Introduction
Breast cancer is one of the most prevalent cancer
worldwide, accounting for 11.7% of all cancer incidence and 6.9% of
total mortality in 2020(1). It
often develops drug resistance and has high recurrence rates
(2). In addition, the high rates
of metastasis during clinical treatment greatly worsens the
prognosis of patients (3).
Therefore, identifying novel molecular markers that can be applied
to effectively predict the progression and prognosis of breast
cancer would be of great imortance for prevention and
treatment.
Yes-associated protein (YAP) is a transcriptional
co-factor of the hippo signaling pathway, the expression of which
has been frequently reported to be upregulated in numerous types of
cancers, such as esophageal, lung, liver and colon cancer (4-7).
YAP is encoded by proto-oncogenes, where its expression can be
enhanced by binding to a combination of transcriptional enhancers
and residue domain transcription factors, which in turn promotes
cell proliferation (8-10).
Aberrant alterations in the hippo signaling pathway have been shown
to promote nuclear localization of YAP to induce gene expression,
leading to the progression of different types of tumors, including
breast cancer (11,12). By contrast, one previous study has
found that the expression levels of YAP in Breast tumor are lower
compared with those in normal breast tissues (13). As a transcriptional regulator, it
lacks a binding domain and cannot directly bind DNA; however, it
can bind other transcription factors to regulate the transcription
and expression of downstream target genes (14). In recent years, YAP has been
reported to be a potential target for the treatment of breast
cancer (12). Owing to its role as
a transcription co-factor, YAP may need to combine with other
regulators to mediate its role as a potential therapeutic
target.
β-catenin and smoothened (SMO) are core proteins of
the Wnt and hedgehog signaling pathways, respectively (15,16).
The activities of these two pathways serve a key role in tumor
physiology (17), since the
self-renewal and differentiation capabilities of breast cancer stem
cells are regulated by these two signaling pathways (18). Furthermore, previous studies have
found the possibility of cross-talk between hippo and Wnt signaling
pathways (19,20). In basal-like breast cancer, YAP and
β-catenin were reported to synergistically regulate tumor stem
cells to drive breast cancer pathology (21). In addition, overexpression of YAP
has been found to suppress the hedgehog signaling pathway, whereas
knocking down YAP expression enhanced its activity (22). Although YAP, β-catenin and SMO all
apparently serve important roles in the occurrence and development
of breast cancer, the relationship among them remains unclear.
Therefore, the present study aimed to elucidate the association
between the expression of these three proteins and breast cancer,
in addition to exploring the significance of this association.
Materials and methods
Tissue samples
A total of 60 patients with breast cancer who
underwent surgical treatment from January 2020 to January 2021 were
selected. All patients had complete medical records with related
clinicopathological data and were diagnosed with invasive breast
cancer by the pathology department of The Second Affiliated
Hospital of Fujian Medical University (Quanzhou, China). All
patients were female, who did not receive neoadjuvant therapy
before surgery (mean age was 49.97±10.25 years). We excluded
patients with advanced breast cancer or breast cancer with other
malignancy. The tumor histological grades and stages in the present
study were determined based on the 2020 diagnostic criteria of the
National Comprehensive Cancer Network (23). Clinicopathological data, such as
the expression status of HER2, estrogen receptor (ER), progesterone
receptor (PR) and Ki-67, were obtained from postoperative patient
reports and determined by referring to the 2021 Chinese Society of
Clinical Oncology guidelines for the diagnosis and treatment of
breast cancer (24). All patients
underwent modified radical mastectomy for breast cancer. Tumor,
tumor-adjacent (breast tissues taken 2 cm from the edge of the
cancerous tissue) and normal tissues (breast tissue taken >5 cm
away from the edge of the cancerous tissue without cancer cell
infiltration as confirmed by pathology) were taken 30 min after the
removal of breast tissue. All tissue specimens were fixed with 4%
paraformaldehyde (room temperature, 20-25℃) and embedded in
paraffin blocks. The present study complied with the Declaration of
Helsinki. The present study was approved by the Ethics committee of
The Second Affiliated Hospital of Fujian Medical University.
Written informed consent was obtained from all patients.
Clinicopathological data of the 60 patients with breast cancer are
presented in Table I.
 | Table IClinicopathological features of the
60 patients with invasive breast cancer. |
Table I
Clinicopathological features of the
60 patients with invasive breast cancer.
| Clinicopathological
feature | N | Percentage |
|---|
| Age, years | | |
|
≥50 | 28 | 46.7 |
|
<50 | 32 | 53.3 |
| Histological
grade | | |
|
I | 1 | 1.7 |
|
II | 27 | 45 |
|
III | 32 | 53.3 |
| Tumor size, cm | | |
|
<2 | 21 | 35 |
|
2-5 | 36 | 60 |
|
>5 | 3 | 5 |
| Lymph node
metastasis | | |
|
0 | 34 | 56.7 |
|
1-3 | 15 | 25 |
|
4-9 | 7 | 11.6 |
|
≥10 | 4 | 6.7 |
| Human epidermal
growth factor receptor 2 | | |
|
Positive | 22 | 36.7 |
|
Negative | 38 | 63.3 |
| Estrogen
receptor | | |
|
Positive | 41 | 68.3 |
|
Negative | 19 | 31.7 |
| Progesterone
receptor | | |
|
Positive | 40 | 66.7 |
|
Negative | 20 | 33.3 |
| Ki-67 | | |
|
Positive | 42 | 70 |
|
Negative | 18 | 30 |
| Tumor stage | | |
|
I | 16 | 26.7 |
|
II | 33 | 55 |
|
III | 11 | 18.3 |
Main reagents and materials
Citric acid (pH 6.0) antigen retrieval solution
(cat. no. G1202), 4% paraformaldehyde (cat. no. G1102), bovine
serum albumin (cat. no. G5001), hematoxylin stain solution (cat.
no. G1004), 3, 3'-diaminobenzidine (DAB) chromogenic reagent (cat.
no. G1211), normal rabbit serum (cat. no. G1209) were all purchased
from Wuhan Servicebio Technology Co., Ltd. Primary antibodies
against YAP (cat. no. ab52771), β-catenin (cat. no. ab231305), SMO
(cat. no. ab235183), universal HRP-conjugated secondary antibody
against rabbit (cat. no. ab205718) were all purchased from
Abcam.
