Introduction
Paget's disease of bone (PDB), also known as
‘osteitis deformans’, is a condition associated with bone fragility
and a high risk of bone fractures in affected patients (1-5).
This disease most often affects older individuals, occurring in
2-3% of the population aged >55 years, and is the second most
common bone remodeling disease after osteoporosis (1,6,7).
Epidemiological studies indicate significant geographical
differences as well as a decreasing prevalence of PDB over the last
20 years (1,6-9).
The causes are not well understood, but possible explanations may
involve genetic variance, changes in lifestyle and diet, and
decreasing exposure to certain viral infections (including
availability of vaccination against measles) (9,10). As
elderly patients are already at a higher risk of accidental falls,
PDB further increases their risk of bone fractures and subsequent
prolonged bed rest and immobilization, which inevitably lead to
further complications (1,3). PDB is characterized by increased
osteoclastic bone resorption, increased but disorganized bone
formation and increased bone vascularity, with abnormal osteoclasts
that secrete high levels of IL-6 and induce exuberant bone
formation (1,2). The altered bone remodeling leads to
the development of unstructured, fibroblastic tissue that is
mechanically frail and brittle (1,11).
Medical research findings suggest that the etiopathogenesis of PDB
may be associated with infectious factors (such as
paramyxoviruses), genetic factors [the sequestosome 1 (SQSTM1) gene
has been found to be an important cause of familial PDB] and
environmental factors (such as arsenic used in pesticides)
(1,10,12).
The majority of patients (≥70% as reported by some
authors) are asymptomatic (12);
however, when clinically overt, PDB is associated with bone pain,
osteoarthritis, musculoskeletal deformities, hypervascularity
resulting in excessive warmth, neurological complications and heart
failure (2,3).
Megaloblastic anemia is characterized by the
presence of large, immature, nucleated cells (megaloblasts) in the
blood (4). Megaloblastic anemia is
mainly caused by vitamin B12 or folic acid deficiency (3,5), which
is most likely due to decreased intake or increased demand (such as
malabsorption), but may also be caused by autoimmune or congenital
intrinsic factor deficiency, which is known as pernicious anemia
(3). ‘Pernicious’ is a term with a
Latin root that means highly injurious or deadly, as this condition
was fatal before identifying the cause and subsequently designing
appropriate therapies (13).
In the present study, a systematic literature
research was performed to identify scarce resources and reports on
the possible association of PDB with megaloblastic anemia (13-15),
as some authors have suggested a common autoimmune mechanism
underlying both diseases (16).
The aim of the present study was to report a rare
case of a patient exhibiting both these conditions, discuss the
clinical management of the patient in a long-term real-life
setting, and also discuss the possible association between the two
based on the findings of the present case and those of the
systematic literature review.
Case report
A 72-year-old male patient who was previously
diagnosed with polyostotic PDB associated with megaloblastic
anemia, spine osteoarthritis, hypertension and type II diabetes,
was admitted in February 2021 at the Department of Internal
Medicine and Rheumatology of ‘St. Maria’ Clinical Hospital
(Bucharest, Romania). The patient complained of low back pain,
chronic pain in the left calf and knee, with genu varus
deformation, asthenia and fatigue (Fig.
1).
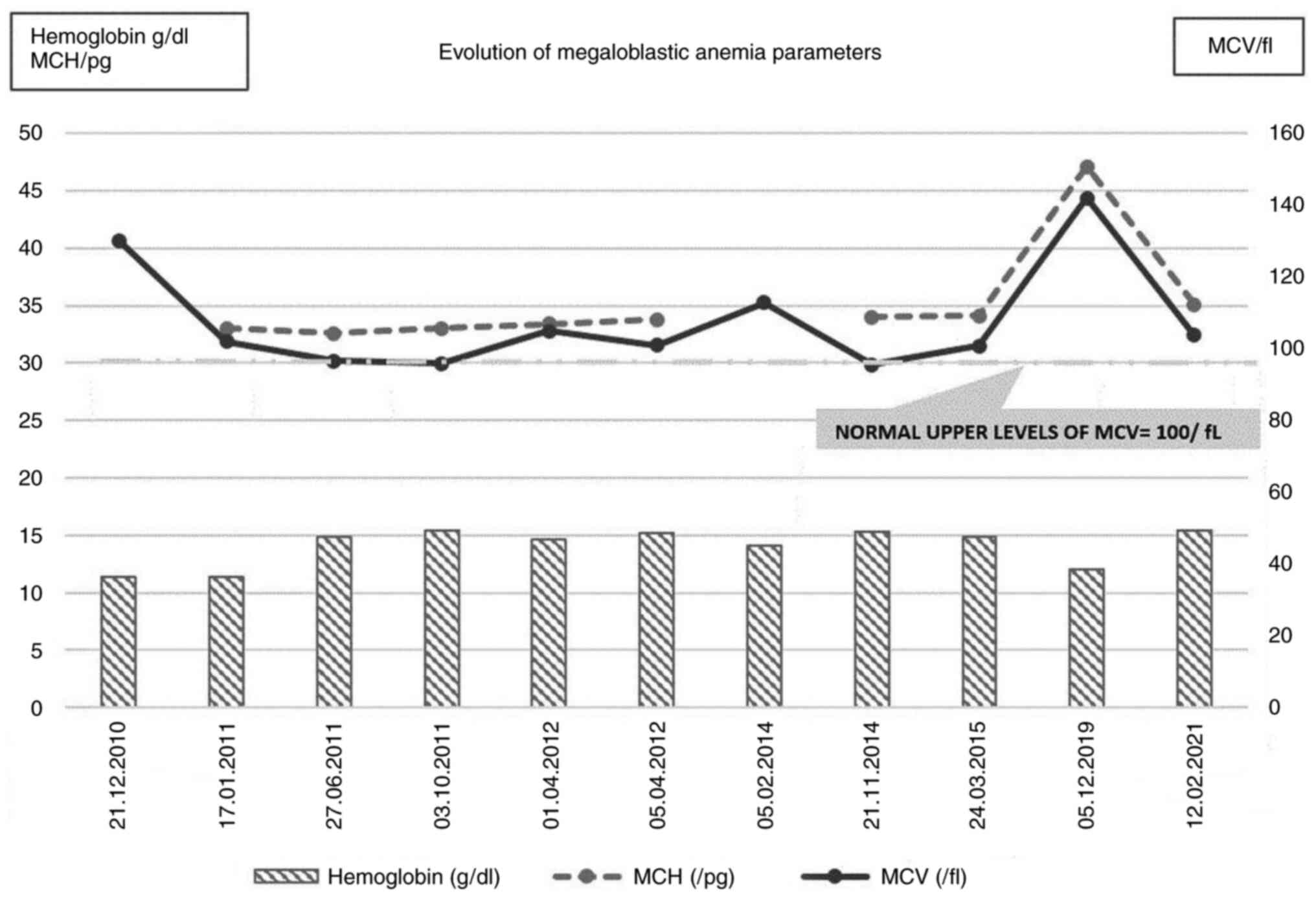
The patient was initially diagnosed in 2010 at
another medical facility, at the age of 62 years, with macrocytic
anemia [hemoglobin (Hb)=11.4 g/dl (normal range, 13.0-17.5 g/dl)
and mean corpuscular volume (MCV)=130 fl, which was 30% above the
upper limit of normal (ULN)] due to hypovitaminosis B12 (<150
pg/ml), hyperostotic disease and hyperemic gastritis. The patient's
symptoms at the time of diagnosis were left knee pain, epigastric
pain and fatigue. The fecal occult blood test was positive and the
radiological examination revealed a thickening of the left femoral
bone and distal epiphysis, with non-homogeneous osteocondensation
and distal lamellar periosteal reaction of the femoral shaft.
