Introduction
Chronic periodontitis is one of the most common oral
diseases, with increasing prevalence with age; ~11.2% of people
worldwide has severe periodontitis (1). Chronic periodontitis is a severe
inflammatory disease, which results in the destruction of the
periodontal tissue. Periodontal tissue, which is composed of the
periodontal ligaments, alveolar bone, gingiva and cementum,
surrounds and supports the teeth, and maintains their function. The
deterioration of chronic periodontitis will eventually cause teeth
to gradually loosen or even fall out (2). The aim of periodontal tissue
regeneration is to concurrently control inflammation and stimulate
stem cells to regenerate new periodontal tissue (3).
The human periodontal ligament tissue is a dense
fibrous tissue, which is highly elastic and can absorb the force
exerted by the teeth whilst chewing. Periodontal ligament cells
comprise periodontal ligament fibroblasts, osteoblasts,
undifferentiated mesenchymal stem cells and cementoblasts (4). Among these cells, undifferentiated
mesenchymal stem cells, also known as human periodontal ligament
stem cells (hPDLSCs), can differentiate into alveolar bone and
periodontal ligament-like tissues and have the potential of
multi-directional differentiation (5). Seo et al (6) successfully isolated hPDLSCs from
periodontal ligament tissue and transplanted hPDLSCs into rodents
with weakened immune functions. The hPDLSCs exhibit the
characteristics of mesenchymal stem cells, including increased
colony formation and a high proliferative ability, and they also
have multiple differentiation capabilities which could be used for
periodontal tissue regeneration (7). However, the differentiation potential
of hPDLSCs in the periodontal tissue of patients with chronic
periodontitis is known to be impaired, leading to a decline in
their regeneration ability (8).
Therefore, it is particularly important to overcome the adverse
effects of inflammation on PDLSCs.
Gastrodin (GAS) is the major bioactive component of
the Chinese herbal medicine Gastrodia elata Blume.
GAS has been reported to exhibit anti-inflammatory, anti-apoptotic
and antioxidant effects in various types of disease. For example,
GAS was demonstrated to ameliorate cerebral ischemic injury by
inhibiting inflammation and apoptosis in animal models (9-12).
In addition, previous studies suggested that GAS may attenuate
lipopolysaccharide (LPS)-induced inflammation and apoptosis in lung
cells both in vitro and in vivo (13,14).
In particular, the protective effect of GAS on bone-related
diseases has also been reported. Zheng et al (15) demonstrated that GAS prevents
steroid-induced osteonecrosis in the femoral head of rats by
inducing some anti-apoptotic effects. GAS also exhibits an
anti-osteoporosis effect by reducing reactive oxygen species levels
(16), and inhibits
osteoclastogenesis by downregulating the nuclear factor of
activated T cells signaling pathway and stimulating
osseointegration in vitro (17). In addition, GAS can prevent and/or
delay dexamethasone-induced osteoporosis by improving osteoblast
function (18). These findings
indicate the regulatory role of GAS on osteocyte biological
functions. However, whether GAS might protect hPDLSCs against
LPS-induced injury has not been elucidated, to the best of our
knowledge.
It has been previously reported that GAS can
attenuate the activation of microglia by regulating the
renin-angiotensin system and sirtuin 3 (SIRT3) signaling pathways
(19). SIRT3 is one of seven
mammalian sirtuins that belong to a conserved family of proteins
that possess NAD+-dependent deacetylase activity
(20). SIRT3, which is primarily
located in the mitochondria, has been demonstrated to bind and
deacetylate metabolic and respiratory enzymes that regulate
important mitochondrial functions (21). It has also been suggested that
SIRT3 might serve a key role in numerous metabolic- and
aging-related diseases, including cardiovascular disease,
age-related hearing loss, cancer, obesity and type 2 diabetes
(22-25).
Bone marrow metabolism has been reported to be closely associated
with systemic metabolism (26).
However, to the best of our knowledge, little is known about the
role of SIRT3 in regulating bone marrow metabolism. A previous
study reported that 8-week-old mice with SIRT3 deficiency present
with osteopenia, indicating that SIRT3 might play a positive role
in the formation of peak bone mass (27).
The present study hypothesized that GAS may play a
protective role in LPS-treated hPDLSCs, and that this mechanism of
action may be associated with SIRT3. The study aimed to elucidate
the mechanisms underlying the effects of GAS, with the intention of
providing insight for developing strategies for the treatment of
chronic periodontitis.
Materials and methods
Cell culture
Fresh decayed premolars were obtained from five
volunteers (all female; age, 18-20 years) who received orthodontic
treatment voluntarily between May 2019 and May 2021 according to
the ethics approval received from The Xingyi People's Hospital
(approval no. 20190512). The patients provided consent for the use
of their samples in scientific research. The collected premolars
were placed in α-minimum essential medium (α-MEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 5% 100 U/ml penicillin
and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
After washing three times with PBS, 1/3 of the periodontal ligament
tissue from the root was removed and cut into small pieces with a
blade. The cut tissues were placed in 3 mg/ml type I collagenase
(Sigma-Aldrich; Merck KGaA) and 4 mg/ml dispase II enzymes (Roche
Diagnostics) and digested at 37˚C for 40 min to obtain a single
cell suspension. The single cell suspension was filtered through a
70-µm cell strainer, seeded into a 10-cm culture dish at the
density of 2x105 cells/cm2 and cultured in
α-MEM supplemented with 20% FBS (Gibco; Thermo Fisher Scientific,
Inc.) at 37˚C. The cell medium was changed every 3 days. Once cells
reached 80-90% confluence, they were harvested with 0.25%
trypsin-0.2% EDTA and passaged at 1:2 for further use. hPDLSCs
between the fourth and sixth passages were used in subsequent
experiments (28).
Short hairpin (sh)RNA
transfection
The sh-negative control (shRNA-NC) and shRNAs
specific for SIRT3 (shRNA-SIRT3-1, shRNA-SIRT3-2) were purchased
from Oligobio. The shRNAs (100 ng) were transfected into hPDLSCs
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Reverse transcription-quantitative (RT-q)PCR was used to verify the
transfection efficiency at 72 h post-transfection.
Cell Counting Kit-8 (CCK-8) assay
hPDLSCs were seeded into 96-well microplates at the
density of 3x103 cells/well and allowed to adhere for 24
h at 37˚C. Cells were pretreated with 0.1, 1, 10, 50 or 100 nM GAS
for 1 h (Sigma-Aldrich; Merck KGaA; purity >98%) and then
treated with 10 µg/ml LPS (Beijing Solarbio Science &
Technology Co., Ltd.) for 24 h at 37˚C. Following treatment, 10 µl
CCK-8 solution (MedChemExpress) was added to each well and
incubated at 37˚C for 2 h in the dark. The absorbance was measured
at a wavelength of 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc.).
Enzyme-linked immunosorbent assay
(ELISA), and the detection of malondialdehyde (MDA) and lactate
dehydrogenase (LDH)
After stimulating hPDLSCs with 50 µM GAS for 1 h
followed by 10 µg/ml LPS for 24 h at 37˚C, the cell culture
supernatant was collected. The concentrations of tumor necrosis
factor-α (TNF-α; cat. no. PT518) and interleukin-6 (IL-6; cat. no.
PI330) were measured using ELISA kits according to the
manufacturers' instructions. The MDA (cat. no. S0131S) and LDH
(cat. no. C0016; both Beyotime Institute of Biotechnology) were
measured using corresponding kits in accordance with the
manufacturers' protocols.