Immunohistochemical staining
Paraffin-embedded tissue sections were
deparaffinized in xylene, rehydrated in a series of ethanol
solutions (100 to 50%) at room temperature. For antigen retrieval,
tissue sections were placed in a box filled with the citric acid
(pH 6.0) antigen retrieval solution in a microwave oven, heated on
medium power for 8 min until boiling. 0.3% hydrogen peroxide
solution for 20 min to block the endogenous peroxidase activity.
Samples were blocked for 30 min at room temperature by using bovine
serum albumin. Next, the sections were incubated overnight with
primary antibodies against YAP, β-catenin and SMO (1:200 dilution)
at 4˚C. Subsequently, the sections were incubated with secondary
antibodies against rabbit (1:400 dilution) for 50 min at room
temperature. The sections were then stained with DAB and
counterstained using hematoxylin stain solution for 3 min at room
temperature. Placed the section in a series of ethanol solutions
(100 to 50%) to dehydrate, and than mounting was performed.
Finally, the stained tissue sections were observed under a
microscope and images were captured for analysis.
Scoring criteria
Each region of the tissue sections was initially
examined with a low magnification (x100), before higher
magnification (x200) was used to observe the local area and to
select a representative area for image capture and analysis. A
comprehensive staining score was created by counting the total
number of cells as well as the number of YAP-, β-catenin- and
SMO-positive cells in the measurement area. Cells were scored
according to the staining intensity and the percentage of stained
cells [(number of stained cells/total number of cells) x100].
Staining scoring criteria were as described previously (25): i) 0, no color; ii) 1, light yellow;
iii) 2, brownish-yellow; and iv) 3, brown. Scoring based on the
extent of stained cells was as follows: i) 0, 0-5%; ii) 1, 6-25%;
iii) 2, 26-50%; iv) 3, 51-75%; and v) 4, >75%. Multiplying the
staining intensity score with the percentage of stained cells
yielded the comprehensive staining score, wherein 0-3 was
considered to be low expression, and a score ≥4 was considered high
expression.
Statistical analysis
SPSS 26.0 (IBM Corp.) and GraphPad Prism 8.0
