Introduction
Osteoarthritis (OA), which is one of the most common
joint diseases diagnosed globally, is characterized by the gradual
degeneration of articular cartilage, secondary paroxysmal synovitis
and bone reconstruction (1).
Inflammatory molecules, specifically IL-1β, are some of the key
mediators involved in OA pathophysiology (2). It is reported that ~10% of men and
18% of women aged >60 years are affected by OA (3). Furthermore, OA contributes to
impaired mobility in the elderly, as well as pain, loss of function
and decline in quality of life (4). To date, the pathophysiology of OA is
poorly understood and the treatment for OA is under investigation
(5). Thus, it is of great urgency
to explore the underlying mechanism of OA and to seek effective
therapeutic options for the treatment of OA.
Ketorolac tromethamine (KT), a novel injectable/oral
non-steroidal anti-inflammatory analgesic that was first
commercialized in the United States in March 1990, has no obvious
opiate receptor activity and can be used alone or combined with
other opiate analgesics to relieve postoperative pain (6,7). It
has been noted that KT has anti-inflammatory effects (8). Shrestha et al (9) reported that the considerable pain and
loss of function resulting from acute gouty arthritis could be
alleviated through the use of KT. It was also revealed that the
consistent administration of KT could relieve the pain of
rheumatoid arthritis (RA) (10).
The viability of cartilage cells was verified to be increased after
exposure to KT, indicating that KT is an ideal therapeutic agent
for the treatment of OA (11). In
view of this, the present study was conducted to investigate our
hypothesis that KT could relieve chondrocyte injury.
Cyclo-oxygenase-2 (COX-2) is an isoform of
cyclooxygenase, which is an enzyme involved in the biosynthesis of
prostaglandin, that plays an indispensable role in relieving pain,
inflammatory response and improving joint function for patients
suffering from RA (12,13). It has been reported that COX-2 is a
target of OA and RA therapy due to its favorable efficacy on
alleviating pain and inflammation (14). Anderson et al (15) reported that COX-2 acted as a
prominent player in the inflammation involved in adjuvant
arthritis, and that the inhibition of COX-2 could improve adjuvant
arthritis (15). Additionally, KT
was reported to relieve the inflammatory response in OA through the
inhibition of COX-2 expression (11). Therefore, in the present study, the
hypothesis that KT could alleviate chondrocyte injury by targeting
COX-2 expression was assessed.
Materials and methods
Cell culture, treatment and
transfection
The chondrogenic cell line, ATDC5, was purchased
from RIKEN BioResource Center and cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C in a humidified atmosphere with 5%
CO2. The cells were stimulated with IL-1β (10 ng/ml) for
24 h and then 5, 10 and 20 mg/ml KT were used to treat these
IL-1β-induced ATDC5 cells at 37˚C for 24 h; IL-1β-induced ATDC5
cells without KT as used as the control.
With the aim of overexpressing COX-2 expression in
ATDC5 cells, the pcDNA3.1 vector containing full-length COX-2
(OV-COX-2) and empty vector (OV-NC) were all designed and
synthesized by Thermo Fisher Scientific, Inc. In addition, the
cells that were not transfected with the plasmid were used as the
control group. The transfection of ATDC5 cells was performed with
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at a concentration of 50 ng/ml.
Following transfection for 48 h at 37˚C, the transfection
efficiency was detected by reverse transcription-quantitative PCR
(RT-qPCR) 48 h post-transfection.
MTT assay
The proliferation of KT-treated ATDC5 cells was
detected by performing an MTT assay (Beyotime Institute of
Biotechnology). The cells were seeded into 96-well plates at a
density of 2x103 cells/well and incubated at 37˚C for 24
h. Following this, 10 µl MTT solution was added into each well and
then the cells were incubated with formazan lysis solution for
another 4 h at 37˚C until all purple crystals were dissolved.
Finally, the absorbance at 490 nm was detected using a
spectrophotometer.
RT-qPCR
The total RNA from ATDC5 cells was extracted with
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
then reverse transcribed into cDNA using PrimeScript Reverse
Transcriptase (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. qPCR was conducted via SYBR-Green PCR
Master Mix kit (Takara Biotechnology Co., Ltd.) on the ABI PRISM
7000 Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
following thermocycling conditions were used for qPCR: 95˚C for 10
min; followed by 40 cycles of denaturation at 95˚C for 10 sec and
annealing/extension at 60˚C for 60 sec. The following primers
(GenScript) were used for qPCR: COX-2 forward,
5'-AGGACTCTGCTCACGAAGGA-3' and reverse, 5'-TGACATGGATTGGAACAGCA-3';
and GAPDH forward, 5'-ACCCTTAAGAGGGATGCTGC-3' and reverse,
5'-CCCAATACGGCCAAATCCGT-3'. Gene expression levels were quantified
using the 2-ΔΔCq method (16) and normalized to the internal
reference gene GAPDH.