Alkaline phosphatase (ALP) assay
hPDLSCs were seeded into a 6-well plate at the
density of 1x105 cells/well and cultured in medium
supplemented with 10% FBS for 24 h at 37˚C. Then, hPDLSCs were
pretreated with 50 µM GAS (10 µg/ml) for 1 h at 37˚C and 10 µg/ml
LPS was added into the osteogenic induction culture medium
(containing 10% FBS, 10-8 mol/l dexamethasone, 50 mg/l
ascorbic acid and 10 mmol/l β-glycerophosphate sodium;
Sigma-Aldrich; Merck KGaA), in which hPDLSCs were cultured for 7
days at 37˚C. Subsequently, cells were permeabilized with 1%
Triton-X at room temperature for 30 min and ALP activity was
determined with an ALP activity kit (cat. no. P0321S; Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. The optical density was determined at a wavelength of
520 nm in the dark.
Alizarin Red S staining
To investigate the osteogenic differentiation
potential of hPDLSCs, cells were seeded into a 6-well plate at the
density of 1x105 cells/well and were pretreated with 50
µM GAS for 1 h at 37˚C. Following pretreatment, 10 µg/ml LPS was
added into the aforementioned osteogenic induction culture medium.
After 21 days induction, Alizarin Red S (Beijing Solarbio Science
& Technology Co., Ltd.) was used to detect the levels of
mineralization according to the manufacturer's protocol. The
absorbance was measured at a wavelength of 490 nm.
Flow cytometry
The hPDLSCs were centrifuged at 700 x g for 5 min at
room temperature and then gently resuspended in 500 µl Annexin
V-FITC solution (Beijing Solarbio Science & Technology Co.,
Ltd.). After being mixed and incubated at room temperature for 10
min in the dark, the cells were centrifuged at 700 x g for 5 min at
room temperature and then gently resuspended in 500 µl Annexin
V-FITC binding solution (Beijing Solarbio Science & Technology
Co., Ltd.). Subsequently, 10 µl PI (Beijing Solarbio Science &
Technology Co., Ltd.) staining solution was added to the cells,
mixed and incubated in an ice bath in the dark for 30 min. Flow
cytometry (BD FACSCalibur; BD Biosciences) and FlowJo v10 (FlowJo,
LLC) were used to detect and analyze the apoptosis rate.
Western blotting
hPDLSCs were pretreated with 50 µM GAS for 1 h and
with 10 µg/ml LPS, which was added into the osteogenic induction
culture medium for 7 days. After treatment, hPDLSCs were lysed
using RIPA lysis buffer (Beyotime Institute of Biotechnology) at
4˚C and the protein concentration was detected using a BCA kit
(Abcam). Proteins (30 µg/lane) were separated by 10% SDS-PAGE and
transferred onto PVDF membranes, which were blocked with 5% skimmed
milk at room temperature for 2 h. The membranes were then incubated
at 4˚C overnight with primary antibodies against ALP (1:1,000; cat.
no. sc-271431; Santa Cruz Biotechnology, Inc.), RUNX family
transcription factor 2 (Runx2; 1:1,000; cat. no. sc-390351; Santa
Cruz Biotechnology, Inc.), osteocalcin (OCN; 1:1,000; cat. no.
sc-365797; Santa Cruz Biotechnology, Inc.), osteopontin (OPN;
1:1,000; cat. no. sc-21742; Santa Cruz Biotechnology, Inc.), SIRT3
(1:1,000; cat. no. sc-365175; Santa Cruz Biotechnology, Inc.),
Bcl-2 (1:1,000; cat. no. sc-7382; Santa Cruz Biotechnology, Inc.),
Bax (1:1,000; cat. no. sc-7480; Santa Cruz Biotechnology, Inc.),
pro-caspase-3 (1:1,000; cat. no. sc-7272; Santa Cruz Biotechnology,
Inc.), pro-caspase-9 (1:1,000; cat. no. sc-56076; Santa Cruz
Biotechnology, Inc.), GAPDH (1:1,000; cat. no. sc-47724; Santa Cruz
Biotechnology, Inc.), cleaved caspase-3 (1:1,000; cat. no. 9661;
Cell Signaling Technology, Inc.) and cleaved caspase-9 (1:1,000;
cat. no. 20750; Cell Signaling Technology, Inc.). GAPDH was used as
the internal loading control. Following the primary antibody
incubation, the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:10,000; cat. no.
ab205718; Abcam) or goat anti-mouse IgG secondary antibody
(1:10,000; cat. no. 43593; Cell Signaling Technology, Inc.) for 2 h
at room temperature. Protein bands were visualized using enhanced
chemiluminescence reagent (Santa Cruz Biotechnology, Inc.) and
exposed to X-ray film (Kodak). Relative expression levels were
normalized to endogenous control GAPDH using GelDox XR system
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0.1 software (GraphPad Software, Inc.). All data were
confirmed to be normally distributed using a D'Agostino-Pearson
test and were presented as the means ± standard deviation of three
independent experiments. Statistical differences between the
various experimental groups were analyzed using a one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
GAS rescues the LPS-induced decreased
viability of hPDLSCs
hPDLSCs derived from periodontal ligament explants
had a long, spindle-like morphology (Fig. 1A). The effect of GAS on hPDLSC
viability was determined and the results revealed that GAS, at
concentrations of 0.1, 1, 10, 50 or 100 µM, did not exert
significant effects on cell viability (Fig. 1B). Subsequently, the effect of 0.1,
1, 10 or 50 µM GAS on the viability of LPS-stimulated hPDLSCs was
evaluated. The results demonstrated that cell viability was
significantly impaired following LPS treatment, while the addition
of 10 or 50 µM GAS effectively restored hPDLSC viability (Fig. 1C). Subsequent experiments were
performed using 50 µM GAS, as the viability of LPS-treated hPDLSCs
was the highest following GAS treatment at this dose. These data
indicated that GAS may rescue the viability of hPDLSCs injured by
LPS.
