Introduction
Infectious disease occurring during surgery is a
serious healthcare problem; in orthopedics, as well as other
surgical specialties, a wound infection causes the healing process
to be delayed, as well as an increase in the clinical burden faced
by the patient. Patients in these cases report greater suffering
than patients that were cured without infection while having the
same pathology (1). Accurate and
prompt diagnosis, as well as proper care, is critical, because
infection of a surgical wound, particularly in the case of
arthroplasties or the use of orthopedic implants, can have a
catastrophic effect in most cases, necessitating the removal of
foreign material (2,3).
According to the literature, an exponential rise in
arthroplasty procedures for maintaining or improving mobility have
been accompanied with an increase in life expectancy (4). Owing to an increase in the amount of
traffic accidents and sports injuries, the use of joint
replacements and orthopedic implants is growing (5). Biocompatible prosthetics are used in
the treatment of degenerative pathologies, as well as for cosmetic
purposes (6,7).
When a foreign body is present, the risk of
infection is substantially higher; nearly half of all nosocomial
infections are linked to medical equipment in some way (8,9).
Infection can originate from an infected implant, as well as
disinfection and sterilization errors. For these reasons, any
infectious outbreaks must be investigated, located and treated in
the case of suspicion of postoperative septic evolution (10). Orthopedic infections are more common
in patients with dental abscesses, skin or digestive infections
(11). Staphylococcus aureus
(S. aureus), coagulase-negative staphylococci,
Escherichia coli (E. coli), Enterobacter spp.,
Klebsiella pneumonie (K. pneumonie). and
Pseudomonas aeruginosa are among the bacteria commonly
present; S. aureus is the most frequently detected of these
(11-13).
Factors that can reduce the risk of infection include the
introduction of flow restriction in the operation room, highly
qualified surgeons with reduced operation time, the application of
prophylactic antibiotics and, when applicable, the use of
antibiotic-charged implants (14,15).
A technique that has found a place in the
ever-changing context of technological evolution is
microcalorimetry. This technique, widely employed in inorganic
chemistry, is only starting to find more widespread use in
medicine. The basis of microcalorimetry is the capacity of bacteria
to produce heat from metabolic activity; a microcalorimeter records
the energy released due to different biochemical reactions, which
can be used to generate growth curves for clinical evaluation
(16-20).
Infectious pathology in the field of orthopedics and
traumatology occurs less frequently than in other medical or
medicosurgical specialties (21);
however, musculoskeletal infections can lead to negative outcomes,
and are relatively difficult to identify and treat. Making a
positive diagnosis and evaluating the options based on the
diagnosis, as well as treating the pathology as soon as possible,
are critical.
An unidentified infection can impair the functioning
of a limb or, if it leads to sepsis, can cause life-threatening
circumstances (22,23). Osteomyelitis is a disease that can
affect individuals of all ages and involve any part of the skeletal
system; it can and should be categorized based on the type of host
response, the length of the infection or the age of the infected
individual in order to select the most optimal therapeutic approach
and determine the prognosis (24).
Concerning onset time, osteomyelitis may be categorized as acute if
it occurs within the first 6 weeks of infection, or chronic if the
onset occurs after 6 weeks. At this stage, localized infection can
occur in one of two ways (23,24).
When a microorganism penetrates from the outside into the bone, it
is known as direct inoculation (following trauma, during surgery or
when a neglected septic process develops in the vicinity of the
bone); alternatively, remote inoculation results from a distant
outbreak area that involves the bone via hematogenous dissemination
of the pathogen (24,25). These cases are frequently
plurimicrobial infections, which affect easily identifiable
anatomical areas and, in the case of trauma, are followed by a
major, quantitative contamination of the wound (23,25).
Infection involves a compromised local environment
with devitalized tissues and necrosis, both of which are conducive
to the development of septic processes due to suboptimal
vascularization that prevents or impairs penetration of
antimicrobial substances (22,24,25).
Following the development of a distant septic process, hematogenous
dissemination of microbial agents occurs, resulting in bacteremia.
During bacteremia, infections of the synovial fluid, cartilage or
bones are possible. Microorganisms are typically restricted to
sites of damage, such as a fracture treated with osteosynthesis or
a joint prosthesis. Diabetes or smoking, which affect the
structures of small vessels, as well as liver failure, kidney
failure and conditions involving immunodeficiency, are all
host-related systemic factors that may promote the spread of
infection (22,24). In these cases, the patient's age is
critical, as infections resulting from this process are heavily
affected by local anatomy and physiology (23,25,26).
To understand the basics, it is necessary to discuss
other technical concepts. Preliminary principles of thermodynamics
discovered by Thomas Johann Seebeck (late 18th century) are often
referred to as the Seebeck effect. Different materials with the
same mass may require different levels of heat to raise their
temperature by 1˚C (27,28). The Seebeck effect (direct
thermoelectric effect) describes the generation of
thermoelectromechanical tensions in a circuit, which are converted
into a temperature difference between two materials with different
structures (27,28).
