Introduction
Periodontitis is a common chronic inflammatory oral
disease (1). With the gradual
onset of inflammation, periodontitis causes destruction of the
periodontal ligament, cementum and alveolar bone (2). If treatment is not applied in a
timely manner, periodontitis will cause further periodontal
detachment and bone loss, eventually leading to early tooth loss
(3). Due to the importance of
periodontal tissues, the objective of periodontal treatment is to
first control the infection and inhibit disease progression, then
to regenerate the damaged periodontal tissue and reconstruct the
missing tooth support structures (4). At present, conventional treatments
for periodontal disease are relatively basic in nature, including
cleansing, curettage and root leveling (5). Although these can control
inflammation and prevent the progression of periodontal disease,
they cannot completely restore the damaged tooth support structure
(5). In addition, although the
emergence of guided tissue regeneration methods has improved the
treatment of periodontal disease, its ultimate effect remains
limited and unstable (6,7). Furthermore, indications for guided
tissue regeneration must be strictly screened and clinical
predictability of its application is poor, limiting its application
further (6,7). Over the previous decade, with the
development of regenerative medicine, stem cell-based tissue
engineering technology has provided an important means of repairing
damaged tissues whilst preserving the highly ordered surrounding
internal environment (8-11).
This observation has been reported by a number of previous
preclinical studies (8-11).
In particular, human periodontal ligament stem cells (hPDLSCs) have
been demonstrated to favorably regenerate supporting tissues
surrounding the tooth in vivo (12). Therefore, hPDLSCs are increasingly
being applied for the regeneration treatment of bone loss caused by
periodontal disease (12).
Stilbene compounds are general names given to a
series of compounds with the stilbene group as the parent backbone,
which serve important roles in pharmacology. For example, several
stilbene-based drugs such as raloxifene, toremifene and tamoxifen,
have been approved clinically for treating various diseases,
including breast cancer (13).
Pterostilbene (PTE; 3, 5-dimethoxy-4'-hydroxystilbene) is a
representative of stilbene compounds that can be found naturally in
blueberries and red sandalwood (14). PTE is a methylated derivative of
resveratrol, both of which confer considerable anti-oxidant,
anti-inflammatory and anticancer effects (15). On account of the presence of two
methoxy groups, PTE has been reported to exert improved
lipophilicity, increased oral absorption and higher bioavailability
compared with resveratrol (16,17).
Therefore, it has been considered to be a potential drug for the
treatment of a number of diseases, such as Alzheimer's disease,
vascular dementia, bladder cancer (16,17).
Previous studies have revealed that PTE can prevent
hypoxia-reoxygenation injury by activating sirtuin 1 in
cardiomyocytes (18), in addition
to improving cardiac function and reducing oxidative stress in
animal models of ischemia-reperfusion injury (19). Another previous study suggested
that PTE reduced high-fat-induced atherosclerosis in mice by
inhibiting various proinflammatory cytokines, including TGF-β,
TNF-α and interleukins (20). In
particular, it was also demonstrated that PTE can reduce the
release of inflammatory factors, including IL-1β, IL-6 and TNF-α by
macrophages induced by oral symbiotic bacteria (21). Therefore, the present study
hypothesized that PTE can prevent the onset of periodontitis.
TNF-α is one of the pivotal endogenous mediators of
periodontitis (22). Therefore,
TNF-α was used to induce hPDLSCs in the present study to assess the
effects of PTE on cell proliferation and differentiation. Results
of the present study may expand the current understanding of the
efficacy of PTE and provide a novel direction for the design of
treatment strategies for periodontitis.
Materials and methods
Cell culture and grouping
hPDLSCs (cat. no. BJ-ATCC0562; primary cells;
Shanghai Bangjing Industry Co., Ltd.) were cultured in α-MEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) in 5% CO2 at 37˚C.
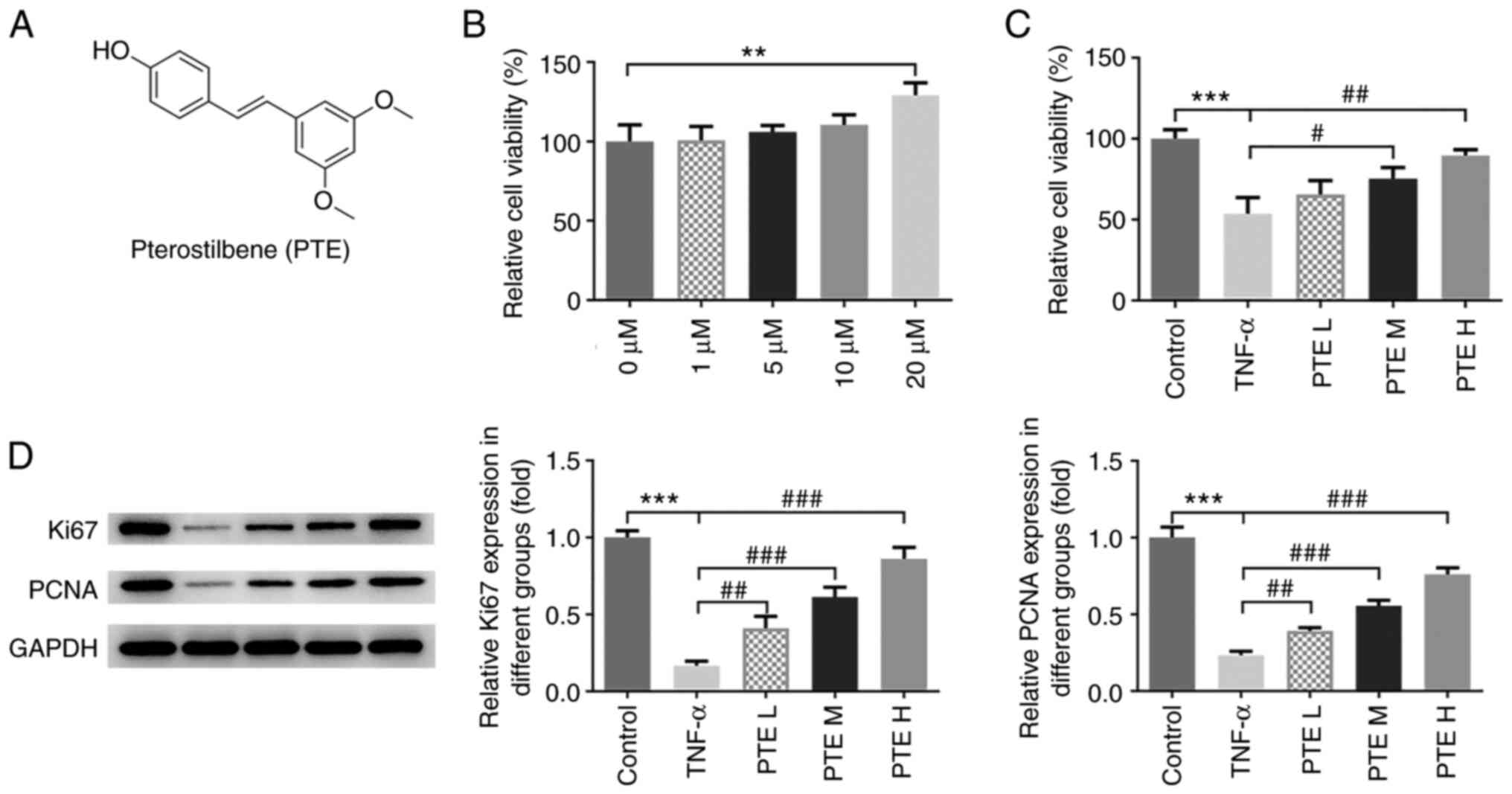
PTE (purity ≥97%; Sigma-Aldrich; Merck KGaA;
Fig. 1A) was dissolved in DMSO
(purity >99.8%; Shanghai Aladdin Biochemical Technology Co.,
Ltd.) and diluted to concentrations of 1, 5, 10 and 20 µM (23). TNF-α (purity ≥95%; Sigma-Aldrich;
Merck KGaA) was dissolved in DMSO and diluted to concentration of
100 ng/ml (24). Following the
determination of PTE concentration, hPDLSCs were divided into the
following five groups: i) Control (untreated); ii) TNF-α (100
ng/ml), iii) PTE low (L; TNF-α + PTE low dose 5 µM); iv) PTE medium
(M; TNF-α + PTE medium dose 10 µM); and v) PTE high (H; TNF-α + PTE
high dose 20 µM).
