Introduction
Diabetes mellitus is a chronic disease, which is
becoming increasingly widespread globally. It causes several
secondary complications, including heart disease, renal failure,
blindness, nerve injury and vascular calcification (VC) (1,2). It
has been reported that the global incidence of diabetes is 8.8%,
and 70-80% of the affected population succumb to cardiovascular
complications, making it the leading cause of mortality in patients
with diabetes (3). VC is an
important sign suggesting a high risk of cardiovascular disease
(4). Although VC was previously
considered to be a manifestation of the aging process, present
research indicates that VC is a strictly regulated and active
process, similar to bone formation (5). However, the mechanism of VC in a
high-glucose (HG) environment is unclear. Macrophages have been
shown to release numerous inflammatory cytokines, including tumor
necrosis factor (TNF)-α, in a HG environment to maintain glucose
and lipid homeostasis. These factors also maintain the body in a
low-intensity inflammatory state, which can activate the
Wnt/β-catenin pathway in vascular smooth muscle cells (VSMCs),
leading to VC (6). In the process
of VC, the osteoblastic differentiation of VSMCs is the most
important step (5). Under normal
conditions, VSMCs secrete a series of endogenous calcium
inhibitors, including osteopontin, osteoprotegerin and matrix Gla
protein, which inhibit osteoblastic differentiation (4). During VC, the levels of these
endogenous calcium inhibitors are reduced by HG and low-intensity
inflammation. In addition, the concentration of intracellular
phosphate is increased by the sodium-dependent phosphate
cotransporter Pit-1, which contributes to the transformation of
VSMCs into osteoblast-like cells. Under the control of core binding
factor A1, these osteoblasts express alkaline phosphatase (ALP) and
bone-related proteins such as osteocalcin and bone morphogenetic
protein-2, which further promote osteoblastic differentiation and
the calcification of VSMCs (4).
Hence, activation of the Wnt/β-catenin pathway in VSMCs under
low-intensity inflammation may be a key cause of VC in HG
conditions.
The Wnt/β-catenin pathway plays an important role in
osteoblastic differentiation (7).
Normally, without the intervention of Wnt proteins, a destruction
complex is formed that is able to bind with and phosphorylate
β-catenin in the cytoplasm and disable it. However, when Wnt
protein is secreted, it combines with cell-surface receptors and
helps the Axin component of the destruction complex to combine with
phosphorylated low-density lipoprotein receptor-related protein,
resulting in the breakdown of the destruction complex and the
accumulation of high levels of β-catenin in the cytoplasm.
β-catenin then enters the nucleus and binds with the DNA-binding T
cell factor transcription factor, which activates Wnt gene
transcription and recruits several transcriptional coactivators and
histone modifiers, such as Brg1, CREB binding protein, Cdc47, Bcl9
and Pygopus, to drive target gene expression (8).
Transforming growth factor-β (TGF-β) is a
multifunctional cytokine, which is highly expressed in most tumor
cells and helps to regulate the Wnt/β-catenin pathway. In addition
to promoting the epithelial-mesenchymal transition, invasion and
metastasis of tumor cells, TGF-β serves an important role in the
regulation of the adaptive immune system (9). TGF-β has been reported to inhibit the
expression of various pro-inflammatory factors in macrophages,
including interleukin (IL)-1β and IL-8, and to regulate TNF-α under
certain conditions (10). Due to
its anti-inflammatory effect, TGF-β may regulate the Wnt/β-catenin
pathway, which is potentially the key cause of the phenotypic
transformation of VSMCs and, therefore, is a potential therapeutic
target.
Long intergenic RNA-erythroid pro-survival
(lincRNA-EPS) activates TGF-β and thus inhibits the Wnt/β-catenin
pathway (11). Therefore,
lincRNA-EPS is indicated to be a regulatory factor that suppresses
the dedifferentiation and transformation of VSMCs. lincRNAs are
RNAs >200 nucleotides in length that control gene transcription
by binding to chromatin-modifying factors, heteronuclear
ribonucleoproteins or transcription factors (11). Although they lack protein-coding
potential, they are important regulatory molecules for gene
expression (12). lincRNA-EPS is
expressed in macrophages and dendritic cells, where it inhibits
macrophage function and the expression of inflammatory genes. A
previous study demonstrated that lincRNA-EPS precisely regulates
macrophages to inhibit the expression of inflammation-related genes
(IRGs); a combination of gain-of-function and rescue experiments
and the transcriptome analysis of mouse macrophages lacking
lincRNA-EPS revealed the role of lincRNA as an inhibitor of IRG
expression and repressor of inflammatory responses (11). Therefore, in the present study, the
role of lincRNA-EPS in the process of VC was explored to determine
its potential as a target for clinical treatment in the management
of diabetic angiopathy.
In the present study, in vivo and in
vitro models were used to study whether lincRNA-EPS affects the
osteoblastic differentiation of mouse VSMCs. In vitro, VSMCs
were transfected with lincRNA-EP empty control, lincRNA-EPS small
interfering RNA (siRNA) or empty control and cultured in a HG
environment. The ALP activity and expression of relevant proteins
were observed using immunofluorescence and western blot analysis.
An in vivo mouse model of diabetes was also established, and
double lincRNA-EPS knockout and overexpression methods were used to
study VC under various conditions.
Materials and methods
Animal experiments
A total of 20 six-week-old male C57BL/6 mice
weighing 19-21 g, purchased from the Animal Center of Army Medical
University were used for adaptive breeding for 1 week. Animal care
and procedures were carried out with the approval of the Animal
Management Committee of the General Hospital of Central Theater
Command and strictly complied with the National Institutes of
Health (NIH) Guide for the Care and Use of Laboratory Animals. The
specific feeding environment was as follows: The temperature was
controlled at 18-22˚C, with humidity at 50-60%. In order to keep
the air fresh, the ammonia concentration did not exceed 20 ppm, and
the ventilation times reached 10-20 air changes/h. The light
intensity at 1 m from the ground was 200 lux, and the light/dark
cycle was 12:12. Food from Jiangsu Xietong Pharmaceutical
Bio-engineering Co., Ltd. was eaten at 3-7 g/day, supplied 3-4
times per week. Water was supplied through drinking bottles. Of
these, 10 randomly selected mice were given normal feed and the
other 10 mice were given high-fat feed (40% fat, 42% carbohydrate
and 18% protein). After 1 month of feeding and fasting for 12 h
(overnight), the mice fed a high-fat diet were injected
intraperitoneally with 100 mg/kg streptozotocin (dissolved in 0.1
mol/l sodium citrate buffer; pH 4.2), while the mice fed a normal
diet were given a similar volume of sodium citrate buffer. After 7
days, blood glucose levels were checked and the mice with fasting
blood glucose levels ≥11.1 mmol/l were considered as a suitable
model for diabetes (13,14). A wild-type group was created by the
random selection of six mice from those that met the criteria for
the diabetic model. A total of 6 mice were randomly selected from
the 10 mice given normal feed to form the control group.
Chemicals
Parathyroid hormone (PTH; cat. no. H207) and ALP
(cat. no. A059-2-2) ELISA kits were purchased from Nanjing
Jiancheng Bioengineering Institute. The fibroblast growth factor-23
(FGF-23) ELISA kit (cat. no. EM0271) was purchased from Wuhan Fine
Biotech Co., Ltd. Fetal bovine serum (FBS; cat. no. 30067334) was
purchased from Gibco (Thermo Fisher Scientific, Inc.). DMEM (cat.
no. SH30021.01), streptomycin (cat. no. SV30010) and penicillin
(cat. no. SV30010) were purchased from HyClone (Cytiva).
TRIzol® reagent (cat. no. 15596018) was purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). The BCA kit (cat. no.
C503021-0500) was purchased from Sangon Biotech Co., Ltd.
VSMC culture
After feeding, 12 male C57BL/6 mice (6-week-old,
weighing 19-21 g) from two groups were euthanized with
CO2. The flow rate of CO2 was 10-30% of the
euthanasia chamber volume/min, and the mice were exposed to 100%
CO2 for 5 min. After their vital signs disappeared, the
mice were observed for 2 min to confirm their death. The common
carotid artery was then surgically removed. The adventitia of the
aorta was removed and cut into 1-mm2 tissue blocks,
which were cultured in primary culture medium for 3-5 generations
to provide VSMCs. The VSMC culture medium comprised α-MEM (cat. no.