Endoscopic examination revealed diffuse hyperemic gastritis with a
single gastric polyp; the biopsy and subsequent histological
examination revealed epithelial tissue without dysplasia.
The patient was then referred to The Department of
Internal Medicine and Rheumatology of ‘Sf. Maria’ Clinical Hospital
in Bucharest (Romania) for further investigations. There was no
evidence of the mechanism underlying the megaloblastic anemia with
vitamin B12 deficiency (parietal cell antibodies or intrinsic
factor determination). After the confirmation of the diagnosis, the
patient was started on vitamin B12 supplementation therapy,
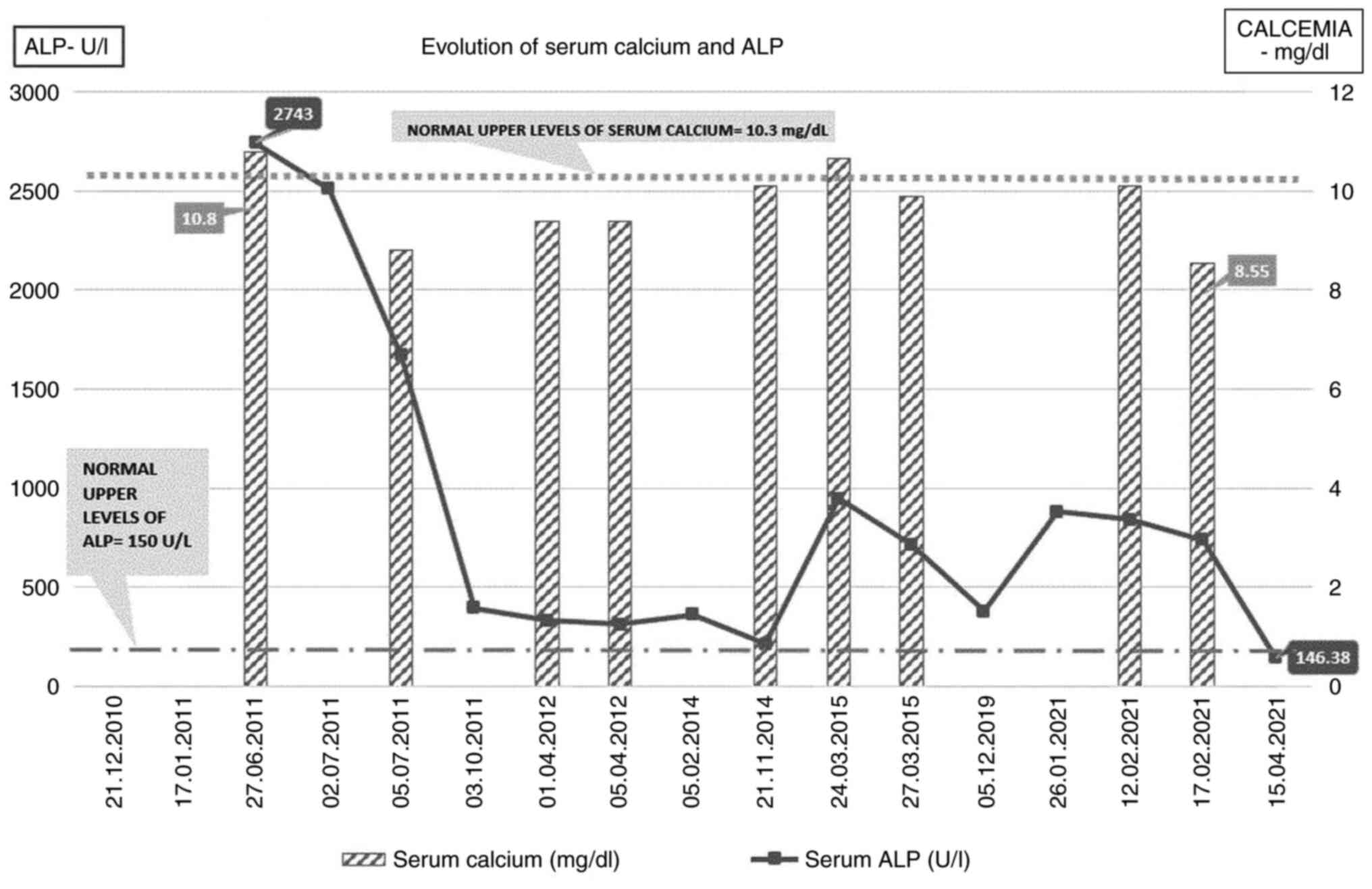
pamidronic acid and calcitonin nasal spray. The serum alkaline
phosphatase (ALP) level at diagnosis was 2,743 UI/l (normal
reference range, 40-150 U/l), and it decreased to 1,553 UI/l after
the first course of therapy with pamidronate 30 mg IV infused over
4 h for 2 consecutive days. At 1 year after the diagnosis and after
two courses of pamidronate, the ALP level had decreased to 394.03
UI/l and the clinical symptomatology related to PDB had
diminished.
