Introduction
Recurrent spontaneous abortion (RSA), also known as
recurrent pregnancy loss or recurrent miscarriage, is a
complication of pregnancy affecting 1-2% of fertile couples
(1). It refers to the occurrence of
three or more consecutive spontaneous abortions and causes severe
physical and mental harm to affected patients (2). Chromosomal abnormalities, pathogenic
infections, immune disorders and genetic mutations are factors that
contribute to RSA (3,4). Nevertheless, for ~50% of patients with
RSAs, the causative factors remain unknown (5). Hence, it is important to investigate
the underlying pathogenesis of RSA and identify available targets
for its therapy.
Long non-coding RNAs (lncRNAs) are a group of
non-coding RNAs with a length >200 nt (6). Previous studies have reported that
lncRNAs affect the progression of RSA at the cellular level. For
example, downregulation of the lncRNA metastasis associated lung
adenocarcinoma transcript 1 inhibited the cell proliferation and
migration, and increased apoptosis of human umbilical vein
endothelial cells (7).
Overexpression of HOX antisense intergenic RNA promoted trophoblast
cell invasion and migration (8).
Notably, the lncRNA nuclear paraspeckle assembly transcript 1
(NEAT1) was reported to affect cell proliferation and migration in
several human cancer types such as endometrial (9), breast (10) and cervical cancer (11). Recently, Wang et al (12) discovered that NEAT1 expression was
significantly reduced in the villi of patients experiencing
recurrent miscarriages. However, the underlying function of NEAT1
in the pathogenesis of RSA has rarely been investigated.
MicroRNAs (miRNAs/miRs) are a type of conserved
non-protein coding RNA (~22 nt) that participate in biological
processes including cell differentiation, growth, apoptosis and
angiogenesis (13,14). Previous studies have revealed that
abnormal expression of miRNAs was associated with increased risk of
RSA. For instance, upregulation of miR-27a (15) and miR-34a (16) and downregulation of miR-146a-5p
(17) may contribute to RSA.
Additionally, elevated miR-125b expression has been observed in
decidual and villi tissues of patients with RSAs and has been
suggested to be associated with RSA development (16,18).
The interactions between lncRNAs and miRNAs, such as lncRNA
SNHG7-1/miR-34a (19) and lncRNA
H19/miR-106a-5p (20), reportedly
affect the development of RSA. Nevertheless, the detailed
regulatory mechanisms of miR-125b and its interaction with NEAT1 in
the progression of RSA remain unclear.
Herein, the expression levels of NEAT1, miR-125b and
BCL-2 in the villus tissues of patients with RSAs was evaluated and
the regulatory mechanism of the NEAT1/miR-125b/BCL-2 axis on RSA
pathogenesis was investigated in vitro. The present study
aimed to elucidate the molecular mechanism underlying RSA and
reveal possible targets for RSA treatment.
Materials and methods
Patients and clinical samples
In this retrospective study, villus tissue samples
were obtained from patients with RSAs and healthy controls who
visited Liaocheng Dongchangfu District Maternal and Child Health
Hospital (Liaocheng, China) between March 2018 and February 2019.
The RSA group included 20 Chinese women (age range, 25-35 years
old; mean age, 29.84±3.44 years old) with a history of three or
more spontaneous abortions. The control group included 20
age-matched women (age range, 24-35 years old; mean age, 29.23±3.01
years old) with the request for termination of pregnancy because of
unplanned pregnancy. The inclusion criteria for both groups were as
follows: i) No chromosomal abnormalities; ii) normal reproductive
endocrinology; iii) no diabetes, thyroid dysfunction or other
systemic diseases; and iv) no organic deformity of the uterus and
genital tract. The villus samples were obtained from cases of
induced abortion at 5-10 gestational weeks. All samples were
cleaned in sterile saline to remove excess blood, mucus and
deciduas, and stored in liquid nitrogen cans. The present study was
approved by the Ethics Committee of Liaocheng Dongchangfu Maternal
and Child Health Hospital and all participants signed informed
consent.
Cell culture and transfection
The human placental choriocarcinoma cell line
(JEG-3) was purchased from the Cell Bank of Type Culture Collection
(Chinese Academy of Sciences). JEG-3 cells were cultured in
Dulbecco's Modified Eagle's Medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) at 37˚C in an incubator containing 5%
CO2.
Short hairpin RNA NEAT1 (sh-NEAT1) and its negative
control (sh-NC), sh-BCL-2, miR-125b mimics
(5'-UCCCUGAGACCCUAACUUGUGA-3') and their corresponding negative
control (mimics NC, 5'-UUCUCCGAACGUGUCACGUTT-3'), miR-125b
inhibitors (5'-UCACAAGUUAGGGUCUCAGGGA-3') and their corresponding
negative control (inhibitor NC, 5'-CAGUACUUUUGUGUAGUACAA-3') and
pcDNA-NEAT1 and its corresponding negative control (pcDNA-NC) were
purchased from Shanghai GenePharma Co., Ltd. JEG-3 cells were
seeded into 6-well plates and allowed to grow until the confluence
reached 80%. The cells were then transfected with the previously
mentioned vectors (all at 20 nM) using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C.
Subsequently, 48 h after transfection, cells were harvested to
perform further experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from villus tissues and JEG-3
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A
volume of 2 µl RNA (1 µg/µl) was reverse-transcribed into cDNA at
42˚C for 45 min using a First-Strand cDNA Synthesis kit (Takara
Biotechnology Co., Ltd.), and qPCR was carried out using the SYBR
Green PCR Kit (Takara Biotechnology Co., Ltd.). The thermocycling
conditions for qPCR were as follows: Initial denaturation at 94˚C
for 5 min; 40 cycles of 94˚C for 15 sec; 60˚C for 30 sec and 72˚C
for 1 min. The expression of NEAT1 and Bcl-2 was normalized to that
of GAPDH, and U6 served as the internal control for miR-125b. The
data were analyzed using the 2-ΔΔCq method (21). The primer sequences are listed in
Table I.
 | Table IPrimers for RT-qPCR. |
Table I
Primers for RT-qPCR.
| Gene | Forward
(5'→3') | Reverse
(5'→3') |
|---|
| NEAT1 |
CTTCCTCCCTTTAACTTATCCATTCAC |
CTCTTCCTCCACCATTACCAACAATAC |
| MiR-125b |
GTCCCTGAGACCCTAACTTG |
AGCCTAACCCGTGGATTT |
| BCL-2 |
CTGCACCTGACGCCCTTCACC |
CACATGACCCCACCGAACTCAAAGA |
| GAPDH |
GCGAGATCGCACTCATCATCT |
TCAGTGGTGGACCTGACC |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
MTT assay
Transfected JEG-3 cells were seeded into 96-well
plates at 3x103 cells/well and incubated with 20 µl of 5
mg/ml MTT reagent (Sigma-Aldrich; Merck KGaA) for 4 h at 37˚C.
