Introduction
Glioblastoma (GBM) is a malignant tumor with one of
the most rapidly increasing morbidity and mortality rates, making
it become a great threat to human life and health (1). To improve the life quality of
patients, the pathologic type and clinical stage of GBM should be
clearly defined (2). It was also
revealed that GBM is prone to early metastasis (3). Several novel therapeutic methods for
tumors have been established, such as immunotherapy and targeted
therapy (4). In recent years,
positive progress has been made towards targeted therapy for GBM,
where multiple targeted therapeutic drugs have been developed
clinically or are currently in clinical trials (5). However, to combat this disease, more
potential therapeutic targets are required.
Mucin 21 (MUC21) is known to be the human
counterpart of mouse epiglycanin (6), which has 98 tandem repeats of 15
amino acids and three exceptional repeats, followed by the stem
domain, transmembrane domain and the cytoplasmic tail (7). It was reported that MUC21 mediates
multiple cellular functions such as cell adhesion (8). Notably, the membranous expression of
MUC21 has been identified in cancer cells (9).
Importantly, the role of MUC21 in the progression
and metastasis of cancer has already been revealed (9). MUC21 has been found to be widely
expressed in multiple types of tissues and highly expressed in the
micropapillary elements of lung adenocarcinomas (9). In addition, MUC21 modulates the
adhesion of tumor cells (7). A
recent study indicated that MUC21 was a critical regulator in the
incohesive growth pattern in lung adenocarcinoma (9). However, the possible effects of MUC21
on GBM cells and the regulatory mechanisms underlying them remain
unclear.
The present study aimed to investigate the
expression of MUC21 in GBM tissues and cell lines, and to explore
its role in GBM progression, further revealing its mechanism.
Materials and methods
Patients
Human GBM tissues and corresponding adjacent
non-cancerous tissues (5 mm from the tumor site; 47 paired) in the
present study were obtained from Chinese patients (Han nationality)
with GBM at the Tianjin Huanhu Hospital (Tianjin, China) who
received only surgical resection with no other chemical or
radiation therapies from February 2018 to January 2021. The present
study was approved by the Ethics Committee of Tianjin Huanhu
Hospital (Tianjin, China). All patients provided written informed
consent. The clinicopathological features, such as patient age,
sex, tumor tumor location (supratentorial or sub-tentorial),
recurrence and isocitrate dehydrogenase [NADP(+)] mutation are
listed in Table I. Written formal
consent was obtained from all recruited patients for the use of
these samples for research purposes.
 | Table IRelationship between MUC21 expression
and clinicopathological characteristics of patients with
glioblastoma (n=47). |
Table I
Relationship between MUC21 expression
and clinicopathological characteristics of patients with
glioblastoma (n=47).
| | Expression level of
MUC21 | |
|---|
| Clinicopathological
characteristic | Total (n=47) | Low | High | Chi-square value | P-value |
|---|
| Sex | | | | 0.002 | 0.966 |
|
Female | 14 | 5 | 9 | | |
|
Male | 33 | 12 | 21 | | |
| Age, years | | | | 0.093 | 0.760 |
|
<60 | 18 | 7 | 11 | | |
|
≥60 | 29 | 10 | 19 | | |
| Tumor
lateralization | | | | 1.877 | 0.171 |
|
Supratentorial | 14 | 3 | 11 | | |
|
Subtentorial | 33 | 14 | 19 | | |
| Recurrence | | | | 4.821 | 0.028 |
|
No | 21 | 4 | 17 | | |
|
Yes | 26 | 13 | 13 | | |
| Isocitrate
dehydrogenase [NADP(+)] mutations | | | | 5.895 | 0.863 |
|
No | 36 | 13 | 23 | | |
|
Yes | 11 | 4 | 7 | | |
Immunohistochemistry (IHC)
To explore the expression levels of MUC21 in tumor
tissues and corresponding adjacent non-cancerous tissues of
patients with GBM, IHC assays were performed. Tumor tissues were
resected, embedded in paraffin and then cut into 5-µm slices. The
sections were deparaffinized with xylene and rehydrated using a
descending ethanol series. Sections were then fixed with 4%
paraformaldehyde (PFA) at 25˚C for 30 min and subsequently
incubated with 2% BSA (Beyotime Institute of Biotechnology) for 30
min at room temperature. Slides were then incubated with MUC21
antibody (1:100; cat. no. NBP3-06591; Novus Biologicals, LLC) for 2
h at room temperature. Following primary incubation, sections were
incubated with biotinylated secondary antibody (1:200; cat. no.
NB7158; Novus Biologicals, LLC) at room temperature for another 1
h. Samples were subsequently stained with diaminobenzidine and
observed under a light microscope (Carl Zeiss AG).
The scoring method was briefly described as follows.
The proportion of cells with positive staining was graded: 0,
negative stained tumor cells; 1, <25% positive stained cells; 2,
25-50% positive stained cells and 3, >50% positive stained
cells. The staining intensity was assessed on a score of 0 (no
staining), 1 (moderate) and 2 (strong). The expression level of
MUC21 was calculated as follows: Positive staining tumor cells
score x staining intensity score. A staining index of 0-2 was
considered as low expression, whereas 3, 4 and 6 were considered as
high expression.
Cell culture and transfection
Human GBM cell lines, U251 (cat. no. MZ-0186), U87
(glioblastoma of unknown origin; cat. no. MZ-2007; both from Ningbo
Mingzhou Biotechnology Co., Ltd.) and U373 (cat. no. CRL-2741™;
American Type Culture Collection) were obtained from the indicated
companies and STR profiling was performed for these cell lines. The
cell lines were maintained in Dulbecco's modified Eagle medium
(DMEM; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) and maintained in a 5% CO2 incubator at 37˚C.
