Introduction
The immune system is made up of two parts: Innate
and acquired immunity. Innate immunity represents the first line of
host defense, and provides resistance against foreign, and
potentially harmful, pathogens or organisms (1). Macrophages are considered important
target cells for immunomodulatory agents among the components of
innate immunity, which include macrophages, monocytes and
granulocytes (2). Immune cell
activation is, in general, a direct and effective way to improve
immunity (3).
Natural polysaccharides have recently attracted a
lot of attention due to their low toxicity and potential
immunomodulatory properties. They activate immune cells either
indirectly or directly to produce immune effects (4). Soybean is the most important oil crop
worldwide and a source of high-protein food, which is widely
planted alongside rice, wheat and corn, as the four major economic
crops. Current research on soybeans has not only focused on
soybean-derived products, but also the development and utilization
of various food industries and functional food materials, such as
soy protein meat, isoflavones, lecithin and peptides, which have
the functions of regulating immunity, lowering cholesterol and
anti-oxidation (5). As a result,
there has been an increasing number of soybean by-products. Soybean
hulls, which are the seed coat of soybeans (~8%), are one of the
major by-products released during the initial cracking process in
the production of soybean oil. The vast majority are discarded or
used in animal feed (6). The
soybean hull contains variable amounts of cellulose (29-51%),
hemicellulose (10-25%), lignin (1-4%), pectins (4-8%) and proteins
(11-15%) (7). Soybean hulls are
considered a source of novel polysaccharides, which have been shown
to exert hypoglycemic and hypolipidemic effects (8). However, few reports have explored the
functional properties of these polysaccharides, especially in
immune regulation.
RAW264.7 murine macrophages are useful to study the
molecular mechanisms of macrophages in immune regulation (8) and the pattern recognition receptors
(PRRs) on the surface of immune cells, such as Toll-like receptors
(TLRs) that recognize pathogen-related molecular patterns (PAMPs).
As a type of PAMP, plant polysaccharides can activate macrophages
by specifically binding to their cell membrane receptors and can
subsequently activate the mitogen-activated protein kinase (MAPK)
and nuclear factor κB (NF-κB) pathways to generate an immune
response. In the present study, SHP was extracted, and three
fractions were separated. The three fractions were obtained and
designated F1, F2 and F3. Their effects on the proliferation and
pinocytosis, as well as nitric oxide (NO) and cytokine secretion,
of RAW264.7 cells were evaluated to study SHP immunomodulatory
activity and to clarify its underlying molecular mechanisms.
Materials and methods
Experimental material
Soybean hulls were purchased from Jinzhou Beiwang
Bean Products Co., Ltd. Lipopolysaccharide (LPS),
phosphate-buffered saline (PBS), trypsin solution and polymyxin B
(PMB) were purchased from Damao Chemical Reagent Factory. Roswell
Park Memorial Institute (RPMI)-1640 medium, Griess reagent, fetal
bovine serum (FBS) and penicillin were purchased from Qingdao
Haiyang Chemical Co. Ltd. Anti-TLR2 (cat. no. ab209216), anti-TLR4
(cat. no. ab22048), anti-Dectin-1 (DC; cat. no. ab169783) and
anti-mannose receptor (MR; cat. no. ab64693) antibodies used to
treat cells and inhibit PRRs were obtained from Abcam. Rabbit
monoclonal antibodies against phosphorylated (p)-Erk at
Thr202/Tyr204 (cat. no. 9101S), p-p38 at Thr180/Tyr182 (cat. no.
9211S), p-SAPK/JNK at Thr183/Tyr185 (cat. no. 4668T), p-p65 (cat.
no. 3033S), p38 MAPK (cat. no. 8690S), NF-κB p65 (cat. no. 8242S),
Erk1/2 (cat. no. 4695S), SAPK/JNK (cat. no. 9252S), α-tubulin (cat.
no. 2144S) and horseradish peroxidase-labeled secondary antibody
(cat. no. 7074P2) were purchased from Cell Signaling Technology,
Inc. All of the antibodies for western blotting were diluted in 5%
skim milk solution at a 1:2,000 dilution from a 1 mg/ml stock
solution.
Preparation of polysaccharides
SHP was extracted from the soybean hull (9). Briefly, dried soybean hull (20 g) was
treated with 85% ethanol (EtOH, 200 ml) overnight at 20˚C with
constant mechanical stirring. The residual part was separated by
centrifugation (10˚C, 1,500 x g, 10 min), rinsed in acetone and
dried at room temperature. The dried biomass (10 g) was extracted
with distilled water (200 ml) and stirred for 2 h at 65˚C. The
extracts were centrifuged at 3,000 x g for 10 min at room
temperature, and the supernatants were collected and concentrated
to ~200 ml using reduced pressure evaporation at 60˚C for 1 h. EtOH
(99%) was added to the supernatants to obtain a final concentration
of 70%, and the solution was incubated at 4˚C overnight. The
polysaccharide was obtained by filtration of the solution through a
membrane (0.45-µm pore size; Cytiva), was washed with EtOH (99%),
followed by acetone and then dried at room temperature for 24 h.
The precipitated polysaccharide was called the crude
polysaccharide, and the yield was calculated (weight of crude
extract/weight of seaweed powder) according to the dried biomass
obtained after treating the milled sample with 85% EtOH and drying
at room temperature for 24 h.
To obtain different fractions, SHP (200 mg) was
dissolved in 10 ml distilled water, filtered through an
ion-exchange chromatography system, equipped with a DEAE Sepharose
Fast Flow column (cat. no. 17-0709-01; Cytiva) and eluted with
distilled water to obtain a non-absorbed fraction F1. Subsequently,
a stepwise NaCl gradient (0.5-1 M) was used to wash the highly
charged anionic macromolecules, for which elution with a 0.5 M NaCl
gradient was performed to obtain fraction F2 and elution with a 1 M
NaCl gradient was performed to obtain fraction F3, and the unbound
samples was washed with 2 M NaCl gradient. The fractions were
obtained at a flow rate of 1.5 ml/min for 7 h. The
carbohydrate-positive fractions were pooled, concentrated, dialyzed
and lyophilized (9).
Chemical composition analysis
Using the phenol-sulfuric acid method and dextrose
as the standard, the total carbohydrate content of SHP was
quantified (10). With FBS as the
standard, the protein content was determined using a Bradford
method (11). The sulfate content
was determined using the BaCl2-gelatin method with
K2SO4 as the standard following SHP
hydrolysis using 0.5 M HCl (12).
The uronic acid content was determined using the
sulfamate/m-hydroxy diphenyl assay with glucuronic acid as the
standard (13).
Monosaccharide composition
analysis
The composition of SHP monosaccharides was
determined as previously described (14). SHP was hydrolyzed using 4 M
trifluoroacetic acid at 100˚C for 6 h, reduced in water using
NaBD4, acetylated with acetic anhydride. TFA was removed
by evaporation with a dried stream of nitrogen (nitrogen gas
temperature, 350˚C; nebuliser pressure, 40 psi; flow rate, 8
l/min). The hydrolysates were injected into the high-performance
liquid chromatography system that consisted of a pump (Waters 510;
Waters Corporation), an injection valve (Model 7010; Rheodyne) with
a 20-l sample loop, a column (carbohydrate analysis column, 4.6x250
mm; Waters Corporation) and a refractive index detector (Waters
2414; Waters Corporation). A mixture of acetonitrile and water
(80:20, v/v) was used as a mobile phase at a flow rate of 2 ml/min.
The following neutral monosaccharides were used as references:
Rhamnose, xylose, mannose, galactose, and glucose.
Cell line and cell culture
The murine macrophage cell line RAW264.7 was
obtained from the Cell Bank of Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences. RAW264.7 macrophages were
cultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. The cells were incubated at
37˚C and 5% CO2 (15).