Subsequently, 200 µl of dimethyl sulfoxide (Sigma-Aldrich; Merck
KGaA) was added to each well for 10 min at 37˚C to dissolve the
formazan crystals. The absorbance of the wells was detected using a
microplate reader at 540 nm.
Flow cytometry
Cell apoptosis assay was measured using an Annexin V
Apoptosis Detection kit (cat. no. BMS500FI-300; Thermo Fisher
Scientific, Inc.). Following 24 h of transfection, JEG-3 cells were
washed with ice cold PBS and centrifuged (450 x g for 20 min at
4˚C). The cells were then resuspended in 1x binding buffer and
incubated with annexin V-FITC and PI (Thermo Fisher Scientific,
Inc.) at 25˚C in the dark for 20 min. Apoptotic cell populations
were assessed using a FACScan™ flow cytometer (BD
Biosciences) and the data were analyzed using FlowJo software
(version 0.9.18, FlowJo LLC).
Dual-luciferase reporter assay
(DLR)
The targeting relationships between miR-125b and
NEAT1 or BCL-2 were analyzed using StarBase database (version 2.0;
http://starbase.sysu.edu.cn). The
predicted wild-type (WT) or mutant (MUT) 3'-untranslated regions of
NEAT1/BCL-2 containing miR-125b binding sites were inserted into
pGL3-basic vectors (Promega Corporation) to produce NEAT1 WT, NEAT1
MUT, BCL-2 WT and BCL-2 MUT plasmids. JEG-3 cells (2,000
cells/well) were cultured in 24-well plates until the confluence
reached 80% and transfected with WT or MUT luciferase reporters and
miR-125b mimics or miR-NC mixed with Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific Inc.) according to the
manufacturer's protocol. After 24 h at 37˚C, luciferase activity
was detected by running a Dual-Luciferase Assay system (Promega
Corporation). Renilla luciferase served as an endogenous
control.
Western blot analysis
JEG-3 cells were lysed in RIPA buffer (Beyotime
Institute of Biotechnology) to obtain total protein. The protein
concentration was detected by the BCA Protein Assay Kit (Abcam). A
total of 50 µg of protein/lane was separated by 10% SDS-PAGE, and
then transferred to PVDF membranes and immersed in 5% skimmed milk
for 1 h at 25˚C to block non-specific binding. The membranes were
subsequently incubated overnight at 4˚C with primary antibodies
against BCL-2 (1:1,000; cat. no. ab32124; Abcam), β-actin (1:1,000,
ab5694, Abcam), pro caspase 3 (1:1,000; cat. no. ab32150; Abcam)
and cleaved caspase 3 (1:1,000; cat. no. ab32042; Abcam). Following
the primary incubation, membranes were incubated with secondary
antibody HRP-conjugated anti-rabbit IgG (1:5,000; cat. no.
ab205718; Abcam) at 37˚C for 1 h. Protein signals were detected
using the ECL Plus reagent (Beyotime Institute of Biotechnology)
and the immunoblots were quantified using ImageJ software (version
4.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analyses were conducted using SPSS
22.0 software (IBM Corp.). The data are presented as the mean ±
standard deviation. Differences between two groups were compared
using unpaired Student's t-test and those between multiple groups
were compared using one-way analysis of variance followed by
Tukey's post hoc test. Variable correlation was evaluated through
Pearson's correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
NEAT1 overexpression enhances
viability and inhibits apoptosis of JEG-3 cells
In order to examine the role of NEAT1 in the
pathogenesis of RSA, NEAT1 expression was detected in the villi of
patients with RSAs and women with normal pregnancies. The mRNA
expression of NEAT1 in the villi of patients with RSAs was found to
be significantly lower than that in the villi of women with normal
pregnancies (Fig. 1A). After
pcDNA-NEAT1 transfection, NEAT1 mRNA expression was enhanced in
JEG-3 cells compared with that in cells transfected with pcDNA-NC,
while NEAT1 knockdown caused the opposite effect (Fig. 1B). The MTT assay demonstrated that
the cell viability was significantly increased in the pcDNA-NEAT1
group compared with the pcDNA-NC group; while compared to the sh-NC
group, cell viability in the sh-NEAT1 was reduced (Fig. 1C). Meanwhile, apoptosis rate was
significantly inhibited in the pcDNA-NEAT1 group compared with the
pcDNA-NC group, while it was promoted in the sh-NEAT1 group
compared with the sh-NC group (Fig.
1D). To validate the observation on apoptosis, western blot
analysis was performed to evaluate the expression of cleaved
caspase 3/pro-caspase-3 ratio. This ratio was significantly
suppressed in JEG-3 cells transfected with pcDNA-NEAT1 compared
with those transfected with pcDNA-NC, whereas it was significantly
elevated after sh-NEAT1 transfection compared with sh-NC
transfection (Fig. 1E). These data
demonstrate that NEAT1 overexpression enhances the viability and
inhibits the apoptosis of JEG-3 cells.
