients with long-term hypertension and diabetes,
the proportion of oxidative muscle fibers is gradually decreased,
with increased proportions of glycolytic muscle fibers (12,13).
Moreover, the levels of sugar and energy metabolism in oxidative
muscle fibers are higher compared with those in glycolytic muscle
fibers (14,15). These observations led to the present
study which hypothesized that VEGF might play a role in the
transformation of skeletal fiber types and help improve vascular
ischemia in DF. Therefore, the aim of the present study was to
investigate the mechanism and effects of VEGF-induced muscle fiber
conversion in angiogenesis and the treatment of DF.
Materials and methods
Animals
All animal experiments were conducted following the
Guide for the Care and Use of Laboratory Animals published by the
NIH (eighth edition, updated 2011) and ARRIVE guidelines (16). In total, 22 male C57BL/6 mice (age,
8 weeks, weight, 23.14±0.65 g) were purchased from Beijing Vital
River Laboratory Animal Technology Co., Ltd. Mice were maintained
at typical temperatures (20-22˚C) and humidity (40-60%) in the
Chinese People's Liberation Army (PLA) General Hospital
experimental Animal Center under specific pathogen free conditions
with a 12-h light/dark cycle and ad libitum access to food
and water. Animal health and behavior were monitored every 2 or 3
days. All experiments were approved by the Ethics Committee of
Chinese PLA General Hospital (approval no. 8157021269; Beijing,
China).
Voluntary exercise training
In total, 12 C57BL/6 mice (age, 10 weeks at the
start of the experiment) were divided into sedentary (sed, n=3) and
voluntary exercise groups (n=9) in cages equipped with a voluntary
running wheel (diameter, 11 cm). The wheel was connected to a
counter for recording (17,18). After 0, 1, 2 and 4 weeks of
exercise, three mice/time point were anesthetized with 3-4%
isoflurane in air and sacrificed by cervical dislocation to obtain
gastrocnemius tissue.
Cells
Mouse C2C12 myoblasts were purchased from Cyagen
Biosciences, Inc. Human umbilical vein endothelial cells (HUVECs),
PUMC-HUVEC-T1, were kindly provided by the Molecular Biology
Laboratory of PLA General Hospital (purchased from the National
Experimental Cell Resource Sharing Platform). C2C12 myoblasts and
PUMC-HUVEC-T1 cells were maintained in DMEM high sugar culture
medium (Hyclone; Cytiva) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 U/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at
37˚C, 5% CO2. To induce myotube formation, C2C12
myoblasts with 60-80% confluence were seeded in DMEM
differentiation medium containing 2% horse serum (Cytiva), 100 U/ml
penicillin and 100 U/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C for 5 days (19). The success of differentiation was
validated by Giemsa staining of myotubes (cat. no. G4507;
Sigma-Aldrich, Merck KGaA) and western blotting using anti-myosin
heavy chain antibody (cat. no. sc-53095, 1:1,000, Santa Cruz
Biotechnology).
For Giemsa staining, cells were fixed in 100%
methanol at room temperature (RT) for 10 min. After air dry, Giemsa
stain was added and stained for 30 min at RT. After rinsing with
deionized water, cells were examined at x20 magnification under a
light microscope (DP73; Olympus Corporation).
Recombinant proteins and
antibodies
Recombinant murine VEGF (rmVEGF) was purchased from
PeproTech, Inc. The anti-cytochrome c oxidase (COX) IV (cat. no.
ab16056, 1:2,000), anti-peroxisome proliferator-activated receptor
γ coactivator 1-α (PGC-1α; cat. no. ab54481, 1:1,000), anti-glucose
transporter member (GLUT) 4 (cat. no. ab654, 1:1,000) antibodies
were purchased from Abcam. The anti-PI3K (cat. no. 4292, 1:1,000),
phosphorylated (p)-PI3K antibody (Tyr458/Tyr199; cat. no. 4228;
1:1,000), AMPKα antibody (cat. no. 2532; 1:1,000), p-AMPKα antibody
(Thr172; cat. no. 2535; 1:500), Akt antibody (cat. no. 4685S;
1:250), p-Akt antibody (Ser473) (cat. no. 4060S; 1:250) antibodies
were purchased from Cell Signaling Technology, Inc. The
anti-β-actin (cat. no. 854-s, 1:2,000) was purchased from
HuaBio.
Immunofluorescence on frozen
sections
The center part of gastrocnemius muscle from mice
trained with voluntary exercise was obtained and immediately
immersed in liquid nitrogen (-196˚C). Tissues were embedded in
optimal cutting temperature compound (cat. no. 4583; Sakura Finetek
USA, Inc.) and cut into 6-µm-thick sections. Sections were then
fixed in 4% polyformaldehyde solution at RT for 10 min,
permeabilized in 0.3% Triton X solution, blocked with goat serum
(cat. no. ZLI-9022; ZSGB-Bio; OriGene Technologies, Inc.) at RT for
30 min, and stained with anti-CD31 (1:50, cat. no. MCA2388, Bio-Rad
Laboratories) and myosin heavy chain IIa (MHCIIa) antibodies
(1:200, cat. no. sc-53095, Santa Cruz Biotechnology) at 4˚C
overnight followed by incubation with FITC- (cat. no. ZF-0315,
1:300; ZSGB-Bio; OriGene Technologies, Inc.) or Alexa Fluor 594-
(cat. no. ZF-0513, 1:300, ZSGB-Bio; OriGene Technologies, Inc.)
conjugated secondary antibodies at RT for 30 min. The images were
captured at x40 magnification using a confocal microscope (TCS SP8;
Leica Microsystems GmbH). The fluorescence intensity was quantified
using ImageJ (version 1.52a; National Institutes of Health)
(20).
ELISA
Gastrocnemius muscle samples from mice trained with
voluntary exercise were cut into small pieces (~5-mm), and quickly
immersed in liquid nitrogen (-196˚C). Tissues were then homogenized
and centrifuged at 8,000 x g for 15 min at 4˚C. The supernatant was
used to assay the concentration of VEGF (cat. no. EK0541; Boster
Biological Technology) and enzyme activity of citric acid synthase
(cat. no. MAK057; Sigma-Aldrich, Merck KGaA) according to
manufacturer's protocols.
Lower limb ischemia model in diabetic
mice and adenovirus transduction
A total of 10 8-week-old male C57BL/6 mice were fed
with high-fat diet (Research Diets, Inc.) for 6 weeks and then
injected with 100 mg/kg streptozocin (STZ, Sigma-Aldrich; Merck
KGaA) on day 0. Diabetic mice were screened for random blood
glucose levels and confirmed by two consecutive tests of tail vein
blood glucose of >11 mmol/l on days 2 and 7 after STZ injection.
On day 8, diabetic mice were intraperitoneally injected with
pentobarbital (50 mg/kg, Sigma-Aldrich; Merck KGaA) and then
received surgery to ligate the femoral artery on the left lower
limb, using the right lower limb as the control (skin not cut open)
(21). On day 9, laser Doppler
imaging apparatus (PeriCam PSI System; PIMSoft version 1.5.4.8078;
Perimed, AB) was used to detect the blood flow perfusion in lower
limbs to ensure the ischemia was successful in seven mice
(monitoring distance, 15 cm; area height, 4.5 cm; width, 5.0 cm;
area, 110 cm2; modeling failed in three mice, which were
sacrificed immediately) (22).