Due to aggravation of the left knee functional
impairment with severe pain, the patient was subjected to
arthroplasty 3 years after PDB was first diagnosed (March
2014).
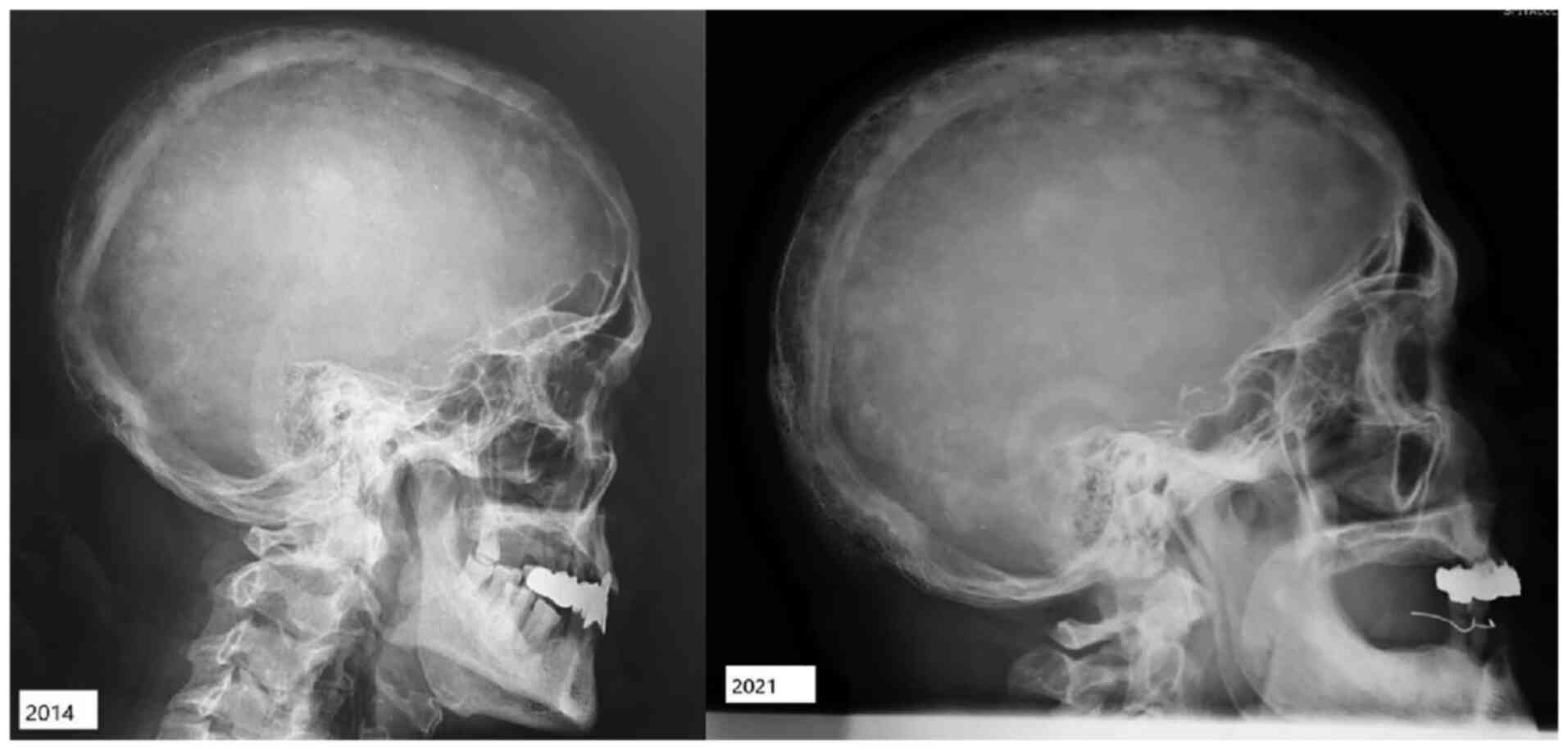
Radiological images obtained during a medical
evaluation in 2014 revealed progressive bone disease at the cranial
level [two osteocondensation zones of the skull cap and flattening
of the skull base with increased dimensions of the sella turcica
(antero-posterior diameter of 15 mm)] and in the right femur and
fibula. The Hb levels were normal (14.1 g/dl), but macrocytosis was
present (MCV=112.8 fl). Another pamidronic acid course was
administered and the continuation of vitamin B12 supplementation
was recommended. Repeated superior digestive endoscopy revealed
persistence of hyperemic gastritis and two sessile gastric polyps
(no dysplasia was observed following biopsy and histological
examination). The patient was referred for a cardiological
examination due to a systolic murmur, which revealed moderate
aortic stenosis.
In March 2015, the patient had only mild
macrocytosis, but the ALP levels had increased again to 947 U/l
(>3 times the ULN) and the serum calcium level was also
increased (10.66 mg/dl; normal range 8.1-10.4 mg/dl). The patient
received another infusion of pamidronate, after which the ALP level
decreased to 715 U/l.
Due to personal reasons, the patient did not attend
the scheduled follow-up visits until December 2019 (intermittent
vitamin B12 supplementation and two courses of pamidronate therapy
were administered between 2015 and 2019), at which time the
laboratory findings revealed macrocytic anemia [Hb=12 g/dl; MCV=142
fl; mean corpuscular Hb (MCH)=47.1 pg (normal range, 27-34 pg)]
with vitamin B12 deficiency (serum level of 95 pg/ml), normal
plasma level of folic acid (23.97 ng/ml) and active PDB, with
ALP=380 U/l (normal range, 40-150 U/l). As regards the
cardiovascular status, the degree of the aortic stenosis had
progressed to ‘severe’, and the patient also suffered from
recurrent chest pain (spontaneous and related to physical effort)
(Fig. 2).
In January 2021, the patient was again admitted to
‘St. Maria’ Clinical Hospital (Bucharest, Romania) at the age of 72
years, with complaints of back pain and bilateral knee pain, with a
significant gait deficit. The laboratory parameters were as
follows: Serum ALP=840 U/l, with normal calcium and Hb levels and
mild macrocytosis (MCV=103.8 fl, MCH=36.4 pg) (Fig. 3).