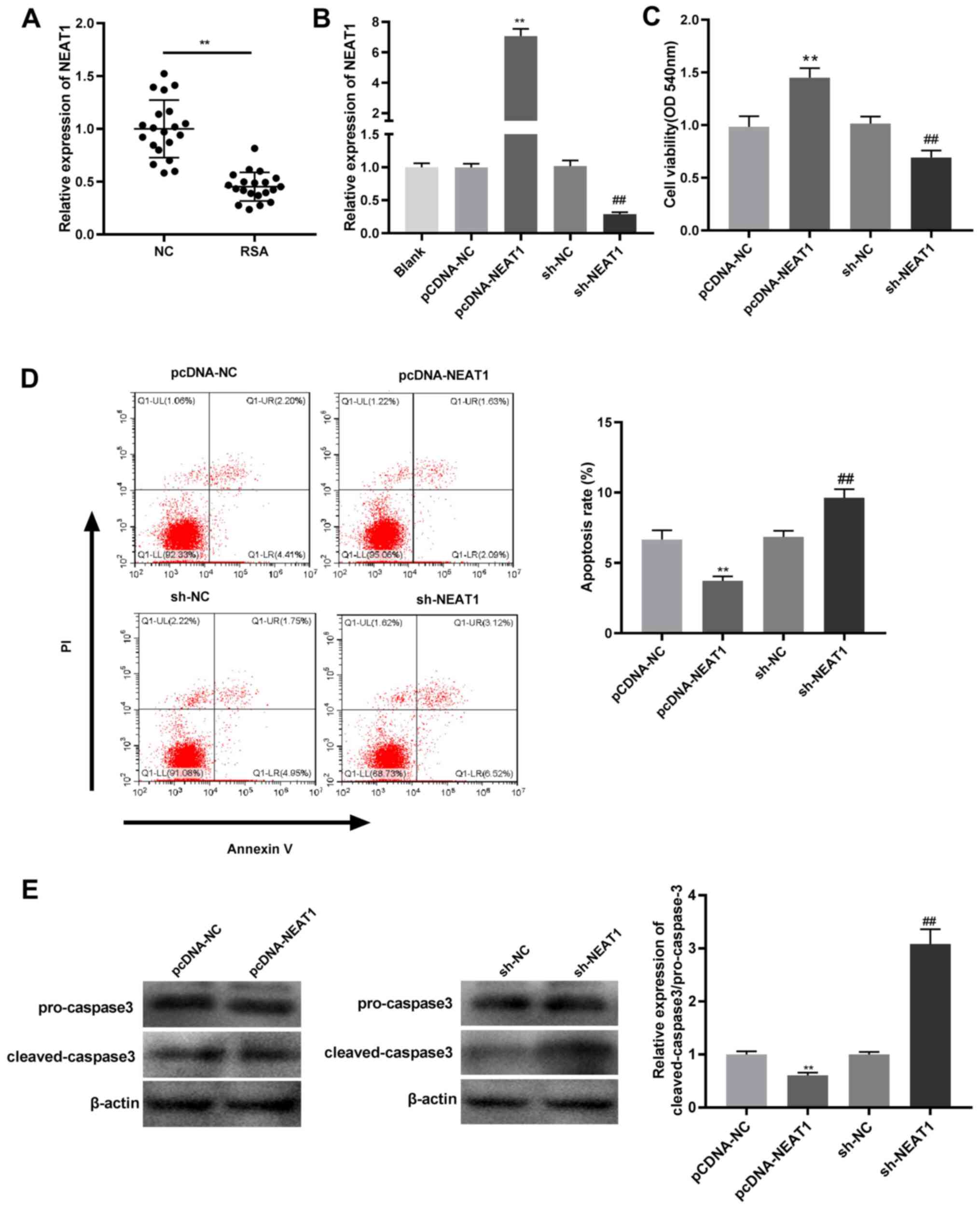 | Figure 1NEAT1 overexpression promotes cell
viability and inhibits apoptosis in JEG-3 cells. (A) NEAT1
expression was detected by RT-qPCR in villi of RSA patients and
normal pregnancy villi. **P<0.01 vs. normal pregnancy
villi. (B) NEAT1 expression was detected by RT-qPCR in JEG-3 cells
transfected with pcDNA-NC, pcDNA-NEAT1, sh-NC or sh-NEAT1.
**P<0.01 vs. pcDNA-NC; ##P<0.01 vs.
sh-NC. (C) Cell viability was detected by MTT assay in JEG-3 cells
transfected with pcDNA-NC, pcDNA-NEAT1, sh-NC or sh-NEAT1.
**P<0.01 vs. pcDNA-NC; ##P<0.01 vs.
sh-NC. (D) Flow cytometry was used to detect the apoptotic rates of
JEG-3 cells transfected with pcDNA-NC, pcDNA-NEAT1, sh-NC or
sh-NEAT1. **P<0.01 vs. pcDNA-NC;
##P<0.01 vs. sh-NC. (E) Ratio of protein expression
of cleaved caspase3/pro-caspase was detected by western blot
analysis in JEG-3 cells transfected with pcDNA-NC, pcDNA-NEAT1,
sh-NC or sh-NEAT1. **P<0.01 vs. pcDNA-NC;
##P<0.01 vs. sh-NC. NEAT1, nuclear paraspeckle
assembly transcript 1; RT-qPCR, reverse transcription-quantitative
PCR; RSA, recurrent spontaneous abortion; NC, negative control; sh,
short hairpin. |
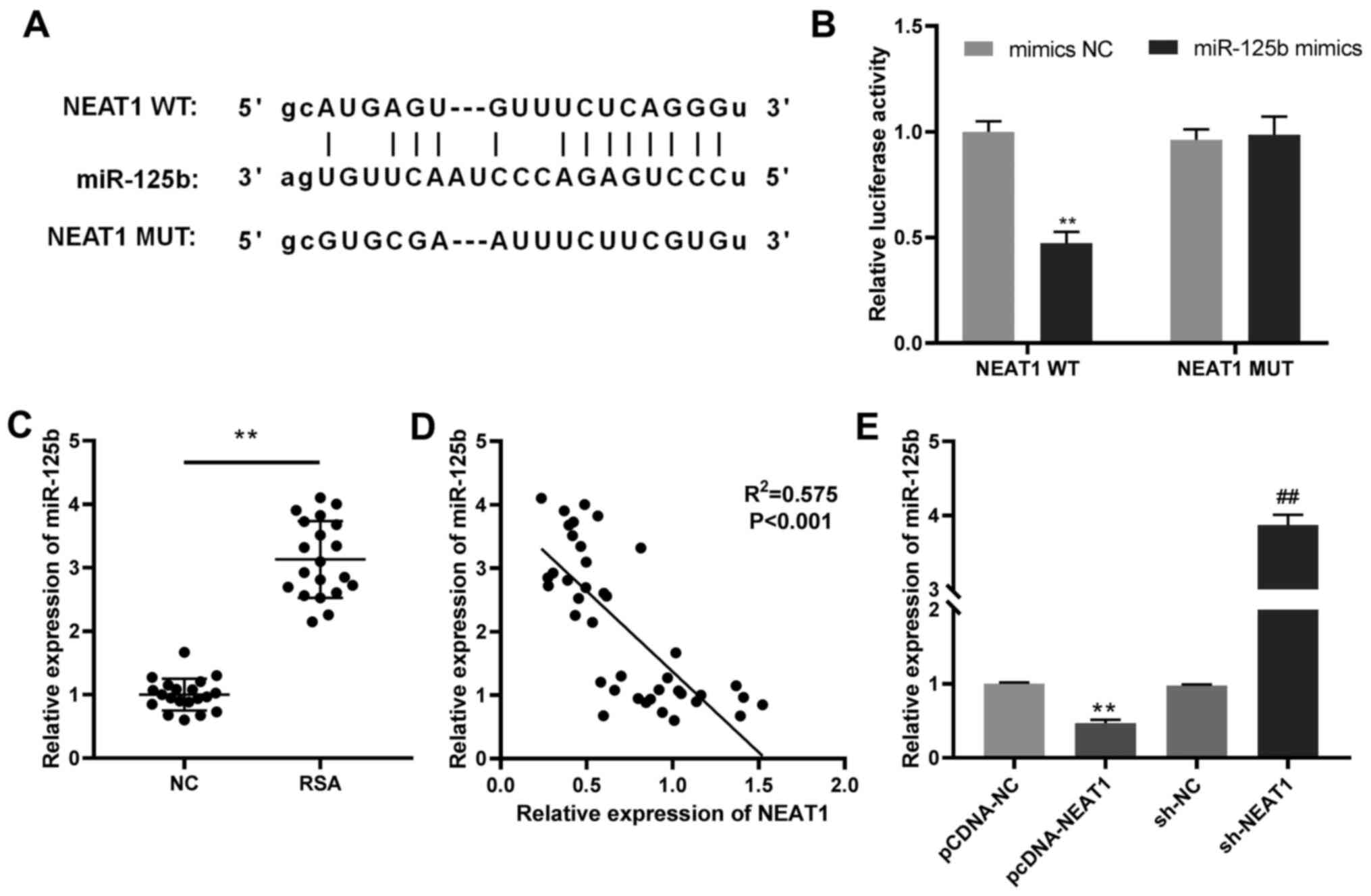
miR-125b acts as a target of
NEAT1
Possible miRNA targets of NEAT1 were studied in
order to understand the regulatory mechanism of NEAT1 in RSA
development. The present study demonstrated that NEAT1 contained
complementary binding sites to miR-125b (Fig. 2A). DLR assay demonstrated that the
luciferase activity in the miR-125b mimics/NEAT1 WT group was
significantly reduced compared with the mimics NC/NEAT1 WT group
(Fig. 2B). In addition, miR-125b
mRNA expression was expressed at significantly higher levels in the
villi of patients with RSAs compared to those in the villi of women
with normal pregnancies (Fig. 2C).
