Introduction
Acute lung injury (ALI) is a clinically severe
respiratory disorder, which is characterized by pulmonary edema,
diffuse alveolar damage, intrapulmonary hemorrhage and impaired gas
exchange. Notably, ALI may progress to its most severe form, acute
respiratory distress syndrome (ARDS) (1-3).
There are numerous pathogenic factors in ALI, including
pancreatitis, pneumonia, sepsis, aspiration of gastric contents and
inhalation of injurious gases (4).
Despite the advances in clinical treatment and management, ALI
remains a major cause of mortality worldwide (5); therefore, the development of
preventative and therapeutic measures for ALI is imperative.
Rutaecarpine (RUT) is a major quinazolino carboline
alkaloid compound from the dry unripe fruit (referred to as
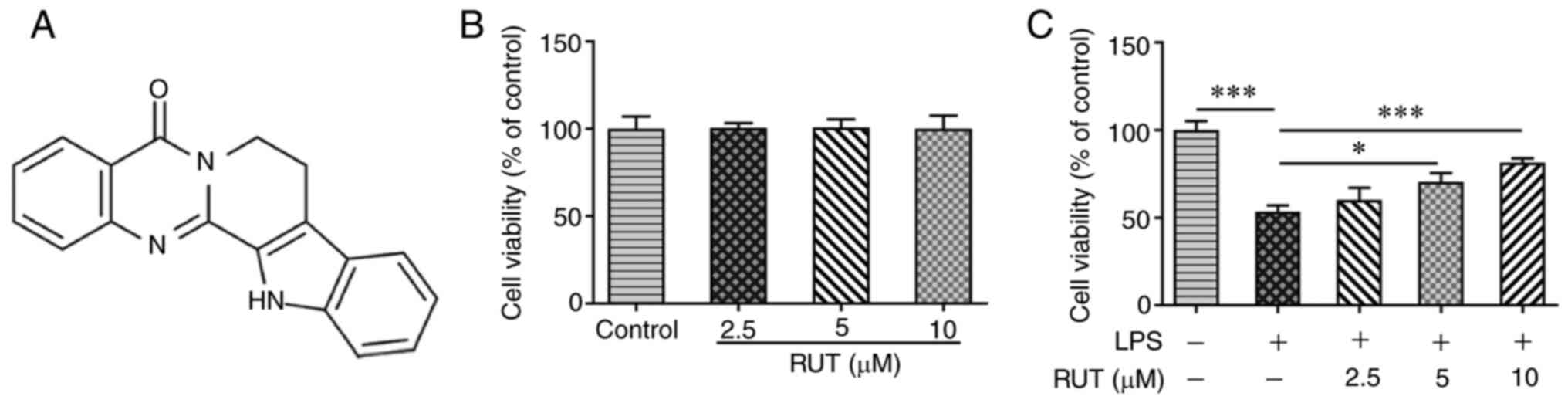
‘Wu-Chu-Yu’ in China) of Tetradium ruticarpum (Fig. 1A) (6). A pharmacological study previously
demonstrated that RUT has a variety of biological and
pharmacological effects, such as anti-inflammatory, antioxidant,
cardiovascular protective and brain function recovery effects
(7). Recently, Ren et al
(8) reported that RUT alleviated
ethanol-induced gastric damage, the ulcer index and
histopathological damage. Additionally, RUT was shown to suppress
the expression and nuclear translocation of NF-κB p65, and the
expression levels of TNF-α, IL-6, IL-1β and myeloperoxidase
(8). Another study revealed that
RUT may reduce H2O2-induced intracellular
reactive oxygen species accumulation and ameliorate dextran sulfate
sodium-induced inflammatory bowel disease by increasing nuclear
factor-erythroid factor 2-related factor 2 (NRF2) nuclear
translocation (9), thus suggesting
that RUT may serve a protective role in inflammatory diseases. It
has been documented that RUT alleviates hyperlipidemia and
hyperglycemia in fat-fed, streptozotocin-treated rats via
regulation of insulin receptor substrate 1/PI3K/Akt, and promotion
of the phosphorylation of AMP-activated protein kinase (AMPK) and
acetyl-CoA carboxylase 2(10). In
addition, Wang et al (11)
reported that dexmedetomidine ameliorated sepsis-induced lung
injury and reduced inflammatory cytokine expression and apoptosis
by activating the AMPK/sirtuin 1 (SIRT1) signaling pathway.
However, to the best of our knowledge, the biological role of RUT
in ALI remains incompletely understood.
The present study aimed to investigate the role of
RUT in LPS-induced ALI and to explore the potential underlying
molecular mechanism through the AMPK/SIRT1 signaling pathway.
Materials and methods
Cell culture and treatment
BEAS-2B human bronchial epithelial cells were
obtained from American Type Culture Collection, and were cultured
in DMEM containing 10% FBS (both from Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(MilliporeSigma) in a humidified atmosphere containing 5%
CO2 at 37˚C. Subsequently, the cells were pretreated
with RUT (2.5, 5 and 10 µM; MedChemExpress) for 24 h at 37˚C
(9,12) and then incubated with LPS (2 µg/ml;
Sigma-Aldrich; Merck KGaA) at 37˚C for a further 24 h. In addition,
compound C (20 µM; Sigma-Aldrich, Merck KGaA) was used as an
inhibitor of the AMPK signaling pathway to treat cells prior to LPS
or RUT treatment for 24 h at 37˚C.
Cell viability assay
BEAS-2B cells were seeded into a 96-well plate at a
density of 5x103 cells/well and cultured for 24 h. RUT
(2.5, 5 and 10 µM) was administered to pretreat BEAS-2B cells
before LPS stimulation. After 24 h of LPS treatment, 10 µl Cell
Counting Kit-8 (CCK-8) solution (Beyotime Institute of
Biotechnology) was added to each well and the plates were incubated
at 37˚C for 2 h. The absorbance at 450 nm was measured using a
microplate reader (Bio-Rad Laboratories, Inc.).
ELISA
The levels of TNF-α, IL-1β and IL-6 in BEAS-2B cells
were determined using TNF-α assay kit (cat. no. H052-1), IL-1β
assay kit (cat. no. H002) and IL-6 assay kit (cat. no. H007-1-1)
(all from Nanjing Jiancheng Bioengineering Institute) according to
the manufacturer's instructions. The absorbance at 450 nm was
detected using a microplate reader (Bio-Rad Laboratories, Inc.).
Six parallel wells were set for ELISAs.
Determination of malondialdehyde
(MDA), superoxide dismutase (SOD) and glutathione peroxidase
(GSH-Px) levels
The MDA content, and the activities of SOD and
GSH-Px in BEAS-2B cells were evaluated using MDA assay kit (cat.
no. A003-1-2), SOD assay kit (cat. no. A001-3-2) and GSH-Px assay
kit (cat. no. A005-1-2) (all from Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's protocols.