SH30265.01; HyClone; Cytiva) supplemented with 10% FBS, 100 U/ml
penicillin and 0.1 mg/ml streptomycin.
LincRNA-EPS fragment transfection into
VSMCs
The cultured VSMCs were used to establish normal,
empty control (empty vector), lincRNA-EPS overexpression vector,
lincRNA-EPS siRNA and si-negative control (NC) groups. The VSMCs
were digested with 0.125% trypsin for ~2 min. After centrifugation
at 100 x g at 25˚C for 10 min, the VSMCs were seeded at a density
of 1x104 cells/cm3 into 6-well plates and
cultured for 24 h. When the cells had grown to 60-70% confluence,
the medium was changed to a serum-free medium. The VSMCs were then
transfected with 200 nM si-negative control (NC) or lincRNA-EPS
siRNA, or with 2.5 µg plasmid containing lincRNA-EPS overexpression
or empty control (Thermo Fisher Scientific, Inc.), using the
Lipofectamine® 2000 transfection kit (cat. no. 11668027;
Thermo Fisher Scientific, Inc.). After 6 h, transfection was
terminated, and culture was continued for 24 h. Reverse
transcription-quantitative PCR (RT-qPCR) was used to detect the
expression of lincRNA-EPS in the RNA extracts from the cells
collected from each group.
The lincRNA-EPS overexpression sequence was
synthesized according to the full-length cDNA of mouse lincRNA-EPS,
and the sequence was synthesized according to its coding sequence
(Sangon Biotech Co., Ltd.). The VSMCs in the empty control group
were transfected with scrambled controls (Thermo Fisher Scientific,
Inc.) that were cloned into an expression vector; specifically, the
complete mouse lincRNA-EPS and scrambled sequences (Appendix S1) were subcloned into the
adenoviral shuttle vector pDC315 (Microbix Biosystems Inc.). The
siRNA sequences used were as follows: si-EPS1 forward,
5'-GGUUUAGCACUCACUGCUAGC-3' and reverse,
5'-UAGCAGUGAGUGCUAAACCGU-3'; si-EPS2 forward,
5'-CGCAUGGUCACUCACCUAAUA-3' and reverse,
5'-UUAGGUGAGUGACCAUGCGUG-3'; si-EPS3 forward,
5'-CAUGGUCACUCACCUAAUAAG-3' and reverse,
5'-UAUUAGGUGAGUGACCAUGCG-3'. si-NC sequences were as follows: si-NC
forward, 5'-UUCUCCGAACGUGUCACGUTT-3' and reverse,
5'-ACGUGACACGUUCGGAGAATT-3' was purchased from General Biosystems
(Anhui) Co., Ltd. All siRNA groups were tested and si-EPS1 was
selected in the end.
LincRNA-EPS knockout mice
Under the control of a phosphoglycerate kinase 1
promoter, the 4-kb lincRNA-EPS genomic site was replaced with a
neomycin cassette to generate lincRNA-EPS knockout mice as
previously described (11). In
brief, a lincRNA-EPS targeting vector was electroporated into
C57BL/6 mice embryonic stem (ES) cells and the lincRNA-EPS-positive
ES cells were injected into blastocyst-stage embryos to produce
chimeric mice. lincRNA-EPS heterozygous mice were obtained by
gamete line transmission after mating the chimeric mice with
wild-type C57BL/6 mice The resulting gene knockout mice were
designated the lincRNA-EPS knockout group
(lincRNA-EPS-/-). The lincRNA-EPS knockout group
contained 6 male mice, which were supplied with a high-fat
diet.
Overexpression of lincRNA-EPS in
mice
Using a WAVE Bioreactor system (GE WAVE 200;
Cytiva), an adeno-associated virus (AAV) with high expression of
lincRNA-EPS was constructed, packaged in 293 cells (cat. no.
CL-0005; Procell Life Science & Technology Co., Ltd.) and
purified as required. In six-well dishes, 1.2x106
subconfluent 293 cells (15) were
transfected with 2.5 µg plasmid
(pAAV-CMV-EGFP-P2A-lincRNA-EPS-3FLAG; designed by OBiO Technology
(Shanghai) Corp., Ltd.) at 4˚C for 20 min. A total of six
6-week-old male C57BL/6 mice weighing 19-21 g were injected with
1x1011 AAV particles via the tail vein (16). These mice were designated as the
lincRNA-EPS overexpression group (lincRNA-EPSTg/+). The
6 mice in the lincRNA-EPS overexpression group were supplied with a
high-fat diet. After 6 weeks, the mice from the lincRNA-EPS
knockout and overexpression groups were euthanized with
CO2, and 5 ml of blood were sampled for the extraction
of macrophages and T cells. The expression of lincRNA-EPS in these
cells was detected using RT-qPCR to confirm the success of the
transfection models (17-19).
Harvesting, cell lysis and
clarification
The mice were euthanized and their spleen was
removed under sterile conditions. The spleen was ground on a filter
screen using the piston of the syringe for 5 min. The grinding
product was put in 4-5 ml Mouse Lymphocyte Separation Medium (cat.
no. 7211011; Dakewe Biotechnology Co., Ltd.), 500 µl RPMI-1640
medium were added. Subsequently, the samples were centrifuged at
800 x g at 25˚C for 30 min. The upper cell suspension was added to
10 ml of RPMI-1640 medium, and centrifuged at 250 x g at 25˚C for
10 min. After the supernatant was removed, lympho Spot™
medium (Dakewe Biotechnology Co., Ltd.) was added to the sediment,
and the T cells were counted.
In order to extract macrophages, a total of 1 ml
starch broth was injected into the abdominal cavity of mice for 3
days, then DMEM medium was injected, fully mixed, sucked out,
centrifuged and resuspended.
RT-qPCR
The VSMCs, macrophages and T cells were lysed using
TRIzol® reagent according to the manufacturer's
instructions. RNA was extracted with isopropanol after centrifuging
the lysate with chloroform at 1,200 x g at 4˚C for 10 min. The
pellet was washed and resuspended in 75% ethanol, then air-dried
for 10 min. Then, 20 µl TE buffer at 60˚C was added. After
incubating for 10 min, the quality of the RNA was checked, and
those samples that passed the quality test were stored at
-70˚C.
The following primers were designed: ALP forward,
5'-GCCGCACCACGACTTGTTCA-3' and reverse, 5'-GCGATCGTGTTGGCGAGAAC-3'
(150 bp); Wnt3 forward, 5'-AGCTGCCTCTACTCGTGACA-3' and reverse,
5'-ATCTTGCTCCCACTGTTGGC-3' (194 bp). TGF-β1 forward,
5'-TGGAGCAACATGTGGAACTC-3' and reverse, 5'-GTCAGCAGCCGGTTACCA-3'
(73 bp). β-catenin forward, 5'-ACAGGGAAGACATCACTGAGCC-3' and
reverse, 5'-CAGTGGGATGGTGGGTGTAAGA-3' (145 bp); lincRNA-EPS
forward, 5'-CGCATTAATGGGGGCATTC-3' and reverse,
5'-CTAAACCGTGTTTTCCCCGC-3'; GAPDH forward, 5'-GAAGGGTGGAGGCAAAAG-3'
and reverse, 5'-ACCAGTGGTTGCAGGGAT-3'. The reverse transcription
kit (cat. no. 11939823001; the Licensing Department of Roche
Molecular Diagnostics, Inc.) was used to synthesize cDNA from the
RNA according to the manufacturer's protocol. The RT-qPCR system
was configured for each group of cDNA samples accordingly. qPCR was
conducted using a SYBR premix Ex Taq™ kit (Takara Bio,
Inc.) according to the manufacturer's instructions. A total of 40
PCR cycles (94˚C for 1 min for denaturation; 55˚C for 30 sec for
annealing; 72˚C for 1 min for extension) were performed and melting
curves of the PCR products were established to determine the
expression of ALP, Wnt3, β-catenin and TGF-β (20).
HG culture and ALP ELISA
According to a previously reported method (21), common carotid artery samples were
taken from 6-week-old mice and the transfected VSMCs were cultured
under HG conditions (25 mM) while the untransfected VSMCs were
cultured under normal glucose conditions. The temperature was 25˚C
and the culturing time was 3 days. Subsequently, the ALP-ELISA kit
was used to determine the ALP levels of the samples.