Adenovirus (Ad)-VEGF-green fluorescent protein (GFP) and Ad-GFP
were produced by Hanbio Biotechnology Co., Ltd. based on the
sequence of VEGF transcript (pAdEasy-EF1-MCS-3flag-CMV-EGFP;
accession no. of VEGF, NM_001025366.2; 1x1010 pfu/ml
each). On day 10, a total of 200 µl Ad-VEGF-GFP or Ad-GFP was
injected at three sites of the left gastrocnemius of ischemic
diabetic mice (three mice/group, one mouse was sacrificed). A pilot
experiment showed that ischemia could be released 2 weeks after
surgery. Therefore, the blood flow perfusion in those mice was
examined by laser Doppler imaging at 1, 3, 7 and 14 days after
adenovirus transduction. The speed of blood flow under the skin was
indicated as perfusion unit in different colors: Red indicated the
most rapid blood flow, dark blue indicated the slowest blood flow,
and yellow indicated moderate speed of blood flow (21,22).
To eliminate the influence of environmental factors and individual
variations, the ratio of blood flow in the ischemic limb and
control limb in each mouse was used to analyze the data. On day 14,
all mice were anesthetized by 3-4% isoflurane in air and sacrificed
by cervical dislocation. The transfection efficiency of Ad
particles was evaluated at x40 magnification under a fluorescence
microscope by observing GFP expression in the frozen slices of
skeletal muscle tissue (23).
Semi-quantitative and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol®
(cat. no. 15596026, Thermo Fisher Scientific, Inc.). In total, 1 µg
RNA was mixed with 1 µl Oligo dT Primer (50 µM), 1 µl dNTP Mixture
(10 mM each) and RNase-free dH2O (fill up to 10 µl
volume) and incubated for 5 min at 65˚C, before cooling immediately
on ice. This 10 µl mixture was added with 4 µl 5X PrimeScript™
Buffer, 0.5 µl RNase Inhibitor (40 U/µl), 1 µl PrimeScript™ RTase
(200 U/µl) and RNase Free dH2O (fill up to 20 µl) and
incubated at 42˚C for 1 h. Inactivation of reverse transcription
was performed by incubating at 95˚C for 5 min and then cooled on
ice (PrimeScript™ 1st strand cDNA Synthesis Kit; Takara
Biotechnology Co., Ltd.).
The expression of VEGFR1 and VEGFR2 in C2C12
myotubes was evaluated by semi-quantitative PCR. The PCR mix
contained 25 µl 2X Power Taq PCR MasterMix (BioTeke Corporation), 1
µl cDNA, 1 µl forward primer (10 µM), 1 µl reverse primer (10 µM)
and 22 µl nuclease-free H2O. PCR reaction was performed
using the following thermocycling conditions: 95˚C for 120 sec,
followed by 35 cycles of 95˚C for 30 sec, 60˚C for 30 sec and 72˚C
for 30 sec, 72˚C for 2 min. The following primers were used: Mouse
VEGFR1 (forward, 5'-AAAGCGCAGCCTACCTCACC-3' and reverse,
5'-AGGAGCCAAAAGAGGGTCGC-3'), mouse VEGFR2 (forward,
5'-GGTGCCTTCGGCCAAGTGAT-3' and reverse, 5'-CGATGCTCGCTGTGTGTTGC-3')
and mouse β-actin (forward, 5'-CCCAGCACAATGAAGATCAAGATCAT-3' and
reverse, 5'-ATCTGCTGGAAGGTGGACAGCGA-3'). Subsequently, 1% agarose
gel with ethidium bromide was used for electrophoresis.
After the formation of myotubes in C2C12 cells, 5,
10, 20 ng/ml rmVEGF was added into cells incubated at 37˚C for
various durations (6, 12 and 24 h). Control cells were treated with
20 ng/ml BSA. qPCR was performed with TB Green Premix Ex Taq (Tli
RNaseH Plus; Takara Biotechnology Co., Ltd.) using the following
thermocycling conditions: 95˚C for 30 sec, 40 cycles of 95˚C for 5
sec and 60˚C for 20 sec. The gene expression levels were quantified
as a fold change against GAPDH using the 2-ΔΔCq method
(24). The following primers were
used: mouse MHCIIa (forward, 5'-CGCAGAATCGCAAGTCAATA-3' and
reverse, 5'-ATATCTTCTGCCCTGCACCA-3'), mouse GAPDH (forward,
5'-CGTGTTCCTACCCCCAATGT-3' and reverse,
5'-TGTCATCATACTTGGCAGGTTTCT-3').
HUVEC migration assay
A total of 200 µl HUVECs (2x105 cells/ml
in the aforementioned DMEM) were seeded into the upper Transwell
chambers in triplicate (24-well plate with 8.0-µm pore insert, cat.
no. 3422, Corning, Inc.). The lower chamber was added 800 µl DMEM
differentiation medium containing either 3x105 cells/ml
untreated C2C12 myotubes, or 3x105 cells/ml VEGF-treated
C2C12 myotubes (20 ng/ml rmVEGF-treated for 12 h at 37˚C, but no
VEGF in Transwell afterwards), or 20 ng/ml rmVEGF for 24 h at 37˚C.
The cells in the lower chamber were fixed with 100% methanol at RT
for 20 min and stained with 0.1% crystal violet solution at room
temperature for 20 min (Beijing Solarbio Science & Technology
Co., Ltd.) (25). A total of eight
fields of view in each chamber were captured at x100 magnification
using a confocal microscope (TCS SP8; Leica Microsystems GmbH).
Tubule formation of HUVECs
A total of 800 µl HUVECs (2x105 cells/ml
in the aforementioned DMEM) were seeded into the lower Transwell
chamber in triplicate (24-well plate with 8.0-µm pore insert; cat.
no. 3422; Corning, Inc.). The upper chamber was added 200 µl DMEM
differentiation medium containing either 3x105 cells/ml
untreated C2C12 myotubes, or 3x105 cells/ml VEGF-treated
C2C12 myotubes (20 ng/ml rmVEGF-treated for 12 h at 37˚C, but no
VEGF in Transwell afterwards), or 20 ng/ml rmVEGF. After 24 h
culture at 37˚C, nine fields of view in each chamber were captured
at x100 magnification under an inverted phase contrast light
microscope (TH4-200; Olympus Corporation). HUVEC tube length was
analyzed using ImageJ software (version 1.52a) (26).
Glucose uptake assay
In total, 2x105 cells/ml C2C12 cells were
induced to differentiate into myotubes in a six-well plate in DMEM
differentiation medium and then starved in serum-free DMEM for 6 h
at 37˚C. Cells were then treated with either 20 ng/ml BSA
(control), or 100 nmol/l insulin (Sigma-Aldrich; Merck KGaA) or 20
ng/ml rmVEGF at 37˚C for 12 h. The level of glucose in the
supernatant was assayed according to manufacturer's instructions
(cat. no. 09000240660; Shanghai Mingdian Biotechnology Co.,
Ltd.).
