Introduction
Septic shock is defined as a subset of sepsis in
which specific and profound circulatory, cellular, coagulation and
metabolic abnormalities are associated with a greater risk of
mortality than with sepsis alone (1,2).
Several markers can be detected in the serum of septic patients and
they reflect endothelial cell activation and endothelium disruption
as well as increased leukocyte-endothelial interactions (3,4). In
particular, angiopoietin 2 (Ang-2) is a molecule that is involved
in the maintenance of endothelial cell barrier function. Increased
Ang-2 is predominantly associated with increased intracellular gap
formation and disseminated intravascular coagulation (DIC)
(5). For instance, Statz et
al (6) reported that serum
Ang-2 levels were related to the prognosis of patients with
sepsis-associated DIC. Overall, prediction of a patient's outcome
is of great importance to clinicians in their management of septic
shock, and the optimal place of novel vasoactive agents in therapy
for circulatory dysfunction should be established with further
confidence. In particular, direct biomarkers of endothelial damage
could also be of great significance during septic shock.
The Sequential Organ Failure Assessment (SOFA) and
Acute Physiology And Chronic Health Evaluation II (APACHE II)
scores are the most widely applied prognostic markers, owing
primarily to their simplicity, accuracy, and integrity of use in
clinical practice (2,7). However, due to variations in
aetiology, intervention, and patient characteristics, a more
suitable marker for sepsis and septic shock must be established and
evaluated. The present study retrospectively investigated Ang-2
mRNA levels in a cohort of patients with septic shock. Typically,
this context of septic shock consists of surgical and nonsurgical
diseases. We hypothesized that Ang-2 mRNA levels are higher in
patients with septic shock than in healthy controls and that Ang-2
mRNA levels will decrease over time if patients receive standard
and effective treatment. Accordingly, we hypothesized that Ang-2
mRNA levels and illness severity scores are higher in non-survivors
than survivors. For Ang-2 to be used in daily practice, equivalent
comparisons with standard currently used predictive clinical
scoring systems are needed. Thus, another objective of this study
was to validate the predictive value of Ang-2 mRNA levels compared
with SOFA and APACHE II scores. Additionally, we hypothesized that
Ang-2 is more accurate than illness severity scores in mortality
outcome prediction.
Patients and methods
Patients and data collection
In this retrospective study, clinical data of
patients admitted to the intensive care unit (ICU) with a diagnosis
of septic shock were collected between January 2020 and October
2020 at Tongji Hospital (Wuhan, Hubei, China). Patients underwent
laboratory tests, including but not limited to, routine blood
tests, biochemical items (including liver and renal function
tests), coagulation factors and inflammation indicators such as
C-reactive protein (CRP) and procalcitonin (PCT). To measure Ang-2
mRNA levels, citrated whole blood samples were collected upon ICU
admission at baseline (day 0), as well as on days 3, 5 and 7 for
those remaining in the ICU at those times. Plasma samples were
stored at -80˚C prior to analysis. The baseline clinical
characteristics of the patients are provided in Table I.
 | Table IMain clinical characteristics of the
patients with sepsis. |
Table I
Main clinical characteristics of the
patients with sepsis.
| Parameters | Healthy controls
(n=10) | All patients
(n=85) | Survivors (n=65) | Non-survivors
(n=20) | P-value |
|---|
| Mean age (range)
(years)a | 53 (30-68) | 55 (30-80) | 55 (30-80) | 57 (33-69) | 0.820 |
| Sex
(male/female)a | 7/3 | 55/30 | 40/25 | 15/5 | 0.300 |
| Ang II level
(ratio)b | 1±0.2 | 10.5±3.7 | 7.8±2.9 | 12.6±2.7 | <0.001 |
| PCT
(ng/ml)b | 0.3±0.1 | 16.39±13.1 | 10.92±8.13 | 21.86±15.95 | 0.007 |
| CRP
(ng/ml)b | 5±2.1 | 135.7±59.58 | 139.6±63.69 | 127.85±44.71 | 0.360 |
| Albumin
(g/l)b | 43.3±3.2 | 26.3±3.7 | 26.3±3.3 | 24.1±3.9 | 0.030 |
| Lactate
(mmol/l)b | 0.8±0.5 | 4.8±2.5 | 3.4±1.6 | 6.1±2.9 | <0.001 |
| PT
(sec)b | 12±1.1 | 13.5±6.2 | 14.3±3.0 | 20.5±5.6 | <0.001 |
| Fibrinogen
(g/l)b | 2.8±1.5 | 1.6±0.8 | 2.0±0.7 | 1.1±0.7 | <0.001 |
| SOFA
scoreb | - | 8.6±3.7 | 6.7±2.4 | 11.0±2.9 | <0.001 |
| APACHE II
scoreb | - | 16.3±7.4 | 12.1±4.5 | 22.2±6.5 | <0.001 |
| Length of ICU stay
(mean ± SD, days)b | - | 14.6±8.6 | 11.2±6.8 | 17.3±10.8 | 0.025 |
Diagnosis and treatment
Septic shock was diagnosed according to the
consensus of Sepsis-3 published in February 2016(1). Clinical criteria for septic shock can
be identified by a clinical construct of sepsis with persisting
hypotension requiring vasopressors to maintain MAP ≥65 mmHg and
having a serum lactate level >2 mmol/l despite adequate volume
resuscitation (2). The SOFA score
was calculated based on available clinical information and measured
respiratory, hematological, hepatic, cardiovascular, central
nervous system and renal function in order to describe clinical
status. The APACHE II system uses a point score based upon initial
values of 12 routine physiological measurements, age and previous
health status to provide a general measure of the severity of
disease. A higher SOFA or APACHE II score is indicative of a worse
prognosis (Table I). All patients
received standard medical or surgical treatment, if needed. The
standard medications included but were not limited to antibiotics,
vasopressors to maintain MAP ≥65 mmHg, respiratory support
including mechanical ventilation, and continuous renal replacement
therapy (CRRT) if indicated. The patients underwent laboratory
tests, including but not limited to, routine blood tests,
biochemical items, coagulation factors and inflammation indicators
such as C-reactive protein (CRP) and procalcitonin (PCT).
