Introduction
Acute kidney injury (AKI) is a clinical syndrome,
which presents with a rapid reduction in renal function over a
short time period and is caused by numerous factors, including
toxic agents, trauma or surgery (1,2). It
is characterized by a decreased glomerular filtration rate,
retention of creatinine and urea nitrogen, a decrease in water and
electrolytes, and impairment of acid-base balance. Severe AKI can
also lead to multiple organ dysfunction syndromes (1,3).
Moreover, AKI causes huge social and economic burdens, especially
in China (4). Renal ischemia
reperfusion (IR)/hypoperfusion leads to the occurrence of oxidative
stress and cell apoptosis, which is a direct result of decreased
renal parenchymal cells, the atrophy of renal tissue structure and
a decrease in renal function (renal insufficiency) (5). Surgical or pathological conditions,
such as major vascular surgery, cardiac and hepatic surgeries,
shock, sepsis, trauma, and kidney transplantation, can lead to
IR-AKI (6). Currently, there is no
effective method to reduce renal tissue injury and promote renal
tissue repair. Renal tubular epithelial cell necrosis caused by
renal hemodynamic changes is one of the pathophysiological
mechanisms of AKI (2).
During apoptosis, several proteolytic enzyme
families are activated, including caspases. Caspases are mainly
activated via two signaling pathways: i) The exogenous death
receptor signaling pathway and ii) the endogenous mitochondrial
signaling pathway, which activate caspase-8 zymogen and caspase-9
zymogen, respectively. Both signaling pathways activate downstream
effector caspase-3 leading to apoptosis (7). Therefore, caspase-3 is a terminal
shear enzyme in the process of apoptosis. Chatterjee et al
(8) reported that caspase
inhibitors reduce the renal AKI response. Excessive levels of
reactive oxygen species (ROS) induce apoptosis of functional cells.
ROS cause tissue damage by triggering the caspase cascade, which
includes activating caspase-3 and inducing cell apoptosis (9). Furthermore, renal tubular epithelial
cells have an antioxidative defense system, which includes the
nuclear factor-E 2-related factor 2 (Nrf2)/heme oxygenase-1
(HO-1)-mediated antioxidative response (10).
Nrf2 is a key regulatory protein in the endogenous
antioxidant defense system. In oxidative stress, Nrf2 performs
nuclear translocation and binds with the antioxidant response
element (ARE) to start the transcription of numerous downstream
antioxidant genes and serve a protective role in antioxidation
(11). The antioxidant enzymes
regulated by Nrf2 include superoxide dismutase (SOD), catalase
(CAT), thioredoxin, peroxiredoxin, glutathione (GSH) peroxidase,
GSH reductase, glutamine cysteine ligase, glutamine cysteine
synthase, NAD(P)H quinone oxidoreductase (NQO1) and HO-1 (12,13).
Neutrophil gelatinase-associated lipocalin (NGAL) is
a lipid carrier protein. It was originally discovered as a small
molecular weight secretory protein in activated neutrophils
(14). Under normal physiological
conditions, NGAL is rarely expressed in the kidneys. When the renal
tubular epithelium is damaged, a large amount of NGAL is secreted
into the blood and urine (15).
Therefore, neutrophils in the infiltrating tubulointerstitium are
induced to transition to the tubules and the protein levels of NGAL
in the urine and serum is increased (16). Thus, NGAL can be used as an
effective biomarker for diagnosing AKI and early diabetic
nephropathy (17,18). Sevoflurane (SEV) is an inhaled
anesthetic widely used in surgery. Previous studies have
demonstrated that SEV has antioxidative stress and
anti-inflammatory activity, and can therefore protect organs from
damage caused by oxidative stress. Research over the past few years
has demonstrated that SEV has different degrees of protection
against renal injury (19,20).
The present study therefore focused on the Nrf2
signaling pathway with the aim of studying the possible mechanism
of SEV preconditioning on renal injury induced by IR in mice.
Oxidative stress, the inflammatory response and apoptosis were all
investigated.
Materials and methods
Animals
In total, 40 healthy 5-week-old male C57BL/6J mice
(weight, 20±2 g; SPF Biotechnology Co., Ltd.), were fed in cages
and kept at 45% humidity and 20˚C under a 12-h light/dark cycle.
All mice had free access to water and food. The mice were randomly
assigned to the following groups: i) Sham operation group (Sham);
ii) IR group + vehicle (IR + vehicle); iii) IR + SEV low-dose
preconditioning (SEV-L; inhalation of 2.2% SEV); and iv) IR + SEV
high-dose preconditioning (SEV-H; inhalation of 3.3% SEV). Each
group contained 10 mice.
The second animal experiment aimed to further
investigate the renal protective effect of SEV via activation of
Nrf2. Another 40 healthy 5-week-old male C57BL/6J mice (weight,
20±2 g; SPF Biotechnology Co., Ltd.), were used (n=8 mice/group).
All mice were fed in cages and kept at 45% humidity and 20˚C under
a 12-h light/dark cycle, with free access to water and food. A
further five groups of mice were involved: i) Sham group; ii) IR
group; iii) IR + brusatol (Selleck Chemicals) group; iv) IR + SEV
(high dose); and v) IR + SEV (high dose) + brusatol group. Brusatol
(30 mg/kg), an Nrf2 inhibitor, was administered orally 2 h before
preconditioning with SEV. Following IR, the levels of blood urea
nitrogen (BUN) and serum creatinine (Scr) were detected.
IR surgery
The mice were anesthetized with an intraperitoneal
injection of 1% sodium pentobarbital (50 mg/kg) and were fixed in
the lateral position on the operating table. Longitudinal abdominal
incisions from the left and right sides were cut until the kidneys
were exposed. Both sides of the incision were covered with normal
saline gauze. The renal pedicle capsule was peeled off and the
separated renal artery on both sides was clamped with a
non-invasive artery clamp. The kidney was returned in the abdominal
cavity following successful clamping. The bilateral artery clamp
was removed 45 min later. The kidneys were returned into their
original place after confirming the recovery of blood perfusion.
Subsequently, the muscle layer was sutured followed by the skin
layer. All experimental animals maintained spontaneous breathing
during the operation. The renal artery was not clamped in the Sham
group, but all other experimental steps were the same as in the
other groups. All animal experiments were conducted in strict
accordance with the guidance provided on the treatment of
experimental animals issued by the Ministry of Science and
Technology of China (21). At the
end of the experiments, all animals were euthanized by cervical
dislocation.
Mice in the SEV group inhaled the mixture of
low-dose or high-dose sevoflurane (Maruishi Pharmaceutical Co.,
Ltd.) and O2 (80%) for 60 min, 30 min prior to IR. The
renal IR model was established following anesthesia. The mice were
placed in a clean environment with a constant temperature of 22˚C
following surgery with free access to food and water. Urine was
collected before mice were sacrificed by cervical dislocation.
Sample collection and serum marker
detection
The mice were sacrificed 24 h following surgery.
Blood samples were extracted from the heart, placed in a 1.5 ml
tube and centrifuged at 1,000 x g at 4˚C for 15 min after standing
at room temperature. The supernatant was taken for further
analysis. BUN and Scr were quantified using an automatic
biochemical instrument (TBA-120FR; Toshiba Corporation). NGAL
levels were assessed using a commercially available ELISA kit (cat.
no. ab119601; Abcam). Serum inflammatory cytokine levels, including
TNF-α (cat. no. ab46105), IL-6 (cat. no. ab222503), and IL-1β (cat.
no. ab197742) were also measured using ELISA kits (all purchased
from Abcam). Intercellular adhesion molecule-1 (ICAM-1; cat. no.
ab100688), monocyte chemoattractant protein-1 (MCP-1; cat. no.
ab100721) and vascular cell adhesion protein-1 (VCAM-1; cat. no.
ab201278) were measured using ELISA kits (all purchased from
Abcam).
