Introduction
Atherosclerosis (AS) is the common pathological
cause of cardiovascular and cerebrovascular diseases (1). Activation and injury of vascular
endothelial cells, sub-endothelial deposition of oxidized
low-density lipoprotein (ox-LDL), formation of foam cells under the
vascular intima and proliferation and migration of vascular smooth
muscle cells (VSMCs) are the main events in the pathogenesis of AS
(2). Aberrant proliferation and
migration of VSMCs are common pathological features of AS,
restenosis after stenting and hypertension (3,4).
Fibulin-5 is a glycoprotein that consists of 448
amino acids and is involved in regulating extracellular matrix
(5). Recent data showed that
fibulin-5 expression was decreased in atherosclerotic plaque
tissues in human aneurysmatic aortas compared with healthy vessels
(6). Another previous study
suggested that fibulin-5 overexpression reduces MMP levels by
inhibiting the Wnt/β-catenin signaling pathway (7). Yin Yang-1 (YY1) is a widely expressed
transcription factor belonging to the Gli-Kruppel class of zinc
finger proteins that can bind transcriptional initiation repeats to
alter the activities of target promoters (8,9). Its
overexpression can cause the abnormal transcription of downstream
genes, participating in disease occurrence, such as diabetes and B
lymphoma (8,10,11).
YY1 was reported to serve an important role in AS by mediating the
proliferation and migration of VSMCs (12). In addition, a previous study
indicated that YY1 was involved in the formation of foam cells in
response to oxLDL (13). The
present study was therefore designed to investigate the potential
interaction between YY1 and the promoter region of fibulin-5, and
the role of fibulin-5 in ox-LDL-treated VSMCs.
Materials and methods
Collection of clinical samples
In the present study, five patients with coronary
heart disease (CHD; male, n=2; female, n=3), with an average age of
65.1±2.4 years, were diagnosed by coronary angiography in The Laixi
Municipal Hospital (Laixi, China) between June 2019 and August
2020. The control group was also composed of five individuals
(male, n=2; female, n=3) with an average age of 62.3±3.1 years, who
were present in the Laixi Municipal Hospital for routine physical
examination. The inclusion criterion for coronary heart disease
cases was the diameter stenosis of at least one major coronary
artery of patients with stable angina (>80%). The exclusion
criteria were as follows: i) Unstable angina pectoris or myocardial
infarction; ii) comorbidity with other organic heart diseases, such
as rheumatic heart disease and myocardiopathy; iii) comorbidity
with severe liver and kidney disease; iv) comorbidity with familial
hypercholesterolemia; v) comorbidity with malignancies; and vi)
comorbidity with inflammatory diseases. A total of 3 ml venous
blood was collected from each participant. Oral informed consent
was obtained from all participants prior to enrollment in the
present study. The present study was approved by the Ethics
Committee of Laixi Municipal Hospital (approval no.
ky-2020-041-03).
Human aortic (HA)-VSMC culture
HA-VSMCs were purchased from the American Type
Culture Collection (cat. no. CRL-1999) and cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) at 37˚C with 5% CO2. Different
concentrations of ox-LDL (Yeasen Biotechnology (Shanghai) Co.,
Ltd., Shanghai, China) dissolved into DMEM medium (0, 50, 100 and
150 µg/ml) were added to induce cell calcification at 37˚C for 48
or 72 h.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA from VSMCs was extracted with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the instructions of the supplier. A total of 1
µg mRNA per sample was reverse transcribed into cDNA using
PrimeScript™ RT Reagent kit (Takara Bio, Inc.) at 37˚C for 15 min
and 85˚C for 5 sec. Subsequently, SYBR Green kit (cat. no. 208054;
Qiagen GmbH) was used to prepare a 20 µl qPCR reaction mix and qPCR
was performed on the StepOne Real Time PCR instrument (Thermo
Fisher Scientific, Inc.). The primer sequences were: Fibulin-5
Forward: 5'-CTCACTGTTACCATTCTGGCTC-3', Reverse:
5'-GACTGGCGATCCAGGTCAAAG-3'. YY1 Forward:
5'-ACGGCTTCGAGGATCAGATTC-3', Reverse: 5'-TGACCAGCGTTTGTTCAATGT-3'.
Smooth muscle α-actin (α-SMA) Forward: 5'-AAAAGACAGCTACGTGGGTGA-3',
Reverse: 5'-GCCATGTTCTATCGGGTACTTC-3'. GAPDH:
5'-GGAGCGAGATCCCTCCAAAAT-3', Reverse:
5'-GGCTGTTGTCATACTTCTCATGG-3'. PCR was performed at 95˚C for 30
sec, followed by 45 cycles of 95˚C for 5 sec and 60˚C for 30 sec.
GAPDH was used as an internal reference. The relative levels of
mRNA were calculated with the 2-ΔΔCq method (14).
Cell Counting Kit-8 (CCK-8) assay
VSMCs (1x104 cells/well) were seeded into
96-well plates and then incubated for 24 h at 37˚C. Following
ox-LDL treatment or transfection of Ov-Fibulin-5 plasmids and
ox-LDL treatment, the medium was replaced with serum-free DMEM
medium. A total of 10 µl CCK-8 solution (Beyotime Institute of
Biotechnology) was added to each well for incubation at 37˚C for 1
h. The absorbance at 450 nm was subsequently detected using a
microplate reader (Thermo Fisher Scientific, Inc.).
Western blotting
Following the x-LDL treatment or transfection of
Ov-Fibulin-5 plasmids plus ox-LDL treatment, or the co-treatment of
transfection of Ov-YY1 and siRNA-Fibulin-5 plus ox-LDL treatment,
VSMCs were collected and lysed by adding RIPA buffer (Beyotime
Institute of Biotechnology) on ice for 30 min. The supernatant was
collected after centrifugation at 4˚C at 14,000 x g for 20 min. The
protein concentration of each group was detected by a BCA kit
(Abcam). A total of 20 µg each protein sample was separated by 10%
SDS-PAGE and then transferred into PVDF membrane. The membranes
were then blocked with 5% BSA (MilliporeSigma) at room temperature
for 2 h and incubated with primary antibodies (Fibulin-5; cat. no.
ab109428, 1:1,000; cyclin D1; cat. no. ab16663, 1:200; CDK2; cat.
no. ab32147, 1:1,000; MMP2; cat. no. ab92536, 1:1,000; MMP9; cat.
no. ab76003, 1:1,000; GAPDH; cat. no. ab8245, 1:5,000. Abcam) at
4˚C overnight. After incubation with a secondary antibody at 37˚C
for 2 h (Goat Anti-Rabbit IgG; cat. no. ab7090, 1:10,000. Abcam,
England), a ECL color development reagent (cat. no. ab133409;
Abcam) was added to the membranes. The density of the protein bands
was analyzed using the ImageJ software (version 1.53b; National
Institutes of Health).
Wound healing assay
HA-VSMCs in a logarithmic proliferation phase were
seeded into six-well plates at 2x104 cells per well.
