Introduction
Breast cancer was the second leading cause of
cancer-related mortality (17-20%) in women worldwide in
2019(1). Based on the sensitivity
to different treatments, prognosis and clinicopathological
characteristics, breast cancer can be divided into several subtypes
(2,3). According to the expression levels of
estrogen receptor (ER), progesterone receptor (PR), human epidermal
growth factor receptor (HER) 2 (HER2) and Ki67 (a proliferation
index marker), various molecular subtypes of breast cancer have
been identified, including luminal A-like, luminal B-like,
HER2-positive, basal-like (mainly triple-negative) and normal
breast-like (4,5). Among these subtypes, luminal A tumors
are defined as ER-positive, PR >20%, HER2-negative and Ki67
<14% (6,7).
Previous epidemiological studies reported that
luminal A breast cancer accounted for >50% of all new diagnosed
cases of breast cancer (8-12).
Endocrine therapy (ET) is the main treatment for almost all luminal
A-subtype breast tumors, unless endocrine resistance occurs
(12,13). In recent years, known ETs and novel
targeted drugs have been combined to reduce tumor resistance to
hormonal therapy (14). These
targeted drugs are divided into two main categories: i) Specific
rapamycin (mTOR)/phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit α inhibitors and ii) cyclin-dependent kinase 4/6
(CDK4/6) inhibitors. However, numerous challenges prevent the
identification of effective treatment for metastatic luminal A
breast cancer. For example, drug resistance can occur with
combinations of CDK4/6 inhibitors and ETs (15). Therefore, there is an urgent need
for identifying effective therapeutic targets for luminal A-subtype
breast cancer.
The accumulation of multiple mutations results in
tumorigenesis, including tumor suppressor gene inactivation and
oncogene activation (16). The
inactivation of tumor suppressor genes is considered to play an
important role in the occurrence of cancer. As the first member of
the BTG/transducer of ERBB2 gene family, BTG2 has two highly
conserved domains (BTG boxes A and B), which are separated by 20-25
non-conserved amino acids (17-19).
As a novel tumor suppressor in malignancies, BTG2 is associated
with numerous cellular functions, such as cell proliferation,
apoptosis and DNA damage repair (20-23).
In pancreatic cancer, microRNA (miR)-27a silencing has been
indicated to inhibit cell proliferation and invasion, and promote
apoptosis through the elevation of BTG2(24). In non-small-cell lung cancer, the
downregulation of nucleolar and spindle-associated protein 1 or
LINC01234 inhibits cell growth, migration and invasion by
increasing the expression of BTG2 (25,26).
In human muscle-invasive bladder cancers, BTG2 also suppresses
muscle invasion via inhibition of DNA methyltransferase 1(27). In ER-positive breast cancer,
downregulation of BTG2 is associated with overexpression of cyclin
D1 protein and with increased tumor grade and size (28). In addition, BTG2 inhibits the
expression of HER ligands and serves an essential role in the
endocrine (tamoxifen) resistance of ER-positive tumors (29). Thus, the suppression or absence of
BTG2 promotes the progression of triple-negative breast cancer.
However, to the best of our knowledge, studies on the function of
BTG2 in luminal A-subtype breast cancer and its association with
these cell processes have not been conducted to date.
The present study aimed to investigate the function
of BTG2 in luminal A-subtype breast cancer using the MCF-7 cell
line due to its positive expression of ER and PR and negative
expression of HER (30,31). MTT, Transwell invasion and wound
healing assays were applied to investigate the function of BTG2 on
MCF-7 cell proliferation, migration and invasion. Finally, the mRNA
and protein level of BTG2 was also confirmed in luminal A breast
tumor tissue.
Materials and methods
Bioinformatics prediction based on
Gene Expression Omnibus (GEO) database
The expression profile dataset GSE20437 was obtained
from the GEO database (http://www.ncbi.nlm.nih.gov/geo/), which is a public
and free database for gene expression data (32,33).
GSE20437 includes 24 healthy and 18 breast cancer tissue samples
(34). GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/)
was used to identify differentially expressed genes (DEGs) between
healthy and breast cancer tissue. P<0.05 and |log
fold-change|>1 were considered the criteria to classify
significant DEGs between healthy and breast cancer tissue
samples.
Protein-protein interaction (PPI)
network construction
A PPI network was constructed using the Search Tool
for the Retrieval of Interacting Genes/Proteins (STRING; https://string-db.org/) and Cytoscape software 3.6.1
(www.cytoscape.org). Hub genes were identified
from the PPI network.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) analyses
To understand the biological significance of DEGs,
GO enrichment (http://geneontology.org/) and KEGG analyses
(https://www.genome.jp/kegg/) were
conducted using Database For Annotation, Visualization And
Integrated Discovery (DAVID; http://david.ncifcrf.gov) (35), which is a free analysis online tool
that provides a convenient method for identifying the biological
role of DEGs.
Survival analysis
The OncoLnc (http://www.oncolnc.org) database was used to perform
survival analysis based on DEGs (36). OncLnc contains clinical data of
8,647 patients from 21 studies on cancer and precomputed survival
analyses for users to explore survival associations in cancer. The
difference in the expression level of BTG2 between healthy and
breast cancer tissues was further analyzed with the Oncomine
database (www.oncomine.org) (37). BTG2 expression was assessed in
breast cancer tissues relative to that in healthy tissues. UALCAN
(http://ualcan.path.uab.edu), which is an
open web-portal for cancer subgroup gene expression, was then used
to perform survival analysis of BTG2 in different cancer subgroups
based on race, menopause status and cancer type (38).
Tissue samples
The luminal A breast cancer and paracarcinoma
tissues (collected >5 mm from the tumor border) were collected
from 8 patients with luminal A breast cancer at the affiliated
Weihai Second Municipal Hospital of Qingdao University (Weihai,
China) between July 2019 and November 2019. All patients were
female and aged between 18 and 60 years with ER-positive,
PR-positive (>20%), HER2-negative and Ki67 (<30%). Patients
were excluded if they had received chemotherapy or radiotherapy
prior to surgical resection. The patients signed an informed
consent form prior to study commencement, and the study was
approved by the Ethics committee of Clinical Trails of the
affiliated Weihai Second Municipal Hospital of Qingdao University
(Weihai, China; approval no. 2019-ER-04).
Cell culture and transfection
The MCF-7 breast cancer cell line representing
luminal A cancer was purchased from the Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences and cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C under 5%
CO2 (27,28). BTG2 overexpression (OE-BTG2) and
empty (OE-NC) vectors were designed and synthesized by Wanleibio
Co., Ltd. Prior to transfection, cells with 70-90% density were
washed twice with serum-free Opti-MEM (Invitrogen; Thermo Fisher
Scientific, Inc.). OE-BTG2 and OE-NC vectors (50 nM) were
subsequently transfected into MCF-7 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C for 4 h according to the
manufacturers protocol. The cells were cultured at 37˚C for 24 h
and then collected for further study.
Reverse transcription-quantitative
(RT-q)PCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from MCF-7
cells or breast cancer/paracarcinoma tissues, according to the
manufacturer's protocol. The extracted RNA was reverse transcribed
into cDNA using a PrimeScript RT Reagent Kit with gDNA Eraser (cat.
no. RR047A; Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. Subsequently, SYBR Green PCR Master Mix
kit (Toyobo Life Science) was used for PCR-mediated amplification.
Relative mRNA expression was calculated using the 2-ΔΔCq
method (39). The primer sequences
used for qPCR were as follows: BTG2-forward (F),
5'-CATCATCAGCAGGGTGGC-3'; BTG2-reverse (R),
5'-CCAATGCGGTAGGACACC-3'; β-actin-F,
5'-CTTAGTTGCGTTACACCCTTTCTTG-3'; and β-actin-R,
5'-CTGTCACCTTCACCGTTCCAGTTT-3'. The reactions were performed using
the following thermocycling conditions: Initial denaturation at
95˚C for 5 min, followed by 32 cycles of 95˚C for 30 sec, 56˚C for
40 sec and 72˚C for 40 sec. All quantifications were normalized to
the internal reference gene β-actin.