Patients with advanced cancer, serious
cardiovascular disease, trauma with haemorrhagic shock and other
severe illnesses with a high risk of short-term mortality were
excluded from the study.
RNA isolation and cDNA synthesis
Cell-free RNA (cf.-RNA) was isolated from 2 ml blood
samples using a commercially available RNA simple kit (Tiangen)
according to the manufacturer's instructions. The amount of total
RNA was quantified by the absorbance at 260 nm using an N-5000
spectrophotometer (Yoke Technologies). RNA integrity was analyzed
by electrophoresis on a 1% agarose gel and stained with ethidium
bromide.
Complementary DNA (cDNA) was synthesized from
cf.-RNA with mRNA-specific stem-loop reverse transcription (RT)
primers by the First-Strand cDNA Synthesis Kit (Tiangen) using the
oligo(dT) primers included in the kit according to the
manufacturer's protocol. A 20-µl reaction mixture for each sample
was prepared containing 5 µl MgCl2, 2 µl 10X PCR buffer,
2 µl dNTPs, 2 µl oligo-deoxythymidine primer, 5 µl RNase-free
dH2O, 2 µl ribonuclease inhibitor, 1 µl of Moloney
murine leukaemia virus reverse transcriptase and 1 µg of total RNA
diluted in 1 µl and placed into a 0.2 ml thin-wall PCR tube. The
reverse transcription (RT) reaction was performed at 42˚C for 50
min and inactivated by heating to 90˚C for 10 min. The first-strand
cDNA was cooled on ice and then stored at 0˚C until PCR was
performed.
Reverse transcription-quantitative PCR
(RT-qPCR)
qPCR was performed on an ABI Prism® 7000
PCR instrument (Applied Biosystems China Inc.) using program
parameters provided by the manufacturer. GAPDH was used as an
internal control for normalization. A total of 100 µl of PCR
mixture was prepared containing 0.2 mmol/l dNTPs and 2.5 U
Taq-polymerase (Tiangen) with the corresponding paired
primers at a concentration of 0.2 µmol/l of each primer. The
following primers for RT-PCR were designed using Primer Express
software (version 5.0; PREMIER Biosoft International): Ang-2-Fwd,
CAGATTTTGGACCAGACCAGTG; Ang-2-Rev, ACTGTATGTTGGATGATGTGCTTG;
GAPDH-Fwd, GAAGGTGAAGGTCGGAGTC; and GAPDH-Rev,
GAAGATGGTGATGGGATTTC. The PCR program was performed according to
the manufacturer's instructions: denaturation (95˚C for 10 min)
followed by amplification (35 cycles at 95˚C for 60 sec, 60˚C for
45 sec and 72˚C for 30 sec). The ∆∆cq value and relative quantity
(RQ) were calculated by SDS 1.4 version software (Applied
Biosystems; Thermo Fisher Scientific, Inc.), RQ=2-∆∆cq
(8). Each sample was tested in
triplicate, and the differences in RNA concentration were
normalized in each sample by quantitation of the transcript of the
housekeeping gene GAPDH. All Ang-2 concentrations are expressed
relative to GAPDH.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism (version 7.0 for Windows, GraphPad Software).
Quantitative data are expressed as mean ± standard deviation (SD).
