Introduction
Inflammation is a complex defensive response to
several stimuli and injury. According to epidemiological and
clinical data, inflammation is a major mechanism underlying chronic
diseases or their complications, such as arthritis (1), cardiovascular disease (2), cancer (3) and type Ⅱ diabetes mellitus (4). Therefore, anti-inflammatory
strategies should be adopted in the treatment of these diseases.
Currently, the anti-inflammatory drugs approved for clinical use
mainly cover non-steroidal anti-inflammatory drugs and
corticosteroid hormones. However, the long-term consumption of
anti-inflammatory drugs triggers adverse reactions in various
organs. Therefore, there is an urgent requirement to discover novel
drugs with stronger curative effects and milder side effects.
Quercetin is a type of flavonoid ubiquitous in
fruits and vegetables (5,6). This flavonoid has antioxidative,
anticancer and anti-inflammatory properties (7). Quercetin inhibits inflammatory
responses in numerous diseases; however, the mechanisms involved
remain to be clarified (8,9).
Network pharmacology is an analytical tool
integrating systems biology, multidirectional pharmacology,
computational biology, network analysis and other emerging concepts
and methods (10). Network
pharmacology has been widely used in the functional analysis of
drugs. In the present study, network pharmacology, molecular
docking technology and in vitro experiments were used to
investigate the molecular mechanisms of the anti-inflammatory
effects of quercetin.
Materials and methods
Analysis of the anti-inflammatory
effects of quercetin Materials and reagents
The RAW264.7 cell line was purchased from the Cell
Bank of the Chinese Academy of Sciences. DMEM and FBS were from
Gibco (Thermo Fisher Scientific, Inc.). Lipopolysaccharide (LPS;
cat. no. L2630) was obtained from Sigma-Aldrich (Merck-KGaA). The
Cell Counting Kit (CCK)-8 assay kit (cat. no. C0037) was purchased
from Beyotime Institute of Biotechnology. The RNA Purification kit
(cat. no. B0004DP), RNA reverse transcription (RT) kit (cat. no.
EZB-RT2GQ) and the fluorescence quantitative PCR (qPCR) kit (cat.
no. A0001-R2) were from EZBioscience. The TNF-α (cat. no. EM3311S),
IL-6 (cat. no. EM3201S) and IL-1β (cat. no. EM3184S) ELISA duo set
kits were purchased from Biotechwell. Antibodies against
phosphorylated (p-)Akt (cat. no. T55561) and Akt (cat. no. T40067)
were obtained from Abmart. Antibodies against TNF-α (cat. no.
11948), IL6 (cat. no. 12912), β-tubulin (cat. no. 2148), GAPDH
(cat. no. 5174) and rabbit horseradish peroxidase-conjugated
secondary antibodies (cat. no. 7074) were purchased from Cell
Signaling Technology, Inc. The antibody against IL-1β (cat. no.
26048-1-AP) was obtained from ProteinTech Group, Inc. The Akt
inhibitor (cat. no. HY-108232) was purchased from
MedChemExpress.
Cell culture
The RAW264.7 cell line was maintained in DMEM
containing 10% FBS and 100 U/ml streptomycin/penicillin at 37˚C in
a humidified incubator with 5% CO2. Cells in the
logarithmic phase were used for the experiments.
Cell viability assay
Cell viability was analyzed using the CCK-8 assay
according to the manufacturer's protocol. In brief, the cells were
seeded into 96-well plates (1x104 cells/ml) for 12 h and
subsequently, quercetin was added to the cells at various
concentrations (0, 5, 10, 20, 40 and 80 µM), followed by incubation
at 37˚C for 24 h. Subsequently, 10 µl CCK-8 solution was added into
each well of the plate and cells were incubated for 3 h at 37˚C.
The optical density was measured at 450 nm using a microplate
reader (BioTek Instruments, Inc.).
RT-qPCR
The RAW264.7 cell line was treated with various
concentrations of quercetin (5, 10 and 20 µM) at 37˚C for 1 h in
6-well microplates and stimulated with LPS (1 µg/ml) at 37˚C for 24
h. The total RNA was extracted according to the manufacturer's
specification (EZBioscience). The concentration of RNA was
evaluated using spectrophotometric analysis. Total RNA (1 µg) was
reverse transcribed into cDNA using 4X EZscript Reverse
Transcription Mix Ⅱ. RT was performed at 42˚C for 15 min. The
fluorescence quantitative PCR was performed using a 2X SYBR-Green
qPCR Master Mix (ROX2 plus). Relative gene expression levels were
obtained following normalization to β-actin. The following
thermocycling conditions were used: Initial denaturation at 95˚C
for 5 min; followed by 40 cycles of 95˚C for 10 sec and 60˚C for 30
sec and the reaction was performed in a 7500 Real Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
2-ΔΔCq method was used to quantify the results (11). The primer sequences used for qPCR
were purchased from Sangon Biotech Co., Ltd. and the sequences of
the primers are presented in Table
I.
 | Table IPrimers used for quantitative PCR. |
Table I
Primers used for quantitative PCR.
| Gene | GenBank accession
no. | Direction | Sequence (5'-3') | Product length
(bp) |
|---|
| β-actin | NM_007393.5 | Forward |
ACTGTCGAGTCGCGTCC | 17 |
| | | Reverse |
CCCACGATGGAGGGGAATAC | 20 |
| TNF-α | NM_001278601.1 | Forward |
CCCTCCAGAAAAGACACCATG | 21 |
| | | Reverse |
CACCCCGAAGTTCAGTAGACAG | 22 |
| IL-6 | NM_001314054.1 | Forward |
GGGACTGATGCTGGTGACAAC | 21 |
| | | Reverse |
CAACTCTTTTCTCATTTCCACGA | 23 |
| IL-1β | XM_006498795.5 | Forward |
GCTTCAGGCAGGCAGTATCA | 20 |
| | | Reverse |
TGCAGTTGCTAATGGGAACG | 20 |
ELISA
Raw264.7 cells (3x105 cells/well) were
cultured in 6-well microplates at 37˚C for 12 h. Thereafter, the
RAW264.7 cell line was treated with various concentrations of
quercetin (5, 10 and 20 µM) at 37˚C for 1 h and stimulated with LPS
(1 µg/ml) at 37˚C for 24 h. The cell culture supernatant was then
obtained to measure the levels of TNF-α, IL-6 and IL-1β using the
ELISA kits according to the manufacturer's protocol.