Furthermore, the mRNA expression of miR-125b was negatively
correlated with that of NEAT1 (Fig.
2D), as NEAT1 mRNA overexpression suppressed miR-125b mRNA
expression in JEG-3 cells but NEAT1 silencing upregulated miR-125b,
compared with their respective negative controls (Fig. 2E). These results reveal that NEAT1
directly targets and negatively modulates the expression of
miR-125b.
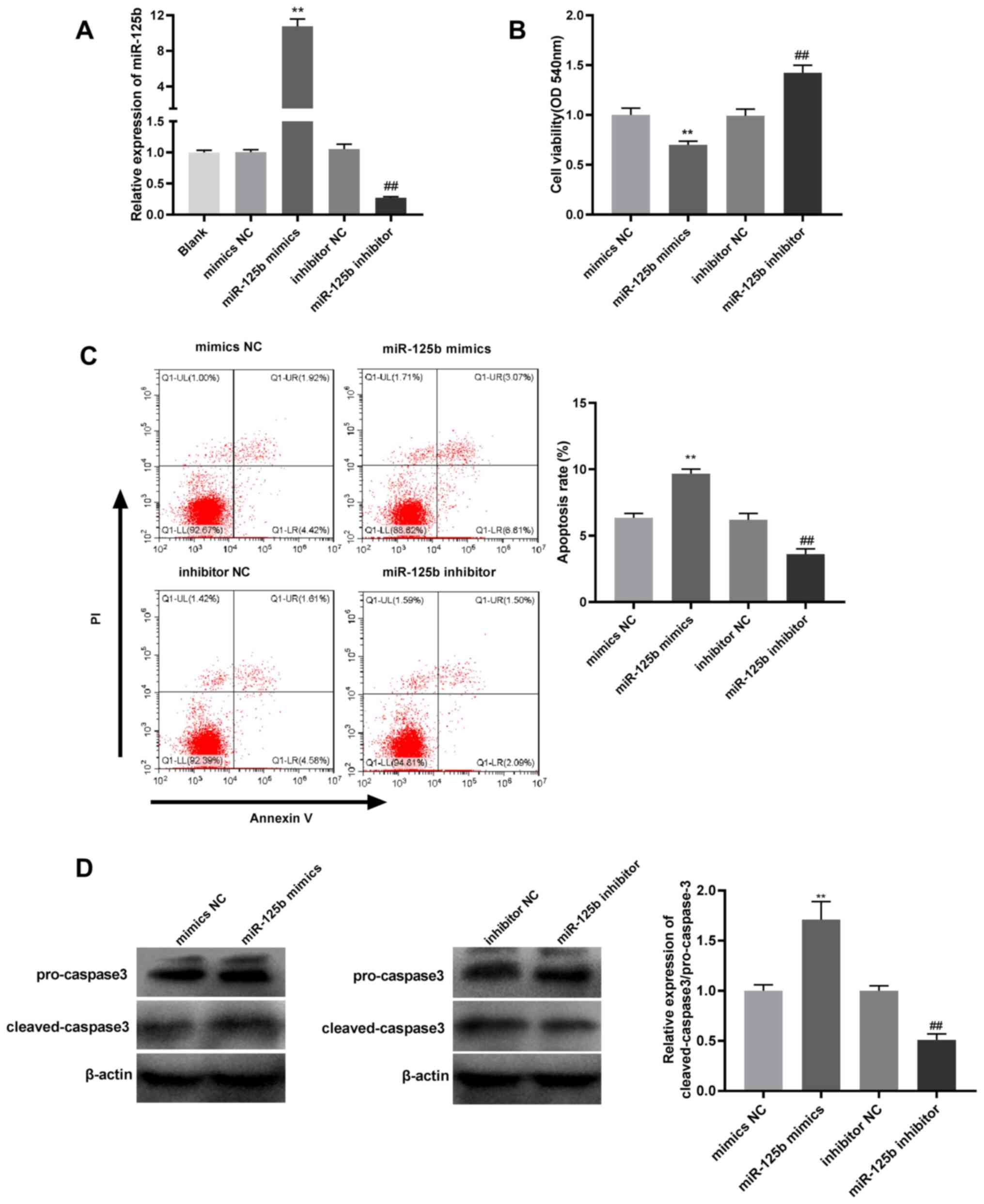
miR-125b overexpression suppresses
viability and promotes apoptosis of JEG-3 cells
To examine the effect of miR-125b in RSA
progression, JEG-3 cells were transfected with miR-125b mimics or
inhibitors, which significantly enhanced and reduced the mRNA
expression levels of miR-125b, respectively, compared with their
respective negative controls (Fig.
3A). The present study demonstrated that miR-125b
overexpression suppressed the viability and promoted the apoptosis
of JEG-3 cells compared with mimics NC; whereas, compared with
inhibitor NC, miR-125b inhibition induced the opposite effect
(Fig. 3B and C). Furthermore, western blot analysis
demonstrated that cleaved caspase 3/pro-caspase-3 ratio was
upregulated by miR-125b overexpression compared with mimics NC and
downregulated by miR-125b inhibition compared with inhibitor NC
(Fig. 3D).