Bioinformatics analysis
TargetNet (www.targetnet.scbdd.com) is a platform that can be
used for drug target prediction (25). The structure of PTE was input with
default parameters (area under curve ≥0.7; fingerprint type:
extended connectivity fingerprint 4) to obtain a report of probable
target proteins.
Molecular docking technology is a theoretical
simulation method used for studying the interactions among
molecules, such as ligands and receptors, in addition to predicting
the nature of their binding mode and affinity (26). The structure of PTE was first
imported into the OpenBabel v2.2.1 software (Free software
foundation, Inc.) for hydrogenation and conversion into its 3D
structure. The 3D structure of various histone deacetylase (HDAC)
proteins, including HDAC2 (PDB ID: 6WBW), HDAC4 (PDB ID: 6FYZ),
HDAC6 (PDB ID: 5EDU) and HDAC8 (PDB ID: 5D1B), were downloaded from
the Protein Data Bank website (https://www.rcsb.org/). To reduce the influence of
binding force prediction, water molecules and existing ligands in
the protein structure were deleted using PyMOL v2.2.0 software
(DeLano Scientific, LLC). The position of the original ligand was
set as a docking site before the docking between PTE and HDACs was
performed using AutoDock v4.2 software (Scripps Institute) via
automatic calculation of binding free energy PTE and HDACs in the
running process of the system. All docking calculations were
performed using a Lamarckian genetic algorithm. The default FlexX
parameters were used.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was measured using CCK-8 assay kit
(Dojindo Molecular Technologies, Inc.). hPDLSCs were seeded into
96-well plates at a density of 3x103 cells/well. After
allowing for cell adherence, the original culture medium was
replaced with medium containing PTE (0, 1, 5, 10 and 20 µM) and/or
TNF-α (100 ng/ml). After 48 h of incubation at 37˚C, 10
µl CCK-8 was added to each well, after which samples were incubated
at 37˚C for an additional 2 h. Optical density (OD)
values were recorded at 450 nm using a microplate reader (Dojindo
Molecular Technologies, Inc.).
Alkaline phosphatase (ALP) activity
assay
hPDLSCs were seeded into six-well plates at a
density of 2x105 cells/well. After cells reached 60-70%
confluence, the culture medium was replaced with osteogenesis
induction medium [α-MEM supplemented with 10% FBS, 10 mM
β-glycerolphosphate (China Pharmaceutical Shanghai Chemical Reagent
Co., Ltd.), 50 mg/l ascorbate and 2 mg/l dexamethasone (both
Sigma-Aldrich; Merck KGaA)] containing TNF-α (100 ng/ml) and PTE
(0, 5, 10 and 20 µM). Briefly, the cells were lysed with 0.05%
Triton X-100 (Sigma-Aldrich) at 4˚C for 2 h. On day 7
following osteogenic induction at 37˚C, the cell lysate
was centrifuged at 10,000 x g for 15 min at 4˚C to
collect the supernatant. ALP activity was measured according to the
manufacturer's protocol of the ALP activity detection kit (cat. no.
P0321; Beyotime Institute of Biotechnology). The OD value was
recorded at 405 nm using a microplate reader (Dojindo Molecular
Technologies, Inc.).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
The apoptosis of hPDLSCs cells was assessed using a
TUNEL assay (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. After 24 h cell culture, transfected
cells were fixed with 1% paraformaldehyde at room temperature for
15 min and 50 µl TUNEL was used to incubate cells for 1 h at 37˚C,
followed by staining of nuclear DNA with 10 µg/ml DAPI at 37˚C for
2-3 min and mounted with glycerol gelatin (Sigma-Aldrich; Merck
KGaA). The cells were analyzed from three fields of view using a
fluorescence microscope (magnification, x200; Olympus
Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
hPDLSCs were incubated in six-well plates at a
density of 2x105 cells/well. TNF-α (100 ng/ml) and PTE
(0, 5, 10 and 20 µM) were added to the plates at 37˚C
until cells reached 60-70% confluence. Cells were subsequently
incubated at 37˚C for 48 h. For the determination of ALP
and runt-related transcription factor 2 (RUNX2) expression levels,
cells were collected on day 7 of osteogenic induction. Total RNA
was extracted from the cultured cells using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol and reversed transcribed into cDNA
using the PrimeScript RT reagent kit (Takara Bio, Inc.) in
accordance with the manufacturer's protocol. qPCR reactions were
performed using the SYBR Green Master Mix (Biosharp Life Sciences)
on a 7500 Real-time system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
thermocycling conditions were as follows: Initial denaturation at
95˚C for 5 min, followed by 45 cycles of 95˚C
for 30 sec, 55˚C for 30 sec, 72˚C for 30 sec
and 72˚C for 7 min. Relative mRNA expression was
quantified using the 2-∆∆Cq method (27) and normalized to GADPH. The primer
sequences used for qPCR are listed in Table I.