Western blot analysis
Raw264.7 cells (3x105 cells/well) were
cultured in 6-well microplates at 37˚C for 12 h. Thereafter, the
RAW264.7 cell line was treated with various concentrations of
quercetin (5, 10 and 20 µM) at 37˚C for 1 h and stimulated with LPS
(1 µg/ml) at 37˚C for 6 h. Ice-cold PBS was used to wash the cells
three times and lysis solution (cat. no. P0013B; Beyotime Institute
of Biotechnology) was used to dissociate the cells on ice for 30
min. The protein concentration was determined using the
bicinchoninic acid method. Total protein (20 µg) was separated
using 10% SDS-PAGE and then transferred onto polyvinylidene
difluoride membranes (cat. no. IPVH00010 PORE; Merck Millipore) for
1 h at 350 mA. After blocking with 5% bovine serum albumin (BSA;
cat. no. 4240GR100; BioFroxx) for 1 h, the protein was incubated
with the primary antibodies [p-Akt (1:1,000 dilution), Akt (1:1,000
dilution), TNF-α (1:500), IL6 (1:500), IL-1β (1:500), β-tubulin
(1:1,000 dilution) or GAPDH (1:1,000 dilution)] overnight at 4˚C.
The membranes were washed four times with Tris-buffered
saline-Tween-20 (TBST) solution for 10 min and then incubated with
rabbit horseradish peroxidase-conjugated secondary antibodies
(1:10,000 dilution) in the presence of BSA for 1 h at room
temperature. Subsequently, the membranes were washed four times
with TBST for 10 min. ImageJ software 1.46 (National Institutes of
Health) was used for grey value analysis.
Network pharmacological analysis Data
collection
The targets of quercetin were obtained from the
Traditional Chinese Medicine database system pharmacology (TCMSP)
and analysis platform (http://tcmspw.com/tcmsp.php) using the key word
‘quercetin’. The ‘Canonical SMILES’ of quercetin was searched using
the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) with the key word
of ‘quercetin’ and then imported into Swiss Target Prediction
(http://www.swisstargetprediction.ch/index.php) to
obtain the targets of quercetin. The targets screened from the two
databases were combined and the overlapped targets were removed.
Subsequently, the targets associated with inflammation were
obtained from the GeneCards database (https://www.genecards.org/), with ‘inflammation’ as
the key word. The targets with a relevance score greater than the
median digit were screened.
Protein-protein interaction (PPI)
network and topology analysis
The targets of quercetin were mapped to those of
inflammation. The overlapped targets were imported into the Search
Tool for the Retrieval of Interacting Genes/proteins database
(https://string-db.org/) to construct the PPI
network. The host species was limited to ‘Homo sapiens’ and
the confidence score for the correlation degree was set to ≥0.4.
Subsequently, Cytoscape v3.7.2 was used for topology analysis.
Enrichment analyses
Gene set enrichment analysis was performed using the
Database for Annotation, Visualization and Integrated Discovery
(DAVID; https://david.ncifcrf.gov/). Gene
Ontology (GO) and Kyoto Encyclopedia Genes and Genomes enrichment
(KEGG) analyses were performed using the DAVID v6.7 database. The
species was set as ‘Homo sapiens’ and the significance
threshold was set as P<0.05.
Molecular docking
The molecular docking analysis was performed using
the TCM Network Pharmacology Analysis System (TCMNPAS; no.
2019SR1127090; http://54.223.75.62:3838/npa4/). The PSOVina
algorithm was used for molecular docking. First, the ‘Canonical
SMILES’ of quercetin was searched using the PubChem database and
the ‘protein database (PDB) ID’ of Akt (3O96) was obtained from the
PDB database (https://www.rcsb.org/). Subsequently,
the ‘Canonical SMILES’ of quercetin and ‘PDB ID’ of Akt were added
to the molecular docking module of TCMNPAS. ‘From ligand’ is an
online method to obtain protein docking pocket parameters based on
the ligand position. The ‘From ligand’ button was then selected.
Finally, the conformation and docking results of the compounds were
downloaded and imported into Pymol3.8 (https://pymol.org) to visualize their
three-dimensional structure.
In vitro experiments Effects of
quercetin on the phosphorylation of Akt
The RAW264.7 cell line was treated with various
concentrations of quercetin (5, 10 or 20 µM) at 37˚C for 1 h in
6-well microplates and stimulated with LPS (1 µg/ml) at 37˚C for 6
h. Other experimental steps were mentioned previously in the
western blot analysis subsection.
Comparison of the efficacy of the Akt
inhibitor MK-2206 and quercetin
The RAW264.7 cell line was preincubated with
quercetin (20 µM) and the AKT inhibitor (MK-2206; 5 µM) at 37˚C for
1 h in 6-well microplates and stimulated with LPS (1 µg/ml) at 37˚C
for 6 or 24 h. Other experimental steps were mentioned previously
in the RT-qPCR and western blot analysis subsections.
Statistical analysis
All the experiments were performed at least 3 times.