Western blot analysis
The total proteins were extracted from VSMCs using
RIPA lysis buffer (cat. no. 2114-100; BioVision, Inc.). The
proteins were then quantified using a BCA kit, denatured at 100˚C
for 10 min and stored at -80˚C. Western blotting was used to
determine the expression levels of ALP, TGF-β, Wnt3 and β-catenin.
A total of 100 µg of protein per sample was loaded on a 10%
SDS-PAGE gel. The proteins were subsequently transferred to PVDF
membranes, then blocked with 5% non-fat milk powder at 25˚C for 60
min. The membranes were washed with PBS buffer containing 0.1%
Tween. GAPDH antibody (cat. no. 51332S; Cell Signaling Technology,
Inc.), ALP antibody (cat. no. ab67228; Abcam), TGF-β antibody (cat.
no. 3711S; Cell Signaling Technology, Inc.), Wnt3 antibody (cat.
no. ab32249; Abcam) and β-catenin antibody (cat. no. AC106;
Beyotime Institute of Biotechnology) were used at 1:1,000 dilution
at 25˚C for 2.5 h. The membranes were subsequently incubated with
HRP-labeled Goat Anti-Mouse IgG (H+L) secondary antibodies (cat.
no. A0216; Beyotime Institute of Biotechnology) diluted by 1:1,000
at 25˚C for 1 h. Antibody detection was performed with an ECL kit
from Shanghai Zeye Biotechnology Co., Ltd. (cat. no. ZY120201).
Luciferase reporter assay
VSMCs were cultured in 24-well plates and
co-transfected with luciferase reporter plasmids and lincRNA-EPS
overexpression vector, inhibitor or their respective controls and
50 ng per well of pRL-TK vector (designed by Promega Corporation)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 25˚C for 20 min according to the
manufacturer's instructions. After co-transfection, VSMCs were
collected 48 h later and lysed prior to the measurement of
luciferase activity with the Dual-Luciferase Reporter Assay System
(Promega Corporation). Renilla luciferase activity was used
for normalization (22).
Alizarin red S staining
To evaluate the inhibitory effect of lincRNA-EPS on
VC in vitro, VSMCs transfected with 200 nM empty control,
lincRNA-EPS overexpression or lincRNA-EPS siRNA using
Lipofectamine® 2000 were cultured in DMEM for 7 days.
After washing, the cells were fixed in 4% formalin at 25˚C for 24
h, then stained with 1% alizarin red S at 37˚C for 30 min. The
VSMCs were washed with PBS to remove the excess stain and
photographic images of the stained matrix were captured using a
digital microscope. The VSMCs were then incubated in
cetyl-pyridinium chloride for 15 min to release the alizarin red S,
and the amount of released dye was measured by spectrophotometry at
570 nm.
Scratch wound assay
A total of 1x106 cells/ml VSMCs (23) were seeded onto 12-well culture
plates and cultured overnight, after which mitomycin (5 µg/ml,
Bio-Rad Laboratories, Inc.) was added for 2 h. Then, a sterile
P-200 pipette tip was used to create an artificial wound in each
well. After 12 h of culture in the presence of 1% FBS, the cells
were stained with crystal violet at 25˚C for 10 min and evaluated
using light microscopy (cat. no. DVM6; Leica Microsystems GmbH).
The results were analyzed using ImageJ software v. 1.8.0.112
(National Institutes of Health).
Morphological and immunofluorescence
analysis
Aortic tissue was fixed at 4˚C overnight in 4%
formalin and embedded in paraffin. The tissues were cut into
40-µm-thick sections. For immunofluorescence analysis, the sections
were first immersed in boiling citric acid buffer (95˚C) for 10 min
to restore antigenicity and then soaked in 0.3%
H2O2 at 95˚C for 30 min to eliminate
endogenous peroxidase. Subsequently, the sections were blocked with
5% goat serum (cat no. KJ-S-0028G; Kejing Biological Technology
Co., Ltd.) at 25˚C for 30 min. Next, after washing with PBS, the
sections were incubated with Runt-related transcription factor 2
(Runx2; 1;1,000; cat. no. ab76956; Abcam) for 2 h at 25˚C. After
washing, the sections were incubated with fluorescent secondary
antibodies (1:1,000; cat. no. AB_2534088; Thermo Fisher Scientific,
Inc.) for 1 h at 25˚C, then counterstained with DAPI for 10 min at
25˚C. Finally, the sections were stained with diaminobenzidine and
visualized using a light microscope (24).
Cytokine analysis
For the detection of cytokines, 0.2 ml of blood was
collected from each mouse under anesthesia (1-1.5% isoflurane;
Abbott Scandinavia AB) by retroorbital vein puncture. This
procedure was only performed once after 6 weeks of feeding. After
the blood was collected, rearing of the mice continued until they
were required for further analysis. ELISA kits were used to detect
the expression of inflammation-associated factors in the serum of
each group, including IL-1β (cat. no. E-EL-M0037c; Elabscience
Biotechnology, Inc.), TNF-α (cat. no. PT512; Beyotime Institute of
Biotechnology), IL-6 (cat. no. E-EL-M0044c; Elabscience
Biotechnology Co., Ltd.) and nitric oxide (NO; cat. no. YM-6178;
Shanghai Yuanmu Biological Technology Co., Ltd.).
Biochemical analysis
The mice were euthanized with CO2, and 5
ml blood and the aortas were retrieved. The aortas of the
experimental mice were rinsed with distilled water, dried and
weighed. The weight of every group was controlled to 3 mg. The
blood was collected into anticoagulation tubes (BD Biosciences).
After 3,000 x g centrifugation for 30 min at 4˚C, the supernatant
of blood was taken as plasma. ELISA kits were used to quantify
FGF-23 and PTH levels in plasma according to the manufacturer's
instructions.
Calcium deposition
The quantification of aortic calcium was carried out
by incubating the tissues at 37˚C overnight in 0.6 M HCl. The
calcium content of the supernatant was then determined using a
QuantiChrom™ Calcium assay kit (BioAssay Systems)
according to the manufacturer's protocol. The total protein
concentration was measured using the Bradford method (Bio-Rad
Laboratories, Inc.). The VSMCs were decalcified for 24 h at 4˚C in
0.6 M HCl. The calcium content was then determined using the
aforementioned QuantiChrom™ Calcium assay kit. The VSMCs
were lysed with 0.1 M NaOH/0.1% SDS. The calcium content was
normalized to the total protein concentration.
Statistical analysis
For multiple group comparisons, one-way ANOVA with
Tukey's post hoc test were performed. P<0.05 was considered to
indicate a statistically significant difference. GraphPad Prism 7
(GraphPad Software, Inc.) was used for graph construction and
statistical analyses (20).