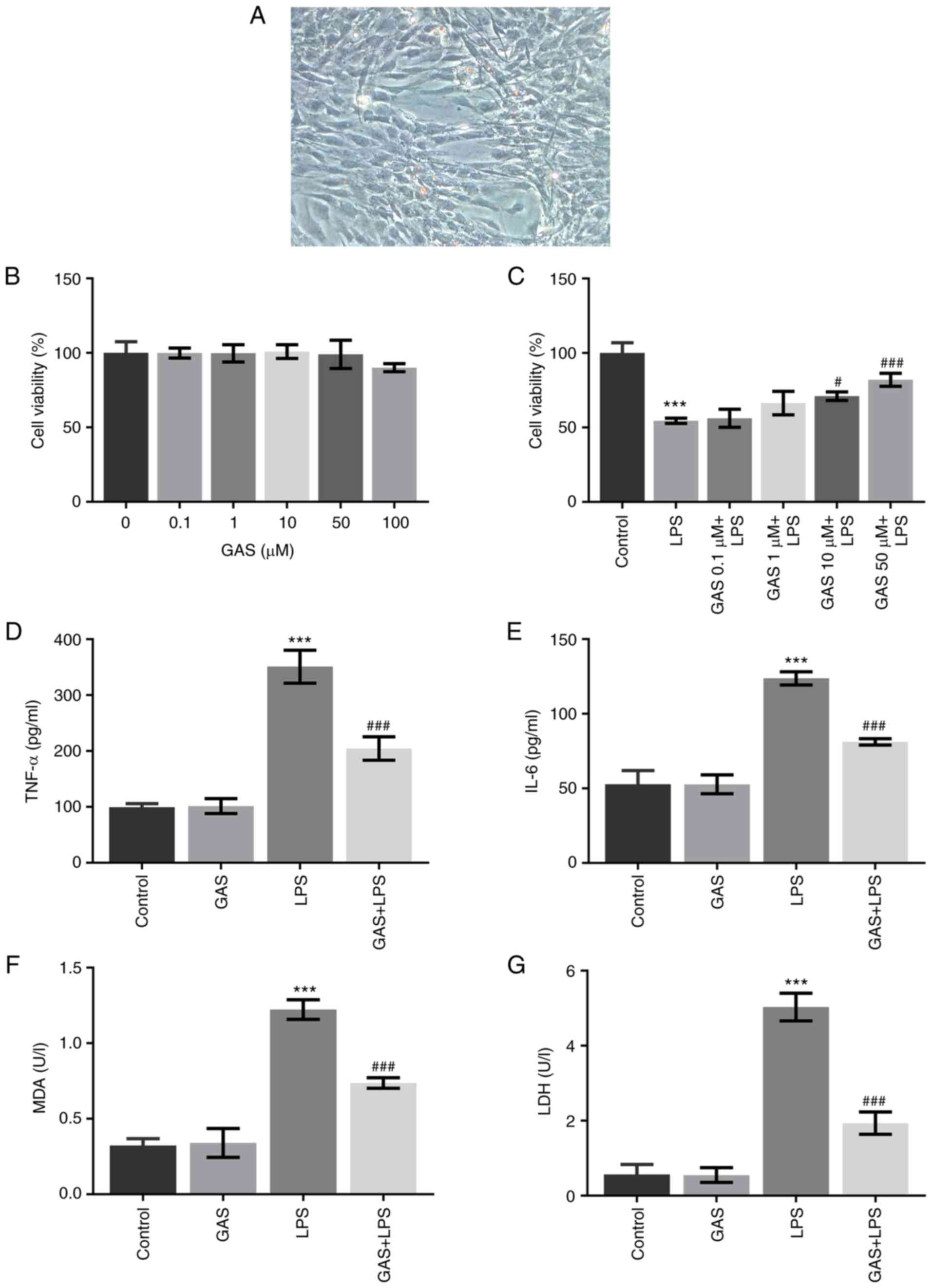 | Figure 1Effect of GAS on LPS-induced
inflammation and oxidative stress in hPDLSCs. (A) hPDLSCs derived
from periodontal ligament explants had a long spindle-like
morphology (magnification, x200). (B) Viability of hPDLSCs exposed
to different concentrations of GAS (0, 0.1, 1, 10, 50 and 100 µM).
(C) Viability of control hPDLSCs or LPS-treated hPDLSCs exposed to
different concentrations of GAS (0, 0.1, 1, 10 and 50 µM). (D and
E) Concentration of TNF-α and IL-6 in the culture medium of
hPDLSCs. (F and G) Concentration of MDA and LDH in the culture
medium of hPDLSCs. ***P<0.001 vs. control.
#P<0.05 and ###P<0.001 vs. LPS. GAS,
gastrodin; hPDLSCs, human periodontal ligament stem cells; IL-6,
interleukin-6; LDH, lactate dehydrogenase; LPS, lipopolysaccharide;
MDA, malondialdehyde; TNF-α, tumor necrosis factor-α. |
GAS inhibits the inflammation and
oxidative stress associated with LPS-treated hPDLSCs
The levels of released proinflammatory factors,
including TNF-α and IL-6, and the levels of markers of oxidative
stress, MDA and LDH, were determined using ELISAs and corresponding
kits. As presented in Fig. 1D and
E, the production of TNF-α and
IL-6 was not affected by 50 µM GAS, but it was markedly enhanced
following LPS treatment. However, the cotreatment of GAS and LPS
significantly reduced TNF-α and IL-6 concentrations compared with
the LPS group. Similarly, the LPS-induced increased contents of MDA
and LDH were also significantly decreased following treatment with
GAS (Fig. 1F and G, respectively). These results suggested
that GAS may suppress LPS-induced inflammation and oxidative stress
in hPDLSCs.
GAS promotes the osteogenic
differentiation of LPS-stimulated hPDLSCs
We investigated whether GAS could promote osteogenic
differentiation in LPS-treated hPDLSCs. The ALP activity was found
to be significantly decreased in the LPS group compared with that
in the control group, whereas ALP activity was significantly
increased in the GAS + LPS group compared with the LPS group
(Fig. 2A). As presented in
Fig. 2B and C, the LPS-induced decrease in the number
of mineralized nodules was significantly restored by the addition
of GAS. The expression of proteins reflecting the osteogenic
differentiation ability, including ALP, Runx2, OCN and OPN, was
also determined. The results demonstrated that GAS alone had no
effect on the expression of these proteins; however, LPS treatment
significantly decreased the expression of ALP, Runx2, OCN and OPN.
Conversely, addition of GAS to LPS-treated cells significantly
upregulated the expression of ALP, Runx2, OCN and OPN (Fig. 2D and E).
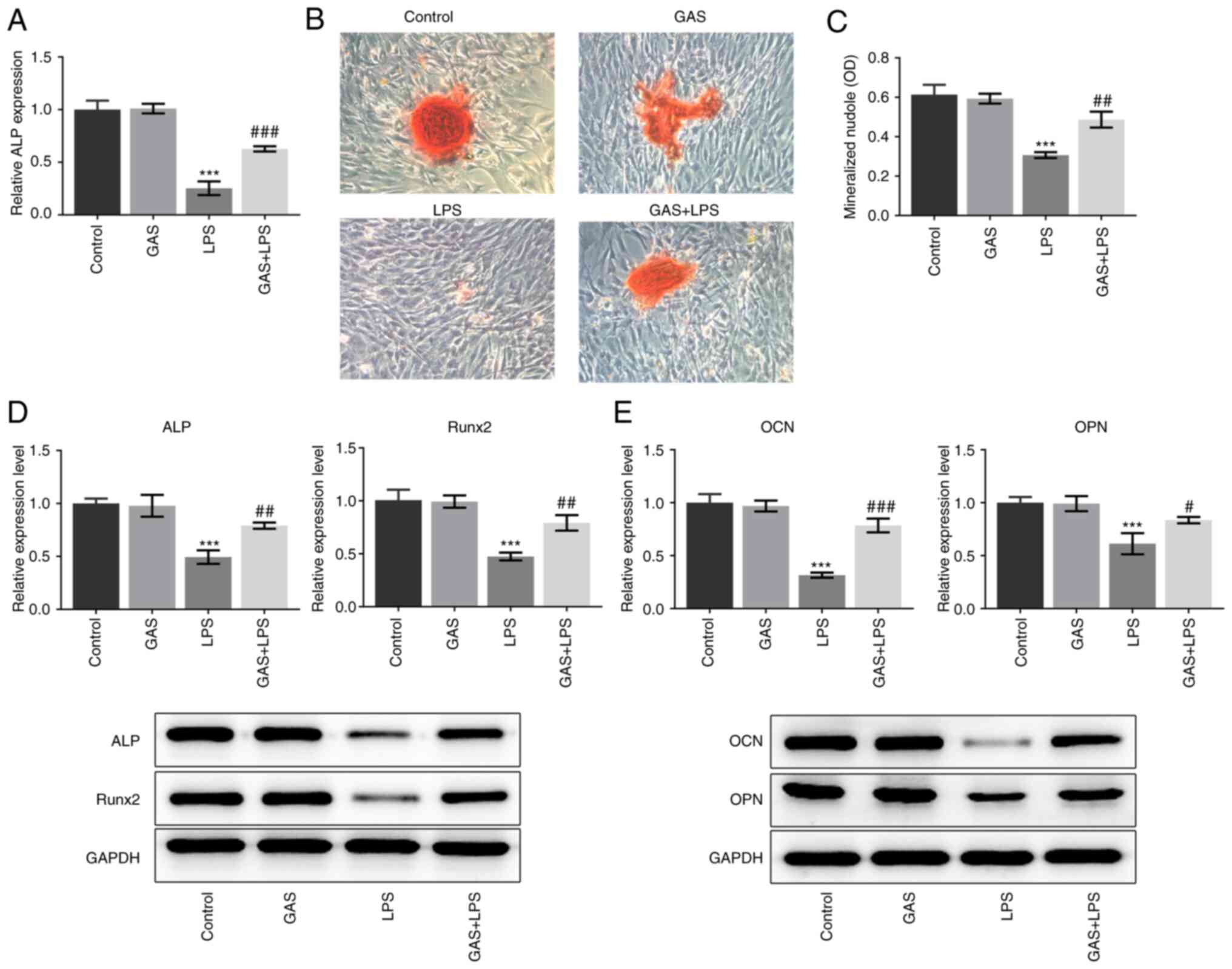 | Figure 2Effect of GAS on osteogenic
differentiation of LPS-stimulated hPDLSCs. (A) ALP expression in
hPDLSCs was measured using ALP kit. (B) Alizarin Red staining
images of hPDLSCs (magnification, x200). (C) Alizarin Red staining
quantification. (D) Protein expression of ALP and Runx2 was
detected by western blotting. (E) Protein expression of OCN and OPN
was detected by western blotting. ***P<0.001 vs.