Western blotting
Total protein was extracted from MCF-7 cells or
breast cancer/paracarcinoma tissues using RIPA lysis buffer (Thermo
Fisher Scientific, Inc.), and quantified using Pierce BCA Protein
Assay Kit (cat. no. 23225; Thermo Fisher Scientific, Inc.). Total
protein (40 µg per lane) was separated by 7.5-15% SDS-PAGE and
transferred to a 0.45-µm PVDF membrane (ABclonal Biotech Co.,
Ltd.). The membrane was pre-blocked with 5% non-fat dry milk for 1
h at room temperature before incubation with the corresponding
primary antibodies, including anti-BTG2 (cat. no. ab197362;
1:1,000; Abcam) and anti-β-actin (cat. no. ab8227; 1:2,000; Abcam)
overnight at 4˚C. After washing the membranes three times with
TBS-0.1%Tween-20, the membranes were incubated with HRP-conjugated
goat anti-rabbit (cat. no. ab205718; 1:5,000; Abcam) at 37˚C for 1
h. Protein bands were visualized with an enhanced chemiluminescence
reagent (Thermo Fisher Scientific, Inc.) using the ChemiDoc™ XRS+
imaging system (Image Lab 4.0; Bio-Rad Laboratories, Inc.).
Proliferation curve of MCF-7
cells
Non-transfected, OE-NC or OE-BTG2 transfected MCF-7
cells (~8,000/well) were seeded in a 96-well plate in 100 µl DMEM
supplemented with 10% FBS and incubated for 24, 48, 72 or 96 h at
37˚C in the presence of 5% CO2. Subsequently, a total of
10 µl MTT (Sigma-Aldrich; Merck KGaA) was added into the 96-well
plate, which was placed in an incubator at 37˚C in the presence of
5% CO2 for 4 h. After removing the medium, 100 µl DMSO
was added to each well to dissolve formazan crystals. Finally, the
plate was placed in a microplate reader (BioTek Instruments, Inc.)
for measurement of the absorbance at 570 nm.
Detection of MCF-7 cell invasion
Cell invasion was calculated based on the number of
cells that passed through the polycarbonate membrane that separated
the upper and lower chambers of an 8.0-µm Transwell chamber
(Corning, Inc.). Briefly, Matrigel was thawed and diluted in
serum-free DMEM (1:3) on ice. The Transwell chambers were placed in
a 24-well plate, and 40 µl diluted Matrigel was added, followed by
incubation at 37˚C for 2 h. Subsequently, 2x105 MCF-7
cells overexpressing BTG2 or transfected with the pcDNA3.1 empty
vector [overexpression (OE)-negative control (NC)] or
non-transfected cells were suspended in 200 µl serum-free DMEM and
added to the upper chamber, while 800 µl DMEM containing 10% FBS
was added to the lower chamber. The 24-well plate was placed in an
incubator at 37˚C in the presence of 5% CO2 for 24 h.
Subsequently, the upper chamber was removed while the lower chamber
was washed with PBS three times. The cells in the lower chamber
surface of the membrane were subsequently stained with 1% crystal
violet at 25˚C for 10 min and the number of invading cells was
counted under a light microscope (magnification, x200; Olympus
Corporation).
Scratch test
A total of 5x105 MCF-7 cells/well were
seeded into a six-well plate. Subsequently, a 1-ml sterile pipette
tip was used to create a linear scratch in the cell monolayer.
Fresh serum-free DMEM was then added to each well, and the cells
were incubated at 37˚C in a 5% CO2 incubator for 24 h. A
light microscope (magnification, x100) was used to observe the
progressive change in the scratch width after 24 h. The migration
distance was measured using ImageJ software 1.8.0 (National
Institutes of Health).
Statistical analysis
Data are presented as the mean ± SEM. All
experiments were duplicated and repeated at least three times.
Statistical analyses were performed using GraphPad Prism 8.0
(GraphPad Software, Inc.). Unpaired Student's t-tests were used for
the comparison between two groups. One-way ANOVA with the post hoc
Tukey's test was used for the comparison of the mean values between
multiple groups. Multiple regression analysis was used for the
survival analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of DEGs and
bioinformatics analysis
In the present study, 236 DEGs were identified in
dataset GSE20437, which comprised epithelial samples from patients
with breast cancer and patients that were cancer-free and receiving
prophylactic mastectomy. The up- and downregulated genes are
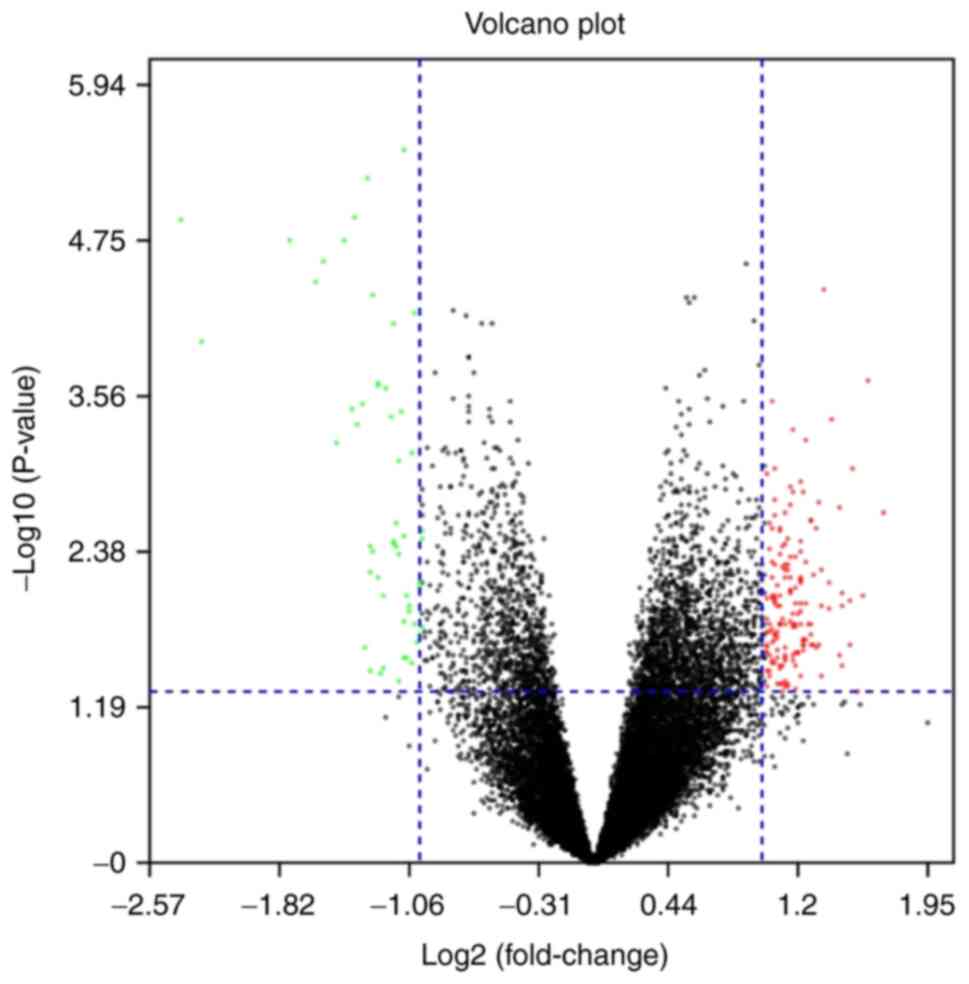
displayed in the volcano plot of Fig.
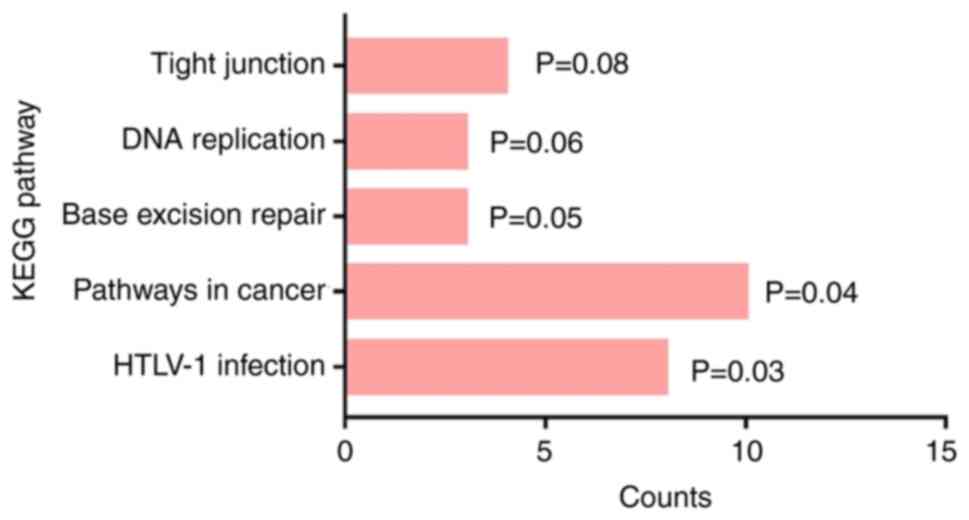
1. Results of the KEGG analysis demonstrated that the DEGs were
enriched in ‘tight junction’, ‘DNA replication’, ‘base excision
repair’, ‘pathways in cancer’ and ‘human T-cell leukemia virus type
1 infection’ (Fig. 2).