After routine overnight culture, the cells received transfection of
Ov-Fibulin-5 plasmids and ox-LDL treatment, or the transfection of
Ov-YY1 and siRNA- Fibulin-5 and ox-LDL treatment, with three
replicates per group. When 80% cell confluence was reached, a
scratch was performed using a horizontal line perpendicular to the
back with a 20-µl pipette tip. The cells were washed by phosphoric
acid buffer three times and cultured in serum-free DMEM medium. PBS
was used to rinse the plates and serum-free DMEM medium was added
at 37˚C. The cell migration was observed under a light microscope
at 0 and 24 h (Olympus Corporation; magnification, x100). The
relative migration was calculated using following formula: (Initial
width at 0 h-final width at 24 h)/width at 0 h.
Transwell assay
The HA-VSMCs received transfection with Ov-Fibulin-5
plasmids and ox-LDL treatment, or transfection of Ov-YY1 and
siRNA-Fibulin-5 and ox-LDL treatment and their concentration was
adjusted to 2x108 cells/ml. Diluted Matrigel (25 µl; BD
Biosciences) with DMEM medium without serum was added to upper
chamber at 37˚C. A total of 200 µl cell suspension prepared with
serum-free medium was added to the upper chambers of a Transwell
insert (cat. no. 3422; 8-µm; Corning). DMEM with 10% FBS was added
to the lower chamber. After 24 h of incubation at 37˚C, the cells
that have gone through the membrane were fixed using 4% methanol
and stained with crystal violet at room temperature for 10 min and
images were captured under an inverted microscope (Olympus
Corporation, magnification, x100).
Plasmid transfection
Lipofectamine® 3000 transfection reagent
(Thermo Fisher Scientific, Inc.) was used according to the
manufacturer's instructions to transfect YY1-overexpressing
plasmids (0.5 µg; pcDNA-YY1; Ov-YY1; Shanghai GenePharma Co., Ltd.)
or its control (0.5 µg; pcDNA), or siRNA targeting fibulin-5 (15
pmol; 5'-GAUUGUUGUGAGGAGUCUAGC-3') or its negative control (15
pmol; siRNA-NC, scrambled RNA, 5'-GUGAUUGCAUGAUUGUGCGGA-3') into
HA-VSMCs, which were constructed and purchased from Shanghai
GenePharma Co., Ltd. After 24 h at 37˚C, the cells were treated
with 100 µg/ml ox-LDL (100 µg/ml ox-LDL significantly promoted cell
proliferation) for 24 h at 37˚C for further experiments.
Bioinformatics
Using PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3,
access date: 10.09.2020) and Transcription Factor DataBase (TFDB;
http://bioinfo.life.hust.edu.cn/HumanTFDB#!/, access
date: 10.09.2020) tools, YY1 was predicted to bind to the promoter
region of fibulin-5.
Chromatin immunoprecipitation
(ChIP)
After chromatin was subjected to ultrasonication,
magnetic beads (60 µl) coated with anti-YY1 or anti-IgG (EMD
Millipore) were added to form immune complex. The immune complex
was washed with wash buffer (low salt wash buffer, high salt wash
buffer, LiCl wash buffer and TE buffer, in sequence) and then was
centrifuged at 4˚C at 16,000 x g for 2 min. Next, collected
immunoprecipitation was eluted with eluent including 10% SDS,
NaHCO3 and ddH2O at 65˚C for 15 min. 20 µl NaCl was added for
incubation at 65˚C overnight. After de-crosslink, 1 µl RNaseA was