Data in this study were statistically processed by SPSS Statistics
22.0 (IBM Corporation). Values are expressed as the mean ± standard
deviation. Significant differences were analyzed using one-way
ANOVA followed by Tukey's post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Anti-inflammatory effects of quercetin
Effects of quercetin on cell viability
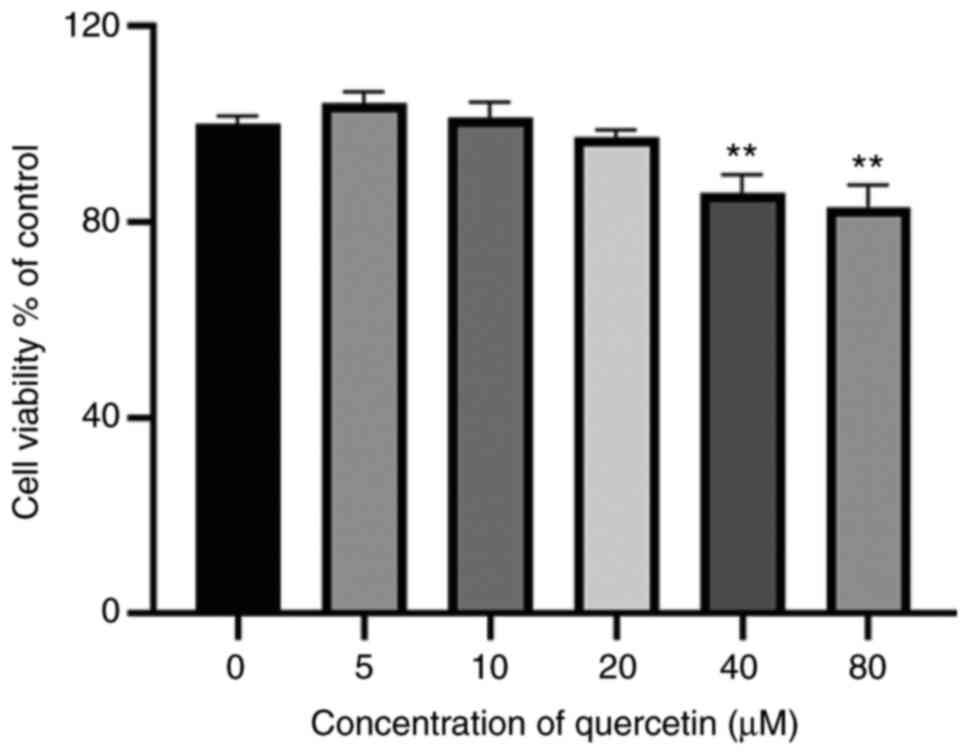
The results of the CCK-8 assay indicated that
treatment with quercetin (5-20 µM) for 24 h exerted no notable
effects on cell viability (Fig.
1). Therefore, 5-20 µM quercetin was used for the subsequent
experiments.
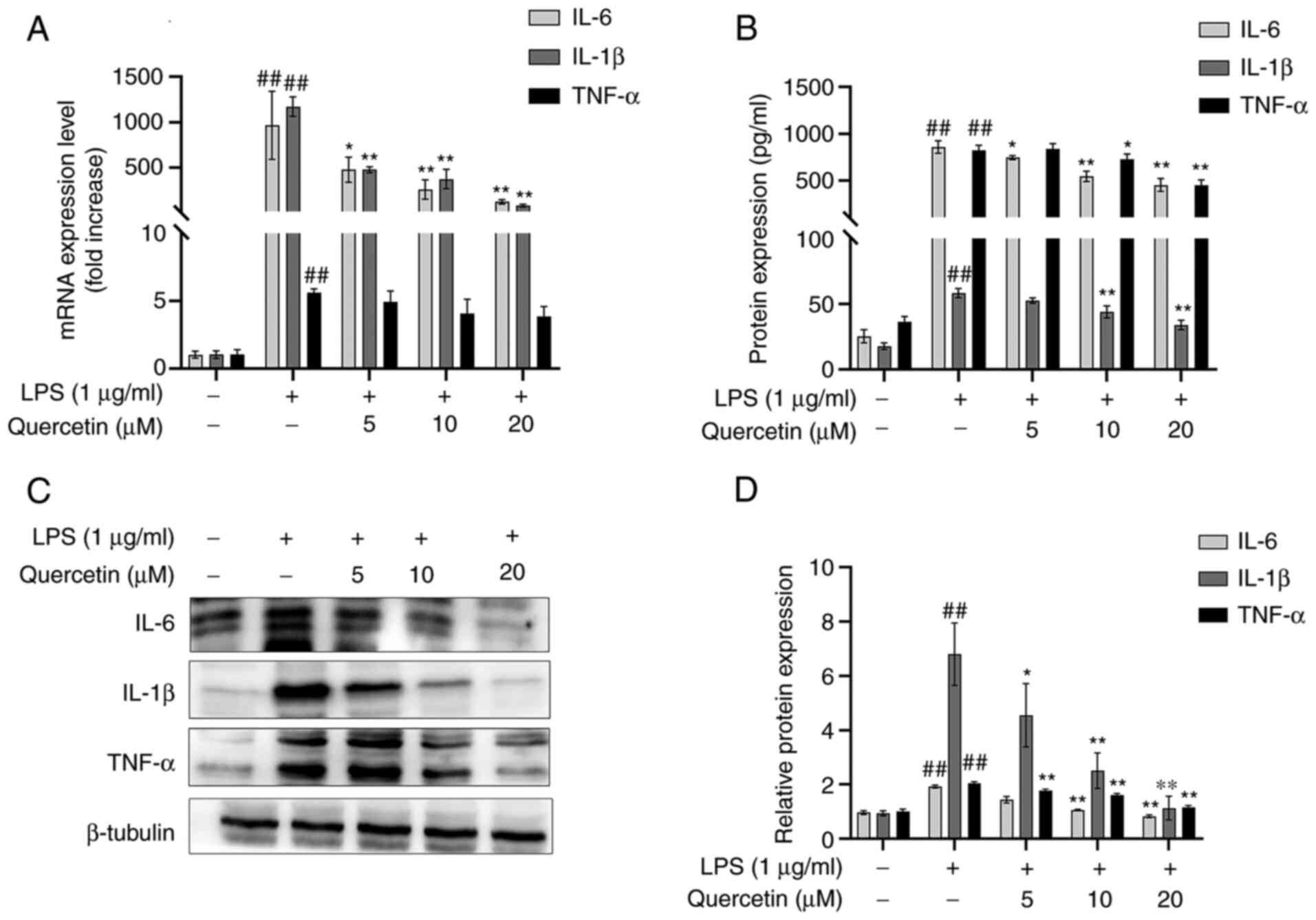
Effects of quercetin on TNF-α, IL-6
and IL-1β
As presented in Fig.