Peltier's effect is the second physical fundamental;
it should be considered an inversion of the previously discussed
effect, as it describes a process in which a potential difference
is converted into a temperature difference (27,28).
This effect, which can be seen in thermoelectric heating and
cooling systems, was discovered in 1834 by French physicist Jean
Charles Athanase Peltier, who demonstrated through experiments that
if an electric current passes through a junction of two different
metals, it can register losses or increases in temperature at that
zone. The value of absorbed warmth is equal to the value of emitted
warmth, based on Peltier's coefficient (27,28).
Modern microcalorimetry dates back to the 19th
century, when physicist Albert Tian built a calorimeter (depth, 7
m) in order to maintain stable temperatures. Peltier and Seebeck
effects are at the heart of microcalorimeter activity, both in the
past and in the present, resulting in two of the most critical
components: The Seebeck effect, which is used to create a
temperature sensor that can convert a temperature difference into
an electric signal; by means of a Peltier-type cell, the applied
electrical energy is transformed in absorbed or released heat
(28).
Tian succeeded in studying insect metabolism using a
microcalorimeter and the Peltier effect in 1922, and Edouard
Calvet, his successor, succeeded in transforming the
microcalorimeter into a laboratory apparatus in 1948 by developing
two twin calorimetric components: The reference and the sample
(28,29). The first microcalorimeter was sold
in 1970(29), and it has evolved
since then due to technological advancements. Microcalorimeters
have been developed with increasing sensitivity, the ability to
acquire a greater quantity of data due to the availability of
multiple channels and computerized technology that can process a
wide variety of data in a short amount of time (30,31).
The present study aimed to demonstrate that this tool could be used
to diagnose infectious pathologies in the field of orthopedics by
evaluating different bacterial strains using the method.
Materials and methods
Theoretical considerations Thermal
analysis
Microcalorimetry is a science that uses a
calorimeter to measure the heat generated by different processes.
This process is called heat flux, and it is based on the physical
principle that when two bodies with different temperatures are
brought together, they will adjust their temperatures until they
reach a thermal equilibrium (32).
To record this occurrence, calorimeters will measure temperatures
and collect data in an indirect manner, as thermal changes will be
converted into electric signals, which will be recorded and
processed through an informatics program that will allow data
interpretation (32).
In order to conduct a thermal analysis, some
temperature changes must occur, which must be recorded; in the
present study, a differential measurement method has been used. For
these experiments, changes in temperature that occur in one probe
compared to a reference when the temperature is changed are used;
for an experiment to be successful, one property must be different
between the two cells (which house the probe and the reference) in
order to properly quantify and analyze the results. It should be
noted that this property can be changed in subsequent experiments
to evaluate various changes. The calorimeter has two rooms that are
equipped with temperature sensors; following the introduction of
the two cells (sample and reference), they regulate the flow of
heat between the two cells, as well as between cells and structures
in the body.
When it comes to twin cells (which, as previously
stated, have similar properties), the amount of energy transferred
into the cell has no effect; only the difference between the two is
recorded, which is known as differential type microcalorimetry
(33,34).
Role of microcalorimetry in
bacteriological research
Numerous studies have been conducted using
microcalorimetry, investigating bacterial metabolism and how
bacteria react to changes in the culture environment, including the
levels of nutritive substances, temperature or the amount of oxygen
in the microcalorimetric cells (31,34).
All of these variables are necessary and must be factored into the
equation, as changes in their order can have a substantial impact
on calorimetric detection and the rate of bacterial growth. The
majority of studies are conducted in closed systems, which contain
a substance in a finite quantity, allowing bacterial culture to
represent a finite growth cycle that can be quantified.