added for incubation for 1 h at 37˚C. RT-qPCR was performed to
analyze precipitated DNA fragments.
Dual-luciferase reporter gene
assay
Luciferase activity was detected after
co-transfection of fibulin-5 promoter site 1 (TCCCAGCCCCCGCACC)
with mutations or wild-type sequence luciferase reporter genes (0.1
µg; pGL3-basic; Promega Corporation) and YY1 overexpressing
plasmids (0.2 µg)or its control plasmids (empty plasmids) into 293T
cells (ATCC, USA) with Lipofectamine® 3000 following the
manufacturer's protocol. Renilla luciferase was used as
control reporter gene. After 48 h, luciferase activity was detected
using Dual luciferase reporter gene assay kit according to the
manufacturer's instructions (cat. no. RG028; Beyotime Institute of
Biotechnology).
Statistical analysis
All data were statistically analyzed by GraphPad
Prism 8 software (GraphPad Software, Inc.). The measurement data
were represented by the mean ± SD and one-way ANOVA was used for
comparison between multiple groups, followed by Tukey's test.
Chi-square test was used to compare the two rates of age, smoking,
drinking, or hypertension between CHD and control. Each experiment
was repeated at least three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
Ox-LDL induces low fibulin-5
expression in HA-VSMCs
The level of high-density lipoprotein in the
peripheral blood samples of patients with CHD was significantly
lower than that in the control group (Table I). However, there was no statistical
significance in the comparison of all other indicators between the
two groups (Table I).
 | Table IClinical characteristics of patients
with CHD. |
Table I
Clinical characteristics of patients
with CHD.
|
Characteristics | CHD (n=5) | Control (n=5) | P-value |
|---|
| Age, years | 65.1±2.4 | 62.3±3.1 | >0.05 |
| Smoking, % | 20 | 20 | >0.05 |
| Drinking, % | 20 | 20 | >0.05 |
| Hypertension,
% | 60 | 60 | >0.05 |
| Cholesterol,
mmol/l | 3.98±0.66 | 4.01±0.42 | >0.05 |
| High-density
lipoprotein, mmol/l | 0.94±0.12 | 1.35±0.29 | <0.01 |
| Low density
lipoprotein, mmol/l | 2.12±0.63 | 2.15±0.64 | >0.05 |
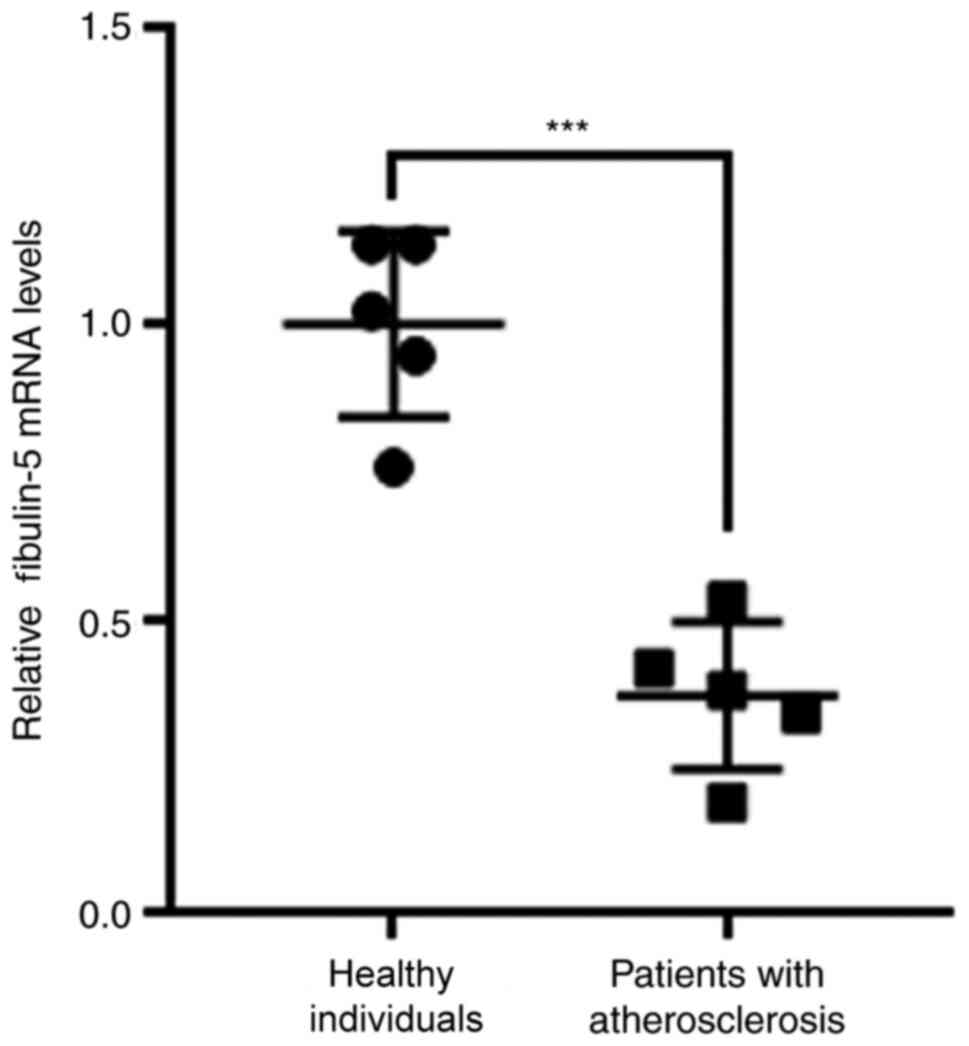
To explore the role of fibulin-5 in AS, fibulin-5
mRNA expression was quantified in the peripheral blood of patients
with AS. Fibulin-5 mRNA expression was significantly increased in
patients with AS compared with that in healthy individuals
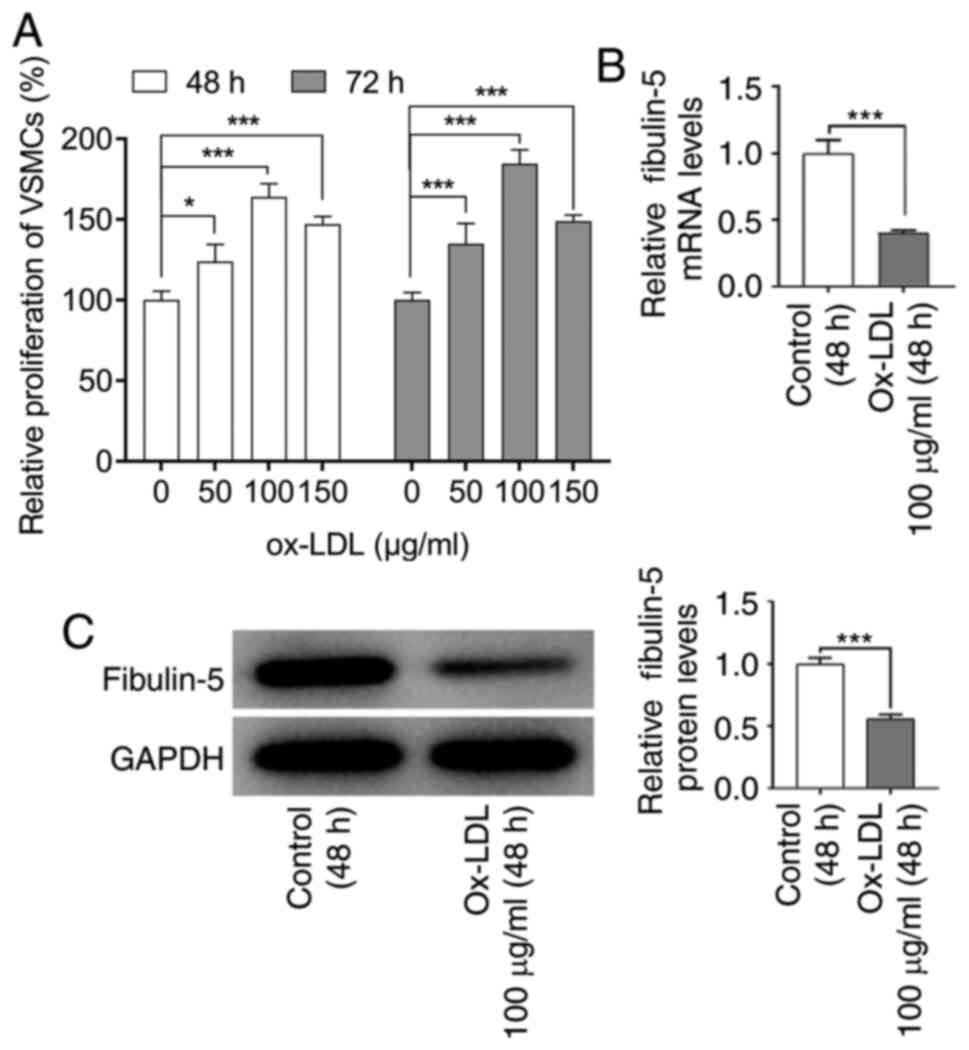
(P<0.001; Fig. 1). VSMCs have
been previously reported to serve an important role in
atherogenesis (15). As indicated
in Fig. 2A, 100 µg/ml ox-LDL was
the concentration that increased VSMC viability the strongest
(P<0.001). This concentration was therefore selected for further
experiments. RT-qPCR and western blotting analyses of fibulin-5
expression in VSMCs indicated that the mRNA and protein levels of
fibulin-5 were significantly reduced after ox-LDL treatment
compared with those in the control group (P<0.001; Fig. 2B and C). To further investigate the role of
fibulin-5, HA-VSMCs treated with ox-LDL were transfected with a
fibulin-5-overexpressing plasmid.