Western blot analysis
The total proteins from transfected ATDC5 cells
treated by IL-1β and KT were extracted with RIPA lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.) and levels
were determined using a BCA protein assay kit (Thermo Fisher
Scientific Inc.). The proteins (30 µg/lane) were separated on a 10%
gel using SDS-PAGE and then transferred onto PVDF membranes.
Membranes were blocked with 5% skimmed milk for 2 h at room
temperature. Subsequently, the membranes were incubated overnight
at 4˚C with the following primary antibodies: Anti-COX-2 (1:5,000;
cat. no. Ab179800), anti-Bcl2 (1:1,000; cat. no. Ab182858),
anti-Bax (1:1,000; cat. no. Ab32503), anti-GAPDH (1:1,000; cat. no.
Ab8245), anti-matrix metallopeptidase (MMP)1 (1:1,000; cat. no.
Ab137332), anti-MMP13 (1:1,000; cat. no. Ab219620), anti-inducible
NO synthase (iNOS) (1:1,000; cat. no. Ab178945), anti-Collagen II
(1:1,000; cat. no. Ab34712), anti-Aggrecan (1:1,000; cat. no.
Ab216965) and anti-β-actin (1:1,000; cat. no. Ab8227; all Abcam).
Following primary incubation, the membranes were incubated with a
goat anti-rabbit horseradish peroxidase-conjugated IgG secondary
antibody (1:5,000; cat. no. Ab6721; Abcam) at room temperature for
4 h. Finally, the images of protein bands were visualized using ECL
reagent (MilliporeSigma). Protein expression levels were
semi-quantified using ImageJ software (version 1.46; National
Institutes of Health) with GAPDH as the loading control.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assay was employed to assess cell viability.
The transfected cells treated by IL-1β and KT were inoculated into
96-well plates at 5x103 cells/well for 24 h and then
incubated with 10 µl CCK-8 reagent (Beyotime Institute of
Biotechnology) at 37˚C for another 2 h. The absorbance value at 450
nm was detected using a microplate reader (BioTek Instruments,
Inc.).
TUNEL
To detect the apoptosis of transfected ATDC5 cells
treated by IL-1β and KT, TUNEL assay kit (C1086; Beyotime Institute
of Biotechnology) were employed according to the manufacturer's
protocol. ATDC5 cells were fixed with 4% paraformaldehyde at room
temperature for 15 min and permeabilized in 0.25% Triton X-100 for
20 min at room temperature. Then, the cells (1x106
cells/well) were incubated with TUNEL reaction solution at 37˚C for
1 h, followed by staining with 1 mg/ml DAPI at 37˚C for 30 min and
mounted in an anti-fade reagent (Beijing Solarbio Science &
Technology Co., Ltd.). Finally, a fluorescence microscope was
applied to observe and capture images of TUNEL-positive cells in
five fields of view selected at random.
Measurement of nitric oxide (NO)
production
The production of NO in transfected ATDC5 cells
treated by IL-1β and KT was detected using a NO assay kit (S0021;
Beyotime Institute of Biotechnology) according to the
manufacturer's specifications. In brief, the cell supernatants were
plated into 96-well plates and then incubated with Griess Reagent
for 15 min at room temperature. Following which, the absorbance was
measured at 540 nm (magnification, x200).
ELISA
ELISA kits (Beyotime Institute of Biotechnology)
were applied according to the manufacturer's protocol to detect the
levels of inflammatory cytokines, including IL-6 (cat. no. P1326)
and TNF-α (cat. no. PT512). The expression level of prostaglandin
E2 (PEG2) was detected using a PGE2 kit (Shanghai Tongwei
Industrial Co., Ltd.; cat. no. TW8620). The reactive oxygen species
(ROS) ELISA Kit (AMEKO, LianShuo Biological; cat. no. DRE901Mu) and
superoxide dismutase (SOD) ELISA Kit (Excell Bio; cat. no.
EMC56-96) were used to evaluate ROS and SOD levels in cells,
respectively. The optical density was recorded using a microplate
reader (Bio-Rad Laboratories, Inc.) under the circumstance of λ=450
nm and the levels of inflammatory cytokines were calculated with
the standard curve.