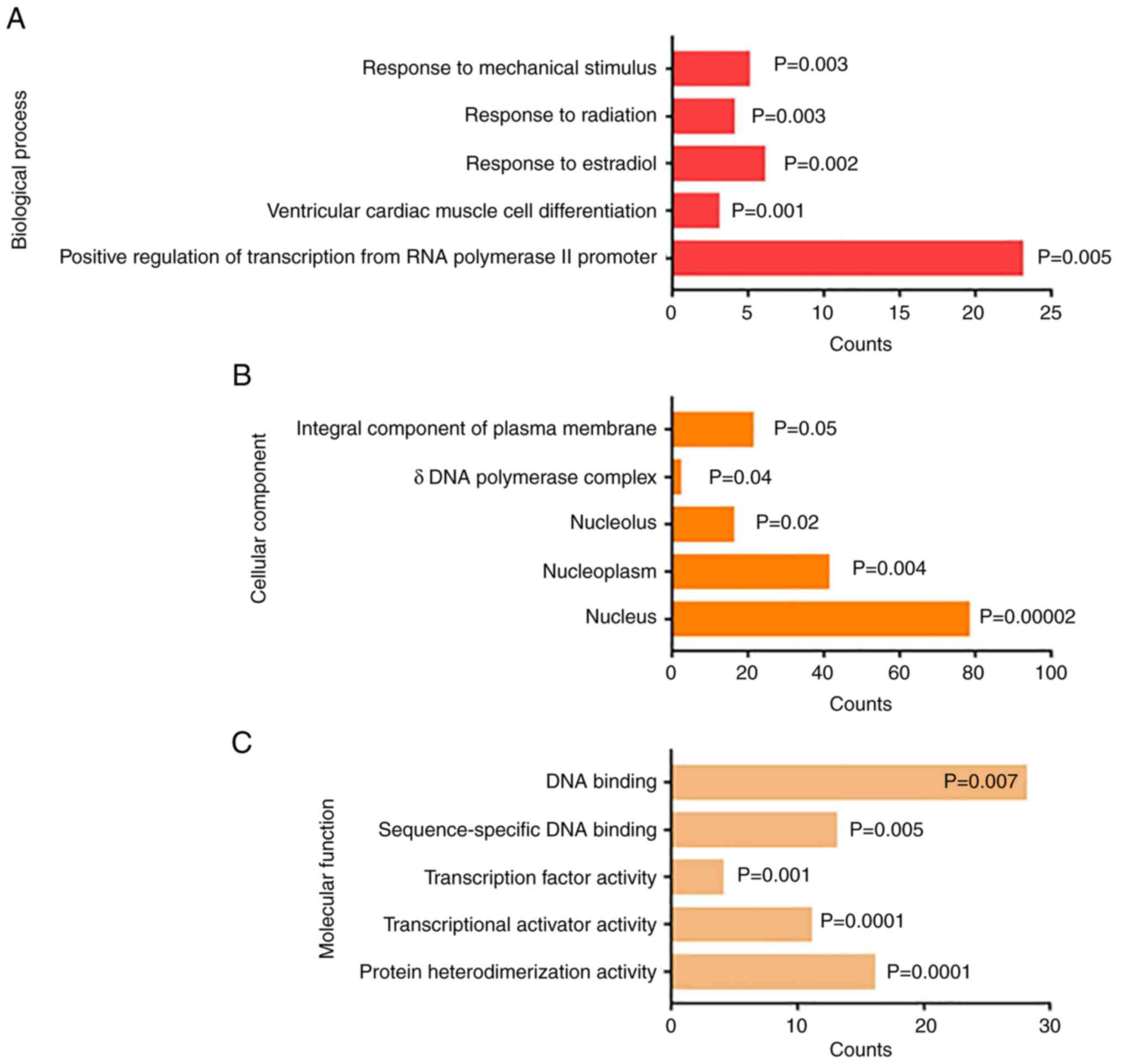
GO analysis demonstrated that the DEGs were enriched
in the category biological process, including ‘response to
mechanical stimulus’, ‘response to radiation’, ‘response to
estradiol’, ‘ventricular cardiac muscle cell differentiation’ and
‘positive regulation of transcription from RNA polymerase II
promoter’ (Fig. 3A). The results
of GO analysis in the category cellular component demonstrated that
the DEGs were enriched in the ‘integral component of plasma
membrane’, ‘d DNA polymerase complex’, ‘nucleolus’, ‘nucleoplasm’
and ‘nucleus’ (Fig. 3B).
Furthermore, results of the GO analysis in the category molecular
function indicated that the DEGs were enriched in ‘DNA binding’,
‘sequence-specific DNA binding’, ‘transcription factor activity’,
‘transcriptional activator activity’ and ‘protein
heterodimerization activity’ (Fig.
3C).
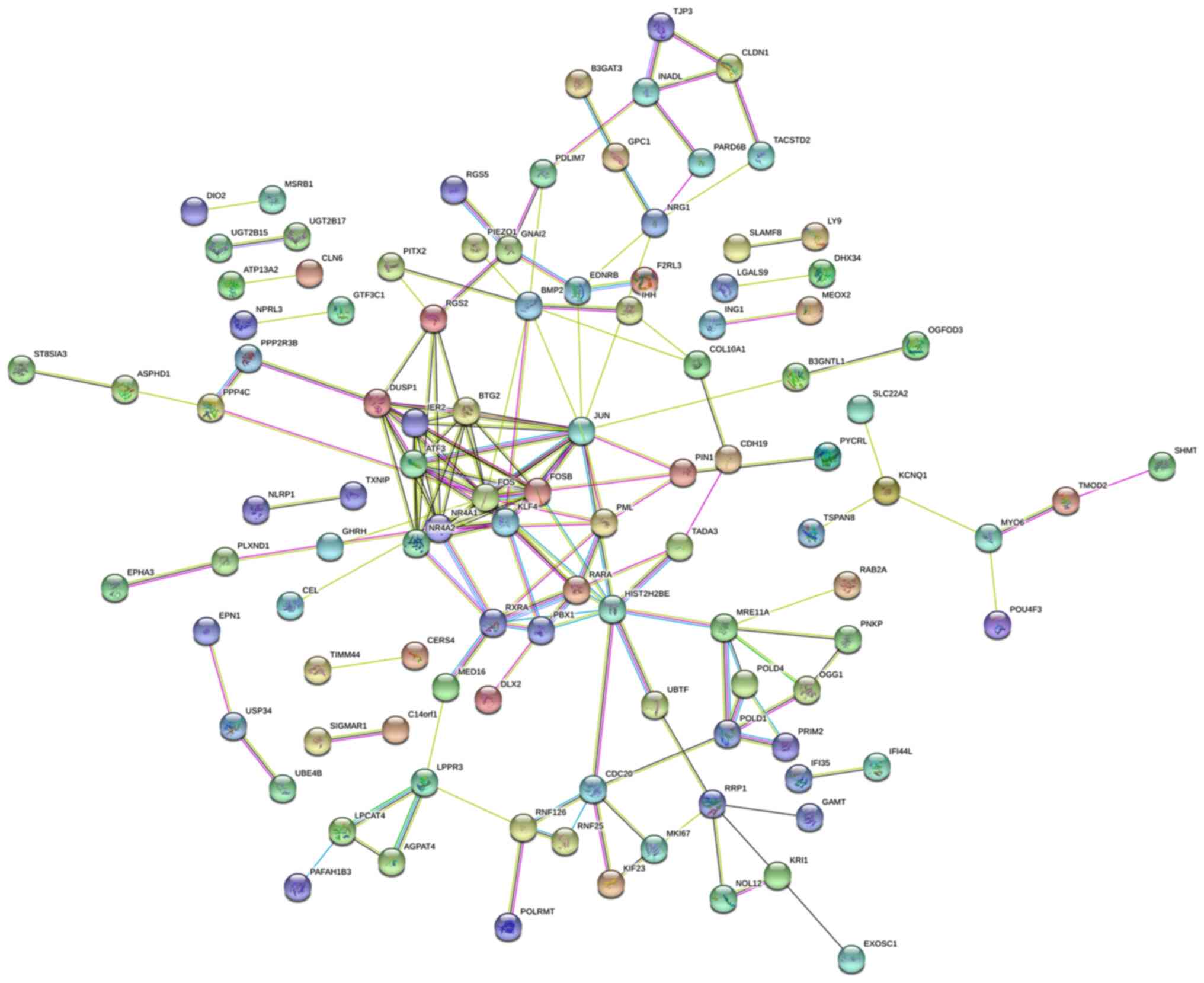
Hub gene analysis
In total, 236 DEGs were uploaded to the STRING
online database, and Cytoscape was subsequently used to identify
the cluster. Among the DEGs, nuclear receptor subfamily 4 group A
member (NR4A)1, immediate early response 2, dual-specificity
phosphatase 1, activating transcription factor 3, NR4A2, protein
FOSB, BTG2, proto-oncogene c-JUN and proto-oncogene c-FOS exhibited
the closest association. The PPI network of these nine genes is
presented in Fig. 4.