MUC21 short hairpin RNA (shRNA; sequence:
5'-GGGTCAGCATAGTCACCAACT-3' and 5'-GCGCTCTGACATGCAGAA-3') and
negative control shRNA (shNC; sequence: 5'-TTCTCCGAACGTGTCACGT-3')
plasmids were purchased from Santa Cruz Biotechnology, Inc. and
transfected into both U251 and U87 cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). shNC was used as a control to compare with MUC21
shRNA (1 µg) transfection. pcDNA3.1-MUC21 was used for
overexpression assays and the pcDNA3.1-vector (Addgene, Inc.) was
used as control. A total of 1x105 cells were seeded into
six-well plates and 0.5 µg plasmids were used for transfection. The
cells were transfected using 10 µl Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) in each well. Cells
were cultured for 4 h with Lipofectamine®/plasmid mix at
37˚C and the transfection was completed. Subsequent assays were
performed after 24 h.
Reverse transcription-quantitative PCR
assay
To extract total RNA from U87 and U251 cells,
TRIzol® (cat. no. 15596026; Invitrogen; Thermo Fisher
Scientific, Inc.) reagent was used. Total RNA was reverse
transcribed into cDNA at 42˚C for 1 h using M-MLV reverse
transcriptase (cat. no. M1701; Promega Corporation, includes M-MLV
5X Reaction Buffer 5 µl, dNTP, 10 mM 1.25 µl, Recombinant
RNasin® Ribonuclease Inhibitor 25 units, M-MLV RT 200
units and Nuclease-Free Water to final volume of 25 µl).
qPCR was then performed using the SYBR Ex Taq kit
(cat. no. 638319; Takara Bio, Inc.) and used according to the
manufacturer's protocol. MUC21 expression levels were normalized to
the relative level of GAPDH. The following primer pairs were used:
MUC21 forward, 5'-CTTCCCATAGTGCATCTACTGC-3' and reverse,
5'-GAACCAGTTAGGACTCCACCTGGGCC-3'; and GAPDH forward,
5'-GGTCGTATTGGGCGCCTGGT-3' and reverse, 5'-TACTCAGCGCCAGCATCGCC-3'.
The relative mRNA level of MUC21 was derived from triplicate
reactions. The following thermocycling conditions were used:
Initial denaturation at 95˚C for 3 min; followed by 30 cycles of
denaturation at 95˚C for 30 sec, annealing at 58˚C for 30 sec and
extension at 72˚C for 30 sec. The 2-ΔΔCq method was used
to quantify the results (10).
Western blotting
Proteins were extracted from U87 cells using RIPA
lysis buffer (Cell Signaling Technology, Inc.). The BCA assay
method was used for protein concentration determination, after
which proteins were separated (20 µg per lane) by 8% SDS-PAGE. The
proteins were transferred onto a PVDF membrane, which was
subsequently blocked in 5% BSA at room temperature for 2 h in TBST
with 0.5% Tween and incubated with specific antibodies against
MUC21 (1:1,000), β-actin (1:10,000; cat. no. ab8227; Abcam),
anti-Ki67 (1:1,000; cat. no. ab15580; Abcam), anti-proliferating
cell nuclear antigen (PCNA; 1:1,000; cat. no. 2586; Cell Signaling
Technology, Inc.), anti-MMP2 (1:1,000; cat. no. 40994; Cell
Signaling Technology, Inc.), anti-MMP9 (1:1,000; cat. no. 13667;
Cell Signaling Technology, Inc.) STAT3 (1:1,000; cat. no. ab68153;
Abcam), p-STAT3 (1:1,000; cat. no. ab267373; Abcam), AKT (1:1,000;
cat. no. ab8805; Abcam) and phosphorylated (p)-AKT (1:1,000; cat.
no. Ab38449; Abcam) at 25˚C for 2 h. The membranes were then
incubated at room temperature with HRP-conjugated anti-mouse IgG
(cat. no. 7076) and anti-rabbit IgG (cat. no. 7074; both from Cell
Signaling Technology, Inc.) secondary antibodies (1:3,000) for 1 h
at room temperature. Signals were visualized using an ECL kit
(Beyotime Institute of Biotechnology). ImageJ (version 1.8.0;
National Institutes of Health) was used for densitometry.
Colony formation assay
U87 and U251 cells (1,000/well) transfected with
control or MUC21 shRNA plasmids were plated into six-well culture
plates. Cells were maintained using DMEM with 10% FBS. Colonies
were considered to consist of >100 cells. After 2 weeks, cell
colonies were fixed with PFA at room temperature for 25 min and
stained with 0.1% crystal violet at 25˚C for 20 min. After washing
with PBS twice, the number of colonies was manually counted.
MTT assay
U87 and U251 cells at a density of ~1,000 cells per
well were plated into 96-well plates and maintained for 24 h. MTT
(0.5 mg/ml) was added into the plate for 4 h at 25˚C and then
removed to extract the stained cells. Dimethyl sulfoxide (200 µl)
was used to dissolve the purple formazan. The OD value in each well
was measured using a microplate reader at a wavelength of 570
nm.
Wound closure assay
U87 and U251 cells were cultured for 24 h, after
which a wound was made using 20-µl pipette tips. Samples were then
washed with PBS to remove debris and serum-free DMEM was added to
stimulate healing. Images were captured at 0 and 24 h timepoints
using a light microscope and the extent of wound closure was
calculated.
Tumor growth assays
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Tianjin Huanhu
Hospital (approval no. 2019-0002). A total of 8 female nude mice (8
weeks old and weighing 18-20 g, 4 mice per group were used) were
purchased from the Experimental Center of Beijing Vital River
Laboratory Animal Technology Co., Ltd. The mice were housed in an
air conditioning-regulated environment (20°C and 40%
humidity). Mice were kept under a 12 h light/dark cycle with ad
libitum access to food and water. The mice were anesthetized in
acrylic chambers. Anesthesia was induced by inhalation of 2.5%
isoflurane and 1% isoflurane was used for maintenance. Then the
mice were placed in the right lateral decubitus position. Briefly,
~5x105 U251 cells stably transfected with control or
MUC21 shRNA plasmids in 50 µl of PBS with 50 µg of growth
factor-reduced Matrigel were injected into the left lateral thorax
of the mice at the lateral dorsal axillary line. Additionally, the
tumor grew subcutaneously in the abdomen of the nude mice, at the
injection site. After 14 days, the tumor volume was monitored every
7 days. After 49 days, mice were weighed, anesthetized with sodium
pentobarbital (60 mg/kg, intraperitoneally) and then sacrificed by
cervical dislocation. Subsequently, all tumors were isolated,
measured, their images were captured and the tumor growth curves
were exhibited and analyzed. Tumor volume was calculated using the
following formula: Volume=(length x width x height)/2.