Mann-Whitney U test was used to compare the medians between
survivors and non-survivors. One-way ANOVA was used to assess the
differences in Ang-2 levels between baseline (Day 0), and Day 3,
Day 5 and Day 7. The Tukey-Kramer post hoc test was performed for
pairwise comparison between groups. The t-tests were applied to
compare the means between healthy controls and patients and the
means between survivors and non-survivors. A χ2 test or
Fisher's exact test was performed for comparison of qualitative
data. Receiver operating characteristic (ROC) procedures were used
to predict mortality outcomes, and the areas under the ROC curves
(AUCs) were calculated. The DeLong method was used to test the
statistical significance of the difference between ROC curves
(Ang-2, SOFA score and APACHE II score). All analyses were
2-tailed, with P-values <0.05 considered to indicate a
statistically significant difference.
Results
Clinical features of the septic shock
patients
Clinical data of 85 patients with septic shock and
10 healthy controls were collected for the study. Differences in
sex and age were not significant between the patients and the
control group. The serum lactate and prothrombin time (PT) were
significantly higher in the non-survivors (6.1±2.9 mmol/l vs.
3.4±1.6 mmol/l; and 20.5±5.6 sec vs. 14.3±3.0 sec, P<0.001,
respectively). However, serum albumin and fibrinogen levels were
significantly higher in the survivors (26.3±3.3 g/l vs. 24.1±3.9
g/l; and 2.0±0.7 g/l vs. 1.1±0.7 g/l, P=0.03 and P<0.001,
respectively). Serum procalcitonin (PCT) and C-reactive protein
(CRP) are generally accepted indicators of inflammation, but only
PCT differed significantly between the survivors and the
non-survivors (10.92±8.13 ng/ml vs. 21.86±15.95 ng/ml, P=0.007).
The non-survivors had higher SOFA (11.0±2.9 vs. 6.7±2.4) and APACHE
II (22.2±6.5 vs. 12.1±4.5) scores (P<0.001). Overall, the
survivors had a shorter length of ICU stay (11.2±6.8 days vs.
17.3±10.8 days, P=0.025). Data are presented in Table I.
Both surgical and nonsurgical diseases can result in
septic shock. However, no significant difference was found in
regards to the mortality between the two groups (P=0.25). Among
sequential sites of infection, the lung, abdomen and urinary tract
were the most frequent sources of infection, with proportions of
30.6, 23.5 and 20%, respectively. There were no significant
differences in mortality in each category (P=0.410, 0.540, and