NO production and cell proliferation
analysis
RAW264.7 cells were seeded in 96-well microplates
(1x106 cells/ml) and cultured in a CO2
incubator. After 24 h, the medium was removed and replaced by 100
µl culture medium containing different concentrations (25, 50 and
100 µg/ml) of SHP (Crude, F1, F2 and F3) or LPS (1 µg/ml); cells
treated with RPMI were used as the negative control group and LPS
as the positive control group. After an additional 24 h of culture,
the cell NO production and cell proliferation were determined using
the Griess reaction and water-soluble tetrazolium-1 (WST-1) assays
(cat. no. ab65475; Abcam), respectively, according to the
manufacturer's instructions. The optical density was measured using
a microplate reader (EL-800; BioTek Instruments, Inc.) (16).
Endotoxin contamination and reactive
oxygen species (ROS) analyses
RAW264.7 cells were seeded in 96-well microplates
(1x105 cells/ml) and cultured in a CO2
incubator. After 24 h, the medium was removed and replaced with 100
µl medium containing SHP (100 µg/ml) or LPS (1 µg/ml); cells
treated with RPMI were used as the negative control group and LPS
as the positive control group, in the presence or absence of PMB
(50 µg/ml) to assess endotoxin contamination. After an additional
24 h of culture, the NO content of the supernatant was measured as
aforementioned (17).
RAW264.7 cells were seeded in a 96-well microplate
(1x105 cells/ml) and cultured in a CO2
incubator. After 24 h, the medium was removed and replaced with 100
µl medium containing different concentrations (25, 50 and 100
µg/ml) of SHP or LPS (1 µg/ml); cells treated with RPMI were used
as the control group. After an additional 24 h of culture, all
media were removed and 100 µl 2',7'-dichlorofluorescin diacetate
(10 µM) was added, and the cells were incubated in the dark for 20
min at 37˚C. The supernatant was removed and the cells were washed
three times with PBS. Fluorescence intensity was immediately
detected and recorded at 488 nm excitation and 525 nm emission
using an Infinite M200 Pro microplate reader (Tecan Group, Ltd.)
(18).
Pinocytic activity analysis
RAW264.7 cells were seeded in a 96-well microplate
(2x104 cells/ml). After 6 h of culture, the medium was
removed and replaced with 100 µl medium containing different
concentrations (25, 50 and 100 µg/ml) of SHP or LPS (1 µg/ml);
cells treated with RPMI were used as the negative control group and
LPS as the positive control group. After an additional 24 h of
culture, the medium was removed, 100 µl PBS containing 0.08%
neutral red (cat. no. ab146365; Abcam) was added, and the cell
plate was incubated for 1 h at 37˚C. Subsequently, the medium was
removed, the cells were washed three times with PBS and 100 µl cell
lysis buffer (acetic acid: ethanol=1:1) was added to each well. The
absorbance was recorded at 540 nm using a VersaMax microplate
reader (Molecular Devices) (19).
Quantitative analysis of mRNA
expression
RAW264.7 cells were seeded in a 24-well microplate
(1x106 cells/ml) and were cultured for 24 h in the
presence of SHP (100 µg/ml) or LPS (1 µg/ml); cells treated with
RPMI were used as the negative control group and LPS as the
positive control group. Total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and cDNA generation was carried out in a total volume of 10
µl, containing 5 µl RNA (100 ng/ml), 0.5 µl 10 pmol oligo-(dT)20
primer, 0.5 µl dNTP (10 mM) and 4 µl superscript III RT (Takara
Biotechnology, Co., Ltd.). The primers used are shown in Table I, and β-actin was used as the
internal standard. Quantitative PCR (qPCR) was performed using the
CFX connect Real-time system (Bio-Rad Laboratories, Inc.) with a
Fast Start DNA Master TB Green II kit (Takara Biotechnology Co.,
Ltd.), with the following program: 1 cycle of initial PCR
denaturation at 95˚C for 15 min, 2 cycles of primer annealing at
60˚C for 0.5 min, 32 cycles of denaturation at 95˚C for 0.5 min, 1
cycle of final extension at 60˚C for 1 min and 1 cycle of melting
curve analysis at 95˚C for 0.5 min. The results were calculated
using the 2-∆∆Cq method (20) and are expressed relative to
β-actin.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR analysis. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR analysis.
| Gene | Primer sequence
(5'-3') |
|---|
| TNF-α | F:
ATGAGCACAGAAAGCATGATC |
| | R:
TACAGGCTTGTCACTCGAATT |
| iNOS | F:
CCCTTCCGAAGTTTCTGGCAGCAGC |
| | R:
GGCTGTCAGAGCCTCGTGGCTTTGG |
| IL-1β | F:
ATGGCAACTATTCCAGAACTCAACT |
| | R:
CAGGACAGGTATAGATTCTTTCCTTT |
| IL-6 | F:
TTCCTCTCTGCAAGAGACT |
| | R:
TGTATCTCTCTGAAGGACT |
| IL-10 | F:
TACCTGGTAGAAGTGATGCC |
| | R:
CATCATGTATGCTTCTATGC |
| β-actin | F:
TGGAATCCTGTGGCATCCATGAAAC |
| | R:
TAAAACGCAGCTCAGTAACAGTCCG |
Inhibition of NO production using
antibodies against PRR
Cells were pretreated with medium containing
different antibodies against PRR (10 µg/ml) for 2 h at 37˚C, prior
to treatment with SHP-F2 (100 µg/ml) or LPS (1 µg/ml) for 24 h at
37˚C to stimulate the macrophages; cells treated with RPMI were
used as the negative control group and LPS as the positive control
group. The levels of NO in the supernatant were measured using the
aforementioned procedure (20).
Flow cytometry
RAW264.7 cells (2x106 cells/ml) were
cultured for 24 h in the presence of SHP (100 µg/ml) or LPS (1
µg/ml); cells treated with RPMI were used as the negative control
group and LPS as the positive control group. Subsequently, cells
were harvested and washed with FACS buffer containing 1% bovine
serum albumin and 0.1% sodium azide (Damao Chemical Reagent
Factory). Cells were then stained with 10 µg/ml anti-mouse CD40
conjugated with APC (cat. no. ab272271) and CD11b conjugated with
FITC (cat. no. ab24874) (both from Abcam) for 30 min at 4˚C
Parallel sets of cells were incubated without the antibodies and
their autofluorescence intensity served as a non-specific negative
control. A total of 100,000 viable cells per treatment (as
determined using light scatter profiles) were analyzed using a FACS
Symphony A5 flow cytometer (BD Biosciences) and CytExpert cytometry
software (version 1.0) (Beckman Coulter, Inc.).
Western blot analysis
RAW264.7 cells (2x06 cells/ml) were
cultured for 24 h in the presence of crude SHP or SHP fractions
(100 µg/ml), or LPS (1 µg/ml); cells treated with RPMI were used as
the negative control group and LPS as the positive control group.
The cells were then washed three times with PBS, and the protein
was extracted using lysis reagents [1 ml RIPA lysis buffer (cat.
no. ab156034) + 1 µl inhibitor cocktail + 1 µl EDTA; Abcam). The
protein content was measured using a BCA protein kit. The extracted
protein (30 µg) was loaded, separated by SDS-PAGE on a 10% gel, and
then transferred onto a PVDF membrane (0.22 µm; MilliporeSigma).
The membranes were incubated with primary antibodies at 4˚C
overnight after blocking with Blocking One (Nacalai Tesque, Inc.)
for 1 h at 4˚C. Finally, the membranes were incubated for 1 h at
room temperature with horseradish peroxidase-labeled secondary
antibodies. Antibodies for western blotting were diluted in 5% skim
milk solution at a 1:2,000 dilution from a 1 mg/ml stock solution.
The protein was detected using an ECL kit (Takara Bio, Inc.).
Statistical analysis
Excel 2016 (Microsoft Corporation) and SPSS software
(v19.0; IBM Corp.) were used for the statistical analysis of the
data, and Origin 8.0 (OriginLab) was used for generating the
related graphics. Data are presented as the mean ± standard
deviation from at least three independent experiments. Significant
differences were statistically analyzed by two-way analysis of
variance (ANOVA) or one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was used to indicate a statistically significant
difference.