Renal histopathological
evaluation
Renal tissues were fixed with 4% paraformaldehyde
(PFA) at room temperature for 24 h, embedded in paraffin, cut into
8-µm thick sections, and stained with H&E at 35˚C for 3 min.
The pathological changes in renal tissue were observed using a
light microscope. A total of two different sections were taken from
each sample to observe the histopathological changes of the kidney.
According to the method introduced by Zhang et al (22,23),
for each animal, at least 10 high-power (magnification, x400)
fields were examined. The percentage of tubules that displayed
cellular necrosis, loss of brush border, cast formation,
vacuolization and tubule dilation was scored as follows: 0 (none),
1 (<10%), 2 (11-25%), 3 (26-45%), 4 (46-75%) and 5 (>76%).
Masson staining was performed at room temperature using a
commercial staining kit (cat. no. G1340; Beijing Solarbio Science
& Technology Co., Ltd.) according to the manufacturer's
protocol, to evaluate collagen fibrils in renal tissues. The
staining was performed according to manufacturer's instruction. A
total of 10 stained fields were randomly selected from each section
and observed using a CX23 RFS2 LED light microscope (magnification,
x400, Olympus Corporation). The images were analyzed by Image-Pro
Plus 6.0 (Media Cybernetics, Inc.). For fibrotic area
semi-quantification, a ratio of blue stained area to the area of
the entire field (including glomeruli, tubule lumina and blood
vessels) was assessed and expressed as a percentage of Masson
staining.
Detection of oxidative stress
A total of 0.2 g kidney tissue was ground,
centrifuged at 1,000 x g for 10 min at 4˚C. The supernatant was
obtained to detect the concentrations of different proteins. The
enzyme activity of SOD (cat. no. A001-3), myeloperoxidase (MPO;
cat. no. A044) and CAT (cat. no. A007), as well as the
concentration of malondialdehyde (MDA; cat. no. A003-1) were
determined using commercial kits (all purchased from Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
protocol.
Immunohistochemistry
Immunohistochemistry was performed using a two-step
method. Briefly, 5-µm thick sections of mouse kidney were dewaxed
and rehydrated using xylene and gradient alcohol, treated with 1%
hydrogen peroxide solution at 37˚C for 10 min, heated and repaired
using an antigen repair solution (1:10; cat. no. P0088; Beyotime
Institute of Biotechnology), cooled to room temperature and
incubated with primary and secondary antibodies. The slide was
incubated with DAB solution (cat. no. P0203; Beyotime Institute of
Biotechnology) for 8 min at room temperature and then sealed for
observation. Positive staining was indicated by yellowish-brown
tissues. The following primary antibodies were used: Caspase-3
(1:100; cat. no. 66470-2-Ig), Nrf2 (1:100; cat. no. 66504-1-Ig),
HO-1 (1: 100; cat. no. 27282-1-AP) and NQO1 (1:100; cat. no.
67240-1-Ig) (all from ProteinTech Group, Inc.). The sections were
examined using a light microscope (DMLS-1000; Leica Microsystems
GmbH) with a high-power field of view (magnification, x400). A
total of five visual fields were randomly selected and the images
were analyzed using Biosens Digital Imaging System Software version
16 (Shanghai Bio-Tech Co., Ltd.). The color of the images was
transformed to separate the positive areas from the background for
automatic measurement. The following formula was used: Positive
target expression index (%)=average optical density of positive
target x positive area/(positive area + negative area).
TUNEL staining
The kidney sections from each group were dewaxed
with xylene and washed with 100% ethanol, rehydrated and washed in
decreasing concentrations of ethanol and were immersed in 0.85%
NaCl, then washed twice with PBS. Apoptosis was detected in tissue
sections using the DeadEnd™ Colorimetric Apoptosis
Detection System (Promega Corporation). Samples were fixed with 4%
PFA for 15 min at 20-25˚C and immersed in PBS twice for 10 min.
Subsequently, samples were permeabilized with 20 µg/ml proteinase K
solution and incubated for 20 min at room temperature. Slides were
washed in PBS and fixed with 4% PFA at room temperature for 5 min,
washed, and equilibrated with equilibration buffer for 10 min.
Then, 100 µl terminal deoxynucleotidyl transferase mix was added to
the tissue sections, which were incubated for 60 min at 37˚C and
then washed in PBS. The slides were immersed in 0.3% hydrogen
peroxide for 3 min and washed in PBS. Subsequently, 100 µl
streptavidin HRP (cat. no. A0303; 1:500; in PBS; Beyotime Institute
of Biotechnology), was added and the samples were incubated for 30
min at room temperature. After the slides were washed in PBS, 100
µl DAB solution (Beyotime Institute of Biotechnology) was added to
stain the slides for 10 min at 15-25˚C. The slides were washed in
deionized water and were mounted using mounting medium.
TUNEL-positive apoptotic cells were visualized using a light
microscope (magnification, x200).
Western blotting
After homogenizing the preserved kidney tissues,
total protein was extracted using a protein extraction kit (Beijing
Solarbio Science & Technology Co., Ltd.) and the protein
concentration was quantified using the BCA method. Total protein,
using 30 µg from each sample, was separated using 10% SDS-PAGE and
transferred onto a PVDF membrane. The membranes were blocked with
5% skimmed milk for 1.5 h at 20-25˚C, incubated overnight at 4˚C
with primary antibodies and were subsequently incubated with a
suitable secondary antibody at room temperature for 1 h. An
Enhanced Chemiluminescence Kit (Amersham; Cytiva) was used to
detect protein bands. β-actin served as a loading control. Bands
were semi-quantified via scanning densitometry using the
Odyssey® CLx Imaging System and Image Studio Version 3.1
software provided by the manufacturer (LI-COR Biosciences). The
primary antibodies included caspase-3 (cat. no. 66470-2-Ig;
1:1,000), Nrf2 (cat. no. 66504-1-Ig; 1: 1,000), HO-1 (cat. no.
27282-1-AP; 1:1,000) and NQO1 (cat. no. 67240-1-Ig; 1:1,000) (all
from ProteinTech Group, Inc.). HRP goat anti-mouse IgG (1:5,000;
cat. no. sc-2005) or HRP goat anti-rabbit IgG (1:4,000; cat. no.
sc-2054) (both from Santa Cruz Biotechnology, Inc.) was used.
Cell culture and in vitro HK-2 cell
model of hypoxia/reoxygenation (H/R)
The human renal proximal tubular HK-2 cell line was
purchased from American Type Culture Collection. Cells were plated
in 100-mm culture dishes and cultured in DMEM supplemented with 10%
FBS (both from Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin, 2 mM glutamine, 100 µg/ml streptomycin and 1 mM HEPES
buffer. Cells were cultured at 37˚C in humidified air containing 5%
CO2. The medium was replaced every other day. For the
H/R group, cells were exposed to hypoxic conditions (5%
CO2, 1% O2 and 94% N2) for 24 h
followed by 12 h of reoxygenation (5% CO2, 21%
O2 and 74% N2). To determine the effect of
SEV, cells were pretreated with either vehicle buffer (0.5% DMSO
solution) or SEV (1 and 2 concentration) for 12 h prior to H/R
treatment. A Nrf2 inhibitor, brusatol (10 nM) was also added to
evaluate whether SEV, serving its protective role, was dependent on
the Nrf2 signaling pathway. The in vitro study contained the
following six groups; i) Control group without H/R; ii) H/R group
treated with vehicle; iii) H/R group treated with 1% SEV; iv) H/R
group treated with 2% SEV; v) H/R group treated with brusatol; and
vi) H/R group treated with brusatol and 2% SEV. An in-line
anesthetic vaporizer (Drägerwerk AG & Co., KGaA) fed by a
supply of a gas containing 21% O2 and 5% CO2,
balanced with N2, was used to deliver SEV at a rate of 2
l/min for at least 5 min until the desired SEV concentration (1-2%)
was achieved. Concentrations of SEV and O2 were
monitored every h using an anesthetic analyzer (Drägerwerk AG &
Co. KGaA). Cells in the vehicle control conditions were placed in
an identical gas chamber under 21% O2 and 5%
CO2, balanced with N2. Once sealed, the
chambers were placed in a 37˚C incubator.