(GraphPad Software, Inc.) were used for the statistical analyses.
Friedman's test and Nemenyi's test were used to compare the
staining scores among each group. The counted data were analyzed
using the χ2 test. Spearman's correlation analysis was
used for the correlation of the IHC data. All data are presented in
this study by mean ± SD, P<0.05 was considered to indicate a
statistically significant difference.
Results
Differential expression of YAP,
β-catenin and SMO in tumor, tumor-adjacent and normal breast
tissues
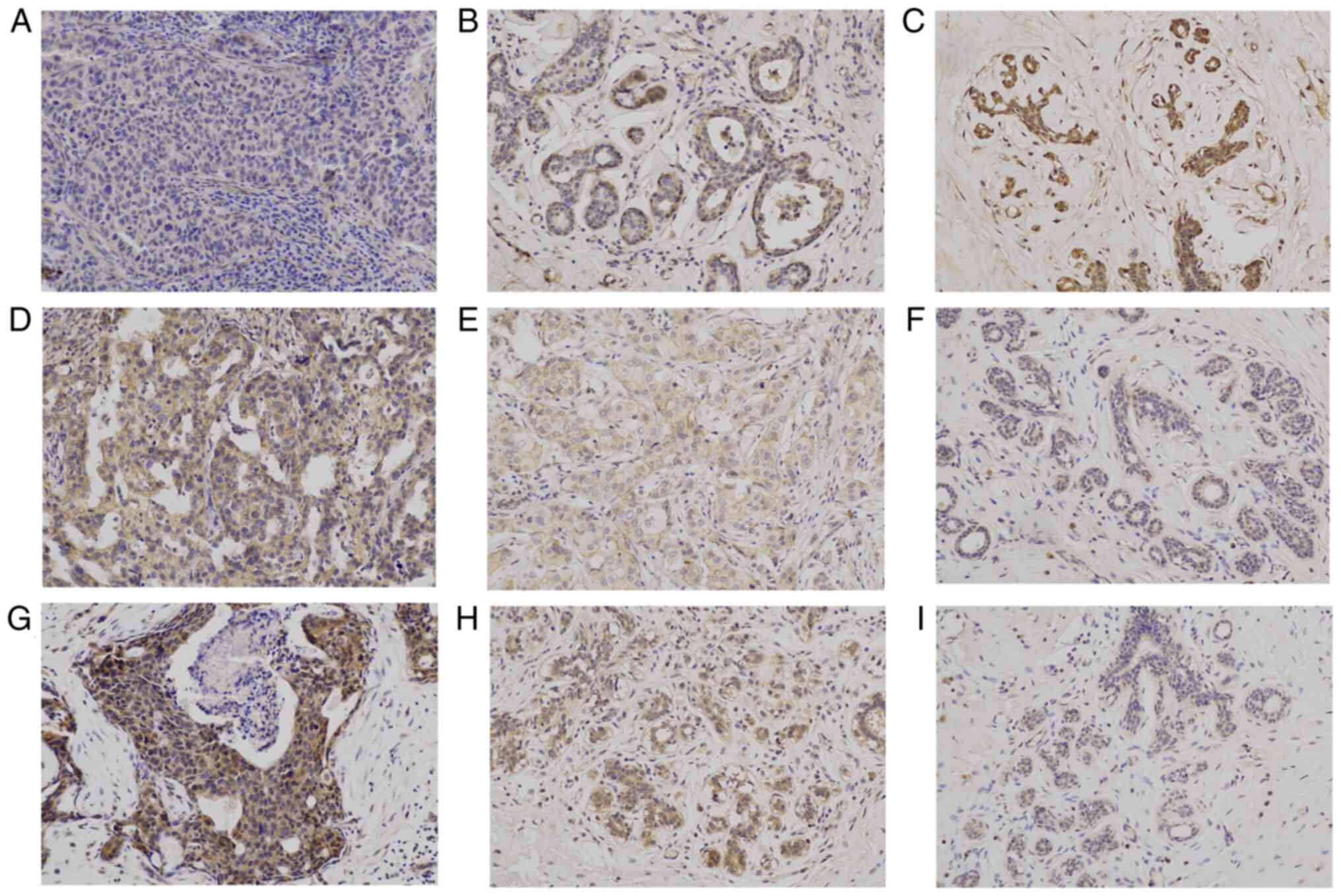
The strongly positive expression of YAP is mainly
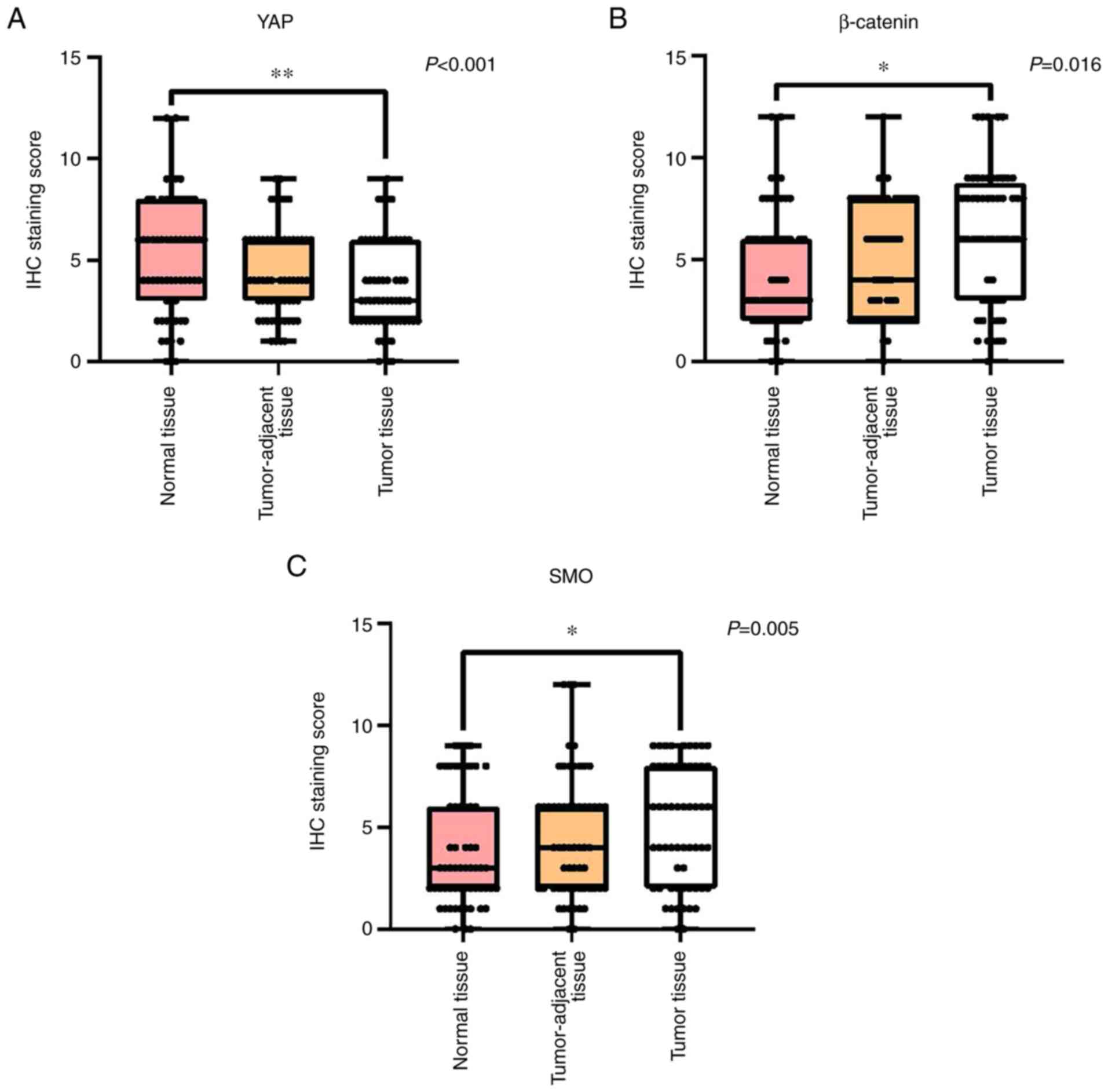
localized to the cytoplasm and nucleus (Fig. 1C). The expression levels of YAP in
the three different types of breast tissue samples were found to be
significantly different (P<0.001; Figs. 2A and 1A-C). Pairwise comparisons revealed
significantly decreased YAP expression levels in the tumor tissues
compared with those in the normal breast tissues (P<0.01). In
addition, the expression levels of YAP in the tumor-adjacent breast
tissues had no significant differences compared with those in the
normal tissues.
The strongly positive expression of β-catenin is
mainly localized to the cytoplasm and cell membrane (Fig. 1C). The expression levels of
β-catenin in the three breast tissue samples were also found to be
significantly different (P=0.016; Figs. 2B and 1D-F). Pairwise comparisons showed
significantly increased expression levels of β-catenin in the tumor
tissues compared with those in the normal breast tissues
(P<0.05). The expression levels of β-catenin in the
tumor-adjacent tissue showed no significant difference compared
with those in the normal breast tissues.
The strong positive expression of β-catenin is
mainly localized to the cytoplasm (Fig. 1G). The expression levels of SMO in
the three breast tissue samples were significantly different
(P=0.005; Fig. 2C and 1G-I). Pairwise comparisons showed
significantly increased expression levels of SMO in the tumor
tissues compared with those in the normal breast tissues
(P<0.05). The expression levels of SMO in the tumor-adjacent
tissues had no significant difference compared with those in the
normal breast tissues.