Survival analysis
To further evaluate the prognostic value of the
aforementioned hub genes, a survival analysis was conducted using
the OncoLnc database. The results revealed that low expression of
BTG2 was significantly associated with low survival rate of
patients with breast cancer (Fig.
5A). In contrast, the low expression of other genes, including
DUSP1, FOS, FOSB, JUN, MR4A1, MR4A2 and ATF3, was not associated
with a low survival rate of patients with breast cancer (Fig. 5B-I). Furthermore, the Oncomine
database revealed that the BTG2 expression was lower in breast
cancer tissues containing luminal breast cancer compared with that
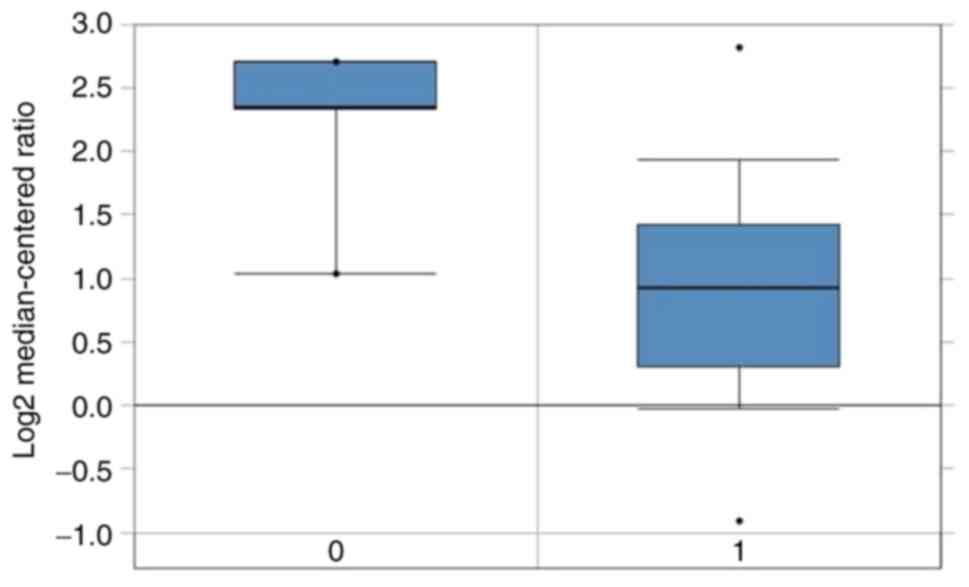
in normal counterparts (Fig. 6).
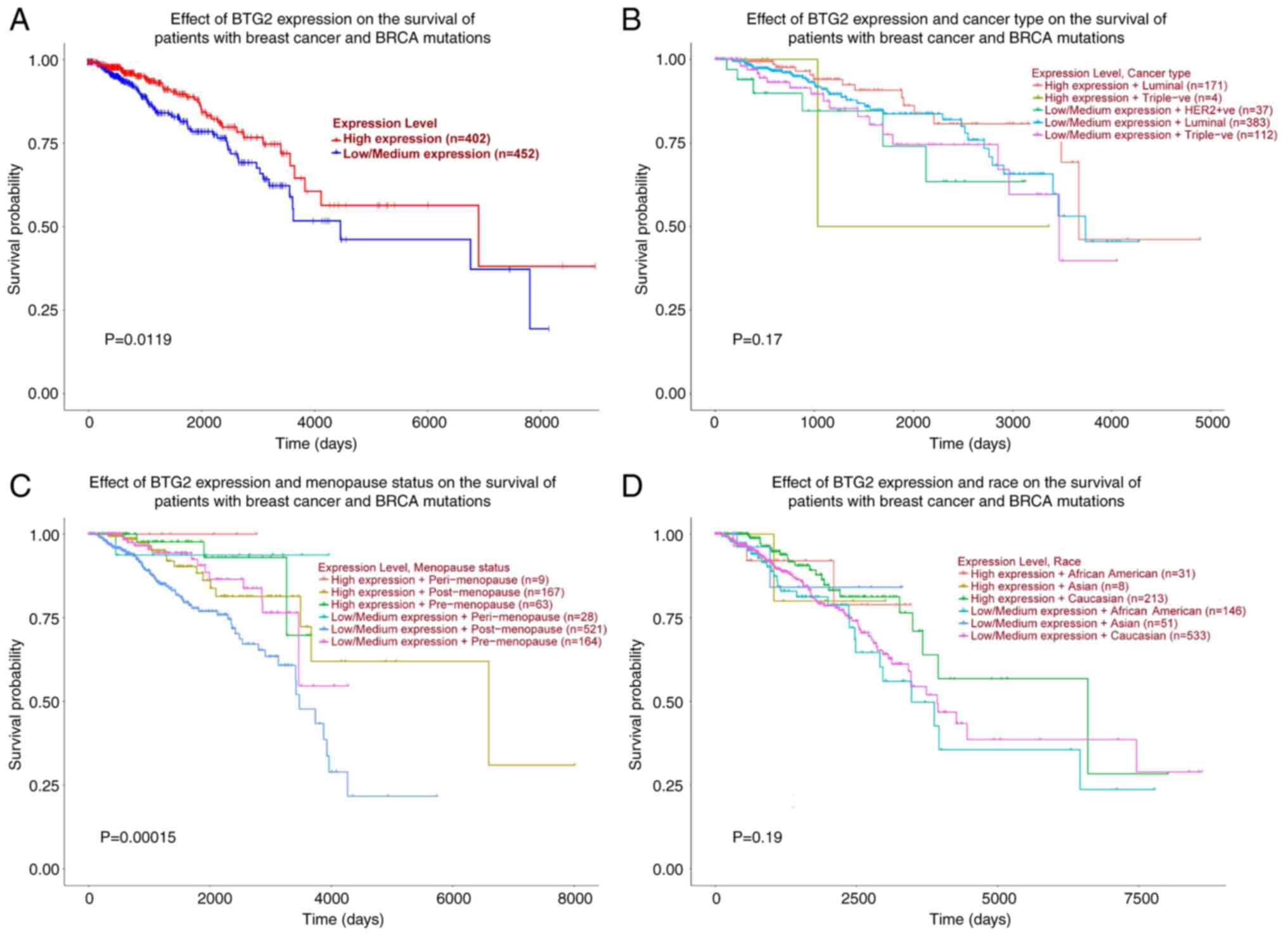
Further survival analysis based on UALCAN revealed that the
expression level of BTG2 and menopause status may have an impact on
the survival of patients with breast cancer that also exhibit
mutations in breast cancer susceptibility protein (Fig. 7A and C). In contrast, the expression level of
BTG2 and cancer type or race were not significantly associated with
the survival of patients with breast cancer (Fig. 7B and D).