Cell viability assay
HK-2 cells were treated with Cell Counting Kit-8
reagent (10 µl/well; Applygen Technologies, Inc.) for 2 h. The
optical density was recorded at 450 nm using a microplate
absorbance reader (Tecan Group, Ltd.).
Statistical analysis
The data were analyzed using GraphPad Prism 8.0
software (GraphPad Software, Inc.). Data are presented as the mean
± SD. Statistical comparisons among the groups were analyzed using
one-way ANOVA followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
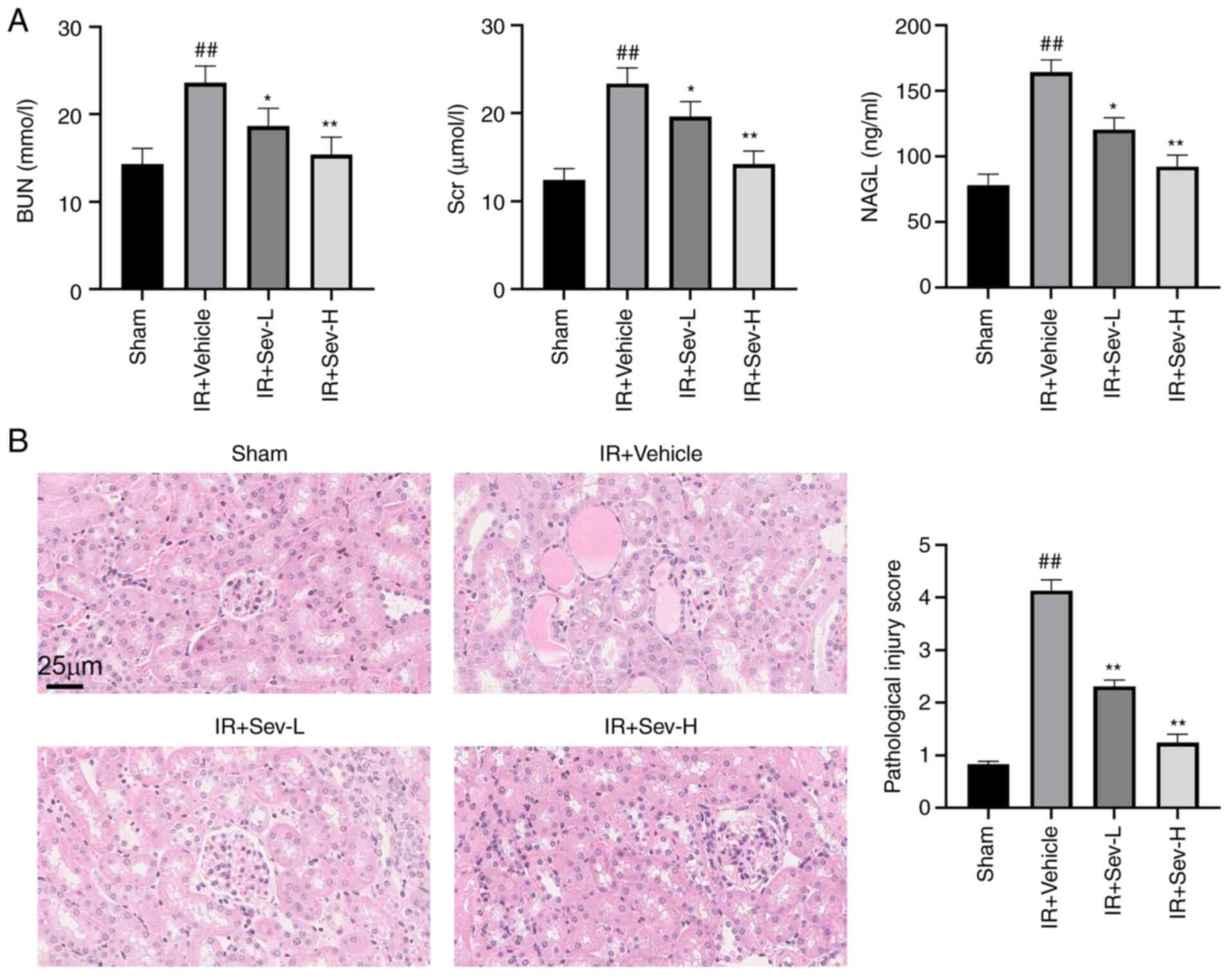
Renal function
Compared with that in sham group, the concentration
of BUN, Scr, and serum NGAL in the IR group were significantly
increased (all P<0.01) (Fig.
1A). Preconditioning with SEV decreased the BUN, Scr and serum
NGAL concentrations in a dose-dependent manner (Fig. 1A). The H&E staining (Fig. 1B) also demonstrated that
preconditioning with SEV attenuated acute renal tubular injury
induced by I/R in a dose-dependent manner, consistent with the
biochemical analysis.
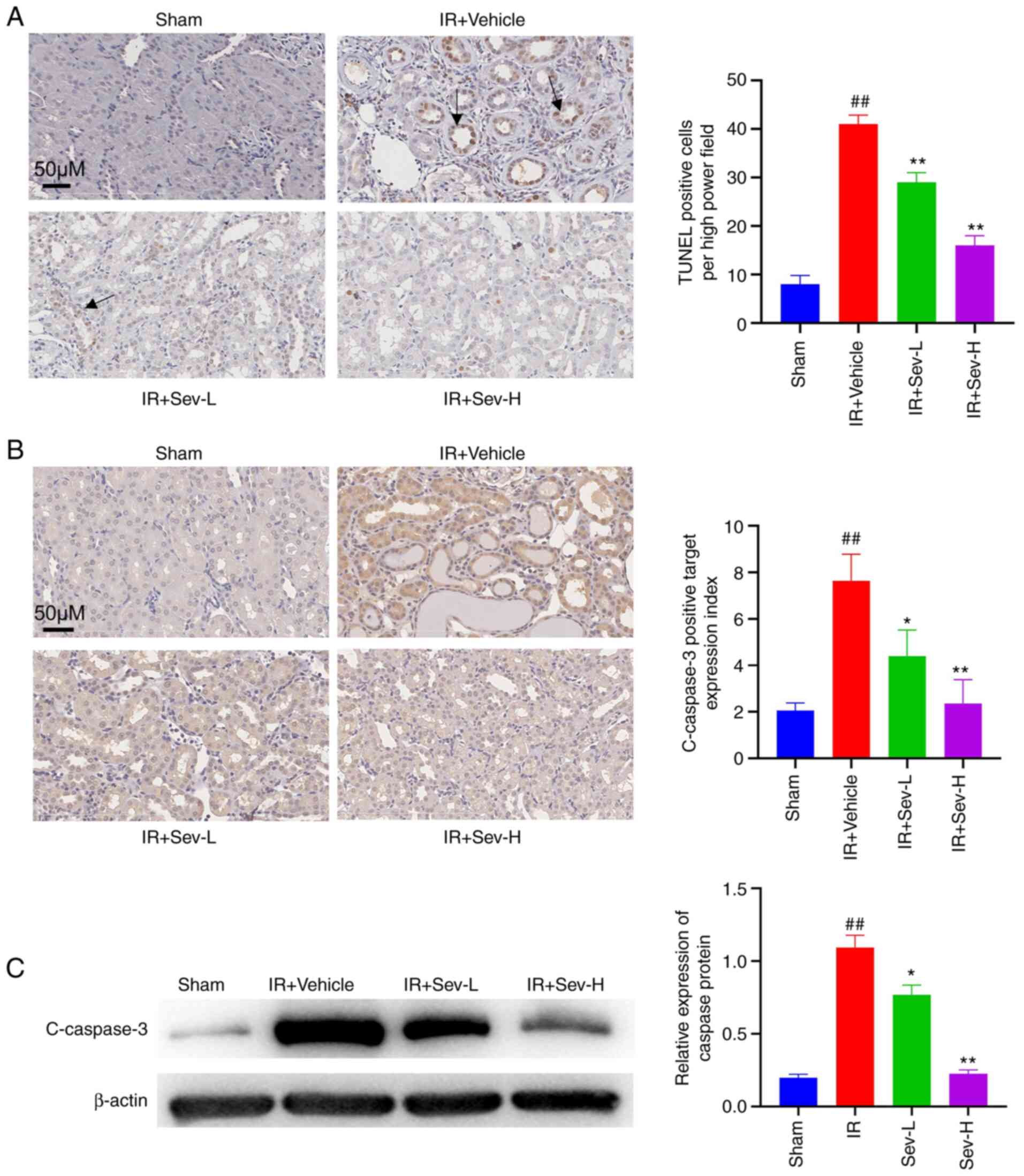
SEV preconditioning decreases tubular
cell apoptosis and cleaved caspase-3 protein expression levels
It was determined via TUNEL staining that
preconditioning with SEV resulted in a significantly reduced
quantity of apoptotic tubular cells in the renal interstitium
compared with that in the vehicle control group (P<0.05;
Fig. 2A). Actively cleaved
caspase-3 protein was mainly expressed in the cytoplasm and nucleus
of impaired renal tubular epithelial cells. The results
demonstrated that the protein expression level of cleaved caspase-3
in the sham group was low, whereas IR-induced AKI increased the
caspase-3 expression levels in the cytoplasm and nucleus of renal
tubular epithelial cells. SEV preconditioning significantly
decreased the caspase-3 protein expression levels in a
dose-dependent manner, especially in the SEV-H group (Fig. 2B). Western blotting (Fig. 2C) also revealed that SEV
preconditioning reduced cleaved caspase-3 protein expression levels
compared with that in the IR vehicle group (P<0.01).