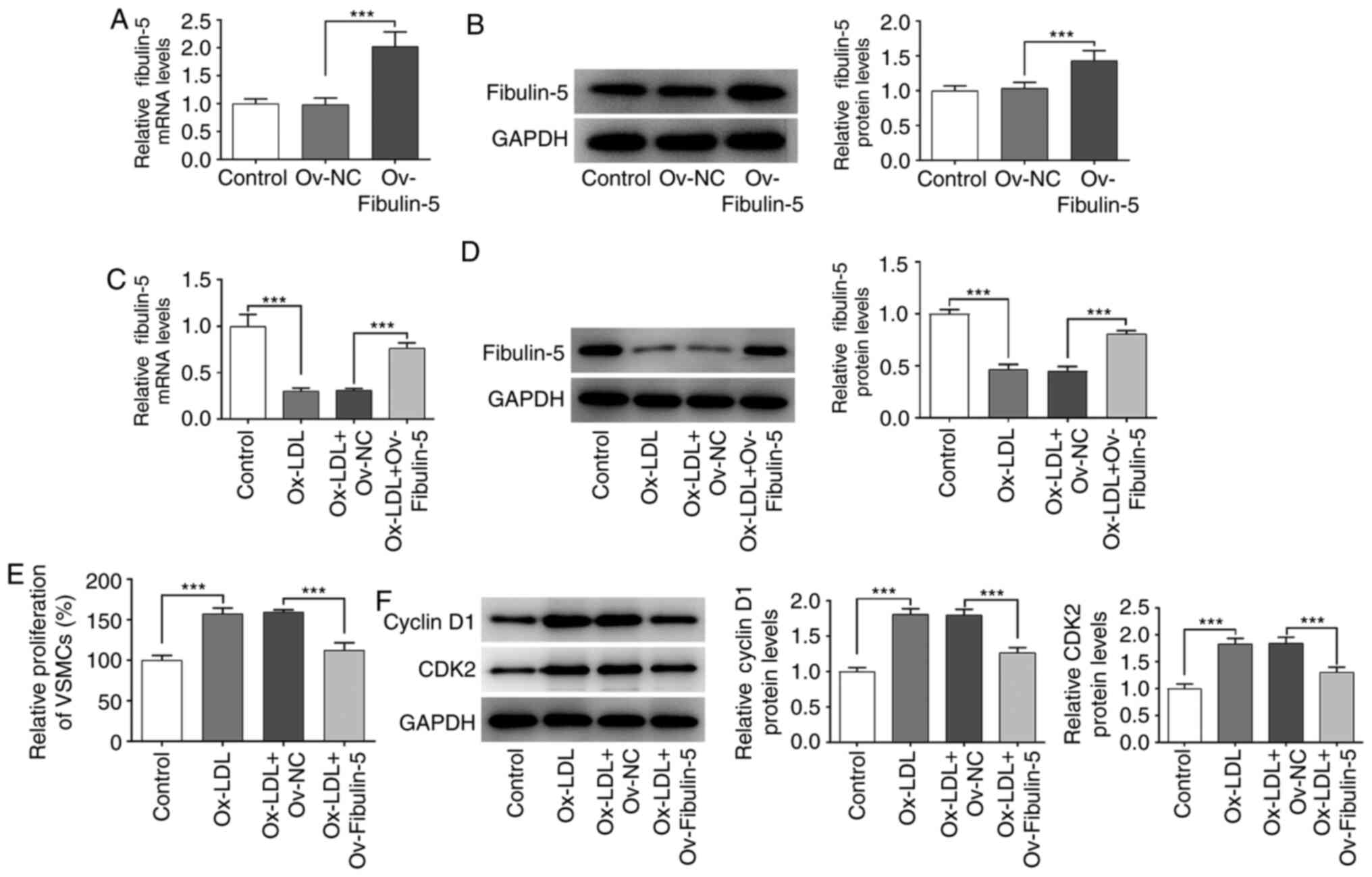
Fibulin-5 overexpression suppresses
HA-VSMC proliferation in response to ox-LDL
Since ox-LDL decreased fibulin-5 expression, it was
hypothesized that fibulin-5 serve a role in HA-VSMCs in response to
ox-LDL. As a transfection control, a vector overexpressing
(Ov)-fibulin-5 significantly increased the mRNA and protein
expression levels of fibulin-5 compared with that in the
Ov-negative control (NC) group (P<0.001; Fig. 3A and B). Analyzes of fibulin-5 expression in
ox-LDL-treated HA-VSMCs demonstrated that the reduced fibulin-5
expression was significantly reversed by fibulin-5 overexpression
(P<0.001; Fig. 3C and D). In VSMCs cycle progression, cyclin D1
and CDK2 are involved in promoting Rb phosphorylation, the
inhibition of which can lead to cycle arrest (16). VSMCs were subsequently subjected to
CCK-8 analysis to measure cell viability (P<0.001; Fig. 3E) whereas western blotting was used
to quantify protein levels of cell cycle proteins cyclin D1 and
CDK2 (P<0.001; Fig. 3F). The
present results demonstrated that the overexpression of fibulin-5
in HA-VSMCs reversed the increase in cell viability induced by
ox-LDL treatment, in addition to reversing the increased protein
expression of cyclin D1 and CDK2 induced by ox-LDL treatment
(P<0.001; Fig. 3E and F).
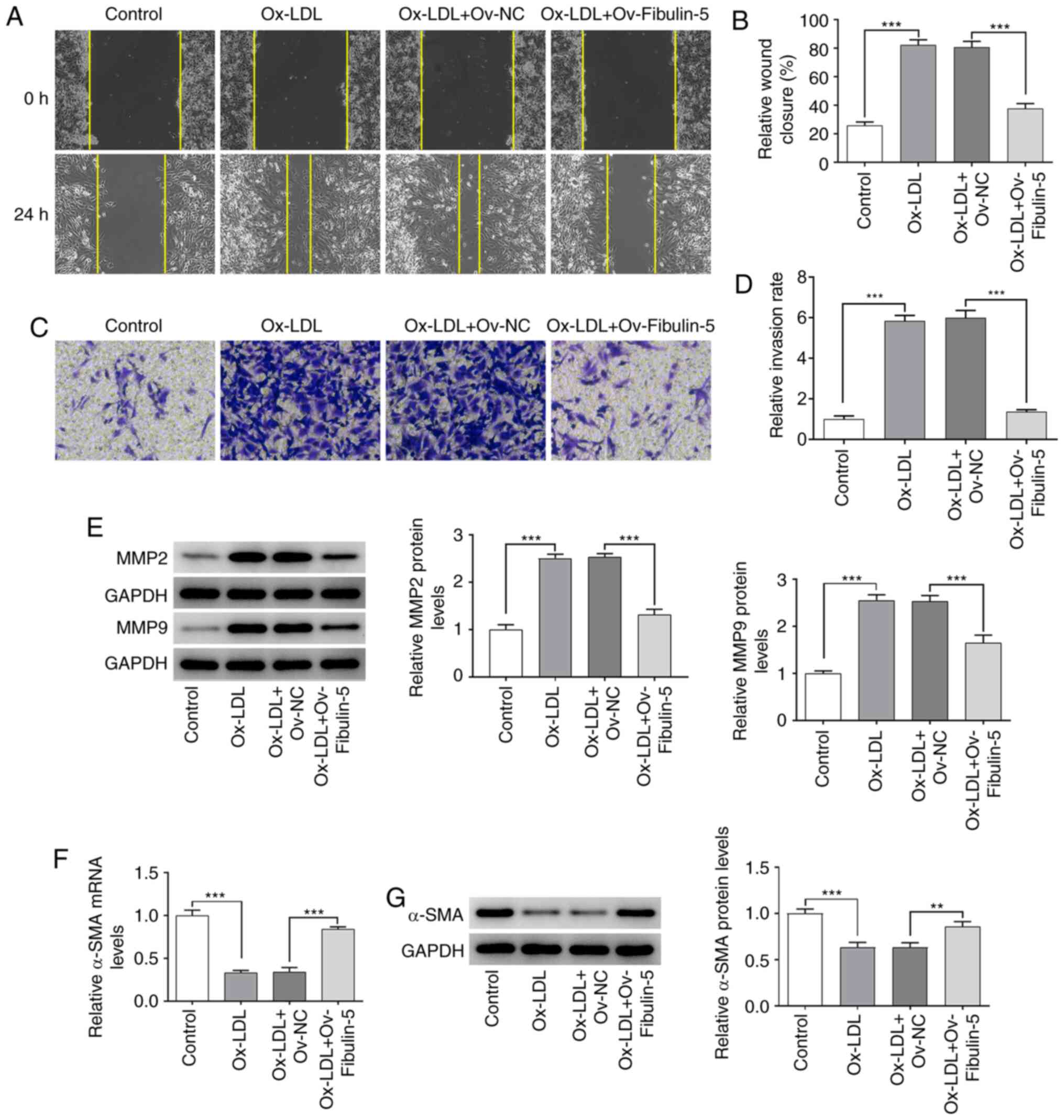
Cell migration and invasion were studied by
performing wound healing and Transwell assays, respectively. The
present results demonstrated that HA-VSMCs exhibited significantly
increased migration and invasion after ox-LDL treatment, which were
significantly reversed following fibulin-5 overexpression (all
P<0.001; Fig. 4A-D). MMP-2 and
MMP-9 expressed in VSMCs, modulated the collagen degradation of the
extracellular matrix and served a vital role in AS progression
(17).