TUNEL assay
The TUNEL assay was performed to assess cell
apoptosis. Briefly, BEAS-2B cells were fixed with 4%
paraformaldehyde for 15 min at room temperature and stained using a
TUNEL kit for 1 h at 37˚C, followed by counterstaining of the
nuclei with 10 µg/ml DAPI at 37˚C for 2-3 min. The cells were then
mounted using an anti-fade reagent (Beijing Solarbio Science &
Technology Co., Ltd.). The labeled BEAS-2B cells were observed from
three random fields under a fluorescence microscope (magnification,
x200; Nikon Eclipse80i; Nikon Corporation).
Western blot analysis
Total proteins were extracted from BEAS-2B cells
using RIPA buffer (Beyotime Institute of Biotechnology) and the
protein concentration was measured using a BCA Protein Assay Kit
(Beijing Dingguo Changsheng Biotechnology Co., Ltd.). An equal
amount of protein (30 µg/lane) was separated by SDS-PAGE on 10%
gels and then transferred to a PVDF membrane (MilliporeSigma).
After blocking with 5% skimmed milk for 1 h at room temperature,
the membranes were probed at 4˚C overnight with the following
primary antibodies: Anti-Bcl-2 (dilution, 1:1,000; cat. no.
ab32124; Abcam), anti-Bax (dilution, 1:1,000; cat. no. ab32503;
Abcam), anti-cleaved caspase-3 (dilution, 1:500; cat. no. ab32042;
Abcam), anti-cleaved caspase-9 (dilution, 1:1,000; cat. no. 20750;
Cell Signaling Technology, Inc.), anti-CHOP (dilution, 1:1,000;
cat. no. 2895; Cell Signaling Technology, Inc.), anti-glucose
regulated protein-78 (GRP78; dilution, 1:1,000; cat. no. ab108615;
Abcam), anti-caspase-12 (dilution, 1:1,000; cat. no. ab62484;
Abcam), anti-activating transcription factor 6 (ATF6; dilution,
1:1,000; cat. no. ab227830; Abcam), anti-phosphorylated-(p-)AMPK
(dilution, 1:1,000; cat. no. ab92701; Abcam), anti-p-SIRT1
(dilution, 1:1,000; cat. no. ab76039; Abcam), anti-AMPK (dilution,
1:1,000; cat. no. ab32047; Abcam), anti-SIRT1 (dilution, 1:1,000;
cat. no. ab189494; Abcam) and anti-GAPDH (dilution, 1:2,500; cat.
no. ab9485; Abcam). The membranes were then incubated with
HRP-conjugated goat anti-rabbit or goat anti-mouse IgG secondary
antibodies (dilution, 1:2,000; cat. nos. #7074 and #7076; Cell
Signaling Technology, Inc.) at room temperature for 1 h. Finally,
the protein bands were visualized using an ECL detection system
(Beyotime Institute of Biotechnology) and the bands were
semi-quantified using ImageJ software 1.8.0 (National Institutes of
Health).
Statistical analysis
SPSS 22.0 software (IBM Corp.) and GraphPad Prism 6
software (GraphPad Software, Inc.) were used to analyze the data
from three independent experimental repeats. All results are
presented as the mean ± SD. Differences among multiple groups were
analyzed by one-way ANOVA followed by a Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RUT restores the viability of
LPS-induced BEAS-2B cells
To explore the effects of RUT on lung epithelial
cells, the present study first examined the effects of RUT on
BEAS-2B cell viability. The results of the CCK-8 assay demonstrated
that 2.5-10 µM RUT had no significant effects on BEAS-2B cell
viability (Fig. 1B). In addition,
LPS treatment markedly inhibited the viability of BEAS-2B cells,
whereas RUT restored cell viability in a dose-dependent manner
(Fig. 1C). These results indicated
that RUT reversed the negative effect of LPS treatment on BEAS-2B
cell viability.
RUT inhibits the inflammatory
response, oxidative stress and apoptosis in LPS-induced BEAS-2B
cells
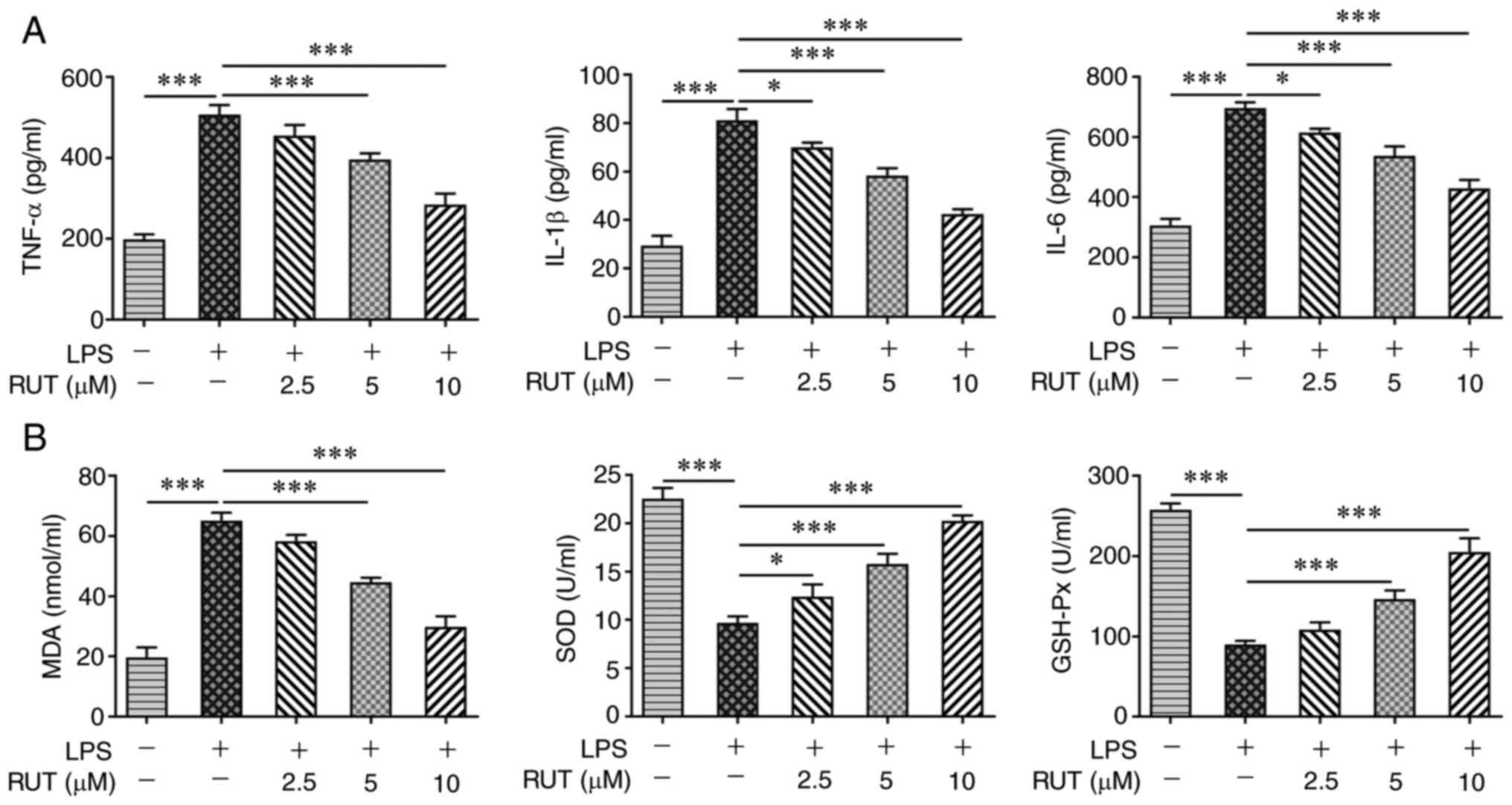
The effects of RUT on the inflammatory response,
oxidative stress and apoptosis in BEAS-2B cells induced by LPS were
further investigated. As shown in Fig.