Results and Discussion
Isolation and chemical composition
analysis of SHP
Soybean hull was extracted using hot water and crude
polysaccharides were obtained using ethanol precipitation (yield,
9.2%). Ion-exchange chromatography on a DEAE Sepharose Fast Flow
column was used for separation and purification to obtain three
fractions: F1 (distilled water), F2 (0.5 M NaCl) and F3 (1 M NaCl).
Chemical analysis of the soybean hull crude polysaccharides
revealed that they were mainly composed of carbohydrates (64.3%),
protein (16.2%) and sulfate (12.5%), as well as a small amount of
uronic acid (3.2%) (Table
II).
 | Table IIThe chemical compositions of
polysaccharides of soybean hull. |
Table II
The chemical compositions of
polysaccharides of soybean hull.
| | SHP |
|---|
| Components | Crude | F1 | F2 | F3 |
|---|
| Yield, % |
9.2±0.4a |
33.4±1.2b |
47.1±0.5c |
20.2±1.3d |
| Total carbohydrate,
% |
64.3±1.4d |
81.2±0.4c |
69.5±0.8b |
51.2±2.1a |
| Protein, % |
16.2±0.3c |
12.5±0.5b |
8.3±0.2a |
11.6±0.6d |
| Uronic acid, % |
3.2±0.5c |
2.3±0.1b |
1.6±0.3d |
3.4±0.2c |
| Sulfate, % |
12.5±0.8c |
11.6±1.2c |
8.6±0.4b |
7.2±0.6b |
| Monosaccharide
content, % | | | | |
|
Arabinose |
9.4±0.6b |
7.2±1.2d |
12.3±0.9c |
6.7±0.6d |
|
Glucose |
36.5±1.3b |
42.3±0.8c |
28.4±1.1d |
28.1±0.5d |
|
Galactose |
18.7±0.3c |
14.3±0.4d |
17.2±0.2b |
14.0±0.5d |
|
Mannose |
41.7±1.1c |
10.2±0.2a |
34.2±0.6b |
22.5±0.4d |
The monosaccharide composition of SHP, F1, F2 and F3
was determined using gas chromatography-mass spectrometry. The
crude polysaccharide and the fractions were primarily composed of
mannose (22.5-41.7%) and glucose (28.1-42.3%), whereas F1 was
primarily composed of glucose (42.3%), galactose (14.3%) and
mannose (10.2%) (Table II;
Fig. S1).
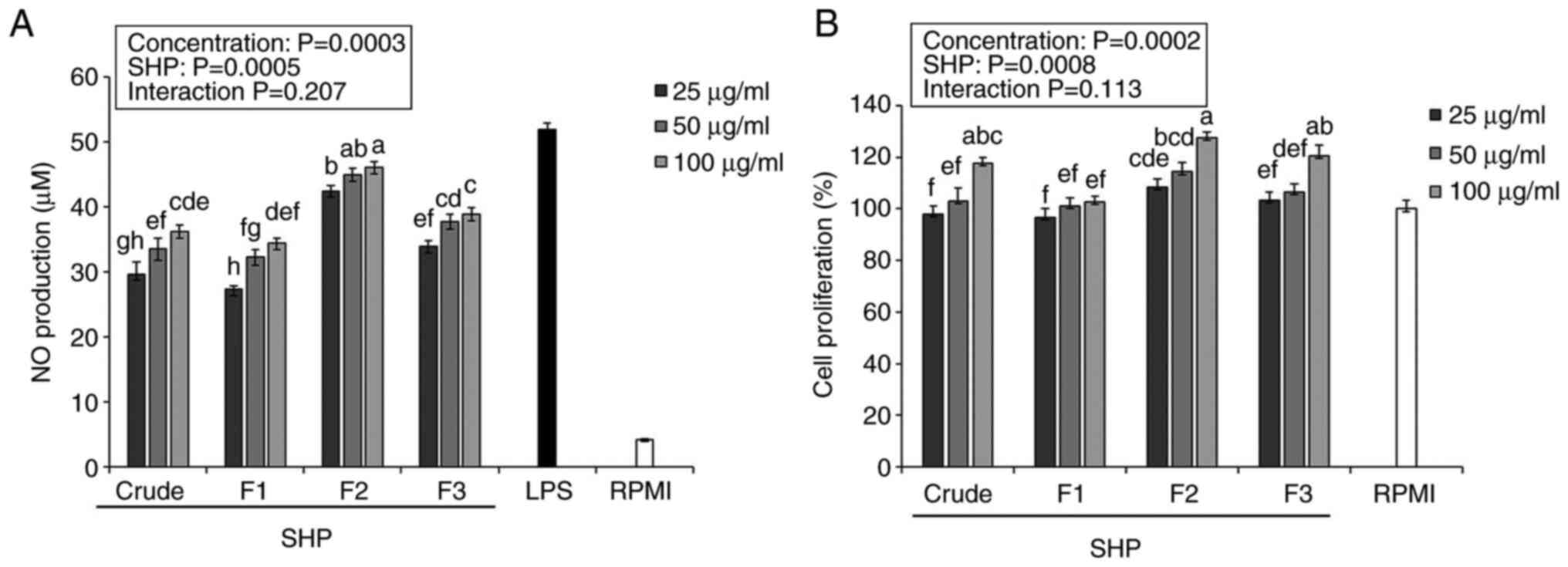
Effects of SHP on NO production and
cell proliferation
In the present study, crude SHP and fractions were
used to stimulate NO production in RAW264.7 cells and assess the
ability of SHP to induce immune activity. Overall, the purified
fraction F2 was more effective than crude SHP at promoting NO
secretion in RAW264.7 cells (Fig.
1A).
Previous reports have indicated that NO may induce
the apoptosis of bacteria, microorganisms and tumor cells in the
body (21,22). The purified component F2 produced
41.21-43.57 µM NO, which was significantly higher than that
produced by crude polysaccharides and the other purified components
(Fig. 1A), indicating that F2 had
the highest ability to stimulate RAW264.7 cell activation.
According to a previous study, the cell membrane is torn during
apoptosis, and the intracellular nutrients are dissolved out,
resulting in a false-positive increase in the measurement of the NO
value (21). To verify whether SHP
increases NO values due to sample apoptosis, a cytotoxicity test
was performed.
Macrophages are known to serve a vital role in
innate and acquired immune responses (23); therefore, active ingredients that
increase macrophage proliferation have a certain significance for
the immune system response. Herein, the proliferation of RAW264.7
cells was stimulated by crude SHP and the isolated fractions
(Fig. 1B). After being treated
with increasing SHP concentrations (25, 50 and 100 µg/ml), cell
proliferation was 100.44-126.02% compared with that in the RPMI
negative control group (100%), which indicated that SHP (25, 50 and
100 µg/ml) exhibited a nontoxic effect on RAW 264.7 cells, further
verifying that soybean hull polysaccharides-induced stimulation of
NO production is not due to sample toxicity leading to cell
apoptosis. Notably, F2 had the highest immunostimulatory potential
among the three fractions, making it a good source of natural
immune modulators.