Statistical analysis
The experimental data are displayed as the mean ± SD
and were assessed with SPSS software 22.0 (IBM Corp). Two-tailed
Student's t-tests (unpaired) were used to compare the differences
between two groups. Comparisons among multiple groups were
performed with one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
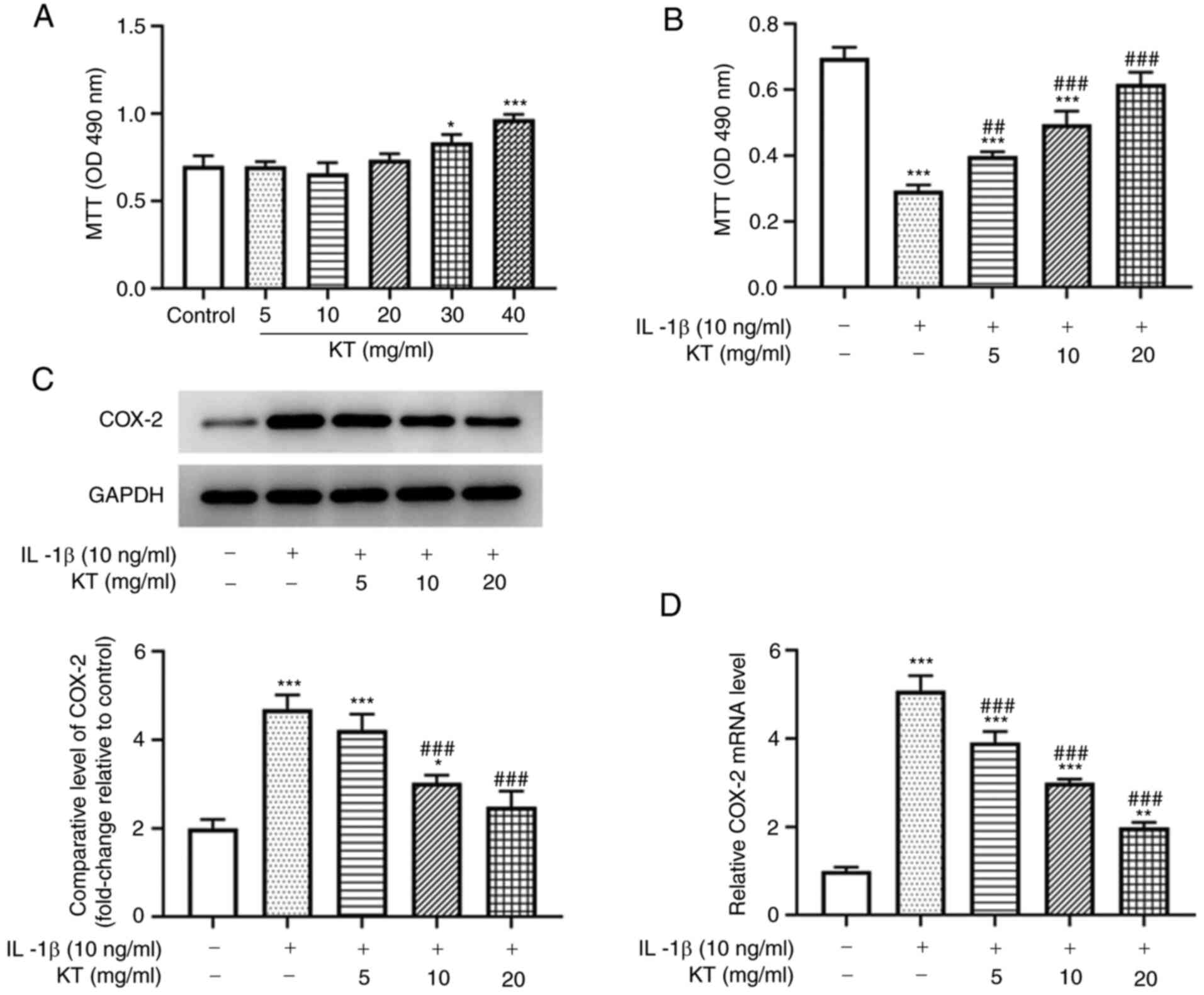
KT increases viability and decreases
COX-2 expression of IL-1β-induced ATDC5 cells
To ensure the findings in the following experiments
were reliable, the viability of ATDC5 cells following the
application of MTT was detected. Overall, KT increased ATDC5 cell
viability in a dose-dependent manner (Fig. 1A). After induction with IL-1β, the
viability of ATDC5 cells was detected with another MTT assay. As
shown in Fig. 1B, the viability of
IL-1β-induced ATDC5 cells treated with KT was increased compared
with that of the IL-1β group. In addition, the relative protein and
mRNA expression levels of COX-2 in IL-1β-induced ATDC5 cells were
decreased after treatment with KT in a concentration-dependent
manner (Fig. 1C and D). Therefore, the concentration of KT
with the best inhibitory effect (20 mg/ml) was selected for the
following experiment.