Statistical analysis
Statistical analyses were performed with GraphPad
Prism software (version 8.3.0; GraphPad Software, Inc.). Data are
presented as the mean ± standard deviation and were analyzed with
unpaired Student's t-test (two-tailed) or one-way analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Voluntary exercise induces VEGF
expression and skeletal fiber type switch
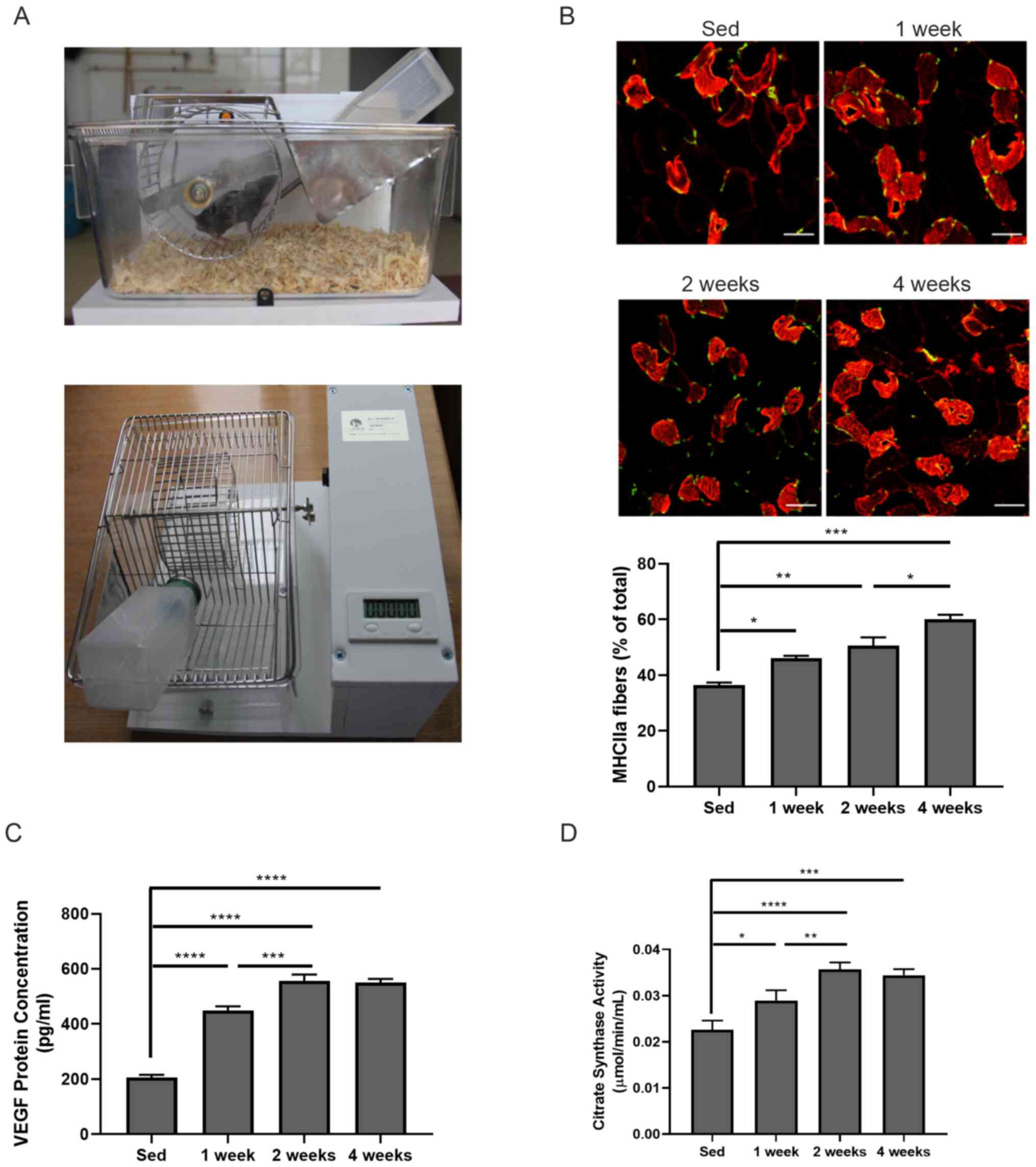
Mouse housing cages were first set up equipped with
a voluntary running wheel (Fig.
1A). After 1, 2 and 4 weeks of exercise, mouse gastrocnemius
muscle tissues were collected to investigate whether there were any
changes in the muscle fiber types in those mice. Immunofluorescence
staining found that the frequency of oxidized muscle fibers (red,
MHCIIa-positive) was significantly increased after 1-week
(46.050±0.919 vs. 36.400±0.990; P<0.05), 2-week (50.600±2.970
vs. 36.400±0.990; P<0.01), 4-week (60.050±1.626 vs.
36.400±0.990; P<0.001) exercise compared with that in sedentary
mice (Fig. 1B). Significantly
increased oxidized muscle fibers were also observed between 2- and
4-week exercise (50.600±2.970 vs. 60.050±1.626; P<0.05; Fig. 1B). The present study proceeded to
measure VEGF levels in gastrocnemius muscle by ELISA. It was found
that VEGF expression in skeletal muscle samples was significantly
increased compared with the sed group even after 1 week of exercise
(205.962±9.712 vs. 449.164±15.280 pg/ml; P<0.0001) and further
increased with longer exercise duration (556.818±22.659 and
549.366±14.410 pg/ml after 2 or 4 weeks of exercise, respectively;
both P<0.0001 vs. sed group; Fig.
1C). Moreover, the enzyme activity of citric acid synthase in
skeletal muscles was also enhanced compared with that in the sed
group after 1 week of exercise (0.029±0.002 vs. 0.023±0.002 pg/ml;
P<0.05) and further increased after 2 week (0.036±0.001 vs.
0.023±0.002 pg/ml; P<0.0001) and 4 week of exercise (0.034±0.001
vs. 0.023±0.002 pg/ml; P<0.001). The enhanced citric acid
synthase activity was also observed between 1 and 2 weeks exercise
(0.029±0.002 vs. 0.036±0.001; P<0.01; Fig. 1D). These data suggested that
skeletal fiber type switch might be involved in VEGF-induced
angiogenesis.