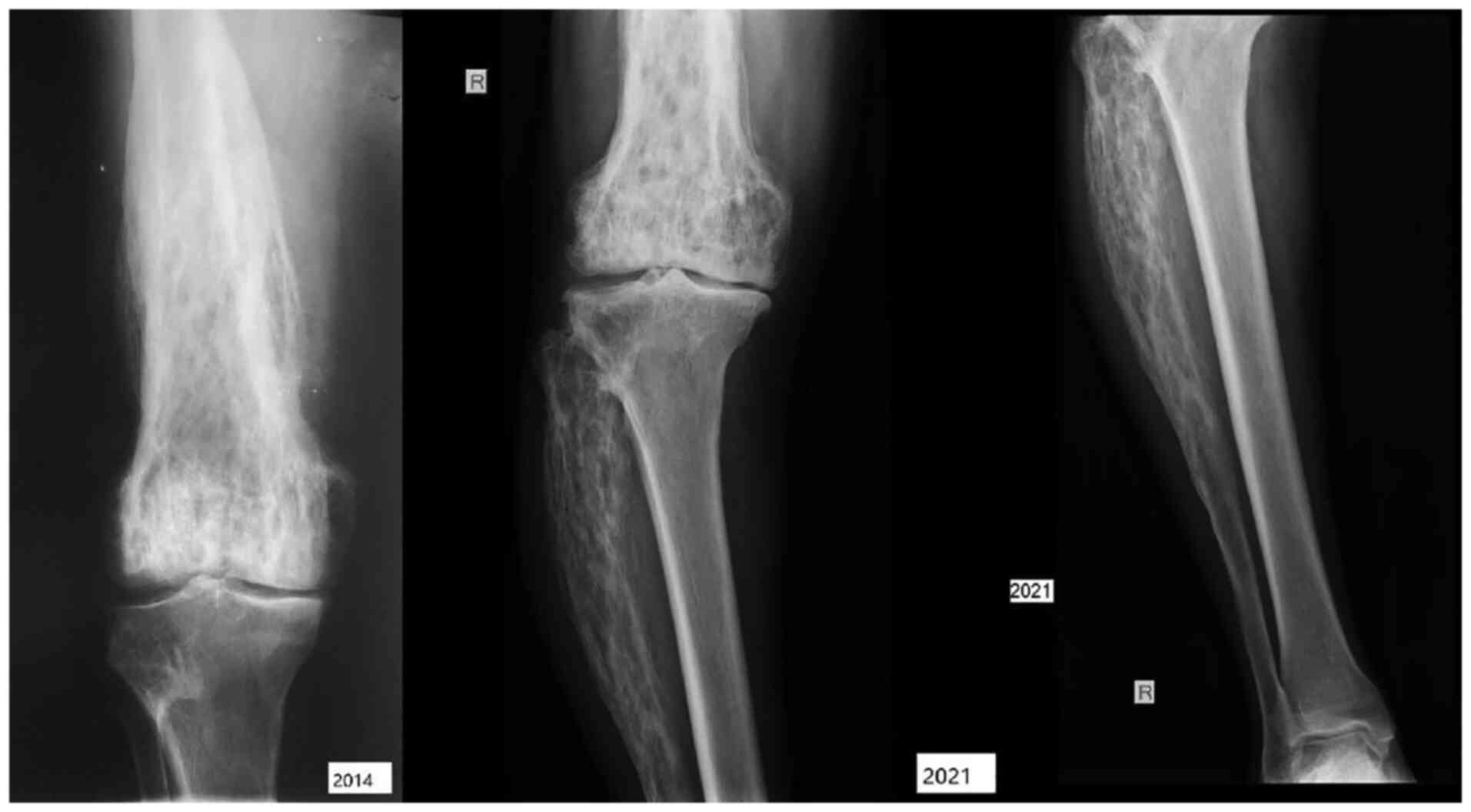
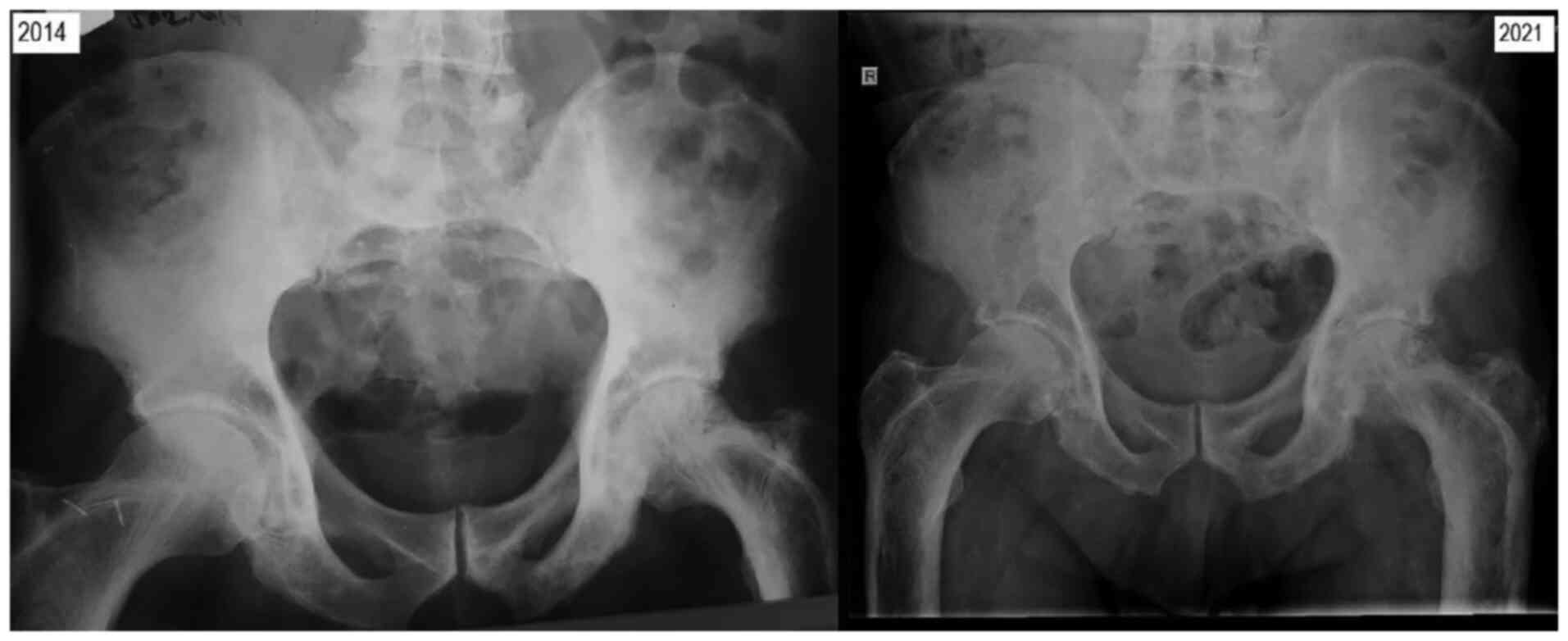
The radiological assessment revealed progression of
the skull osteolytic lesions, as well as specific coarsening of the
trabeculae and cortical thickening of the femoral and fibular
bones, with the most notable lesions observed in the right calf.