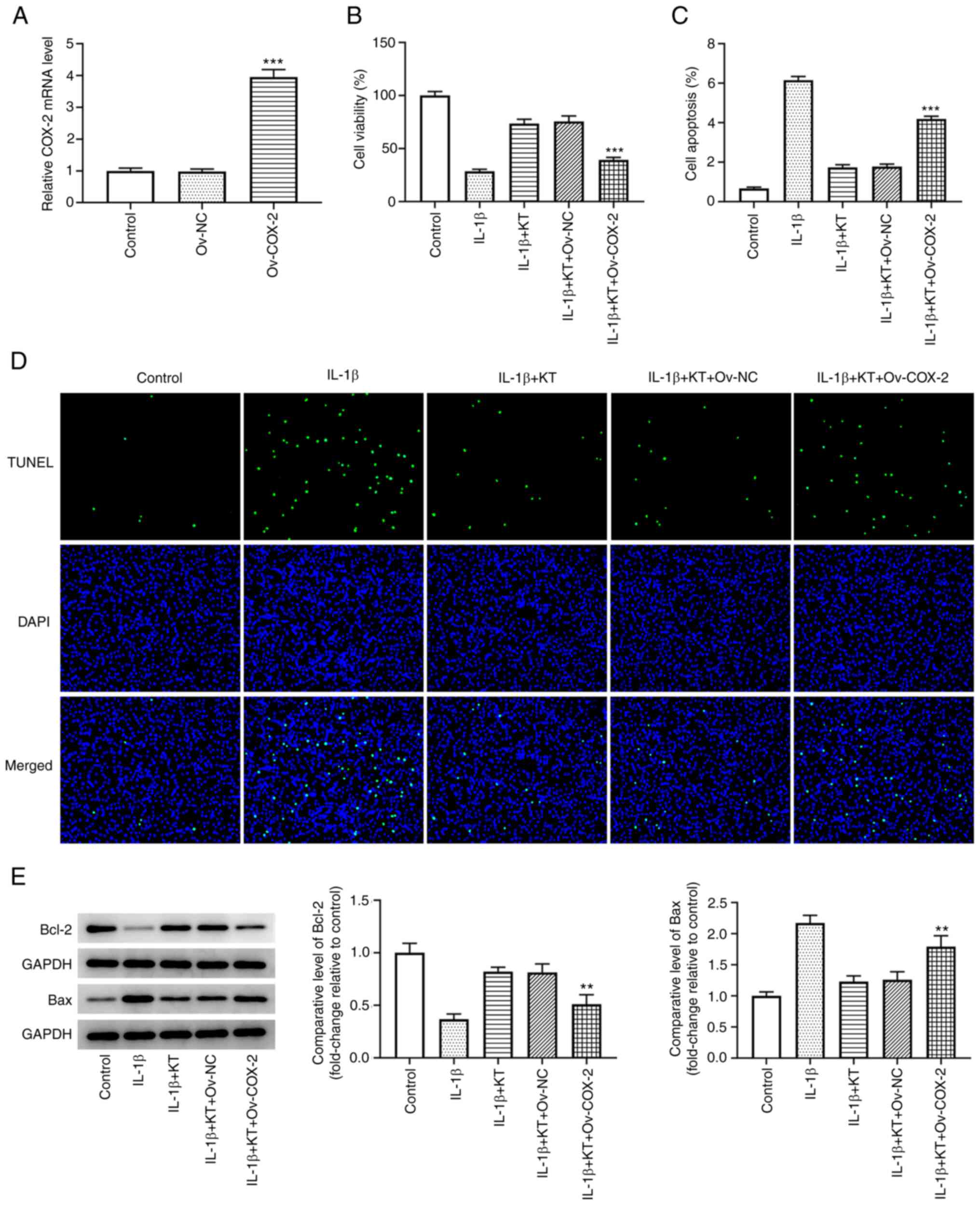
KT promotes IL-1β-induced ATDC5 cell
proliferation and suppresses apoptosis by inhibiting COX-2
expression
As revealed in Fig.
2A, the expression of COX-2 in IL-1β-induced ATDC5 cells was
significantly upregulated following transfection with COX-2
overexpression plasmids. Results from the CCK-8 assay revealed that
the viability of IL-1β-induced ATDC5 cells was markedly increased
after treatment with KT compared with that of the IL-1β group.
However, the increased cell viability was then decreased by COX-2
overexpression (Fig. 2B). In
addition, the decreased apoptosis of IL-1β-induced ATDC5 cells
treated with KT was increased by COX-2 overexpression, suggesting
that COX-2 overexpression reversed the inhibitory effects of KT on
IL-1β-induced ATDC5 cells (Fig. 2C
and D). Compared with that in the
IL-1β + KT + Ov-negative control (NC) group, the expression of
Bcl-2 was downregulated by COX-2 overexpression, while the
expression of Bax was upregulated (Fig. 2E).