SEV preconditioning alleviates
inflammation and oxidative stress
The results demonstrated that IR led to the
increased levels of cytokines and chemokines, including TNF-α,
IL-6, IL-1β, ICAM-1, MCP-1 and VCAM-1 compared with in the sham
group (Fig. 3). SEV
preconditioning significantly reduced cytokine and chemokine levels
in the peripheral blood compared with in the IR + vehicle group,
especially in the SEV-H group. Similarly, SEV preconditioning
significantly decreased concentrations of MDA and MPO, whereas the
activity of SOD and CAT was significantly increased as shown in
Fig. 4.
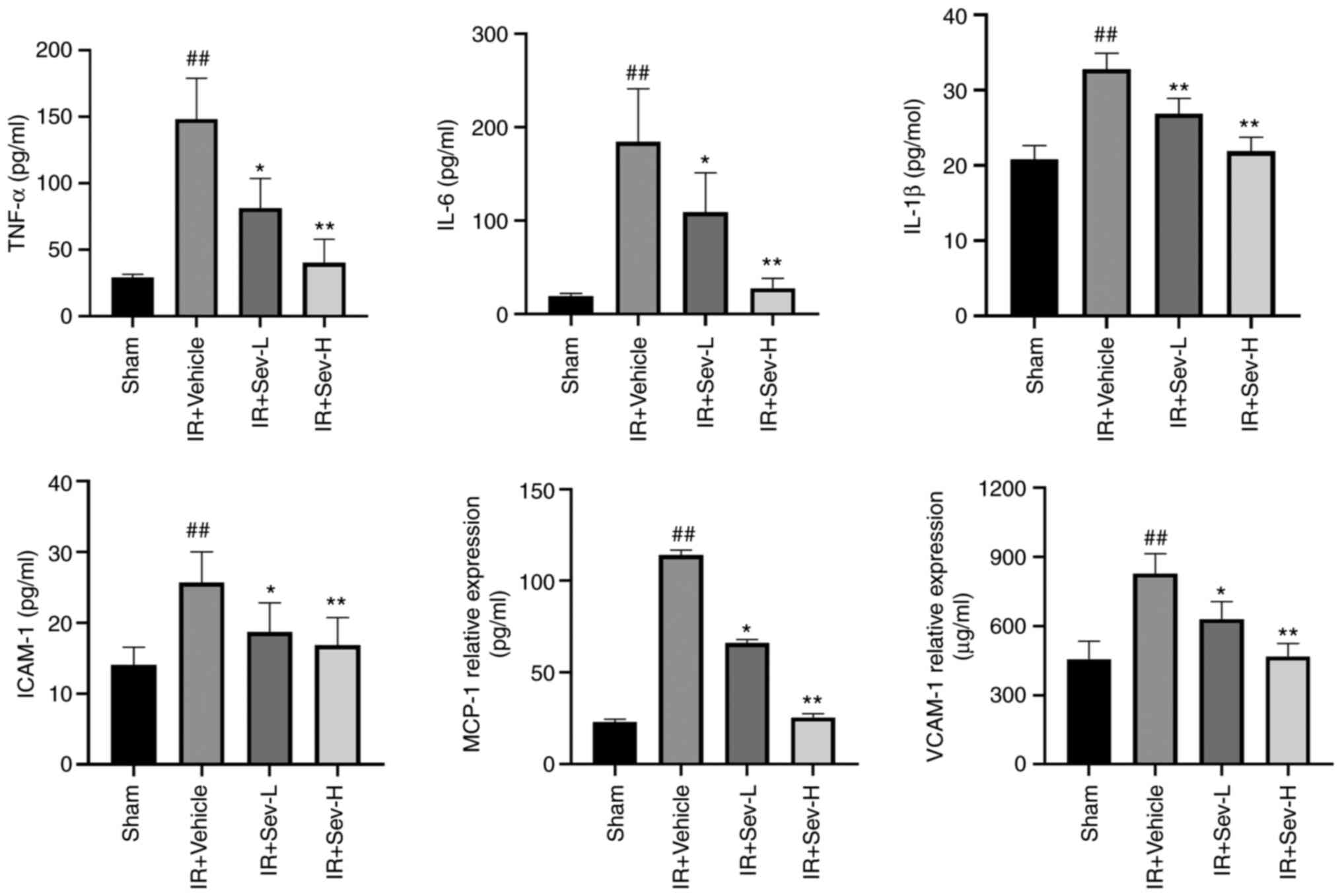 | Figure 3SEV preconditioning significantly
reduces cytokine and chemokine levels. The concentration of
inflammatory factors TNF-α, IL-6, IL-1βICAM-1, MCP-1 and VCAM-1 in
the peripheral blood were determined by ELISA kits.