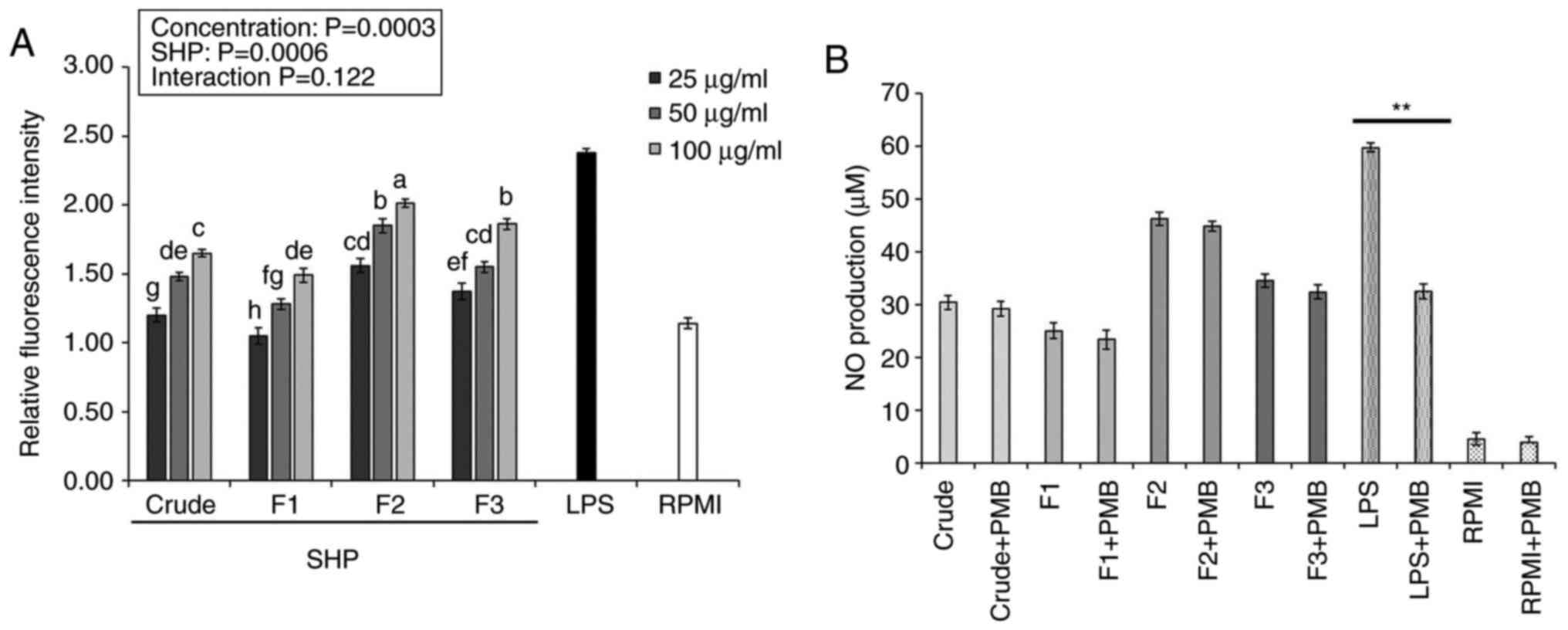
Effects of SHP on ROS production and
endotoxin contamination
ROS are important intracellular signaling molecules
that participate in the secretion and synthesis of inflammatory
factors in macrophages, making them important in the
pathophysiology of inflammation, immune diseases and pathogen
defense. Therefore, analysis of ROS is one of the main interests
for several researchers (24,25).
ROS content produced by the RAW264.7 macrophages, as measured by
fluorescence intensity, increased as the concentrations of crude
SHP and SHP fractions increased (25-100 µg/ml), with significant
differences observed between the SHP-treated and negative control
(RPMI) groups (Fig. 2A). Following
treatment with crude SHP and SHP fractions, ROS content in cells
treated with F2 (100 µg/ml) was ~2-fold higher than that in the
control group. The results indicated that SHP could mediate the
upregulation of intracellular ROS production, with the F2 fraction
having the greatest impact.
A recent study indicated that polysaccharides
isolated from different natural sources have the high possibility
of being contaminated by LPS and show false-positive results in
immune-stimulation assays (17).
To overcome this limitation, some studies have demonstrated that
PMB blocks the biological effects of gram-negative LPS through
binding to lipid A, the toxic component of LPS, which is negatively
charged (26). Therefore, in the
present study, to rule out the possibility of LPS (endotoxin)
contamination in SHP, RAW 264.7 cells were treated for 24 h with
SHP or LPS (positive control group) in the absence or presence of
PMB. As shown in Fig. 2B, the
presence of PMB did not affect NO production in SHP-treated
macrophages. PMB, on the other hand, significantly inhibited
LPS-induced NO production in macrophages, indicating that
SHP-induced activation of macrophages was not caused by endotoxin
contamination.
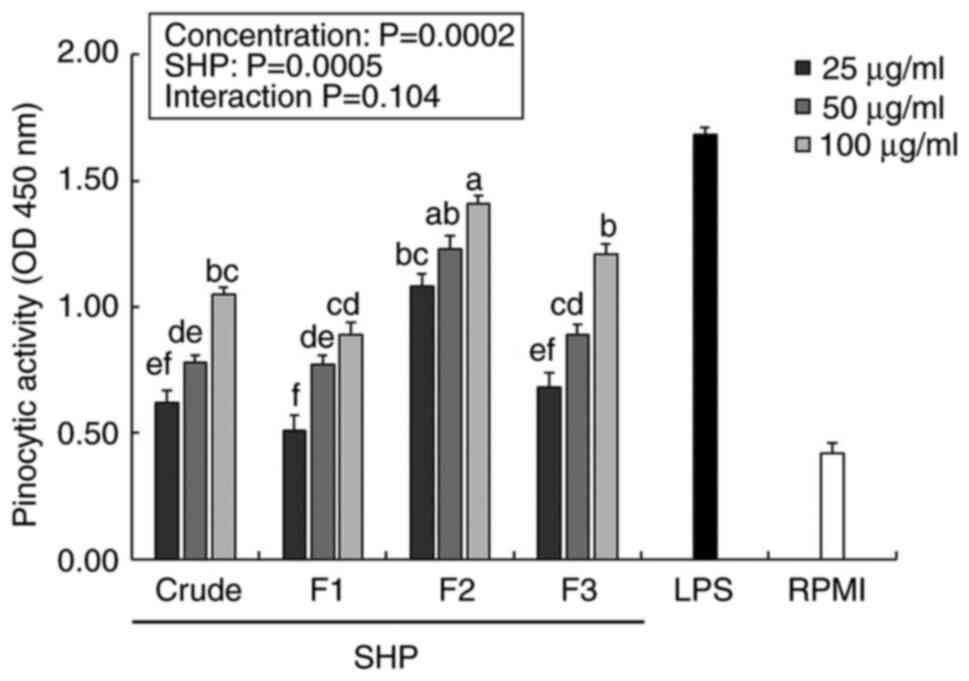
Effect of SHP on pinocytic
activity
Macrophages play an immunomodulatory role in the
body through biological activities, such as phagocytosis or
pinocytosis (27). Therefore,
enhancement of the pinocytic activity of macrophages is an
important sign of macrophage activation, which can be indirectly
evaluated by the absorption of neutral red solution (28). The pinocytic indexes of the
SHP-treated groups were significantly higher than those of the
negative control (RPMI) samples in a dose-dependent manner
(Fig. 3). Following treatment with
25 µg/ml F2, the pinocytotic indices of RAW264.7 cells exceeded
1.0, and F2 upregulated the pinocytotic activity of RAW264.7 cells
in a dose-dependent manner. In addition, LPS (positive control
group) significantly promoted pinocytosis at a lower concentration
(1 µg/ml) when compared with RPMI group, and the F2-enhanced cell
pinocytosis index was significantly higher than that in the crude,
F1 and F3 groups.