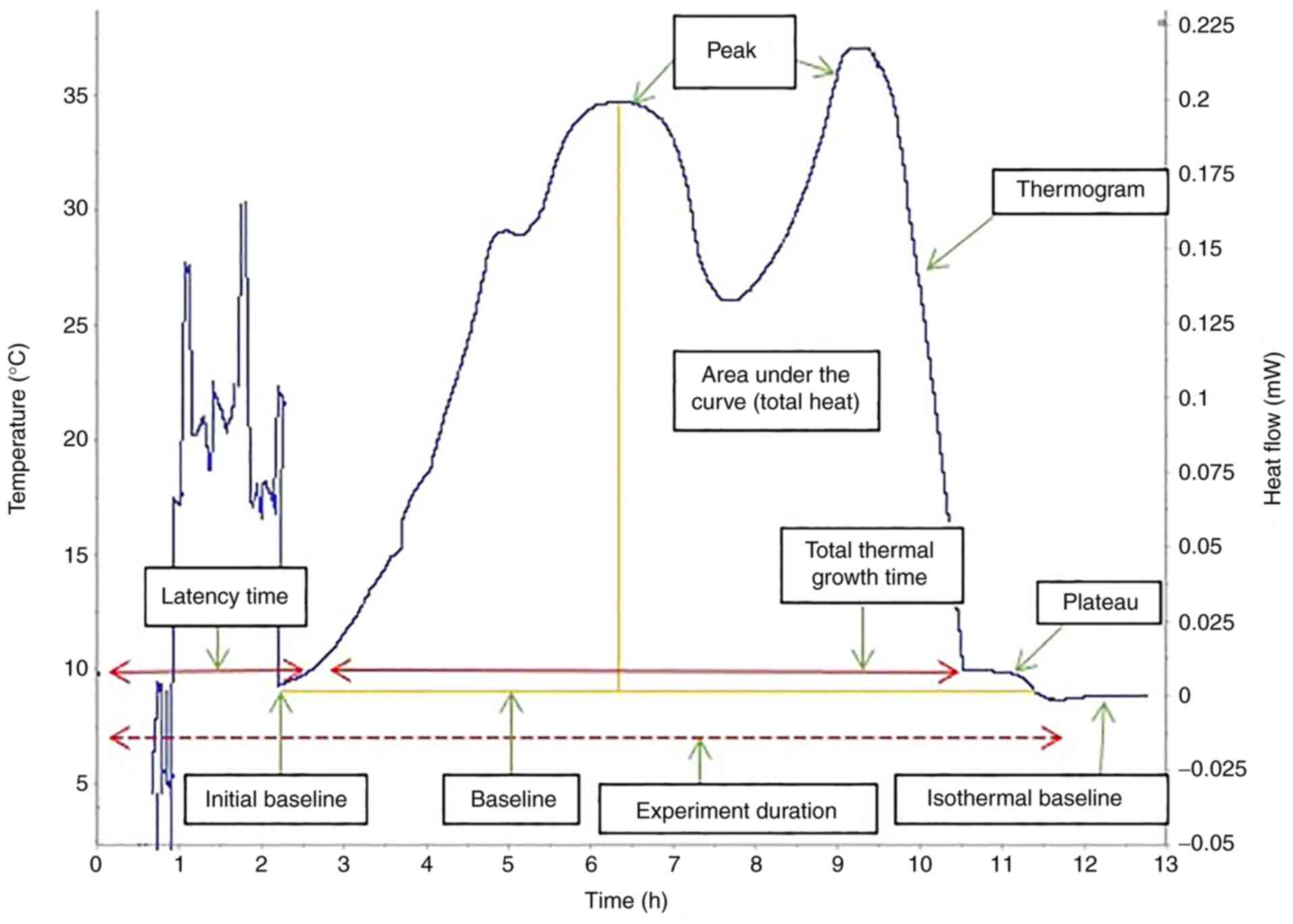
A bacterial culture has four stages of growth
(Fig. 1) (35,36):
i) Lag phase/latency phase, during which the bacteria adapt to the
environment in which they were inoculated, with protein synthesis
and low-intensity metabolic reactions, but no division; ii)
exponential growth phase, during which bacteria grow as long as
environmental conditions are favorable (the bacterial growth rate
can be calculated using an exponential function with base 2, X =
X02n, as they multiply via binary division;
this evolution can also be expressed as a function of time, as the
generation time is represented by the interval between two
successive multiplications); iii) stationary phase, during which
the rate of bacterial generation becomes equal to that of bacterial
death due to the increase in the quantity of inhibitors and
decrease in substances necessary for growth; and iv) decline phase,
at the end of which most bacteria are destroyed as a result of the
conditions in the stationary phase, leaving a low number of
bacteria in a non-multiplicative state that can resume the cycle if
they are offered favorable environment (37).
Demonstrating the variability and
reproducibility of the process using American type culture
collection (ATCC) reference strains
To conduct superimposable experiments, the phases of
experimental planning must be conducted in the same manner with
each experimental repeat. Preparing a liquid culture of the
infectious agent to be studied is the first step in conducting an
experiment. As the amount of non-viable bacteria accumulates in the
investigated environment over time, an elevated artificial
nephelometric index may be misleading; thus, cultures must be
freshly prepared for each experiment, as the bacteria used have the
capacity to grow at room temperature (13,19,21,32).
Therefore, the second important step is to prepare the samples and
insert them into the microcalorimeter as soon as possible. When an
experiment is performed after a long period of time has elapsed
since the sample was prepared, the initial growth stage may be lost
from the recording timeline.
Another critical consideration when using a
microcalorimeter is maintaining a stable ambient temperature, as
changes in the room can create artifacts in the bacterial growth
curve. Considering the above, it may be assumed that establishing a
working procedure and standard rules of conduct for
microcalorimetry is crucial for efficient experiments.