Results
LincRNA-EPS transfection inhibits
HG-induced osteoblastic differentiation in mouse VSMCs by reducing
ALP levels
Previous studies have shown that ALP activity is an
indicator of the osteoblastic differentiation of VSMCs (25,26)
and that ALP is a significant factor in the regulation of this
process; higher ALP levels are associated with higher osteoblastic
differentiation (25,26). Experiments were performed using
mouse VSMCs transfected with lincRNA-EPS overexpression vector or
siRNA, and RT-qPCR analysis verified the transfection efficiency
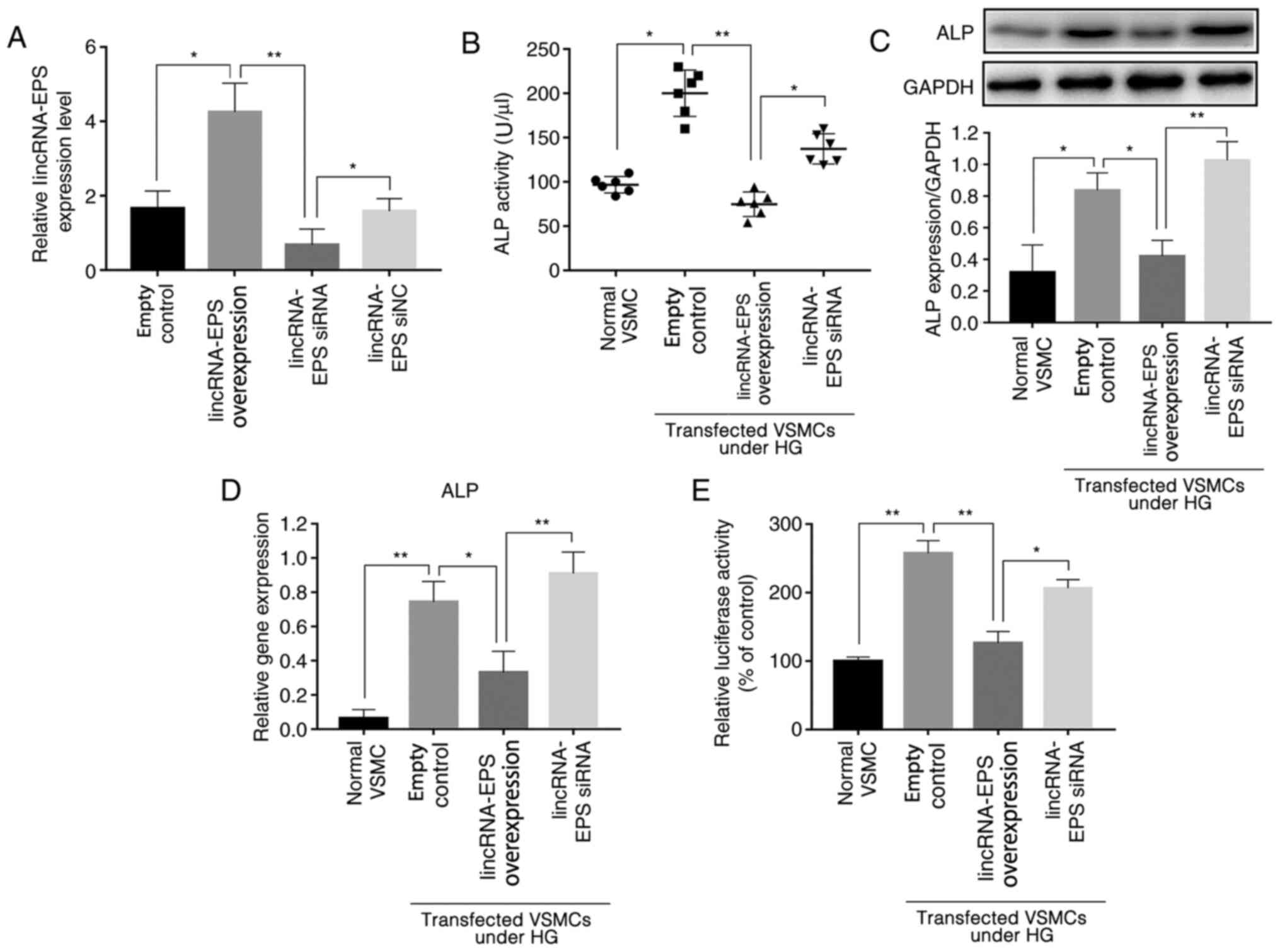
(Fig. 1A). The ALP activity of
various wild-type mouse VSMCs was tested using an ALP ELISA kit,
and the results showed that, under HG conditions, the ALP activity
of VSMCs transfected with lincRNA-EPS overexpression vector was
reduced by an average of 62.5% compared with that of cells
transfected with the empty control, and of 35% in the lincRNA-EPS
siRNA group (Fig. 1B). For further
analysis, western blotting was used to detect the expression levels
of ALP protein, and the results showed that expression of ALP in
VSMCs after transfection with lincRNA-EPS overexpression vector was
reduced by an average of 50% compared with that of cells
transfected with the empty control while, contrarily, an increase
by 25% was observed in the lincRNA-EPS siRNA group (Fig. 1C). The expression of ALP mRNA was
also detected using RT-qPCR (Fig.
1D), and the results revealed that the average expression of
ALP was reduced by 57% by the lincRNA-EPS overexpression vector,
and by 20% by the lincRNA-EPS siRNA compared with the empty
control. In addition, the luciferase reporter assay showed that the
ALP gene promoter activity in wild-type VSMCs transfected with
lincRNA-EPS overexpression vector was downregulated by 50% compared
with that in the empty control group, while ALP gene expression in
VSMCs transfected with lincRNA-EPS overexpression vector was
downregulated by 50% compared to that in the control group and no
obvious downregulation was observed in the lincRNA-EPS siRNA group
(Fig. 1E). The present findings
suggest that the transfection of lincRNA-EPS has a significant
inhibitory effect on the expression of ALP, indicating that it may
affect the osteoblastic differentiation of VSMCs.
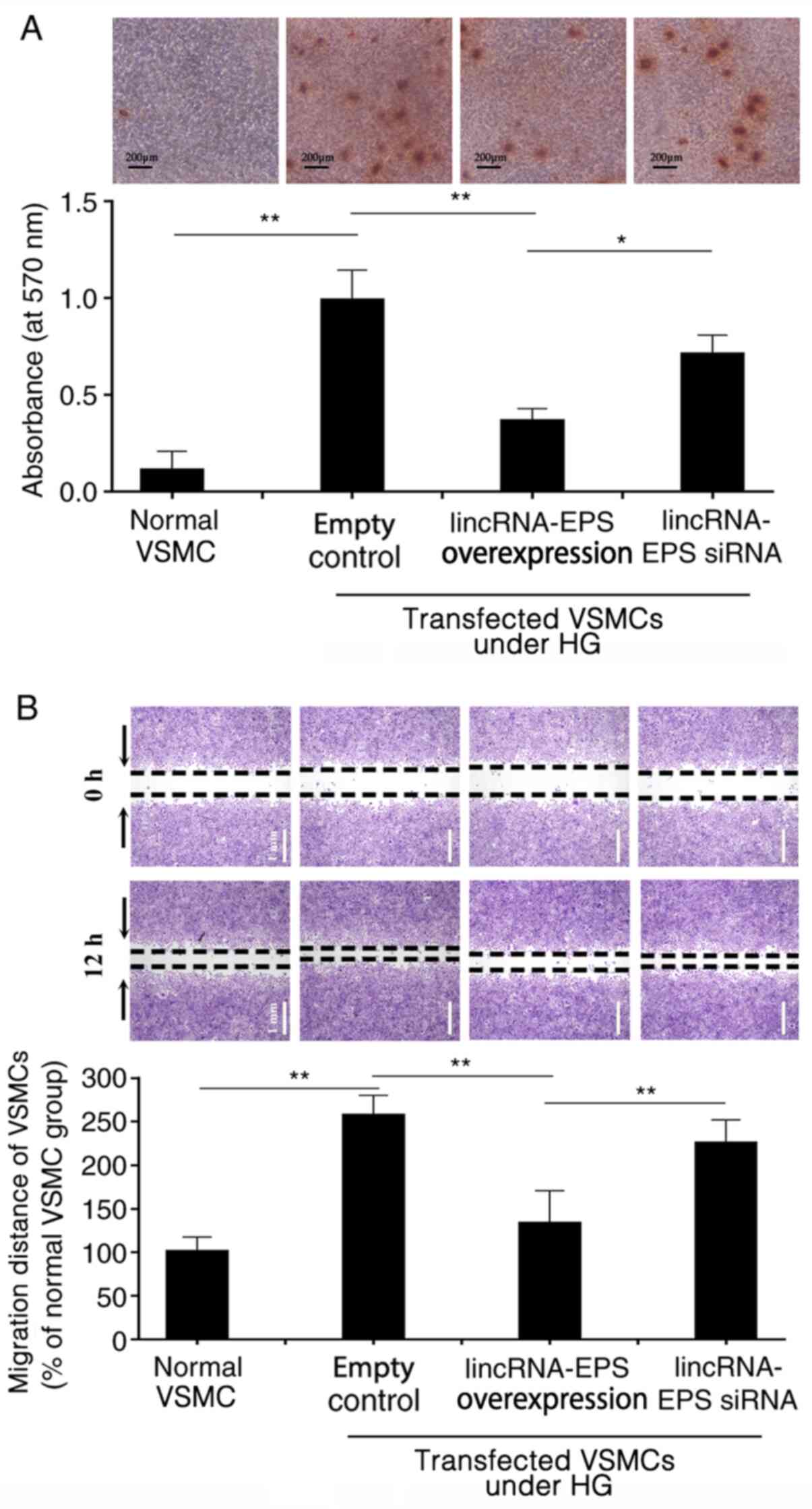
LincRNA-EPS transfection inhibits
HG-induced VSMC migration and calcification
HG triggers calcium deposition in wild-type VSMCs,
which may result from the osteoblastic differentiation of VSMCs
(27,28). Alizarin red S staining revealed
that, in HG conditions, calcium deposition was significantly
inhibited in VSMCs following transfection with lincRNA-EPS
overexpression vector compared with empty control. However, calcium
deposition remained at higher levels in the VSMCs transfected with
the lincRNA-EPS siRNA (Fig. 2A).