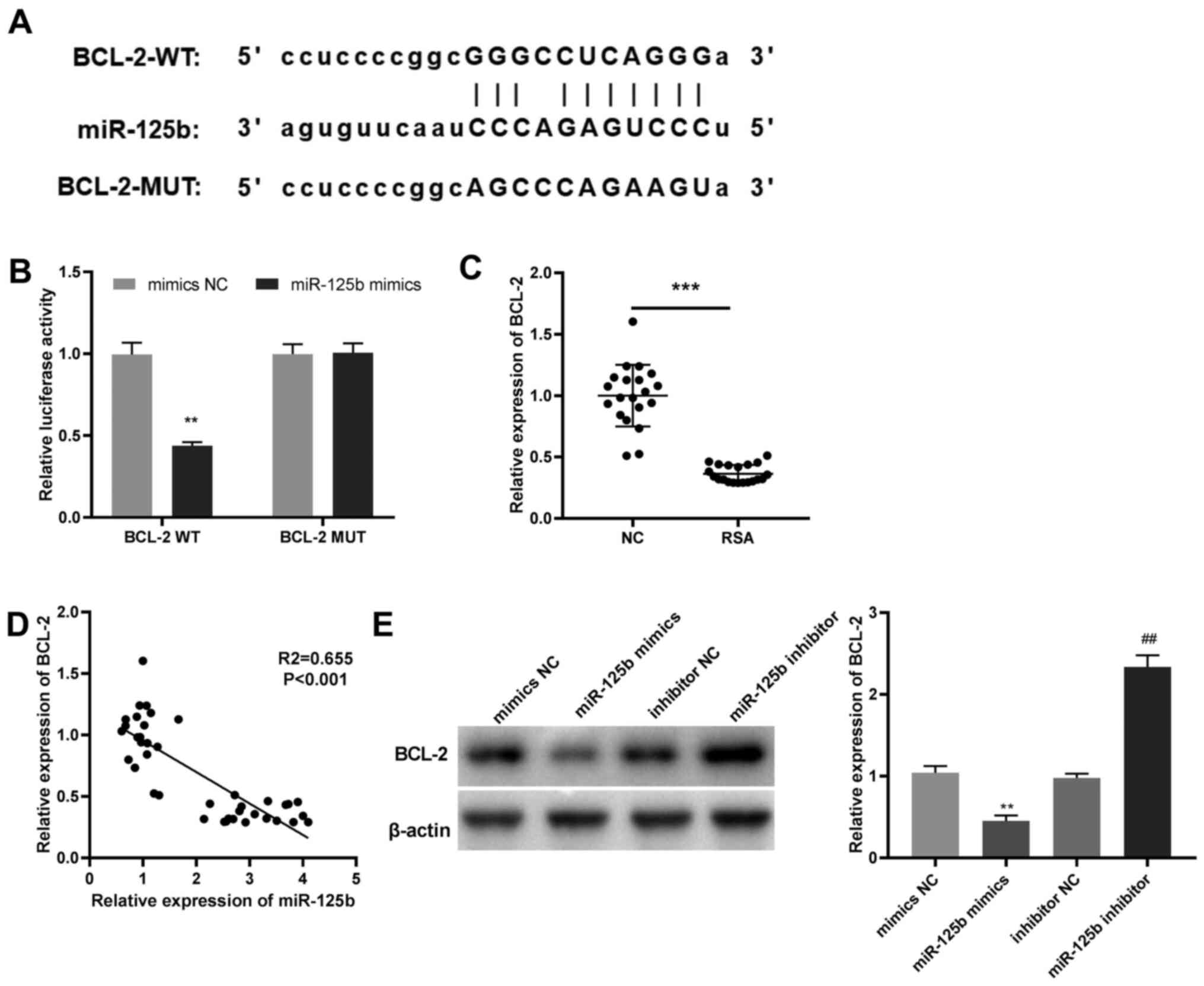
MiR-125b directly targets BCL-2
The potential target site between BCL-2 and miR-125b
was predicted using Starbase (Fig.
4A). DLR assay revealed that the luciferase activity in the
miR-125b mimics/BCL-2 WT group was significantly reduced compared
with the mimics NC/BCL-2 WT group (Fig.
4B) in JEG-3 cells. Moreover, the mRNA expression of BCL-2 in
the villi of patients with RSAs as markedly lower than that in the
villi of normal pregnancies (Fig.
4C) and BCL-2 mRNA expression was negatively correlated with
that of miR-125b (Fig. 4D).
MiR-125b overexpression significantly downregulated BCL-2 compared
with mimics NC; whereas, compared with inhibitor NC, miR-125b
inhibition significantly enhanced BCL-2 protein expression in JEG-3
cells (Fig. 4E). These data confirm
that miR-125b directly targets BCL-2 and negatively regulates BCL-2
expression.
NEAT1 overexpression enhances cell
viability and inhibits apoptosis by regulating the miR-125b/BCL-2
axis
The transfection efficiency of sh-BCL-2 was detected
by RT-qPCR. The mRNA expression of BCL-2 in JEG-3 cells transfected
with sh-BCL-2 was significantly decreased compared with sh-NC
(Fig. 5A), suggesting that sh-BCL-2
was transfected successfully. Rescue experiments were subsequently
conducted to examine the association between NEAT1 and the
miR-125b/BCL-2 axis in JEG-3 cells. NEAT1 mRNA overexpression
significantly increased the protein expression of BCL-2 compared
with pcDNA-NC, but this effect was inhibited by miR-125b
overexpression and BCL-2 knockdown in JEG-3 cells (Fig. 5B). Compared with pcDNA-NEAT1,
overexpression of miR-125b and silencing of BCL-2 attenuated both
the NEAT1-induced increase in viability and decrease in apoptosis
in JEG-3 cells (Fig. 5C and
D). Meanwhile, the results of the
western blot analysis demonstrated that both miR-125b
overexpression and BCL-2 suppression reversed the inhibitory effect
of NEAT1 overexpression on the protein expression of cleaved
caspase 3 (Fig. 5E). Overall, these
findings suggest that NEAT1 overexpression enhances the viability
and inhibits the apoptosis of JEG-3 cells by regulating the
miR-125b/BCL-2 axis.