 | Table ISequences of the primers. |
Table I
Sequences of the primers.
| Gene | Sequence
(5'-3') | NCBI Reference
sequence | Product size |
|---|
| IL-1β | | NM_000576.3 | 153 |
|
Forward |
CTACCTGTCCTGCGTGTTGA | | |
|
Reverse |
GGGAACTGGGCAGACTCAAA | | |
| IL-6 | | NM_000600.5 | 233 |
|
Forward |
CCTTCGGTCCAGTTGCCTTCT | | |
|
Reverse |
CAGTGCCTCTTTGCTGCTTTC | | |
| IL-10 | | NM_000572.3 | 148 |
|
Forward |
AGACAGACTTGCAAAAGAAGGC | | |
|
Reverse |
TCGAAGCATGTTAGGCAGGTT | | |
| ALP | | NM_000478.6 | 231 |
|
Forward |
CTTGTGCCTGGACGGACC | | |
|
Reverse |
CGCCAGTACTTGGGGTCTTT | | |
| RUNX2 | | NM_001015051.4 | 129 |
|
Forward |
CGAATGGCAGCACGCTATTAA | | |
|
Reverse |
GTCGCCAAACAGATTCATCCA | | |
| GAPDH | | NM_001256799.3 | 138 |
|
Forward |
GCACCGTCAAGGCTGAGAAC | | |
|
Reverse |
TGGTGAAGACGCCAGTGGA | | |
Western blotting
The treatment of hPDLSCs was the same as that
described in RT-qPCR. Protein lysates from hPDLSCs were prepared
using RIPA lysis buffer (Beyotime Institute of Biotechnology) and
protein concentration was measured using a BCA kit (Beijing
Solarbio Science & Technology, Co., Ltd.). Samples (20 µg per
lane) were subjected to 10 or 12% SDS-PAGE and transferred onto
PVDF membranes. After blocking with 5% non-fat milk at room
temperature for 1 h, blots were incubated with the following
primary antibodies overnight at 4˚C: Ki67 (1:100; cat.
no. ab16667; Abcam), phosphorylated (p)-IκBα (1:1,000; cat. no.
2859; Cell Signaling Technology, Inc.), IκBα (1:1,000; cat. no.
4812; Cell Signaling Technology, Inc.), cleaved caspase 3 (1:500;
cat. no. ab32042; Abcam), ALP (1:500, cat. no. ab65834; Abcam),
runt-related transcription factor 2 (RUNX2; 1:1,000; cat. no.
ab23981; Abcam), GAPDH (1:10,000; cat. no. ab181602; Abcam),
proliferating cell nuclear antigen (PCNA; 1:1,000; cat. no. 13110;
Cell Signaling Technology, Inc.), Bcl2 (1:1,000; cat. no. 4223;
Cell Signaling Technology, Inc.), Bax (1:1,000; cat. no. 5023; Cell
Signaling Technology, Inc.), HDAC2 (1:1,000; cat. no. 57156; Cell
Signaling Technology, Inc.), HDAC4 (1:1,000; cat. no. 15164; Cell
Signaling Technology, Inc.), HDAC6 (1:1,000; cat. no. 7558; Cell
Signaling Technology, Inc.) and HDAC8 (1:1,000; cat. no. 66042;
Cell Signaling Technology, Inc.). The membranes were then incubated
with HRP-conjugated goat anti-rabbit (1:10,000; cat. no. ab6721;
Abcam) secondary antibodies for 2 h at room temperature and
developed using an ECL kit (Biosharp Life Sciences). ImageJ v1.8.0
software (National Institutes of Health) was applied for image
analysis and relative protein expression was normalized to
GAPDH.
Alizarin red staining
hPDLSCs were seeded into 12-well plates at a density
of 1x105 cells/well. After the cells reached 60-70%
confluence, the culture medium was replaced with osteogenesis
induction medium containing TNF-α (100 ng/ml) and PTE (0, 5, 10 and
20 µM). At 21 days after osteogenic induction at 37˚C,
cells were washed once with PBS and fixed with 4% paraformaldehyde
for 20 min at room temperature. 0.04 M Alizarin Red S staining
solution (cat. no. C0148S; Beyotime Institute of Biotechnology) was
subsequently added for 30 min at room temperature. Images were
observed under a light microscope (magnification, x200; Olympus,
Corporation).
Statistical analysis
All experimental data are presented as the mean ± SD
from three independent experiments. GraphPad Prism 8.0 statistical
software (GraphPad Software, Inc.) was used to analyze the data.
Additionally, the data were in accordance with the normal
distribution by Shapiro-Wilk test. One-way ANOVA and Tukey's post
hoc test was conducted to compare differences between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PTE promotes TNF-α-induced hPDLSC
viability and alleviates inflammation
The effect of different concentrations of PTE (0, 1,
5, 10 and 20 µM) on the viability of hPDLSCs was determined using a
CCK-8 assay. The results revealed that PTE at 20 µM increased cell
viability compared with control untreated cells (Fig. 1B). Since PTE at a concentration of
1 µM did not have a significant effect on cells, PTE at 5, 10 and
20 µM concentrations were used in subsequent experiments and
labeled as L, M and H groups, respectively. Following TNF-α
induction, the effects of these three concentrations of PTE on cell
viability were assessed using a CCK-8 assay. The results indicated
that cell viability was significantly decreased in the TNF-α group
compared with the control group, but subsequent PTE treatment
reversed this reduction in cell viability in a
concentration-dependent manner (Fig.
1C). The expression levels of Ki67 and PCNA were then measured
using western blotting. The data revealed that the expression
levels of each protein were significantly decreased following TNF-α
treatment. However, PTE significantly reversed these reductions in
Ki67 and PCNA expression (Fig.
1D). These results suggested that PTE prevented hPDLSCs from
TNF-α-induced damage and upregulated cell viability.