The absorbance of Alizarin red from the VSMCs in each group was
measured using spectrophotometry to evaluate the osteogenic
differentiation of VSMCs in each group. The absorbance value of the
empty control group was set at 1.0 and the relative absorbance
value of the lincRNA-EPS overexpression transfection group was
0.35. The absorbance of the VSMCs transfected with lincRNA-EPS
overexpression vector was lower than those transfected with empty
control and the lincRNA-EPS siRNA group (Fig. 2A). In the wound healing assay,
microscopy was used to observe the migration of VSMCs in each
group. HG increased the migration of VSMCs by 175% compared with
that of VSMCs under normal conditions. However, migration was
reduced by 50% following transfection with lincRNA-EPS
overexpression vector compared with the empty control (Fig. 2B).
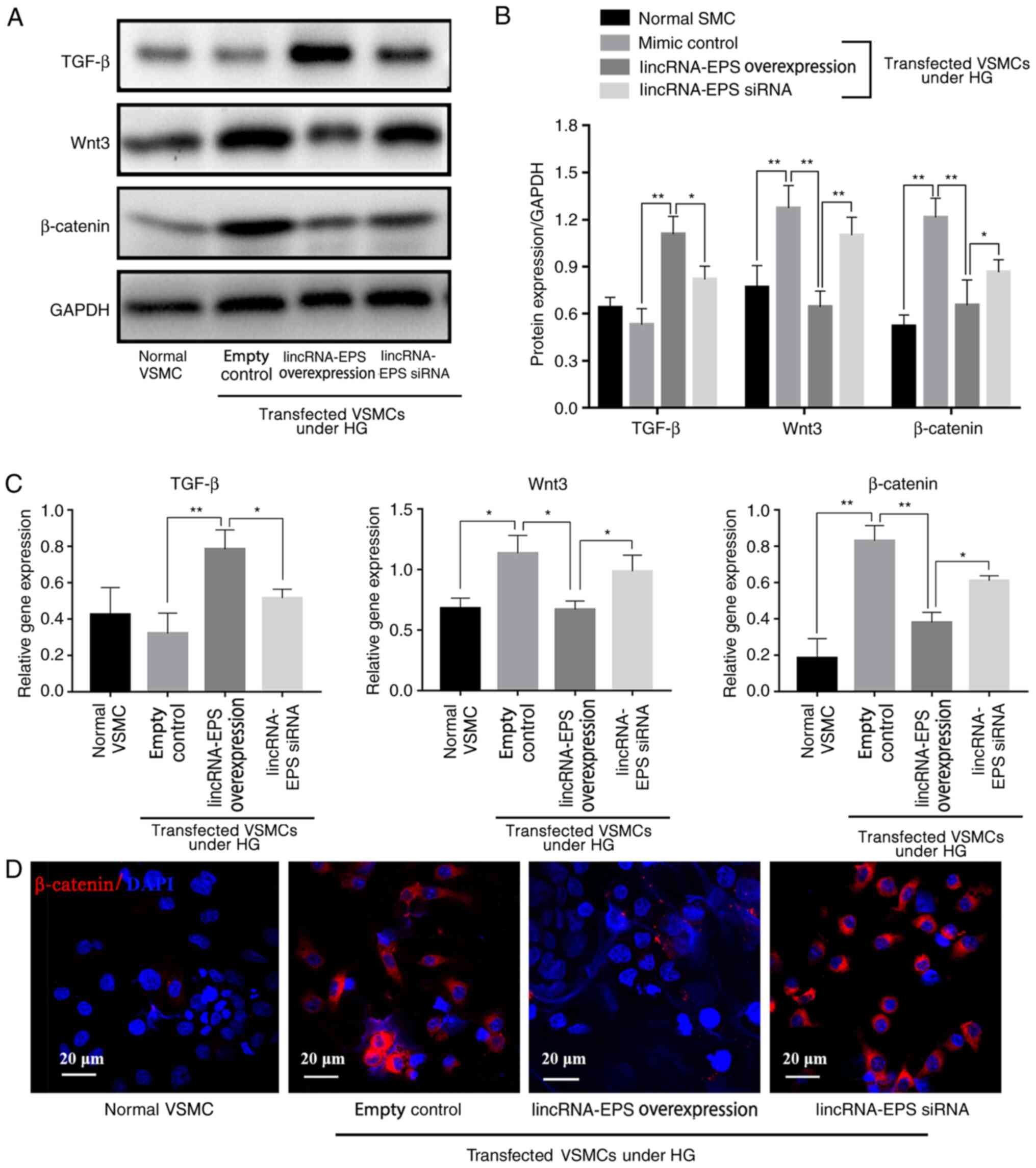
LincRNA-EPS activates TGF-β to
suppress inflammation and inhibits HG-induced Wnt pathway
activation
Results from western blot analysis showed that,
under HG conditions, Wnt3 expression decreased by 50%, β-catenin
expression decreased by 46% and TGF-β expression increased by 70%
in the lincRNA-EPS overexpression group compared with the empty
control group, indicating that lincRNA-EPS significantly inhibited
the expression of Wnt3 and β-catenin proteins and increased that of
TGF-β (Fig. 3A and B). RT-qPCR results showed that, in the
lincRNA-EPS overexpression group, Wnt3 levels were reduced by 36%,
β-catenin levels were reduced by 50% and TGF-β levels were
increased by 128% compared with those in the empty control group,
suggesting that lincRNA-EPS significantly inhibited Wnt3 and
β-catenin protein expression and enhanced TGF-β expression due to
the upregulation of the mRNA expression of TGF-β and downregulation
of the mRNA expression of Wnt3 and β-catenin in VSMCs (Fig. 3C). Immunofluorescence staining
(Fig. 3D) further demonstrated that
transfection with lincRNA-EPS overexpression vector reduced the
expression of β-catenin.
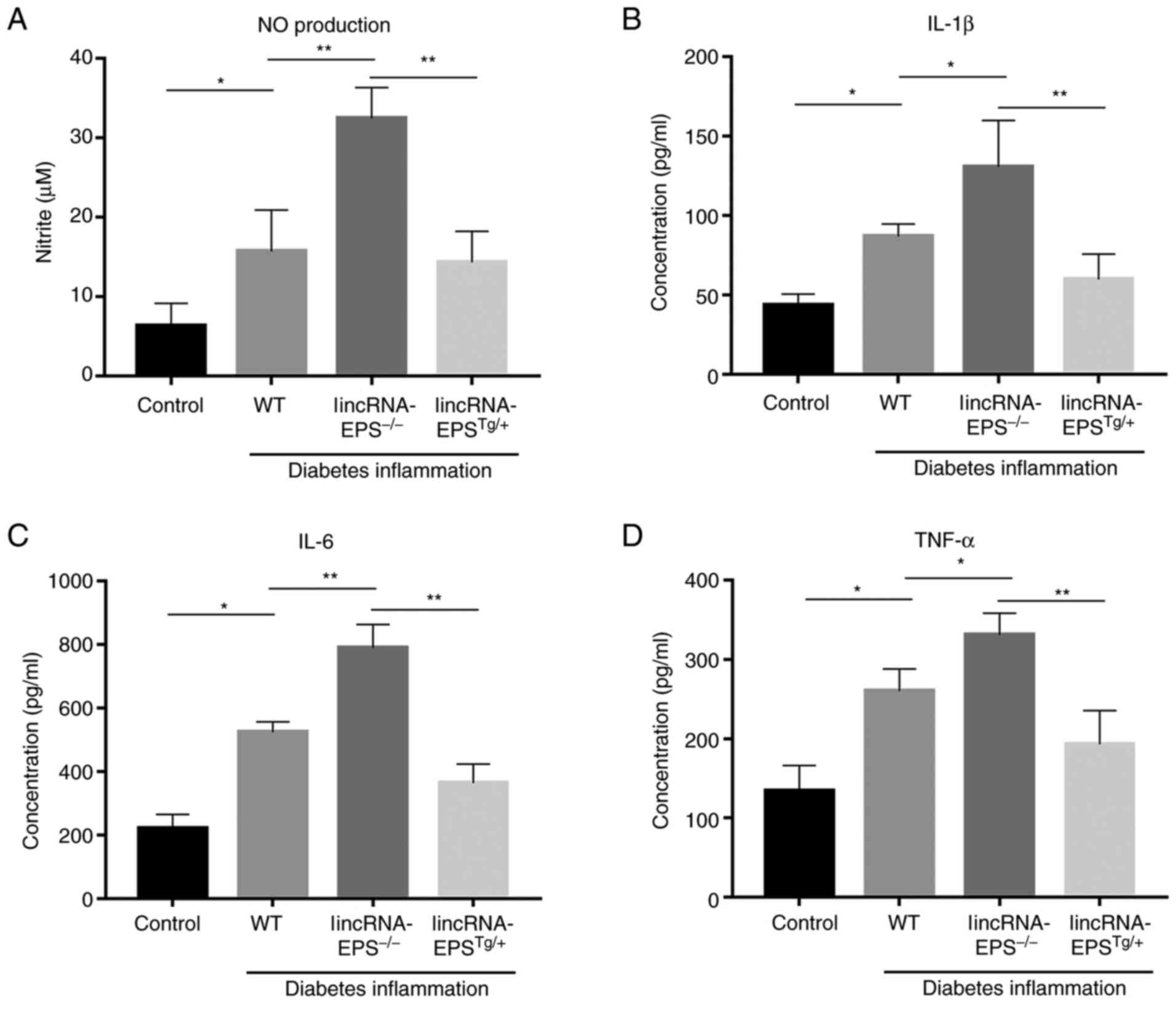
LincRNA-EPS reduces the levels of
inflammatory factors in the plasma of diabetic mice
After knocking out lincRNA-EPS from mice, there was
a significant increase in the levels of several inflammatory
factors, including NO, IL-1β, IL-6 and TNF-α. Among them, NO
increased by 100% (Fig. 4A), IL-1β
by 56% (Fig. 4B), IL-6 by 60%
(Fig. 4C) and TNF-α by 28%
(Fig. 4D) compared with those in
wild-type mice. In the mice with lincRNA-EPS overexpression, the
levels of these inflammatory factors were noticeably reduced
compared with those of the wild-type control. The present results
indicated the significance of lincRNA-EPS in the suppression of
inflammation.