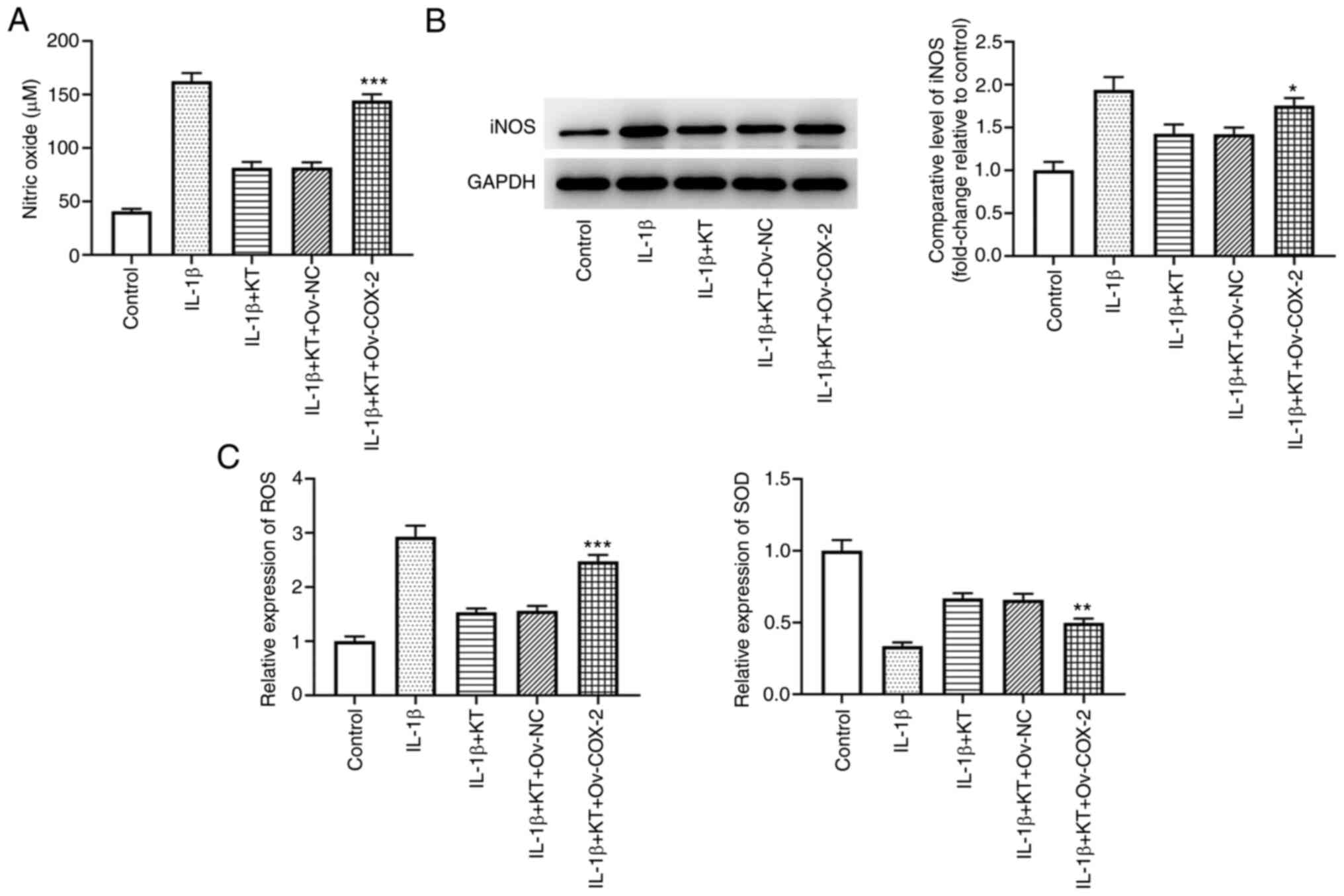
KT suppresses oxidative stress in
IL-1β-induced ATDC5 cells by inhibiting COX-2 expression
As presented in Fig.
3A, the decreased NO expression caused by co-treatment of IL-1β
and KT was increased by COX-2 overexpression when compared with
that in the IL-1β + KT + Ov-NC group. The relative levels of iNOS
were found to be downregulated in IL-1β-induced ATDC5 cells after
treatment with KT. Nevertheless, COX-2 overexpression reversed the
inhibitory effects of KT, evidenced by the increased iNOS
expression compared with the IL-1β + KT + Ov-NC group (Fig. 3B). Additionally, compared with the
IL-1β + KT + Ov-NC group, COX-2 overexpression upregulated ROS
expression, but downregulated SOD expression (Fig. 3C).