Relationship between the expression of
YAP, β-catenin and SMO and the clinicopathological characteristics
of patients with breast cancer
The expression of YAP in the tumor tissues of the
patients with breast cancer was not found to be associated with
age, tumor histological grade and size, lymph node metastasis,
Ki-67 expression index or tumor stage (Table II). However, it was significantly
associated with the expression of HER2 (χ2=8.735;
P=0.003), PR (χ2=5.735; P=0.017) and ER
(χ2=4.45; P=0.035).
 | Table IIRelationship between the expression
levels of YAP, β-catenin and SMO and the clinicopathological
features of patients with breast cancer. |
Table II
Relationship between the expression
levels of YAP, β-catenin and SMO and the clinicopathological
features of patients with breast cancer.
| Group | N | High YAP
expression, n (%) | P-value | High β-catenin
expression, n (%) | P-value | High SMO
expression, n (%) | P-value |
|---|
| Age, years | | | | | | | |
|
≥50 | 28 | 14(50) | 0.33 | 22 (78.6) | 0.55 | 18 (64.3) | 0.91 |
|
<50 | 32 | 12 (37.5) | | 23 (71.9) | | 21 (65.6) | |
| Histological
grade | | | | | | | |
|
I-II | 28 | 14(50) | 0.33 | 19 (67.9) | 0.37 | 15 (53.6) | 0.083 |
|
III | 32 | 12 (37.5) | | 25 (78.1) | | 24(75) | |
| Tumor size, cm | | | | | | | |
|
≤2 | 21 | 8 (38.1) | 0.55 | 15 (71.4) | 0.81 | 16 (76.2) | 0.18 |
|
>2 | 39 | 18 (46.2) | | 29 (74.4) | | 23(59) | |
| Lymph node
metastasis | | | | | | | |
|
No | 34 | 18 (52.9) | 0.09 | 19 (55.9) | 0.98 | 20 (58.8) | 0.25 |
|
Yes | 26 | 8 (30.8) | | 26(100) | | 19 (73.1) | |
| Human epidermal
growth factor receptor 2 | | | | | | | |
|
Positive | 22 | 15 (68.2) | 0.003 | 22(100) | <0.001 | 10 (45.5) | 0.016 |
|
Negative | 38 | 11 (28.9) | | 23 (60.5) | | 29 (76.3) | |
| Estrogen
receptor | | | | | | | |
|
Positive | 41 | 14 (34.1) | 0.035 | 30 (73.2) | 0.63 | 27 (65.9) | 0.84 |
|
Negative | 19 | 12 (63.2) | | 15 (78.9) | | 12 (63.2) | |
| Progesterone
receptor | | | | | | | |
|
Positive | 40 | 13 (32.5) | 0.017 | 28(70) | 0.21 | 27 (67.5) | 0.57 |
|
Negative | 20 | 13(65) | | 17(85) | | 12(60) | |
| Ki-67 | | | | | | | |
|
Positive | 42 | 20 (47.6) | 0.65 | 32 (76.2) | 0.9 | 27 (64.3) | 0.32 |
|
Negative | 18 | 6 (33.3) | | 13 (72.2) | | 12 (66.7) | |
| Tumor stage | | | | | | | |
|
I | 16 | 6 (37.5) | 0.09 | 11 (68.8) | 0.55 | 12(75) | 0.16 |
|
II | 33 | 15 (45.5) | | 26 (78.8) | | 18 (54.5) | |
|
III | 11 | 9 (81.8) | | 8 (72.7) | | 9 (81.8) | |
The expression of β-catenin in the tumor tissue of
the patients with breast cancer was not associated with age, tumor
histological grade and size, lymph node metastasis, Ki-67
expression index, ER, PR or tumor stage (Table II). However, it was significantly
associated with the expression of HER2 (χ2=11.579;
P<0.001).
The expression levels of SMO in the tumor tissues of
the patients with breast cancer were not associated with age, tumor
histological grade, size, lymph node metastasis, Ki-67 expression
index, ER, PR or tumor staging (Table
II). However, a significant association was observed between
SMO and HER2 expression (χ2=5.833; P=0.016).
Correlation of YAP with β-catenin and
SMO expression in the tumor tissues
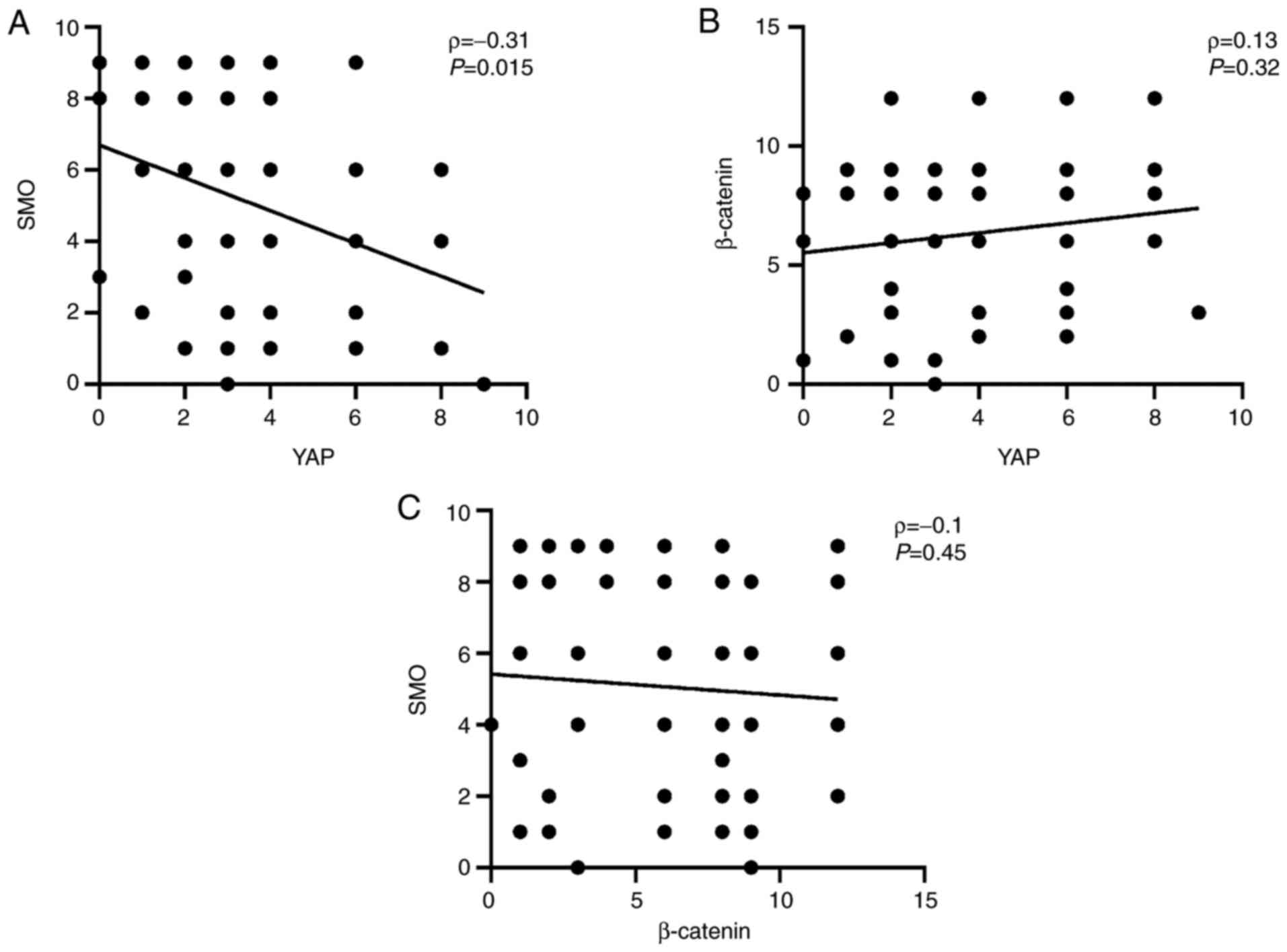