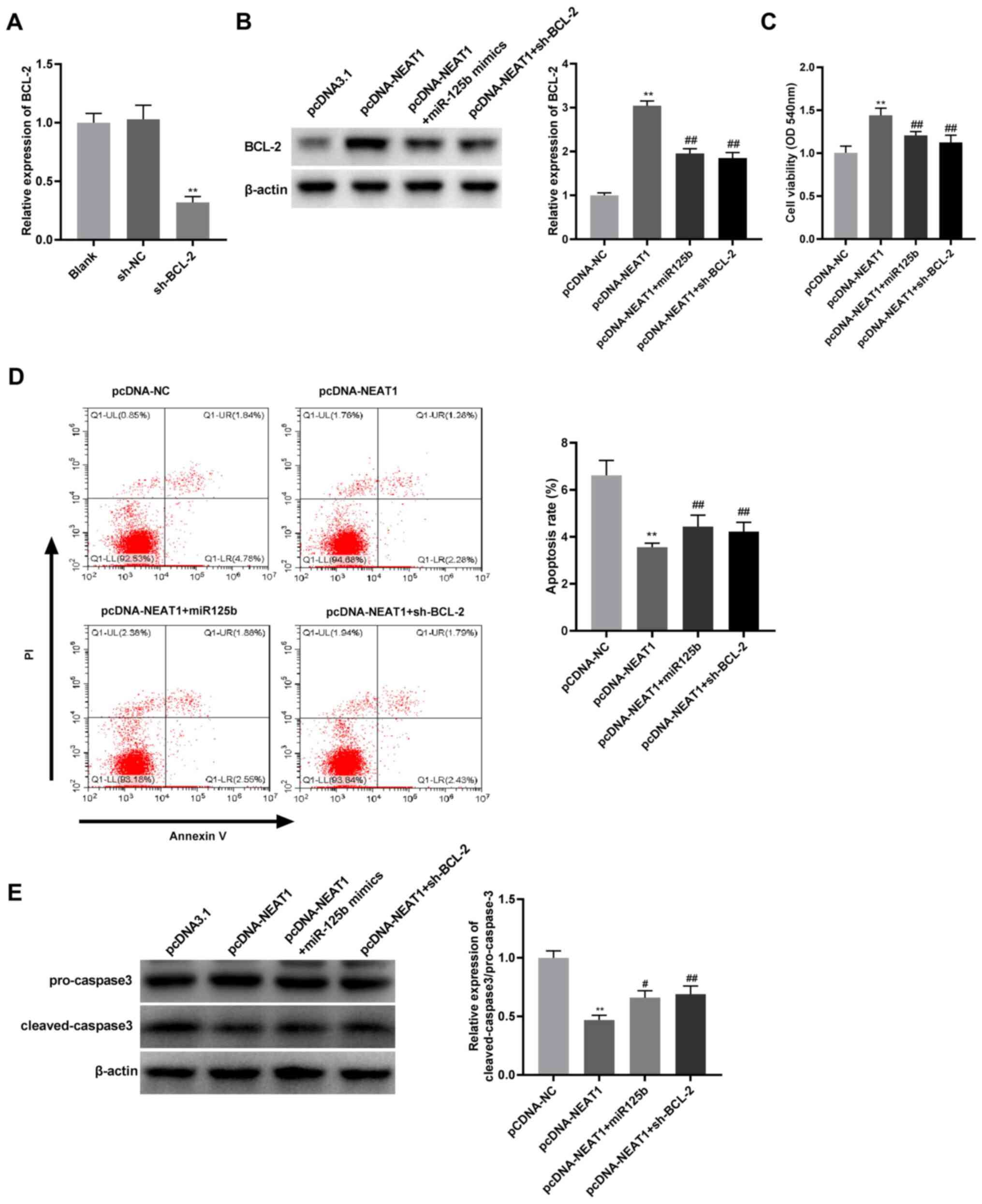 | Figure 5NEAT1 overexpression promotes cell
viability and inhibits apoptosis by regulating miR-125b/BCL-2 axis.
(A) The expression of BCL-2 was measured by RT-qPCR in JEG-3 cells
transfected with sh-BCL-2 or sh-NC. **P<0.01 vs.
sh-NC. (B) The protein expression of BCL-2 was detected by western
blot analysis in JEG-3 cells transfected with pcDNA-NC,
pcDNA-NEAT1, pcDNA-NEAT1 + miR-125b mimics or pcDNA-NEAT1 +
sh-BCL-2. **P<0.01 vs. pcDNA-NC;
##P<0.01 vs. pcDNA-NEAT1. (C) Cell viability was
detected by MTT assay in JEG-3 cells transfected with pcDNA-NC,
pcDNA-NEAT1, pcDNA-NEAT1 + miR-125b mimics or pcDNA-NEAT1 +
sh-BCL-2. **P<0.01 vs. pcDNA-NC;
##P<0.01 vs. pcDNA-NEAT1. (D) Flow cytometry was used
to detect the apoptotic rates of JEG-3 cells transfected with
pcDNA-NC, pcDNA-NEAT1, pcDNA-NEAT1 + miR-125b mimics, or
pcDNA-NEAT1 + sh-BCL-2. **P<0.01 vs. pcDNA-NC;
##P<0.01 vs. pcDNA-NEAT1. (E) Ratio of protein
expression of cleaved caspase3/pro-caspase 3 was detected by
western blot analysis in JEG-3 cells transfected with pcDNA-NC,
pcDNA-NEAT1, pcDNA-NEAT1 + miR-125b mimics or pcDNA-NEAT1 +
sh-BCL-2. **P<0.01 vs. pcDNA-NC;
#P<0.05, ##P<0.01 vs. pcDNA-NEAT1.
NEAT1, nuclear paraspeckle assembly transcript 1; miR, microRNA;
RT-qPCR, reverse transcription-quantitative PCR; sh, short hairpin;
NC, negative control. |
Discussion
RSA is a common complication during pregnancy which
has a complex etiology (5). Normal
trophoblast cells play a critical role in fetal growth, but failure
in trophoblast transformation or impaired trophoblast function can
lead to RSA (22). In the present
study, NEAT1 (an RSA-associated lncRNA) was identified as a
potential target for RSA therapy. The present study demonstrated
that NEAT1 expression was decreased in the villi of patients with
RSAs and NEAT1 overexpression enhanced the viability and inhibited
the apoptosis of JEG-3 cells by interacting with the miR-125b/BCL-2
axis.
Numerous lncRNAs such as MALAT1(7), HOTAIR (8) and TCL6(23) have been reported to be abnormally
expressed in RSA. In the present study, NEAT1 expression was
downregulated in the villi of RSA patients. Similarly, a recent
study demonstrated that NEAT1 expression was reduced in the villi
of patients experiencing recurrent miscarriages (12). These data therefore suggest that
altered NEAT1 expression may be associated with RSA pathogenesis.
In addition, NEAT1 is a critical lncRNA that regulates cell
processes, such as apoptosis and proliferation in human cancers
(24-26).
Wang et al (25) reported
that NEAT1 overexpression increased the viability of endometrial
cancer cells and Yuan et al (26) revealed that NEAT1 knockdown
decreased the viability and enhanced the apoptosis of cervical
cancer cells. Herein, it was demonstrated that NEAT1 overexpression
enhanced the viability and suppressed the apoptosis of JEG-3 cells.