The following microorganisms were used for the
experiments intended to establish reproducibility: E. coli
(ATCC 25922) and K. pneumonie (ATCC 700603), which were
accessed with the goodwill of the medical staff at the Cantacuzino
National Institute of Research and Development for Microbiology and
Immunology. The bacteria were grown on trypticase soy agar
[TSB; mixture of pancreatic digest of casein (17 g), NaCl (5 g),
papaic digest of soybean meal (3 g), K2HPO4)
(2.5 g) and dextrose (1.8 g), diluted to 1 liter and pH 7.3±0.2 at
25˚C] and Sabouraud dextrose agar [SDA; mixture of mycological
peptone (10 g), dextrose (40 g) and agar (15 g), diluted to 1 liter
and pH 5.6 at 25˚C], which were composed in-house.
Procedure
Experiments were conducted as follows. First, 3,000
µl of sterile medium (SDA or TSB, depending on the experiment) was
added to a nephelometric tube, and the McFarland index was measured
using a nephelometer. Then, 15 µl pathological produce was
dispersed in 300 µl medium, ensuring that the microorganisms were
homogeneously dispersed. Then, 2 µl solution was repeatedly
pipetted in the nephelometric tube until the McFarland index rose
by 1.0. Sample cells were filled with 600 µl inoculated medium at
room temperature and hermetically sealed using a silicon o-ring. A
batch cell containing 600 µl sterile TSB or SDA was used as the