Spearman's analysis revealed a negative correlation
between expression levels of YAP and SMO in the tumor tissue
(ρ=-0.31; P=0.015; Fig. 3A). No
correlation was identified between the expression levels of YAP and
β-catenin (ρ=0.13; P=0.32; Fig.
3B) or between the β-catenin and SMO (ρ=-0.1; P=0.45; Fig. 3C).
Relationship between the combined
expression changes of YAP, β-catenin and SMO in tumor tissue and
the clinicopathological characteristics
Compared with individual changes in each of YAP
(Decreased expression: IHC staining Score<4), β-catenin and SMO
expression (Increased expression: IHC staining Score≥4), their
combined changes were found to be significantly associated with the
tumor histological grade (P=0.013; Table III). In particular, a significant
association was found with grade III (low differentiation) compared
with grades I-II (medium and high differentiation). There was no
significant association with age, tumor size, lymph node
metastasis, HER2, ER, PR, Ki-67 or tumor stage. This observation
suggested that the combined expression changes of these three
proteins may be associated with the histological grade of breast
cancer.
 | Table IIIRelationship between the combined
changes in tumor expression of YAP, β-catenin SMO and the
clinicopathological characteristics. |
Table III
Relationship between the combined
changes in tumor expression of YAP, β-catenin SMO and the
clinicopathological characteristics.
| Group | N |
YAP-/β-catenin+/SMO+ | P-value |
|---|
| Age, years | | | |
|
≥50 | 28 | 8 (28.6) | 0.82 |
|
<50 | 32 | 10 (31.3) | |
| Histological
grade | | | |
|
I-II | 28 | 4 (14.3) | 0.013 |
|
III | 32 | 14 (43.8) | |
| Tumor size, cm | | | |
|
≤2 | 21 | 5 (23.8) | 0.44 |
|
>2 | 36 | 13 (36.1) | |
| Lymph node
metastasis | | | |
|
No | 34 | 7 (20.6) | 0.07 |
|
Yes | 26 | 11 (42.3) | |
| Human epidermal
growth factor receptor 2 | | | |
|
Positive | 22 | 4 (18.2) | 0.13 |
|
Negative | 38 | 14 (36.8) | |
| Estrogen
receptor | | | |
|
Positive | 41 | 15 (36.6) | 0.1 |
|
Negative | 19 | 3 (15.8) | |
| Progesterone
receptor | | | |
|
Positive | 40 | 14(35) | 0.23 |
|
Negative | 20 | 4(20) | |
| Ki-67 | | | |
|
Positive | 42 | 12 (28.6) | 0.71 |
|
Negative | 18 | 6 (33.3) | |
| Tumor stage | | | |
|
I | 16 | 3 (18.8) | 0.51 |
|
II | 33 | 11 (33.3) | |
|
III | 11 | 4 (36.4) | |
Discussion
A number of studies have previously reported that
YAP expression is upregulated in malignant tumors, such as
esophageal, lung, liver and colon cancers (4-7).
Therefore, YAP is considered to be an oncogenic protein. However,
data regarding YAP from previous studies on breast cancer remain
controversial, since the expression profile of YAP in this type of
cancer is ambiguous (13,26). Results from the present study
revealed that the expression levels of YAP in normal breast tissues
is higher compared with those in tumor tissues, suggesting that YAP
may be a tumor suppressor in invasive breast cancer. Yuan et
al (27) previously reported
that YAP may serve as a tumor suppressor in breast cancer. They
found that YAP expression was decreased in breast cancer, such that
the metastatic and invasive capabilities of breast cancer cells
without YAP expression were increased.