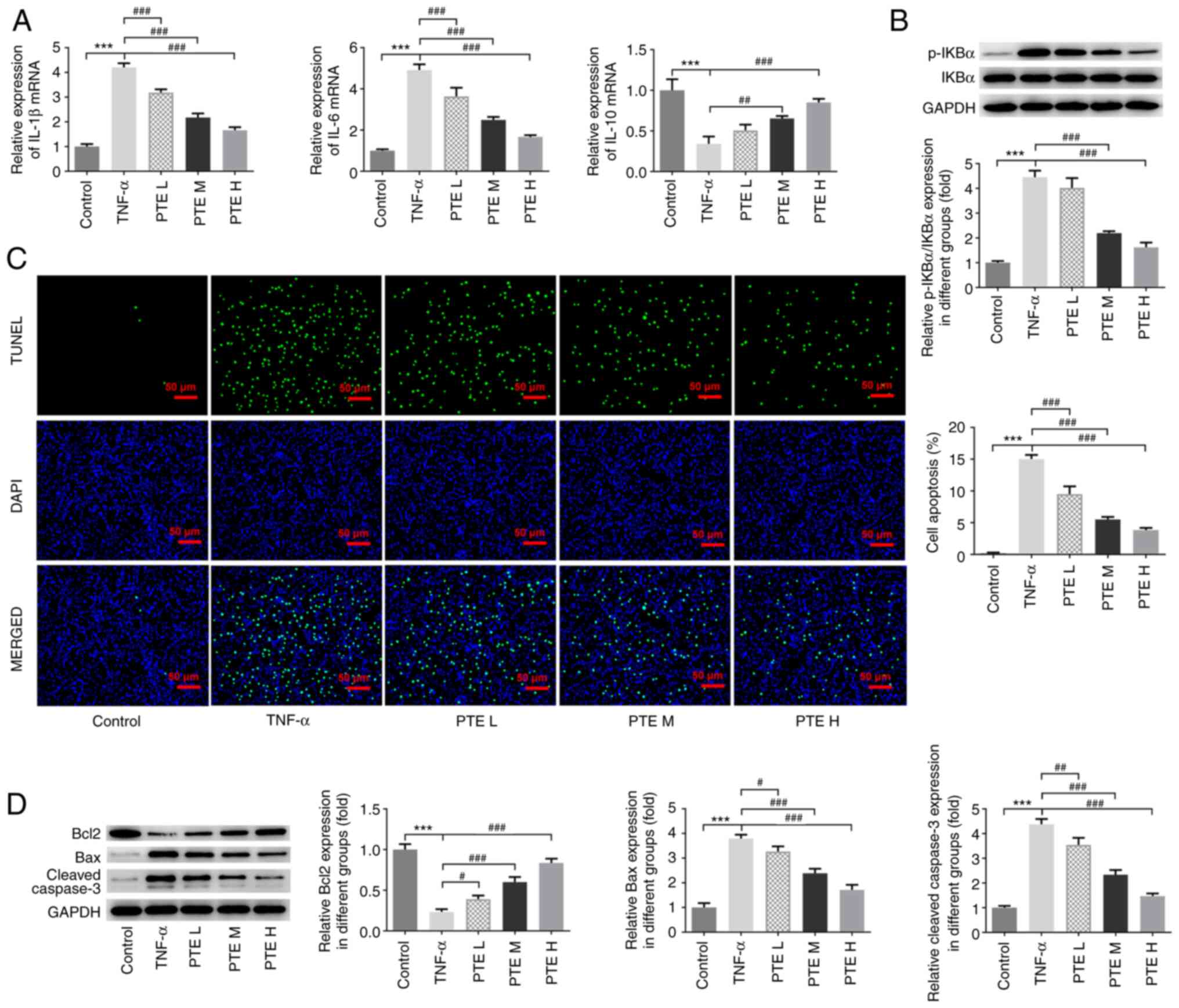
The expression levels of certain inflammatory
factors, including IL-1β, IL-6 and IL-10, were next assessed in
hPDLSCs by performing RT-qPCR. The results revealed that the levels
of IL-1β and IL-6 were significantly increased, whilst that of
IL-10 was significantly decreased, in the TNF-α group compared with
the control group. Furthermore, PTE treatment significantly
downregulated IL-1β and IL-6 levels whilst upregulating IL-10
expression compared with the TNF-α group (Fig. 2A). In addition, western blotting
revealed that PTE inhibited the TNF-α-induced phosphorylation of
p65, with M and H groups reaching significance (Fig. 2B).
PTE reduces apoptosis and promotes
TNF-α-induced hPDLSC differentiation
Cell apoptosis was assessed using TUNEL and western
blotting assays. The number of apoptotic cells emitting green
fluorescence in the TNF-α group was increased significantly
compared with the control group. Additionally, as the PTE
concentration increased, the number of apoptotic cells decreased
significantly (Fig. 2C).
Furthermore, the expression levels of Bax and cleaved caspase 3
were significantly increased in the TNF-α group compared with the
control. However, they were significantly decreased in the
PTE-treated groups compared with the TNF-α group (Fig. 2D). Bcl2 expression levels
demonstrated the opposite trend following TNF-α and PTE treatment
(Fig. 2D). The results of these
assays suggested that PTE alleviated TNF-α-induced hPDLSC
apoptosis.
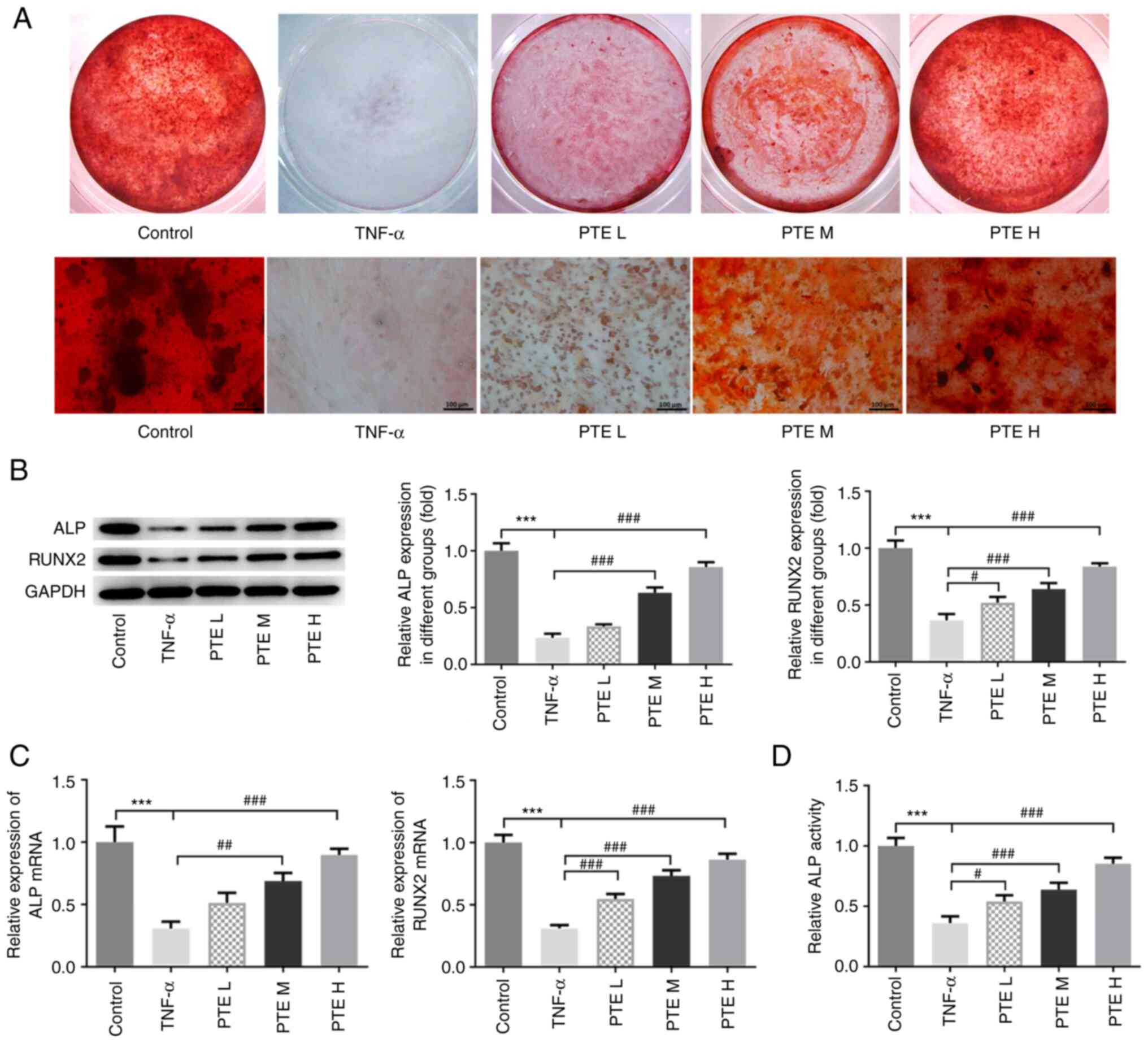
The role of PTE in the mineralization capacity of
hPDLSCs was subsequently evaluated using alizarin red staining.