reference for differential scanning microcalorimetry. Both cells
were sealed and then inserted into the microcalorimeter.
After both the sample and the reference cell were
inserted, the acquisition program was initiated and set to maintain
a temperature of 37˚C. The recording was terminated and analyzed
after the curve reached the isoelectric line for 2-3 h, which is
the point where the bacterial culture no longer produced energy.
Only experiments conducted at 37˚C with a loading volume of 600 µl
were included in the present study.
The tracking of bacterial growth curves and the
primary processing of data obtained by the microcalorimeter was
performed using Calisto (v1.493; Setaram; KEP Technologies), and R
(v3.5.0) (38) and RStudio server
packages (v1.1.456) (39) were used
for the processing, comparison and statistical analysis of
data.
Results
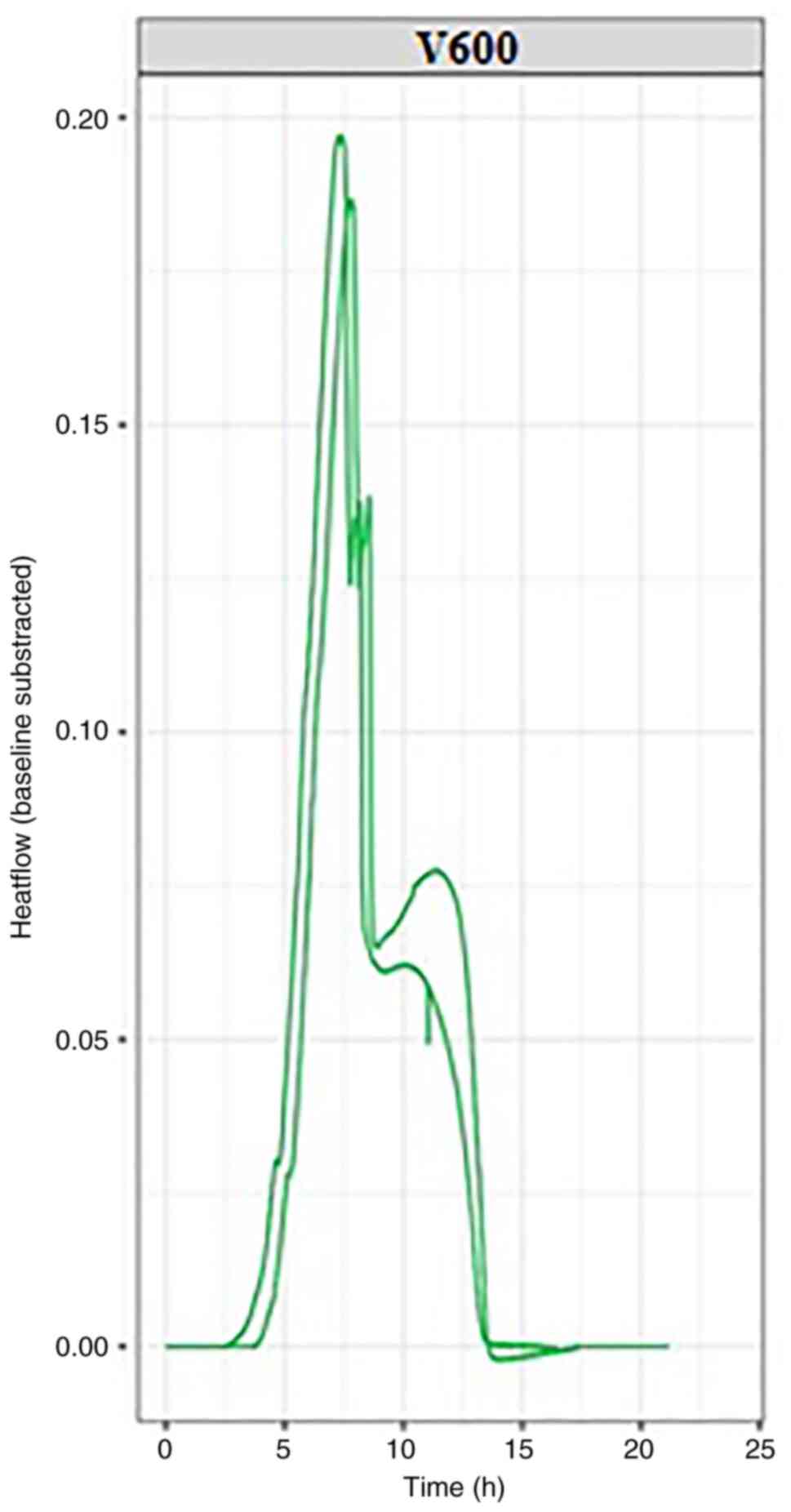
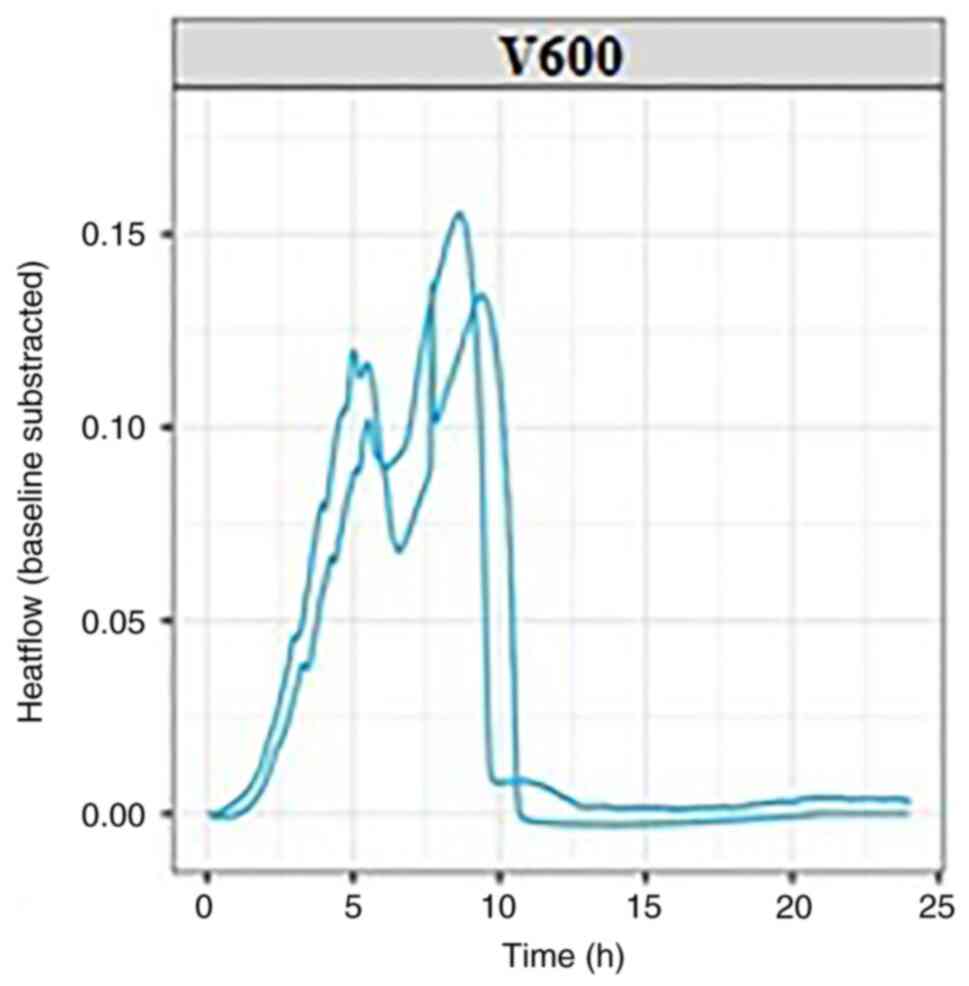
In the present study, two separate experiments were
conducted with each bacterial strain, K. pneumonie (Fig. 2) and E. coli (Fig. 3); each experiment was conducted at
37˚C, the normal temperature at which the bacteria develop. The
thermograms can be evaluated based on key points determined by a
set of quantitative parameters, including thermal signal detection,
establishment of the exponential growth, the peak maximum and the
return to baseline (Fig. 1).