2A, a notable increase in the production of TNF-α, IL-1β and
IL-6 was observed in LPS-induced cells compared with in the control
cells. However, RUT treatment reduced the LPS-induced elevated
levels of TNF-α, IL-1β and IL-6 in BEAS-2B cells. Furthermore, LPS
enhanced the MDA levels, but inhibited the activities of SOD and
GSH-Px, whereas RUT reversed the effects of LPS on MDA, SOD and
GSH-Px, indicating the suppressive effect of RUT on oxidative
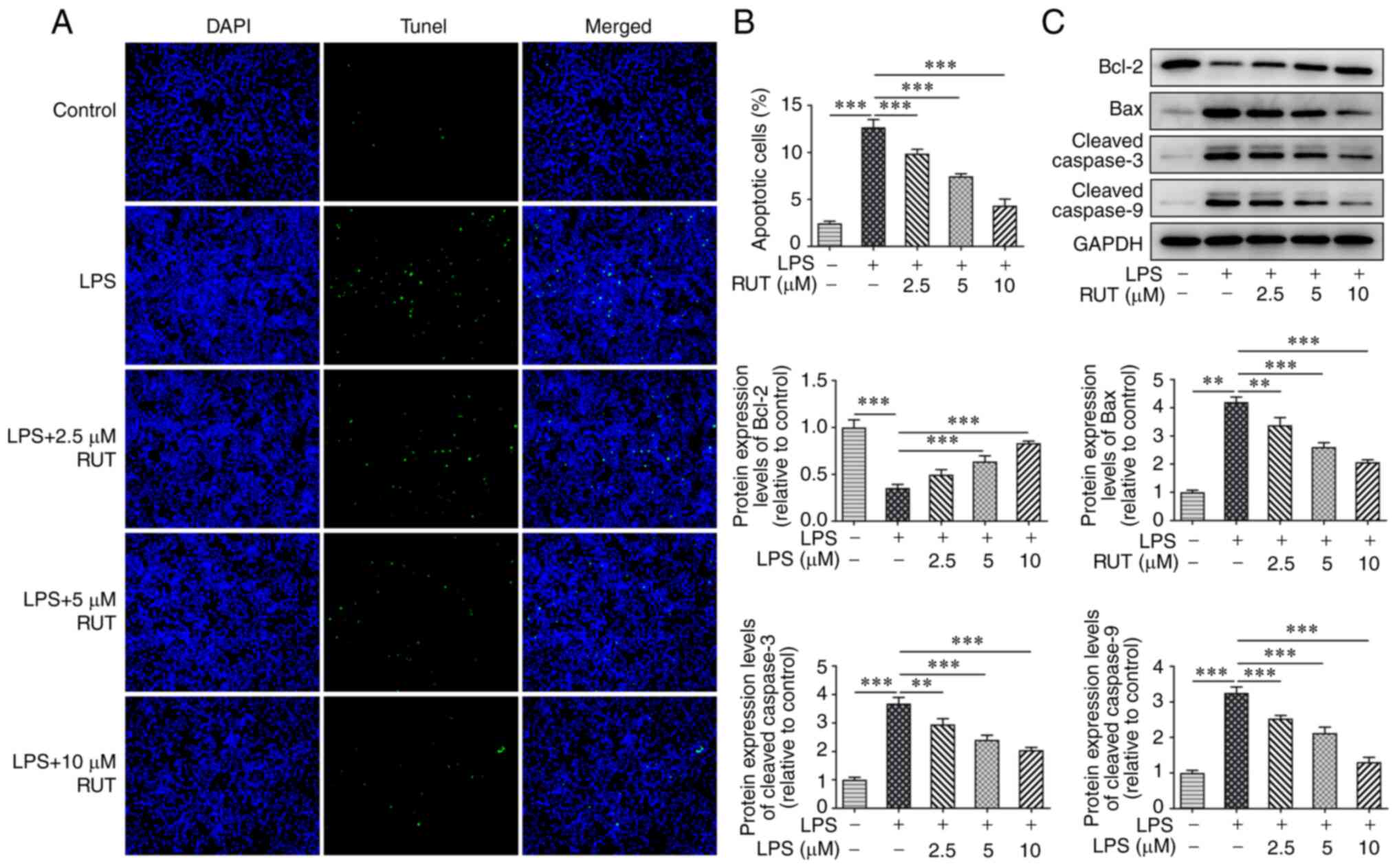
stress in LPS-induced BEAS-2B cells (Fig. 2B). Furthermore, the cell apoptotic
rate was significantly increased in the LPS group compared with in
the control group, and RUT pretreatment suppressed cell apoptosis
under LPS stimulation in a dose-dependent manner (Fig. 3A and B). Western blot analysis further
demonstrated that the protein expression levels of Bcl-2 were
decreased, whereas the protein expression levels of Bax, cleaved
caspase-3 and cleaved caspase-9 were markedly increased in BEAS-2B
cells following stimulation with LPS, whereas the opposite trends
in the expression levels of these proteins were observed following
pretreatment with RUT (Fig. 3C).
These data suggested that RUT pretreatment suppressed the
LPS-induced inflammation, oxidative stress and apoptosis of BEAS-2B
cells.