Statistical analysis
GraphPad 5.0 software (GraphPad Software, Inc.) was
used for statistical analysis in the present study. Results were
presented as the mean ± SD (n=3). The associations between
clinicopathological features of patients with GBM and MUC21
expression were analyzed using χ2 analysis. Statistical
differences between two groups were analyzed by paired student's
t-test. ANOVA followed by Dunnett's post hoc test was used for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
MUC21 levels are upregulated in GBM
tissues and are associated with clinical features
To analyze the role of MUC21 in the progression of
GBM, MUC21 expression levels in tumor tissues and adjacent tissues
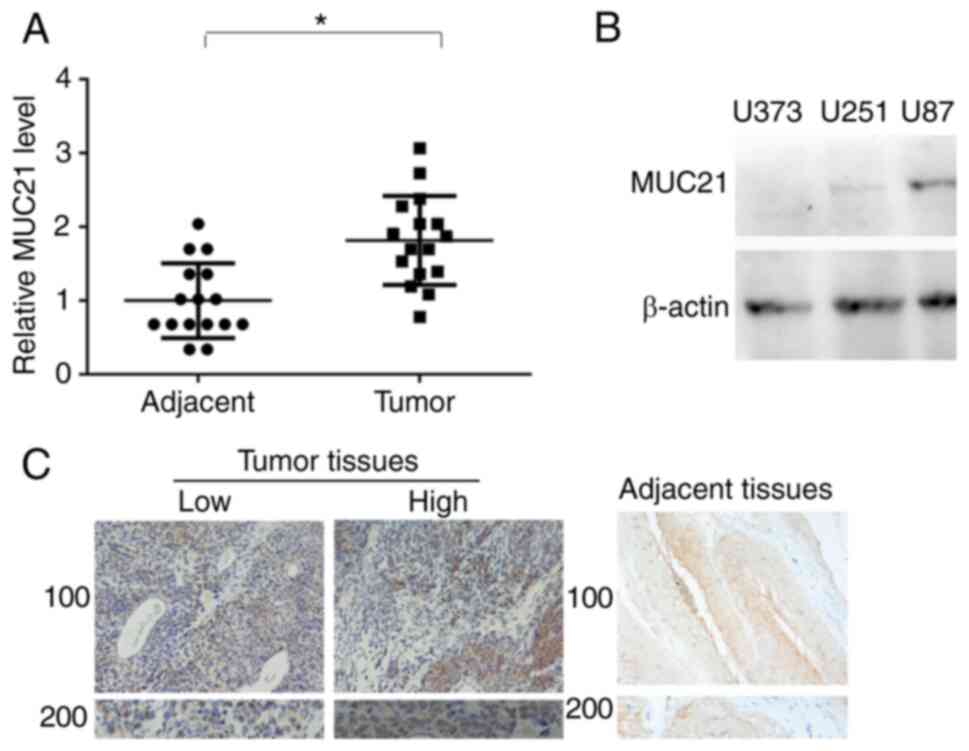
from patients with GBM were detected (Fig. 1A). The results revealed that GBM
tissue had higher MUC21 expression levels compared with normal
adjacent tissue. Subsequently, the levels of MUC21 in three types
of GBM cells (U251, U87 and U373) were investigated. It was
revealed that MUC21 protein levels were markedly enhanced in U251
and U87 cells, compared with U373 cells (Fig. 1B). In addition, the IHC assay
revealed high MUC21 expression levels in GBM tissues, with arrows
indicating high and low expression areas (Fig. 1C). Compared with patients with low
expression of MUC21, no significant association between the sex,
age, tumor lateralization and isocitrate dehydrogenase mutations of
patients and the expression level of MUC21 was observed. By
contrast, MUC21 expression was significantly associated with
recurrence (P=0.028; Table I).
Therefore, the enhanced MUC21 expression in GBM tissues and its
association with clinical features of patients with GBM was
deduced.