0.750, respectively) (Table II),
with P<0.05 indicating a significant difference.
 | Table IIInfection localization of septic
shock. |
Table II
Infection localization of septic
shock.
| Parameters | All patients
(n=85) | Survivors,
(n=65) | Non-survivors
(n=20) | P-value |
|---|
| Surgical
intervention | 22 (25.9) | 17 (26.2) | 5 (22.7) | 0.250 |
| Infection
location | | | | |
|
Pulmonary | 26 (30.6) | 18 (27.7) | 8(40) | 0.410 |
|
Abdominal | 20 (23.5) | 15 (23.1) | 3(15) | 0.540 |
|
Liver
abscess | 3 (3.5) | 2 (3.1) | 1(5) | 0.560 |
|
Biliary
tract | 3 (3.5) | 2 (3.1) | 1(5) | 0.560 |
|
Urinary
tract | 17(20) | 14 (21.5) | 3(15) | 0.750 |
|
Trauma | 8 (9.4) | 7 (10.8) | 1(5) | 0.670 |
|
Central
nervous system | 5 (5.9) | 3 (4.6) | 2(10) | 0.590 |
|
Undefined | 3 (3.5) | 2 (3.1) | 1(5) | 0.560 |
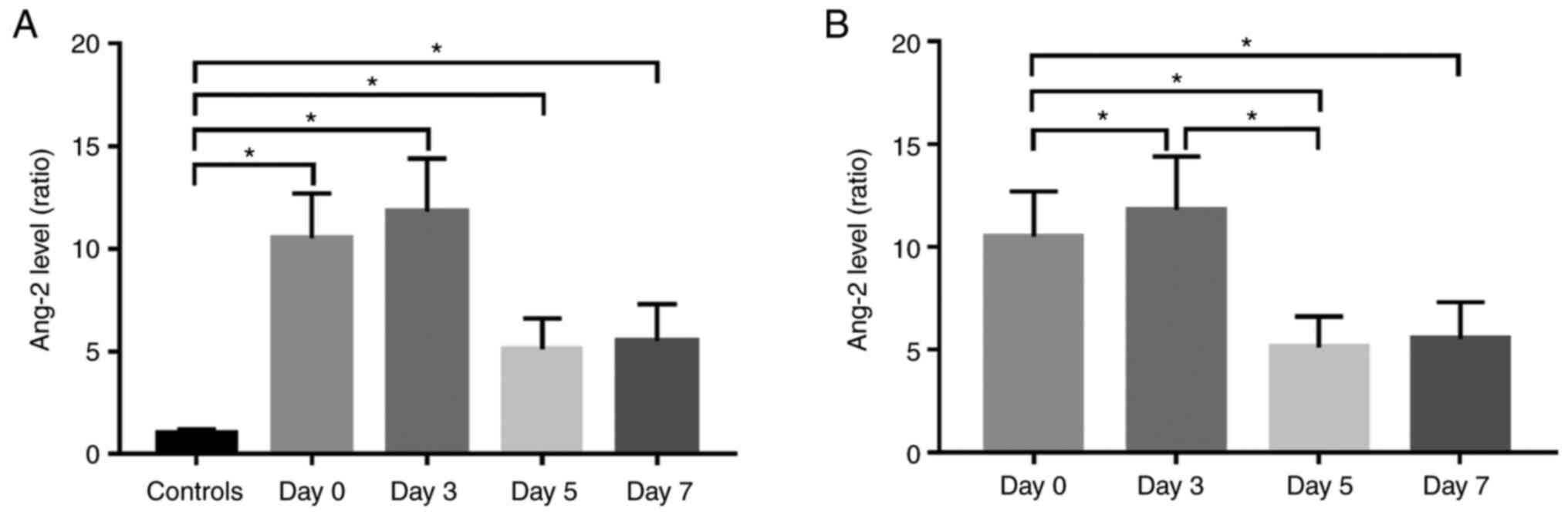
Downward trend of serum Ang-2 levels
over time
The mean Ang-2 mRNA levels in all patients with
septic shock at baseline (Day 0), Day 3, Day 5, and Day 7 were
compared to those in the normal controls. Mean Ang-2 levels in
patients with septic shock at Day 0, Day 3, Day 5, and Day 7 were
significantly higher than those in the controls (Fig. 1A, P<0.001). However, the Ang-2
mRNA level at Day 3 was significantly elevated compared to the
baseline (Day 0) (P<0.001), and decreased rapidly at Day 5 and 7
(P<0.001). The Ang-2 mRNA levels were measured in healthy
controls at 1.0±0.2 and in patients at 10.5±2.2 at Day 0, 11.8±2.6
at Day 3, 5.1±1.5 at Day 5, and 5.5±1.8 at Day 7. Generally, the
serum Ang-2 levels showed a significant downward trend over time
after standard treatment (Fig. 1B,
P<0.001).
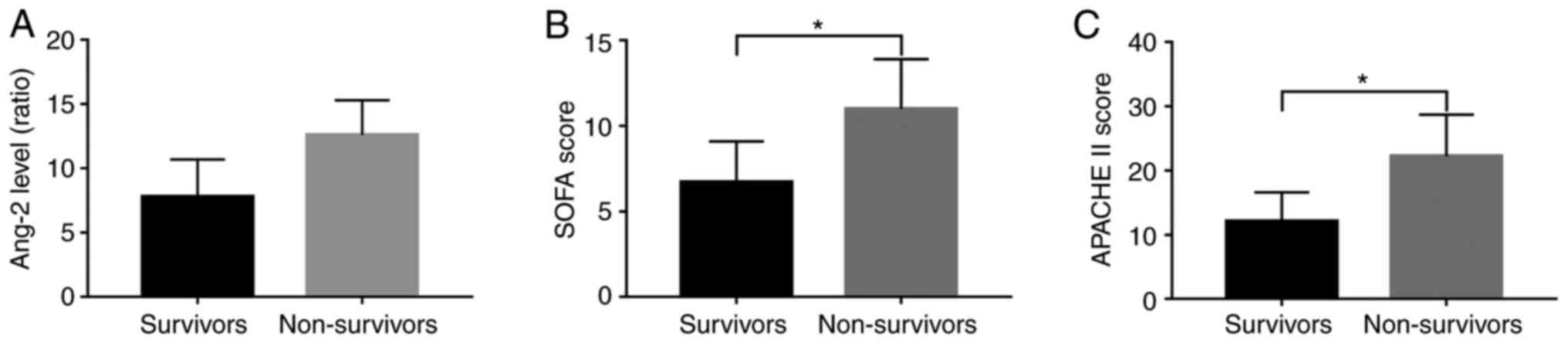
Ang-2 level, SOFA and APACHE II scores
indicate illness severity and prognosis
Of the 85 patients for which mortality data were
reported, 65 survived and 20 died, giving a 28-day mortality rate
of 23.57%. The mean Ang-2 mRNA levels at admission and baseline
clinical measures of illness severity, including SOFA and APACHE II
scores, were significantly lower in survivors than in non-survivors
(Fig. 2A-C, P<0.001). The Ang-2
mRNA level in the survivors was 7.8±2.9 compared to 12.6±2.7 in the
non-survivors. SOFA score was 6.7±2.4 in the survivors compared to
11.0±2.9 in the non-survivors. The APACHE II score was 12.1±4.5 in
the survivors compared to 22.2±6.5 in the non-survivors (Table I, P<0.001).