control; #P<0.05, ##P<0.01 and
###P<0.001 vs. LPS. ALP, alkaline phosphatase; GAS,
gastrodin; hPDLSCs, human periodontal ligament stem cells; LPS,
lipopolysaccharide; OCN, osteocalcin; OD, optical density; OPN,
osteopontin; Runx2, RUNX family transcription factor 2. |
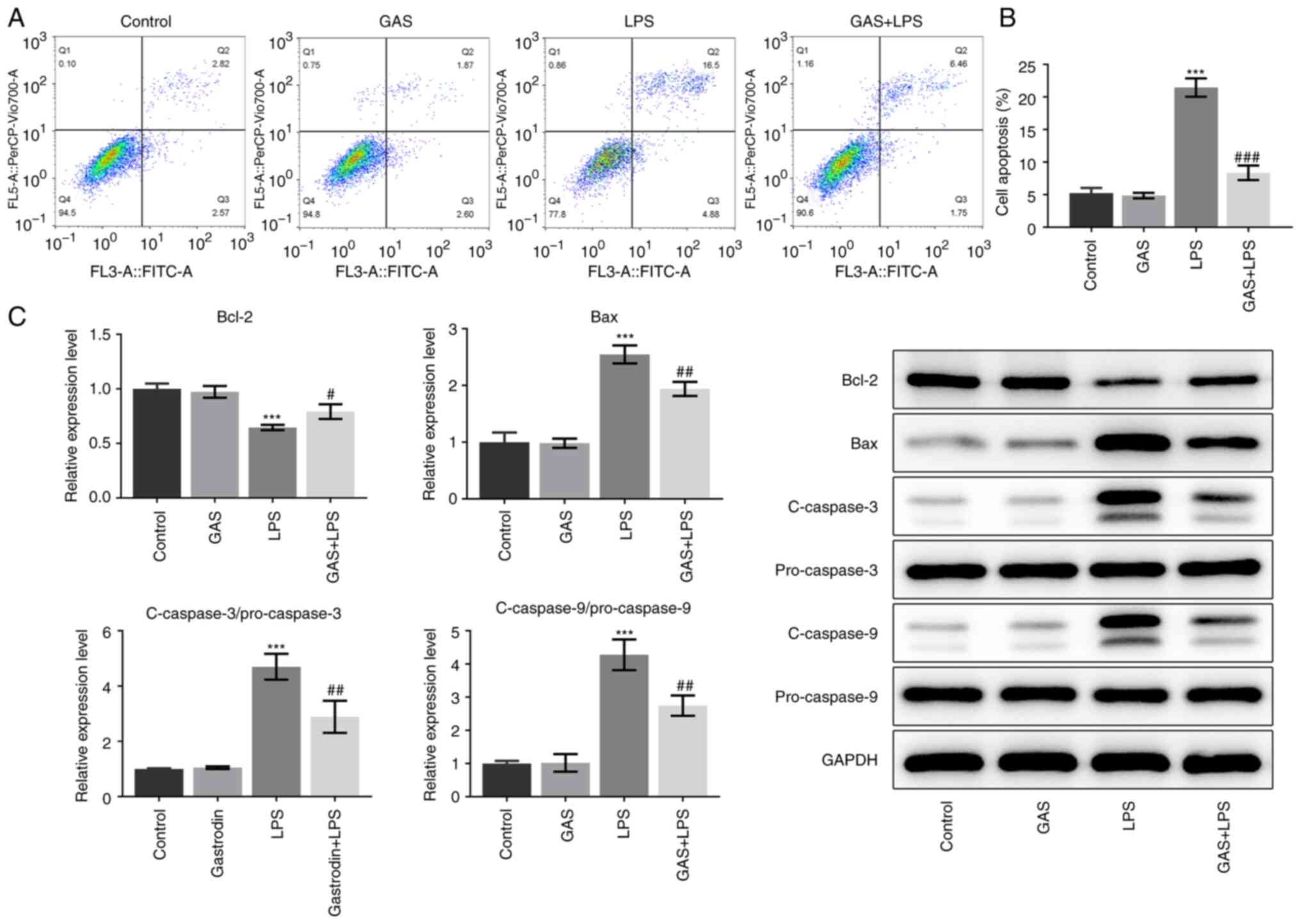
GAS inhibits the apoptosis of
LPS-treated hPDLSCs
The changes in hPDLSC apoptosis were measured
following different treatments. As presented in Fig. 3A and B, the results from flow cytometry
revealed that LPS significantly increased the cell apoptotic rate,
which was then significantly decreased following cotreatment with
GAS. Furthermore, as seen in Fig.
3C, GAS had no effect on Bcl-2, Bax and cleaved caspase-3/9
expression, while LPS stimulation significantly decreased Bcl-2
expression and increased Bax and cleaved caspase-3/9 expression.
Additionally, co-treatment with GAS and LPS significantly
attenuated the effects of LPS alone in hPDLSCs, suggesting that
inhibition of apoptosis by GAS was partially a result of modulation
of these apoptosis-associated proteins.