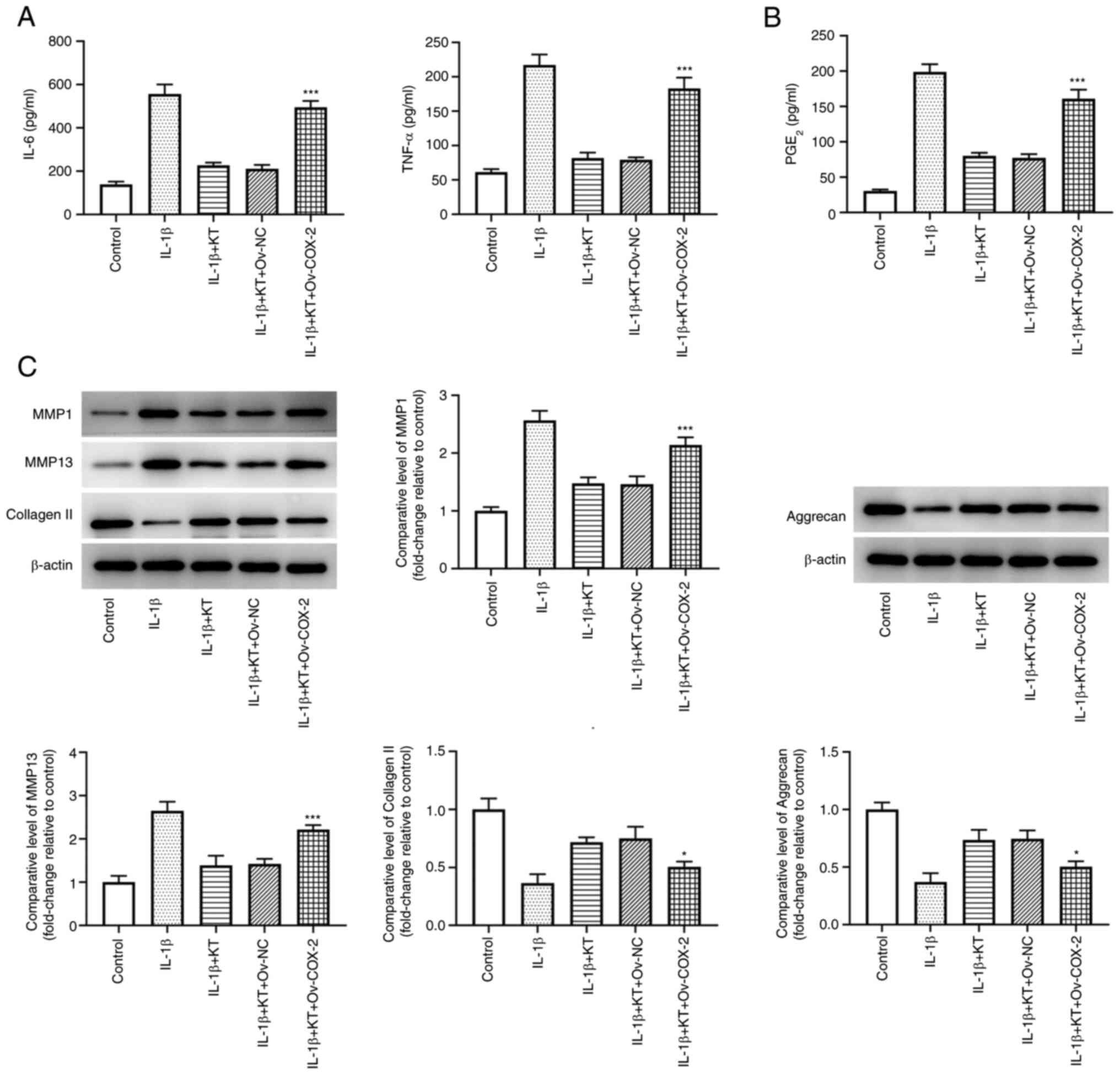
KT suppresses the inflammatory
response and extracellular matrix (ECM) degradation of
IL-1β-induced ATDC5 cells by inhibiting COX-2 expression
Results from Fig.
4A suggested that the increased levels of IL-6 and TNF-α in
IL-1β-induced ATDC5 cells were decreased by KT treatment. However,
COX-2 overexpression partially abolished the inhibitory effects of
KT on IL-1β-induced ATDC5 cells, evidenced by the increased levels
of IL-6 and TNF-α in comparison with those in the IL-1β + KT +
Ov-NC group. In addition, KT treatment effectively reduced the
level of PGE2 in IL-1β-induced ATDC5 cells, while COX-2
overexpression also reversed this positive effect (Fig. 4B). Additionally, compared with the
IL-1β + KT + Ov-NC group, COX-2 overexpression upregulated the
expression levels of MMP1 and MMP13, but downregulated the
expression levels of type II collagen (collagen II) and aggrecan
(Fig. 4C).
Discussion
OA, which is regulated by multi-level and complex
biological interactions, is the most common form of arthritis
diagnosed in the musculoskeletal system (17,18).
OA is characterized by pain, swelling and stiffness of the joints,
and contributes to disability worldwide (19). Furthermore, OA has caused a large
number of direct and indirect social and economic costs worldwide
(20). At present, the exact
underlying mechanism of OA remains unclear and currently no
effective cure exists (5).
Therefore, it is of great importance to identify a more effective
therapy for the treatment of OA.