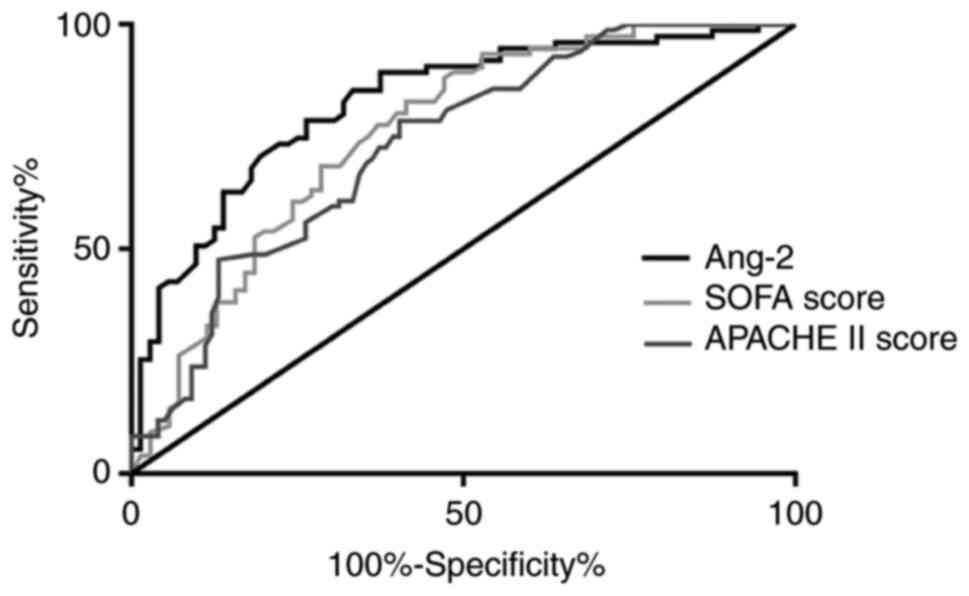
Ang-2 level predicts the outcome of
septic shock
Fig. 3 shows the
ROC curves for predicting the 28-day mortality based on baseline
parameters. The different parameters (AUC, SEM, and P-values) for
Ang-2 (0.82, 0.03, P<0.01), for SOFA score (0.76, 0.04,
P<0.01), and APACHE II score (0.73, 0.04, P<0.01) were
calculated. This indicates that the Ang-2 level at admission to the
ICU may be more accurate than current clinical illness scores at
predicting 28-day mortality in patients with septic shock.
Discussion
In the critical setting, the upregulation of
angiopoietin 2 (Ang-2) is a physiologic and potentially lifesaving
response and is significantly associated with the severity of
disease. Ang-2 has been proven to increase in the context of sepsis
(6,9,10).
Ang-2 mRNA is directly or indirectly induced by infection across a
broad spectrum of diseases (9,11).
Several features of endothelial function are significantly
correlated with the severity or outcome of sepsis, such as
endothelial-originated anticoagulants, represented by protein C;
indicators of endothelial injury, represented by vWF; and
regulators of vascular function and permeability, represented by
Ang-2 (12,13). In the present study, elevated Ang-2
expression was confirmed by measuring the serum Ang-2 mRNA level in
the patients with septic shock and by correlation with survival
outcome. Both surgical and nonsurgical patients were enrolled, and
patients presenting with multiple infection sites (lung, abdomen
and urinary tract) were included. As determined by the present
study, a downward trend of Ang-2 mRNA levels over time after
intensive treatment indicated an improvement in circulation
deterioration. Through continuous intensive and effective treatment
in the ICU, the majority of patients improved and were discharged
from the ICU. However, longer stays in the ICU indicated a more
severe illness, which influenced the serum Ang-2 mRNA level on Day
7 compared with Day 5.
Sepsis is defined as a life-threatening multiple
organ dysfunction caused by a dysregulated host response to
infection (14). A National Heart,
Lung and Blood Institute report also proposed the unifying concept
of sepsis as a severe endothelial dysfunction syndrome that causes
multiple organ failure in response to intravascular or
extravascular microbial agents, which calls for a better
understanding of endothelial function (15). Accumulating evidence suggests that
the breakdown of endothelial barrier function plays a central role
in the pathogenesis of sepsis (14,16,17).
Although microcirculatory dysfunction may arise to various degrees
in most clinical conditions, automatic regulatory mechanisms of
microvascular function are the most severely impaired during
sepsis, suggesting that microcirculatory dysfunction is a
predominant pathophysiological manifestation of sepsis syndrome
(18). Systemic and localized
infections can induce disruptions of the integrity of the
microcirculation in multiple organs. The severity of the infection
is due to an activation cascade that leads to auto-amplification of
cytokine production: the cytokine storm (16,19).