VEGF alone is sufficient to induce
skeletal fiber type switch
The aforementioned results indicated that voluntary
exercise induced VEGF expression and fiber type switch in the
skeletal muscle. Subsequent experiments aimed to confirm whether
VEGF or other factors were responsible for the skeletal fiber type
switch. C57BL/6 mice were fed with high fat diet and then treated
with 100 mg/kg STZ to induce diabetes. Femoral artery ligation
surgery in the left lower limb was performed in diabetic mice
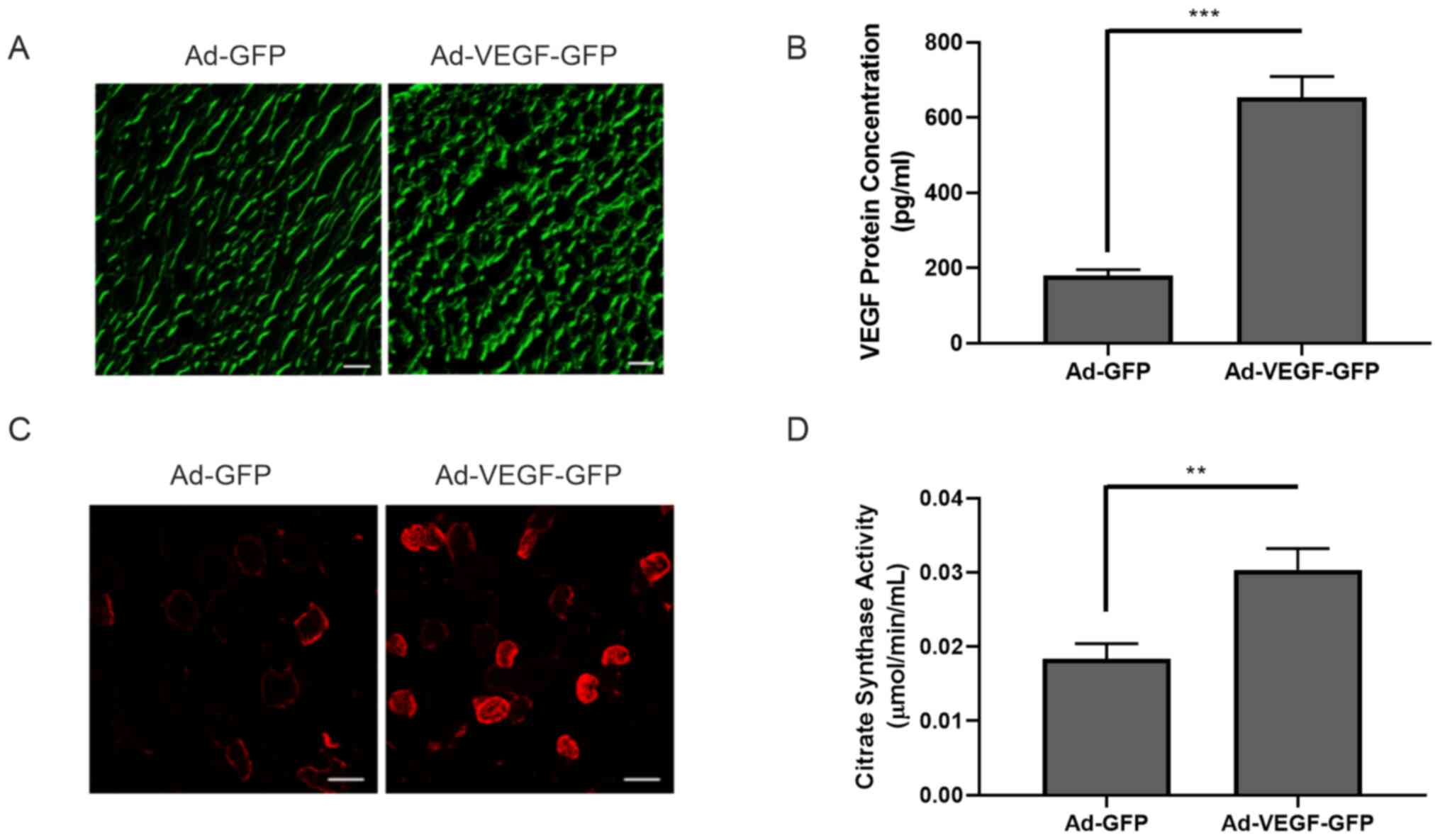
(Fig. S1). Ad-GFP or Ad-VEGF-GFP
particles were injected into the gastrocnemius muscle. VEGF
overexpression was examined by GFP expression and further confirmed
using ELISA (180.339±15.000 vs. 653.373±55.348 pg/ml; P<0.001)
(Fig. 2A and B). At 14 days after adenovirus
intramuscular injection, the content of oxidized muscle fibers
(red, MHCIIa) markedly increased following VEGF overexpression
(Fig. 2C). Similarly, the activity
of citric acid synthase in skeletal muscle was also significantly
enhanced (0.018±0.002 vs. 0.030±0.003 µmol/min/ml; P<0.01;
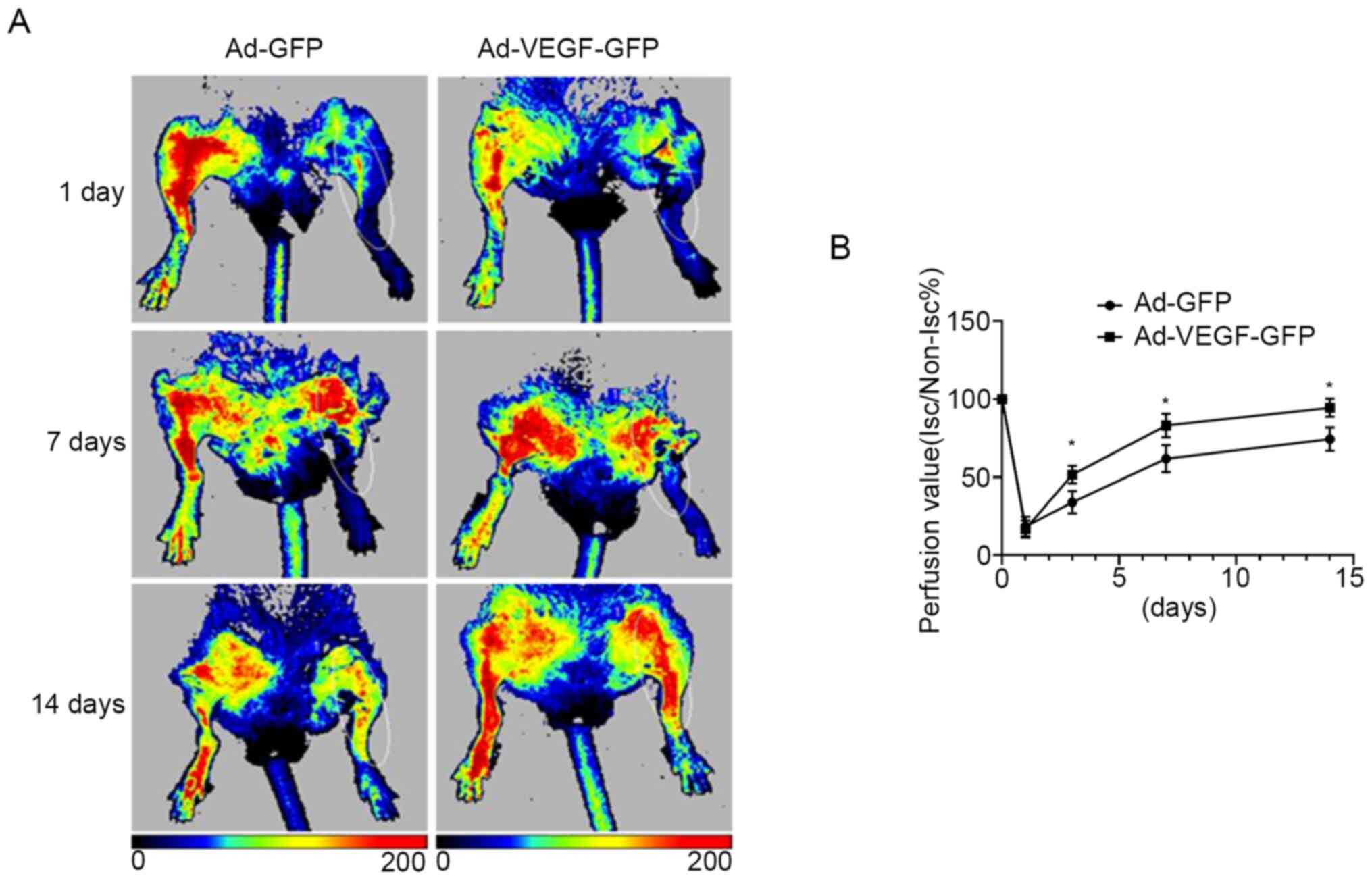
Fig. 2D). Of note, blood perfusion
in the ischemic limb was significantly improved in the Ad-VEGF-GFP
group compared with that in the Ad-GFP mice at days 3, 7 and 14
(all P<0.05), suggesting that VEGF alone was sufficient to
induce muscle fiber type switch and improved ischemia in a diabetic
murine model of hind limb ischemia (Fig. 3A and B).
VEGF-induced oxidized muscle fibers
promote the migration and tubule formation of HUVECs
The present study has showed that VEGF improved
blood perfusion and induced muscle fiber type switch. Subsequently,
whether the changes of muscle fiber types could influence
angiogenesis was assessed using C2C12 myoblasts and HUVECs. C2C12
myoblasts were cultured and differentiated into myotubes, which was
validated by the changes in morphology, which changed from round
shape to elongated tubule shape (Fig.