LincRNA-EPS inhibits VC in the
diabetic mouse model
Runx2 is a transcription factor that can induce Sp7,
another important transcription factor, which is able to promote
the osteoblastic differentiation of VSMCs through the Wnt pathway
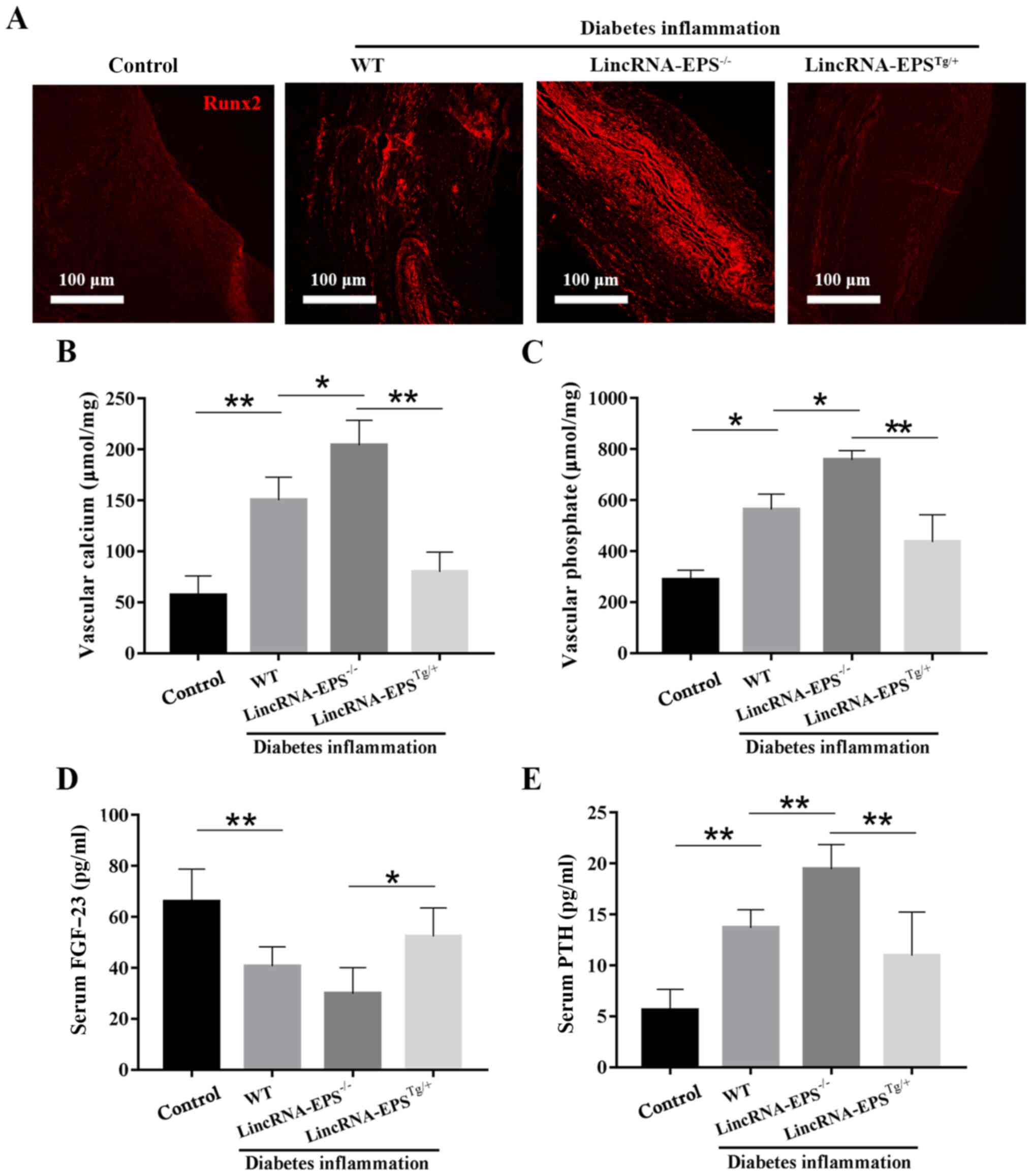
(28). As shown in Fig. 5A, Runx2 expression was low in the
aorta of the control group and abundant in the
lincRNA-EPS-/- group, but notably reduced in the
lincRNA-EPSTg/+ group compared with that in the
wild-type diabetic group. These results suggest that lincRNA-EPS
may affect the osteoblastic differentiation of VSMCs by suppressing
the expression of Runx2. As illustrated in Fig. 5B-E, compared with the wild-type
diabetic group, the levels of vascular calcium, vascular phosphate
and serum PTH in the lincRNA-EPSTg/+ group were notably
reduced and the levels of serum FGF-23, a blood
phosphorus-regulating hormone that promotes the excretion of
phosphate, were increased. Specifically, vascular calcium decreased
by 50%, vascular phosphate by 18% and serum PTH by 7%, while serum
FGF-23 increased by 25%. The present results confirmed the positive
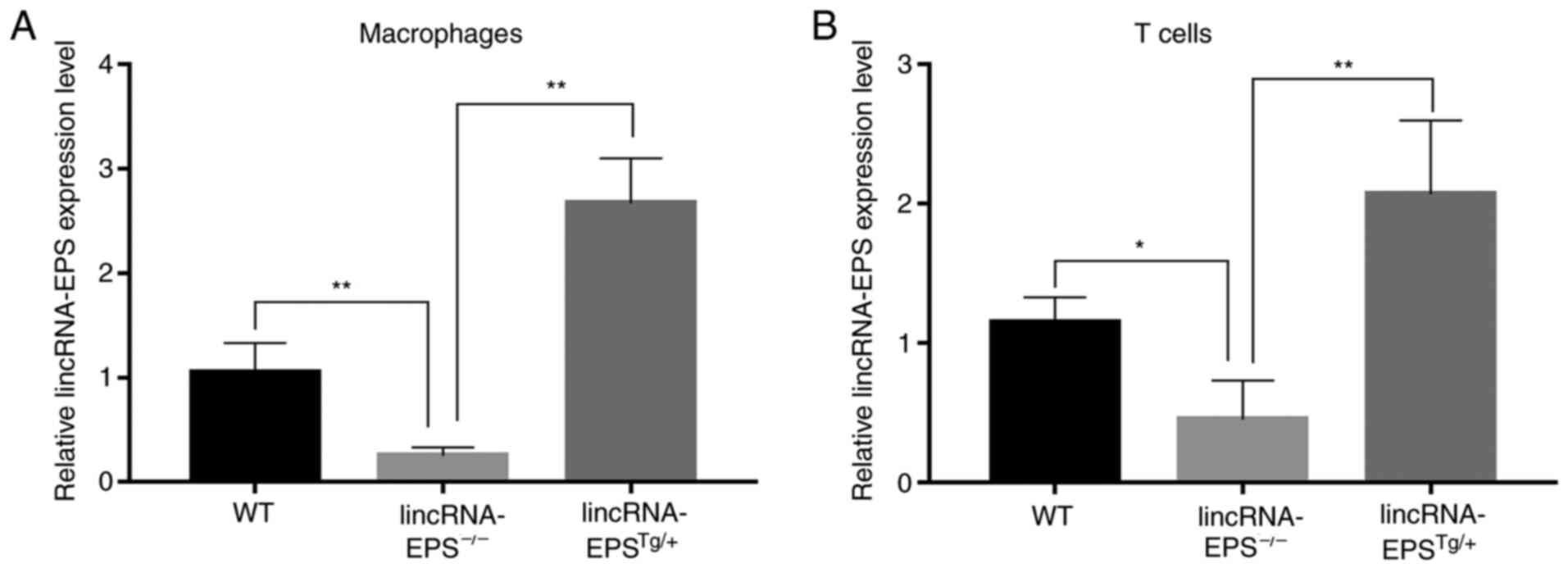
impact of lincRNA-EPS on VC in diabetic mice. Finally, the relative
lincRNA-EPS expression levels of macrophages and T cells in the
lincRNA-EPS-/- and lincRNA-EPSTg/+ groups
were measured and compared with those in the wild-type group to
confirm that the mouse models had been successfully established
(Fig. 6).