Additionally, the protein expression of MMP2 and
MMP9, which participate in phenotype transition (18,19),
was quantified by western blotting, whilst the mRNA and protein
expression of α-SMA was measured by RT-qPCR and western blotting,
respectively. MMP2 and MMP9 protein expression was significantly
increased after ox-LDL treatment, an effect that was significantly
reversed by fibulin-5 overexpression (all P<0.001; Fig. 4E). Similarly, α-SMA mRNA and protein
expression were significantly decreased after ox-LDL treatment
(P<0.001), which was significantly reversed by fibulin-5
overexpression (P<0.01 and P<0.001; Fig. 4E-G).
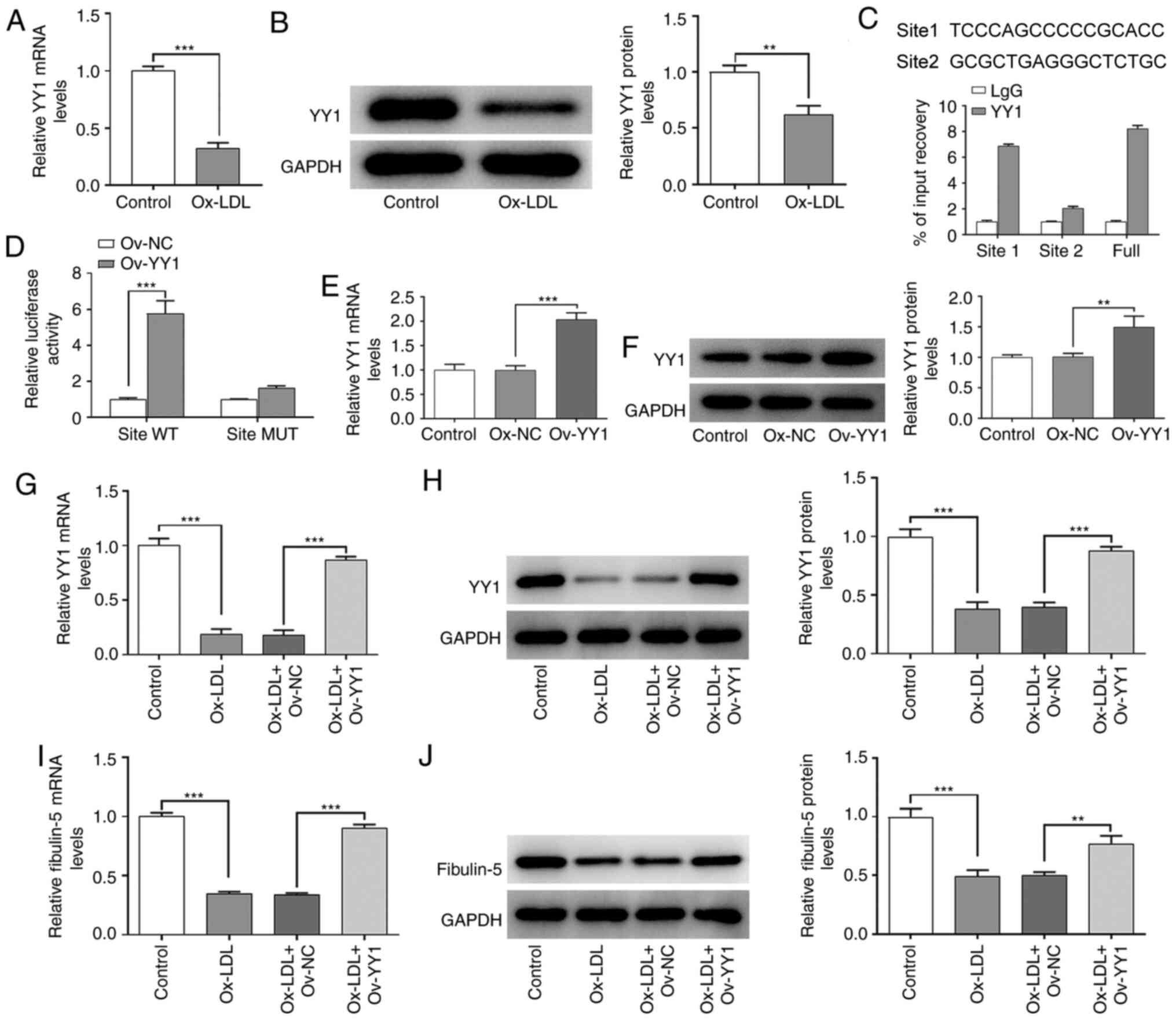
YY1 binds to the promoter of fibulin-5
to induce its upregulation in HA-VSMCs
To further explore the role of fibulin-5 in AS,
PROMO and TFDB databases were used to predict whether YY1 can bind
to the promoter region of fibulin-5 to regulate fibulin-5
expression in ox-LDL-treated HA-VSMCs. The mRNA and protein
expression levels of YY1 were significantly decreased in
ox-LDL-treated HA-VSMCs compared with those in the control group
(P<0.01 and P<0.001; Fig. 5A
and B). Next, the two promoter
sites and the overall promoter region level of fibulin-5, were
detected by qPCR in an immunoprecipitation complex formed by using
YY1 antibody. Promoter site 1 was shown to be markedly enriched
(Fig. 5C). The luciferase activity
of the pGL4 luciferase reporter vectors containing the promoter
region of fibulin-5 with binding site (site 1) for YY1 was
significantly increased when YY1-overexpressing plasmids were
co-transfected into HA-VSMCs, compared with that in cells
co-transfected with the Ov-NC plasmid (P<0.001, Fig. 5D). When the pGL4 luciferase reporter
vectors with muted sites and Ov-NC or Ov-YY1 were co-transfected
into 293T cells, there was no significant difference in luciferase
activities between Ov-NC and Ov-YY1 (Fig. 5D). These results suggest that YY1
can bind to site 1 of the fibulin-5 promoter. The transfection
efficiency of the YY1-overexpressing plasmid was tested in Fig. 5E and F. As shown in Fig. 5E and F, cells transfected with Ov-YY1 showed
significantly higher YY1 expression levels compared with those in
cells transfected with Ov-NC (P<0.01 and P<0.001). As
indicated in Fig. 5G and H, overexpression of YY1 in the HA-VSMCs
significantly reversed the decreased mRNA and protein expression of
YY1 induced by ox-LDL treatment (P<0.001). Similarly, YY1
overexpression also significantly restored the expression of
fibulin-5, which was decreased after ox-LDL treatment (P<0.01
and P<0.001; Fig. 5I and
J).