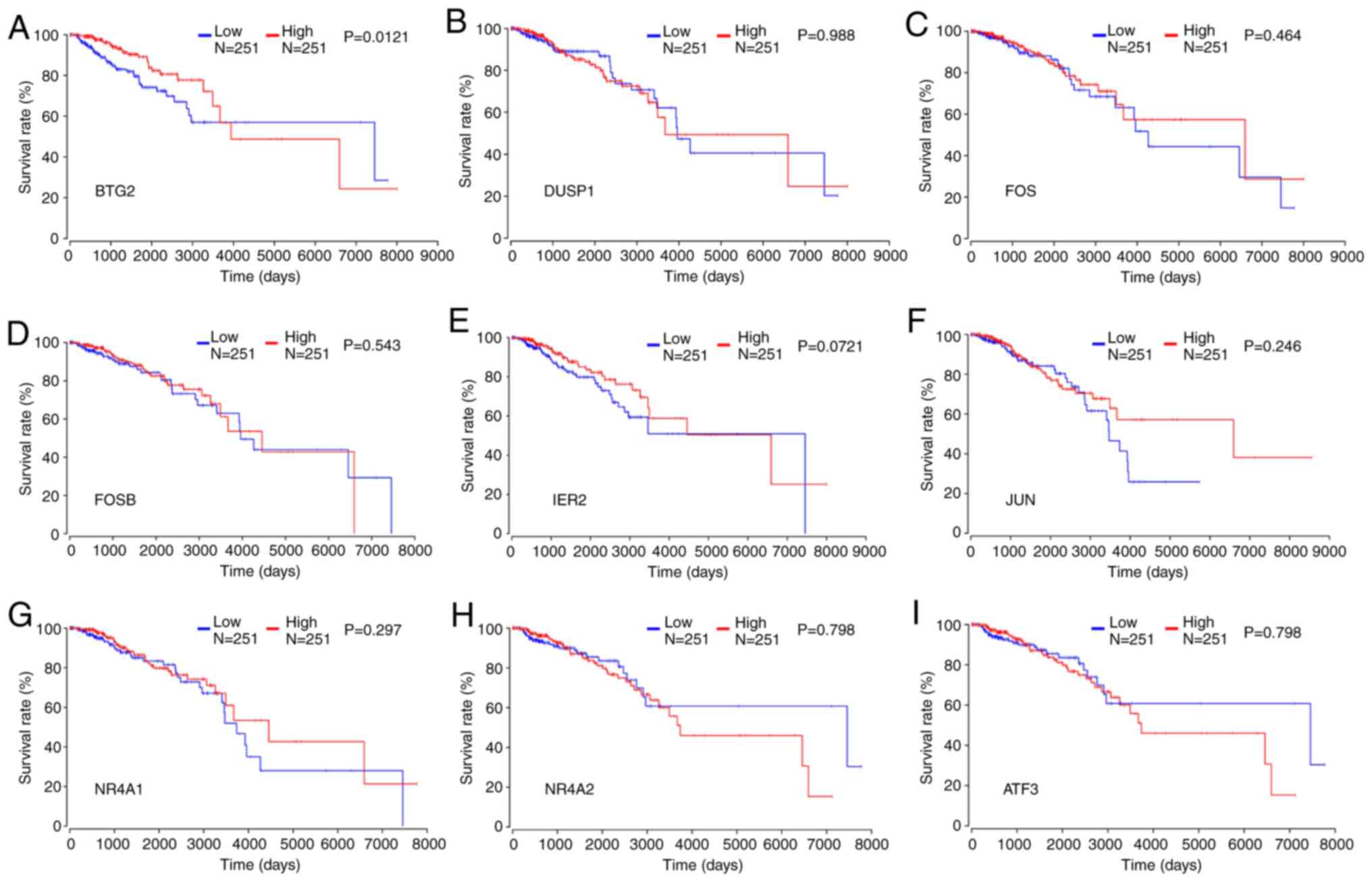 | Figure 5Impact of the expression of key
protein-coding genes on the survival of patients with all types of
breast cancer. Survival analysis of (A) BTG2, (B) DUSP1, (C) FOS,
(D) FOSB, (E) IER2, (F) JUN, (G) NR4A1, (H) NR4A2 and (I) ATF3 in
patients with all types of breast cancer. BTG2, B-cell
translocation gene 2; DUSP1, dual specificity phosphatase 1; FOS,
proto-oncogene c-FOS; FOSB, protein FOSB; JUN, proto-oncogene
c-Jun; NR4A, nuclear receptor subfamily 4 group A member; ATF3,
activating transcription factor 3. |
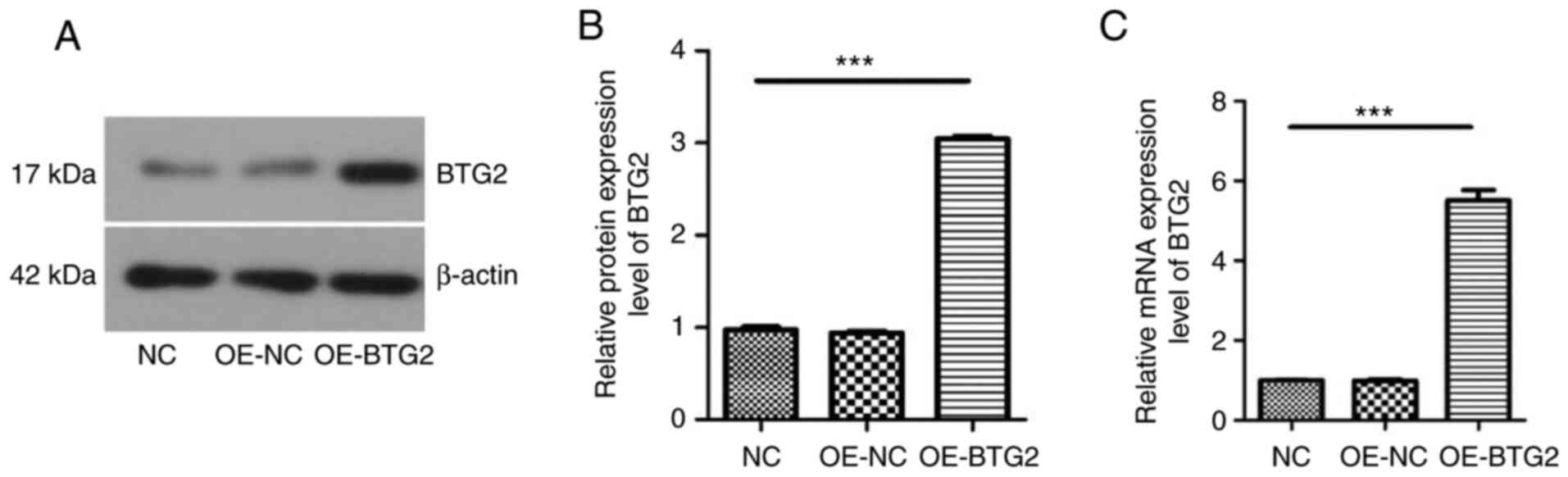
BTG2 expression in MCF-7 cells
Western blotting and RT-qPCR were carried out to
identify the expression levels of BTG2 in MCF-7 cells following
transfection of BTG2. As presented in Fig. 8, the protein and mRNA expression
level of BTG2 was low in MCF-7 cells of the OE-NC and control
groups, while high BTG2 expression was detected in
BTG2-overexpressing MCF-7 cells. Thus, the transfection of plasmids
overexpressing BTG2 in MCF-7 cells was successful, and the
transfected cells were used for further experiments.