2A, after treatment with LPS, the mRNA expression levels of
TNF-α, IL-6 and IL-1β were significantly increased compared with
those in the blank group. In comparison, pre-treatment with
quercetin decreased the mRNA expression levels of TNF-α, IL-6 and
IL-1β in the RAW264.7 cell line in a dose-dependent manner.
The results of the ELISA suggested that after
stimulation with LPS, the concentrations of TNF-α, IL-6 and IL-1β
were significantly increased and this increase was attenuated in
the groups pre-treated with quercetin (Fig. 2B).
Western blot analysis also indicated that after
stimulation with LPS, the protein expression levels of TNF-α, IL-6
and IL-1β were significantly increased. However, in comparison,
pre-treatment with quercetin decreased the protein expression
levels of TNF-α, IL-6 and IL-1β in the RAW264.7 cell line (Fig. 2C and D).
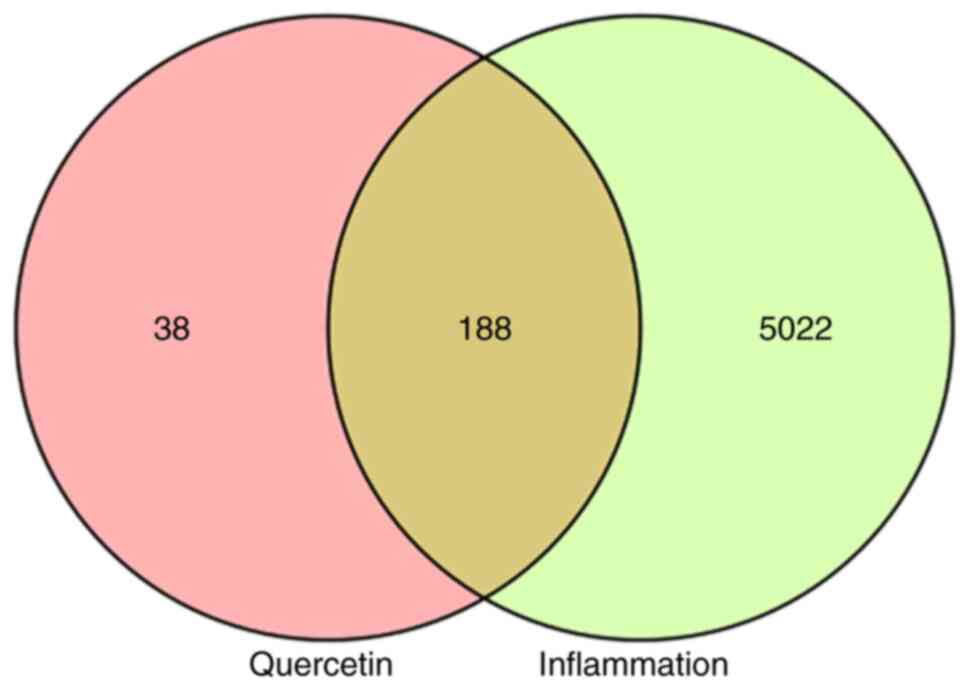
Results of network pharmacological
analysis Targets of quercetin and inflammation
A total of 226 potential targets of quercetin were
obtained using the TCMSP and Swiss Target Prediction databases. A
total of 10,454 differentially expressed genes were obtained from
the GeneCards database and 5,210 targets with a Relevance Score
>0.947288 were screened. Finally, 188 targets were selected for
enrichment analyses from an overlap in the Venn diagram (Fig. 3).