S2A-D) and the expression of MHC (Fig. S2E). The expression of VEGFR1 in
C2C12 myotubes was confirmed by semi-quantitative PCR (Fig. S3A). Different concentrations (5, 10
and 20 ng/ml) of the recombinant murine VEGF proteins (rmVEGF) were
added into C2C12 myotubes for various durations to screen for the
optimal treatment protocol. RT-qPCR showed treatment of 20 ng/ml
VEGF for 12 h had the greatest effect on MHCIIa expression
(Fig. S3B), which was used in
subsequent experiments. The expression of oxidized muscle fibers
(MHCIIa, red) in C2C12 myotubes also increased after treating with
20 ng/ml VEGF for 12 h, but not with 20 ng/ml BSA (Fig. S3C).
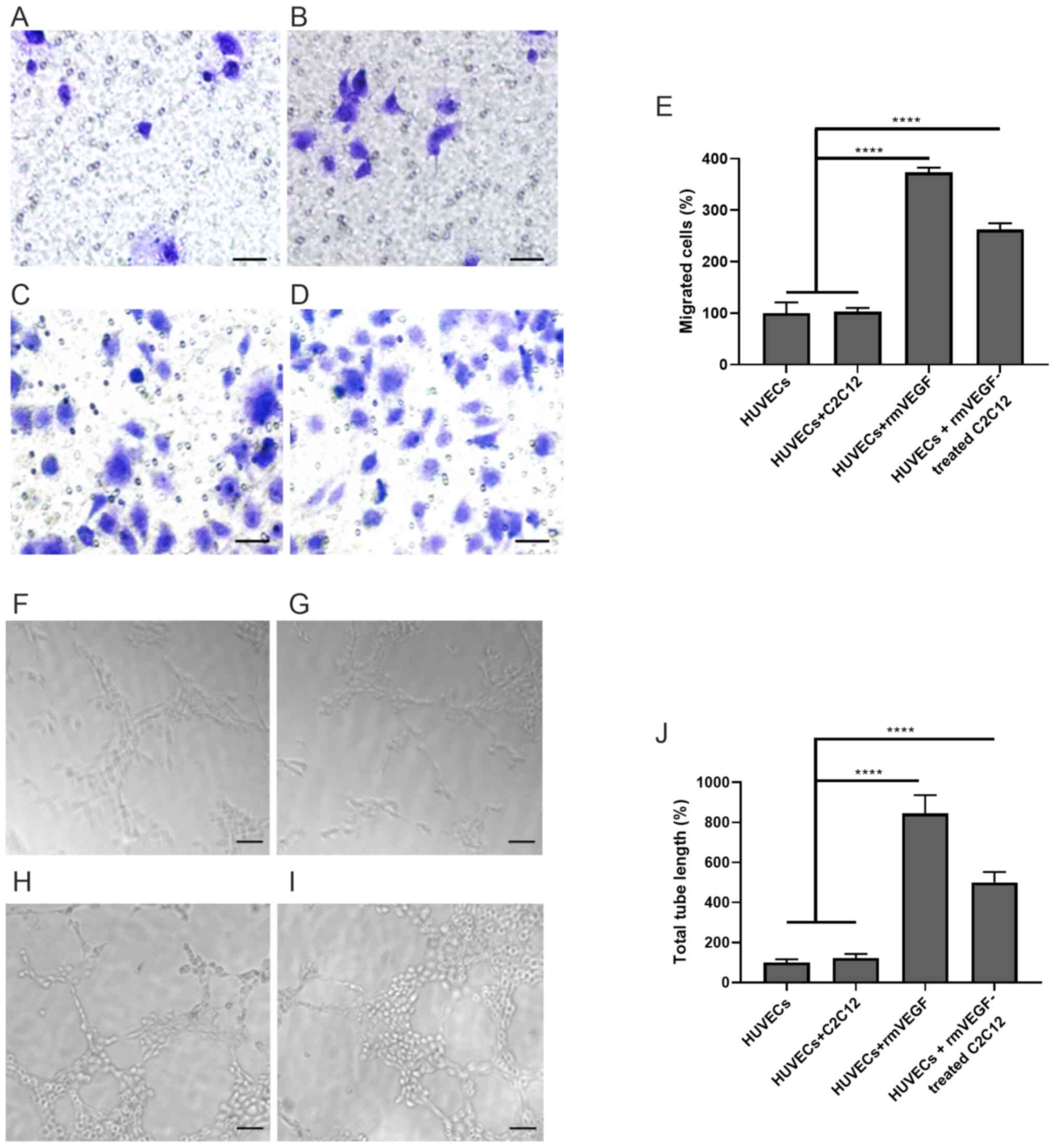
HUVECs were cultured alone, with C2C12 myotubes,
VEGF (positive control) or VEGF-treated C2C12 myotubes in Transwell
chambers. The migrated HUVECs were then quantified and normalized
(HUVECs, 100±20.84%; HUVECs + C2C12, 103.1±7.07%; HUVECs + rmVEGF,
373.5±9.184%; HUVECs + rmVEGF-treated C2C12, 262.2±12.37%).
Treatment of VEGF significantly promoted HUVEC migration
(P<0.0001, HUVECs + rmVEGF vs. HUVECs or HUVECs + C2C12;
Fig. 4A-E). Co-culturing of
VEGF-treated C2C12 myotubes also significantly enhanced HUVEC
migration (P<0.0001; HUVECs + rmVEGF-treated C2C12 vs. HUVECs or
HUVECs + C2C12; Fig. 4A-E).
Similarly, the tubule formation of HUVEVs were
quantified and normalized (HUVECs, 100±16.67%; HUVECs + C2C12,
122.2±20.97%; HUVECs + rmVEGF, 844.4±91.41%; HUVECs +
rmVEGF-treated C2C12, 500.0±52.04%). Both VEGF treatment
(P<0.0001, HUVECs + rmVEGF vs. HUVECs or HUVECs + C2C12) and
co-culture with VEGF-treated C2C12 myotubes (P<0.0001 for HUVECs
+ rmVEGF-treated C2C12 vs. HUVECs or HUVECs + C2C12 group)
significantly enhanced HUVEC tubule formation (Fig. 4F-J).