There was also posterior vertebral osteophytosis at L3-L4 and
L5-S1, slight retrolisthesis at L2, alterations of the bone
structure in the sacrum significant for PDB, with condensation of
trabeculae at the periphery (Fig.
4).
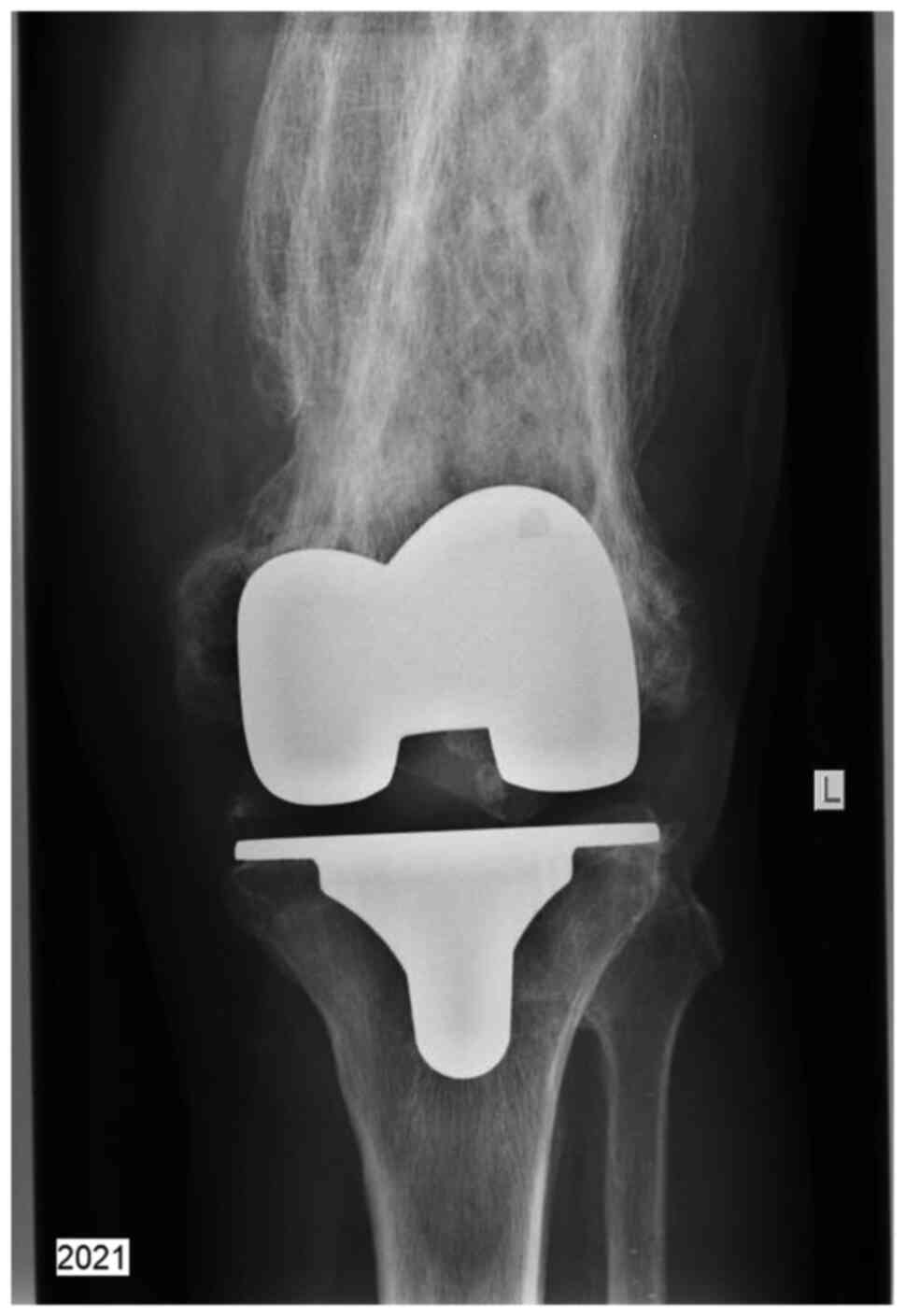
The left knee prosthesis was well-positioned and
functional, despite the significant deterioration of the leg bones
(Fig. 5).
Following an infusion course of zoledronic acid (a
single 4 mg intravenous infusion) and analgesics (paracetamol 1 g
daily for 5 days), the ALP level slightly decreased to 739 U/l and
the symptoms improved, so the patient was discharged with
recommendations for specific therapy (iron supplement 100 mg daily,
vitamin D 2000 IU daily, metoprolol 50 mg daily) at home for anemia
and hypertension, as well as vitamin B12 supplementation. After 3
months, the patient underwent laboratory tests with the family
physician, and the serum ALP level was 146.38 U/l (~20% over the
ULN), while he reported improvement of the bone-related symptoms
(Fig. 6).
Discussion
PDB is usually diagnosed in patients aged >50
years, most of whom are asymptomatic (1,2,17). The
prevalence of PDB exhibits notable geographic variability, with the
highest prevalence documented in Europe (predominantly England,
France and Germany) (7-9,17,18).
The geographic distribution of the disease may be explained by
genetic transmission and dissemination by population migration.
Studies have reported a positive family history in 12.3-22.8% of
the cases (7,9). In addition, a 7- to 10-fold increase
in the incidence of PDB was observed in relatives of patients
diagnosed with this condition compared with control groups
(10). Numerous studies have
described families exhibiting autosomal dominant inheritance
(2,12). Studies of potential genetic markers
for PDB have identified an association between human leukocyte
antigen (HLA)-A, HLA-B and HLA-C (class I) and clinical evidence of
the disease, but other trials reported an increased frequency of
DQW1 and DR2 antigens (class II HLA) (2,19-22).