While clear red mineralized nodules were observed in the control
group, no staining could be observed in the TNF-α group. However,
PTE treatment promoted the formation of mineral nodules in
TNF-α-induced hPDLSCs (Fig. 3A).
The expression levels of mineralization indices were determined
using western blotting and RT-qPCR. The protein and mRNA expression
levels of ALP and RUNX2 were both significantly downregulated in
the TNF-α group, which was significantly reversed in the three PTE
groups (Fig. 3B and C). The activity of ALP was similarly
decreased significantly by TNF-α, which was also significantly
reversed by all three doses of PTE (Fig. 3D).
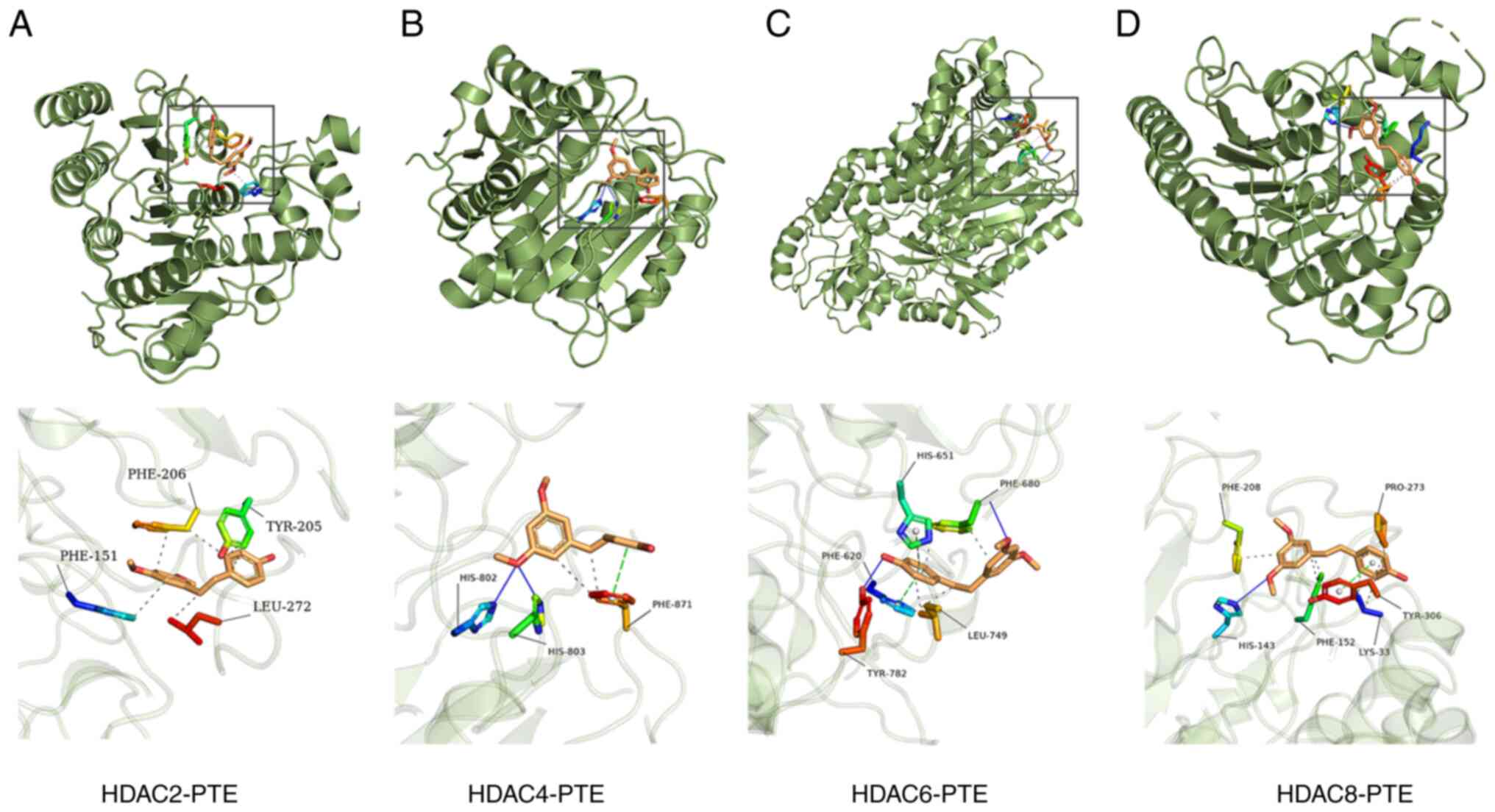
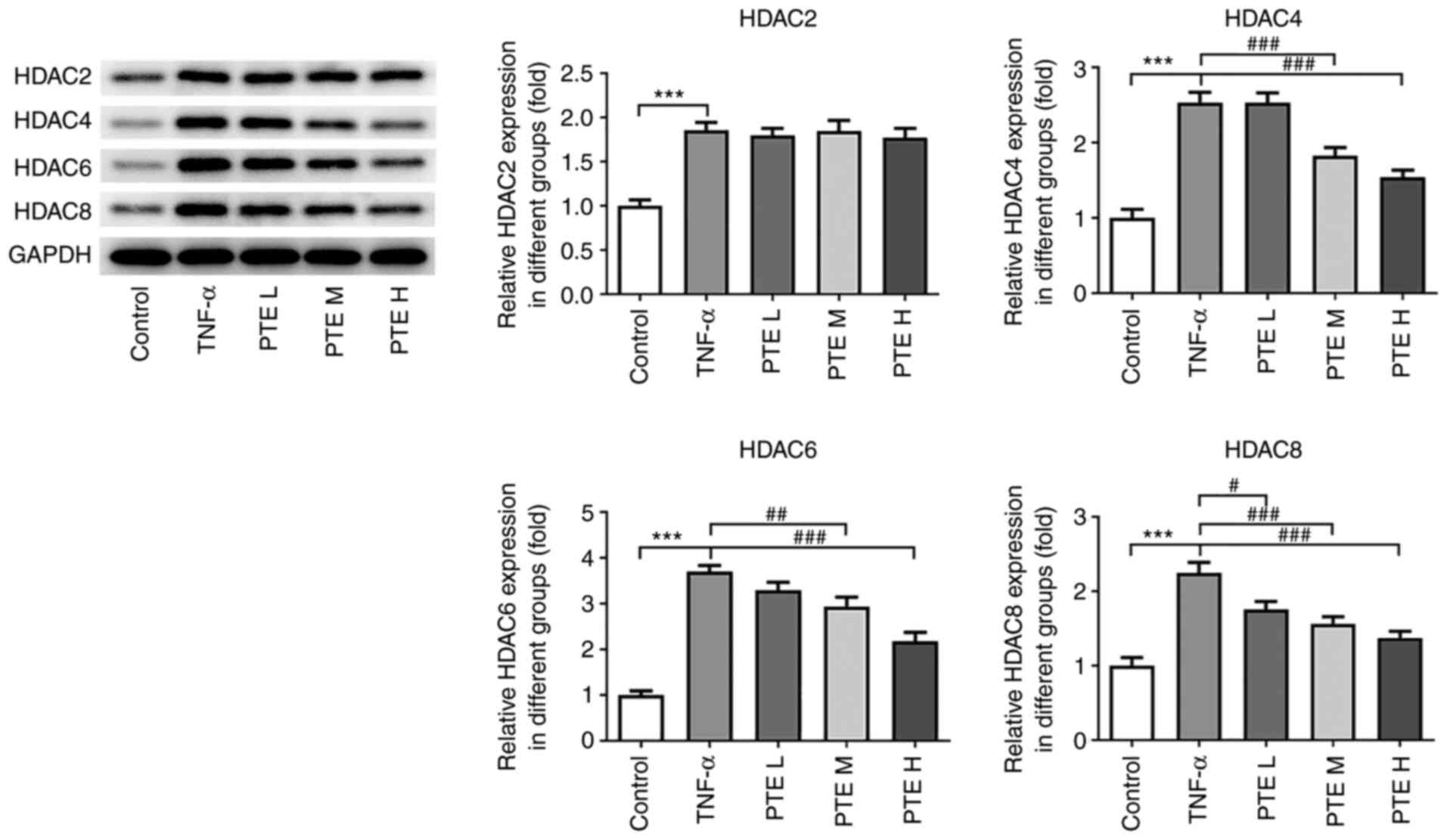
HDACs are probable targets of PTE
The targets of PTE were predicted using the
Targetnet database. The results determined HDAC2, 4, 6 and 8 to be
probable targets of PTE. Therefore, the possible association
between PTE and these proteins were calculated using the molecular
docking module. The whole protein was presented in green, whilst
the main skeleton of PTE was presented in brown. The blue solid
line represented hydrogen bonds, the green dashed line represented
π-stacking and the gray dashed line represented hydrophobic
interactions. The docking values between PTE and HDAC2, 4, 6 and 8
were -6.0, -7.3, -6.4 and -6.4 kcal/mol, respectively, which
indicated that the complex formation is thermodynamically
spontaneous and implied the high stability of PTE-HDAC2, 4, 6 and 8
complexes (Fig. 4). Following
computer prediction, the expression levels of these four proteins
were determined using western blotting. TNF-α significantly
increased the expression levels of all four of the HDAC isoforms
tested, whilst PTE treatment markedly decreased HDAC4, 6 and 8
expression in a concentration-dependent manner compared with the
TNF-α group. However, no significant difference could be observed
for HDAC2 between the TNF-α and PTE groups (Fig. 5).
Discussion
hPDLSCs derived from human periodontal tissues are a