Septic shock can lead to ischemic and inflammatory injuries to the
lungs, kidney, heart, brain, and other organs and is a barrier to
advances in the medical and surgical management of multiple
diseases.
Genetic and molecular studies spanning thousands of
human subjects have linked imbalances of Ang-2 levels to major
adverse clinical events arising from bacterial sepsis and other
severe infections (9,11). Ang-2 acts on endothelial cells,
increasing endothelial permeability (20). Under normal physiological
conditions, Ang-2 levels are low but upregulated during
inflammation or cancer. Upon Ang-2 binding, Tie2 heterodimerizes
with Tie1, and they can both form complexes with integrins. Ang-2
can also bind to integrins independently of Tie2, inducing
endothelial cell migration and sprout formation (21,22).
In addition, Ang-2 can induce a rapid loss of the endothelial
glycocalyx in human sepsis. Inhibition of Ang-2 or Tie2 activation
completely abolished endothelial glycocalyx damage (23). These studies on the regulation of
the vascular endothelium have raised optimism concerning the
development of methods to affect the progression of shock and
multiorgan dysfunction.
In recent years, increasing empirical evidence has
shown that extensive cross-talk between inflammation and
coagulation systems plays a pivotal role in the pathogenesis of
microvascular failure and subsequent multiple organ failure as a
result of severe infection (24,25).
For clinical operationalization, patients with septic shock can be
identified by a vasopressor requirement to maintain a mean arterial
pressure of 65 mmHg or greater and serum lactate level greater than
2 mmol/l (>18 mg/dl) in the absence of hypovolemia. This
combination is associated with hospital mortality rates greater
than 40% (19). The high morbidity
and mortality of sepsis are the consequences of multiple organ
dysfunction/failure, including acute respiratory distress syndrome
(ARDS) or disseminated intravascular coagulation (DIC), which are
due primarily to septic injury and dysfunction of the
microvasculature, especially microvascular endothelial cells.
Septic microvascular dysfunction of both pulmonary and systemic
vascular beds is of clinical importance, as it occurs early in the
course of sepsis (26,27).
The key pathophysiology underlying this
life-threatening disease is the preponderant inflammatory host
response to pathogen infections leading to the overexpression of
inflammatory mediators. Sepsis-induced acute lung injury (ALI) is
characterized by injury and dysfunction of the pulmonary
microvasculature and pulmonary microvascular endothelial cells
(28). Septic pulmonary
microvascular dysfunction is primarily induced by the effects of
septic inflammation on pulmonary microvascular endothelial cells
(12,27). Sepsis-associated coagulopathy is
also a result of the inflammation-induced activation of coagulation
pathways. Although large clinical trials of sepsis-related
anticoagulant therapies failed to show survival benefits, post hoc
analysis of databases showed beneficial effects of anticoagulants
in subgroups of patients with early sepsis-induced disseminated
intravascular coagulation (29).
As stated above, the pathophysiological changes in sepsis are
entirely mediated by microvascular dysfunction.
As septic shock can occur across a spectrum of
severities, it is of great importance to quantify disease severity
in any study population. Our data showed that among the 85 septic
patients involved in surgical and nonsurgical diseases, the lung,
abdomen and urinary tract were the most frequent sites of
infection, with proportions of 30.6, 23.5 and 20%, respectively.
However, there were no significant differences in the mortality
rate among the infection sites. Surgical intervention had no impact
on mortality. Sequential Organ Failure Assessment (SOFA) scores
were calculated daily in the ICU and are recommended as clinical
criteria, combining the axes of the respiratory, hematologic,
hepatic, cardiovascular, and central nervous systems, and renal
function, to describe the clinical status. Organ dysfunction can be
indicated by an increase in the SOFA score of 2 points or more,
associated with an in-hospital mortality greater than 10% (30). The Acute Physiology and Chronic
Health Evaluation (APACHE II) scoring system consists of three
parts: the acute physiology score, age, and chronic health
evaluation, and it is the most widely applied predictive model in
the ICU. Likewise, the APACHE II score can be used as an indicator
of sepsis severity. A higher APACHE II score is indicative of a
worse prognosis. The APACHE II and SOFA scores incorporate similar
but not identical criteria with which to identify septic shock. In
the present study, the non-survivors were found to have higher SOFA
and APACHE II scores (P<0.001).
In a prospective study, Ang-2 expression was found
to be significantly upregulated in sepsis-associated coagulopathy,
and this biomarker was used to stratify patients with sepsis into
non-overt DIC and overt DIC (6).
Studies have also shown that Ang-2 is a promising biomarker with
which to evaluate the gastrointestinal status and function in acute
pancreatitis and it could serve as a useful tool for clinicians
evaluating disease severity and prognosis (31,32).