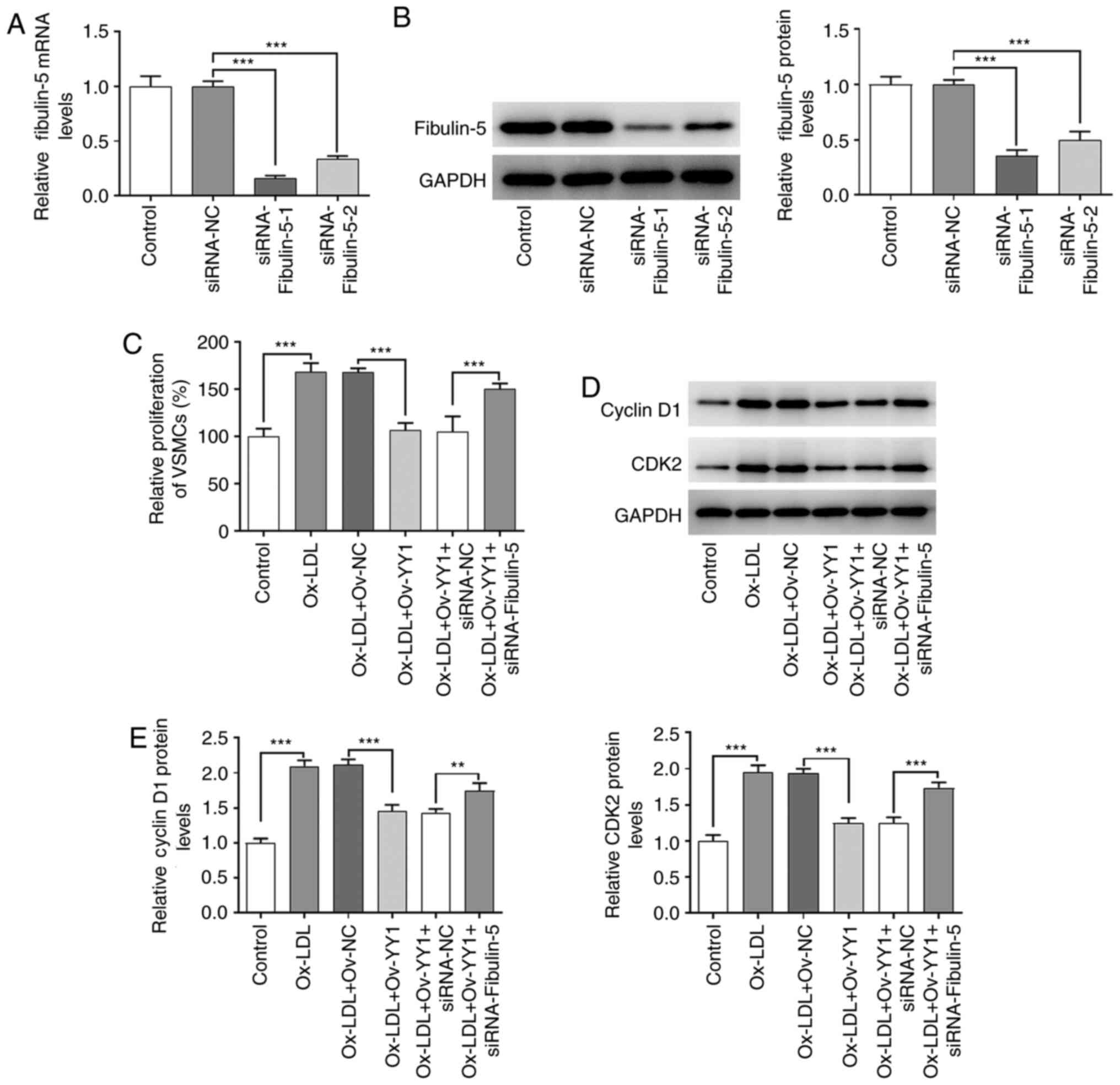
YY1 mediates the effects of fibulin-5
on proliferation, migration and invasion in HA-VSMCs stimulated
with ox-LDL
The present study next aimed to determine whether
fibulin-5 mediated the effects of YY1 in cultured HA-VSMCs
stimulated with ox-LDL. First, the transfection efficiency of small
interfering (si)RNA-fibulin-5 was tested in the HA-VSMCs. Analyzes
of fibulin-5 expression in HA-VSMCs demonstrated that it was
effectively knocked down by siRNA-Fibulin-5 transfection compared
with cell transfected with siRNA-NC (P<0.001; Fig. 6A and B). Since it was the most efficient in
knocking down fibuin-5 expression, si-fibulin-1 was selected for
further experiments. Based on the present results, it was
hypothesized that YY1 interacted with the promoter sites of
fibulin-5 to regulate cell proliferation, migration and invasion in
VSMCs treated with ox-LDL. First, cell viability was compared
between VSMCs transfected with siRNA-NC and with siRNA-fibulin-5.
Fibulin-5 silencing induced a significant reversal of the
potentiating effects due to YY1 overexpression on cell viability,
cyclin D1 and CDK2 expression (P<0.01 and P<0.001; Fig. 6C-E). Significant differences were
also observed in the cell migration and invasion of HA-VSMCs, and
the expression of MMP2, MMP9 and α-SMA between Ov-YY1 vs. Ov-NC and
between Ov-YY1+siRNA-NC and with Ov-YY1+siRNA-fibulin-5 (P<0.05,
P<0.01 and P<0.001; Fig.
7A-G). This suggests that YY1 participated in mediating the
effects of fibulin-5 on cell viability, migration and invasion
induced by ox-LDL.
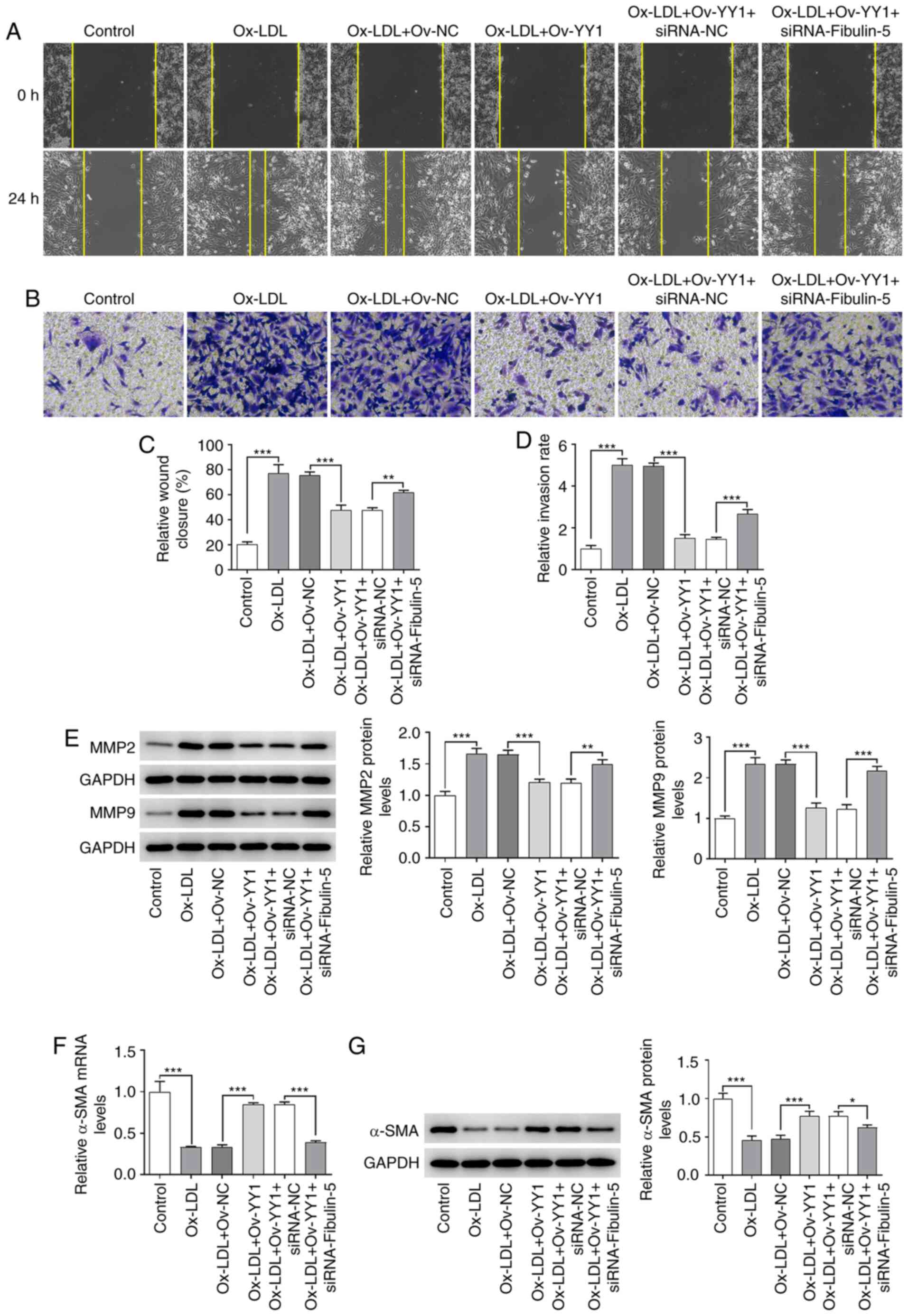 | Figure 7The effects of YY1 overexpression
migration and invasion were reversed by fibulin-5 silencing.