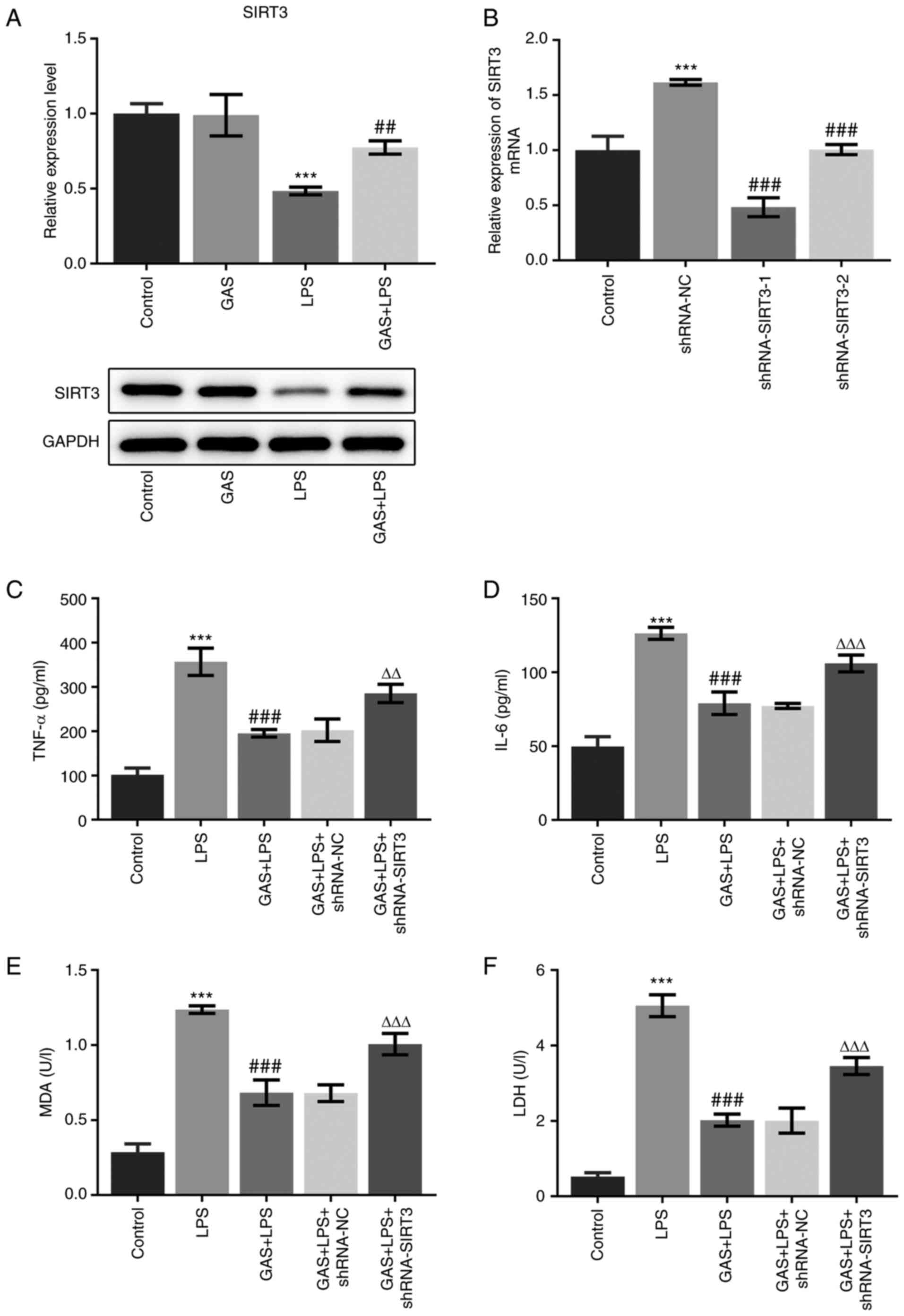
SIRT3 knockdown suppresses the
protective effect of GAS on LPS-induced hPDLSC injury
We aimed to confirm the role of SIRT3 in the
protective effect of GAS against LPS-induced hPDLSC injury. The
expression of SIRT3 in hPDLSCs cultured in control medium and
medium supplemented with GAS, LPS or GAS + LPS, was detected. As
illustrated in Fig. 4A, SIRT3
expression was not altered in the GAS group, while it was
significantly decreased in the LPS group compared with the control
group. Furthermore, SIRT3 expression was found to be significantly
increased in the GAS + LPS group compared with the LPS group,
indicating that the addition of GAS may upregulate the expression
of SIRT3.
To further investigate the regulatory effect of
SIRT3, its gene expression was silenced using targeting shRNA.
shRNA-SIRT3-1 was selected to knockdown SIRT3, as it exerted the
highest transfection efficiency (Fig.
4B). The effect of GAS on the LPS-induced production of TNF-α,
IL-6, MDA and LDH was significantly suppressed following SIRT3
knockdown (Fig. 4C-F). In
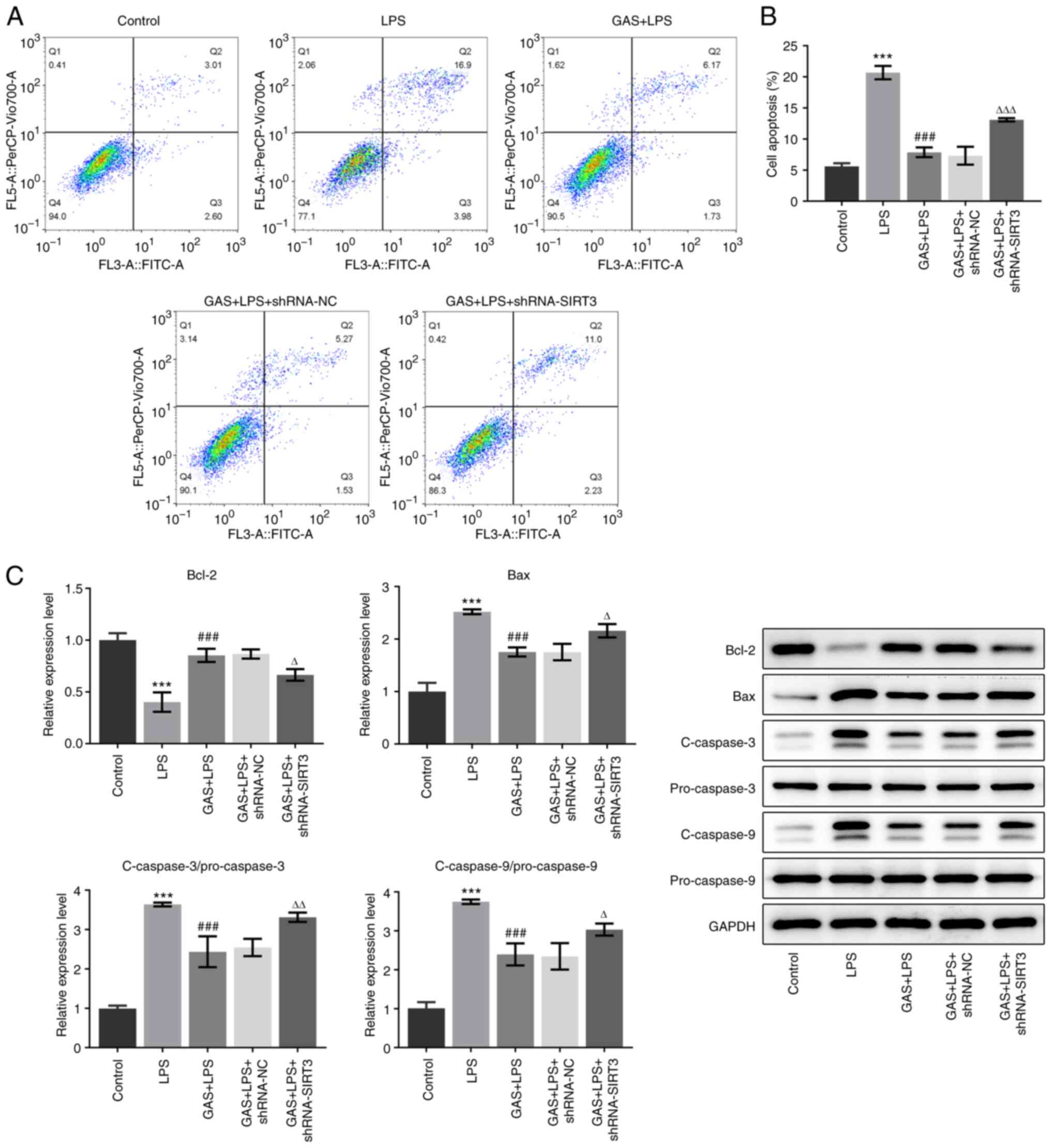
addition, compared with the LPS group, the increased activity of
ALP and number of mineralized nodules in the GAS + LPS group were
significantly diminished following SIRT3 silencing (Fig. 5A-C). The results presented in
Fig. 5D and E demonstrated that the effects of GAS on
ALP, Runx2, OCN and OPN expression in LPS-stimulated hPDLSCs were
significantly weakened by SIRT3 knockdown. Furthermore, compared
with the LPS group, the GAS-induced decrease in the apoptotic cell
rate (Fig. 6A and B), alongside its effects on the
expression of proteins associated with apoptosis in LPS-treated
hPDLSCs (Fig. 6C), were also
prevented by SIRT3 knockdown. These findings indicated that the
protective effect of GAS against LPS-induced hPDLSC injury may be
mediated by SIRT3 upregulation.