RUT reduces endoplasmic reticulum (ER)
stress in LPS-induced BEAS-2B cells
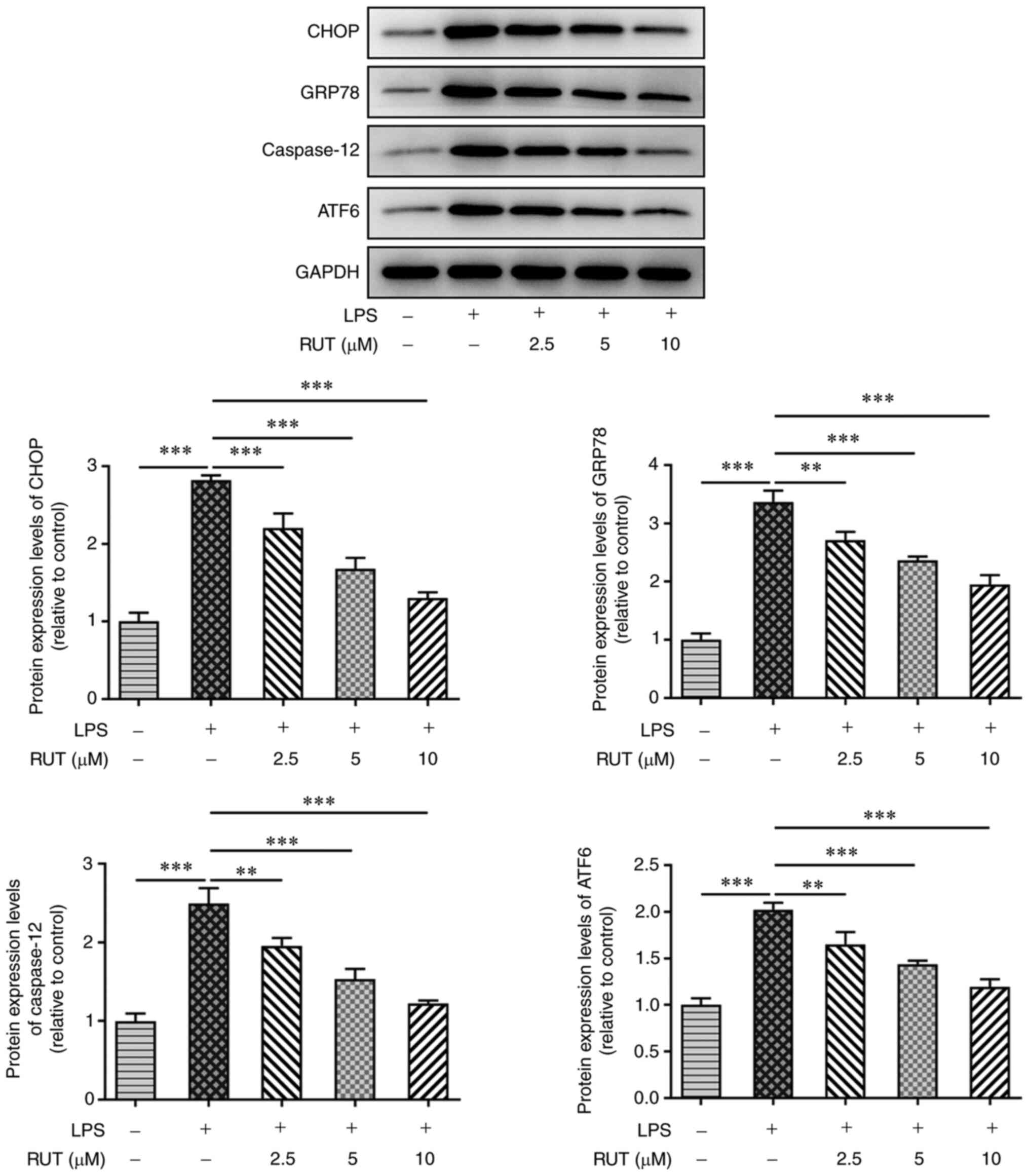
The present study explored the potential mechanisms
underlying the protective effects of RUT on LPS-exposed BEAS-2B
cells. As shown in Fig. 4, western
blot analysis revealed that LPS significantly promoted the
expression levels of ER stress-related proteins, including CHOP,
GRP78, caspase-12 and ATF6. However, the expression levels of these
proteins were dose-dependently reduced by treatment with different
concentrations of RUT. These results indicated that RUT
pretreatment had an inhibitory effect on LPS-induced ER stress in
BEAS-2B cells.
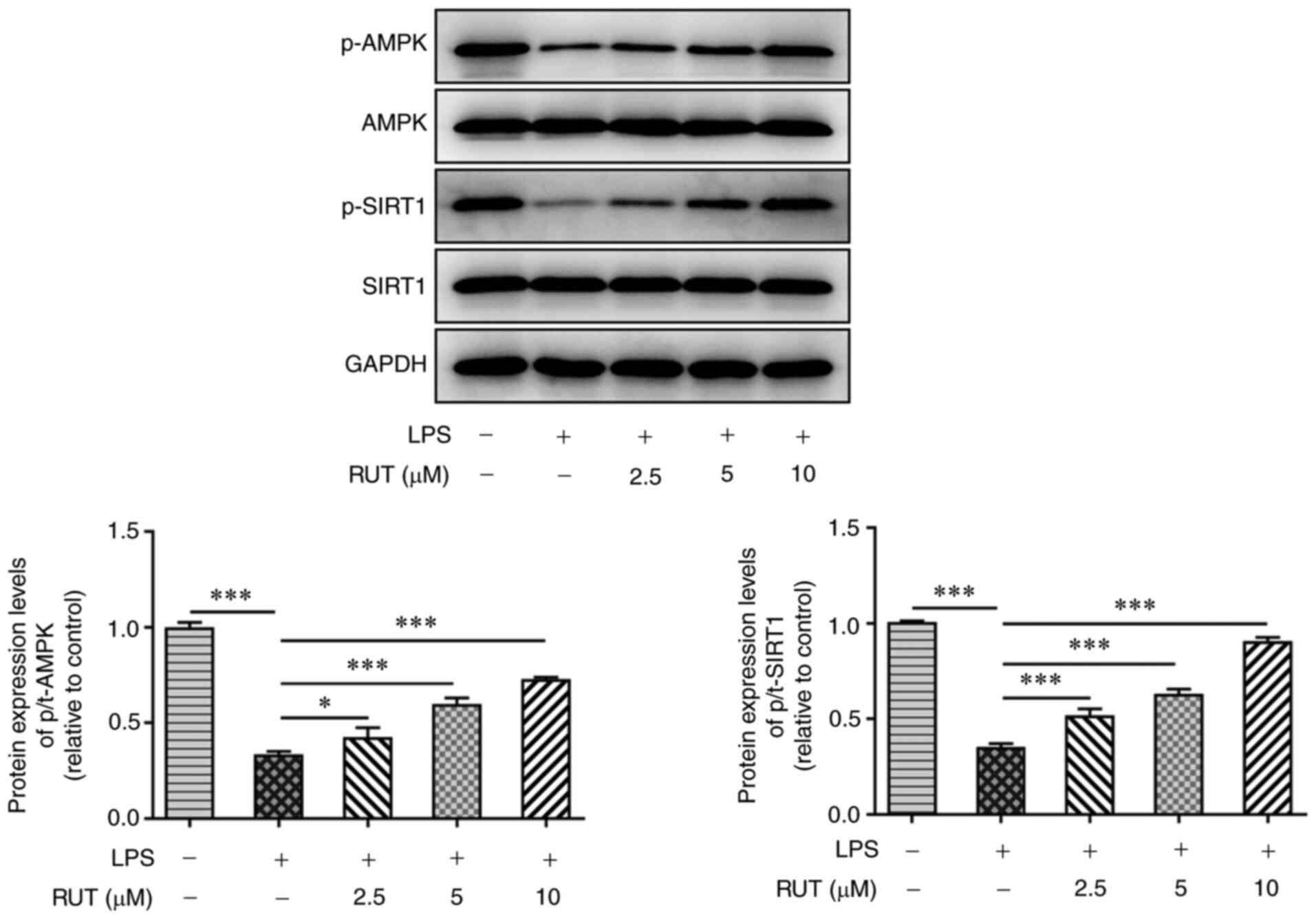
RUT activates the AMPK/SIRT1 signaling
pathway
To investigate the molecular mechanism underlying
the effects of RUT on LPS-treated cells, the expression levels of
proteins involved in the AMPK/SIRT1 signaling pathway were detected
by western blotting. As shown in Fig.