type of mesenchymal stem cell that is readily obtainable in
clinical practice (28). They can
be obtained following wisdom teeth extraction or premolars that are
extracted by orthodontists using minimally invasive methods
(29). This availability render
hPDLSCs attractive sources of autologous stem cells. A previous
study reported that the addition of hPDLSCs to biological materials
is beneficial to promote bone healing in rat skull defect models
(30). In addition, a number of
in vitro and in vitro studies have demonstrated that
hPDLSCs exhibit potent self-renewal and multi-lineage
differentiation capacities (31,32).
Their ability to differentiate into osteoblasts makes them
considerably superior options to stem cells derived from other oral
tissues, including the dental pulp, gums and dental follicles
(33). These characteristics also
mean that hPDLSCs have been extensively applied for the
regeneration treatment of periodontal disease-induced periodontal
cord and bone loss (12).
Therefore, the present study selected hPDLSCs to assess the
possible role of PTE in periodontitis, which revealed that PTE
increased the viability of hPDLSCs in a concentration-dependent
manner.
During the progression of periodontitis, the
increased expression of TNF-α further promotes the secretion of
other inflammatory mediators, adhesion molecules and cytokines that
are closely associated with bone resorption (22). This aggravates the clinical
symptoms of periodontitis (22).
TNF-α was used in the present study to treat hPDLSCs, after which
the effects of PTE on the cell viability, inflammatory factor
release, apoptosis and differentiation of hPDLSCs were assessed.
PTE was revealed to upregulate the expression levels of
proliferation markers Ki67 and PCNA, and reduce IL-1β and IL-6
levels, whilst increasing IL-10 levels. It has been previously
demonstrated that IL-10 serves an anti-inflammatory role by
strongly inhibiting the synthesis of IL-1, IL-6, IL-8 and TNF-α on
a transcriptional level, regardless of whether it is endogenous or
exogenous (34-36).