HA-VSMCs after ox-LDL treatment, YY1 overexpression and/or
fibulin-5 knockdown were subjected to (A) wound healing and (B)
Transwell assays to compare (C) cell migration and (D) invasion.
Magnification, x100. (E) Western blot analyses were performed after
ox-LDL treatment, YY1 overexpression and/or fibulin-5 knockdown to
measure the protein expression of MMP2 and MMP9. (F) Reverse
transcription-quantitative PCR and (G) western blotting were
performed to determine α-SMA expression levels. Data are presented
as the mean ± SD. *P<0.05, **P<0.01 and
***P<0.001. HA-VSMC, human aortic-vascular smooth
muscle cell; ox-LDL, oxidized low-density lipoprotein; YY1, Yin
Yang-1; siRNA, small interfering RNA; NC, negative control; α-SMA,
smooth muscle α-actin. |
Discussion
The present study demonstrated that fibulin-5
participated in ox-LDL-induced cell viability, invasion and
migration in VSMCs. A previous study on the fibulin-5 mechanism
revealed that fibulin-5 was involved in arterial stiffening in
patients with AS (20). The present
study indicated that YY1 regulated the promoter activity and
expression levels of fibulin-5 in VSMCs under ox-LDL treatment,
which provided a new insight in the understanding of the role of
fibulin-5 in AS.
The role of fibulin-5 in AS was found to involve the
binding of extracellular superoxide dismutase to the vascular
tissue to modulate vascular superoxide production, which was
identified to serve a vital role in AS (21). As for the regulatory mechanism of
fibulin-5 promoter activity, a previous report indicated that SOX9
could regulate the fibulin-5 promoter activity in VSMCs under the
stimulus of TNF-α (22). In the
present study, a YY1 binding sequence on the fibulin-5 promoter was
identified and predicted, where it was verified further that YY1
could directly bind to the fibulin-5 promoter and regulate its
expression. This translated downstream to the regulation of the
biological processes of VSMCs in response to ox-LDL. However, the
molecular mechanism by which YY1 binding activates fibulin-5
transcription was not uncovered in the present study, which was
predicted to be associated with YY1 involvement in transcriptional
activation by forming complexes with some proteins, such as INO80
or Sp1(23) in the promoter of
fibulin-5. YY1 has been reported to be a transcriptional activator
of some genes, such as Xist in ES cells and CDC6 in HEK293 cells,
(24,25). For instance, YY1 initiated the
activity of the X-inactive specific transcript (Xist) promoter by
directly binding to the Xist 5' region in ES cells (24). Downstream, YY1 was also revealed to
be involved in the pathophysiology of AS by regulating cell
proliferation and apoptosis (12,26,27).
For example, a previous study reported that YY1 was involved in the
promoting effects of miR-544 on the maturation and antioxidative
effects of stem cell-derived endothelial-like cells (28). A previous mechanistic analysis of
YY1 in ox-LDL-treated macrophages of AS demonstrated that YY1 can
translocate into the nucleus and increase the expression of miR-29a
by binding to its transcriptional promoter region (27).
In addition to its regulatory role in cell
viability, invasion and migration, fibulin-5 overexpression
markedly reduced the expression levels of cyclin D1 and CDK2, which
regulate G1-phase cell cycle progression and
subsequently proliferation in VSMCs (29,30). A
previous study found that the expression levels of YY1, which is a
negative regulator of cyclin D1, could be regulated by miR-29a
(31). The present results suggest
that cyclin D1 and CDK2 can participate in the regulation of
YY1/fibulin-5 during cell proliferation. Although the present study
revealed that fibulin-5/YY1 affected the viability, invasion and
migration of HA-VSMCs stimulated with ox-LDL, whether this axis is
involved in the pathogenesis of AS requires further study, which is
a limitation of the present study.
In conclusion, the present study presented evidence
that fibulin-5, regulated by YY1, can suppress the viability,
invasion and migration of HA-VSMCs stimulated with ox-LD. The
present results provided a new insight for the further
investigation of the mechanism of AS. Targeting fibulin-5/YY1 can
be a potential therapeutic option for AS. However, further studies
should be performed, mainly on the molecular mechanism by which the
binding of YY1 can activate fibulin-5 transcription. In addition,
the mechanism of YY1/fibulin-5 would need to be validated in
vivo.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and YZ made substantial contributions to the
conception and design of the study. LW collected clinical samples
and interpreted the patient data regarding fibulin-5 expression.
LW, CL, CF and YZ performed other experiments and collected data.
LW and YZ interpreted the data, drafted and revised the manuscript
for important intellectual content. LW and YZ confirm the
authenticity of the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Laixi Municipal Hospital (approval no.
ky-2020-041-03). Oral informed consent was obtained from all
participants prior to enrollment in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao Q, Guo Z, Du S, Ling H and Song C:
Circular RNAs in the pathogenesis of atherosclerosis. Life Sci.
255(117837)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kattoor AJ, Goel A and Mehta JL: LOX-1:
Regulation, signaling and its role in atherosclerosis. Antioxidants
(Basel). 8(218)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cheng YC, Sheen JM, Hu WL and Hung YC:
Polyphenols and oxidative stress in atherosclerosis-related
ischemic heart disease and stroke. Oxid Med Cell Longev.