In the present study, association analysis between
YAP expression and the clinicopathological characteristics of
patients with breast cancer revealed that it was associated with
HER2, ER and PR expression. This suggested that YAP may mediate an
important role during the endocrine or targeted therapy of invasive
breast cancer. A study by Zhu et al (28) previously revealed that YAP serves
as co-regulators of ERα for estrogen-regulated enhancer activation,
which suggests YAP to be a potential therapeutic target for
ER-positive breast cancer. In addition, a recent study found that
after targeting HER2 with trastuzumab, a monoclonal antibody, the
expression of YAP was significantly higher in trastuzumab-sensitive
BT474 cell lines compared with that in their trastuzumab-resistant
counterparts, suggesting its involvement in preventing trastuzumab
resistance in HER2-positive breast cancer cells (29). Therefore, YAP can be used as a
prognostic marker for trastuzumab neoadjuvant therapy in patients
with HER2-positive breast cancer (29). The present study demonstrated that
YAP may serve a key role in the treatment of invasive breast
cancer. Previous studies have shown that the expression levels of
YAP in different breast cancer subtypes also differs (13,30),
meaning that the biological role of YAP in breast cancer cells is
likely to be dependent on their pathological subtypes or
microenvironments. Therefore, the viability of YAP as a potential
target for the treatment of breast cancer remains unclear and
further research is needed to identify the specific factors and
pathways that can regulate YAP expression in different subtypes of
breast cancer.
In the present study, immunohistochemistry results
revealed that β-catenin was expressed at a higher levels in the
tumor tissues compared with those in the normal breast tissues,
which was mainly localized to the cell membrane and cytoplasm.
After the Wnt signaling pathway is activated, β-catenin enters the
cytoplasm from the cell membrane and undergoes a series of
reactions, resulting in its accumulation in the cytoplasm.
Eventually, nuclear translocation occurs, activating the
transcription of target genes associated with tumor development and
metastasis (15). These results
are consistent with those reported by previous studies. For
example, Jang et al (31)
found that Wnt/β-catenin signaling activity was enhanced in the
breast tumors compared with that in the normal breast tissues. In
addition, mouse models of breast cancer were used in this study to
demonstrate that inhibition of the Wnt/β-catenin signaling pathway
suppressed breast cancer stem cell activity, thereby reducing the
metastatic potential of breast cancer cells (31). Upregulation of the Wnt/β-catenin
signaling pathway was also previously found to increase the
metastatic ability of primary breast tumors (32). Therefore, β-catenin can be
considered to be an oncogene involved in the occurrence and
metastasis of breast cancer.
β-catenin expression was found to associate with the
expression levels of HER2 in the present study, which suggested the
potential of targeting the Wnt/β-catenin signaling pathway for
breast cancer therapy. A previous study reported that HER2
activated Wnt/β-catenin signaling pathway through its downstream
regulators, AKT and MAPK; this can inhibit glycogen synthase
kinase-3 expression, resulting in the translocation of β-catenin to
the nucleus, thereby promoting the transcription of target genes
(33). Another study found that
Wnt3 ligand-mediated activation of the Wnt/β-catenin signaling
pathway induced epithelial-mesenchymal transition and trastuzumab
resistance in HER2-positive breast cancer cells (34). At present, no Wnt inhibitors have
been approved for breast cancer treatment. Therefore, further
research on the Wnt/β-catenin signaling pathway can potentially
provide solutions for the clinical treatment of breast cancer.
SMO is one of the core components of the hedgehog
signaling pathway (16). The
hedgehog signaling pathway is involved in the regulation of mammary
gland development during embryogenesis, the development of duct
structures and the differentiation of the breast during lactation
(35). During the early stages of
embryogenesis, the hedgehog pathway is inhibited to allow breast
parenchyma formation. Subsequently, ductal morphology typically
develops during puberty, when the hedgehog signaling pathway must
be activated to promote elongation of the terminal buds. Shortly
after puberty, its activity decreases again in the mammary glands
(36). Aberrant activation of the
hedgehog signaling pathway can lead to the development and
metastasis of breast cancer (37).
The present results revealed that the expression levels of SMO in
tumor breast tissues is higher compared with those in normal
tissues. Therefore, findings from the present study suggested that
SMO may be an oncogenic gene product that can mediate the abnormal
activation of the hedgehog pathway to promote the progression of
breast cancer.
Association analysis between SMO and the
clinicopathological characteristics of patients with breast cancer
showed that SMO was associated with HER2, which suggested that SMO
is important for the targeted therapy of invasive breast cancer.
Several studies have previously shown that SMO inhibitors can be
combined with other inhibitors or chemotherapeutic agents to
effectively inhibit breast cancer progression (38-40).
In particular, a clinical trial targeted SMO in the hedgehog
signaling pathway in female patients with breast cancer (41). The results of these trials
suggested that targeting this pathway can be an effective treatment
option for patients with cancer (41).
The present study demonstrated that the expressions
of YAP and SMO in breast cancer tissues were negatively associated,
suggesting that there may have been an interaction between the
hippo and hedgehog signaling pathways. Tariki et al
(22) previously revealed that the
overexpression of YAP blocked the hedgehog signaling pathway,
whereas knocking down YAP expression using siRNA enhanced its
activity, demonstrating a negative regulatory relationship between
these two pathways; however, hedgehog signaling pathway can enhance
YAP activity through a post-transcriptional mechanism, which in
turn forms a negative feedback loop to turn off hedgehog
signaling.
Zheng et al (42) revealed an oncogenic function of YAP
in reprogramming glucose metabolism, The lncRNA breast cancer
anti-estrogen resistance 4 (BCAR4) is required for YAP-dependent
glycolysis. Mechanistically, The overexpression of YAP leads to
therapeutic efficacy of BCAR4-targeted locked nucleic acids (LNA)
by inducing the transcription of BCAR4 in order to promote the
expression of BCAR4, which coordinated with the hedgehog signaling
pathway to enhance the expression of glycolysis activators
hexokinase-2 and 6-phosphofructo-2-kinase/fructose-2,
6-bisphosphatase 3. This resulted in the therapeutic delivery of
LNA, which attenuated YAP-dependent glycolysis and breast tumor
growth, suggesting that YAP, the core protein of the hippo
signaling pathway in breast cancer, has both inhibitory and
potentiation effects on the hedgehog pathway. When the hedgehog
signaling pathway is aberrantly activated, the activity of YAP may
be enhanced, thereby decreasing the activity of the hedgehog
pathway. Therefore, it was speculated that YAP may regulate the
growth and development of normal breast cells by stabilizing
protein expression in the hedgehog signaling pathway. The absence
of YAP in cells may lead to the abnormal activation of the hedgehog
signaling pathway to promote the occurrence and development of
breast cancer.