5, LPS stimulation markedly inhibited the phosphorylation of
AMPK and SIRT1; however, different doses of RUT dose-dependently
increased the protein expression levels of p-AMPK and p-SIRT1.
Additionally, the total protein levels of both AMPK and SIRT1 were
not significantly different among the groups. These results
indicated that RUT may activate the AMPK/SIRT1 signaling pathway in
ALI.
RUT alleviates damage and ER stress by
activating the AMPK/SIRT1 signaling pathway in LPS-induced BEAS-2B
cells
To identify the role of AMPK/SIRT1 signaling in
RUT-treated BEAS-2B cells, the AMPK inhibitor compound C was used.
As shown in Fig. 6A, pretreatment
with compound C (20 µM) markedly enhanced the levels of TNF-α,
IL-1β and IL-6 in BEAS-2B cells co-treated with RUT and LPS. In
addition, compound C increased the levels of MDA, and reduced the
activities of SOD and GSH-Px compared with those in the RUT + LPS
group (Fig. 6B). Additionally, the
decreased percentage of apoptotic cells was enhanced by compound C,
and the protein expression levels of Bcl-2, Bax, cleaved caspase-3
and cleaved caspase-9 were reversed by compound C in BEAS-2B cells
co-treated with RUT and LPS (Fig.
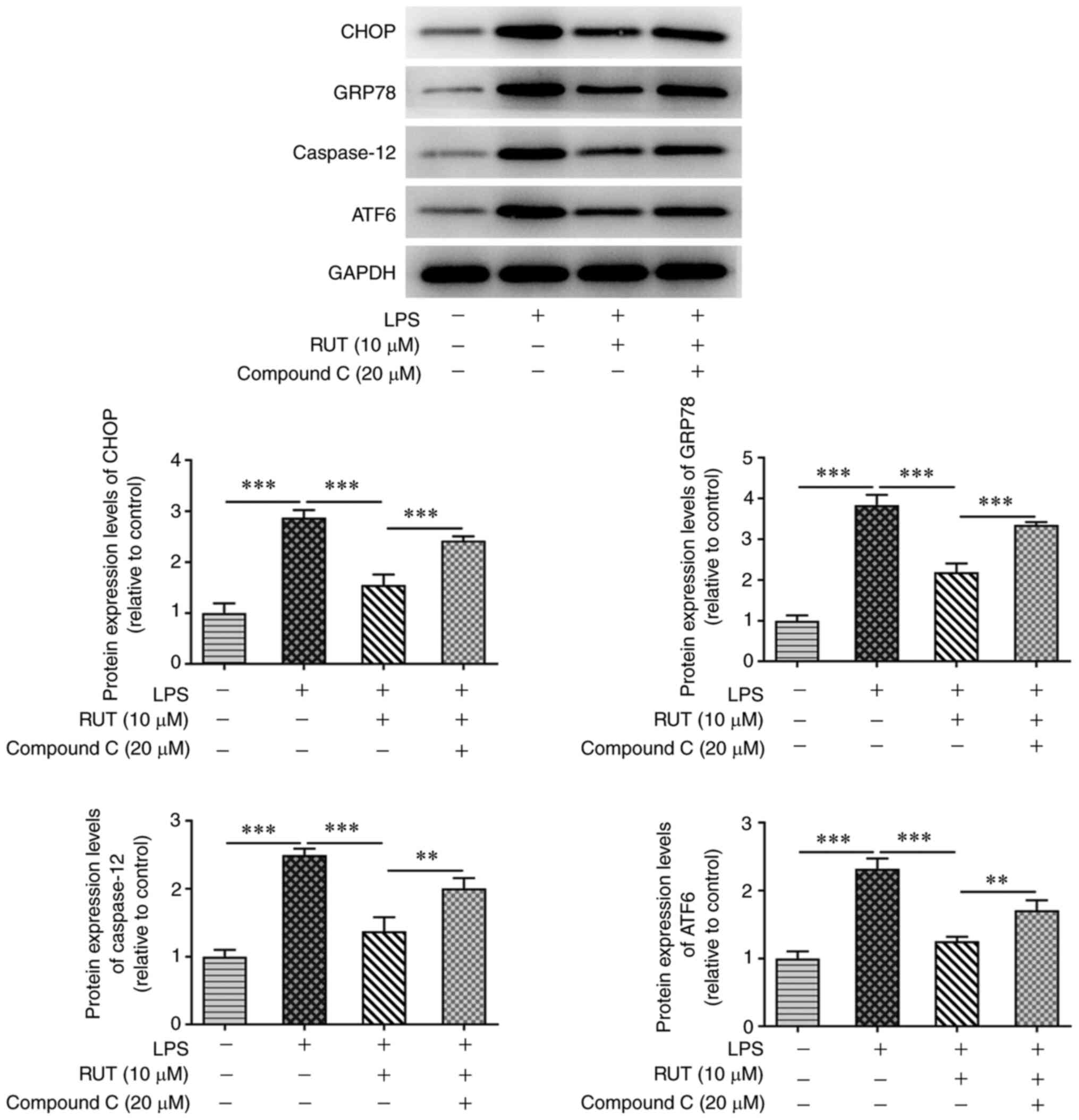
6C-E). Furthermore, compound C markedly exacerbated the ER
stress process by increasing the expression levels of CHOP, GRP78,
caspase-12 and ATF6 in BEAS-2B cells co-treated by RUT and LPS
(Fig. 7). These data suggested
that RUT suppressed LPS-induced cell injury and ER stress via
activation of the AMPK/SIRT1 signaling pathway in BEAS-2B
cells.