##P<0.05 vs. sham; **P<0.01,
*P<0.05 vs. IR vehicle control. SEV, sevoflurane;
MCP-1, monocyte chemoattractant protein-1; ICAM-1, intercellular
adhesion molecule-1; VCAM-1, vascular cell adhesion protein-1; IR,
ischemia reperfusion; L, low; H, high. |
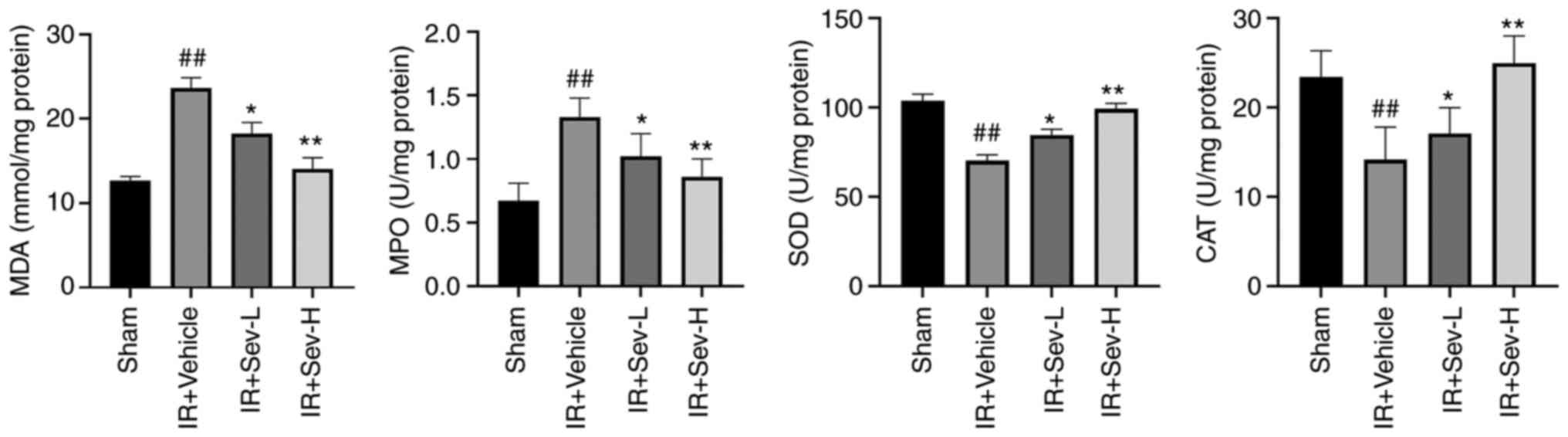 | Figure 4Effect of SEV preconditioning on
oxidative stress. Quantitative analysis indicate the concentrations
of MDA and MPO, as well as SOD and CAT in the kidney tissues from
IR mouse model. ##P<0.05 vs. sham;
**P<0.01, *P<0.05 vs. IR vehicle
control. SEV, sevoflurane; MDA, malondialdehyde; MPO,
myeloperoxidase; SOD, superoxide dismutase; CAT, catalase; IR,
ischemia reperfusion; L, low; H, high. |
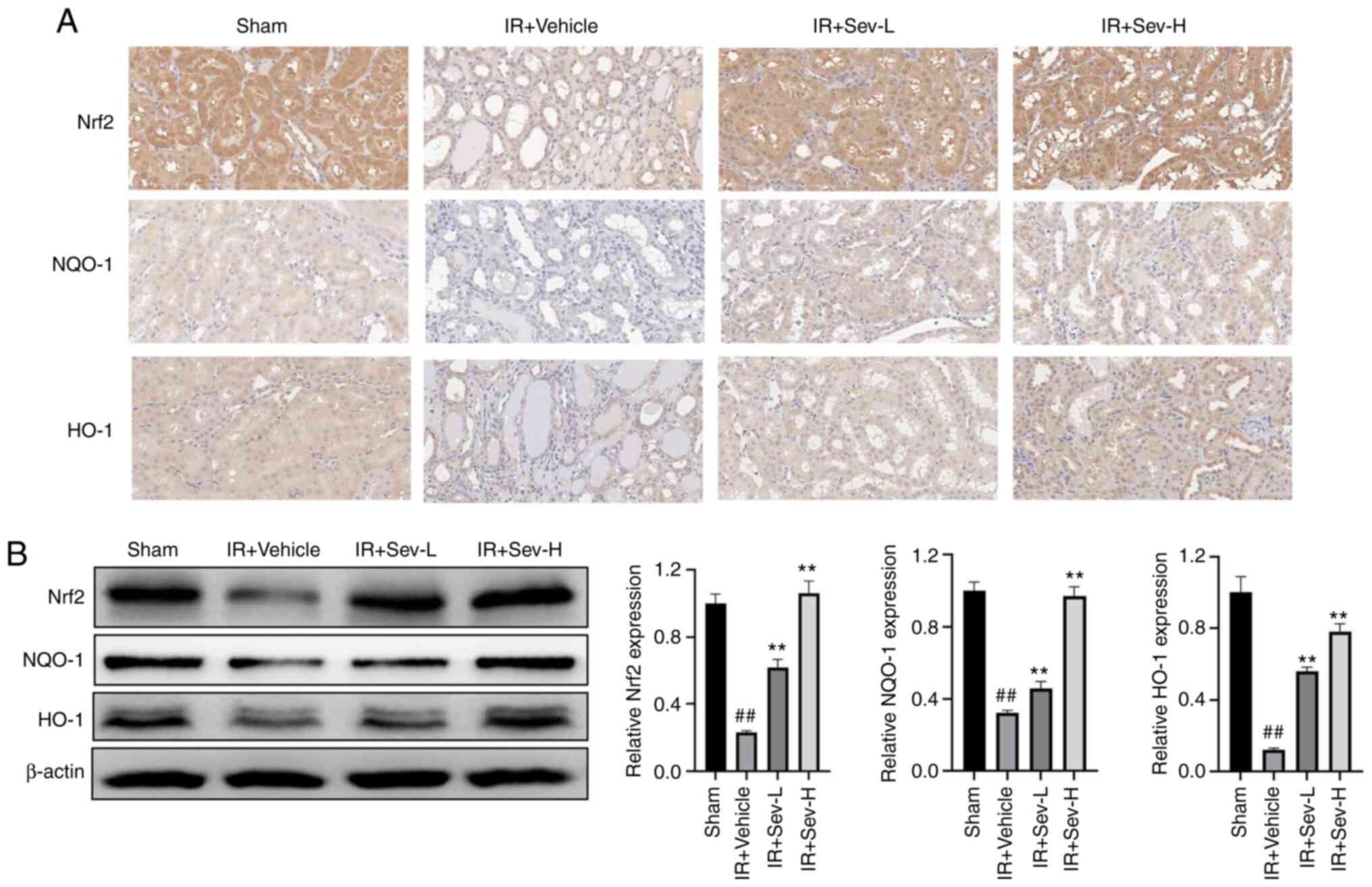
SEV preconditioning activated the
expression of Nrf2 in the kidney
Due to the antioxidant properties of SEV
preconditioning as demonstrated in the aforementioned results, it
was hypothesized that SEV preconditioning might activate the
endogenous antioxidant defense system and that the Nrf2 signaling
pathway was a possible target. By performing immunohistochemistry
it was determined that the Nrf2 protein expression levels in kidney
tissue in the preconditioning groups were notably increased
compared with that in the IR group. Moreover, its downstream
proteins HO-1 and NQO-1 were also notably upregulated by SEV
preconditioning (Fig. 5A). Western
blotting demonstrated a similar trend to the immunohistochemistry
results (Fig. 5B), which further
confirmed that SEV preconditioning may activate the Nrf2 signaling
pathway in the kidneys from the mouse model of IR.