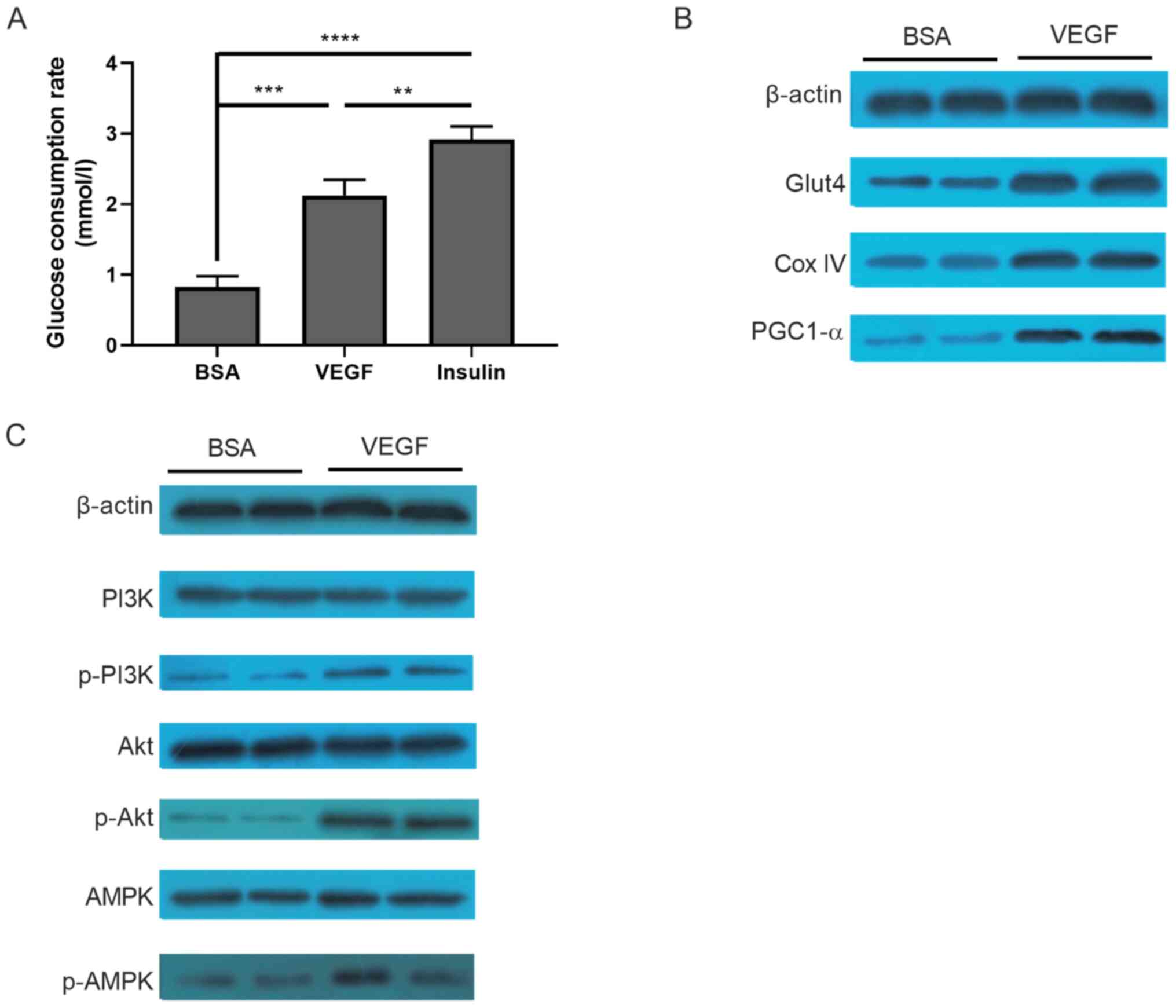
Glucose consumption and PI3K/Akt/AMPK
signaling pathways are involved in the VEGF-induced oxidization of
C2C12 myotubes
The molecular mechanism underlying VEGF-induced
oxidization of C2C12 myotubes was subsequently investigated. Since
skeletal muscle is one of the most important organs involved in
glucose consumption, it was first evaluated whether VEGF treatment
altered glucose consumption in C2C12 tubule. Compared with the
control group (BSA treated), glucose consumption in the VEGF group
was significantly increased (0.829±0.150 vs. 2.126±0.220 mmol/l;
P<0.001; Fig. 5A), which was
significantly lower compared with the positive control insulin
group (2.918±0.182 mmol/l). The expression of molecules involved in
glucose consumption, including PGC-1α, GLUT4 and COX IV, was
markedly increased following VEGF treatment (Fig. 5B). Furthermore, several pathways,
including PI3K, Akt and AMPK signaling, were activated in
VEGF-treated cells, but not in BSA-treated cells (Fig. 5C).
Discussion
The present study indicated that VEGF could induce
the switch of muscle fiber types by promoting the formation of
oxidative fibers in vivo and in vitro. Furthermore,
the present study showed that VEGF-induced oxidization of muscle
fibers improved migration and tubule formation of HUVECs. In
addition, the present study provided the molecular mechanism for
VEGF-induced oxidization of muscle fibers. These data may provide a
novel insight into VEGF-mediated therapy in DF.
Voluntary exercise in the running wheel for 1 week
was sufficient to induce the increase in the proportion of
oxidative skeletal muscle fibers and the activity of citric acid
synthase, which is consistent with a previous study showing that
exercise could promote skeletal muscle type transformation and
vascular angiogenesis (27).
However, multiple myokines, including VEGF, are also influenced by
exercise (28-30).
Therefore, in subsequent experiments, adenoviruses were injected
into the skeletal muscle to locally upregulate VEGF expression.
Ameliorated ischemia in mice with DF was observed, suggesting that
VEGF in the muscle alone is sufficient to induce angiogenesis.
Skeletal muscles consist of a variety of fiber types
with different functions. Based on the expression of the main
myosin heavy chain subtypes, rodent fibers present with I, IIa,
IId/x and IIb subtypes, while humans present with I, IIa, and IId/x
subtypes (4). According to the
oxidation capacity, rodent skeletal muscles are divided into
oxidized fibers (I and IIa) and glycolytic fibers (IIb and IId/x).
Under normal conditions, the number of oxidized and glycolytic
skeletal muscle fibers is balanced but these fiber types may be
readily transformed into each other in a number of settings. For
example, aerobic exercise can induce the transformation of
glycolytic muscle fibers into oxidized muscle fibers, characterized
by a higher mitochondrial content, which was also observed in the
present study (31-33).
VEGF deficiency in mouse skeletal muscle abrogated exercise-induced
angiogenesis and the increase in the proportion of oxidative
fibers, indicating the importance of VEGF originating from muscles
(32).
It was further determined whether the fiber type of
skeletal muscle had an effect on vascular endothelial cell
activity. Due to the lack of VEGF knockout mice, the present study
was unable to fully analyze the mechanism of VEGF-mediated muscle
fiber type switch and vascular angiogenesis in vivo.
Instead, the commonly used myoblast (C2C12 cells) and epithelial
(PUMC-HUVEC-T1 cells) cell lines were used to investigate the role
of oxidized muscle fibers on vascular angiogenesis. It was found
that VEGF-oxidized C2C12 myoblasts promoted the migration and
tubule formation of HUVECs, confirming the close association
between oxidative muscle fibers and angiogenesis (34).
There are four muscle fiber types found in mice.
Different subtypes of muscle fibers have distinct mitochondrial
characteristics (35,36). MHCIIa-positive oxidative fibers
exhibit high mitochondrial content and fusion rates (35). PGC-1α is an essential regulator in
mitochondrial biogenesis (37). In
addition, it was demonstrated that it plays an important role in
exercise-induced adaptation of skeletal muscle (31,38).
Systemic or muscle-specific knockout PGC-1α resulted in a decrease
in the ratio of oxidized muscle fibers (39). In addition, specific expression of
PGC-1α in skeletal muscle induced oxidized muscle fibers (40). To the best of our knowledge, a
limited number of studies have examined the effects of VEGF on
PGC1-α and mitochondrial biogenesis. In adipose and brain tissues,
treatment with VEGF increased PGC1-α expression and promoted
mitochondrial biogenesis (41,42).
However, VEGF facilitated the cytoplasmic localization of PGC1-α in
endothelial cells (43). The
present study observed increased expression of PGC-1α in
VEGF-treated C2C12 myoblasts, suggesting that the changes of
skeletal muscle type mediated by VEGF were possibly mediated by
PGC-1α. Meanwhile, an increased mitochondria content in oxidative
muscle fibers was also suggested by the enhanced activity of citric
acid synthase and increased COX IV expression.