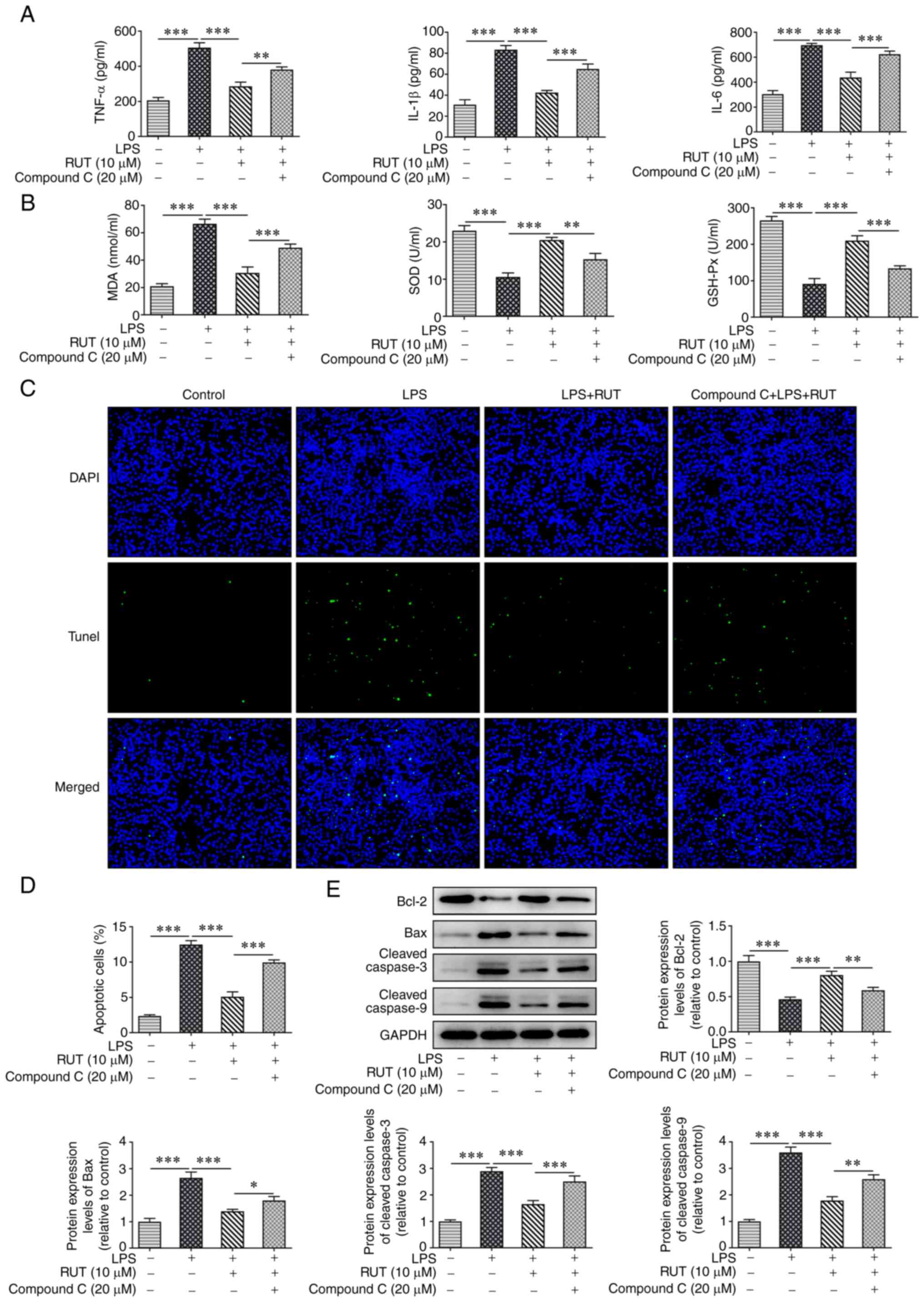 | Figure 6RUT protects against LPS-induced
BEAS-2B cell damage by activating the AMPK/SIRT1 signaling pathway.
(A) Levels of TNF-α, IL-1β and IL-6 in RUT-treated cells induced by
LPS with or without compound C. (B) MDA levels, and the activities
of SOD and GSH-Px in RUT-treated cells induced by LPS with or
without compound C. (C and D) Apoptosis was detected by TUNEL
assay. Magnification, x200. (E) Protein expression levels of Bcl-2,
Bax, cleaved caspase-3 and cleaved caspase-9 were measured by
western blotting. Results are presented as the mean ± SD.
*P<0.05, **P<0.01,
***P<0.001. GSH-Px, glutathione peroxidase; LPS,
lipopolysaccharide; MDA, malondialdehyde; RUT, rutaecarpine; SOD,
superoxide dismutase. |
Discussion
ALI is the direct cause of ARDS, which has a heavy
medical burden on individuals and society (13). It is well known that inflammatory
factors, such as IL-6, IL-1β and TNF-α, are closely associated with
ALI and ARDS (14). Despite the
high morbidity and mortality rates of lung injury in humans, there
are few effective treatments for ALI (15). The present study provided evidence
indicating that RUT exerted protective effects against LPS-induced
lung injury through the improvement of cell viability, and the
inhibition of the inflammatory response and oxidative stress in
BEAS-2B lung epithelial cells subjected to LPS treatment. The
present data also demonstrated that RUT diminished LPS-triggered
apoptosis and ER stress. In addition, mechanistic investigation
revealed that RUT attenuated LPS-induced lung injury by activating
the AMPK/SIRT1 signaling pathway.
Traditional Chinese medicine (TCM) has been
developed and used for thousands of years to treat a variety of
human diseases, including lung injury (16,17).