The results of the present study are consistent with the previous
literature. The ROC curves for predicting 28-day mortality based on
the baseline parameters of Ang-2, SOFA and APACHE II scores were
calculated in the present study. Higher mortality was associated
with higher Ang-2 mRNA levels as well as SOFA and APACHE II scores.
Consequently, the Ang-2 mRNA level was found to be a valuable
disease severity indicator and a predictive biomarker for mortality
outcomes.
However, the inflammatory indicator of C-reactive
protein (CRP) did not differ between the survivors and the
non-survivors in this study. A possible explanation for this
phenomenon is that prolonged septic shock leads to severe
endothelial dysfunction and extensive leukocyte insufficiency as
well as consumption of inflammatory factors. In general, the
indeterminacy of clinical applications of synthetic human Ang-2
requires more clinical evidence.
In summary, the present study confirmed that the
serum Ang-2 mRNA level is a prognostic indicator that is superior
to SOFA and APACHE II scores currently used in the ICU. Since
sepsis is not a specific illness but rather a syndrome encompassing
a still uncertain pathology, additional studies to explore
therapeutic interventions in regards to serum angiopoietin 2 are of
great importance for the clinical management of septic shock.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JF and LW designed the study and reviewed the
manuscript, and share first authorship. YF and GY performed the
laboratory examinations, acquired the majority of the data and
drafted the manuscript. DZ and JW confirmed the authenticity of all
the raw data, analyzed the data and revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Data collection and analysis were undertaken in
compliance with the approval and supervision of the Tongji Hospital
Research Institutional Review Board (approval no. TJ-IRB20211252;
Wuhan, China). The local ethics committee classified this study as
a retrospective study based on data analysis, and patient consent
was not required.
Patient consent for publication
Patient consent for publication was not necessary
because this retrospective study did not contain any personal
information that could lead to the identification of the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shankar-Hari M, Phillips GS, Levy ML,
Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD and
Singer M: Sepsis Definitions Task Force. Developing a new
definition and assessing new clinical criteria for septic shock:
For the third international consensus definitions for sepsis and
septic shock (sepsis-3). JAMA. 315:775–787. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kazune S, Caica A, Volceka K, Suba O,
Rubins U and Grabovskis A: Relationship of mottling score, skin
microcirculatory perfusion indices and biomarkers of endothelial
dysfunction in patients with septic shock: An observational study.
Crit Care. 23(311)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Muller RB, Ostrowski SR, Haase N,
Wetterslev J, Perner A and Johansson PI: Markers of endothelial
damage and coagulation impairment in patients with severe sepsis
resuscitated with hydroxyethyl starch 130/0.42 vs Ringer acetate. J
Crit Care. 32:16–20. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thamm K and David S: Role of
angiopoietin-2 in infection-A double-edged sword? Cytokine.
83:61–63. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Statz S, Sabal G, Walborn A, Williams M,
Hoppensteadt D, Mosier M, Rondina M and Fareed J: Angiopoietin 2
levels in the risk stratification and mortality outcome prediction
of sepsis-associated coagulopathy. Clin Appl Thromb Hemost.
24:1223–1233. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haniffa R, Mukaka M, Munasinghe SB, De
Silva AP, Jayasinghe KSA, Beane A, de Keizer N and Dondorp AM:
Simplified prognostic model for critically ill patients in resource
limited settings in South Asia. Crit Care. 21(250)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu XW, Ma T, Liu W, Cai Q, Wang L, Song
HW, Yuan L and Liu Z: Sustained increase in angiopoietin-2,
heparin-binding protein, and procalcitonin is associated with
severe sepsis. J Crit Care. 45:14–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Szederjesi J, Almasy E, Lazar A, Hutanu A
and Georgescu A: The role of angiopoietine-2 in the diagnosis and
prognosis of sepsis. J Crit Care Med (Targu Mures). 1:18–23.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gutbier B, Neuhauß AK, Reppe K, Ehrler C,
Santel A, Kaufmann J, Scholz M, Weissmann N, Morawietz L, Mitchell
TJ, et al: Prognostic and pathogenic role of angiopoietin-1 and -2
in pneumonia. Am J Respir Crit Care Med. 198:220–231.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hou PC, Filbin MR, Wang H, Ngo L, Huang
DT, Aird WC, Yealy DM, Angus DC, Kellum JA, Shapiro NI and ProCESS
Investigators: Endothelial permeability and hemostasis in septic
shock: Results from the ProCESS trial. Chest. 152:22–31.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu T, Jing M, Gao Y, Liu C, Liu L, Jia H,
Liu P and Chang M: Study on the relationship between
hyperthyroidism and vascular endothelial cell damage. Sci Rep.
10(6992)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chousterman BG, Swirski FK and Weber GF:
Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol.