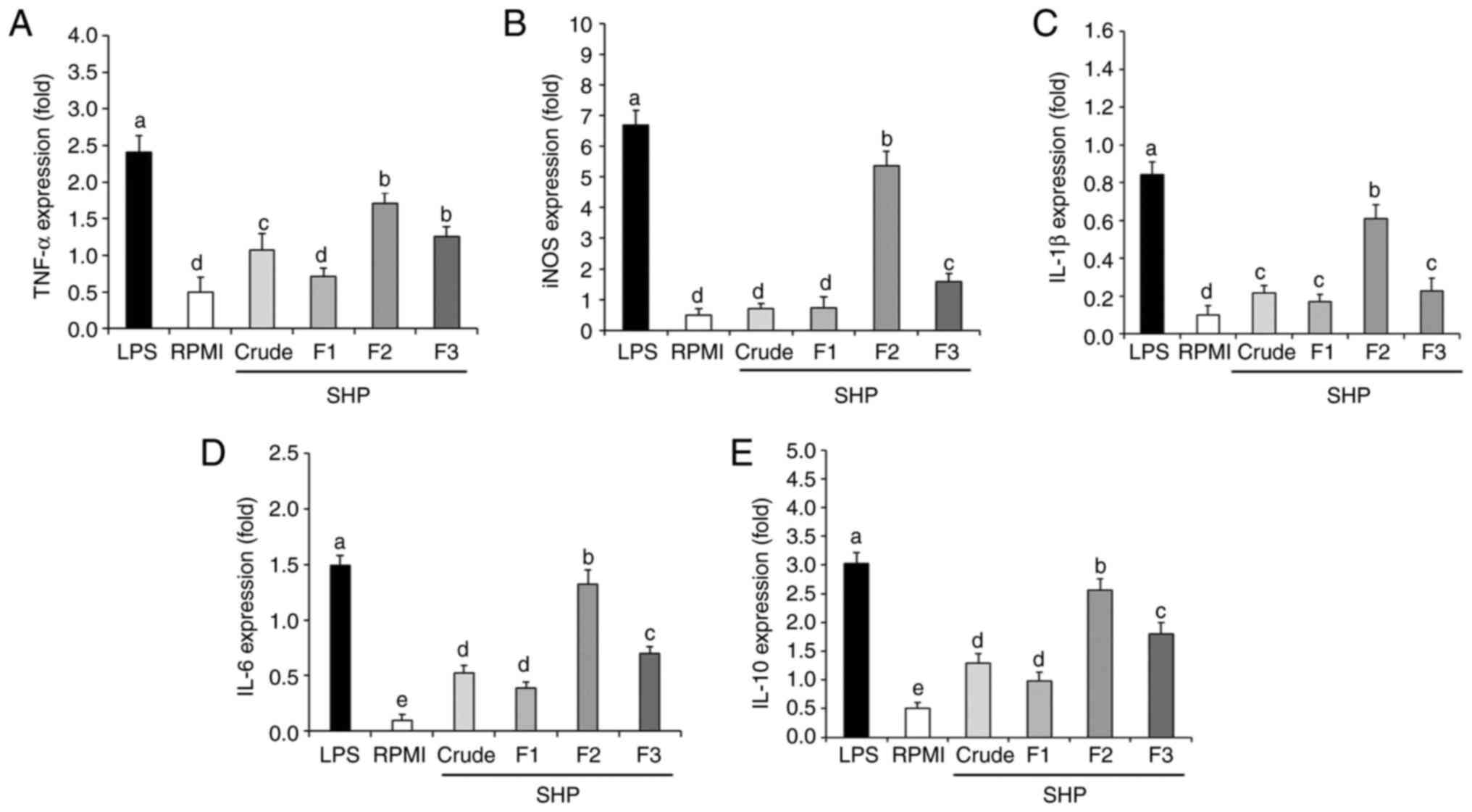
Effect of SHP on the mRNA expression
levels of inflammatory factors
To further explore the effect of SHP on RAW264.7
cells at the molecular level, qPCR was used to analyze the gene
expression of inflammatory factors. Inducible nitric oxide synthase
(iNOS) is one of the three key enzymes that catalyze the production
of NO from L-arginine (29).
Proinflammatory cytokines have been shown to play an important role
in immune regulation. When foreign antigens are detected by the
host immune cells, proinflammatory factors aid T cells in
developing an immune response and quickly initiate the innate
immune defense (30). Therefore,
the expression levels of TNF-α, IL-1β, IL-6, and IL-10 were
determined in the present study. The results showed that the SHP
fractions were able to upregulate the mRNA expression levels of
inducible nitric oxide synthase (iNOS), as well as those of tumor
necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-10
(Fig. 4). Compared with in the
RPMI (negative control group), the mRNA expression levels of iNOS
and cytokines were significantly increased following SHP (F2 and
F3) or LPS (positive control group) stimulation. Notably, although
the inflammatory cytokines (TNF-α, IL-1β, IL-6) are beneficial at
appropriate amounts, their excessive production in a deregulated
fashion is toxic and may cause severe inflammatory responses
(30). As an anti-inflammatory
agent, IL-10 can protect cells from enduring severe inflammatory
reactions and cell death by inhibiting the overexpression of
inflammatory factors such as TNF-α, IL-1β and IL-6. Therefore, the
potential production of IL-10 was examined in the present study and
the gene expression levels were significantly increased (Fig. 4), indicating the capability of SHP
to tightly mediate the inflammatory process. Specifically, the mRNA
expression levels of iNOS, IL-6, IL-1β, TNF-α, and IL-10 following
treatment with 100 µg/ml F2 were 9.72-, 12.2-, 5.10-, 2.42- and
4.12-fold higher than those in the negative control group (RPMI),
but were lower than those in the positive control group (LPS).
These results indicated that F2 could upregulate the secretion and
related gene expression of NO and cytokines in murine
macrophages.
Effect of specific antibodies against
PRR on NO production of SHP-stimulated macrophages
PRRs are known to be used by macrophages to identify
pathogens and exert immune effects. In particular, glycans and
glycol-conjugates (as PAMPs) are recognized by PRRs on macrophages
(29), which include TLRs, DC and
MR. The activation of PRRs causes cells to transmit related
signals, thus stimulating the secretion of inflammatory factors and
macrophage activation (30). SHP
was used as a PAMP to verify whether it could be recognized by
specific PRRs to exert immune effects. Following the addition of
inhibitors of TLR2, TLR4, DC and MR during RAW264.7 cell culture,
cells were stimulated with the F2 fraction and NO production was
detected, with cells treated with RPMI being used as a negative
control group. The addition of a TLR2 inhibitor to the culture
medium of F2-stimulated RAW264.7 cells significantly reduced NO
production compared with that in cells not exposed to the inhibitor
(Fig. 5). Hence, these results
suggested that the F2 fraction (100 µg/ml) stimulated the
production of NO through signals from TLR2. This finding differed
from the results of a previous study (31), which may be related to the
structural characteristics of the polysaccharides, as TLR2 is more
easily recognized by polysaccharides with a high content of glucose
and mannose (18). These findings
indicated that the F2 fraction, as a type of PAMP, was specifically
recognized by TLR2.