Discussion
VC is a key risk index of cardiovascular disease and
a common cause of mortality in patients with diabetes. However, the
drugs currently used to treat VC in patients with diabetes lack
efficacy, which is problematic for patients and doctors.
Previous studies have provided evidence to show that
the osteoblastic differentiation of VSMCs is an important mechanism
for the development of VC (29-31).
In the present study, the results of western blot analysis revealed
the low expression of TGF-β and high expression of Wnt3 and
β-catenin in empty control-transfected VSMCs under HG conditions.
However, in the HG environment, transfection with lincRNA-EPS
increased the expression of TGF-β and decreased the expression of
Wnt3 and β-catenin. In addition, a series of experiments
demonstrated that ALP decreased significantly in VSMCs transfected
with lincRNA-EPS overexpression vector and increased in those
transfected with lincRNA-EPS siRNA. In addition, using Alizarin red
S staining, it was shown that lincRNA-EPS-transfected VSMCs had
less calcium deposition than the empty control-transfected VSMCs
when cultured in a HG environment. Furthermore, the cell-migration
distance of the lincRNA-EPS-transfected VSMCs was smaller than that
of the empty control VSMCs. Based on the results of the present
experiments, it may be inferred that TGF-β played a role in VC.
Additionally, when levels of TGF-β are higher osteoblasts are less
likely to differentiate. This is because high levels of TGF-β
interfere with the Wnt/β-catenin pathway in VSMCs activated by
continuous inflammation, which is responsible for the osteoblastic
differentiation of VSMCs (22).
However, the results of the present study indicated that the high
expression of lincRNA-EPS regulated the Wnt/β-catenin pathway and
affected the osteoblastic differentiation of VSMCs by promoting the
expression of TGF-β and reducing Wnt3 and β-catenin expression.
In the present study, the overexpression of
lincRNA-EPS in VSMCs was indicated to inhibit the osteoblastic
differentiation of VSMCs and reduce the deposition of calcium and
phosphate, thus significantly reducing the degree of VC. As
mentioned above, the present study revealed that, with an increase
in TGF-β levels, both Wnt3 and β-catenin were downregulated in
VSMCs overexpressing lincRNA-EPS. In addition, significantly
increased levels of IL-1β, IL-6 and TNF-α were detected in the
plasma of lincRNA-EPS knockout diabetic mice. A previous study has
demonstrated that TGF-β downregulated several pro-inflammatory
factors in cells, including VSMCs, in atherosclerosis (32). In addition, TGF-β can promote the
transformation of VSMCs from a quiescent, contractile state to an
active state in which they can repair themselves (32). Several inflammatory factors promote
the upregulation of inducible nitric oxide synthase expression in
inflammatory cells (33) to produce
large quantities of NO, as observed in the lincRNA-EPS knockout
diabetic mice. NO can react with superoxide anions to form
peroxynitrite (33) and can also
increase the permeability of the microvasculature to promote the
exudation of cells and serum via IL-2, both of which may cause
sustained low-level inflammation (34,35).
In the present study, serum analysis revealed significantly
decreased FGF-23 levels and significantly increased PTH levels in
lincRNA-EPS knockout diabetic mice, which are indicative of a
significant disorder of calcium and phosphorus metabolism in the
mice. Therefore, it may be concluded that the persistent low-level
inflammation in the lincRNA-EPS knockout diabetic mice was
associated with a disorder of calcium and phosphorus metabolism.
FGF-23 promotes phosphate excretion by reducing the reabsorption of
renal phosphate and reducing the level of PTH, a hormone that can
induce VC, by reducing parathyroid secretion (36,37).
When blood vessel samples from the mice were analyzed using Runx2
immunostaining, the results indicated that the degree of VC in the
lincRNA-EPS knockout diabetic mice was higher than that in
wild-type diabetic mice, while that in the lincRNA-EPS
overexpressing diabetic mice was lower. These results collectively
suggest that the high expression of lincRNA-EPS ameliorates VC;
therefore, it may have a bright future in the management of
diabetes. However, any adverse effects of the high expression of
lincRNA-EPS require further exploration.
In conclusion, the present study demonstrated the
effect of the overexpression of lincRNA-EPS in diabetes-induced VC.
lincRNA-EPS was shown to regulate the Wnt/β-catenin pathway by
promoting the expression of TGF-β and interfering with the
expression of Wnt3 and β-catenin, thereby inhibiting the
osteoblastic differentiation of VSMCs. These findings may
potentially help to reduce or prevent VC in patients with diabetes
and lower the risk of cardiovascular disease. In the future,
lincRNA-EPS overexpression may be used as a novel treatment
approach or preventive drug to delay the development of VC in
patients with diabetes.
The timely resolution of inflammation in patients
with diabetes is necessary to reduce the activation of the
Wnt/β-catenin pathway resulting from the disordered calcium and
phosphorus metabolism caused by inflammation. This may prevent the
osteoblastic differentiation of VSMCs and the deposition of calcium
and phosphorus in blood vessels. In addition, the present study
suggested that lincRNA-EPS inhibits the expression of various
inflammatory factors by increasing TGF-β levels and can inhibit the
Wnt/β-catenin pathway to reduce inflammation, thereby potentially
helping to prevent VC. In patients with diabetes, inflammation is
generally the key cause of VC. Therefore, this study may serve as
an innovative and useful starting point for the treatment of VC in
patients with diabetes.
Supplementary Material
Sequence of lincRNA-EPS and scramble
control. lincRNA-EPS, long intergenic non-coding RNA-erythroid
pro-survival.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 81372975).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CT, YL and ZY conceptualized the study. ZX, CY and
YL investigated the related references. YL, ZY, ZX and CY performed
experiments and wrote the methods. CY and CT analyzed the results.
ZX wrote the original draft. CT. YL and ZY confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was conducted with the approval
from the Animal Management Committee of the Army Medical University
(approval no. SYXK2017002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association. Diagnosis
and classification of diabetes mellitus. Diabetes Care. 36 (Suppl
1):S67–S74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang C, Zhang K, Huang F, Feng W, Chen J,
Zhang H, Wang J, Luo P and Huang H: Exosomes, the message
transporters in vascular calcification. J Cell Mol Med.