Genome-wide association studies (GWAS) have identified several loci
involved in the development of the disease, with two regions
studied in detail: The optineurin and Ras and Rab interactor 3
genes (10,19,23).
It has been proven that environmental factors play
an important role in PDB (10,12).
Altered cytokine expression was identified in cases of PDB
(19,23). One hypothesis is based on an
unidentified viral infection that acts through upregulation of IL-6
and the IL-6 receptor genes (19,20,23);
however, this has not been shown conclusively. The slow virus
theory is the most widely accepted hypothesis for an infectious
etiology in PDB (21,22). According to this theory, progenitors
of osteoclasts from the bone marrow are infected by a virus,
resulting in abnormal activation of osteoclast growth (21,22,24).
Osteoclast precursors in patients with PDB also
appear to be hyperresponsive to vitamin D and calcitonin (11). The treatment efficacy of
bisphosphonates in PDB appears to be associated with suppression of
receptor activator of NF-κΒ ligand (RANKL)-induced bone resorption
(1,11), with lower RANKL levels and increased
production of osteoprotegerin (22,25).
Other suggested etiologies, based on strong clinical
and research evidence, include an inflammatory cause, which is also
supported by evidence of clinical improvement after treatment with
anti-inflammatory agents (21,22).
Elevated parathyroid hormone levels have been observed in PDB
(26), while autoimmune, connective
tissue and vascular disorders or an osteogenic mechanism have been
also proposed (3,12,22).
Case reports of PDB in the literature have presented
other associated pathologies (16,26-28),
including vitiligo, chronic kidney disease (29), rheumatoid arthritis (16), tumor and tumor-like conditions;
specifically, <5% of patients with PDB will undergo malignant
transformation to sarcoma (30),
but more extensive disease and a more prolonged course are
associated with higher risk of eventually developing a sarcoma
(31) (Fig. 7).
During the course of PDB the patients may develop
complications and comorbidities (12), such as fractures, neuromuscular
syndromes, autoimmune thyroiditis (28), Dupuytren contracture,
chondrocalcinosis, hyperparathyroidism (26), cardiovascular abnormalities
(22), hypoacusia (2) and osteosarcoma (30). The patient presented herein
developed several of those over time as follows: i) Dupuytren
contracture in both hands was noted at diagnosis in 2011, with the
severity progressing from the 5th finger to grade 2-3 in fingers
2-5 bilaterally; ii) the patient reported progressive hearing loss
over the last 6 years; iii) severe gonarthrosis, particularly in
the left knee, requiring an arthroplasty 3 years after diagnosis;
and iv) cardiovascular symptoms that became progressively more
severe, particularly stage II hypertension preexisting to the PDB
diagnosis in 2010; in 2019 a moderate aortic stenosis was
documented, together with a mild left ventricular hypertrophy; the
latter is commonly seen in PDB (2)
and is associated with increased cardiac output.
Megaloblastic anemia was also present at the time of
PDB diagnosis in 2010, documented by a complete blood count
revealing anemia with macrocytosis and megaloblasts in the
peripheral blood due to a deficit of vitamin B12; due to
intermittent cyanocobalamin supplementation, the vitamin B12
deficiency was recurrent. Vitamin B12 and folic acid deficiency is
the leading cause of megaloblastic anemia (13), but dietary deficiency due to
decreased intake did not appear to be the case for our patient.
Specific medical evaluation after the initial diagnosis revealed
diffuse hyperemic gastritis with the presence of benign gastric
polyps, and several years later a hiatal hernia was identified
during routine endoscopy. It was therefore hypothesized that
impaired absorption of vitamin B12 may have been the cause of this
chronic deficit.
A systematic literature review was performed using
the key words ‘Paget's disease of bone’, ‘macrocytic anemia’,
‘megaloblastic anemia’ and ‘vitamin B12’ using PubMed/MEDLINE and
Google Scholar. A total of 4,651 articles were identified, which
were analyzed following PRISMA criteria (32) with focus on those referring to
anemia associated with PDB. There is limited available evidence on
the association between PDB and megaloblastic anemia, with <20
cases in total cited in the literature to date, including an 8-case
series report published in 1968(33). The authors of this 1968 article
aimed to draw attention to the malabsorption syndrome associated
with PDB and introduced an interesting theory of impaired folate
absorption due to reduced splanchnic blood flow with relative
ischemia of the bowel, in the context of the significant increase
of the bone blood flow during extensively active PDB (33). Prevalent comorbidities reported in
PDB are associated with the manifestations of the disease in
elderly patients, with the most frequent associations being with
cardiovascular pathologies, such as hypertension, atrial
fibrillation and other arrhythmias, ischemic heart disease and
stroke (12,22), and neurodegenerative disorders, such
as Parkinson's Disease, cranial nerve palsies and frontotemporal
dementia (11).
Evidence regarding the rare comorbidity of Biermer's
anemia and PDB also comes from a short list of relevant
publications: A case report of an icteroedematous form of
pernicious anemia and PDB in 1951(27), a review of the available literature
at the time of publication of a possible association of PDB and
pernicious anemia in 1970(13), as
well as a case report of a patient with PDB, pernicious anemia and
vitiligo in 1986(14). All these
reports promote the hypothesis that PDB may be an autoimmune
disease sharing some common underlying mechanisms with other
immune-mediated disorders (13,14,34-36).
A literature review showed evidence of the
association among vitamin B12 and/or folate, homocysteine (HCY) and
osteoblastic and osteoclastic activity, and suggested the use of
these biomarkers to assess bone metabolism (e.g., in osteosarcoma)
(37-39).
An analysis published in 1964(40)
focused on the decrease in ALP serum levels following vitamin B12
supplementation therapy in patients with pernicious anemia, based
on the case of a patient suffering from both pernicious anemia and
PDB. The authors noted that the patient had normal serum levels of
ALP, which increased after the initiation of the cyanocobalamin
therapy, while vitamin B12 supplementation suppressed osteoblastic
activity (40).