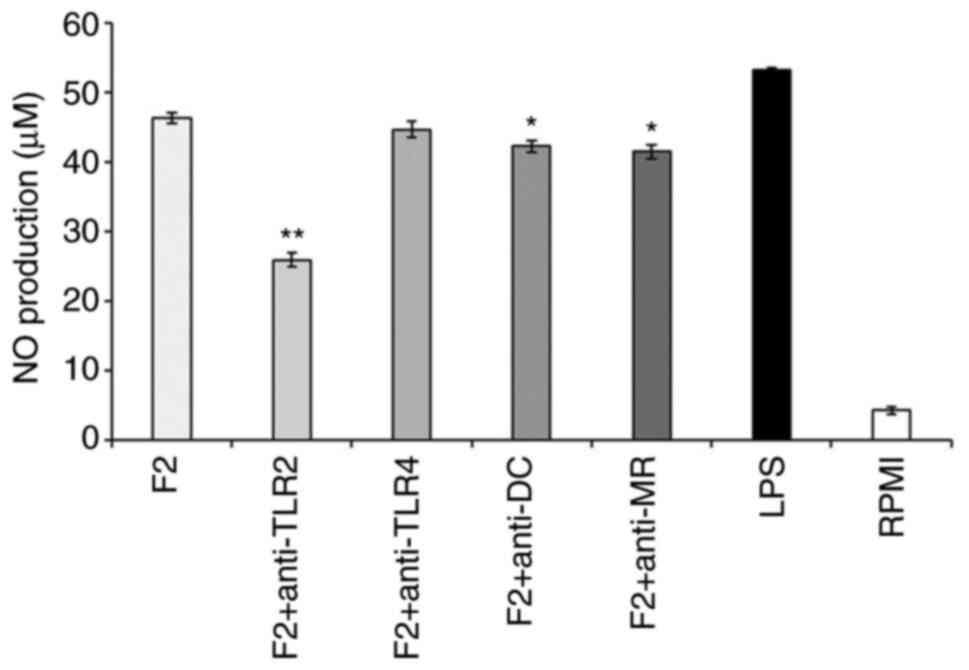 | Figure 5Effect of specific PRR inhibitors on
the production of NO by SHP-stimulated macrophages. RAW264.7 cells
were pretreated with PRR-specific inhibitors (10 µg/ml) for 2 h,
before adding the SPH sample (100 µg/ml), RPMI or LPS (1 µg/ml) to
stimulate the macrophages *P<0.05;
**P<0.01 vs. F2 group. Values are expressed as the
mean ± standard deviation (n=3). PRR, pattern recognition
receptors; F2, fraction 2; NO, nitric oxide; TLR2, Toll-like
receptor 2; TLR4, Toll-like receptor 4; DC, dectin-1; MR, mannose
receptor; LPS, lipopolysaccharide;. RPMI, Roswell Park Memorial
Institute. |
Effect of SHP on the expression of
surface molecules in RAW264.7 cells
CD11b and CD40 are important inflammatory regulators
that not only protect the activity of immune cells but also induce
cytokine expression (32). CD40 is
a member of the TNF receptor family that is expressed in
antigen-presenting cells (33).
Therefore, the high-intensity signals from CD11b and CD40 indicate
that macrophages have been activated to induced an inflammatory
responses.
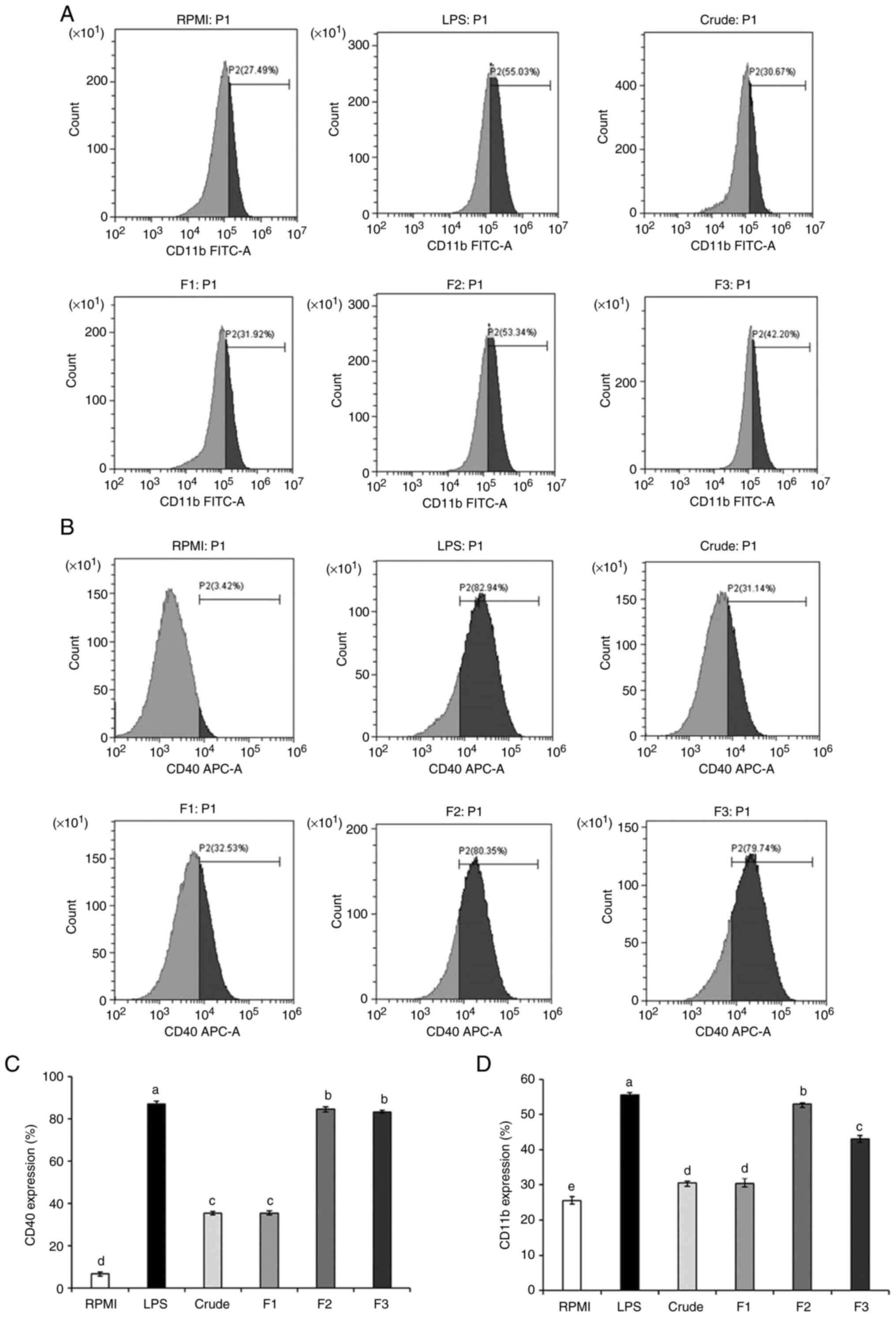
Expression of CD11b and CD40 in macrophages was
increased in response to stimulation with crude SHP and SHP
fractions when compared with the RPMI group (negative control), but
was significantly lower than that detected in the LPS group
(positive control) (Fig. 6A-D).
Notably, no difference in the expression of the inflammatory
regulator CD40 was observed between F2- and F3-treated cells;
however, CD11b levels were significantly different when the F2
group was compared with the F3-treated group. These findings
indicated that CD11b and CD40 may be involved in the SHP-promoted
immune regulation of RAW264.7 cells.
Effects of SHP on the activation of
MAPK and NF-κB signaling pathways in macrophages
A previous study demonstrated that PRRs can
specifically recognize PAMPs and activate the NF-κB pathway,
thereby inducing the transcription of downstream genes, as well as
promoting the production of inflammatory factors and NO (27). IκB-α, as an inhibitor of NF-κB,
encapsulates NF-κB to form a complex; thus, macrophages will be
inactive. When IκB-α is phosphorylated, it is degraded, which, in
turn, releases NF-κB and enhances the translocation of p65 from the
cytoplasm to the nucleus (34).
MAPKs are important regulators of cell proliferation,
differentiation and stress response, as well as the secretion of
cytokines, chemical factors and other regulators. Thus,
phosphorylation of MAPKs directly affects the activated stress
response of macrophages (35).