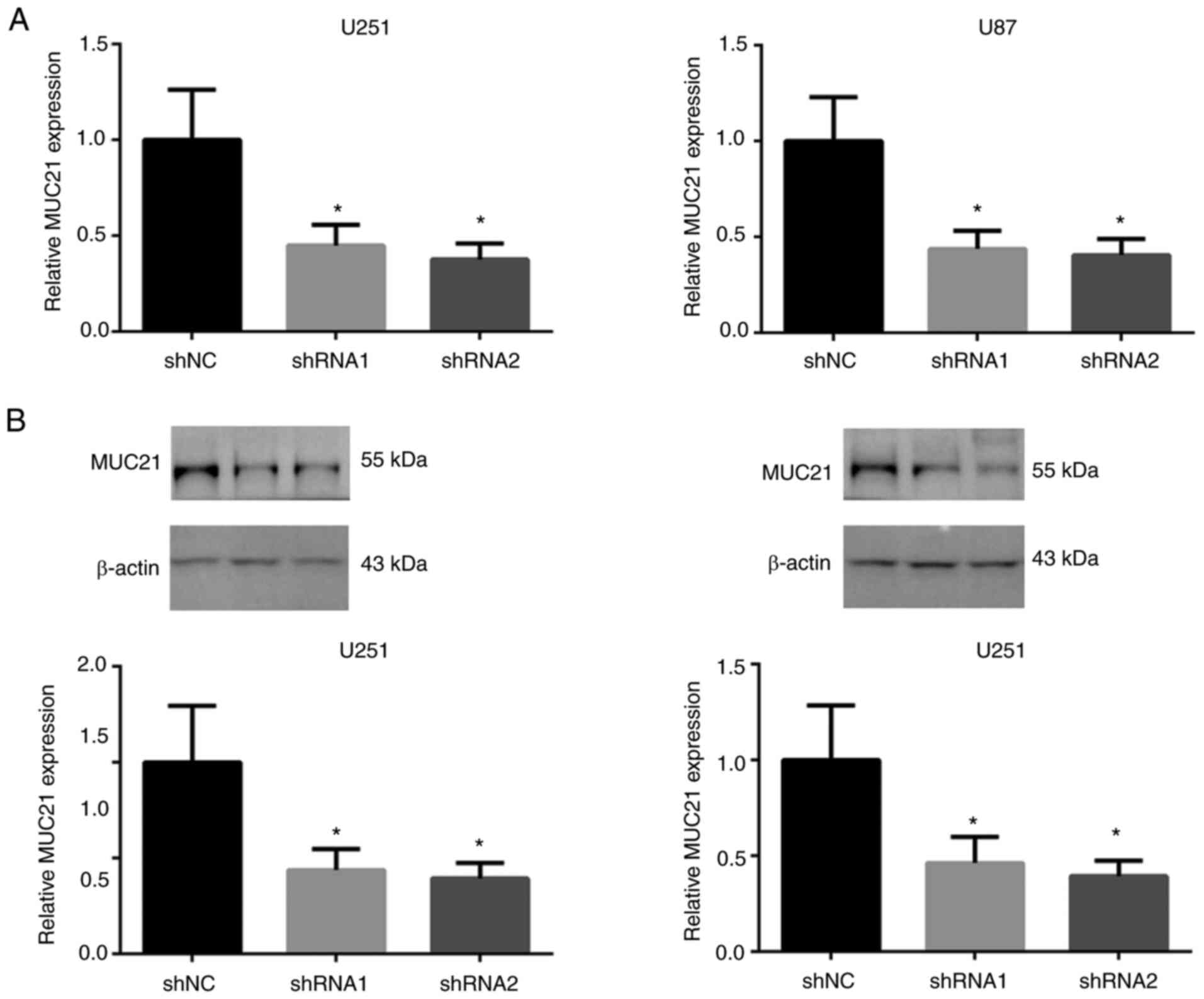
MUC21 expression is downregulated in
U251 and U87 cells with MUC21 shRNA transfection
To determine the potential role of MUC21 in GBM
progression, MUC21 expression was decreased by shRNA plasmids to
reveal its biological function in GBM cell lines. After
transfecting MUC21 or control shRNA into U251 and U87 cells,
RT-qPCR analysis revealed that MUC21 expression levels were reduced
(Fig. 2A). Similarly, western blot
analysis demonstrated a reduction of MUC21 protein levels following
the transfection of MUC21 shRNA plasmids (Fig. 2B). Therefore, transfection of MUC21
shRNA effectively downregulated MUC21 mRNA and protein levels.
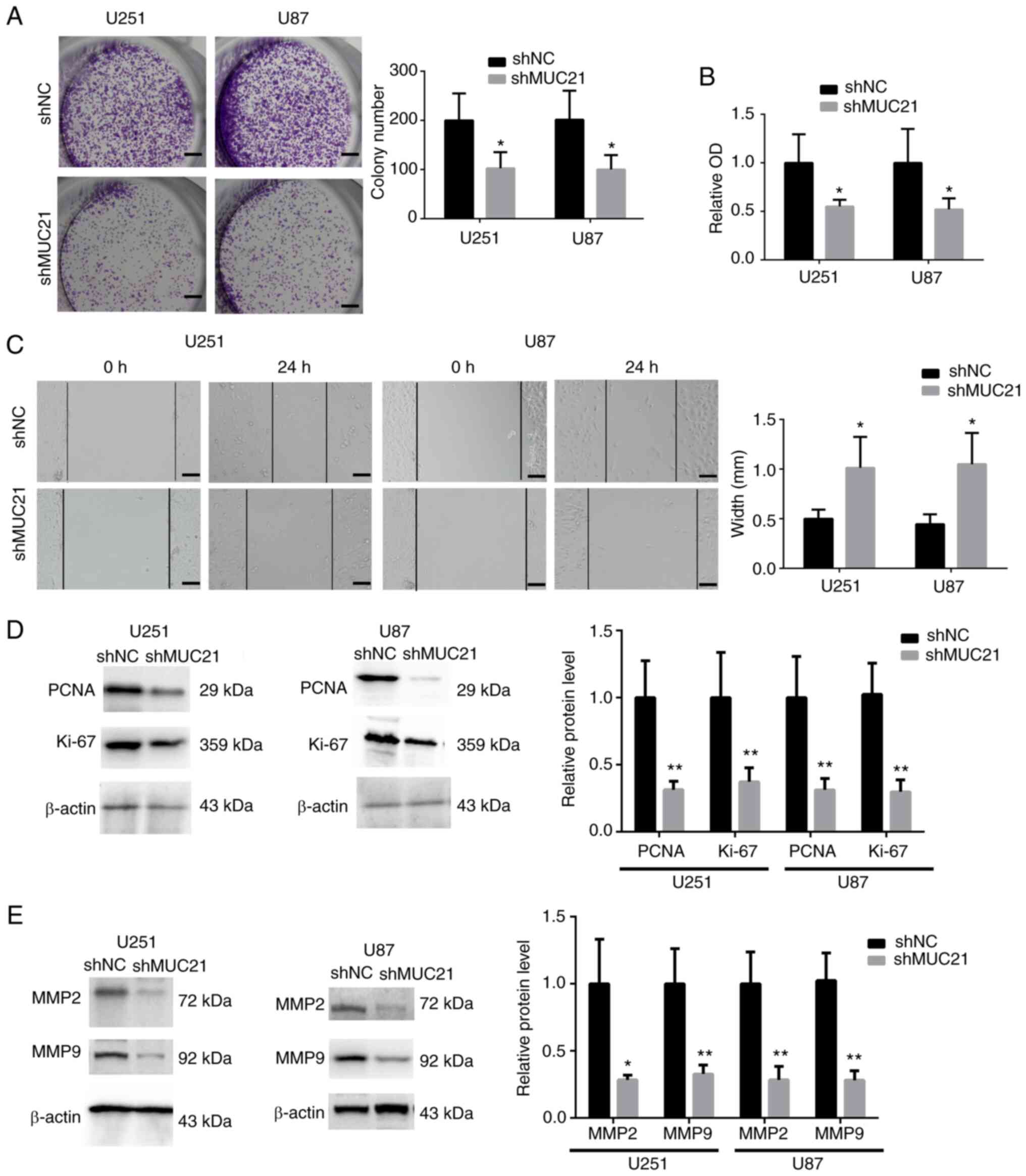
MUC21 promotes GBM cell viability and
migration in vitro
To assess the effects of MUC21 in GBM progression,
the viability of U251 and U87 cells was evaluated by colony
formation and MTT assays after silencing MUC21. The results
revealed that cell viability was significantly reduced after the
depletion of MUC21, with the decreased colony number and OD value
at 570 nm (Fig. 3A and B). Subsequently, wound closure assays
revealed the effects of MUC21 on the migration of GBM cells. It was
observed that MUC21 silencing significantly reduced wound closure
in U251 and U87 cells (Fig. 3C).