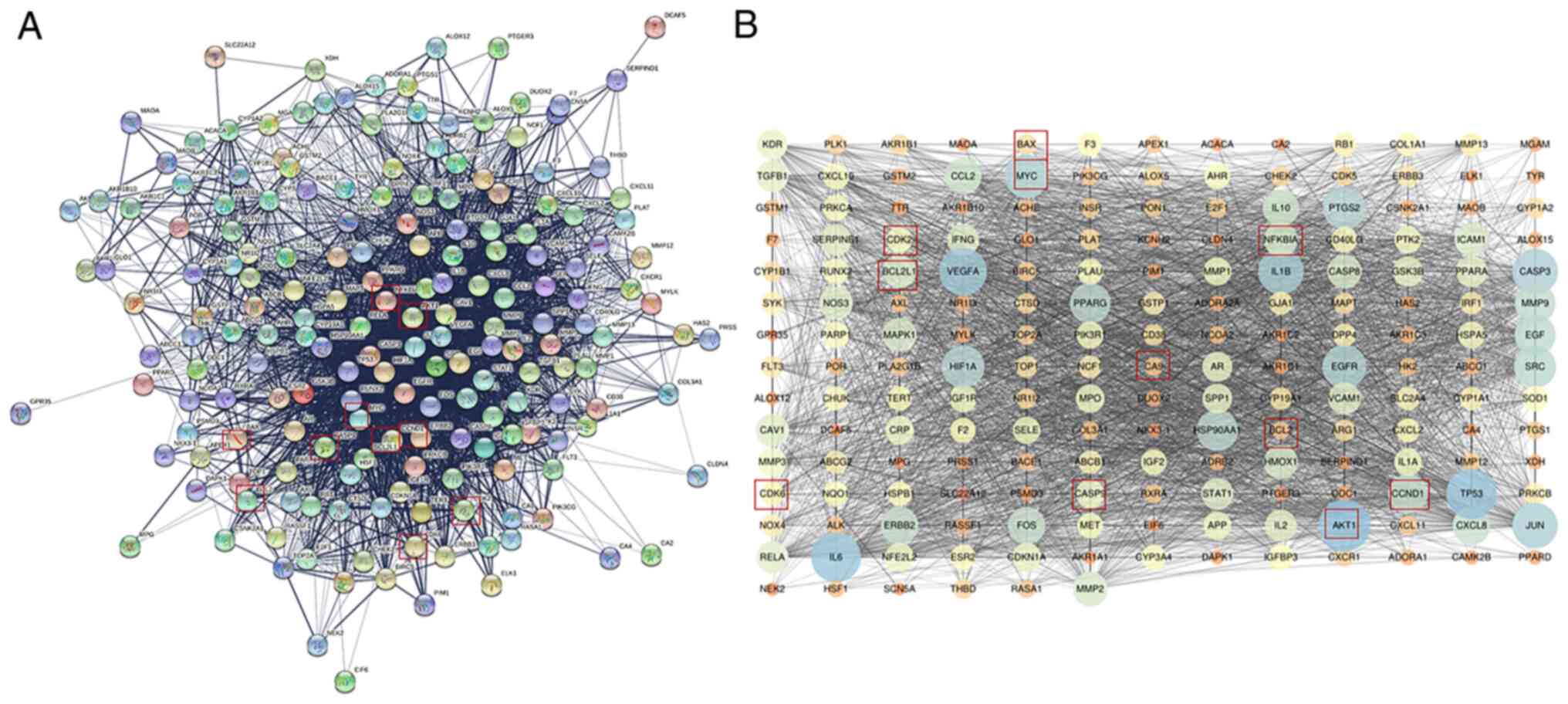
Network construction and topology
analysis
Fig. 4A presents
the PPI network of the potential targets of quercetin treatment to
counteract inflammation. The PPI network was constructed and
visualized using Cystoscape. The nodes and edges are presented in
Fig. 4B. All the Akt targets are
displayed in the figure. The average node degree value in the PPI
network was 34.556. The top 10 nodes with the largest degree of
connectivity values were AKT1, TP53, IL6, VEGFA, IL1B, CASP3, JUN,
MYC, EGFR and PTGS2.
Enrichment analysis of key
targets
A total of 188 selected key targets were submitted
for GO and KEGG enrichment analyses and screened with a cutoff of
P<0.05. A total of 651 targets were identified in the category
biological process (BP), 65 in cellular component (CC) and 144 in
the category molecular function (MF), while there were 122 enriched