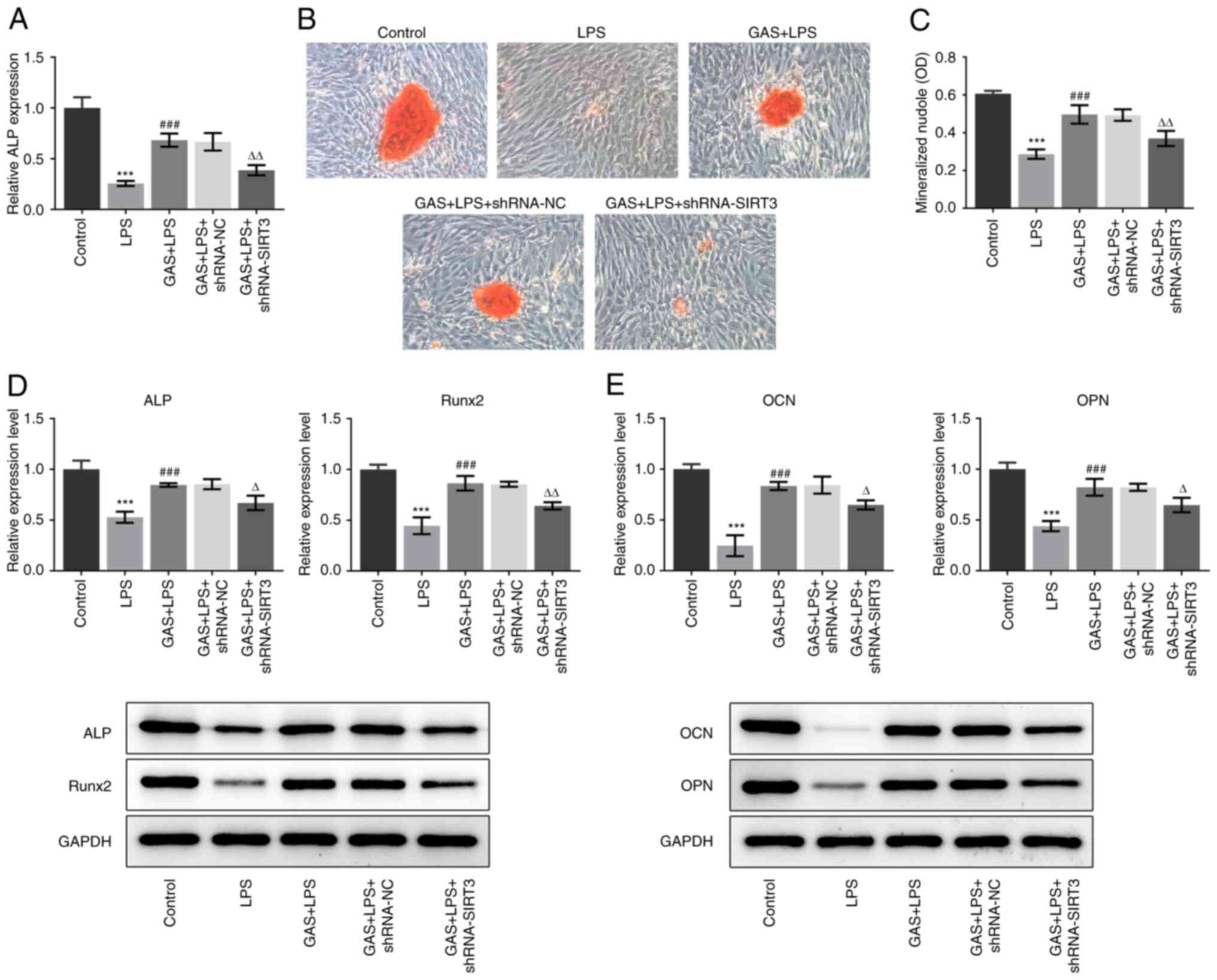 | Figure 5Effect of SIRT3 silencing on GAS
promotion of osteogenic differentiation in LPS-stimulated hPDLSCs.
(A) ALP expression in hPDLSCs was measured using ALP kit. (B)
Alizarin Red staining images of hPDLSCs (magnification, x200). (C)
Alizarin Red staining quantification. (D) Expression of ALP and
Runx2 was detected by western blotting. (E) Expression of OCN and
OPN was detected by western blotting. ***P<0.001 vs.
control; ###P<0.001 vs. LPS; ∆P<0.05,
∆∆P<0.01 vs. GAS + LPS + shRNA-NC. ALP, alkaline
phosphatase; GAS, gastrodin; hPDLSCs, human periodontal ligament
stem cells; LPS, lipopolysaccharide; NC, negative control; OCN,
osteocalcin; OD, optical density; OPN, osteopontin; Runx2, RUNX
family transcription factor 2; sh, short hairpin; SIRT3, sirtuin
3. |
Discussion
Chronic periodontitis is a severe inflammatory
disease, which results in the destruction of periodontal tissues.
LPS is an active pathogenic substance of gram-negative bacteria
that serves an important role in the occurrence and development of
periodontitis. Previous studies have reported that the LPS content
in gingival crevicular fluid is closely associated with the degree
of inflammation in periodontal tissue, and the higher the LPS
content, the more severe the inflammation of the periodontal tissue
(29-31).
LPS can cause local allergic reactions in the periodontal tissue by
activating monocytes to produce cytokines and activating complement
to release allergic mediators, thereby leading to the destruction
of the periodontal tissue (32).