In functional studies, there are some similarities between
trophoblasts and cancer cells that may be related to their
migration and invasion capabilities (27). The results of the present study
corroborate those in previous studies suggesting that NEAT1
overexpression may protect patients against RSA by enhancing cell
viability and inhibiting apoptosis.
Increasing evidence has demonstrated that miR-125b
expression is increased in decidual (16) and villus tissues (18) of patients with RSAs. In the current
study, the level of miR-125b was also discovered to be upregulated
in the villi of patients with RSAs, suggesting that dysregulation
of miR-125b expression may be associated with increased risk of
RSA. Meanwhile, accumulating data have implicated the significant
role of miR-125b in regulating cellular processes. For instance,
miR-125b overexpression induced the apoptosis of trophoblast cells
(28). In another study, increased
miR-125b expression reduced cell viability and impaired the
invasion and migration capacities of extra-villous trophoblastic
cells (29). In the current study,
miR-125b overexpression reduced the viability and promoted the
apoptosis of JEG-3 cells. Notably, lncRNAs act as sponges of miRNAs
and compete for complementary binding with miRNAs, thereby
inhibiting their function (30).
Previous studies have reported that NEAT1 regulated cell motility
by targeting miR-361-5p (31),
miR-101-3p (32) and
miR-37(33). In this research,
miR-125b was identified as a direct target of NEAT1 and was also
negatively regulated by NEAT1. Therefore, it was hypothesized that
miR-125b may interact with NEAT1 to modulate RSA progression.
Rescue experiments confirmed that upregulation of miR-125b reversed
the enhancing effect of NEAT1 overexpression on cell viability, and
the inhibitory effect on apoptosis further validated this
assumption.
During early pregnancy, both fetal and maternal
tissues experience cell death caused by apoptosis and susceptivity
to RSA is associated with the balance of cell death and
proliferation (34). BCL-2, an
anti-apoptotic protein, plays a key role in regulating endometrial
cell turnover (35). A recent study
reported that BCL-2 expression was reduced in trophoblastic tissues
of patients with RSAs (36).
Similarly, a reduction in BCL-2 expression was discovered in the
villi of patients with RSAs, suggesting that low BCL-2 expression
may be associated with RSA. Previous studies have demonstrated that
overexpression of BCL-2 can enhance cell viability and suppress
apoptosis and miRNAs such as miR-34a (37) and miR-195-5p (38) regulated this effect by targeting
BCL-2. In the current study, BCL-2 was identified as a downstream
target gene of miR-125b. Based on previous data showing that NEAT1
overexpression attenuated the malignant behavior of RSA by
regulating miR-125b, it was hypothesized that BCL-2 may be involved
in RSA pathogenesis by modulating the NEAT1/miR-125b axis.
Transfection of sh-BCL-2 significantly reversed the enhancing
effect of pcDNA-NEAT1 on cell viability and the suppressive effect
on apoptosis in JEG-3 cells. In conclusion, NEAT1 overexpression
enhanced the viability and inhibited the apoptosis of JEG-3 cells
by interacting with the miR-125b/BCL-2 axis.
There are several limitations in the present study.
First, the effects of the NEAT1/miR-125b/BCL-2 axis on biological
processes such as cell migration and invasion, in addition to cell
viability and apoptosis, need to be investigated in RSA. Second,
the interactions between this regulatory axis and relevant
downstream signaling pathways remain unclear. Third, the present
study only focused on elucidating the mechanism behind the
NEAT1/miR-125b/BCL-2 axis at the cellular level and in vivo
experiments will be required to supplement the present results.
Further studies are required in order to address these issues.
In summary, the present study revealed that NEAT1
expression was reduced in the villi of patients with RSAs and that
NEAT1 overexpression regulated the viability and apoptosis of JEG-3
cells by targeting the miR-125b/BCL-2 axis. These findings offer
new insights into the etiology of RSA and may aid in identifying
potential targets for RSA treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and HZ are mainly responsible for the design of
articles, data analysis, methodology, project management and
modification of important contents and drafting of manuscripts. LS,
BX and JL are responsible for resource integration, experimental
data analysis, software, visualization, investigation and
literature query, manuscript modification and editing. All authors
have been involved in writing, editing, reading and approving the
current version. All authors confirmed the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Liaocheng Dongchangfu Maternal and Child Health
Hospital (Liaocheng, China; approval ID: 2020-02) and all
participants undersigned the informed consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rai R and Regan L: Recurrent miscarriage.
Lancet. 368:601–611. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li X, Yin M, Gu J, Hou Y, Tian F and Sun
F: Metabolomic profiling of plasma samples from women with
recurrent spontaneous abortion. Med Sci Monit. 24:4038–4045.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Krieg SA, Fan X, Hong Y, Sang QX, Giaccia
A, Westphal LM, Lathi RB, Krieg AJ and Nayak NR: Global alteration
in gene expression profiles of deciduas from women with idiopathic
recurrent pregnancy loss. Mol Hum Reprod. 18:442–450.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pereza N, Ostojić S, Kapović M and
Peterlin B: Systematic review and meta-analysis of genetic
association studies in idiopathic recurrent spontaneous abortion.
Fertil Steril. 107:150–159.e2. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Christiansen OB, Steffensen R, Nielsen HS
and Varming K: Multifactorial etiology of recurrent miscarriage and
its scientific and clinical implications. Gynecol Obstet Invest.
66:257–267. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pauli A, Rinn JL and Schier AF: Non-coding
RNAs as regulators of embryogenesis. Nat Rev Genet. 12:136–149.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Wang Y, Liu HZ, Liu Y, Wang HJ, Pang WW
and Zhang JJ: Downregulated MALAT1 relates to recurrent pregnancy
loss via sponging miRNAs. Kaohsiung J Med Sci. 34:503–510.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Y, Jin F, Li XC, Shen FJ, Ma XL, Wu
F, Zhang SM, Zeng WH, Liu XR, Fan JX, et al: The YY1-HOTAIR-MMP2
signaling axis controls trophoblast invasion at the maternal-fetal
interface. Mol Ther. 25:2394–2403. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang W, Ge L, Xu XJ, Yang T, Yuan Y, Ma XL
and Zhang XH: LncRNA NEAT1 promotes endometrial cancer cell
proliferation, migration and invasion by regulating the
miR-144-3p/EZH2 axis. Radiol Oncol. 53:434–442. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xiong Y, Liu Z, Li Z, Wang S, Shen N, Xin
Y and Huang T: Long non-coding RNA nuclear paraspeckle assembly
transcript 1 interacts with microRNA-107 to modulate breast cancer
growth and metastasis by targeting carnitine
palmitoyltransferase-1. Int J Oncol. 55:1125–1136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang L and Zhu H: Long non-coding nuclear
paraspeckle assembly transcript 1 acts as prognosis biomarker and
increases cell growth and invasion in cervical cancer by
sequestering microRNA-101. Mol Med Rep. 17:2771–2777.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang Y, Liu HZ, Liu Y, Wang HJ, Pang WW
and Zhang JJ: Disordered p53-MALAT1 pathway is associated with
recurrent miscarriage. Kaohsiung J Med Sci. 35:87–94.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Urbich C, Kuehbacher A and Dimmeler S:
Role of microRNAs in vascular diseases, inflammation, and
angiogenesis. Cardiovasc Res. 79:581–588. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang CY, Wang SG, Wang JL, Zhou LY, Liu HJ
and Wang YF: Effect of miRNA-27a and leptin polymorphisms on risk
of recurrent spontaneous abortion. Med Sci Monit. 22:3514–3522.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li D and Li J: Association of
miR-34a-3p/5p, miR-141-3p/5p, and miR-24 in decidual natural killer
cells with unexplained recurrent spontaneous abortion. Med Sci
Monit. 22:922–929. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao L, Li J and Huang S: Patients with
unexplained recurrent spontaneous abortion show decreased levels of
microrna-146a-5p in the deciduae. Ann Clin Lab Sci. 48:177–182.