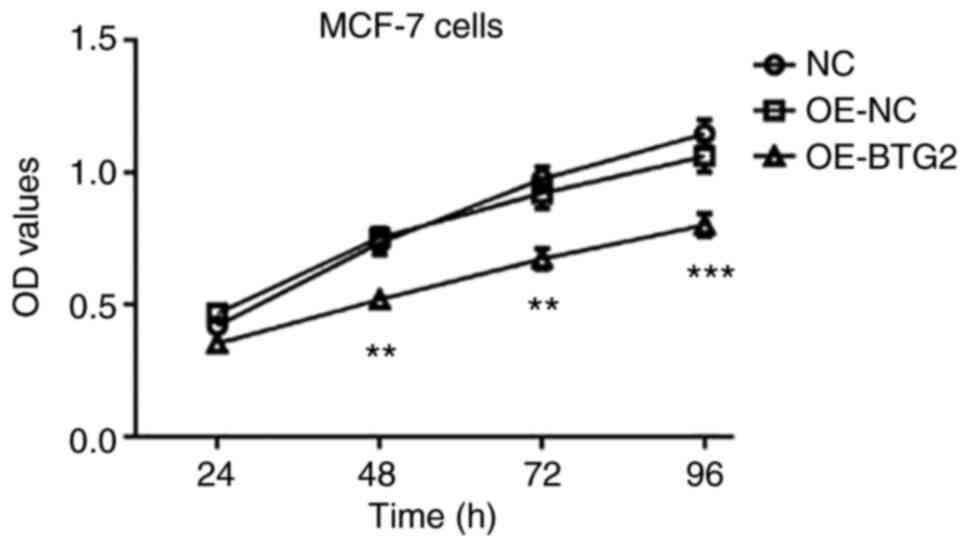
BTG2 suppresses the proliferation of
MCF-7 cells
Subsequently, the effect of BTG2 overexpression on
MCF-7 cell proliferation was investigated. As demonstrated in
Fig. 9, the proliferation of MCF-7
cells in the OE-BTG2 group was significantly lower than that of the
OE-NC and control groups. In addition, there was no significant
difference between the OE-NC and control groups. These results
demonstrated that overexpression of BTG2 suppressed the
proliferation of MCF-7 cells.
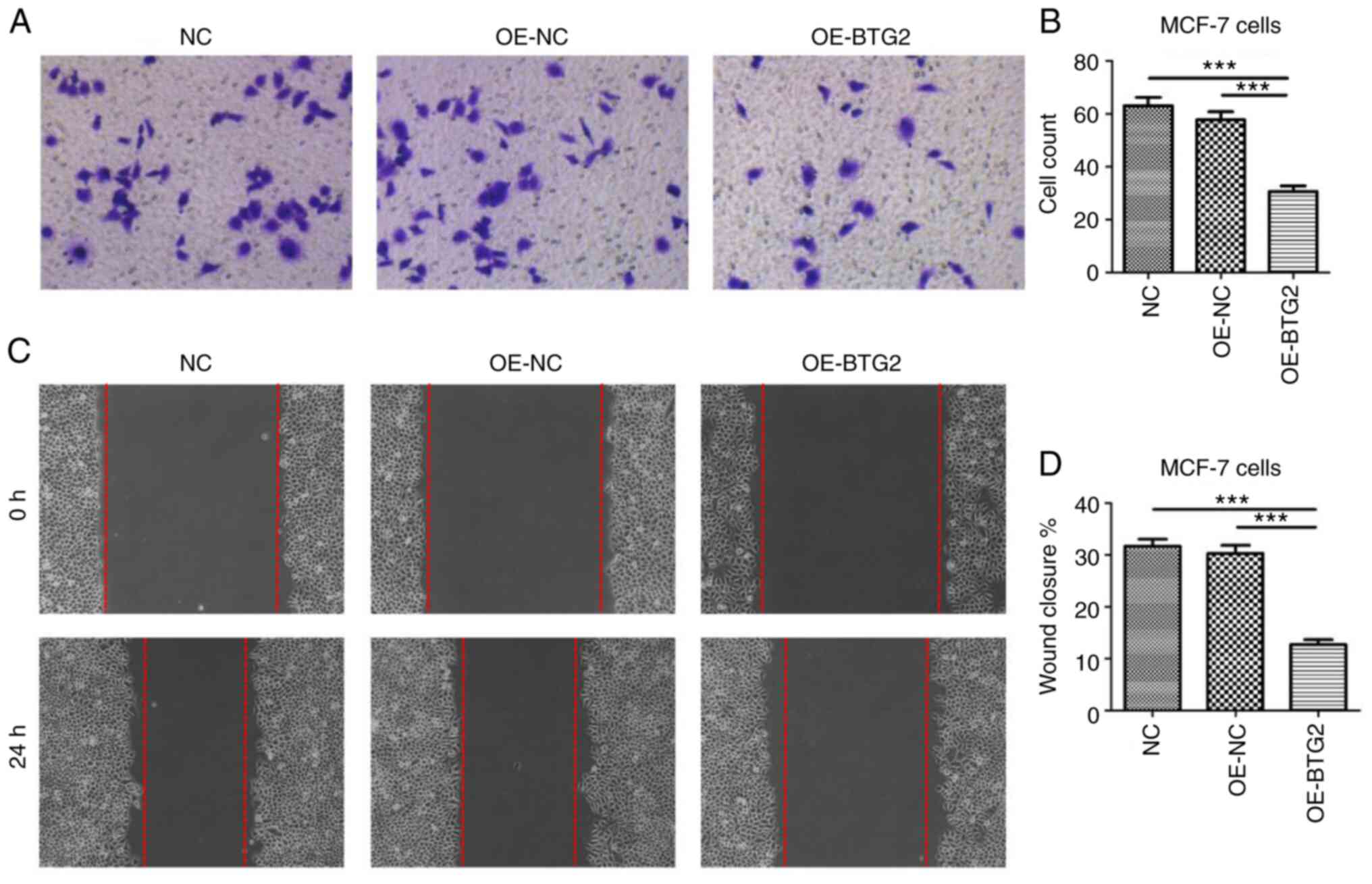
BTG2 suppresses the invasion and
migration of MCF-7 cells
Crystal violet staining demonstrated that the number
of MCF-7 cells that crossed the polycarbonate membrane of the
Transwell invasion chamber in the OE-BTG2 group was significantly
reduced, compared with the empty vector and blank control groups
(Fig. 10A and B).
Furthermore, a scratch assay was used to further
identify the effect of BTG2 overexpression on the migration of
MCF-7 cells. Results displayed in Fig. 10C and D revealed that the wounded scratch area
of MCF-7 cells in the OE-BTG2 group was markedly larger than that
of the OE-NC and control groups after 24 h. These results suggested
that the expression level of BTG2 may be associated with the
inhibition of invasion and migration in MCF-7 cells.
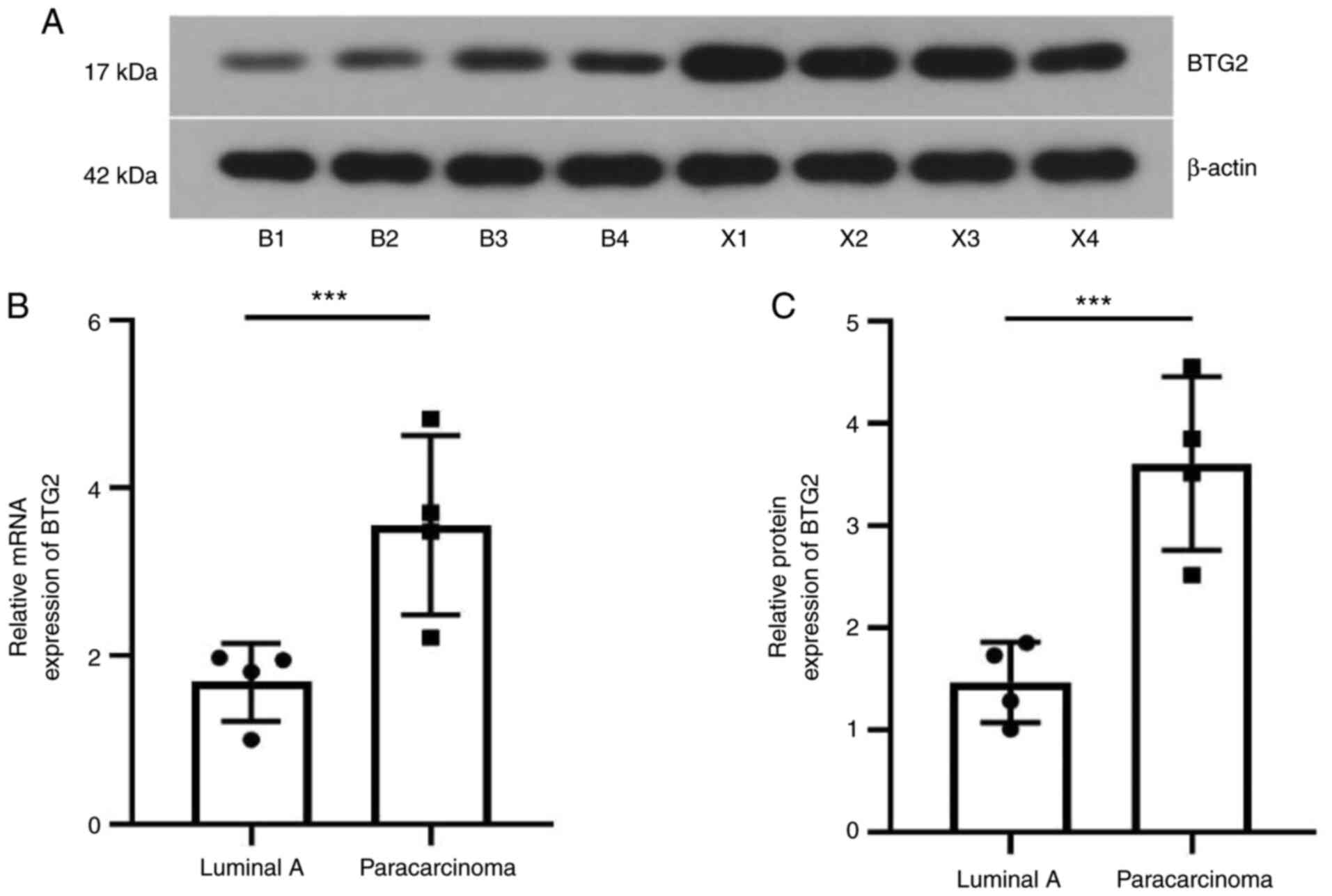
Expression of BTG2 in luminal A breast
cancer tissue
Finally, the protein and mRNA expression of BTG2 in
luminal A breast cancer tissue was investigated. As demonstrated in
Fig. 11, compared with the
paracarcinoma tissues of patients, the expression of BTG2 in
luminal A breast tumor tissue was downregulated at the mRNA and
protein level, which was consistent with the results in
vitro. These results indicated that BTG2 may be a promising
target and biomarker for luminal A breast cancer therapy in the
future.