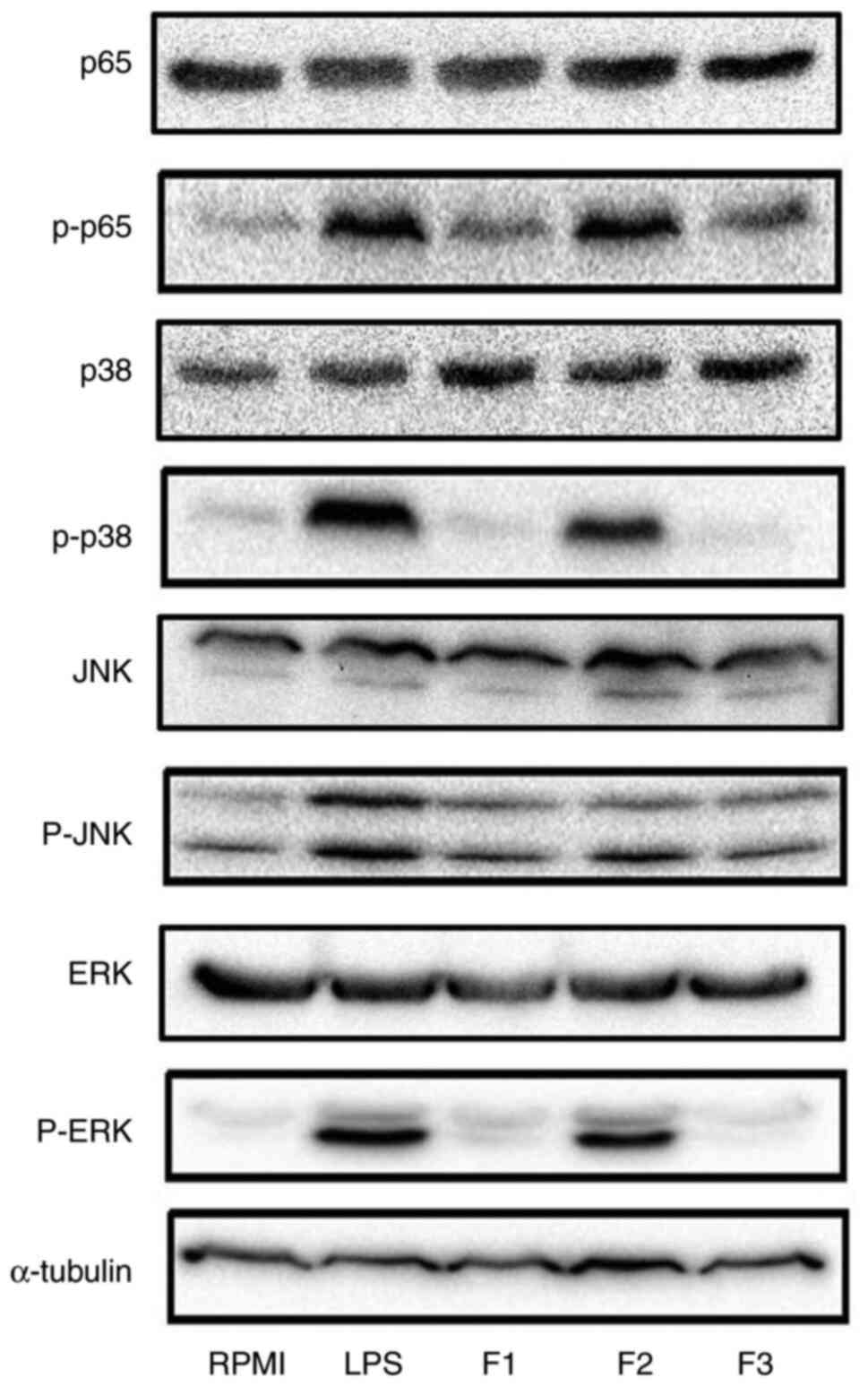
Under SHP fraction stimulation, the levels of p-p65
were increased when compared with those in the RPMI group (negative
control), indicating that SHP activated RAW264.7 cells via the
NF-κB pathway to exert its immune activity (Fig. 7). Moreover, the F2 group exhibited
markedly increased protein expression levels of p-JNK, p-ERK and
p-p38 compared with those in the negative control group (RPMI), but
not as much as positive control group (LPS) (Fig. 7). Furthermore, the expression
levels of these proteins were higher in F2-treated cells than in
cells treated with the other fractions, indicating that F2 had
higher immunostimulatory ability. Collectively, these results
demonstrated that SHP activated macrophages through the NF-κB and
MAPK pathways to support its immune effects. In the present study,
a detailed analysis of these pathways indicated that SHP induced
macrophages to secrete inflammatory factors via TLR2 and consequent
activation of the NF-κB and MAPK pathways.
In summary, following extraction of natural plant
polysaccharides from soybean hull and purification of fractions
(F1, F2 and F3), monosaccharide composition analysis demonstrated
that glucose and mannose were the repeating unit of the
polysaccharide backbone of SHP after hot water extraction (Table II), which is consistent with the
findings of Li et al (36).
SHP-purified component F2 was able to regulate the immune activity
of murine macrophages, by promoting cell proliferation, enhancing
pinocytosis, and inducing the secretion of NO and proinflammatory
cytokines, such as TNF-α. In addition, in comparison with a
previous study, F2 was shown to exert stronger immunostimulatory
effects on macrophages compared with rice bran; for example, high
levels of NO and cytokines were secreted in response to low F2
concentrations (37). Moreover,
CD11b and CD40 were found to be involved in the SHP-mediated immune
regulation of RAW264.7 cells. TLR4 is considered an essential
receptor in the binding of β-glucan to the macrophage surface, and
in activating the MAPK and NF-κB signaling pathways; however, in
the present study, following incubation of cells with SHP and
antibodies against PRRs, TLR2 was shown to activate the immune
response in macrophages through the NF-κB and MAPK pathways, which
in turn may induce the production of NO and the proinflammatory
cytokines, including iNOS, IL-1β and IL-6. The results of this
experiment differ from those in the study by Hsu et al
(38), which may be due to the
different experimental materials used: Hsu et al (38) extracted polysaccharides from
Ganoderma lucidum (reishi or lingzhi), whereas
the present study used soybean hulls as a source of
polysaccharides. In addition, DC was investigated in the present
study and the results regarding this PRR were similar to those
found in the study by Zheng et al (39), which indicated that RAW264.7 cells
only express low levels of DC. However, another study demonstrated
the synergistic stimulation of primary human monocytes and
macrophages by combined stimulation with TLR-4 and DC ligands
(40). Even though the possibility
is low, the immune responses activated by SHP could be amplified
through the synergy between DC and TLR4, which requires further
investigation.
Supplementary Material
Monosaccharide content of the
polysaccharides extracted from soybean hull. (A) Crude, (B) F1, (C)
F2 and (D) F3 extracts.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Key R&D
Projects (grant no. 2018YFD1000905), the Research Initiation Plan
for Talent Introduction (grant no. XYB202011), the Key Scientific
Research Projects of Heilongjiang Farms and Land Reclamation
administration (grant no. HKKY190206-1), the School start-up plan
(grant no. XBD-2017-03) and the Applied Technology Research and
Development Project of Heilongjiang Province (grant no.
GA19B101-02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW was involved in the conceptualization,
investigation and writing the original draft of this study; CF was
involved in the investigation, and the data validation and
analysis; MZ conceived and designed the experiments, and wrote,
reviewed and edited the manuscript; YZ and LC were involved in the
conceptualization, methodology, supervision, writing, reviewing and
editing the manuscript, and funding acquisition. MW and LC
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rungelrath V, Kobayashi SD and DeLeo FR:
Neutrophils in innate immunity and systems biology-level
approaches. Wiley Interdiscip Rev Syst Biol Med.
12(e1458)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ren D, Lin D, Alim A, Zheng Q and Yang X:
Chemical characterization of a novel polysaccharide ASKP-1 from
Artemisia sphaerocephala Krasch seed and its macrophage activation
via MAPK, PI3k/Akt and NF-κB signaling pathways in RAW264.7 cells.
Food Funct. 8:1299–1312. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Beutler B, Hoebe K, Du X and Ulevitch RJ:
How we detect microbes and respond to them: The toll-like receptors
and their transducers. J Leukoc Biol. 74:479–485. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li WH and Huang HY: Soybean utilization
value and high-yield cultivation techniques. Anhui Agric Sci Bull.
16:209–210. 2011.(In Chinese).
|
|
6
|
Lin Q, Yang L, Han L, Wang Z, Luo M, Zhu
D, Liu H, Li X and Feng Y: Effects of soy hull polysaccharide on
dyslipidemia and pathoglycemia in rats induced by a
high-fat-high-sucrose diet. Food Science and Human Wellness.
11:49–57. 2022.
|
|
7
|
Yoo J, Alavi S, Vadlani P and Amanor-Boadu
V: Thermo-mechanical extrusion pretreatment for conversion of
soybean hulls to fermentable sugars. Bioresour Technol.