Glucose is the major energy source for skeletal
muscles and skeletal muscle is the organ which consumes the largest
amount of glucose in the body (44). Several GLUTs are expressed in
muscles to facilitate the transportation of glucose into cells
(45). GLUT1 and GLUT3 are
selectively expressed in fetal and regenerating fibers (46,47).
GLUT4 is the main glucose transporter in skeletal muscle, and
highly expressed in oxidative fibers (48). It was found that oxidized muscle
fibers expressed higher levels of GLUT4, indicating the increased
glucose uptake capacity of skeletal muscle to improve the
hyperglycemic conditions. The present study observed an elevated
expression level of GLUT4 protein in C2C12 myotubes following VEGF
treatment, which might facilitate the consumption of glucose and
mitochondrial biogenesis.
Although the present study found that an increase of
VEGF in the skeletal muscle could promote angiogenesis in
vitro and alleviate lower limb ischemia in the diabetic mice,
several limitations exist. First, the present study did not use a
tissue-specific promoter in the adenovirus, which means that rather
than myofibers, other cells could have been transfected with the
VEGF gene. Although the present study performed in vitro
experiments to assess the effects of VEGF-treated myofibers on
angiogenesis, considering the complexity in vivo, further
studies, such as utilizing adenoviruses containing a skeletal
muscle specific promoter or transgenic mice with skeletal
muscle-specific VEGF overexpression, are required for further
clarification. Second, the present study used femoral artery
ligation to induce ischemia. Although it is widely used to mimic
ischemia in the lower limb of patients with DF, this acute lesion
may not fully mimic the chronic ischemia in patients. Further
studies are required to utilize a novel mouse model to better mimic
the process of PAD in diabetic patients.
In conclusion, the present study extended prior
observations of the protective effects of VEGF in DF by inducing
the conversion of skeletal muscle fiber types and supported the
potential therapeutic utility of VEGF in the future.
Supplementary Material
Generation of the ischemic model. (A)
Femoral artery ligation operation. (B) Blood flow on the 1st day
after operation.
Verification of C2C12 cells. (A) C2C12
myoblasts seeded at a low density. (B) After 3 days of stimulation
with 20 ml/l horse serum, fusion of cells and formation of myotubes
were observed. (C) On day 5, longer and larger myotubes were
observed. (D) Myotubes were stained with Giemsa on day 5. Data are
presented as the mean ± standard deviation and are representative
of two independent experiments. Scale Bar, 200 μm. (E) Anti-MHC
western blotting. MHC, myosin heavy chain.
rmVEGF could induce the oxidative
fiber switch in C2C12 myotubes. (A) Reverse transcription
semi-quantitative PCR analysis of VEGF receptor 1 and 2 mRNA
expression in C2C12 myotubes on day 5 after differentiation. (B)
Induction of MHCIIa mRNA expression after treatment with various
concentration of rmVEGF in murine C2C12 myotubes, as determined by
reverse transcription-quantitative PCR. (C) Identification of
oxidative fibers by MHCIIa immunofluorescence in C2C12 cells
treated with 20 ng/ml VEGF for 12 h. Data are presented as the mean
± standard deviation and are representative of two independent
experiments in triplicate. Scale bars, 100 μm.
*P<0.05 vs. cells treated with 20 ng/ml rmVEGF for 24
h. rh, recombinant human; conc, concentration; MHCIIa, myosin heavy
chain IIa.
Acknowledgements
Not applicable.
Funding
Funding: This work is supported by the Shenzhen Municipal
Science and Technology Innovation Committee Project (grant no.
KCXFZ202002011010445, JCYJ20160422150209240), China Postdoctoral
Science Foundation (grant no. 2014M562547), National Natural
Science Foundation of China (grant no. 81550035) and Natural
science foundation of Guangdong province (grant no.
2020A1515010085).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LK and XF discussed and designed the study, and
critically edited the manuscript. LJ and HBW conducted the
experiments and analyzed the data. PZ and HW analyzed the data and
wrote the manuscript. LJ and HBW confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of Chinese PLA General Hospital (approval no. 8157021269;
Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boulton AJ, Vileikyte L,
Ragnarson-Tennvall G and Apelqvist J: The global burden of diabetic
foot disease. Lancet. 366:1719–1724. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Johannesson A, Larsson GU, Ramstrand N,
Turkiewicz A, Wiréhn AB and Atroshi I: Incidence of lower-limb
amputation in the diabetic and nondiabetic general population: A
10-year population-based cohort study of initial unilateral and
contralateral amputations and reamputations. Diabetes Care.
32:275–280. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Prompers L, Schaper N, Apelqvist J,
Edmonds M, Jude E, Mauricio D, Uccioli L, Urbancic V, Bakker K,
Holstein P, et al: Prediction of outcome in individuals with
diabetic foot ulcers: Focus on the differences between individuals
with and without peripheral arterial disease. The EURODIALE study.
Diabetologia. 51:747–755. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Prompers L, Huijberts M, Apelqvist J, Jude
E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A,
Mauricio D, et al: High prevalence of ischaemia, infection and
serious comorbidity in patients with diabetic foot disease in
Europe. Baseline results from the Eurodiale study. Diabetologia.
50:18–25. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
American Diabetes Association. Peripheral
arterial disease in people with diabetes. Diabetes Care.
26:3333–3341. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Guo X, Shi Y, Huang X, Ye M, Xue G and
Zhang J: Features analysis of lower extremity arterial lesions in
162 diabetes patients. J Diabetes Res. 2013(781360)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Adams V and Linke A: Impact of exercise
training on cardiovascular disease and risk. Biochim Biophys Acta
Mol Basis Dis. 1865:728–734. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brevetti G, Silvestro A, Schiano V and
Chiariello M: Endothelial dysfunction and cardiovascular risk
prediction in peripheral arterial disease: Additive value of
flow-mediated dilation to ankle-brachial pressure index.
Circulation. 108:2093–2098. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Daiber A, Steven S, Weber A, Shuvaev VV,
Muzykantov VR, Laher I, Li H, Lamas S and Münzel T: Targeting
vascular (endothelial) dysfunction. Br J Pharmacol. 174:1591–1619.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Senger DR, Galli SJ, Dvorak AM, Perruzzi
CA, Harvey VS and Dvorak HF: Tumor cells secrete a vascular
permeability factor that promotes accumulation of ascites fluid.
Science. 219:983–985. 1983.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Germani A, Di Carlo A, Mangoni A, Straino
S, Giacinti C, Turrini P, Biglioli P and Capogrossi MC: Vascular
endothelial growth factor modulates skeletal myoblast function. Am
J Pathol. 163:1417–1428. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yan Z, Okutsu M, Akhtar YN and Lira VA:
Regulation of exercise-induced fiber type transformation,
mitochondrial biogenesis, and angiogenesis in skeletal muscle. J
Appl Physiol (1985). 110:264–274. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oberbach A, Bossenz Y, Lehmann S, Niebauer
J, Adams V, Paschke R, Schön MR, Blüher M and Punkt K: Altered
fiber distribution and fiber-specific glycolytic and oxidative
enzyme activity in skeletal muscle of patients with type 2
diabetes. Diabetes Care. 29:895–900. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Picard M, Hepple RT and Burelle Y:
Mitochondrial functional specialization in glycolytic and oxidative
muscle fibers: Tailoring the organelle for optimal function. Am J
Physiol Cell Physiol. 302:C629–C641. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
van Weel V, Deckers MML, Grimbergen JM,
van Leuven KJ, Lardenoye JH, Schlingemann RO, van Nieuw Amerongen
GP, van Bockel JH, van Hinsbergh VW and Quax PH: Vascular
endothelial growth factor overexpression in ischemic skeletal
muscle enhances myoglobin expression in vivo. Circ Res. 95:58–66.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McGrath JC and Lilley E: Implementing
guidelines on reporting research using animals (ARRIVE etc.): New
requirements for publication in BJP. Br J Pharmacol. 172:3189–3193.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Novak CM, Burghardt PR and Levine JA: The
use of a running wheel to measure activity in rodents: Relationship
to energy balance, general activity, and reward. Neurosci Biobehav
Rev. 36:1001–1014. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Waters RE, Rotevatn S, Li P, Annex BH and
Yan Z: Voluntary running induces fiber type-specific angiogenesis
in mouse skeletal muscle. Am J Physiol Cell Physiol.