2017(8526438)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lu QB, Wan MY, Wang PY, Zhang CX, Xu DY,
Liao X and Sun HJ: Chicoric acid prevents PDGF-BB-induced VSMC
dedifferentiation, proliferation and migration by suppressing
ROS/NF-κB/mTOR/P70S6K signaling cascade. Redox Biol. 14:656–668.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ito S, Yokoyama U, Nakakoji T, Cooley MA,
Sasaki T, Hatano S, Kato Y, Saito J, Nicho N, Iwasaki S, et al:
Fibulin-1 integrates subendothelial extracellular matrices and
contributes to anatomical closure of the ductus arteriosus.
Arterioscler Thromb Vasc Biol. 40:2212–2226. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu BF, Liu R, Huang CX, He BS, Li GY, Sun
HS, Feng ZP and Bao MH: Identification of key genes in ruptured
atherosclerotic plaques by weighted gene correlation network
analysis. Sci Rep. 10(10847)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gao JB, Lin L, Men XQ, Zhao JB, Zhang MH,
Jin LP, Gao SJ, Zhao SQ and Dong JT: Fibulin-5 protects the
extracellular matrix of chondrocytes by inhibiting the
Wnt/β-catenin signaling pathway and relieves osteoarthritis. Eur
Rev Med Pharmacol Sci. 24:5249–5258. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Meliala ITS, Hosea R, Kasim V and Wu S:
The biological implications of Yin Yang 1 in the hallmarks of
cancer. Theranostics. 10:4183–4200. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu H, Schmidt-Supprian M and Shi Y,
Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J,
Rajewsky K and Shi Y: Yin Yang 1 is a critical regulator of B-cell
development. Genes Dev. 21:1179–1189. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Blättler SM, Cunningham JT, Verdeguer F,
Chim H, Haas W, Liu H, Romanino K, Rüegg MA, Gygi SP, Shi Y and
Puigserver P: Yin Yang 1 deficiency in skeletal muscle protects
against rapamycin-induced diabetic-like symptoms through activation
of insulin/IGF signaling. Cell Metab. 15:505–517. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sui G, Affar el B and Shi Y, Brignone C,
Wall NR, Yin P, Donohoe M, Luke MP, Calvo D, Grossman SR and Shi Y:
Yin Yang 1 is a negative regulator of p53. Cell. 117:859–872.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yue Y, Lv W, Zhang L and Kang W: miR-147b
influences vascular smooth muscle cell proliferation and migration
via targeting YY1 and modulating Wnt/β-catenin activities. Acta
Biochim Biophys Sin (Shanghai). 50:905–913. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tian FJ, An LN, Wang GK, Zhu JQ, Li Q,
Zhang YY, Zeng A, Zou J, Zhu RF, Han XS, et al: Elevated
microRNA-155 promotes foam cell formation by targeting HBP1 in
atherogenesis. Cardiovasc Res. 103:100–110. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Grootaert MOJ, Moulis M, Roth L, Martinet
W, Vindis C, Bennett MR and De Meyer GRY: Vascular smooth muscle
cell death, autophagy and senescence in atherosclerosis. Cardiovasc
Res. 114:622–634. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kavurma MM and Khachigian LM: Sp1 inhibits
proliferation and induces apoptosis in vascular smooth muscle cells
by repressing p21WAF1/Cip1 transcription and cyclin
D1-Cdk4-p21WAF1/Cip1 complex formation. J Biol Chem.
278:32537–32543. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nasiri-Ansari Ν, Dimitriadis GK,
Agrogiannis G, Perrea D, Kostakis ID, Kaltsas G, Papavassiliou AG,
Randeva HS and Kassi E: Canagliflozin attenuates the progression of
atherosclerosis and inflammation process in APOE knockout mice.
Cardiovasc Diabetol. 17(106)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Terzuoli E, Nannelli G, Giachetti A,
Morbidelli L, Ziche M and Donnini S: Targeting
endothelial-to-mesenchymal transition: The protective role of
hydroxytyrosol sulfate metabolite. Eur J Nutr. 59:517–527.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Viedma-Rodríguez R, Martínez-Hernández MG,
Martínez-Torres DI and Baiza-Gutman LA: Epithelial mesenchymal
transition and progression of breast cancer promoted by diabetes
mellitus in mice are associated with increased expression of
glycolytic and proteolytic enzymes. Horm Cancer. 11:170–181.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Paapstel K, Zilmer M, Eha J, Tootsi K,
Piir A and Kals J: Association between Fibulin-1 and aortic
augmentation index in male patients with peripheral arterial
disease. Eur J Vasc Endovasc Surg. 51:76–82. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nguyen AD, Itoh S, Jeney V, Yanagisawa H,
Fujimoto M, Ushio-Fukai M and Fukai T: Fibulin-5 is a novel binding
protein for extracellular superoxide dismutase. Circ Res.
95:1067–1074. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Orriols M, Varona S, Martí-Pàmies I, Galán
M, Guadall A, Escudero JR, Martín-Ventura JL, Camacho M, Vila L,
Martínez-González J and Rodríguez C: Down-regulation of Fibulin-5
is associated with aortic dilation: role of inflammation and
epigenetics. Cardiovasc Res. 110:431–442. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sarvagalla S, Kolapalli SP and
Vallabhapurapu S: The two sides of YY1 in cancer: A friend and a
foe. Front Oncol. 9(1230)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Makhlouf M, Ouimette JF, Oldfield A,
Navarro P, Neuillet D and Rougeulle C: A prominent and conserved
role for YY1 in Xist transcriptional activation. Nat Commun.
5(4878)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cai Y, Jin J, Yao T, Gottschalk AJ,
Swanson SK, Wu S, Shi Y, Washburn MP, Florens L, Conaway RC and
Conaway JW: YY1 functions with INO80 to activate transcription. Nat
Struct Mol Biol. 14:872–874. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Wei SY, Shih YT, Wu HY, Wang WL, Lee PL,
Lee CI, Lin CY, Chen YJ, Chien S and Chiu JJ: Endothelial Yin Yang
1 phosphorylation at S118 induces atherosclerosis under flow. Circ
Res. 129:1158–1174. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jian D, Dai B, Hu X, Yao Q, Zheng C and
Zhu J: ox-LDL increases microRNA-29a transcription through
upregulating YY1 and STAT1 in macrophages. Cell Biol Int.
41:1001–1011. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guo J, Xiang Q, Xin Y, Huang Y, Zou G and
Liu T: miR-544 promotes maturity and antioxidation of stem
cell-derived endothelial like cells by regulating the YY1/TET2
signalling axis. Cell Commun Signal. 18(35)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qin L, Yang YB, Yang YX, Gong YZ, Li XL,
Li GY, Luo HD, Xie XJ, Zheng XL and Liao DF: Inhibition of smooth
muscle cell proliferation by ezetimibe via the cyclin D1-MAPK
pathway. J Pharmacol Sci. 125:283–291. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vadiveloo PK, Filonzi EL, Stanton HR and
Hamilton JA: G1 phase arrest of human smooth muscle cells by
heparin, IL-4 and cAMP is linked to repression of cyclin D1 and
cdk2. Atherosclerosis. 133:61–69. 1997.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Li YH, Liu C, Nie CJ, Zhang XH,
Zheng CY, Jiang W, Yin WN, Ren MH, Jin YX, Liu SF, et al: miR-29a
regulates vascular neointimal hyperplasia by targeting YY1. Cell
Prolif. 50(e12322)2017.PubMed/NCBI View Article : Google Scholar
|