In addition, LPS has been demonstrated to inhibit the osteogenic
differentiation of hPDLSCs in vitro (33). In the present study, hPDLSCs were
cultured with an appropriate concentration of LPS simulate the
inflammatory microenvironment. Consistent with findings from a
previous study (34), we observed
that LPS stimulation impaired cell viability, induced the
production of cytokines involved in the inflammatory response and
led to an increased oxidative stress. The activity of ALP, the
number of mineralized nodules and the protein expression of ALP,
Runx2, OCN and OPN were also decreased following LPS stimulation.
In addition, LPS triggered the apoptosis of hPDLSCs. These results
confirmed the extent of injury and destruction caused by LPS onto
hPDLSCs.
GAS has been widely reported to exhibit beneficial
effects in a variety of neurological diseases and psychiatric
disorders, such as Alzheimer's disease, Parkinson's disease and
affective disorders, by inhibiting oxidation, inflammation and
apoptosis, suppressing microglial activation and regulating
mitochondrial cascades (35).
Although a previous study revealed the protective effect of GAS on
bone-related diseases (36),
research into the specific effects and underlying mechanisms of
action of GAS in LPS-induced hPDLSC injury is unknown to the best
of our knowledge. The present study demonstrated that GAS could
protect hPDLSCs against LPS-induced decrease in cell viability. The
concentration of proinflammatory cytokines and MDA levels in
LPS-stimulated hPDLSCs were also decreased following GAS treatment.
In particular, the LPS-induced inhibition of ALP activity,
mineralized nodule formation and ALP, Runx2, OCN and OPN
expression, were all rescued by GAS. Enhanced ALP activity is known
to increase the concentration of calcium and phosphorus ions in the
bone tissue, promote the deposition of calcium and phosphorus in
the bone tissue and thus promote bone tissue mineralization
(37). Runx2 appears to be the
master gene involved in the process of osteogenesis, as increased
Runx2 can promote the expression of OPN and OCN, which are both
osteogenesis-related markers and required for terminal osteoblast
differentiation (38). The results
of the present study indicated that GAS may promote the osteogenic
differentiation of hPDLSCs in response to LPS. Furthermore, GAS
could prevent LPS-induced cell apoptosis, which further confirmed
the anti-apoptotic effect of GAS.
Mechanistically, the results of the present study
revealed that LPS could downregulate SIRT3 expression, whereas
addition of GAS partially reversed this effect, suggesting that GAS
may exert its beneficial effect on hPDLSCs via upregulating SIRT3
expression. To validate this hypothesis, SIRT3 knockdown
experiments were performed. The results demonstrated that the
inhibitory effect of GAS on LPS-induced hPDLSC viability was
diminished, and the inhibition of inflammation, oxidative stress
and osteogenic differentiation, and increased level of cell
apoptosis induced by GAS, were effectively suppressed by SIRT3
silencing. A previous study reported that, following SIRT3
knockdown, the expression levels of bone formation-related genes
are significantly downregulated, which might be associated with
peroxisome proliferator-activated receptor γ coactivator 1-α
(PGC-1α)/superoxide dismutase 2 (SOD2)-induced regulation of
mitochondrial function (39). In
addition to the influence of SIRT3 silencing on the effects of GAS
on bone formation-related genes, the present study also
demonstrated that SIRT3 could affect MDA levels, which is a
prominent marker of oxidative stress (40). Therefore, the underlying mechanisms
of GAS in osteogenic differentiation and oxidative stress may be
associated with the SIRT3-induced regulation of PGC-1α/SOD2. Taken
together, the findings from the present study indicated that GAS
may partly exert its effect on LPS-induced hPDLSC injury by
upregulating SIRT3.
In summary, to the best of our knowledge, the
present study was the first to demonstrate the protective effect of
GAS against inflammation, oxidative stress and apoptosis in
LPS-treated hPDLSCs, where it ultimately promoted osteogenic
differentiation. NADH levels have been used to reflect the
mitochondrial function of hPDLSCs (41), and this will be further
investigated to determine the underlying mechanism of GAS. This
study showed that the effects of GAS may, at least in part, depend
on the upregulation of SIRT3. These results not only enhanced the
current understanding of the beneficial effects of GAS, but also
provided evidence for the therapeutic application of GAS in
alleviating or treating periodontitis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QF contributed to the conception and design of the
work and the acquisition, analysis and interpretation of data. QF
drafted the manuscript and revised it critically for important
intellectual content, and confirms the authenticity of all raw
data. QF read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Xingyi People's Hospital (approval no. 20190512). The patients
provided consent for the use of their samples in scientific
research.
Patient consent for publication
Not applicable.
Competing interests
The author declares that there are no competing
interests.
References
|
1
|
Kumar S: Evidence-based update on
diagnosis and management of gingivitis and periodontitis. Dent Clin
North Am. 63:69–81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Smith MM, Knight ET, Al-Harthi L and
Leichter JW: Chronic periodontitis and implant dentistry.
Periodontol 2000. 74:63–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hoare A, Soto C, Rojas-Celis V and Bravo
D: Chronic inflammation as a link between periodontitis and
carcinogenesis. Mediators Inflamm. 2019(1029857)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tomokiyo A, Wada N and Maeda H:
Periodontal ligament stem cells: Regenerative potency in
periodontium. Stem Cells Dev. 28:974–985. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cen LP, Ng TK, Liang JJ, Zhuang X, Yao X,
Yam GH, Chen H, Cheung HS, Zhang M and Pang CP: Human periodontal
ligament-derived stem cells promote retinal ganglion cell survival
and axon regeneration after optic nerve injury. Stem Cells.
36:844–855. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu Y, Zheng Y, Ding G, Fang D, Zhang C,
Bartold PM, Gronthos S, Shi S and Wang S: Periodontal ligament stem
cell-mediated treatment for periodontitis in miniature swine. Stem
Cells. 26:1065–1073. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zheng W, Wang S, Wang J and Jin F:
Periodontitis promotes the proliferation and suppresses the
differentiation potential of human periodontal ligament stem cells.
Int J Mol Med. 36:915–922. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang HS, Liu MF, Ji XY, Jiang CR, Li ZL
and OuYang B: Gastrodin combined with rhynchophylline inhibits
cerebral ischaemia-induced inflammasome activation via upregulating
miR-21-5p and miR-331-5p. Life Sci. 239(116935)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sui Y, Bian L, Ai Q, Yao Y, Yu M, Gao H,
Zhang A, Fu X, Zhong L and Lu D: Gastrodin inhibits inflammasome
through the STAT3 signal pathways in TNA2 astrocytes and reactive
astrocytes in experimentally induced cerebral ischemia in rats.
Neuromolecular Med. 21:275–286. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qiu CW, Liu ZY, Zhang FL, Zhang L, Li F,
Liu SY, He JY and Xiao ZC: Post-stroke gastrodin treatment
ameliorates ischemic injury and increases neurogenesis and restores
the Wnt/β-catenin signaling in focal cerebral ischemia in mice.
Brain Res. 1712:7–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu B, Li F, Shi J, Yang D, Deng Y and
Gong Q: Gastrodin ameliorates subacute phase cerebral
ischemia-reperfusion injury by inhibiting inflammation and
apoptosis in rats. Mol Med Rep. 14:4144–4152. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xi Z, Qiao Y, Wang J, Su H, Bao Z, Li H,
Liao X and Zhong X: Gastrodin relieves inflammation injury induced
by lipopolysaccharides in MRC-5 cells by up-regulation of miR-103.