The results of the present study revealed that PTE increased the
viability of TNF-α-induced hPDLSCs and reduced the release of
inflammatory cytokines. In addition, results from TUNEL staining
and western blotting demonstrated that PTE significantly inhibited
the apoptosis of TNF-α-induced hPDLSCs. A previous study also
revealed that PTE inhibited inflammation and apoptosis in mouse
models of pulmonary fibrosis (37). ALP has been considered to be an
early osteogenic marker, whilst RUNX2 regulates the differentiation
of pluripotent stem cells into osteoblasts (38). In the present study, PTE
significantly upregulated the expression of ALP and RUNX2 on day 7
of osteogenic induction in TNF-α-induced hPDLSCs, suggesting that
PTE promoted hPDLSC differentiation.
Using the Targetnet database, PTE was predicted to
target histone family protein HDACs. Previous studies have revealed
that HDAC inhibitors can promote the proliferation of human dental
pulp stem cells and the differentiation of odontoblasts (39,40).
Furthermore, HDAC2 inhibition has been demonstrated to reduce
cytokine production and osteoclast bone resorption (41). A previous study also reported that
microRNA-22 targets HDAC6 to promote hPDLSC differentiation
(42). In the present study,
according to computer simulation software, it was determined that
PTE could bind to HDACs through intermolecular forces.
Additionally, the docking value between PTE and HDAC4 was the most
optimal among all HDACs. Western blotting revealed that PTE
treatment significantly downregulated the protein expression levels
of HDAC4, 6 and 8, with HDAC4 and 6 exhibiting relatively higher
concentration-dependence. These results suggest that PTE may
participate in the regulation of periodontitis by downregulating
HDAC expression. The downregulation of HDACs reduces the removal of
acetylated groups on the lysine residues of histones, destabilizing
the binding between histones and DNA (43). This detachment promotes the
combination of the DNA promoter regions with transcription factors
(44), which increases the
transcription of genes related to proliferation and differentiation
(45,46). This may be the mechanism through
which PTE can regulate proliferation and differentiation via HDACs.
However, results of the present study are limited to the
preliminary exploration of this mechanism. Additional experiments
are required to verify the association between PTE and HDACs in
periodontitis. Furthermore, in addition to osteogenic
differentiation, cartilage and adipogenic multidirectional
differentiation are also important indices of the differentiation
potential of stem cells. Future studies on the effects of PTE on
these two aspects also warrant further investigation.
In conclusion, the present study revealed that PTE
promoted TNF-α-induced hPDLSC viability and differentiation, whilst
alleviating inflammation and apoptosis. PTE also inhibited the
expression of HDACs, which may be its mechanism of action in
periodontitis. It is hoped that findings of the present study may
promote the development of novel treatment strategies for
periodontitis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY, GW and YL conceived this study. MY, GW and JN
performed the experiments. MP analyzed the data and YL performed
bioinformatics analysis. YL revised the manuscript for important
intellectual content. All authors have read and approved the final
manuscript. MY and GW confirm the authenticity of the raw data.
Ethics approval and consent to
participate
The Ethics Committee of Shanghai Huangpu District
2nd Dental Disease Prevention and Treatment Institute (Shanghai,
China) waived the requirement for ethics approval for using the
purchased human periodontal ligament stem cells.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoare A, Soto C, Rojas-Celis V and Bravo
D: Chronic inflammation as a link between periodontitis and
carcinogenesis. Mediators Inflamm. 2019(1029857)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gasner NS and Schure RS: Periodontal
disease. In: StatPearls. StatPearls Publishing, Treasure Island,
FL, 2021.
|
|
3
|
Liu J, Ruan J, Weir MD, Ren K, Schneider
A, Wang P, Oates TW, Chang X and Xu HHK: Periodontal
bone-ligament-cementum regeneration via scaffolds and stem cells.
Cells. 8(537)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Graziani F, Karapetsa D, Alonso B and
Herrera D: Nonsurgical and surgical treatment of periodontitis: How
many options for one disease? Periodontol 2000. 75:152–188.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deas DE, Moritz AJ, Sagun RS Jr, Gruwell
SF and Powell CA: Scaling and root planing vs. conservative surgery
in the treatment of chronic periodontitis. Periodontol 2000.
71:128–139. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reynolds MA, Kao RT, Camargo PM, Caton JG,
Clem DS, Fiorellini JP, Geisinger ML, Mills MP, Nares S and Nevins
ML: Periodontal regeneration-intrabony defects: A consensus report
from the AAP regeneration workshop. J Periodontol. 86 (Suppl
2):S105–S107. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rojas MA, Marini L, Pilloni A and Sahrmann
P: Early wound healing outcomes after regenerative periodontal
surgery with enamel matrix derivatives or guided tissue
regeneration: A systematic review. BMC Oral Health.
19(76)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gao F, Chiu SM, Motan DA, Zhang Z, Chen L,
Ji HL, Tse HF, Fu QL and Lian Q: Mesenchymal stem cells and
immunomodulation: Current status and future prospects. Cell Death
Dis. 7(e2062)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moradi SL, Golchin A, Hajishafieeha Z,
Khani MM and Ardeshirylajimi A: Bone tissue engineering: Adult stem
cells in combination with electrospun nanofibrous scaffolds. J Cell
Physiol. 233:6509–6522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ouchi T and Nakagawa T: Mesenchymal stem
cell-based tissue regeneration therapies for periodontitis. Regen
Ther. 14:72–78. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yoshida T, Washio K, Iwata T, Okano T and
Ishikawa I: Current status and future development of cell
transplantation therapy for periodontal tissue regeneration. Int J
Dent. 2012(307024)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang GT, Gronthos S and Shi S:
Mesenchymal stem cells derived from dental tissues vs. those from
other sources: Their biology and role in regenerative medicine. J
Dent Res. 88:792–806. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pecyna P, Wargula J, Murias M and Kucinska
M: More than resveratrol: New insights into stilbene-based
compounds. Biomolecules. 10(1111)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cassiano C, Eletto D, Tosco A, Riccio R,
Monti MC and Casapullo A: Determining the effect of pterostilbene
on insulin secretion using chemoproteomics. Molecules.
25(2885)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim H, Seo KH and Yokoyama W: Chemistry of
pterostilbene and its metabolic effects. J Agric Food Chem.
68:12836–12841. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lange KW and Li S: Resveratrol,
pterostilbene, and dementia. Biofactors. 44:83–90. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma Z, Zhang X, Xu L, Liu D, Di S, Li W,
Zhang J, Zhang H, Li X, Han J and Yan X: Pterostilbene: Mechanisms
of its action as oncostatic agent in cell models and in vivo
studies. Pharmacol Res. 145(104265)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo Y, Zhang L, Li F, Hu CP and Zhang Z:
Restoration of sirt1 function by pterostilbene attenuates
hypoxia-reoxygenation injury in cardiomyocytes. Eur J Pharmacol.