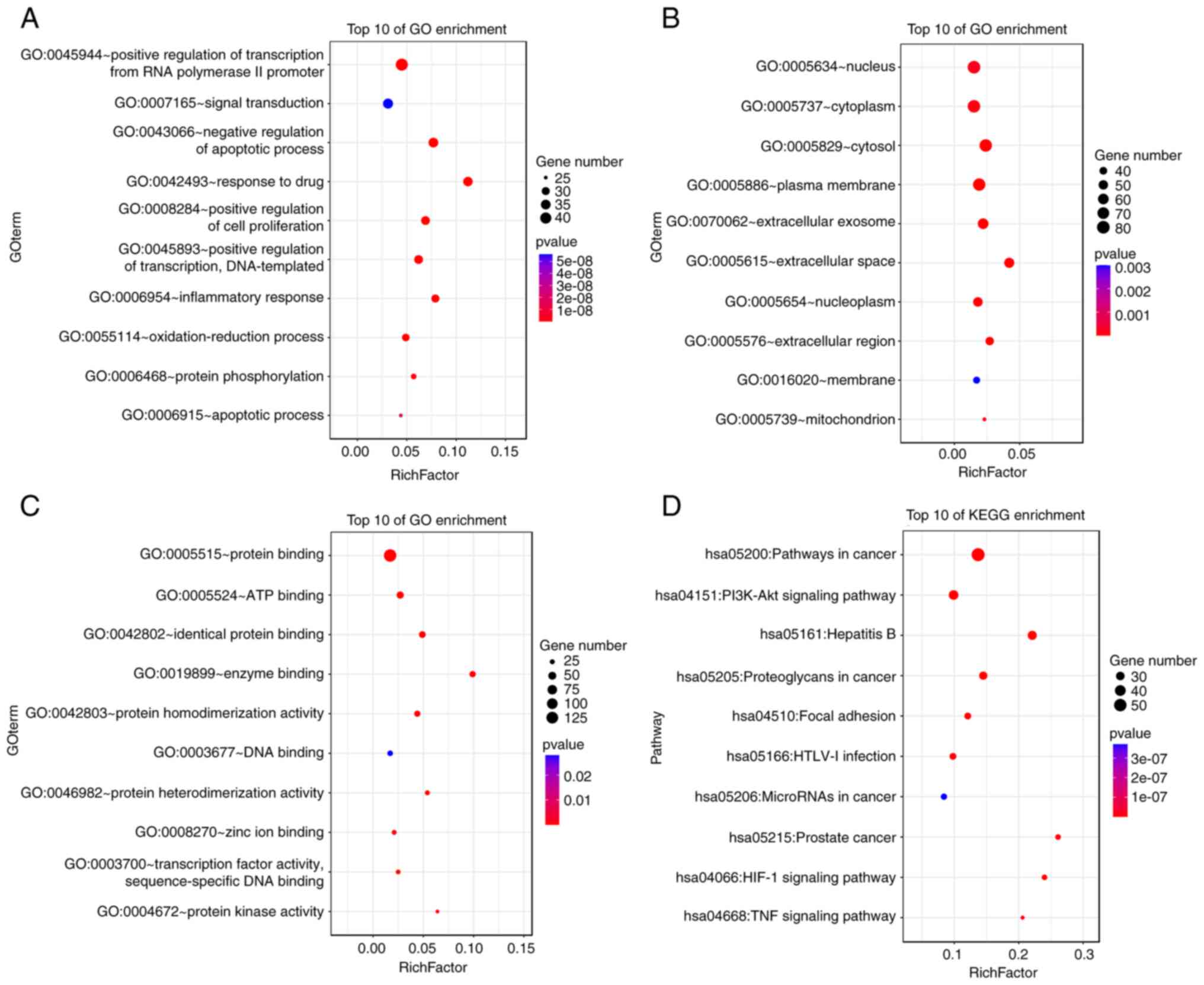
KEGG pathways. The top 10 enriched GO and KEGG pathway terms are
presented in Fig. 5. The BP
targets included positive regulation of transcription from RNA
polymerase II promoter, signal transduction and negative regulation
of apoptotic process. The CC targets included nucleus, cytoplasm
and cytosol. The MF targets included protein binding, ATP blinding
and identical protein binding. The KEGG pathways included cancer,
PI3K/AKT signaling pathway and the Hepatitis B signaling
pathway.
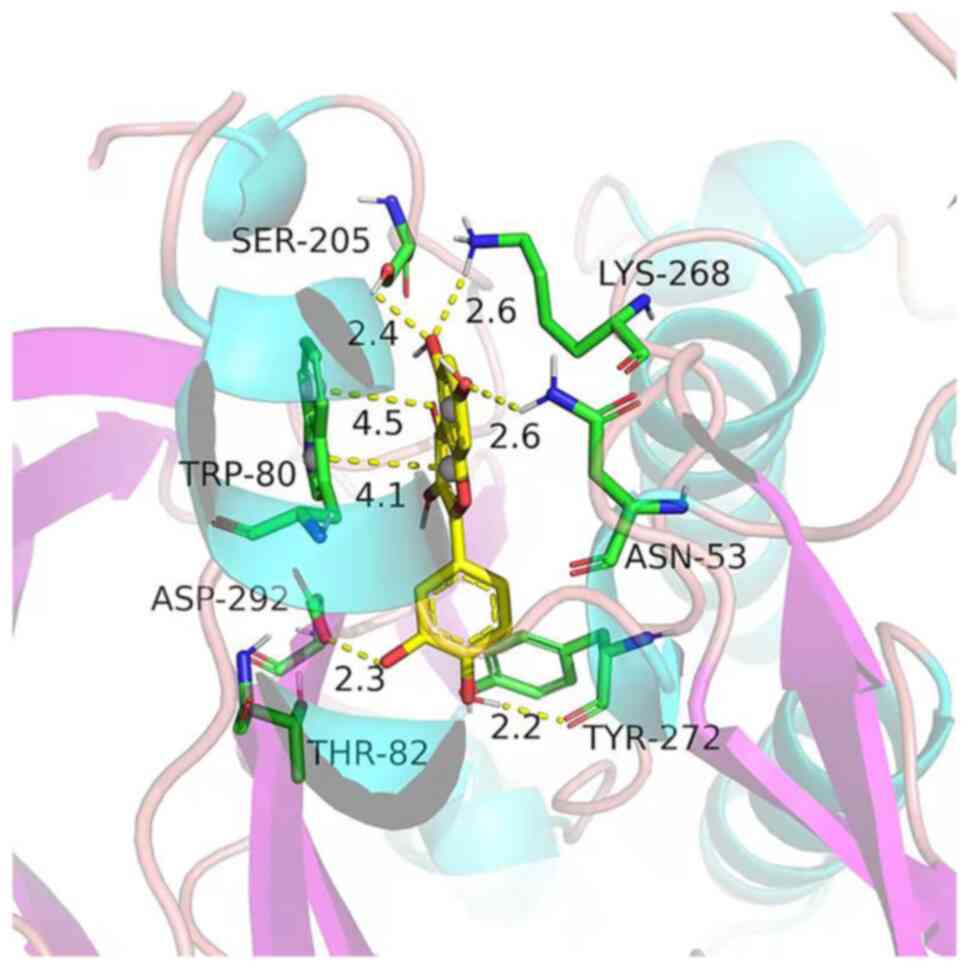
Molecular docking analysis
Molecular docking analysis was performed to estimate
the binding between quercetin and Akt1. The results indicated that
the binding force of quercetin with Akt1 was-8.9 kJ/mol, indicating
that they had a good affinity. The three-dimensional structure of
the molecular docking diagram is provided in Fig. 6.