By analyzing the relationship between the combined
changes in the expression of YAP (decreased), β-catenin (increased)
and SMO (increased) in breast tumors and the clinicopathological
characteristics of the patients, the present study found that these
changes in expression were significantly associated with the tumor
histological grade. Specifically, grade III (low differentiation)
showed higher association compared with grades I-II (medium and
high differentiation). These findings suggested that the
downregulation of YAP and the upregulation of β-catenin and SMO in
breast cancer can promote disease progression. In addition, the
present data suggested that the possibility of recurrence or
malignant transformation in tissues adjacent to tumors can be
determined by detecting the combined expression levels of these
three proteins in the tumor-adjacent tissues. This can provide a
basis for determining the surgical margin and scope of preserving
healthy breast tissues when planning radical mastectomy surgeries.
However, further research is required to verify this
hypothesis.
In summary, YAP, β-catenin and SMO were found to
serve key roles in the occurrence, development, diagnosis and
treatment of breast cancer in the present study. The association
between YAP and SMO provides a new direction for exploring the
mechanisms in the physiology of invasive breast cancer. Because the
expression of YAP is downregulated, whereas β-catenin and SMO are
upregulated in breast cancer, this may promote the progression of
this disease. Therefore, analyzing the expression profile of all
three of these may provide important information on the physiology
of breast cancer. However, the follow-up time of patients in the
present study was short, meaning that it was not possible to
evaluate the relationship between the expression levels of these
proteins and the prognosis of the patients. In future studies, the
prognostic information of patients are also required, which should
be combined to assess the importance of these three proteins in
invasive breast cancer.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Quanzhou Science and
Technology Program (grant no. 2019C065R).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL and JZ conceived and designed the project. PL and
GY provided materials and patient samples and collected the data.
PL and GY performed IHC staining. PL analyzed and interpretated the
data. PL and JZ confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Fujian Medical
University [Quanzhou, China; approval no. (2019)(196)]. Written
informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kaur S, Najm MZ, Khan MA, Akhter N,
Shingatgeri VM, Sikenis M and Sadaf and Aloliqi AA: Drug-resistant
breast cancer: Dwelling the hippo pathway to manage the treatment.
Breast Cancer (Dove Med Press). 13:691–700. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sharma P: Biology and management of
patients with triple-negative breast cancer. Oncologist.
21:1050–1062. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Muramatsu T, Imoto I, Matsui T, Kozaki K,
Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T and Inazawa J: YAP
is a candidate oncogene for esophageal squamous cell carcinoma.
Carcinogenesis. 32:389–398. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xiao L, Zhou H, Li XP, Chen J, Fang C, Mao
CX, Cui JJ, Zhang W, Zhou HH, Yin JY and Liu ZQ: MicroRNA-138 acts
as a tumor suppressor in non small cell lung cancer via targeting
YAP1. Oncotarget. 7:40038–40046. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pu J, Huang Y, Fang Q, Wang J, Li W, Xu Z,
Wu X, Lu Y and Wei H: Hypoxia-induced Fascin-1 upregulation is
regulated by Akt/Rac1 axis and enhances malignant properties of
liver cancer cells via mediating actin cytoskeleton rearrangement
and hippo/YAP activation. Cell Death Discov. 7(385)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Avruch J, Zhou D and Bardeesy N: YAP
oncogene overexpression supercharges colon cancer proliferation.
Cell Cycle. 11:1090–1096. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lamar JM, Stern P, Liu H, Schindler JW,
Jiang ZG and Hynes RO: The hippo pathway target, YAP, promotes
metastasis through its TEAD-interaction domain. Proc Natl Acad Sci
USA. 109:E2441–2450. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guo L, Chen Y, Luo J, Zheng J and Shao G:
YAP1 overexpression is associated with poor prognosis of breast
cancer patients and induces breast cancer cell growth by inhibiting
PTEN. FEBS Open Bio. 9:437–445. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao B, Kim J, Ye X, Lai ZC and Guan KL:
Both TEAD-binding and WW domains are required for the growth
stimulation and oncogenic transformation activity of yes-associated
protein. Cancer Res. 69:1089–1098. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rigiracciolo DC, Nohata N, Lappano R,
Cirillo F, Talia M, Scordamaglia D, Gutkind JS and Maggiolini M:
IGF-1/IGF-1R/FAK/YAP transduction signaling prompts growth effects
in triple-negative breast cancer (TNBC) cells. Cells.
9(1010)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu L and Yang X: Targeting the hippo
pathway for breast cancer therapy. Cancers (Basel).
10(422)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jaramillo-Rodríguez Y, Cerda-Flores RM,
Ruiz-Ramos R, López-Márquez FC and Calderón-Garcidueñas AL: YAP
expression in normal and neoplastic breast tissue: An
immunohistochemical study. Arch Med Res. 45:223–228.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yao CB, Zhou X, Chen CS and Lei QY: The
regulatory mechanisms and functional roles of the hippo signaling
pathway in breast cancer. Yi Chuan. 39:617–629. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu X, Zhang M, Xu F and Jiang S: Wnt
signaling in breast cancer: Biological mechanisms, challenges and
opportunities. Mol Cancer. 19(165)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gan GN and Jimeno A: Emerging from their
burrow: Hedgehog pathway inhibitors for cancer. Expert Opin
Investig Drugs. 25:1153–1166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Toh TB, Lim JJ and Chow EK: Epigenetics in
cancer stem cells. Mol Cancer. 16(29)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Karamboulas C and Ailles L: Developmental
signaling pathways in cancer stem cells of solid tumors. Biochim
Biophys Acta. 1830:2481–2495. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang T, Zhou H, Wang K, Wang X, Wang M,
Zhao W, Xi X, Li Y, Cai M, Zhao W, et al: Role, molecular mechanism
and the potential target of breast cancer stem cells in breast
cancer development. Biomed Pharmacother. 147(112616)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Valenti G, Quinn HM, Heynen GJJE, Lan L,
Holland JD, Vogel R, Wulf-Goldenberg A and Birchmeier W: Cancer
stem cells regulate cancer-associated fibroblasts via activation of
hedgehog signaling in mammary gland tumors. Cancer Res.