KT, a derivative of heteroaryl acetic acid, is a
non-steroidal anti-inflammatory drug and an inhibitor of
non-selective COX (21). A number
of previous studies have reported that KT can protect against
arthritis and that the use of KT is an optimal choice for the
treatment of chondrocyte injury (9,22).
In the present study, it was found that the decreased viability of
IL-1β-induced ATDC5 cells was increased following treatment with
KT. It was also revealed that KT suppressed oxidative stress,
inflammatory response and ECM degradation of IL-1β-induced ATDC5
cells by inhibiting COX-2 expression, revealing that KT exerted
protective effects on IL-1β-induced ATDC5 cells by targeting
COX-2.
The inflammatory response has an important role in
OA. As cytokines regulate the biological function of cells and the
degeneration process of cartilage, they act as important regulators
in the pathogenesis of OA (23,24).
As a critical inflammatory cytokine, TNF-α has been shown to
promote the occurrence of initial events in the process of OA and
has roles in the synthesis of other cytokines, such as
IL-6(25). Furthermore, IL-6 has
been reported to activate the immune system and strengthen the
inflammatory response (26). In
the present study, it was discovered that the levels of IL-6 and
TNF-α were markedly increased following use of IL-1β compared with
those of the control group. However, the levels of IL-6 and TNF-α
declined following treatment with KT. Yin et al (27) demonstrated that COX-2 has a
critical role in the pathological process of inflammation due to
its functions in promoting pro-inflammatory mediators and
cytokines. In the present study, it was identified that COX-2
overexpression increased the levels of IL-6 and TNF-α in comparison
with the IL-1β + KT + Ov-NC group.
Oxidative stress is another important factor in OA.
It has been revealed that ROS can destroy chondrocytes and the ECM
of chondrocytes. Excessive ROS production results in chondrocyte
senescence and death, as well as the dysfunction of subchondral
bone (28,29). Srivastava et al (30) reported that Curcuma longa
extract alleviates OA of the knee by reducing oxidative stress
biomarkers. Similarly, the present study discovered that the
increased ROS expression stimulated by IL-1β was significantly
decreased after treatment with KT.
ECM degradation has also been revealed to be
involved in OA. Daheshia and Yao (31) suggested that pro-inflammatory
cytokines could induce cartilage degradation by activating MMPs.
MMPs, which are a type of proteolytic enzyme, can degrade the ECM
components in OA (32). Cartilage
ECM structure, which is primarily composed of collagen II and
aggrecan, serves as a vital player in supporting joint movements
(33,34). In the present study, it was
determined that the expression levels of collagen II and aggrecan
were downregulated by IL-1β, while the expression levels of MMP1
and MMP13 were upregulated, indicating that IL-1β could induce ECM
degradation. Nevertheless, the degradation of collagen II and
aggrecan, as well as the upregulation of MMP1 and MMP13, were
notably reversed by treatment with KT.
In conclusion, KT suppressed the oxidative stress,
inflammatory response and ECM degradation of IL-1β-induced ATDC5
cells, while COX-2 overexpression reversed the inhibitory effects
of KT on IL-1β-induced ATDC5 cells. These results revealed that KT
could relieve chondrocyte injury by targeting COX-2
overexpression.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL conceptualized and designed the present study. YC
acquired, analyzed and interpreted data. CL and YC drafted the
manuscript and revised it critically for important intellectual
content. CL and YC confirm the authenticity of all the raw data.
All authors read and approved the final manuscript and agreed to be
held accountable for the current study in ensuring questions
related to the integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sacitharan PK: Ageing and osteoarthritis.
Subcell Biochem. 91:123–159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abramoff B and Caldera FE: Osteoarthritis:
Pathology, diagnosis, and treatment options. Med Clin North Am.
104:293–311. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xia B, Di C, Zhang J, Hu S, Jin H and Tong
P: Osteoarthritis pathogenesis: A review of molecular mechanisms.
Calcif Tissue Int. 95:495–505. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Redden RJ: Ketorolac tromethamine: An
oral/injectable nonsteroidal anti-inflammatory for postoperative
pain control. J Oral Maxillofac Surg. 50:1310–1313. 1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vadivelu N, Gowda AM, Urman RD, Jolly S,
Kodumudi V, Maria M, Taylor R Jr and Pergolizzi JV Jr: Ketorolac
tromethamine-routes and clinical implications. Pain Pract.
15:175–193. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jadhav PA and Yadav AV: Design,
development and characterization of ketorolac tromethamine
polymeric nanosuspension. Ther Deliv. 10:585–597. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shrestha M, Chiu MJ, Martin RL, Cush JJ
and Wainscott MS: Treatment of acute gouty arthritis with
intramuscular ketorolac tromethamine. Am J Emerg Med. 12:454–455.