Numerous bioactive ingredients used in TCM have exhibited
anti-inflammatory, anti-apoptotic and antioxidant effects on lung
injury (18,19). For example, Lu et al
(20) reported that forsythoside A
ameliorated ALI pathological damage, and inhibited the generation
of inflammatory cytokines and the activation of STAT3 to prevent
LPS-induced ALI. Additionally, Wang and Xiao (21) demonstrated that isochlorogenic acid
A attenuated LPS-induced ALI via inhibition of lung active markers
and inflammatory factors via the NF-κB/NLR family pyrin
domain-containing 3 signaling pathway. RUT is an alkaloid isolated
from Tetradium ruticarpum, which has been reported to exert
antioxidant and anti-inflammatory effects (22,23).
It has been reported that RUT suppresses cerebral
ischemia/reperfusion (CI/R)-induced neuronal damage in a
dose-dependent manner, and can alleviate CI/R-induced apoptosis,
inflammatory response and oxidative stress (24). Another study revealed that
pretreatment with RUT markedly mitigated pancreatic inflammatory
damage and increased the serum levels of the anti-inflammatory
cytokine IL-10, whereas it reduced the concentrations of the
pro-inflammatory cytokines IL-6 and TNF-α (23). Consistently, the present study
revealed that RUT markedly increased the viability of BEAS-2B cells
treated with LPS, and reduced the inflammatory response by
suppressing the production of TNF-α, IL-1β and IL-6. Furthermore,
LPS-induced oxidative stress and apoptosis were observed to be
inhibited following treatment with different doses of RUT. These
data indicated the protective role of RUT against LPS-induced ALI
through antioxidant, anti-inflammatory and anti-apoptotic
effects.
ER stress may induce physical dysfunction of the ER
and lead to pathological imbalance in ER homeostasis (25). Accumulating evidence has indicated
that ER stress is closely implicated in the occurrence and
development of ALI (26,27). Du et al (28) revealed that pirfenidone reduced the
LPS-induced apoptosis of alveolar epithelial type II cells via the
inhibition of ER stress and mitochondrial injury. Bi et al
(29) demonstrated that helix B
surface peptide reduced the levels of inflammatory factors in lung
tissues, and suppressed oxidative stress and ER stress in lung
epithelial cells by activating the NRF2/heme oxygenase-1 signaling
pathway. In addition, Li et al (30) revealed that RUT ameliorated
sepsis-induced apoptosis and the inflammatory response in
peritoneal resident macrophages by inhibiting the ER
stress-mediated caspase-12 and NF-κB signaling pathways. In the
present study, LPS treatment markedly induced the abnormal
production of ER stress-related proteins; however, RUT suppressed
LPS-induced ER stress of BEAS-2B cells by hindering the protein
expression levels of CHOP, GRP78, caspase-12 and ATF6, which was
consistent with the aforementioned findings.
It has been reported that RUT may prevent
endothelial dysfunction and benefit cardiovascular health by
promoting nitric oxide synthesis and endothelial nitric oxide
synthase phosphorylation via the
calmodulin/Ca2+/calmodulin-dependent protein kinase
kinase β/AMPK signaling pathways, which indicates that RUT could
activate the AMPK signaling pathway in certain pathological
conditions (31). AMPK/SIRT1
signaling is considered to be a crucial pathway that participates
in the regulation of ALI (32,33).
A previous study demonstrated that irisin alleviated pulmonary
epithelial barrier dysfunction in sepsis-induced ALI by suppressing
inflammation and apoptosis via activation of the AMPK/SIRT1
signaling pathways (34). The
present study revealed that the addition of the AMPK inhibitor
compound C markedly reversed the effects of RUT on inflammatory
factors, oxidative stress and apoptosis, and even ER stress levels
in LPS-induced BEAS-2B cells, suggesting that the AMPK/SIRT1
signaling pathway may be involved in the protective effect of RUT
against LPS-induced lung injury.
In conclusion, the present study indicated that RUT
ameliorated LPS-induced lung cell injury by inhibiting
inflammation, oxidative stress, apoptosis and ER stress by
activating AMPK/SIRT1 signaling. These results provide evidence
that RUT may be considered a functional therapeutic agent for
patients with ALI. However, the present study also had limitations.
For example, the effects of RUT on ALI in vivo have not been
assessed. Additionally, considering that the activation of
Toll-like receptors is involved in the inflammatory response
following ALI (35), the effect of
RUT on the regulation of Toll-like receptors remains unclear and
will be explored in future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ, HZ and KZ conceived and designed the
experimental study. XZ and YD performed the experiments. BZ and WM
collected and interpreted the data. HZ and KZ drafted and FZ
revised the manuscript. HZ, XZ and BZ confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matthay MA, Ware LB and Zimmerman GA: The
acute respiratory distress syndrome. J Clin Invest. 122:2731–2740.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Abedi F, Hayes AW, Reiter R and Karimi G:
Acute lung injury: The therapeutic role of Rho kinase inhibitors.
Pharmacol Res. 155(104736)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yan J, Wang A, Cao J and Chen L:
Apelin/APJ system: An emerging therapeutic target for respiratory
diseases. Cell Mol Life Sci. 77:2919–2930. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Standiford TJ and Ward PA: Therapeutic
targeting of acute lung injury and acute respiratory distress
syndrome. Transl Res. 167:183–191. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chambers ED, White A, Vang A, Wang Z,
Ayala A, Weng T, Blackburn M, Choudhary G, Rounds S and Lu Q:
Blockade of equilibrative nucleoside transporter 1/2 protects
against pseudomonas aeruginosa-induced acute lung injury and NLRP3
inflammasome activation. FASEB J. 34:1516–1531. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao Z, Gong S, Wang S and Ma C: Effect
and mechanism of evodiamine against ethanol-induced gastric ulcer
in mice by suppressing Rho/NF-кB pathway. Int Immunopharmacol.
28:588–595. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jia S and Hu C: Pharmacological effects of
rutaecarpine as a cardiovascular protective agent. Molecules.
15:1873–1881. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ren S, Wei Y, Wang R, Wei S, Wen J, Yang
T, Chen X, Wu S, Jing M, Li H, et al: Rutaecarpine ameliorates
ethanol-induced gastric mucosal injury in mice by modulating genes
related to inflammation, oxidative stress and apoptosis. Front
Pharmacol. 11(600295)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Y, Yan T, Sun D, Xie C, Wang T, Liu
X, Wang J, Wang Q, Luo Y, Wang P, et al: Rutaecarpine inhibits
KEAP1-NRF2 interaction to activate NRF2 and ameliorate dextran
sulfate sodium-induced colitis. Free Radic Biol Med. 148:33–41.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nie XQ, Chen HH, Zhang JY, Zhang YJ, Yang
JW, Pan HJ, Song WX, Murad F, He YQ and Bian K: Rutaecarpine
ameliorates hyperlipidemia and hyperglycemia in fat-fed,
streptozotocin-treated rats via regulating the IRS-1/PI3K/Akt and
AMPK/ACC2 signaling pathways. Acta Pharmacol Sin. 37:483–496.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang R, Xie Y, Qiu J and Chen J: The
effects of dexmedetomidine in a rat model of sepsis-induced lung
injury are mediated through the adenosine monophosphate-activated
protein kinase (AMPK)/silent information regulator 1 (SIRT1)
pathway. Med Sci Monit. 26(e919213)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bao MH, Dai W, Li YJ and Hu CP:
Rutaecarpine prevents hypoxia-reoxygenation-induced myocardial cell
apoptosis via inhibition of NADPH oxidases. Can J Physiol
Pharmacol. 89:177–186. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Luo X, Lin B, Gao Y, Lei X, Wang X, Li Y
and Li T: Genipin attenuates mitochondrial-dependent apoptosis,
endoplasmic reticulum stress, and inflammation via the PI3K/AKT
pathway in acute lung injury. Int Immunopharmacol.