2018.PubMed/NCBI
|
|
18
|
Dong F, Zhang Y, Xia F, Yang Y, Xiong S,
Jin L and Zhang J: Genome-wide miRNA profiling of villus and
decidua of recurrent spontaneous abortion patients. Reproduction.
148:33–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xiang H, Yan H, Sun B, Feng F and Chen P:
Decreased expression of long non-coding RNA SNHG7 cause recurrent
spontaneous abortion through suppression proliferation and invasion
of trophoblast cells via miR-34a. Am J Transl Res. 11:463–472.
2019.PubMed/NCBI
|
|
20
|
Zeng H, He D, Xie H, Zhao Y, Peng Z, Deng
H, Hu J, Jiang B and Liu N: H19 regulates angiogenic capacity of
extravillous trophoblasts by H19/miR-106a-5p/VEGFA axis. Arch
Gynecol Obstet. 301:671–679. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Brosens I, Pijnenborg R, Vercruysse L and
Romero R: The ‘Great Obstetrical Syndromes’ are associated with
disorders of deep placentation. Am J Obstet Gynecol. 204:193–201.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu LP and Gong YB: LncRNA-TCL6 promotes
early abortion and inhibits placenta implantation via the EGFR
pathway. Eur Rev Med Pharmacol Sci. 22:7105–7112. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luo Z, Yi ZJ, Ou ZL, Han T, Wan T, Tang
YC, Wang ZC and Huang FZ: RELA/NEAT1/miR-302a-3p/RELA feedback loop
modulates pancreatic ductal adenocarcinoma cell proliferation and
migration. J Cell Physiol. 234:3583–3597. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang J, Zhao X, Guo Z, Ma X, Song Y and
Guo Y: Regulation of NEAT1/miR-214-3p on the growth, migration and
invasion of endometrial carcinoma cells. Arch Gynecol Obstet.
295:1469–1475. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yuan LY, Zhou M, Lv H, Qin X, Zhou J, Mao
X, Li X, Xu Y, Liu Y and Xing H: Involvement of NEAT1/miR-133a axis
in promoting cervical cancer progression via targeting SOX4. J Cell
Physiol. 234:18985–18993. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Piechowski J: Trophoblastic-like
transdifferentiation: A key to oncogenesis. Crit Rev Oncol Hematol.
101:1–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gu Y, Zhao S, Wan J, Meng J, Zuo C, Wang
S, Zhou Y, Li H and Wang X: Hsa-miRNA-125b may induce apoptosis of
HTR8/SVneo cells by targeting MCL1. Reprod Biol. 19:368–373.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tang J, Wang D, Lu J and Zhou X: MiR-125b
participates in the occurrence of preeclampsia by regulating the
migration and invasion of extravillous trophoblastic cells through
STAT3 signaling pathway. J Recept Signal Transduct Res. 41:202–208.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Du Z, Sun T, Hacisuleyman E, Fei T, Wang
X, Brown M, Rinn JL, Lee MG, Chen Y, Kantoff PW and Liu XS:
Integrative analyses reveal a long noncoding RNA-mediated sponge
regulatory network in prostate cancer. Nat Commun.
7(10982)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yu X, Liu X, Wang R and Wang L: Long
non-coding RNA NEAT1 promotes the progression of hemangioma via the
miR-361-5p/VEGFA pathway. Biochem Biophys Res Commun. 512:825–831.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu C, Feng Z, Chen T, Lv J, Liu P, Jia L,
Zhu J, Chen F, Yang C and Deng Z: Downregulation of NEAT1 reverses
the radioactive iodine resistance of papillary thyroid carcinoma
cell via miR-101-3p/FN1/PI3K-AKT signaling pathway. Cell Cycle.
18:167–203. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou ZW, Zheng LJ, Ren X, Li AP and Zhou
WS: LncRNA NEAT1 facilitates survival and angiogenesis in
oxygen-glucose deprivation (OGD)-induced brain microvascular
endothelial cells (BMECs) via targeting miR-377 and upregulating
SIRT1, VEGFA, and BCL-XL. Brain Res. 1707:90–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Michita RT, Zambra FMB, Fraga LR,
Sanseverino MT, Schuler-Faccini L, Chies JAB and Vianna P: The role
of FAS, FAS-L, BAX, and BCL-2 gene polymorphisms in determining
susceptibility to unexplained recurrent pregnancy loss. J Assist
Reprod Genet. 36:995–1002. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lea RG, al-Sharekh N, Tulppala M and
Critchley HO: The immunolocalization of bcl-2 at the maternal-fetal
interface in healthy and failing pregnancies. Hum Reprod.
12:153–158. 1997.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Elsalam SA, Mansor AE, Sarhan MH, Shalaby
AM, Gobran MA and Alabiad MA: Evaluation of apoptosis,
proliferation, and adhesion molecule expression in trophoblastic
tissue of women with recurrent spontaneous abortion and infected
with toxoplasma gondii. Int J Gynecol Pathol. 40:124–133.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Su G, Sun G, Liu H, Shu L and Liang Z:
Downregulation of miR-34a promotes endothelial cell growth and
suppresses apoptosis in atherosclerosis by regulating Bcl-2. Heart
Vessels. 33:1185–1194. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jin J, Wang C, Ouyang Y and Zhang D:
Elevated miR-195-5p expression in deep vein thrombosis and
mechanism of action in the regulation of vascular endothelial cell
physiology. Exp Ther Med. 18:4617–4624. 2019.PubMed/NCBI View Article : Google Scholar
|