1994.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shahi P, Kumari N and Pathak K:
Microspheres and tablet in capsule system: A novel
chronotherapeutic system of ketorolac tromethamine for site and
time specific delivery. Int J Pharm Investig. 5:161–170.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Beitzel K, McCarthy MB, Cote MP,
Apostolakos J, Russell RP, Bradley J, ElAttrache NS, Romeo AA,
Arciero RA and Mazzocca AD: The effect of ketorolac tromethamine,
methylprednisolone, and platelet-rich plasma on human chondrocyte
and tenocyte viability. Arthroscopy. 29:1164–1174. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fan HW, Liu GY, Zhao CF, Li XF and Yang
XY: Differential expression of COX-2 in osteoarthritis and
rheumatoid arthritis. Genet Mol Res. 14:12872–12879.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lo V, Meadows SE and Saseen J: When should
COX-2 selective NSAIDs be used for osteoarthritis and rheumatoid
arthritis? J Fam Pract. 55:260–262. 2006.PubMed/NCBI
|
|
14
|
Bingham CO III: Development and clinical
application of COX-2-selective inhibitors for the treatment of
osteoarthritis and rheumatoid arthritis. Cleve Clin J Med. 69
(Suppl 1):SI5–S12. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Anderson GD, Hauser SD, McGarity KL,
Bremer ME, Isakson PC and Gregory SA: Selective inhibition of
cyclooxygenase (COX)-2 reverses inflammation and expression of
COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest.
97:2672–2679. 1996.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ratneswaran A, Rockel JS and Kapoor M:
Understanding osteoarthritis pathogenesis: A multiomics
system-based approach. Curr Opin Rheumatol. 32:80–91.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lambova SN and Müller-Ladner U:
Osteoarthritis-current insights in pathogenesis, diagnosis and
treatment. Curr Rheumatol Rev. 14:91–97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Johnson CI, Argyle DJ and Clements DN: In
vitro models for the study of osteoarthritis. Vet J. 209:40–49.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Geyer M and Schonfeld C: Novel insights
into the pathogenesis of osteoarthritis. Curr Rheumatol Rev.
14:98–107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sinha VR, Kumar RV and Singh G: Ketorolac
tromethamine formulations: An overview. Expert Opin Drug Deliv.
6:961–975. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ketorolac for Pain Management: A Review of
the Clinical Evidence. CADTH Rapid Response Reports. Canadian
Agency for Drugs and Technologies in Health, Ottawa, ON, 2014.
|
|
23
|
Wojdasiewicz P, Poniatowski LA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rahmati M, Mobasheri A and Mozafari M:
Inflammatory mediators in osteoarthritis: A critical review of the
state-of-the-art, current prospects, and future challenges. Bone.
85:81–90. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lv FH, Yin HL, He YQ, Wu HM, Kong J, Chai
XY and Zhang SR: Effects of curcumin on the apoptosis of
cardiomyocytes and the expression of NF-κB, PPAR-γ and Bcl-2 in
rats with myocardial infarction injury. Exp Ther Med. 12:3877–3884.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hunter CA and Jones SA: Corrigendum: IL-6
as a keystone cytokine in health and disease. Nat Immunol.
18(1271)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yin J, Xia W, Li Y, Guo C, Zhang Y, Huang
S, Jia Z and Zhang A: COX-2 mediates PM2.5-induced apoptosis and
inflammation in vascular endothelial cells. Am J Transl Res.
9:3967–3976. 2017.PubMed/NCBI
|
|
28
|
Facchini A, Stanic I, Cetrullo S, Borzi
RM, Filardo G and Flamigni F: Sulforaphane protects human
chondrocytes against cell death induced by various stimuli. J Cell
Physiol. 226:1771–1779. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lepetsos P and Papavassiliou AG:
ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys
Acta. 1862:576–591. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Srivastava S, Saksena AK, Khattri S, Kumar
S and Dagur RS: Curcuma longa extract reduces inflammatory and
oxidative stress biomarkers in osteoarthritis of knee: A
four-month, double-blind, randomized, placebo-controlled trial.
Inflammopharmacology. 24:377–388. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Daheshia M and Yao JQ: The interleukin
1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol.
35:2306–2312. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nummenmaa E, Hamalainen M, Moilanen T,
Vuolteenaho K and Moilanen E: Effects of FGF-2 and FGF receptor
antagonists on MMP enzymes, aggrecan, and type II collagen in
primary human OA chondrocytes. Scand J Rheumatol. 44:321–330.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wieland HA, Michaelis M, Kirschbaum BJ and
Rudolphi KA: Osteoarthritis-an untreatable disease? Nat Rev Drug
Discov. 4:331–344. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Gooljarsingh LT, Lakdawala A, Coppo F, Luo
L, Fields GB, Tummino PJ and Gontarek RR: Characterization of an
exosite binding inhibitor of matrix metalloproteinase 13. Protein
Sci. 17:66–71. 2008.PubMed/NCBI View Article : Google Scholar
|