39:517–528. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Goldenberg NM, Steinberg BE, Slutsky AS
and Lee WL: Broken barriers: A new take on sepsis pathogenesis. Sci
Transl Med. 3(88ps25)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yamakawa K, Umemura Y, Hayakawa M, Kudo D,
Sanui M, Takahashi H, Yoshikawa Y, Hamasaki T and Fujimi S: Japan
Septic Disseminated Intravascular Coagulation (J-Septic DIC) study
group. Benefit profile of anticoagulant therapy in sepsis: A
nationwide multicentre registry in Japan. Crit Care.
20(229)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Skibsted S, Jones AE, Puskarich MA, Arnold
R, Sherwin R, Trzeciak S, Schuetz P, Aird WC and Shapiro NI:
Biomarkers of endothelial cell activation in early sepsis. Shock.
39:427–432. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Harrois A, Baudry N, Huet O, Kato H, Lohez
M, Ziol M, Duranteau J and Vicaut E: Synergistic deleterious effect
of hypoxemia and hypovolemia on microcirculation in intestinal
villi*. Crit Care Med. 41:e376–e384. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ruiz-Mesa JD, Marquez-Gomez I, Sena G,
Buonaiuto VA, Mora-Ordoñez J, Salido M, Plata Ciézar A, Valiente-De
Santis L, Mediavilla C and Colmenero JD: Factors associated with
severe sepsis or septic shock in complicated pyelonephritis.
Medicine (Baltimore). 96(e8371)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Parikh SM: Angiopoietins and Tie2 in
vascular inflammation. Curr Opin Hematol. 24:432–438.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Akwii RG, Sajib MS, Zahra FT and Mikelis
CM: Role of angiopoietin-2 in vascular physiology and
pathophysiology. Cells. 8(471)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fejza A, Poletto E, Carobolante G, Camicia
L, Andreuzzi E, Capuano A, Pivetta E, Pellicani R, Colladel R,
Marastoni S, et al: Multimerin-2 orchestrates the cross-talk
between endothelial cells and pericytes: A mechanism to maintain
vascular stability. Matrix Biol Plus. 11(100068)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Drost CC, Rovas A, Kusche-Vihrog K, Van
Slyke P, Kim H, Hoang VC, Maynes JT, Wennmann DO, Pavenstädt H,
Linke W, et al: Tie2 activation promotes protection and
reconstitution of the endothelial glycocalyx in human sepsis.
Thromb Haemost. 119:1827–1838. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dalainas I: Pathogenesis, diagnosis, and
management of disseminated intravascular coagulation: A literature
review. Eur Rev Med Pharmacol Sci. 12:19–31. 2008.PubMed/NCBI
|
|
25
|
Gando S, Kameue T, Matsuda N, Hayakawa M,
Morimoto Y, Ishitani T and Kemmotsu O: Imbalances between the
levels of tissue factor and tissue factor pathway inhibitor in ARDS
patients. Thromb Res. 109:119–124. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Trzeciak S, Dellinger RP, Parrillo JE,
Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S,
Hollenberg SM, et al: Early microcirculatory perfusion derangements
in patients with severe sepsis and septic shock: Relationship to
hemodynamics, oxygen transport, and survival. Ann Emerg Med.
49:88–98, 98 e81-e82. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fang Y, Li C, Shao R, Yu H and Zhang Q:
The role of biomarkers of endothelial activation in predicting
morbidity and mortality in patients with severe sepsis and septic
shock in intensive care: A prospective observational study. Thromb
Res. 171:149–154. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gill SE, Rohan M and Mehta S: Role of
pulmonary microvascular endothelial cell apoptosis in murine
sepsis-induced lung injury in vivo. Respir Res.
16(109)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Scarlatescu E, Tomescu D and Arama SS:
Anticoagulant therapy in sepsis. The importance of timing. J Crit
Care Med (Targu Mures). 3:63–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Haas LEM, Termorshuizen F, de Lange DW,
van Dijk D and de Keizer NF: Performance of the quick SOFA in very
old ICU patients admitted with sepsis. Acta Anaesthesiol Scand.
64:508–516. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang Q, Wu Z, Chi C, Wu C, Su L, Zhang Y,
Zhu J and Liu Y: Angiopoietin-2 is an early predictor for acute
gastrointestinal injury and intestinal barrier dysfunction in
patients with acute pancreatitis. Dig Dis Sci. 66:114–120.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Buddingh KT, Koudstaal LG, van Santvoort
HC, Besselink MG, Timmer R, Rosman C, van Goor H, Nijmeijer RM,
Gooszen H, Leuvenink HG, et al: Early angiopoietin-2 levels after
onset predict the advent of severe pancreatitis, multiple organ
failure, and infectious complications in patients with acute
pancreatitis. J Am Coll Surg. 218:26–32. 2014.PubMed/NCBI View Article : Google Scholar
|