Furthermore, western blotting revealed that the expression levels
of two proliferation cell markers, Ki67 and PCNA, were decreased in
both U251 and U87 cells after MUC21 shRNA transfection (Fig. 3D). Decreased expression levels of
MMP2 and MMP9, which are necessary for tumor cell migration, were
also revealed after MUC21 depletion (Fig. 3E). Collectively, it was
demonstrated that MUC21 expression contributes to cell
proliferation and migration of GBM in vitro.
MUC21 contributes to the progression
of GBM via the STAT3 and AKT signaling pathway
To investigate the signaling proteins that are
involved in MUC21-mediated GBM progression, the impact of MUC21
depletion on the STAT3 and AKT pathway in GBM cells was examined.
U251 cells were transfected with MUC21 shRNA or MUC21
overexpressing plasmid for 72 h, after which phosphorylated STAT3
and AKT were assessed by western blot analysis. MUC21 shRNA
transfection resulted in the decrease of phosphorylated STAT3 and
AKT. In addition, the total protein levels of STAT3 and AKT were
not altered upon MUC21 depletion in GBM cells (Fig. 4A). Conversely, MUC21-overexpressing
U251 cells exhibited accumulated levels of phosphorylated tyrosine
proteins STAT3 and T308 phosphorylated AKT (Fig. 4B). These results suggested that
MUC21 modulated STAT3 and AKT activation in GBM cells.
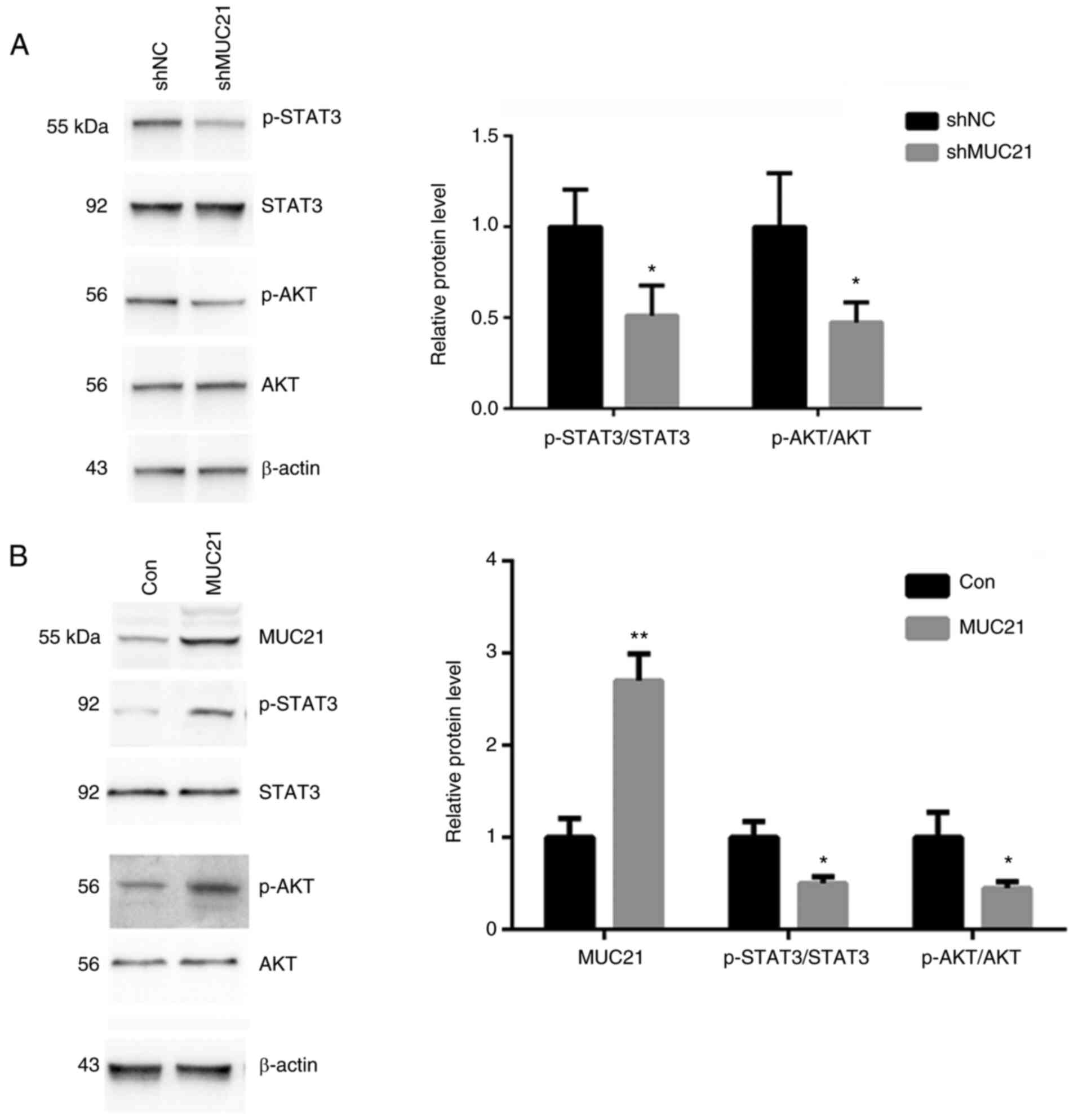 | Figure 4MUC21 contributes to GBM progression
via the STAT3 and AKT signaling pathway. (A) The protein levels of
p-STAT3, STAT3, p-AKT and AKT in shMUC21-transfected or control
U251 cells were detected by immunoblot assays. (B) The protein
levels of MUC21, p-STAT3, STAT3, p-AKT and AKT in
MUC21-overexpressing or control U251 cells were detected by
immunoblot assays. The relative expression was analyzed.