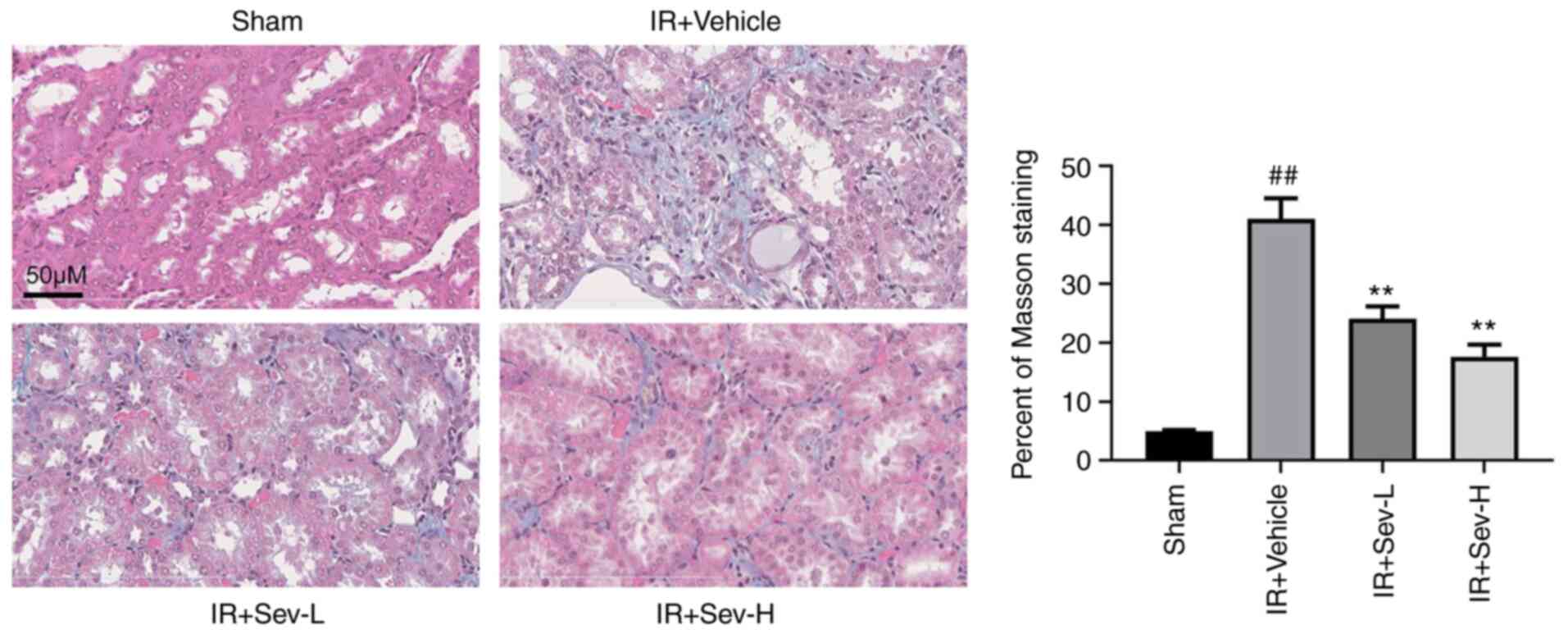
SEV preconditioning improves renal
fibrosis caused by IR
In clinical practice, IR may cause renal
interstitial fibrosis in the later stages and consequently, may
affect the prognosis of AKI. A similar situation was discovered in
animal models (24). In the
present study, IR resulted in significant fibrosis of the renal
interstitium in mice two weeks after IR, which was assessed using
the ratio of Masson staining in high resolution fields (Fig. 6). As well as fibrosis, widening of
the renal interstitium and atrophy of renal tubules were also
evident in the microscopic sections. The degree of fibrosis was
significantly reduced following SEV preconditioning, as well as
amelioration of widening of the renal interstitium and atrophy of
renal tubules.
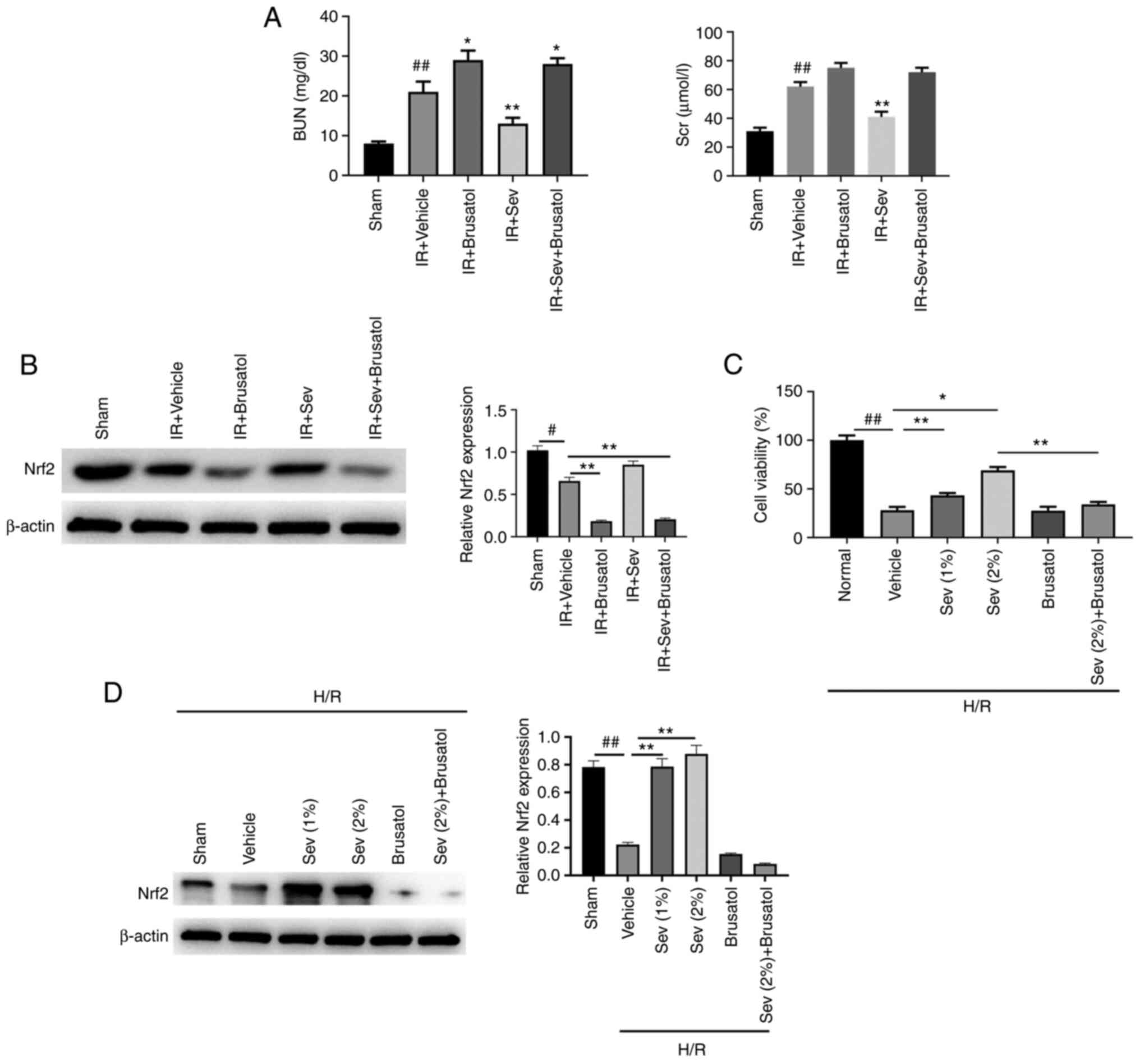
Renal protective effect of SEV
decreases following Nrf2 signaling pathway inhibition both in vivo
and in vitro
To further confirm the renal protective effect of
SEV via the activation of Nrf2, another five groups of animal
experiments were conducted. Brusatol (30 mg/kg), an Nrf2 inhibitor,
was orally administered 2 h prior to SEV preconditioning to the
allocated groups. Following IR, the BUN and Scr levels were
detected. The results demonstrated that brusatol administration
slightly aggravated renal injury in IR mice (Fig. 7A). Moreover, the renal protective
effect of SEV was almost abolished by pretreatment with brusatol.
The Nrf2 protein expression levels in kidney tissues were
significantly reduced by Brusatol treatment as shown in Fig. 7B. Similar results were obtained
using the in vitro HK-2 H/R cell model. The results
demonstrated that H/R treatment markedly reduced HK-2 cell
viability. SEV pretreatment increased cell viability in a
dose-dependent manner, however, its protective effect was
significantly reduced when the cells were also treated with
brusatol (Fig. 7C). Western blot
also confirmed that Nrf2 protein expression level in HK-2 cells was
markedly reduced by brusatol treatment (Fig. 7D). These results indicated that SEV
may exert renal protection, which is dependent on the activation of
Nrf2.
Discussion
IR/hypoperfusion leads to the occurrence of
oxidative stress and can lead to AKI. The pathophysiological
mechanism of renal IR injury is complex and involves numerous
pathways and factors, including the overproduction of oxygen free
radicals, calcium overload, lipid peroxidation injury, the
inflammatory response and cell apoptosis (6,25).