77:2134–2147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Quinn HM, Vogel R, Popp O, Mertins P, Lan
L, Messerschmidt C, Landshammer A, Lisek K, Château-Joubert S,
Marangoni E, et al: YAP and β-catenin cooperate to drive
oncogenesis in basal breast cancer. Cancer Res. 81:2116–2127.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tariki M, Dhanyamraju PK, Fendrich V,
Borggrefe T, Feldmann G and Lauth M: The Yes-associated protein
controls the cell density regulation of hedgehog signaling.
Oncogenesis. 3(e112)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
NCCN: The NCCN breast cancer clinical
practice guidelines in oncology (version 3.2020)[EB/OL]. Fort
Washington: NCCN [2020-03-09], 2020.
|
|
24
|
Li Q, Liu J, Jiang Z and Liu Q: CSCO
breast cancer guideline: Precise, economical and oriental. Sci
China Life Sci. 63:1410–1412. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xie P, Zhang M, He S, Chen Y, Xing G, Lu
Y, Liu P, Li Y, Wang S, Chai N, et al: The covalent modifier Nedd8
is critical for the activation of Smurf1 ubiquitin ligase in
tumorigenesis. Nat Commun. 5(3733)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Calvo F, Ege N, Grande-Garcia A, Hooper S,
Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary
E, Charras G and Sahai E: Mechanotransduction and YAP-dependent
matrix remodelling is required for the generation and maintenance
of cancer-associated fibroblasts. Nat Cell Biol. 15:637–646.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Yuan M, Tomlinson V, Lara R, Holliday D,
Chelala C, Harada T, Gangeswaran R, Manson-Bishop C, Smith P,
Danovi SA, et al: Yes-associated protein (YAP) functions as a tumor
suppressor in breast. Cell Death Differ. 15:1752–1759.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu C, Li L, Zhang Z, Bi M, Wang H, Su W,
Hernandez K, Liu P, Chen J, Chen M, et al: A non-canonical role of
YAP/TEAD is required for activation of estrogen-regulated enhancers
in breast cancer. Mol Cell. 75:791–806.e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cao L, Yao M, Sasano H, Sun PL and Gao H:
YAP increases response to trastuzumab in HER2-positive breast
cancer by enhancing P73-induced apoptosis. J Cancer. 11:6748–6759.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cao L, Sun PL, Yao M, Jia M and Gao H:
Expression of YES-associated protein (YAP) and its clinical
significance in breast cancer tissues. Hum Pathol. 68:166–174.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jang GB, Kim JY, Cho SD, Park KS, Jung JY,
Lee HY, Hong IS and Nam JS: Blockade of Wnt/β-catenin signaling
suppresses breast cancer metastasis by inhibiting CSC-like
phenotype. Sci Rep. 5(12465)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen Y, Shi HY, Stock SR, Stern PH and
Zhang M: Regulation of breast cancer-induced bone lesions by
β-catenin protein signaling. J Biol Chem. 286:42575–42584.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yamaguchi H, Chang SS, Hsu JL and Hung MC:
Signaling cross-talk in the resistance to HER family receptor
targeted therapy. Oncogene. 33:1073–1081. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu Y, Ginther C, Kim J, Mosher N, Chung S,
Slamon D and Vadgama JV: Expression of Wnt3 activates Wnt/β-catenin
pathway and promotes EMT-like phenotype in trastuzumab-resistant
HER2-overexpressing breast cancer cells. Mol Cancer Res.
10:1597–1606. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hui M, Cazet A, Nair R, Watkins DN,
O'Toole SA and Swarbrick A: The hedgehog signalling pathway in
breast development, carcinogenesis and cancer therapy. Breast
Cancer Res. 15(203)2013.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Riobo-Del Galdo NA, Lara Montero Á and
Wertheimer EV: Role of hedgehog signaling in breast cancer:
Pathogenesis and therapeutics. Cells. 8(375)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rajurkar M, De Jesus-Monge WE, Driscoll
DR, Appleman VA, Huang H, Cotton JL, Klimstra DS, Zhu LJ, Simin K,
Xu L, et al: The activity of Gli transcription factors is essential
for Kras-induced pancreatic tumorigenesis. Proc Natl Acad Sci USA.
109:E1038–E1047. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Benvenuto M, Masuelli L, De Smaele E,
Fantini M, Mattera R, Cucchi D, Bonanno E, Di Stefano E, Frajese
GV, Orlandi A, et al: In vitro and in vivo inhibition of breast
cancer cell growth by targeting the Hedgehog/GLI pathway with SMO
(GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget. 7:9250–9270.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Doheny D, Sirkisoon S, Carpenter RL,
Aguayo NR, Regua AT, Anguelov M, Manore SG, Arrigo A, Jalboush SA,
Wong GL, et al: Combined inhibition of JAK2-STAT3 and
SMO-GLI1/tGLI1 pathways suppresses breast cancer stem cells, tumor
growth, and metastasis. Oncogene. 39:6589–6605. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Stathis A, Hess D, von Moos R, Homicsko K,
Griguolo G, Joerger M, Mark M, Ackermann CJ, Allegrini S, Catapano
CV, et al: Phase I trial of the oral smoothened inhibitor sonidegib
in combination with paclitaxel in patients with advanced solid
tumors. Invest New Drugs. 35:766–772. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Garcia N, Ulin M, Al-Hendy A and Yang Q:
The role of hedgehog pathway in female cancers. J Cancer Sci Clin
Ther. 4:487–498. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zheng X, Han H, Liu GP, Ma YX, Pan RL,
Sang LJ, Li RH, Yang LJ, Marks JR, Wang W and Lin A: LncRNA wires
up hippo and hedgehog signaling to reprogramme glucose metabolism.
EMBO J. 36:3325–3335. 2017.PubMed/NCBI View Article : Google Scholar
|