Vitamin B12 deficiency is more prevalent in elderly
patients (37,38,41,42),
causing an increase in the serum levels of HCY and methylmalonic
acid (37). An interesting study
published in 2010(43) evaluated
the serum levels of HCY, folate and vitamin B12 in patients with
PDB, and examined the effect of zoledronic acid on these serum
levels. Existing data suggest that hyperhomocysteinemia (and,
indirectly, deficiency of folate and vitamin B12 through increased
serum HCY levels) may constitute an important risk factor for
osteoporosis and associated fractures, as it adversely affects bone
quality by stimulating bone resorption and affecting the physiology
of collagen crosslinking (24,43).
In the aforementioned study (42),
higher serum HCY and folate levels were observed in the patients
with PDB compared with the control group.
In the present case, no genetic tests were available
and the patient was unable to recall any relevant family history.
The literature review showed that, to date, 7 loci have been
associated with PDB, with SQSTM1 mutations found by GWAS in 20-50%
of the patients (22). Vitamin B12
deficiency may also lead to increased osteoclast formation
indirectly through increasing HCY levels (37). It has been demonstrated that
combined treatment with folate and vitamin B12 was effective in
reducing serum HCY and the risk of hip fractures in elderly
patients after stroke, without affecting bone mineral density
(24). Hyperhomocysteinemia is a
common age-related finding (24).
The main causes of hyperhomocysteinemia in elderly individuals
include folate and vitamin B12 deficiency, as well as deterioration
of renal function (37,38). HCY is known to modulate this process
via several known mechanisms, such as increase in osteoclast
activity, decrease in osteoblast activity and direct action on bone
matrix (43-45).
The patient in the present case exhibited no renal impairment and
the onset of both anemia and bone-related symptoms was dated to
before the initiation of bisphosphonate therapy.
As regards PDB, research should focus on in-depth
analysis of the mechanisms underlying this disorder, identifying
any possible correlations with other comorbidities, immune-related
or other (21,43), and broadening the opportunities of
identifying a possible adjuvant therapy beyond bisphosphonates
(44-46).
There is a pressing need to fully elucidate the pathogenesis of
this illness and to find modalities to prevent long-term
complications.
PDB is the second most common metabolic bone
disorder after osteoporosis, with a high prevalence among elderly
patients (12). Due to the limited
and non-specific symptoms that may not appear until late in the
course of the disease, it remains underdiagnosed and undertreated
(11,17). Although a significant proportion of
patients who present with the pathognomonic features related to PDB
are asymptomatic (1,17), we herein present a case of a
symptomatic patient with associated macrocytic anemia, both
manifesting specific characteristics at the time of initial
diagnosis. The primary cause of the anemia was identified as
hypovitaminosis B12, but with no proven underlying cause. A trigger
for PDB onset or a relevant family history was not reported for
this patient. Common underlying mechanisms linking these two
disorders have yet to be definitively determined, but possible
associations have been suggested and hypotheses have been made
based on similar case reports in the literature (13-16,27,43,44).
Special attention should be paid to associated anemia in elderly
patients diagnosed with PDB, irrespective of the presence or
absence of bone-related symptoms. The 10-year-long real-life
medical history of the present case is indicative of the
significance of the adherence of patients with chronic conditions
to medical follow-up in association with symptomatology, and the
impact on their quality of life. Further research focused on the
etiopathogenesis of both PDB and megaloblastic anemia may help
improve therapy outcomes for such patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VDO, VCB, MB and ALT wrote the case report,
diagnosed and monitored the patient, and conceived the work. VDO,
VCB and ARB confirm the authenticity of the raw data. VCB, ARB and
AR interpreted the data and critically revised the manuscript for
important intellectual content. All the authors have read and
approved the final manuscript and agree to be accountable for all
aspects of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of anonymized data and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shaker JL: Paget's disease of bone: A
review of epidemiology, pathophysiology and management. Ther Adv
Musculoskelet Dis. 1:107–125. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ralston SH, Corral-Gudino L, Cooper C,
Francis RM, Fraser WD, Gennari L, Guañabens N, Javaid MK, Layfield
R, O'Neill TW, et al: Diagnosis and management of Paget's disease
of bone in adults: A clinical guideline. J Bone Miner Res.
34:579–604. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Appelman-Dijkstra NM and Papapoulos SE:
Paget's disease of bone. Best Pract Res Clin Endocrinol Metab.
32:657–668. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mohamed M, Thio J, Thomas RS and Phillips
J: Pernicious anaemia. BMJ. 369(m1319)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aslinia F, Mazza JJ and Yale SH:
Megaloblastic anemia and other causes of macrocytosis. Clin Med
Res. 4:236–241. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Doyle T, Gunn J, Anderson G, Gill M and
Cundy T: Paget's disease in New Zealand: Evidence for declining
prevalence. Bone. 31:616–619. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Poór G, Donáth J, Fornet B and Cooper C:
Epidemiology of Paget's disease in Europe: The prevalence is
decreasing. J Bone Miner Res. 21:1545–1549. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abdulla O, Naqvi MJ, Shamshuddin S,
Bukhari M and Proctor R: Prevalence of Paget's disease of bone in
Lancaster: Time for an update. Rheumatology (Oxford). 57:931–932.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Michou L and Orcel P: Has Paget's bone
disease become rare? Joint Bone Spine. 86:538–541. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Singer FR: Paget's disease of bone-genetic
and environmental factors. Nat Rev Endocrinol. 11:662–671.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cook SJ and Wall C: Paget's disease of
bone: A clinical update. Aust J Gen Pract. 50:23–29.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Paul Tuck S, Layfield R, Walker J,
Mekkayil B and Francis R: Adult Paget's disease of bone: A review.
Rheumatology (Oxford). 56:2050–2059. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Govindaraj M and Krishnan MU: Possible
association of Paget's disease of bone and pernicious anaemia.
Gerontol Clin (Basel). 12:94–98. 1970.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mavrikakis ME, Athanassiades P,
Baltopoulos G, Rassidakis A and Rokas S: Paget's bone disease,
anaemia and vitiligo: A case report. Clin Exp Rheumatol. 4:57–59.
1986.PubMed/NCBI
|
|
15
|
Beickert A and Heinicke HJ: Simultaneous
occurrence of Biermer's disease and Paget's disease. Observations
of 5 associated cases. 1 Of them with vitiligo. Dtsch Gesundheitsw.
26:579–583. 1971.PubMed/NCBI(In German).
|
|
16
|
Borz-Baba C, Sachan Y, Sapers B and
Georgescu L: A case of rheumatoid arthritis and Paget disease of
bone. Am J Case Rep. 20:764–769. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hadjipavlou AG, Gaitanis IN and Kontakis
GM: Paget's disease of the bone and its management. J Bone Joint
Surg Br. 84:160–169. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Menéndez-Bueyes LR and Soler Fernández MC:
Paget's disease of bone: Approach to its historical origins.