*P<0.05 and **P<0.01 vs. shNC or Con.
Con, control; MUC21, mucin 21; NC, negative control; p-,
phosphorylated; shRNA, short hairpin RNA. |
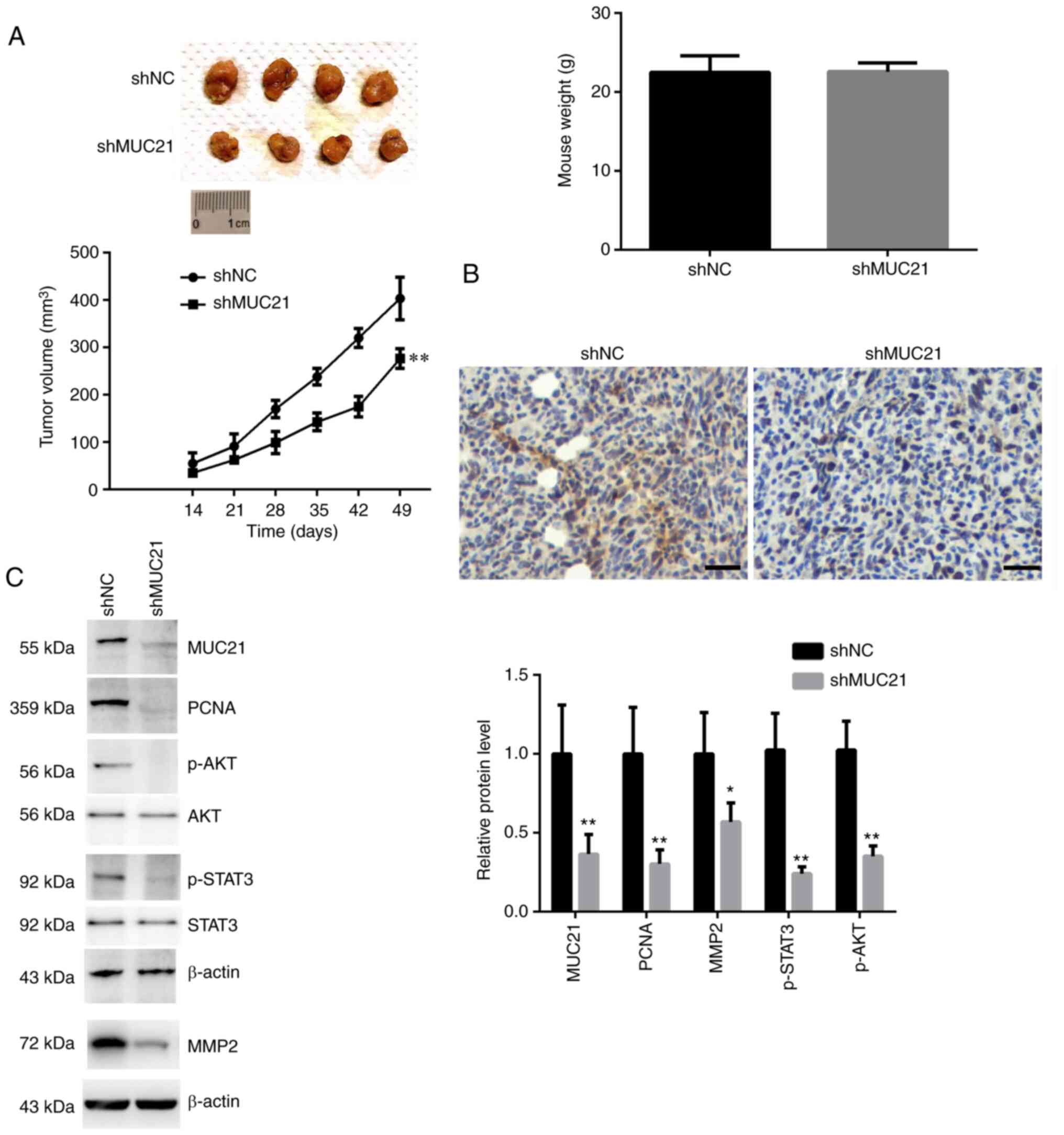
MUC21 stimulates GBM progression
through the STAT3 and AKT pathways in vivo
To evaluate the importance of MUC21 in GBM
progression in vivo, a xenograft assay was performed to
explore the role of MUC21 in the tumor growth of mice. Control or
MUC21-depleted U251 cells were injected into nude mice to induce
tumor growth. The tumor volume was monitored every week from the
2nd to 7th week. Finally, tumors were isolated and images were
captured. No difference in murine weight was observed between the
two groups (Fig. 5A). As exhibited
in Fig. 5A, tumor images were
displayed. A relatively slow tumor growth rate was observed in
MUC21-depleted group compared with the control group (Fig. 5A). There was no significant
difference of the murine body weight between the two groups after
the experiment (data not shown). The knockdown efficiency of MUC21
in isolated tumors was further confirmed through an IHC assay
(Fig. 5B).
Similarly, decreased expression levels of MUC21,
MMP2, PCNA, p-AKT and p-STAT3 were identified following MUC21
knockdown in tumor tissues (Fig.