22:4024–4033. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Neuenschwander M, Ballon A, Weber KS,
Norat T, Aune D, Schwingshackl L and Schlesinger S: Role of diet in
type 2 diabetes incidence: Umbrella review of meta-analyses of
prospective observational studies. BMJ. 366(l2368)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li XY, Li QM, Fang Q, Zha XQ, Pan LH and
Luo JP: Laminaria japonica polysaccharide inhibits vascular
calcification via preventing osteoblastic differentiation of
vascular smooth muscle cells. J Agric Food Chem. 66:1821–1827.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhan JK, Wang YJ, Wang Y, Tang ZY, Tan P,
Huang W and Liu YS: The protective effect of GLP-1 analogue in
arterial calcification through attenuating osteoblastic
differentiation of human VSMCs. Int J Cardiol. 189:188–193.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pedersen BK: Anti-inflammatory effects of
exercise: Role in diabetes and cardiovascular disease. Eur J Clin
Invest. 47:600–611. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nagano A, Arioka M, Takahashi-Yanaga F,
Matsuzaki E and Sasaguri T: Celecoxib inhibits osteoblast
maturation by suppressing the expression of Wnt target genes. J
Pharmacol Sci. 133:18–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ravi R, Noonan KA, Pham V, Bedi R,
Zhavoronkov A, Ozerov IV, Makarev E, V Artemov A, Wysocki PT, Mehra
R, et al: Bifunctional immune checkpoint-targeted antibody-ligand
traps that simultaneously disable TGFβ enhance the efficacy of
cancer immunotherapy. Nat Commun. 9(741)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bierie B and Moses HL: Transforming growth
factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth
Factor Rev. 21:49–59. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Atianand MK, Hu W, Satpathy AT, Shen Y,
Ricci EP, Alvarez-Dominguez JR, Bhatta A, Schattgen SA, McGowan JD,
Blin J, et al: A long noncoding RNA lincRNA-EPS acts as a
transcriptional brake to restrain inflammation. Cell.
165:1672–1685. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ke Z, Lu J, Zhu J, Yang Z, Jin Z and Yuan
L: Down-regulation of lincRNA-EPS regulates apoptosis and autophagy
in BCG-infected RAW264.7 macrophages via JNK/MAPK signaling
pathway. Infect Genet Evol. 77(104077)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Heydemann A: An overview of murine high
fat diet as a model for type 2 diabetes mellitus. J Diabetes Res.
2016(2902351)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Azushima K, Gurley SB and Coffman TM:
Modelling diabetic nephropathy in mice. Nat Rev Nephrol. 14:48–56.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schwarz H, Zhang Y, Zhan C, Malm M, Field
R, Turner R, Sellick C, Varley P, Rockberg J and Chotteau V:
Small-scale bioreactor supports high density HEK293 cell perfusion
culture for the production of recombinant Erythropoietin. J
Biotechnol. 309:44–52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun X, Li W, Zhang X, Qi M, Zhang Z, Zhang
XE and Cui Z: In vivo targeting and imaging of atherosclerosis
using multifunctional virus-like particles of simian virus 40. Nano
Lett. 16:6164–6171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Grieger JC, Soltys SM and Samulski RJ:
Production of recombinant adeno-associated virus vectors using
suspension HEK293 cells and continuous harvest of vector from the
culture media for GMP FIX and FLT1 clinical vector. Mol Ther.
24:287–297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chandler RJ, LaFave MC, Varshney GK,
Trivedi NS, Carrillo-Carrasco N, Senac JS, Wu W, Hoffmann V,
Elkahloun AG, Burgess SM and Venditti CP: Vector design influences
hepatic genotoxicity after adeno-associated virus gene therapy. J
Clin Invest. 125:870–880. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Wei J, Ran G, Wang X, Jiang N, Liang J,
Lin X, Ling C and Zhao B: Gene manipulation in liver ductal
organoids by optimized recombinant adeno-associated virus vectors.
J Biol Chem. 294:14096–14104. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Y, Wan S, Liu G, Cai W, Huo D, Li G,
Yang M, Wang Y, Guan G, Ding N, et al: Netrin-1 promotes
inflammation resolution to achieve endothelialization of
small-diameter tissue engineering blood vessels by improving
endothelial progenitor cells function in situ. Adv Sci (Weinh).
4(1700278)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jiao L, Zhuang Y, Jiang M, Zhou JH, Wu M,
Chen ZJ, Fang JH and Deng YS: Angiopoietin-like 2 has auxo-action
in atherosclerosis by promoting atherosclerotic calcification. Int
J Clin Exp Pathol. 10:9084–9091. 2017.PubMed/NCBI
|
|
22
|
Fang M, Wang CG, Zheng C, Luo J, Hou S,
Liu K and Li X: Mir-29b promotes human aortic valve interstitial
cell calcification via inhibiting TGF-β3 through activation of
wnt3/β-catenin/Smad3 signaling. J Cell Biochem. 119:5175–5185.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang B, Li Q, Jia S, Li F, Li Q and Li J:
LincRNA-EPS in biomimetic vesicles targeting cerebral infarction
promotes inflammatory resolution and neurogenesis. J Transl Med.
18(110)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zou Y, Zhang Y, Church J and Liu X:
Comparison of β-catenin and LEF1 immunohistochemical stains in
desmoid-type fibromatosis and its selected mimickers, with
unexpected finding of LEF1 positivity in scars. Appl
Immunohistochem Mol Morphol. 26:648–653. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Q, Wang G, Wang B and Yang H:
Activation of TGR5 promotes osteoblastic cell differentiation and
mineralization. Biomed Pharmacother. 108:1797–1803. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
He J, Zhang N, Zhang J, Jiang B and Wu F:
Migration critically meditates osteoblastic differentiation of bone
mesenchymal stem cells through activating canonical Wnt signal
pathway. Colloids Surf B Biointerfaces. 171:205–213.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shioi A and Ikari Y: Plaque calcification
during atherosclerosis progression and regression. J Atheroscler
Thromb. 25:294–303. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chiarella E, Aloisio A, Scicchitano S,
Lucchino V, Montalcini Y, Galasso O, Greco M, Gasparini G, Mesuraca
M, Bond HM and Morrone G: ZNF521 represses osteoblastic
differentiation in human adipose-derived stem cells. Int J Mol Sci.
19(4095)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu Y, Lin F, Fu Y, Chen W, Liu W, Chi J,
Zhang X and Yin X: Cortistatin inhibits calcification of vascular
smooth muscle cells by depressing osteoblastic differentiation and
endoplasmic reticulum stress. Amino Acids. 48:2671–2681.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rong S, Zhao X, Jin X, Zhang Z, Chen L,
Zhu Y and Yuan W: Vascular calcification in chronic kidney disease
is induced by bone morphogenetic protein-2 via a mechanism
involving the Wnt/β-catenin pathway. Cell Physiol Biochem.
34:2049–2060. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nagashima M, Sakai A, Uchida S, Tanaka S,
Tanaka M and Nakamura T: Bisphosphonate (YM529) delays the repair
of cortical bone defect after drill-hole injury by reducing
terminal differentiation of osteoblasts in the mouse femur. Bone.
36:502–511. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rudijanto A: The role of vascular smooth
muscle cells on the pathogenesis of atherosclerosis. Acta Med
Indones. 39:86–93. 2007.PubMed/NCBI
|
|
33
|
Guzik TJ, Korbut R and Adamek-Guzik T:
Nitric oxide and superoxide in inflammation and immune regulation.
J Physiol Pharmacol. 54:469–487. 2003.PubMed/NCBI
|
|
34
|
Beck PL, Xavier R, Wong J, Ezedi I,
Mashimo H, Mizoguchi A, Mizoguchi E, Bhan AK and Podolsky DK:
Paradoxical roles of different nitric oxide synthase isoforms in
colonic injury. Am J Physiol Gastrointest Liver Physiol.
286:G137–G147. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Niedbala W, Besnard AG, Nascimento DC,
Donate PB, Sonego F, Yip E, Guabiraba R, Chang HD, Fukada SY,
Salmond RJ, et al: Nitric oxide enhances Th9 cell differentiation
and airway inflammation. Nat Commun. 5(4575)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
de Seigneux S and Martin PY: Phosphate and
FGF23 in the renoprotective benefit of RAAS inhibition. Pharmacol
Res. 106:87–91. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jüppner H: Phosphate and FGF-23. Kidney
Int Suppl. 79:S24–S27. 2011.PubMed/NCBI View Article : Google Scholar
|