776:26–33. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wu M, Lu S, Zhong J, Huang K and Zhang S:
Protective effects of pterostilbene against myocardial
ischemia/reperfusion injury in rats. Inflammation. 40:578–588.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Y and Zhang Y: Pterostilbene, a
novel natural plant conduct, inhibits high fat-induced
atherosclerosis inflammation via NF-κB signaling pathway in
Toll-like receptor 5 (TLR5) deficient mice. Biomed Pharmacother.
81:345–355. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lim YRI, Preshaw PM, Lim LP, Ong MMA, Lin
HS and Tan KS: Pterostilbene complexed with cyclodextrin exerts
antimicrobial and anti-inflammatory effects. Sci Rep.
10(9072)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kitaura H, Kimura K, Ishida M, Kohara H,
Yoshimatsu M and Takano-Yamamoto T: Immunological reaction in
TNF-α-mediated osteoclast formation and bone resorption in vitro
and in vivo. Clin Dev Immunol. 2013(181849)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu H, Wu X, Luo J, Wang X, Guo H, Feng D,
Zhao L, Bai H, Song M, Liu X, et al: Pterostilbene attenuates
astrocytic inflammation and neuronal oxidative injury after
ischemia-reperfusion by inhibiting NF-κB phosphorylation. Front
Immunol. 10(2408)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Meng T, Zhou Y, Li J, Hu M, Li X, Wang P,
Jia Z, Li L and Liu D: Azithromycin promotes the osteogenic
differentiation of human periodontal ligament stem cells after
stimulation with TNF-α. Stem Cells Int.
2018(7961962)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yao ZJ, Dong J, Che YJ, Zhu MF, Wen M,
Wang NN, Wang S, Lu AP and Cao DS: TargetNet: A web service for
predicting potential drug-target interaction profiling via
multi-target SAR models. J Comput Aided Mol Des. 30:413–424.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li J, Fu A and Zhang L: An overview of
scoring functions used for protein-ligand interactions in molecular
docking. Interdiscip Sci. 11:320–328. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xia Y, Tang HN, Wu RX, Yu Y, Gao LN and
Chen FM: Cell responses to conditioned media produced by
patient-matched stem cells derived from healthy and inflamed
periodontal ligament tissues. J Periodontol. 87:e53–e63.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen FM, Sun HH, Lu H and Yu Q: Stem
cell-delivery therapeutics for periodontal tissue regeneration.
Biomaterials. 33:6320–6344. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pizzicannella J, Gugliandolo A, Orsini T,
Fontana A, Ventrella A, Mazzon E, Bramanti P, Diomede F and
Trubiani O: Engineered extracellular vesicles from human
periodontal-ligament stem cells increase VEGF/VEGFR2 expression
during bone regeneration. Front Physiol. 10(512)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bright R, Hynes K, Gronthos S and Bartold
PM: Periodontal ligament-derived cells for periodontal regeneration
in animal models: A systematic review. J Periodontal Res.
50:160–172. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang Y, Xing Y, Jia L, Ji Y, Zhao B, Wen
Y and Xu X: An in vitro comparative study of multisource derived
human mesenchymal stem cells for bone tissue engineering. Stem
Cells Dev. 27:1634–1645. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Trubiani O, Pizzicannella J, Caputi S,
Marchisio M, Mazzon E, Paganelli R, Paganelli A and Diomede F:
Periodontal ligament stem cells: Current knowledge and future
perspectives. Stem Cells Dev. 28:995–1003. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fang D and Zhu J: Molecular switches for
regulating the differentiation of inflammatory and IL-10-producing
anti-inflammatory T-helper cells. Cell Mol Life Sci. 77:289–303.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Munshi S, Parrilli V and Rosenkranz JA:
Peripheral anti-inflammatory cytokine Interleukin-10 treatment
mitigates interleukin-1β-induced anxiety and sickness behaviors in
adult male rats. Behav Brain Res. 372(112024)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ng A, Tam WW, Zhang MW, Ho CS, Husain SF,
McIntyre RS and Ho RC: IL-1β, IL-6, TNF-α and CRP in elderly
patients with depression or Alzheimer's disease: Systematic review
and meta-analysis. Sci Rep. 8(12050)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang H, Hua C, Yang X, Fan X, Song H, Peng
L and Ci X: Pterostilbene prevents LPS-induced early pulmonary
fibrosis by suppressing oxidative stress, inflammation and
apoptosis in vivo. Food Funct. 11:4471–4484. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Newton AH and Pask AJ: CHD9 upregulates
RUNX2 and has a potential role in skeletal evolution. BMC Mol Cell
Biol. 21(27)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jin H, Park JY, Choi H and Choung PH: HDAC
inhibitor trichostatin A promotes proliferation and odontoblast
differentiation of human dental pulp stem cells. Tissue Eng Part A.
19:613–624. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu Z, Chen T, Han Q, Chen M, You J, Fang
F, Peng L and Wu B: HDAC inhibitor LMK-235 promotes the odontoblast
differentiation of dental pulp cells. Mol Med Rep. 17:1445–1452.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Algate K, Haynes D, Fitzsimmons T, Romeo
O, Wagner F, Holson E, Reid R, Fairlie D, Bartold P and Cantley M:
Histone deacetylases 1 and 2 inhibition suppresses cytokine
production and osteoclast bone resorption in vitro. J Cell Biochem.
121:244–258. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yan GQ, Wang X, Yang F, Yang ML, Zhang GR,
Wang GK and Zhou Q: MicroRNA-22 promoted osteogenic differentiation
of human periodontal ligament stem cells by targeting HDAC6. J Cell
Biochem. 118:1653–1658. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Leonova K, Safina A, Nesher E, Sandlesh P,
Pratt R, Burkhart C, Lipchick B, Gitlin I, Frangou C, Koman I, et
al: TRAIN (Transcription of Repeats Activates INterferon) in
response to chromatin destabilization induced by small molecules in
mammalian cells. Elife. 7(e30842)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Elmer JJ, Christensen MD, Barua S, Lehrman
J, Haynes KA and Rege K: The histone deacetylase inhibitor
entinostat enhances polymer-mediated transgene expression in cancer
cell lines. Biotechnol Bioeng. 113:1345–1356. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang Q, Zuo H, Yu S, Lin Y, Chen S, Liu H
and Chen Z: RUNX2 co-operates with EGR1 to regulate osteogenic
differentiation through Htra1 enhancers. J Cell Physiol.
235:8601–8612. 2020.PubMed/NCBI View Article : Google Scholar
|