Reumatol Clin. 13:66–72. 2017.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
19
|
Hughes AE, Shearman AM, Weber JL, Barr RJ,
Wallace RG, Osterberg PH, Nevin NC and Mollan RA: Genetic linkage
of familial expansile osteolysis to chromosome 18q. Hum Mol Genet.
3:359–361. 1994.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Niculet E, Chioncel V, Elisei AM, Miulescu
M, Buzia OD, Nwabudike LC, Craescu M, Draganescu M, Bujoreanu F,
Marinescu E, et al: Multifactorial expression of IL-6 with update
on COVID-19 and the therapeutic strategies of its blockade
(Review). Exp Ther Med. 21(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Galson DL and Roodman GD: Pathobiology of
Paget's disease of bone. J Bone Metab. 21:85–98. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alonso N, Calero-Paniagua I and Del
Pino-Montes J: Clinical and genetic advances in Paget's disease of
bone: A review. Clin Rev Bone Miner Metab. 15:37–48.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hoyland JA, Freemont AJ and Sharpe PT:
Interleukin-6, IL-6 receptor, and IL-6 nuclear factor gene
expression in Paget's disease. J Bone Miner Res. 9:75–80.
1994.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Van Wijngaarden JP, Doets EL, Szczecińska
A, Souverein OW, Duffy ME, Dullemeijer C, Cavelaars AE, Pietruszka
B, Van't Veer P, Brzozowska A, et al: Vitamin B12, folate,
homocysteine, and bone health in adults and elderly people: A
systematic review with meta-analyses. J Nutr Metab.
2013(486186)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Drake MT, Clarke BL and Khosla S:
Bisphosphonates: Mechanism of action and role in clinical practice.
Mayo Clin Proc. 83:1032–1045. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Panuccio VA and Tripepi R: Paget's disease
and secondary hyperparathyroidism: Is healing possible? Front Cell
Dev Biol. 8(399)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Malamud T and Rosenfeld I: Icteroedematous
form of pernicious anemia in Paget's disease of the bone. Prensa
Med Argent. 38:1164–1166. 1951.PubMed/NCBI(In Undetermined Language).
|
|
28
|
Punzi L, Avossa M, De Zambiasi P, Volpe A,
Cesaro G, Schiavon F and Todesco S: Paget's disease of bone
associated with autoimmune thyroiditis and joint chondrocalcinosis.
Rev Rhum Ed Fr. 61:354–356. 1994.PubMed/NCBI(In French).
|
|
29
|
Chan PK, Lyu SY and Lu CC: Paget disease
of bone in an elderly patient with chronic renal disease and weight
loss: A case report. Medicine (Baltimore).
98(e17458)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bojincă V and Janta I: Rheumatic diseases
and malignancies. Maedica (Bucur). 7:364–371. 2012.PubMed/NCBI
|
|
31
|
Davies A, Pluot E and James S: Tumour and
tumour-like conditions associated with Paget's disease of bone. In:
Imaging of Bone Tumors and Tumor-Like Lesions. Davies A, Sundaram M
and James S (eds). Springer, Berlin, Heidelberg, pp515-530,
2009.
|
|
32
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Somayaji BN: Malabsorption syndrome in
Paget's disease of bone. Br Med J. 4:278–280. 1968.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tatu AL and Ionescu MA: Multiple
autoimmune syndrome type 3-thyroiditis, vitiligo and alopecia
areata. Acta Endocrinol (Buchar). 13:124–125. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mihăilă B, Dinică RM, Tatu AL and Buzia
OD: New insights in vitiligo treatments using bioactive compounds
from Piper nigrum. Exp Ther Med. 17:1039–1044. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tatu AL and Nwabudike LC: Male genital
lichen sclerosus-a permanent therapeutic challenge. J Am Acad
Dermatol. 79 (Suppl 1)(AB185)2018.
|
|
37
|
Vaes BL, Lute C, Blom HJ, Bravenboer N, de
Vries TJ, Everts V, Dhonukshe-Rutten RA, Muller M, de Groot LC and
Steegenga WT: Vitamin B(12) deficiency stimulates
osteoclastogenesis via increased homocysteine and methylmalonic
acid. Calcif Tissue Int. 84:413–422. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Toh BH: Pathophysiology and laboratory
diagnosis of pernicious anemia. Immunol Res. 65:326–330.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kharb S, Kumar S and Kundu ZS:
Homocysteine, a biomarker of osteosarcoma. J Can Res Ther.
11:51–53. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Van Dommelen CKV and Klaassen CHL:
Cyanocobalamin-dependent depression of the serum alkaline
phosphatase level in patients with pernicious anemia. N Engl J Med.
271:541–544. 1964.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hanna S, Lachover L and Rajarethinam RP:
Vitamin B12 deficiency and depression in the elderly: Review and
case report. Prim Care Companion J Clin Psychiatry. 11:269–270.
2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wolters M, Ströhle A and Hahn A:
Age-associated changes in the metabolism of vitamin B(12) and folic
acid: Prevalence, aetiopathogenesis and pathophysiological
consequences. Z Gerontol Geriatr. 37:109–135. 2004.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
43
|
Polyzos SA, Anastasilakis AD, Efstathiadou
Z, Litsas I, Kita M, Panagiotou A, Papatheodorou A, Arsos G,
Moralidis E, Barmpalios G, et al: Serum homocysteine, folate and
vitamin B12 in patients with Paget's disease of bone: The effect of
zoledronic acid. J Bone Miner Metab. 28:314–319. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Căruntu C, Zurac SA, Jugulete G and Boda
D: Extramammary Paget's disease in an HIV-positive patient. Rom J
Morphol Embryol. 58:1009–1015. 2017.PubMed/NCBI
|
|
45
|
Rabjohns EM, Hurst K, Ghosh A, Cuellar MC,
Rampersad RR and Tarrant TK: Paget's disease of bone:
Osteoimmunology and osteoclast pathology. Curr Allergy Asthma Rep.
21(23)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Roodman GD and Windle JJ: Paget disease of
bone. J Clin Invest. 115:200–208. 2005.PubMed/NCBI View Article : Google Scholar
|