The overproduction of ROS serves an important role in AKI
initiation and progression. Xanthine dehydrogenase is transformed
into xanthine oxidase during ischemia and the resulting production
of free radicals causes damage (26). Furthermore, following the
reconstruction of the blood supply to ischemic organs, a large
quantity of fresh oxygen flows in and produces excessive ROS, which
oxidizes and modifies carbohydrates, proteins, lipoproteins and
nucleic acids in tissues or cells, directly damaging their normal
functions and leads to cell death (27,28).
This explains why caspase-3 is significantly upregulated in a mouse
model of IR. Caspase-3 is the most common apoptotic signaling
pathway and serves a key role in cell apoptosis (29). The present study demonstrated that
SEV preconditioning decreased caspase-3 protein expression levels
in renal tubular epithelial cells induced by renal IR injury, which
is one of the major mechanisms of the renal protective effect.
The results of the present study also demonstrated
that following IR, oxidative stress in mouse kidneys was
significantly upregulated and SEV preconditioning significantly
reduced MDA and MPO levels, which may be related to SEV
preconditioning resulting in increased activities of the
antioxidant enzymes SOD and CAT. SOD is considered to be the most
powerful antioxidant in the cell (30). CAT is a common antioxidant enzyme
present in almost all living tissues that utilize oxygen (22). Both SOD and CAT are important
endogenous antioxidant enzymes that act as components of the first
line of defense against ROS. In the present study, the activation
of SOD and CAT may have resulted from the upregulation of Nrf2
protein expression levels. Furthermore, immunohistochemistry
demonstrated that SEV preconditioning significantly activated Nrf2
protein expression level in the kidney tissues impaired by IR. Nrf2
is a basic leucine zipper stress-responsive transcription factor
that maintains cellular redox homeostasis. Nrf2 is a key regulator
of the cellular redox response and acts as a signal for ROS
scavenging (31).
Kelch-like ECH-associated protein 1 (Keap1) exists
in the cytoplasm and binds with Nrf2, which mediates Nrf2
inhibition. When in vivo oxidative stress increases, Nrf2
dissociates from the Keap1 protein and translocates to the nucleus,
binds with ARE and induces the increased expression of
Nrf2-regulated genes (e.g., HO-1, GST, NQO-1) (32).
Nrf2/ARE/HO-1 is one of the classic antioxidant
signal axes. Ruan et al (33) reported that inhibiting Nrf2/HO-1
reduces the activities of SOD and GSH, and induces serious
oxidative damage. Moreover, activation of the Nrf2/ARE/HO-1
signaling pathway can improve oxidative stress damage caused to
cells. This conclusion is also confirmed in the present study.
However, the effect of SEV on nitrification stress was not
investigated in the present study. A follow-up study can further
clarify whether SEV preconditioning involves the anti-nitrification
stress mechanism. Leukocyte-endothelial cell adhesion is induced by
proinflammatory factors during the course of inflammatory
responses. When serum proinflammatory factors are dominant and the
level of anti-inflammatory factors is relatively low, the
inflammatory response will be amplified. Moreover, the incomplete
repair of renal tubules and a continuous inflammatory reaction in
tubulointerstitial tissues often occurs following renal IR, which
can cause renal tissue fibrosis (34). The present study demonstrated that
SEV preconditioning improved the post-IR fibrosis of renal tubules
in mice. It can therefore be hypothesized that this slowing effect
is mainly due to decreased inflammation and oxidative stress,
whereby the activation of the Nrf2/HO-1 signaling pathway by SEV is
one of the underlying mechanisms.
Oxidative stress triggers inflammation. TNF-α is one
of the important inflammatory mediators in the inflammatory
response. It can activate neutrophils and lymphocytes, increase the
permeability of vascular endothelial cells, and regulate the
metabolic activities of other tissues (35,36).
TNF-α can also promote the synthesis and release of other
cytokines. TNF-α has a direct cytotoxic effect on renal intrinsic
cells and induces apoptosis and cell death, whereby TNF-α activates
NADPH oxidase and induces ROS and cell damage (35). IL-6 can induce T cell activation,
proliferation and differentiation, and participates in the immune
response (37). IL-6 and other
inflammatory factors are promoters of the inflammatory response
(35,37). In the present study, renal IR
resulted in the significant upregulation of inflammatory factor
expression, which may be mainly caused by the oxidative stress
response in the renal tissue.
With the increase of clinical application,
researchers found that the role of SEV in human body has two
aspects: Potential cognitive damage and potential vascular
protection (38,39). However, further investigation is
required to determine its function on different organs and pathways
to fully understand the mechanism involved. The present study also
demonstrated that SEV preconditioning significantly inhibited the
production of chemokines, including MCP-1, ICAM-1 and VCAM-1
induced by IR in vivo. A previous study has demonstrated
that chemokines serve important roles in IR-induced AKI (40). For example, knocking down ICAM-1
using short hairpin RNA significantly protects renal function
(31) and inhibiting MCP-1
improves renal function in IR mice (16,31).
MCP-1 is a key chemokine involved in regulating monocyte migration
and infiltration. Previous studies (41,42)
have reported that increased MCP-1 expression recruits macrophages.
The uptake of apoptotic cells by macrophages promotes tolerance by
suppressing the release of proinflammatory cytokines and increasing
the release of anti-inflammatory cytokines, such as TGF-β1
(43,44), a key pro-fibrosis mediator.
The potential translational outcome of the present
research is that SEV preconditioning may benefit patients with
chronic kidney disease undergoing surgery and may become a standard
treatment strategy before surgery for patients with chronic kidney
disease in the future. However, the limitation of the present study
is that it is an animal study, not a human one; therefore, it is
not fully consistent with the human physical condition. Therefore,
care must be taken to translate these findings into clinical
practice.
In conclusion, the present study indicated that
renal IR may lead to a significant increase in the oxidative stress
response and the upregulation of the cellular inflammatory
response. Following SEV preconditioning, the renal injury of IR
mice was significantly improved due to the antiapoptotic,
antioxidation and anti-inflammatory properties of SEV. Moreover,
the results suggested that the upregulation of Nrf2 expression may
contribute towards the renal protective effect of SEV in IR.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by Beijing Natural Science
Foundation (grant no. 7202138).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WXW and YBS were responsible for the conception and
design of the study. WXW, ZRZ and YB were responsible for the
acquisition and analysis of data. YXL and XNG conducted
pathological analysis and evaluation. SZ performed TUNEL and in
vitro hypoxia/reoxygenation analysis. YBS drafted the
manuscript. SZ and YBS revised the manuscript. WXW and YBS confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The animal experiment was approved by the Ethics
Committee of Laboratory Animals of Chengde Medical College (Hebei,
China; approval no. P2020145).
Patient consent for participation
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Verma S and Kellum JA: Defining acute
kidney injury. Crit Care Clin. 37:251–266. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bidani A and Churchill PC: Acute renal
failure. Dis Mon. 35:57–132. 1989.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kulvichit W, Kellum JA and Srisawat N:
Biomarkers in acute kidney injury. Crit Care Clin. 37:385–398.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang L, Xing G, Wang L, Wu Y, Li S, Xu G,
He Q, Chen J, Chen M, Liu X, et al: Acute kidney injury in China: A
cross-sectional survey. Lancet. 386:1465–1471. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hilton R: Acute renal failure. BMJ.