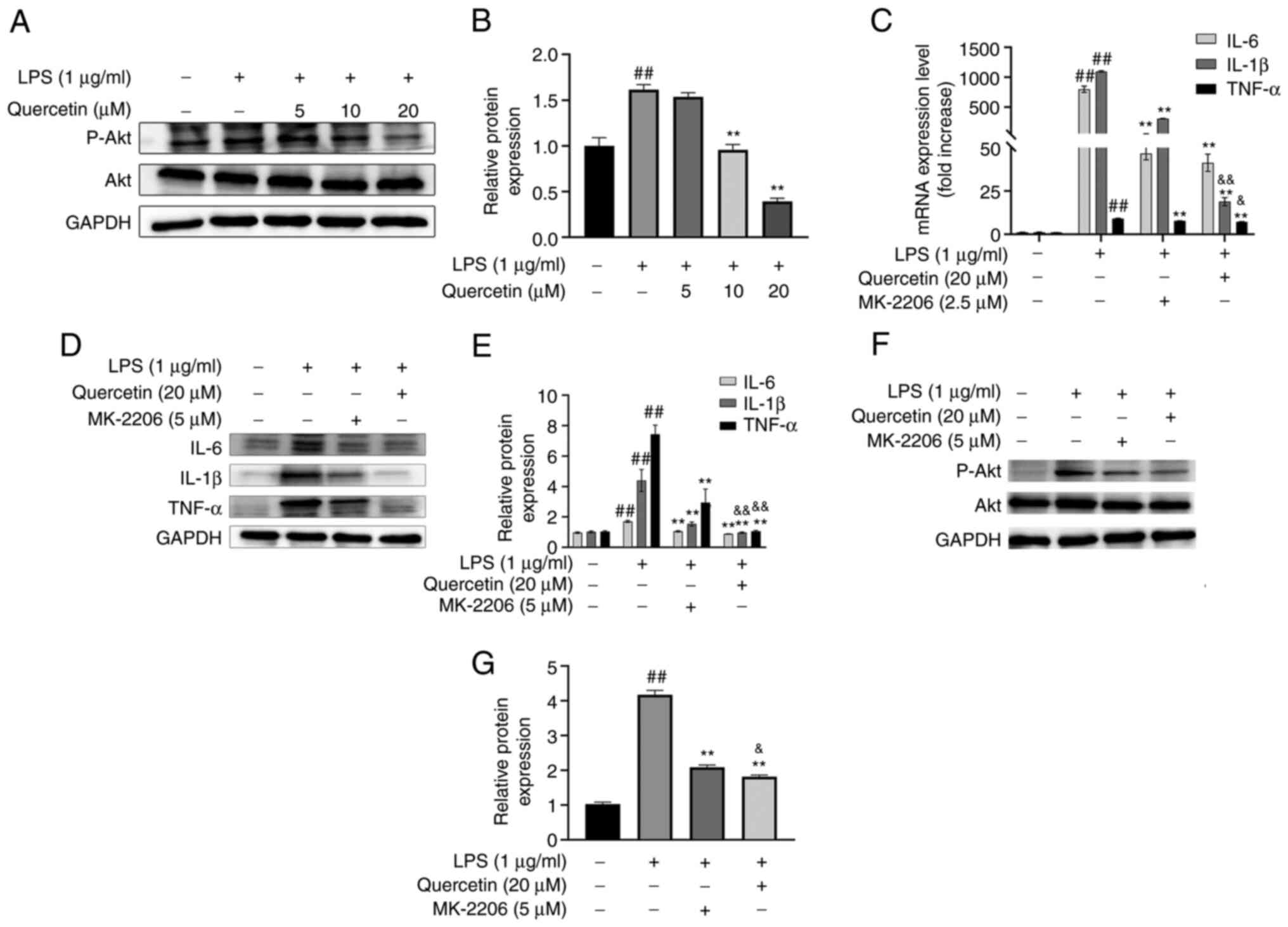
Experimental verification
As presented in Fig.
7A and B, quercetin
dose-dependently inhibited the phosphorylation of Akt, suggesting
that quercetin counteracted inflammation by repressing Akt
phosphorylation.
As indicated in Fig.
7C-E, MK-2206 and quercetin decreased the expression levels of
TNF-α, IL-6 and IL-1β, and quercetin had a stronger efficacy than
MK-2206.
As presented in Fig.
7F and G, MK-2206 and
quercetin inhibited the phosphorylation of Akt and quercetin had a
stronger efficacy than MK-2206.
Discussion
Based on RAW264.7 macrophages, LPS-induced
inflammation models may be constructed to analyze the pathogenesis
of inflammation-related diseases. In the present study, RT-qPCR,
ELISA and western blot analysis indicated that LPS significantly
increased the concentration of TNF-α, IL-6 and IL-1β. It was also
confirmed that quercetin exhibited anti-inflammatory effects by
reducing the expression levels of TNF-α, IL-6 and IL-1β in
LPS-induced RAW264.7 macrophages. Shen et al (12) indicated that quercetin was able to
decrease the secretion of IL-6, TNF-α and IL-1β in the serum and
ankle joint tissues. In addition, another previous study suggested
that quercetin attenuated copper sulfate-induced upregulation of
TNF-α, IL-6 and IL-1β (13). The
aforementioned results are consistent with those observed in the
present study, indicating that quercetin has an anti-inflammatory
effect.
PPI network topology analysis suggested that Akt was
the target of quercetin, with the largest degree of connectivity
value in the network. KEGG analysis indicated that the
anti-inflammatory effects of quercetin may be mediated via the
PI3K-Akt signaling pathway. A binding energy of >4.25 is
considered fair, 5≤ binding energy <7 is considered good and
binding energy ≥7 is considered excellent; this scoring is
conventionally used to classify ligand binding activity (14). The binding energy of quercetin with
Akt1 was-8.9 kJ/mol, indicating that they had a good affinity.
Taken together, it was hypothesized that Akt may be an important
target involved in the mechanisms of the anti-inflammatory action
of quercetin. As expected, western blot analysis confirmed that
quercetin exerted anti-inflammatory effects by repressing the
activity of Akt in vitro.
Akt, also known as protein kinase B, is a major
downstream effector of PI3K. Akt is divided into three subtypes
(Akt1, Akt2 and Akt3). Previous studies have indicated that Akt is
associated with inflammation (15-17).