Discussion
Breast cancer is considered to be a complex disease;
based on the expression level of immunohistochemistry markers, such
as PR, ER, HER2 and the proliferation index marker Ki67, breast
cancer can be molecularly divided into luminal A, luminal B,
HER2-enriched, basal and normal breast-like subtypes (40-43).
As the most common subtype, luminal A breast cancer exhibits the
following characteristics: ER-positive, PR >20%, HER2-negative
and Ki67 <14%. To the best of our knowledge, luminal A tumors
are sensitive to ET and insensitive to chemotherapy, and patients
with luminal A subtype exhibit a better prognosis. However, ET
often causes severe side effects and endocrine resistance, leading
to poor prognosis (15,44-46).
Therefore, there is an urgent need to identify effective treatment
strategies and therapeutic targets for luminal A breast cancer.
The BTG2 gene is widely expressed in numerous organs
and tissues, such as the lung, intestines, pancreas and prostate,
and is involved in various biological activities in cancer cells as
a tumor suppressor (47). It has
been reported that BTG2 serves an important role in cell
proliferation, DNA damage repair and apoptosis. Overexpression of
BTG2 inhibits cell proliferation in pancreatic and lung cancer
cells (48). However,
overexpression of BTG2 also promotes the migration of bladder
cancer cells and causes poor survival rates in patients with
bladder cancer, indicating that the biological functions of BTG2 as
a tumor suppressor may be cancer type-dependent (49). A previous study on the function of
BTG2 on breast cancer mainly focused on triple-negative breast
cancer (50), while few studies on
BTG2 and luminal A breast cancer have been reported to date, to the
best of our knowledge. Therefore, the identification of the
biological function of BTG2 in luminal A breast cancer may
accelerate the development of effective therapeutic targets for
luminal A breast cancer.
The present study used bioinformatics analysis to
identify key target genes associated with breast cancer, and it was
revealed that low expression of BTG2 was significantly associated
with the low survival rate of patients with breast cancer,
indicating that BTG2 may serve as a potential biomarker in breast
cancer. Considering the potential cancer-type dependent role of
BTG2, it is necessary to understand the function of BTG2 in
different subtypes of breast cancer, such as luminal A breast
cancer. Therefore, MCF-7 cells were used in the present study due
to their positive expression of ER and PR and their negative
expression of HER, which is similar to the molecular expression
profile of luminal A breast cancer.
Initially, the pcDNA3.1-BTG2 vector was constructed
and transfected into MCF-7 cells. Western blotting and RT-qPCR were
subsequently performed to determine the expression of BTG2 in MCF-7
cells, with or without transfection. Overexpression of BTG2 was
confirmed in BTG2-transfected MCF-7 cells, while a low level of
BTG2 expression was observed in the OE-NC and control groups. An
MTT assay was utilized to determine the effect of BTG2
overexpression on the proliferation of MCF-7 cells. Results of the
present study demonstrated that overexpression of BTG2
significantly inhibited the proliferation of MCF-7 cells, compared
with that of the OE-NC and control groups. Additionally, the effect
of BTG2 overexpression on the migration and invasion of MCF-7 cells
was investigated. Transwell invasion and scratch assays revealed
that BTG2 overexpression suppressed the migration and invasion of
MCF-7 cells.
Results of a previous study highlighted that
miR-25-3p was upregulated in the triple-negative breast cancer cell
lines MDA-MB-231 and SUM-1315, and miR-25-3p promoted cell
proliferation (15,44-46).
Moreover, suppression of miR-25-3p induced cell apoptosis. The
aforementioned processes were mediated through regulation of BTG2
and the subsequent activation of the AKT and ERK-MAPK signaling
pathways (50). In addition,
miR-92a-3p expression was elevated in triple-negative breast cancer
cell line MDA-MB-231 and luminal cell line MCF, and miR-92a-3p
promoted cell proliferation and metastasis via BTG2 downregulation
(51). Further investigations into
the miR-92a-3p/BTG2 axis may lead to the development of an
effective strategy for the treatment of luminal breast cancer.
In conclusion, the results of the present study
demonstrated that BTG2 was a key targeted gene associated with
breast cancer, and overexpression of BTG2 may suppress cell
proliferation, invasion and migration in luminal A breast cancer.
Thus, BTG2 may serve as a novel target for the treatment of luminal
A breast cancer; however, further studies are required to fully
elucidate the mechanisms underlying its specific function.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RuW, JW and RoW were responsible for the conception
and design of the present study. RoW, TW and HW carried out
administrative support. RuW, JT and JW obtained the study
materials. HT, TW and HW were responsible for data acquisition, and
JT, HT and RuW were responsible for data analysis and
interpretation. RuW wrote the manuscript. RuW and RoW confirm the
authenticity of all raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
All patients signed an informed consent form, and
the experiments were approved by the Affiliated Weihai Second
Municipal Hospital of Qingdao University's Ethics Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Azamjah N, Soltan-Zadeh Y and Zayeri F:
Global trend of breast cancer mortality rate: A 25-year study.
Asian Pac J Cancer Prev. 20:2015–2020. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Perou CM: Molecular stratification of
triple-negative breast cancers. Oncologist. 16 (Suppl 1):S61–S70.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members. Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel members.
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2013. Ann Oncol.
24:2206–2223. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rosenberg PS, Barker KA and Anderson WF:
Estrogen receptor status and the future burden of invasive and in
situ breast cancers in the United States. J Natl Cancer Inst.
107(djv159)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jatoi I, Anderson WF, Jeong JH and Redmond
CK: Breast cancer adjuvant therapy: Time to consider its
time-dependent effects. J Clin Oncol. 29:2301–2304. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sweeney C, Bernard PS, Factor RE, Kwan ML,
Habel LA, Quesenberry CP Jr, Shakespear K, Weltzien EK, Stijleman
IJ, Davis CA, et al: Intrinsic subtypes from PAM50 gene expression
assay in a population-based breast cancer cohort: Differences by
age, race, and tumor characteristics. Cancer Epidemiol Biomarkers
Prev. 23:714–724. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Anderson WF, Rosenberg PS and Katki HA:
Tracking and evaluating molecular tumor markers with cancer
registry data: HER2 and breast cancer. J Natl Cancer Inst.