333:786–790. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han SJ and Lee HT: Mechanisms and
therapeutic targets of ischemic acute kidney injury. Kidney Res
Clin Pract. 38:427–440. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jan R and Chaudhry GE: Understanding
apoptosis and apoptotic pathways targeted cancer therapeutics. Adv
Pharm Bull. 9:205–218. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chatterjee PK, Todorovic Z, Sivarajah A,
Mota-Filipe H, Brown PA, Stewart KN, Cuzzocrea S and Thiemermann C:
Differential effects of caspase inhibitors on the renal dysfunction
and injury caused by ischemia-reperfusion of the rat kidney. Eur J
Pharmacol. 503:173–183. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xiao Z, Shan J, Li C, Luo L, Lu J, Li S,
Long D and Li Y: Mechanisms of cyclosporine-induced renal cell
apoptosis: A systematic review. Am J Nephrol. 37:30–40.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu TJ, Hsieh YJ, Lu CW, Lee CJ and Hsu BG:
Linagliptin protects against endotoxin-induced acute kidney injury
in rats by decreasing inflammatory cytokines and reactive oxygen
species. Int J Mol Sci. 22(11190)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vomund S, Schäfer A, Parnham MJ, Brüne B
and von Knethen A: Nrf2, the master regulator of anti-oxidative
responses. Int J Mol Sci. 18(2772)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Abdo S, Zhang SL and Chan JS: Reactive
oxygen species and nuclear factor erythroid 2-related factor 2
activation in diabetic nephropathy: A hidden target. J Diabetes
Metab 6: 10.4172/2155-6156.1000547, 2015.
|
|
13
|
Ishii T, Itoh K, Takahashi S, Sato H,
Yanagawa T, Katoh Y, Bannai S and Yamamoto M: Transcription factor
Nrf2 coordinately regulates a group of oxidative stress-inducible
genes in macrophages. J Biol Chem. 275:16023–16029. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bolignano D, Donato V, Coppolino G, Campo
S, Buemi A, Lacquaniti A and Buemi M: Neutrophil
gelatinase-associated lipocalin (NGAL) as a marker of kidney
damage. Am J Kidney Dis. 52:595–605. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rhee H, Shin N, Shin MJ, Yang BY, Kim IY,
Song SH, Lee DW, Lee SB, Kwak IS and Seong EY: High serum and urine
neutrophil gelatinase-associated lipocalin levels are independent
predictors of renal progression in patients with immunoglobulin A
nephropathy. Korean J Intern Med. 30:354–361. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Albert C, Zapf A, Haase M, Röver C,
Pickering JW, Albert A, Bellomo R, Breidthardt T, Camou F, Chen Z,
et al: Neutrophil gelatinase-associated lipocalin measured on
clinical laboratory platforms for the prediction of acute kidney
injury and the associated need for dialysis therapy: A systematic
review and meta-analysis. Am J Kidney Dis. 76:826–841.e1.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Naunova-Timovska S, Cekovska S, Sahpazova
E and Tasić V: Neutrophil gelatinase-associated lipocalin as an
early biomarker of acute kidney injury in newborns. Acta Clin
Croat. 59:55–62. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Albeladi FI and Algethamy HM: Urinary
neutrophil gelatinase-associated lipocalin as a predictor of acute
kidney injury, severe kidney injury, and the need for renal
replacement therapy in the intensive care unit. Nephron Extra.
7:62–77. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jose RL, Damayanathi D, Unnikrishnan KP
and Suneel PR: A comparison of sevoflurane versus
sevoflurane-propofol combination on renal function in patients
undergoing valvular heart surgery-A prospective randomized
controlled pilot study. Ann Card Anaesth. 24:172–177.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li H, Weng Y, Yuan S, Liu W, Yu H and Yu
W: Effect of sevoflurane and propofol on acute kidney injury in
pediatric living donor liver transplantation. Ann Transl Med.
7(340)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ministry of Science and Technology of the
PRC. Available from: https://www.puh3.net.cn/images/dwsyzx/zc/2020/5/30/227A82A9E2B44976986A1EB84BB39836.pdf.
|
|
22
|
Zhang S, Xin H, Li Y, Zhang D, Shi J, Yang
J and Chen X: Skimmin, a coumarin from hydrangea paniculata, slows
down the progression of membranous glomerulonephritis by
anti-inflammatory effects and inhibiting immune complex deposition.
Evid Based Complement Alternat Med. 2013(819296)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang S, Ma J, Sheng L, Zhang D, Chen X,
Yang J and Wang D: Total coumarins from hydrangea paniculata show
renal protective effects in lipopolysaccharide-induced acute kidney
injury via anti-inflammatory and antioxidant activities. Front
Pharmacol. 8(872)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Han SJ, Kim JH, Kim JI and Park KM:
Inhibition of microtubule dynamics impedes repair of kidney
ischemia/reperfusion injury and increases fibrosis. Sci Rep.
6(27775)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gonul Y, Ozsoy M, Kocak A, Ozkececi ZT,
Karavelioglu A, Bozkurt MF, Cartilli O, Keles I, Kocak H and Celik
S: Antioxidant, antiapoptotic and inflammatory effects of
interleukin-18 binding protein on kidney damage induced by hepatic
ischemia reperfusion. Am J Med Sci. 351:607–615. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Linas SL, Whittenburg D and Repine JE:
Role of xanthine oxidase in ischemia/reperfusion injury. Am J
Physiol. 258:F711–F716. 1990.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Granger DN, Rutili G and McCord JM:
Superoxide radicals in feline intestinal ischemia.
Gastroenterology. 81:22–29. 1981.PubMed/NCBI
|
|
29
|
Nuñez G, Benedict MA, Hu Y and Inohara N:
Caspases: The proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ighodaro OM and Akinloye OA: First line
defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GPX): Their fundamental role in the entire
antioxidant defence grid. Alex J Med. 54:287–293. 2018.
|
|
31
|
Silva-Islas CA and Maldonado PD: Canonical
and non-canonical mechanisms of Nrf2 activation. Pharmacol Res.
134:92–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ruan H, Luo J, Wang L, Wang J, Wang Z and
Zhang J: Sika deer antler protein against acetaminophen-induced
nephrotoxicity by activating Nrf2 and inhibition FoxO1 via PI3K/Akt
signaling. Int J Biol Macromol. 141:961–987. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
Inflammatory processes in renal fibrosis. Nat Rev Nephrol.
10:493–503. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun L and Kanwar YS: Relevance of TNF-α in
the context of other inflammatory cytokines in the progression of
diabetic nephropathy. Kidney Int. 88:662–665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Griffin GK, Newton G, Tarrio ML, Bu DX,
Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW,
Croce KJ and Lichtman AH: IL-17 and TNF-α sustain neutrophil
recruitment during inflammation through synergistic effects on
endothelial activation. J Immunol. 188:6287–6299. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6(a016295)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Neag MA, Mitre AO, Catinean A and Mitre
CI: An overview on the mechanisms of neuroprotection and
neurotoxicity of isoflurane and sevoflurane in experimental
studies. Brain Res Bull. 165:281–289. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fang FQ, Sun JH, Wu QL, Feng LY, Fan YX,
Ye JX, Gao W, He GL and Wang WJ: Protective effect of sevoflurane
on vascular endothelial glycocalyx in patients undergoing heart
valve surgery: A randomised controlled trial. Eur J Anaesthesiol.
38:477–486. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sen Z, Jie M, Jingzhi Y, Dongjie W,
Dongming Z and Xiaoguang C: Total coumarins from hydrangea
paniculata protect against cisplatin-induced acute kidney damage in
mice by suppressing renal inflammation and apoptosis. Evid Based
Complement Alternat Med. 2017(5350161)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chung CH, Fan J, Lee EY, Kang JS, Lee SJ,
Pyagay PE, Khoury CC, Yeo TK, Khayat MF, Wang A and Chen S: Effects
of tumor necrosis factor-α on podocyte expression of monocyte
chemoattractant protein-1 and in diabetic nephropathy. Nephron
Extra. 5:1–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Haller H, Bertram A, Nadrowitz F and Menne
J: Monocyte chemoattractant protein-1 and the kidney. Curr Opin
Nephrol Hypertens. 25:42–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Voll RE, Herrmann M, Roth EA, Stach C,
Kalden JR and Girkontaite I: Immunosuppressive effects of apoptotic
cells. Nature. 390:350–351. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Fadok VA, Bratton DL, Konowal A, Freed PW,
Westcott JY and Henson PM: Macrophages that have ingested apoptotic
cells in vitro inhibit proinflammatory cytokine production through
autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J
Clin Invest. 101:890–898. 1998.PubMed/NCBI View Article : Google Scholar
|