Measurements determined based on these points (including the
corresponding positions of, or intervals between events on the time
scale, as well as heat flow values) can be used to characterize raw
bacterial growth thermograms, as well as to differentiate the
microorganisms.
By evaluating the thermograms for both E.
coli and K. pneumonie, similarities were observed
for both experimental repeats for each bacterial strain, including
in the time required for bacterial growth and the heat flow
generated by their growth. The bacterial growth and imprint unique
to each strain used in the experiment can be followed in the
graphs, which can be viewed in real-time. For each bacterial
strain, the growth curves reached the isoelectric line within 10-15
h from the start of the experiment.
Discussion
Bacterial microcalorimetry has a variety of
benefits, and should be regarded as a means of rapid and accurate
diagnosis. Sensitivity is a valuable attribute; when only a few
microorganisms are present, microcalorimetric signs of bacterial
multiplication can be observed (40,41).
The generated curve has features that help identify the pathogen
involved, in addition to demonstrating the presence of
microorganisms. It is worth noting that this approach provides
real-time data, the progression of bacterial growth can be tracked
at any time using standard laboratory equipment or portable
devices. In addition to the efficiency with which data can be
processed, a microcalorimetric experiment allows researchers to
evaluate a greater number of variables (temperature, culture
medium, recording method) that correspond with knowledge already
available in the literature, providing greater options for
diagnosis and targeted care (33,34,40).
Any approach that promotes targeted treatment should be sponsored
and developed in a world where the threat of increasing
microorganism resistance to antibiotics and chemotherapeutics is
widely recognized. Within the context of orthopedics, the chronic
pain caused by a pathology, whether surgically treated or not, will
lower patient satisfaction, regardless of whether or not
comorbidities are present. Inadequate or painful mobility can lead
to depression and exacerbation of other pathologies, so effective
treatment of infection should be considered in any situation
(10,24,42,43).
The microcalorimetric method of identifying bacteria
based on their growth curves is still in progress, with no
substantial body of research at present. The probability of
widespread use should be considered, but research this stage will
require extensive research, involving processing with specialized
software in order to develop of a widely applicable algorithm. A
thorough investigation (including kinetic analysis) of a
reproducible thermal signal of bacterial growth could lead to the
creation of new methods for quickly identifying bacteria. Our
research is limited to a small field within infectious medical
pathology, but it is hypothesized that microcalorimetry has the
potential to be applied in a wide range of medical fields.
In the graphs presented in this article, two growth
curves can be observed for each pathogen. Within these growth
curves, the growth pattern is identical but minimal changes in the
amount of energy released can be observed. These differences occur
as experiments are performed successively without using the same
culture medium prepared for the first experiment in the series,
resulting in minute differences between experimental repeats; this
will be addressed in future by purchasing newer generation devices,
but it also emphasizes the sensitivity of the microcalorimetry
method, which can detect otherwise imperceptible differences.
Wound infection and the release of pro-inflammatory
modulators leads to local discomfort and delayed healing.
Pain-related stress impairs the immune response to infection,
further impairing wound healing (44,45).
With this in mind, the development of innovative and quick
diagnostic methods is important for providing rapid care that will
alleviate patient suffering, reduce hospitalization times and
decrease the burden on the medical system.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MP drafted the manuscript, and made substantial
contributions to conception and design. BS and SI designed the
study. AM and BC acquired data. AC analyzed data, whereas VP
interpreted the data. CGS was involved in revising the manuscript
and interpreted data. CO and CC provided final approval of the
manuscript, as well as making substantial contributions to
conception and design. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soon K and Acton C: Pain-induced stress: A
barrier to wound healing. Wounds UK. 2:92–101. 2006.
|
|
2
|
Preoteasa CT, Preoteasa E, Popa M,
Pircalabrioru Gradisteanu G, Grigore R and Arutescu L: In vitro
characterization of microbial biofilm on soft materials used in
overdentures retained by mini implants. Rom Biotechnol Lett.
24:10–19. 2019.
|
|
3
|
Varnum C, Pedersen AB, Rolfson O, Rogmark
C, Furnes O, Hallan G, Mäkelä K, de Steiger R, Porter M and
Overgaard S: Impact of hip arthroplasty registers on orthopaedic
practice and perspectives for the future. EFORT Open Rev.