5C). In conclusion, it was revealed that MUC21 stimulates GBM
progression through the STAT3 and AKT pathway in vivo.
Discussion
Recently, a series of advances have been made in the
targeted therapy of GBM. There are specific therapeutic targets
that are available for the different pathological types of GBM,
which have been established clinical use or are currently in
clinical trials (11). For this
type of malignant tumor, which carries a high morbidity and
mortality, targeted therapy is undoubtedly the most effective
treatment to supplement or even replace traditional treatment, such
as surgical resection, chemo- and radiotherapy (12). To improve the survival of patients
with GBM at advanced stages, the development of new targeted
therapeutic drugs are urgently required. In the present study, it
was revealed that an MUC family protein, MUC21, was highly
expressed in patients with GBM and was associated with the tumor
recurrence of patients. The present study suggested the involvement
of MUC21 in GBM progression and it was considered that MUC21 could
serve as a potential therapeutic target for the disease.
MUC21, previously named epiglycanin, was identified
as a glycoprotein located on the surface of TA3-Ha cells. It was
also determined to be a critical member of Mucin family, which
contains several tumor regulators such as MUC15(13). Mucins have been revealed to play
key roles in lung cancer progression and affect the diagnostic,
prognostic and therapeutic implications of multiple types of tumors
(6,9,14).
MUC21, having a specific glycosylation status, may be involved in
the progression of EGFR-mutated lung cancer, particularly at the
stage where tumors are transforming from pure lepidic to
micropapillary tumors through low papillary lepidic lesions
(9). Specific expression of MUC21
in micropapillary elements of lung adenocarcinomas has been
revealed, which suggests an associaton between MUC21 expression and
the progression of EGFR-mutated lung adenocarcinomas (15). MUC21 inhibitors have potential to
serve as drugs in the treatment of multiple types of cancers, such
as lung cancer and esophageal carcinomas (15). However, the precise mechanism
requires further study. Additionally, MUC21 expression was also
found in the cytoplasm of GBM cells via developing a specific
antibody targeting the cytoplasmic tail of MUC21(16). Through this antibody, the
aforementioned study revealed the high expression of MUC21 in GBM
tissues compared with adjacent normal tissues. Similarly, the
aberrantly high expression of MUC21 in human GBM tissues was also
observed in the present study, and it was identified that MUC21
expression was positively associated with the clinicopathological
features of patients with GBM, in consistency with the previous
data (15). Therefore, these data,
together with the present findings, revealed that MUC21 has
clinical potential as a serum biomarker and therapeutic target for
patients with GBM.
Overexpression experiments have been previously
performed to verify the effects of MUC21 on GBM cells. Notably, it
was found that MUC21 promoted GBM cell proliferation and migration
(Wang et al, unpublished data). In the present study, focus
was addressed on the influence of MUC21 depletion on GBM cells,
suggesting that it may be used as a potential therapeutic target
for GBM.
Through MTT and colony formation assays, the effects
of MUC21 on GBM cell viability were identified. The present data
further confirmed that MUC21 promoted the migration of GBM cells.
These results revealed the oncogenic role of MUC21 in GBM
progression in vitro. In addition, through the present in
vivo study, it was demonstrated that the depletion of MUC21
inhibited the tumor growth of GBM cells in mice, further confirming
in vitro data. Furthermore, it was determined that MUC21
promoted the progression of GBM by regulating the AKT pathway.
The decreased degree of cell adhesion may affect
tumor metastasis by promoting cell invasion and spreading through
the regulation of lymphatics (16). Notably, Yi et al (7) revealed that MUC21 inhibited
cell-extracellular matrix interactions and interfered with
intercellular adhesions, which suggested that MUC21 suppressed
intercellular adhesion molecules and surface integrins (6). Additionally, MUC21 also affected the
immune response (9,17,18).
In the present study, the effects of MUC21 on the migration and
invasion of GBM cells, possibly through the effect on cell
adhesion, were also identified.
The STAT3/AKT axis is widely involved in the
regulation of various physiological and pathological processes
(19,20). It has multiple effects on a variety
of tumors, including lung cancer (21). In addition, the STAT3/AKT axis has
been determined to mediate the migration, invasion and metastasis
of tumors (10,20,21-23).
Notably, the present data revealed that MUC21 promoted the
migration and invasion of GBM cells via the STAT3/AKT axis, which
provided new evidence of MUC21 as an effective therapeutic target
for GBM.
Nevertheless, the lack of examination of the
expression of p-STAT3 and p-AKT on patient tissues is a potential
limitation of the present study. In fact, the tumor samples that
were kept were paraffin specimens. Due to the lack of fresh
samples, western blot assays were not performed.
Several studies have previously revealed that MUC21
was abnormally expressed in human GBM tissues and was associated
with poor patient prognosis (9,17,18).
However, to the best of our knowledge, no studies have provided
evidence that MUC21 inhibitors were developed. In future studies,
inhibitors of MUC21 should be developed and their possible effects
on GBM progression should be investigated.
In the present study, the high expression of MUC21
in human GBM tissues and cells was revealed. The expression of
MUC21 was associated with the tumor recurrence of patients with
GBM. It was further found that MUC21 contributed to the viability,
migration and invasion of GBM cells via the STAT3/AKT axis and that
it promoted tumor growth in mice. Collectively, it was considered
that MUC21 could serve as a promising therapeutic target for GBM
treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW, XZ, JL and QL carried out molecular biology
experiments and drafted the manuscript. LW, XZ, JL and QL designed
the study and performed statistical analysis. LW, XZ, JL and QL
conceived the study, participated in its design, coordinated the
study and drafted the manuscript. JL and QL confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal procedures and patient-derived tissue
experiments performed in the current study were approved by the
Ethics Committee of Tianjin Huanhu Hospital (Tianjin, China).
Written informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Park JE, Kim HS, Lee J, Cheong EN, Shin I,
Ahn SS and Shim WH: Deep-learned time-signal intensity pattern
analysis using an autoencoder captures magnetic resonance perfusion
heterogeneity for brain tumor differentiation. Sci Rep.