287:C1342–C1348. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Thinakaran G, Ojala J and Bag J:
Expression of c-jun/AP-1 during myogenic differentiation in mouse
C2C12 myoblasts. FEBS Lett. 319:271–276. 1993.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jensen EC: Quantitative analysis of
histological staining and fluorescence using ImageJ. Anat Rec
(Hoboken). 296:378–381. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Padgett ME, McCord TJ, McClung JM and
Kontos CD: methods for acute and subacute murine hindlimb Ischemia.
J Vis Exp. 112(54166)2016.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Tokudome T, Kishimoto I, Yamahara K, Osaki
T, Minamino N, Horio T, Sawai K, Kawano Y, Miyazato M, Sata M, et
al: Impaired recovery of blood flow after hind-limb ischemia in
mice lacking guanylyl cyclase-A, a receptor for atrial and brain
natriuretic peptides. Arterioscler Thromb Vasc Biol. 29:1516–1521.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jockusch H and Eberhard D: Green
fluorescent protein as a tracer in chimeric tissues: The power of
vapor fixation. Methods Mol Biol. 411:145–154. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ariyanti AD, Sisjayawan J, Zhang J, Zhang
JQ, Wang GX, Miyagishi M, Wu SR and Kasim V: Elevating VEGF-A and
PDGF-BB secretion by salidroside enhances neoangiogenesis in
diabetic hind-limb ischemia. Oncotarget. 8:97187–97205.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu N, Ding D, Hao W, Yang F, Wu X, Wang
M, Xu X, Ju Z, Liu JP, Song Z, et al: hTERT promotes tumor
angiogenesis by activating VEGF via interactions with the Sp1
transcription factor. Nucleic Acids Res. 44:8693–8703.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Delavar H, Nogueira L, Wagner PD, Hogan
MC, Metzger D and Breen EC: Skeletal myofiber VEGF is essential for
the exercise training response in adult mice. Am J Physiol Regul
Integr Comp Physiol. 306:R586–R595. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Huh JY: The role of exercise-induced
myokines in regulating metabolism. Arch Pharm Res. 41:14–29.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pedersen BK and Febbraio MA: Muscles,
exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev
Endocrinol. 8:457–465. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wrann CD, White JP, Salogiannnis J,
Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME and
Spiegelman BM: Exercise induces hippocampal BDNF through a
PGC-1α/FNDC5 pathway. Cell Metab. 18:649–659. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Birot OJG, Koulmann N, Peinnequin A and
Bigard XA: Exercise-induced expression of vascular endothelial
growth factor mRNA in rat skeletal muscle is dependent on fibre
type. J Physiol. 552:213–221. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cannon DT, Rodewohl L, Adams V, Breen EC
and Bowen TS: Skeletal myofiber VEGF deficiency leads to
mitochondrial, structural, and contractile alterations in mouse
diaphragm. J Appl Physiol (1985). 127:1360–1369. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Leick L, Hellsten Y, Fentz J, Lyngby SS,
Wojtaszewski JF, Hidalgo J and Pilegaard H: PGC-1alpha mediates
exercise-induced skeletal muscle VEGF expression in mice. Am J
Physiol Endocrinol Metab. 297:E92–E103. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Matsakas A, Yadav V, Lorca S, Evans RM and
Narkar VA: Revascularization of ischemic skeletal muscle by
estrogen-related receptor-γ. Circ Res. 110:1087–1096.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mishra P, Varuzhanyan G, Pham AH and Chan
DC: Mitochondrial dynamics is a distinguishing feature of skeletal
muscle fiber types and regulates organellar compartmentalization.
Cell Metab. 22:1033–1044. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hood DA, Memme JM, Oliveira AN and Triolo
M: Maintenance of skeletal muscle mitochondria in health, exercise,
and aging. Annu Rev Physiol. 81:19–41. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fernandez-Marcos PJ and Auwerx J:
Regulation of PGC-1α, a nodal regulator of mitochondrial
biogenesis. Am J Clin Nutr. 93 (Suppl):884S–890S. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chinsomboon J, Ruas J, Gupta RK, Thom R,
Shoag J, Rowe GC, Sawada N, Raghuram S and Arany Z: The
transcriptional coactivator PGC-1alpha mediates exercise-induced
angiogenesis in skeletal muscle. Proc Natl Acad Sci USA.
106:21401–21406. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Handschin C, Chin S, Li P, Liu F,
Maratos-Flier E, Lebrasseur NK, Yan Z and Spiegelman BM: Skeletal
muscle fiber-type switching, exercise intolerance, and myopathy in
PGC-1alpha muscle-specific knock-out animals. J Biol Chem.
282:30014–30021. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss
O, Michael LF, Puigserver P, Isotani E, Olson EN, et al:
Transcriptional co-activator PGC-1 alpha drives the formation of
slow-twitch muscle fibres. Nature. 418:797–801. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao Y, Li X, Yang L, Eckel-Mahan K, Tong
Q, Gu X, Kolonin MG and Sun K: Transient overexpression of vascular
endothelial growth factor a in adipose tissue promotes energy
expenditure via activation of the sympathetic nervous system. Mol
Cell Biol. 38:e00242–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu X, Chu B, Jin S, Li M, Xu Y, Yang H,
Feng Z, Bi J and Wang P: Vascular endothelial growth factor
alleviates mitochondrial dysfunction and suppression of
mitochondrial biogenesis in models of Alzheimer's disease. Int J
Neurosci. 131:154–162. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wright GL, Maroulakou IG, Eldridge J, Liby
TL, Sridharan V, Tsichlis PN and Muise-Helmericks RC: VEGF
stimulation of mitochondrial biogenesis: Requirement of AKT3
kinase. FASEB J. 22:3264–3275. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mészáros K, Lang CH, Bagby GJ and Spitzer
JJ: Contribution of different organs to increased glucose
consumption after endotoxin administration. J Biol Chem.
262:10965–10970. 1987.PubMed/NCBI
|
|
45
|
Evans PL, McMillin SL, Weyrauch LA and
Witczak CA: Regulation of skeletal muscle glucose transport and
glucose metabolism by exercise training. Nutrients.
11(2432)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gaster M, Beck-Nielsen H and Schroder H:
Regenerating human muscle fibres express GLUT3 protein. Pflügers
Arch. 445:105–114. 2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gaster M, Franch J, Staehr P, Beck-Nielsen
H, Smith T and Schroder H: Induction of GLUT-1 protein in adult
human skeletal muscle fibers. Am J Physiol Endocrinol Metab.
279:E1191–E1195. 2000.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Goodyear LJ, Hirshman MF, Smith RJ and
Horton ES: Glucose transporter number, activity, and isoform
content in plasma membranes of red and white skeletal muscle. Am J
Physiol Endocrinol Metab. 261:E556–E561. 1991.PubMed/NCBI View Article : Google Scholar
|