J Cell Mol Med. 24:1451–1459. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang Z, Zhou J, Song D, Sun Y, Liao C and
Jiang X: Gastrodin protects against LPS-induced acute lung injury
by activating Nrf2 signaling pathway. Oncotarget. 8:32147–32156.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zheng H, Yang E, Peng H, Li J, Chen S,
Zhou J, Fang H, Qiu B and Wang Z: Gastrodin prevents
steroid-induced osteonecrosis of the femoral head in rats by
anti-apoptosis. Chin Med J (Engl). 127:3926–3931. 2014.PubMed/NCBI
|
|
16
|
Huang Q, Shi J, Gao B, Zhang HY, Fan J, Li
XJ, Fan JZ, Han YH, Zhang JK, Yang L, et al: Gastrodin: an ancient
Chinese herbal medicine as a source for anti-osteoporosis agents
via reducing reactive oxygen species. Bone. 73:132–144.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou F, Shen Y, Liu B, Chen X, Wan L and
Peng D: Gastrodin inhibits osteoclastogenesis via down-regulating
the NFATc1 signaling pathway and stimulates osseointegration in
vitro. Biochem Biophys Res Commun. 484:820–826. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu S, Fang T, Yang L, Chen Z, Mu S and Fu
Q: Gastrodin protects MC3T3-E1 osteoblasts from
dexamethasone-induced cellular dysfunction and promotes bone
formation via induction of the NRF2 signaling pathway. Int J Mol
Med. 41:2059–2069. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu SJ, Liu XY, Li JH, Guo J, Li F, Gui Y,
Li XH, Yang L, Wu CY, Yuan Y and Li JJ: Gastrodin attenuates
microglia activation through renin-angiotensin system and Sirtuin3
pathway. Neurochem Int. 120:49–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yi X, Guo W, Shi Q, Yang Y, Zhang W, Chen
X, Kang P, Chen J, Cui T, Ma J, et al: SIRT3-dependent
mitochondrial dynamics remodeling contributes to oxidative
stress-induced melanocyte degeneration in vitiligo. Theranostics.
9:1614–1633. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Giralt A and Villarroya F: SIRT3, a
pivotal actor in mitochondrial functions: Metabolism, cell death
and aging. Biochem J. 444:1–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
He X, Zeng H and Chen JX: Emerging role of
SIRT3 in endothelial metabolism, angiogenesis, and cardiovascular
disease. J Cell Physiol. 234:2252–2265. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen J, Wang A and Chen Q: SirT3 and p53
deacetylation in aging and cancer. J Cell Physiol. 232:2308–2311.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kanwal A, Pillai VB, Samant S, Gupta M and
Gupta MP: The nuclear and mitochondrial sirtuins, Sirt6 and Sirt3,
regulate each other's activity and protect the heart from
developing obesity-mediated diabetic cardiomyopathy. FASEB J.
33:10872–10888. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kitada M, Ogura Y, Monno I and Koya D:
Sirtuins and type 2 diabetes: Role in inflammation, oxidative
stress, and mitochondrial function. Front Endocrinol (Lausanne).
10(187)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hawkes CP and Mostoufi-Moab S: Fat-bone
interaction within the bone marrow milieu: Impact on hematopoiesis
and systemic energy metabolism. Bone. 119:57–64. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huh JE, Shin JH, Jang ES, Park SJ, Park
DR, Ko R, Seo DH, Kim HS, Lee SH, Choi Y, et al: Sirtuin 3 (SIRT3)
maintains bone homeostasis by regulating AMPK-PGC-1β axis in mice.
Sci Rep. 6(22511)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao Y, Liu H, Xi X, Chen S and Liu D:
TRIM16 protects human periodontal ligament stem cells from
oxidative stress-induced damage via activation of PICOT. Exp Cell
Res. 397(112336)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liljestrand JM, Paju S, Buhlin K, Persson
GR, Sarna S, Nieminen MS, Sinisalo J, Mäntylä P and Pussinen PJ:
Lipopolysaccharide, a possible molecular mediator between
periodontitis and coronary artery disease. J Clin Periodontol.
44:784–792. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen Q, Liu X, Wang D, Zheng J, Chen L,
Xie Q, Liu X, Niu S, Qu G, Lan J, et al: Periodontal
inflammation-triggered by periodontal ligament stem cell pyroptosis
exacerbates periodontitis. Front Cell Dev Biol.
9(663037)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nakajima Y, Furuichi Y, Biswas KK,
Hashiguchi T, Kawahara K, Yamaji K, Uchimura T, Izumi Y and
Maruyama I: Endocannabinoid, anandamide in gingival tissue
regulates the periodontal inflammation through NF-kappaB pathway
inhibition. FEBS Lett. 580:613–619. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jekabsone A, Sile I, Cochis A,
Makrecka-Kuka M, Laucaityte G, Makarova E, Rimondini L, Bernotiene
R, Raudone L, Vedlugaite E, et al: Investigation of antibacterial
and antiinflammatory activities of proanthocyanidins from
pelargonium sidoides DC root extract. Nutrients.
11(2829)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu H, Zheng J, Zheng T and Wang P:
Exendin-4 regulates Wnt and NF-κB signaling in
lipopolysaccharide-induced human periodontal ligament stem cells to
promote osteogenic differentiation. Int Immunopharmacol.
75(105801)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhong JY, Cui RR, Lin X, Xu F, Zhu T, Li
F, Wu F, Zhou E, Yi L and Yuan LQ: Aberrant DNA methylation of
synaptophysin is involved in adrenal cortisol-producing adenoma.
Aging (Albany NY). 11:5232–5245. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu Y, Gao J, Peng M, Meng H, Ma H, Cai P,
Xu Y, Zhao Q and Si G: A review on central nervous system effects
of gastrodin. Front Pharmacol. 9(24)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zheng B, Shi C, Muhammed FK, He J,
Abdullah AO and Liu Y: Gastrodin alleviates bone damage by
modulating protein expression and tissue redox state. FEBS Open
Bio. 10:2404–2416. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sardiwal S, Magnusson P, Goldsmith DJ and
Lamb EJ: Bone alkaline phosphatase in CKD-mineral bone disorder. Am
J Kidney Dis. 62:810–822. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yin N, Zhu L, Ding L, Yuan J, Du L, Pan M,
Xue F and Xiao H: MiR-135-5p promotes osteoblast differentiation by
targeting HIF1AN in MC3T3-E1 cells. Cell Mol Biol Lett.
24(51)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ding Y, Yang H, Wang Y, Chen J, Ji Z and
Sun H: Sirtuin 3 is required for osteogenic differentiation through
maintenance of PGC-1α-SOD2-mediated regulation of mitochondrial
function. Int J Biol Sci. 13:254–264. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yu X, Meng X, Xu M, Zhang X, Zhang Y, Ding
G, Huang S, Zhang A and Jia Z: Celastrol ameliorates cisplatin
nephrotoxicity by inhibiting NF-κB and improving mitochondrial
function. EBioMedicine. 36:266–280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tabrizi R, Moosazadeh M, Lankarani KB,
Akbari M, Heydari ST, Kolahdooz F and Asemi Z: The effects of
synbiotic supplementation on glucose metabolism and lipid profiles
in patients with diabetes: A systematic review and meta-analysis of
randomized controlled trials. Probiotics Antimicrob Proteins.
10:329–342. 2018.PubMed/NCBI View Article : Google Scholar
|