10(21485)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dai S, Yan Y, Xu Z, Zeng S, Qian L, Huo L,
Li X, Sun L and Gong Z: SCD1 confers temozolomide resistance to
human glioma cells via the Akt/GSK3β/β-catenin signaling axis.
Front Pharmacol. 8(960)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kig C, Beullens M, Beke L, Van Eynde A,
Linders JT, Brehmer D and Bollen M: Maternal embryonic leucine
zipper kinase (MELK) reduces replication stress in glioblastoma
cells. J Biol Chem. 288:24200–24212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mahasenan KV and Li C: Novel inhibitor
discovery through virtual screening against multiple protein
conformations generated via ligand-directed modeling: A maternal
embryonic leucine zipper kinase example. J Chem Inf Model.
52:1345–1355. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhao Y, Zhao Z, Cui Y, Chen X, Chen C, Xie
C, Qin B and Yang Y: Redox-responsive glycosylated combretastatin
A-4 derivative as novel tubulin polymerization inhibitor for glioma
and drug delivery. Drug Develop Res. 82:1063–1072. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yoshimoto T, Matsubara D, Soda M, Ueno T,
Amano Y, Kihara A, Sakatani T, Nakano T, Shibano T, Endo S, et al:
Mucin 21 is a key molecule involved in the incohesive growth
pattern in lung adenocarcinoma. Cancer Sci. 110:3006–3011.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yi Y, Kamata-Sakurai M, Denda-Nagai K,
Itoh T, Okada K, Ishii-Schrade K, Iguchi A, Sugiura D and Irimura
T: Mucin 21/epiglycanin modulates cell adhesion. J Biol Chem.
285:21233–21240. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tian Y, Denda-Nagai K, Kamata-Sakurai M,
Nakamori S, Tsukui T, Itoh Y, Okada K, Yi Y and Irimura T: Mucin 21
in esophageal squamous epithelia and carcinomas: Analysis with
glycoform-specific monoclonal antibodies. Glycobiology.
22:1218–1226. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kai Y, Amatya VJ, Kushitani K, Kambara T,
Suzuki R, Tsutani Y, Miyata Y, Okada M and Takeshima Y: Mucin 21 is
a novel, negative immunohistochemical marker for epithelioid
mesothelioma for its differentiation from lung adenocarcinoma.
Histopathology. 74:545–554. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guo P, Yu Y, Li H, Zhang D, Gong A, Li S,
Liu W, Cheng L, Qiu Y, Yao W, et al: TGF-â1-induced miR-503
controls cell growth and apoptosis by targeting PDCD4 in
glioblastoma cells. Sci Rep. 7(11569)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ganguly R, Hong CS, Smith LG, Kornblum HI
and Nakano I: Maternal embryonic leucine zipper kinase: Key kinase
for stem cell phenotype in glioma and other cancers. Mol Cancer
Ther. 13:1393–1398. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen T, Chen Z, Lian X, Wu W, Chu L, Zhang
S and Wang L: MUC 15 Promotes osteosarcoma cell proliferation,
migration and invasion through livin, MMP-2/MMP-9 and Wnt/β-catenin
signal pathway. J Cancer. 12:467–473. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ratan C, Cicily K D D, Nair B and Nath LR:
MUC Glycoproteins: Potential biomarkers and molecular targets for
cancer therapy. Curr Cancer Drug Targets. 21:132–152.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matsumura M, Okudela K, Nakashima Y,
Mitsui H, Denda-Nagai K, Suzuki T, Arai H, Umeda S, Tateishi Y,
Koike C, et al: Specific expression of MUC21 in micropapillary
elements of lung adenocarcinomas-implications for the progression
of EGFR-mutated lung adenocarcinomas. PLoS One.
14(e0215237)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cao H, Ye D, Zhao Q, Luo J, Zhang S and
Kong J: A novel aptasensor based on MUC-1 conjugated CNSs for
ultrasensitive detection of tumor cells. Analyst. 139:4917–4923.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang L, Strouthos CG, Wang Z and
Deisboeck TS: Simulating brain tumor heterogeneity with a
multiscale agent-based model: Linking molecular signatures,
phenotypes and expansion rate. Math Comput Model. 49:307–319.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Itoh Y, Kamata-Sakurai M, Denda-Nagai K,
Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M and
Irimura T: Identification and expression of human
epiglycanin/MUC21: A novel transmembrane mucin. Glycobiology.
18:74–83. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Z, Sun C, Li C, Jiao X, Griffin BB,
Dongol S, Wu H, Zhang C, Cao W, Dong R, et al: Upregulated MELK
leads to doxorubicin chemoresistance and M2 macrophage polarization
via the miR-34a/JAK2/STAT3 pathway in uterine leiomyosarcoma. Front
Oncol. 10(453)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang M, Liu Q, Mi S, Liang X, Zhang Z, Su
X, Liu J, Chen Y, Wang M, Zhang Y, et al: Both miR-17-5p and
miR-20a alleviate suppressive potential of myeloid-derived
suppressor cells by modulating STAT3 expression. J Immunol.
186:4716–4724. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lv X, Wang L and Zhu T: MiR-20a-5p
suppressed TGF-β1-triggered apoptosis of human bronchial epithelial
BEAS-2B cells by targeting STAT3. Mol Cell Probes.
50(101499)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shang J, Li Y, Yin G, Li Z, Jiang L and
Bai Q: Phosphatidylinositol 3,4,5-trisphosphate-dependent rac
exchanger 2 protein facilitates glioma progression via Akt and
Stat3 signaling. J Mol Neurosci. 71:1674–1682. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim HI, Lee SJ, Choi YJ, Kim MJ, Kim TY
and Ko SG: Quercetin induces apoptosis in glioblastoma cells by
suppressing Axl/IL-6/STAT3 signaling pathway. Am J Chin Med.
49:767–784. 2021.PubMed/NCBI View Article : Google Scholar
|