4:368–376. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Conen A, Fux CA, Vajkoczy P and Trampuz A:
Management of infections associated with neurosurgical implanted
devices. Expert Rev Anti Infect Ther. 15:241–255. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hexter AT, Hislop SM, Blunn GW and Liddle
AD: The effect of bearing surface on risk of periprosthetic joint
infection in total hip arthroplasty: A systematic review and
meta-analysis. Bone Joint J. 100-B:134–142. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jiang B, Liang S, Peng ZR, Cong H, Levy M,
Cheng Q, Wang T and Remais JV: Transport and public health in
China: The road to a healthy future. Lancet. 390:1781–1791.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Katz NP, Paillard FC and Ekman E:
Determining the clinical importance of treatment benefits for
interventions for painful orthopedic conditions. J Orthop Surg Res.
10(24)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shao J, Chang H, Zhu Y, Chen W, Zheng Z,
Zhang H and Zhang Y: Incidence and risk factors for surgical site
infection after open reduction and internal fixation of tibial
plateau fracture: A systematic review and meta-analysis. Int J
Surg. 41:176–182. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Richards MJ, Edwards JR, Culver DH and
Gaynes RP: Nosocomial infections in medical intensive care units in
the United States. National nosocomial infections surveillance
system. Crit Care Med. 27:887–892. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gheorghe A, Moran G, Duffy H, Roberts T,
Pinkney T and Calvert M: Health utility values associated with
surgical site infection: A systematic review. Value Health.
18:1126–1137. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Triantafyllopoulos G, Stundner O,
Memtsoudis S and Poultsides LA: Patient, surgery, and hospital
related risk factors for surgical site infections following total
hip arthroplasty. ScientificWorldJournal.
2015(979560)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gallo J, Holinka M and Moucha CS:
Antibacterial surface treatment for orthopaedic implants. Int J Mol
Sci. 15:13849–13880. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Trampuz A, Salzmann S, Antheaume J and
Daniels AU: Microcalorimetry: A novel method for detection of
microbial contamination in platelet products. Transfusion.
47:1643–1650. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
De Vries FEE, Wallert ED, Solomkin JS,
Allegranzi B, Egger M, Dellinger EP and Boermeester MA: A
systematic review and meta-analysis including GRADE qualification
of the risk of surgical site infections after prophylactic negative
pressure wound therapy compared with conventional dressings in
clean and contaminated surgery. Medicine (Baltimore).
95(e4673)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mattiassich G, Ortmaier R, Rittenschober F
and Hochreiter J: Diagnostic parameters in periprosthetic
infections: The current state of the literature. Eur J Orthop Surg
Traumatol. 28:1573–1580. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Graves N, Wloch C, Wilson J, Barnett A,
Sutton A, Cooper N, Merollini K, McCreanor V, Cheng Q, Burn E, et
al: A cost-effectiveness modelling study of strategies to reduce
risk of infection following primary hip replacement based on a
systematic review. Health Technol Assess. 20:1–144. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Popa M, Cîrstoiu M, Cîrstoiu C, et al:
Algic syndrome in osteoarticular infectious pathology; detection
and rapid treatment of the causative agent using microcalorimetry.
Filodiritto Editore-Proceedings, pp589-594, 2018.
|
|
18
|
Braissant O, Wirz D, Göpfert B and Daniels
AU: Biomedical use of isothermal microcalorimeters. Sensors
(Basel). 10:9369–9383. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zaharia DC, Iancu C, Steriade AT, Muntean
AA, Balint O, Popa VT, Popa MI and Bogdan MA: MicroDSC study of
staphylococcus epidermidis growth. BMC Microbiol.
10(322)2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Popa MG, Muntean A, Popa VT, Dragomirescu
CC, Eremia I, Nica S, Popa MI and Cîrstoiu C: Microcalorimetric
growth evaluation of Candida albicans in different conditions. Rom
Biotechnol Lett. 25:2140–2147. 2020.
|
|
21
|
Uçkay I, Hoffmeyer P, Lew D and Pitted D:
Prevention of surgical site infections in orthopaedic surgery and
bone trauma: State-of-the-art update. J Hosp Infect. 84:5–12.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schwarz EM, Parvizi J, Gehrke T, Aiyer A,
Battenberg A, Brown SA, Callaghan JJ, Citak M, Egol K, Garrigues
GE, et al: 2018 International consensus meeting on musculoskeletal
infection: Research priorities from the general assembly questions.
J Orthop Res. 37:997–1006. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rak M, KavčIč M, Trebše R and CőR A:
Detection of bacteria with molecular methods in prosthetic joint
infection: Sonication fluid better than periprosthetic tissue. Acta
Orthop. 87:339–345. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Scherping SC Jr and Aaron AD: Orthopedic
Infections. In: Essentials of Orthopedic Surgery. Wiesel SW and
Delahay JN (ed) 3rd edition. Springer, pp84-105, 2007.
|
|
25
|
Bori G, McNally MA and Athanasou N:
Histopathology in periprosthetic joint infection: When will the
morphomolecular diagnosis be a reality? Biomed Res Int.