The present study suggested that the anti-inflammatory effects of
quercetin may arise from the inactivation of Akt. Gulati et
al (18) reported that
quercetin inhibited Akt activation in human breast cancer cells,
which is consistent with the results of the present study. Akt is
widely known to be the downstream target of a vast number of
cellular processes (19,20), so it is probable that there are
also numerous downstream/indirect pathways through which quercetin
affects Akt, not only by direct binding. This is also indicated by
the high network connectivity of Akt, which suggests a hub gene
function of Akt and its key role/involvement in multiple pathways,
supporting that the mechanism of quercetin comprises not just 1
direct binding interaction with Akt.
In conclusion, the present study revealed that Akt
may be an important target involved in the anti-inflammatory
mechanisms of quercetin. These findings provide a new direction for
investigating the anti-inflammatory mechanisms of quercetin.
Acknowledgements
The authors would like to thank Dr Ming Yang, Office
of Good Clinical Practice, Longhua Hospital, Shanghai University of
Traditional Chinese Medicine (TCM) (Shanghai, China), who developed
the TCM Network Pharmacology Analysis System (TCMNPAS) as a major
contributor. The molecular docking analysis of the present study
was performed using the TCMNPAS (registration no. 2019SR1127090,
http://54.223.75.62:3838/npa4/).
Funding
Funding: This research was supported by grants from the Shanghai
Committee of Science and Technology Research Projects (grant nos.
19140904600 and 18401900800) and the 2018-2020 Three-year Action
Plan for Traditional Chinese Medicine Further Development in
Shanghai (grant no. ZY2018-2020-CCCX-2002-08).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The authors' responsibilities were as follows: JZ
and HongL designed the study; JZ and HongyanL performed the
experiments; WW analyzed the data; JZ wrote the manuscript, and
HongyanL helped edit the paper; JZ and WW checked and confirmed the
authenticity of the raw data; HongL revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Noack M and Miossec P: Selected cytokine
pathways in rheumatoid arthritis. Semin Immunopathol. 39:365–383.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Poledne R and Lesná IK: Inflammation and
atherosclerosis. Vnitr Lek. 64:1142–1146. 2019.PubMed/NCBI
|
|
3
|
Diakos IC, Charles KA, McMillan DC and
Clarke SJ: Cancer-related inflammation and treatment effectiveness.
Lancet Oncol. 15:e493–e503. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lemmers RFH, van Hoek M, Lieverse AG,
Verhoeven AJM, Sijbrands EJG and Mulder MT: The anti-inflammatory
function of high-density lipoprotein in type II diabetes: A
systematic review. J Clin Lipidol. 11:712–724.e5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Williamson G and Manach C: Bioavailability
and bioefficacy of polyphenols in humans. II. Review of 93
intervention studies. Am J Clin Nutr. 81 (Suppl 1):243S–255S.
2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mennen LI, Walker R, Bennetau-Pelissero C
and Scalbert A: Risks and safety of polyphenol consumption. Am J
Clin Nutr. 81 (1 Suppl):326S–329S. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Woodman OL and Chan EC: Vascular and
anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol
Physiol. 31:786–790. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lu S, Zhou S, Chen J, Zheng J, Ren J, Qi
P, Zhu Z and Li Z: Quercetin nanoparticle ameliorates
lipopolysaccharide-triggered renal inflammatory impairment by
regulation of Sirt1/NF-κB pathway. J Biomed Nanotechnol.
17:230–241. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li CY, Cheng SE, Wang SH, Wu JY, Hsieh CW,
Tsou HK and Tsai MS: The anti-inflammatory effects of the bioactive
compounds isolated from alpinia officinarum hance mediated by the
suppression of NF-κB and MAPK signaling. Chin J Physiol. 64:32–42.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Berger SI and Iyengar R: Network analyses
in systems pharmacology. Bioinformatics. 25:2466–2472.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shen P, Lin W, Ba X, Huang Y, Chen Z, Han
L, Qin K, Huang Y and Tu S: Quercetin-mediated SIRT1 activation
attenuates collagen-induced mice arthritis. J Ethnopharmacol.
279(114213)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Peng X, Dai C, Zhang M and Das Gupta S:
Molecular mechanisms underlying protective role of quercetin on
copper sulfate-induced nephrotoxicity in mice. Front Vet Sci.
7(586033)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hsin K, Ghosh S and Kitano H: Combining
machine learning systems and multiple docking simulation packages
to improve docking prediction reliability for network pharmacology.
PLoS One. 8(e83922)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Seshadri VD: Brucine promotes apoptosis in
cervical cancer cells (ME-180) via suppression of inflammation and
cell proliferation by regulating PI3K/AKT/mTOR signaling pathway.
Environ Toxicol. 36:1841–1847. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim MJ, Kim DH, Bang E, Noh SG, Chun P,
Yokozawa T, Moon HR and Chung HY: PPARα Agonist, MHY3200,
alleviates renal inflammation during aging via regulating
ROS/Akt/FoxO1 signaling. Molecules. 26(3197)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao HN, Hu H, Wen PC, Lian S, Xie XL, Song
HL, Yang ZN and Ren FZ: Yak milk-derived exosomes alleviate
lipopolysaccharide-induced intestinal inflammation by inhibiting
PI3K/AKT/C3 pathway activation. J Dairy Sci. 104:8411–8424.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gulati N, Laudet B, Zohrabian VM, Murali R
and Jhanwar-Uniyal M: The antiproliferative effect of quercetin in
cancer cells is mediated via inhibition of the PI3K-Akt/PKB
pathway. Anticancer Res. 26:1177–1181. 2006.PubMed/NCBI
|
|
19
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: Implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014.PubMed/NCBI View Article : Google Scholar
|