76(105842)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie X, Sun S, Zhong W, Soromou LW, Zhou X,
Wei M, Ren Y and Ding Y: Zingerone attenuates
lipopolysaccharide-induced acute lung injury in mice. Int
Immunopharmacol. 19:103–109. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yeh CC, Lin CC, Wang SD, Chen YS, Su BH
and Kao ST: Protective and anti-inflammatory effect of a
traditional Chinese medicine, Xia-Bai-San, by modulating lung local
cytokine in a murine model of acute lung injury. Int
Immunopharmacol. 6:1506–1514. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Deng G, He H, Chen Z, OuYang L, Xiao X, Ge
J, Xiang B, Jiang S and Cheng S: Lianqinjiedu decoction attenuates
LPS-induced inflammation and acute lung injury in rats via
TLR4/NF-κB pathway. Biomed Pharmacother. 96:148–152.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fu PK, Wu CL, Tsai TH and Hsieh CL:
Anti-inflammatory and anticoagulative effects of paeonol on
LPS-induced acute lung injury in rats. Evid Based Complement
Alternat Med. 2012(837513)2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang KL, Chen CS, Hsu CW, Li MH, Chang H,
Tsai SH and Chu SJ: Therapeutic effects of baicalin on
lipopolysaccharide-induced acute lung injury in rats. Am J Chin
Med. 36:301–311. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lu Z, Yang H, Cao H, Huo C, Chen Y, Liu D,
Xie P, Zhou H, Liu J and Yu L: Forsythoside A protects against
lipopolysaccharide-induced acute lung injury through up-regulating
microRNA-124. Clin Sci (Lond). 134:2549–2563. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Q and Xiao L: Isochlorogenic acid A
attenuates acute lung injury induced by LPS via Nf-κB/NLRP3
signaling pathway. Am J Transl Res. 11:7018–7026. 2019.PubMed/NCBI
|
|
22
|
Jin SW, Hwang YP, Choi CY, Kim HG, Kim SJ,
Kim Y, Chung YC, Lee KJ, Jeong TC and Jeong HG: Protective effect
of rutaecarpine against t-BHP-induced hepatotoxicity by
upregulating antioxidant enzymes via the CaMKII-Akt and Nrf2/ARE
pathways. Food Chem Toxicol. 100:138–148. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan L, Li QF, Rong YT, Chen YH, Huang ZH,
Wang ZZ and Peng J: The protective effects of rutaecarpine on acute
pancreatitis. Oncol Lett. 15:3121–3126. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Han M, Hu L and Chen Y: Rutaecarpine may
improve neuronal injury, inhibits apoptosis, inflammation and
oxidative stress by regulating the expression of ERK1/2 and
Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion
injury. Drug Des Devel Ther. 13:2923–2931. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shumin C, Wei X, Yunfeng L, Jiangshui L,
Youguang G, Zhongqing C and Tao L: Genipin alleviates vascular
hyperpermeability following hemorrhagic shock by up-regulation of
SIRT3/autophagy. Cell Death Discov. 4(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khan MM, Yang WL, Brenner M, Bolognese AC
and Wang P: Cold-inducible RNA-binding protein (CIRP) causes
sepsis-associated acute lung injury via induction of endoplasmic
reticulum stress. Sci Rep. 7(41363)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hu R, Chen ZF, Yan J, Li QF, Huang Y, Xu
H, Zhang XP and Jiang H: Endoplasmic reticulum stress of
neutrophils is required for ischemia/reperfusion-induced acute lung
injury. J Immunol. 195:4802–4809. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Du Y, Zhu P, Wang X, Mu M, Li H, Gao Y,
Qin X, Wang Y, Zhang Z, Qu G, et al: Pirfenidone alleviates
lipopolysaccharide-induced lung injury by accentuating BAP31
regulation of ER stress and mitochondrial injury. J Autoimmun.
112(102464)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bi XG, Li ML, Xu W, You JY, Xie D, Yuan XF
and Xiang Y: Helix B surface peptide protects against acute lung
injury through reducing oxidative stress and endoplasmic reticulum
stress via activation of Nrf2/HO-1 signaling pathway. Eur Rev Med
Pharmacol Sci. 24:6919–6930. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Z, Yang M, Peng Y, Gao M and Yang B:
Rutaecarpine ameliorated sepsis-induced peritoneal resident
macrophages apoptosis and inflammation responses. Life Sci.
228:11–20. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee GH, Kim CY, Zheng C, Jin SW, Kim JY,
Lee SY, Kim MY, Han EH, Hwang YP and Jeong HG: Rutaecarpine
increases nitric oxide synthesis via eNOS phosphorylation by
TRPV1-dependent CaMKII and CaMKKβ/AMPK signaling pathway in human
endothelial cells. Int J Mol Sci. 22(9407)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
He Y, Xu K, Wang Y, Chao X, Xu B, Wu J,
Shen J, Ren W and Hu Y: AMPK as a potential pharmacological target
for alleviating LPS-induced acute lung injury partly via NLRC4
inflammasome pathway inhibition. Exp Gerontol.
125(110661)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang N, Li P, Lin H, Shuo T, Ping F, Su L
and Chen G: IL-10 ameliorates PM2.5-induced lung injury by
activating the AMPK/SIRT1/PGC-1α pathway. Environ Toxicol
Pharmacol. 86(103659)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li X, Jamal M, Guo P, Jin Z, Zheng F, Song
X, Zhan J and Wu H: Irisin alleviates pulmonary epithelial barrier
dysfunction in sepsis-induced acute lung injury via activation of
AMPK/SIRT1 pathways. Biomed Pharmacother.
118(109363)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen X, Wang T, Song L and Liu X:
Activation of multiple Toll-like receptors serves different roles
in sepsis-induced acute lung injury. Exp Ther Med. 18:443–450.
2019.PubMed/NCBI View Article : Google Scholar
|