102:7583–7590. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin Q, Yang L, Han L, Wang Z, Luo M, Zhu
D, Liu H, Li X and Feng Y: Effects of soy hull polysaccharide on
dyslipidemia and pathoglycemia in rats induced by a
high-fat-high-sucrose die. Food Sci Hum Well. 11:49–57. 2022.
|
|
9
|
Hartley JW, Evans LH, Green KY, Naghashfar
Z, Macias AR, Zerfas PM and Ward JM: Expression of infectious
murine leukemia viruses by RAW264.7 cells, a potential complication
for studies with a widely used mouse macrophage cell line.
Retrovirology. 5(1)2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dubois M, Gilles AK, Hamilton JK, Rebers
PA and Smith F: Colorimetric method for determination of sugars and
related substances. Anal Chem. 28:350–356. 1956.
|
|
11
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Aanl Biochem. 72:248–254.
1976.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dodgson KS and Price RG: A note on the
determination of the ester sulphate content of sulphated
polysaccharides. Biochem J. 84:106–110. 1962.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Filisetti-Cozzi TM and Carpita NC:
Measurement of uronic acids without interference from neutral
sugars. Aanl Biochem. 197:157–162. 1991.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tabarsa M, Lee SJ and You S: Structural
analysis of immunostimulating sulfated polysaccharides from Ulva
pertusa. CCarbohydr Res. 361:141–147. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yelithao K, Surayot U, Lee C, Palanisamy
S, Prabhu NM, Lee J and You S: Studies on structural properties and
immune-enhancing activities of glycomannans from schizophyllum
commune. Carbohyd Polym. 218:37–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li C, Talapphet N, Palanisamy S, Ma N, Cho
ML and You S: The relationship between structural properties and
activation of RAW264.7 and natural killer (NK) cells by sulfated
polysaccharides extracted from astragalus membranaceus roots.
Process Biochem. 97:140–148. 2020.
|
|
17
|
Jiang S, Yin H, Qi X, Song W, Shi W, Mou J
and Yang J: Immunomodulatory effects of fucosylated chondroitin
sulfate from stichopus chloronotus on RAW 264.7 cells. Carbohydr
Polym. 251(117088)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu F, Zhou C, Zhou D, Ou S and Huang H:
Structural characterization of a novel polysaccharide fraction from
hericium erinaceus and its signaling pathways involved in
macrophage immunomodulatory activity. J Funct Foods. 37:574–585.
2017.
|
|
19
|
Gao X, Qi J, Ho CT, Li B, Mu J, Zhang Y,
Hu H, Mo W, Chen Z and Xie Y: Structural characterization and
immunomodulatory activity of a water-soluble polysaccharide from
ganoderma leucocontextum fruiting bodies. Carbohydr Polym.
249(116874)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bahramzadeh S, TabarSe M, You S, Li C and
Bita S: Purification, structural analysis and mechanism of murine
macrophage cell activation by sulfated polysaccharides from
cystoseira indica. Carbohydr Polym. 205:261–270. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li J, Qian W, Xu Y, Chen G, Wang G, Nie S,
Shen B, Zhao Z, Liu C and Chen K: Activation of RAW 264.7 cells by
a polysaccharide isolated from antarctic bacterium pseudoaltermonas
sp. S-5. Carbohydr Polym. 130:97–103. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liao W, Luo Z, Liu D, Ning Z, Yang J and
Ren J: Structure characterization of a novel polysaccharide from
dictyophora indusiata and its macrophage immunomodulatory
activities. J Agric Food Chem. 63:535–544. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang G, Zhu L, Yu B and Chen K, Liu B, Li
J, Qin G, Liu C, Liu H and Chen K: Exopolysaccharide from
trichoderma pseudokoningii induces macrophage activation. Carbohydr
Polym. 149:112–120. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lee JS, Kwon DS, Lee KR, Park JM, Ha SJ
and Hong EK: Mechanism of macrophage activation induced by
polysaccharide from cordyceps militaris culture broth. Carbohydr
Polym. 120:29–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu Q, Nie SP, Wang JQ, Yin PF, Huang DF,
Li WJ and Xie MY: Toll-like receptor 4-mediated ROS signaling
pathway involved in ganoderma atrum polysaccharide-induced tumor
necrosis factor-α secretion during macrophage activation. Food Chem
Toxicol. 66:14–22. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schepetkin IA and Quinn MT: Botanical
polysaccharides: Macrophage immunomodulation and therapeutic
potential. Int Immunopharmacol. 6:317–333. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Maverakis E, Kim K, Shimoda M, Gershwin
ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR and Lebrilla CB:
Glycans in the immune system and the altered glycan theory of
autoimmunity: A critical review. J Autoimmun. 57:1–13.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang CL, Lu CY, Pi CC, Zhuang YJ, Chu CL,
Liu WH and Chen CJ: Extracellular polysaccharides produced by
ganoderma formosanum stimulate macrophage activation via multiple
pattern-recognition receptors. BMC Complement Altern Med.
12(119)2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chi DS, Qui M, Krishnaswamy G, Li C and
Stone W: Regulation of nitric oxide production from macrophages by
lipopolysaccharide and catecholamines. Nitric Oxide. 8:127–132.
2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang Y, Zhao XL, Li J, Jiang H, Shan X,
Wang Y, Ma W, Hao J and Yu G: A β-glucan from durvillaea antarctica
has immunomodulatory effects on RAW264.7 macrophages via toll-like
receptor 4. Carbohydr Polym. 191:255–265. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang D, Nie S, Jiang L and Xie M: A novel
polysaccharide from the seeds of plantago asiatica L. induces
dendritic cells maturation through toll-like receptor 4. Int
Immunopharmacol. 18:236–243. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Andrade RM, Wessendarp M, Gubbels MJ,
Striepen B and Subauste CS: CD40 induces macrophage anti-toxoplasma
gondii activity by triggering autophagy-dependent fusion of
pathogen-containing vacuoles and lysosomes. J Clin Invest.
116:2366–2377. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Park YM, Won JH, Yun KJ, Ryu JH, Han YN,
Choi SK and Lee KT: Preventive effect of Ginkgo biloba extract
(GBB) on the lipopolysaccharide-induced expressions of inducible
nitric oxide synthase and cyclooxygenase-2 via suppression of
nuclear factor-kappaB in RAW 264.7 cells. Biol Pharm Bull.
29:985–990. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bi S, Jing Y, Zhou Q, Hu X, Zhu J, Guo Z,
Song L and Yu R: Structural elucidation and immunostimulatory
activity of a new polysaccharide from cordyceps militaris. Food
Funct. 9:279–293. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li S, Gao A, Dong S, Chen Y, Sun S, Lei Z
and Zhang Z: Purification, antitumor and immunomodulatory activity
of polysaccharides from soybean residue fermented with Morchella
esculenta. Int J Biol Macromol. 96:26–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang L, Li Y, Zhu L, Yin R, Wang R, Luo X,
Li Y, Li Y and Chen Z: Antitumor activities and immunomodulatory of
rice bran polysaccharides and its sulfates in vitro. Int J Biol
Macromol. 88:424–432. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hsu HY, Hua KF, Lin CC, Lin CH, Hsu J and
Wong CH: Extract of Reishi polysaccharides induces cytokine
expression via TLR4-modulated protein kinase signaling pathways. J
Immunol. 173:5989–5999. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zheng X, Zou S, Xu H, Liu Q, Song J, Xu M,
Xu X and Zhang L: The linear structure of β-glucan from baker's
yeast and its activation of macrophage-like RAW264.7 cells.
Carbohydr Polym. 148:61–68. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ferwerda G, Meyer-Wentrup F, Kullberg BJ,
Netea MG and Adema GJ: Dectin-1 synergizes with TLR2 and TLR4 for
cytokine production in human primary monocytes and macrophages.
Cell Microbiol. 10:2058–2066. 2008.PubMed/NCBI View Article : Google Scholar
|