2018(141270)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cursaru A, Cretu B, Serban B, Lupu AG,
Iacobescu G, Popa M, Cursaru R and Cirstoiu C: Mechanical safety
study and antibiotic-loaded polymethylmethacrylate spacers
threshold, manufactured intraoperatively in orthopaedic surgery.
Mater Plast. 57:317–324. 2021.
|
|
27
|
Higuera-Guisset J, Rodríguez-Viejo J,
Chacón M, Muñoz FJ, Vigués N and Mas J: Calorimetry of microbial
growth using a thermopile based microreactor. Thermochim Acta.
427:187–191. 2005.
|
|
28
|
Calvet EJP: Calorimeter. In. Edited by
Patent US, vol. 3059471, G01N25/48 ed. United States: Calvet,
Edouard J.P., 1962. https://patents.google.com/patent/US3059471A/en.
|
|
29
|
Calvet E and Prat H: Recent Progress in
Microcalorimetry. Macmillan, New York, NY, 1963.
|
|
30
|
Chen HL, Yao J, Wang L, Wang F, Bramanti
E, Maskow T and Zaray G: Evaluation of solvent tolerance of
microorganisms by microcalorimetry. Chemosphere. 74:1407–1411.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zaharia DC, Muntean A, Popa MG, Steriade
AT, Balint O, Micut R, Iftene C, Tofolean I, Popa VT, Baicus C, et
al: Comparative analysis of Staphylococcus aureus and
Escherichia coli microcalorimetric growth. BMC Microbiol.
13(171)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wadsö L: Isothermal microcalorimetry.
Current problems and prospects. J Therm Anal Calorim. 64:75–84.
2001.
|
|
33
|
Wadsö I: Isothermal microcalorimetry in
applied biology. Thermochim Acta. 394:305–311. 2002.
|
|
34
|
Braissant O, Keiser J, Meister I, Bachmann
A, Wirz D, Göpfert B, Bonkat G and Wadsö I: Isothermal
microcalorimetry accurately detects bacteria, tumorous
microtissues, and parasitic worms in a label-free well-plate assay.
Biotechnol J. 10:460–468. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Borriello SP, Murray PR and Funke G (eds):
Topley and Wilson's Microbiology and Microbial Infections.
Bacteriology. Vol. 1. 10th edition. Hodder Arnold, London,
2005.
|
|
36
|
Madigan MT: Brock Biology of
Microorganisms. Benjamin Cummings, San Francisco, CA, 2012.
|
|
37
|
Kong W, Wang J, Xing X, Jin C, Xiao X,
Zhao Y, Zhang P, Zang Q and Li Z: Screening for novel antibacterial
agents based on the activities of compounds on metabolism of
Escherichia coli: A microcalorimetric study. J Hazard Mater.
185:346–352. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
R Core Team: R: A language and environment
for statistical computing. R Foundation for Statistical Computing,
Vienna, Austria, 2013. http://www.R-project.org/.
|
|
39
|
RStudio Team: RStudio: Integrated
development for R. RStudio, PBC, Boston, MA, 2020. http://www.rstudio.com/.
|
|
40
|
Popa MI, Cursaru A, Popa TV, Muntean AA,
Bogdan S, Crețu B, Iordache S and Cirstoiu C: Study of bacterial
proliferation using a method that shows bacterial growth depending
on the heat released-microcalorimetry. Proc Rom Acad Series B.
23:197–203. 2021.
|
|
41
|
Popa MIG, Cursaru A, Serban B, Cretu B,
Muntean AA, Popa TV, Chifiriuc MC and Cirstoiu C:
Microcalorimetry-versatile method of describing bacterial growth.
Appl Sci. 11(9740)2021.
|
|
42
|
Braissant O, Wirz D, Gopfert B and Daniels
AU: Use of isothermal microcalorimetry to monitor microbial
activities. FEMS Microbiol Lett. 303:1–8. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Popa M, Popa V, Şerban B, Nedelcu R, Creţu
B, Cursaru A and Cîrstoiu C: Utility of microcalorimetru in
describing the growth curve of Candida albicans at different
temperatures-identitying the optimal growth temperature. Rom J
Orthop Surg Traumatol. 2:69–74. 2019.
|
|
44
|
Knight R, Spoors LM, Costa ML and Dutton
SJ: Wound healing in surgery for trauma (WHIST): Statistical
analysis plan for a randomised controlled trial comparing standard
wound management with negative pressure wound therapy. Trials.
20(186)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Matthew L, Achten J, Knight J, Bruce J,
Dutton SJ, Madan J, Dritsaki M, Parsons N, Fernandez M, Grant R, et
al: Effect of incisional negative pressure wound therapy vs.
standard wound dressing on deep surgical site infection after
surgery for lower limb fractures associated with major trauma: The
WHIST randomized clinical trial. JAMA. 323:519–526. 2020.PubMed/NCBI View Article : Google Scholar
|