106(dju093)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
El Hachem G, Gombos A and Awada A: Recent
advances in understanding breast cancer and emerging therapies with
a focus on luminal and triple-negative breast cancer. F1000Res 8:
F1000 Faculty Rev-591, 2019.
|
|
13
|
Cardoso F, Senkus E, Costa A, Papadopoulos
E, Aapro M, André F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh
J, et al: 4th ESO-ESMO international consensus guidelines for
advanced breast cancer (ABC 4)†. Ann Oncol. 29:1634–1657.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rasha F, Sharma M and Pruitt K: Mechanisms
of endocrine therapy resistance in breast cancer. Mol Cell
Endocrinol. 532(111322)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mavratzas A and Marme F: Treatment of
luminal metastatic breast cancer beyond CDK4/6 inhibition: Is there
a standard of care in clinical practice? Breast Care (Basel).
16:115–128. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Andrysik Z, Bender H, Galbraith MD and
Espinosa JM: Multi-omics analysis reveals contextual tumor
suppressive and oncogenic gene modules within the acute hypoxic
response. Nat Commun. 12(1375)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rouault JP, Falette N, Guéhenneux F,
Guillot C, Rimokh R, Wang Q, Berthet C, Moyret-Lalle C, Savatier P,
Pain B, et al: Identification of BTG2, an antiproliferative
p53-dependent component of the DNA damage cellular response
pathway. Nat Genet. 14:482–486. 1996.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rouault JP, Prevot D, Berthet C, Birot AM,
Billaud M, Magaud JP and Corbo L: Interaction of BTG1 and
p53-regulated BTG2 gene products with mCaf1, the murine homolog of
a component of the yeast CCR4 transcriptional regulatory complex. J
Biol Chem. 273:22563–22569. 1998.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Stoica GE, Franke TF, Moroni M, Mueller S,
Morgan E, Iann MC, Winder AD, Reiter R, Wellstein A, Martin MB and
Stoica A: Effect of estradiol on estrogen receptor-alpha gene
expression and activity can be modulated by the ErbB2/PI 3-K/Akt
pathway. Oncogene. 22:7998–8011. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mao B, Zhang Z and Wang G: BTG2: A rising
star of tumor suppressors (review). Int J Oncol. 46:459–464.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Huang H, Wu K, Wang M and Wu B:
Impact of BTG2 expression on proliferation and invasion of gastric
cancer cells in vitro. Mol Biol Rep. 37:2579–2586.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Coppola V, Musumeci M, Patrizii M,
Cannistraci A, Addario A, Maugeri-Saccà M, Biffoni M,
Francescangeli F, Cordenonsi M, Piccolo S, et al: BTG2 loss and
miR-21 upregulation contribute to prostate cell transformation by
inducing luminal markers expression and epithelial-mesenchymal
transition. Oncogene. 32:1843–1853. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chu TY, Yang JT, Huang TH and Liu HW:
Crosstalk with cancer-associated fibroblasts increases the growth
and radiation survival of cervical cancer cells. Radiat Res.
181:540–547. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shang D, Xie C, Hu J, Tan J, Yuan Y, Liu Z
and Yang Z: Pancreatic cancer cell-derived exosomal microRNA-27a
promotes angiogenesis of human microvascular endothelial cells in
pancreatic cancer via BTG2. J Cell Mol Med. 24:588–604.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen Z, Chen X, Lu B, Gu Y, Chen Q, Lei T,
Nie F, Gu J, Huang J, Wei C, et al: Up-regulated LINC01234 promotes
non-small-cell lung cancer cell metastasis by activating VAV3 and
repressing BTG2 expression. J Hematol Oncol. 13(7)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu Z, Wang Y, Xiong J, Cui F, Wang L and
Peng H: NUSAP1 knockdown inhibits cell growth and metastasis of
non-small-cell lung cancer through regulating BTG2/PI3K/Akt
signaling. J Cell Physiol. 235:3886–3893. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Devanand P, Kim SI, Choi YW, Sheen SS, Yim
H, Ryu MS, Kim SJ, Kim WJ and Lim IK: Inhibition of bladder cancer
invasion by Sp1-mediated BTG2 expression via inhibition of DNA
methyltransferase 1. FEBS J. 281:5581–5601. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kawakubo H, Brachtel E, Hayashida T, Yeo
G, Kish J, Muzikansky A, Walden PD and Maheswaran S: Loss of B-cell
translocation gene-2 in estrogen receptor-positive breast carcinoma
is associated with tumor grade and overexpression of cyclin d1
protein. Cancer Res. 66:7075–7082. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Takahashi M, Hayashida T, Okazaki H, Miyao
K, Jinno H and Kitagawa Y: Loss of B-cell translocation gene 2
expression in estrogen receptor-positive breast cancer predicts
tamoxifen resistance. Cancer Sci. 105:675–682. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hamadneh L, Abu-Irmaileh B, Al-Majawleh M,
Bustanji Y, Jarrar Y and Al-Qirim T: Doxorubicin-paclitaxel
sequential treatment: Insights of DNA methylation and gene
expression changes of luminal A and triple negative breast cancer
cell lines. Mol Cell Biochem. 476:3647–3654. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang Q, Zahid KR, Chen J, Pang X, Zhong
M, Huang H, Pan W, Yin J, Raza U, Zeng J, et al: KIN17 promotes
tumor metastasis by activating EMT signaling in luminal-A breast
cancer. Thorac Cancer. 12:2013–2023. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Barrett T and Edgar R: Mining microarray
data at NCBI's gene expression omnibus (GEO)*. Methods
Mol Biol. 338:175–190. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Edgar R, Domrachev M and Lash AE: Gene
Expression omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Graham K, de las Morenas A, Tripathi A,
King C, Kavanah M, Mendez J, Stone M, Slama J, Miller M, Antoine G,
et al: Gene expression in histologically normal epithelium from
breast cancer patients and from cancer-free prophylactic mastectomy
patients shares a similar profile. Br J Cancer. 102:1284–1293.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Anaya J: OncoLnc: Linking TCGA survival
data to mRNAs, miRNAs, and lncRNAs. PeerJ Computer Sci.
2(e67)2016.
|
|
37
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
van de Vijver MJ, He YD, van't Veer LJ,
Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C,
Marton MJ, et al: A gene-expression signature as a predictor of
survival in breast cancer. New Engl J Med. 347:1999–2009.
2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. New Engl J Med. 351:2817–2826.
2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ettl J: Luminal metastatic breast cancer:
Current concepts and future approaches. Breast Care (Basel).
16:99–100. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gonzalez-Conde M, Yanez-Gomez C,
Lopez-Lopez R and Costa C: Liquid biopsy: A new tool for overcoming
CDKi resistance mechanisms in luminal metastatic breast cancer. J
Pers Med. 11(407)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Luftner D, Hartkopf AD, Lux MP, Overkamp
F, Tesch H, Titzmann A, Pöschke P, Wallwiener M, Müller V, Beckmann
MW, et al: Challenges and Opportunities for Real-world evidence in
metastatic luminal breast cancer. Breast Care (Basel). 16:108–114.
2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Melamed J, Kernizan S and Walden PD:
Expression of B-cell translocation gene 2 protein in normal human
tissues. Tissue Cell. 34:28–32. 2002.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wei S, Hao C, Li X, Zhao H, Chen J and
Zhou Q: Effects of BTG2 on proliferation inhibition and
anti-invasion in human lung cancer cells. Tumour Biol.
33:1223–1230. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wagener N, Bulkescher J, Macher-Goeppinger
S, Karapanagiotou-Schenkel I, Hatiboglu G, Abdel-Rahim M,
Abol-Enein H, Ghoneim MA, Bastian PJ, Müller SC, et al: Endogenous
BTG2 expression stimulates migration of bladder cancer cells and
correlates with poor clinical prognosis for bladder cancer
patients. Br J Cancer. 108:973–982. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang YJ, Wei L, Liu M, Li J, Zheng YQ,
Gao Y and Li XR: BTG2 inhibits the proliferation, invasion, and
apoptosis of MDA-MB-231 triple-negative breast cancer cells. Tumour
Biol. 34:1605–1613. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jinghua H, Qinghua Z, Chenchen C, Lili C,
Xiao X, Yunfei W, Zhengzhe A, Changxiu L and Hui H: MicroRNA
miR-92a-3p regulates breast cancer cell proliferation and
metastasis via regulating B-cell translocation gene 2 (BTG2).
Bioengineered. 12:2033–2044. 2021.PubMed/NCBI View Article : Google Scholar
|