Introduction
Bile duct hamartoma in the liver (LBDH), also known
as von Mayenburg complex (VMC) and polycystic bile duct hamartoma,
is a benign malformation of the intrahepatic bile duct associated
with bile duct plate defects (1,2). The
clinical symptoms and signs of disease in patients are often
atypical, so these tumours are usually incidentally found during
physical examination, exploratory laparotomy or autopsy. Some cases
have mild pain in the right upper abdomen, and the blood
α-fetoprotein (AFP) and carcinoembryonic antigen (CEA) levels are
generally normal (3). LBDH is
considered to be caused by abnormal development of intrahepatic
bile ducts. Histologically, it consists of inflammatory cells, bile
ducts and fibrosis. Macroscopically, LBDH appears as multiple small
greyish-white nodules scattered below the liver capsule and around
the portal vein. These lesions usually do not communicate with the
bile duct tree. Microscopically, the bile duct consists of a series
of dilated branching cystic bile ducts lined with a single cuboid
epithelial cell layer surrounded by a rich fibrocollagen matrix.
The diameter of each lesion is 0.1-1.5 cm. The lumen of the bile
duct usually contains bile-stained granular matter (4). LBDH is extremely rare, and its
incidence at autopsy is 0.6-5.6% (5). At the same time, due to the lack of
an adequate understanding of LBDH ultrasonic manifestations,
misdiagnosis easily occurs. The ultrasound misdiagnosis rate of
this disease is high at ~80%. LBDH must mainly be differentiated
from the following diseases: Liver cysts, chronic liver disease,
cirrhosis, diffuse liver parenchymal lesions, Caroli disease,
multiple hepatic hemangiomas and multiple intrahepatic metastases
(6); therefore, investigations
into how to effectively improve the imaging diagnosis level of
LBDHs are warranted. At the same time, LBDH has a certain tendency
toward malignant transformation, and the clinical and imaging
manifestations lack specificity. By comparing the differences in
imaging manifestations on enhanced computed tomography (CT) and
contrast-enhanced ultrasonography (CEUS), the imaging diagnostic
performance can be improved to a great extent.
Case report
Patient
An elderly male, aged 63 years, was found to have
space-occupying lesions ~2 cm in size in the liver during a routine
physical examination at an external hospital. Therefore, the
patient was admitted to The Second Hospital of Wuxi Affiliated to
Nanjing Medical University (Wuxi, China) in January 2021. The
patient was generally in good condition without obvious symptoms of
discomfort and no yellow staining of the skin or sclera. The
patient had a flat and soft abdomen, and no abnormal masses could
be palpated. Laboratory examination revealed that the tumour marker
levels, including that of AFP, CEA, carbohydrate antigen (CA)-125
and CA-199, were within the normal ranges (data not shown). The
results for hepatitis virus markers were negative.
Ultrasound
The patient was subjected to a routine liver
ultrasound examination. Entire liver sections were scanned with a
conventional US. When a target lesion was found, the maximum cross
section of the tumour diameter and blood supply were examined and
recorded. The ultrasound examination showed diffuse chronic liver
disease and cirrhosis. Two space-occupying lesions were found in
the right lobe of the liver: One close to the liver capsule and
located in segment six (S6), and the other 1.5 cm away from the
liver capsule and located in segment five (S5). Both masses were
hyperechoic and had uneven internal echoes, unclear boundaries and
no obvious capsules. If benign, these masses could be hepatic
haemangiomas, while if malignant, they may be hepatocellular
carcinoma (HCC). Therefore, the ultrasonographer suggested further
examinations to determine the nature of the masses.
Enhanced CT
The abdomen was scanned by a Toshiba Aquilion ONE64
slice spiral CT scanner. The scanning parameters were 200 mAsec,
120 kV and a slice thickness of 0.5 mm. The contrast agent injected
for enhanced scanning was ioversol (320 mg I/ml). The injection
flow rate was 3.0 ml/sec and the dose applied was 1.5 ml/kg of body
weight. Arterial phase scanning was performed 25 sec after
injection, venous phase scanning was performed 60 sec later and
delayed phase scanning was performed 120 sec after this.
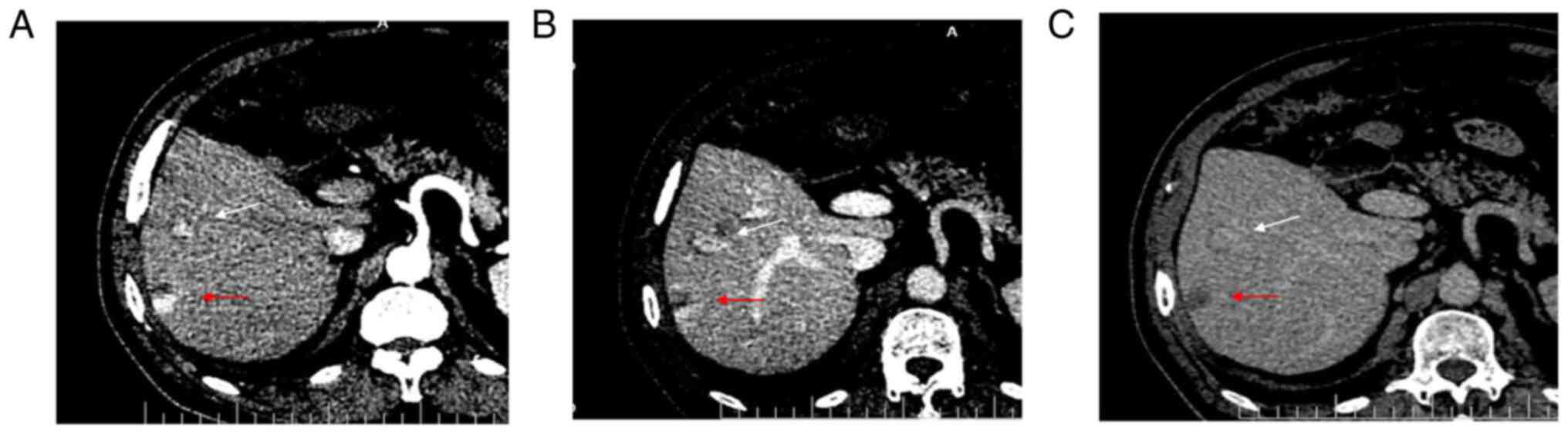
Enhanced CT showed local enhancement in the arterial
phase (Fig. 1A) and continuous
enhancement in the portal (Fig.
1B) and delayed (Fig. 1C)
phases, without obvious clearance. Enhanced CT showed a
strong-equal-equal enhancement mode, suggesting a benign hepatic
hemangioma. The results of the enhanced CT showed that the mass was
a benign hepatic hemangioma.
CEUS
At the suggestion of the ultrasound doctor, the
patient underwent CEUS. CEUS was initiated using a Resona 7
(Shenzhen Mindray Bio-Medical Electronics Co., Ltd.) ultrasound
instrument. After a bolus injection of 1.6 ml SonoVue (Bracco
Group) through a peripheral venous cannula, a 5-ml saline flush was
used and the timer on the sonography was started. Observations were
made until the microbubbles cleared from circulation (usually up to
5 min). All video clips were recorded and then transferred to a
hard disk.
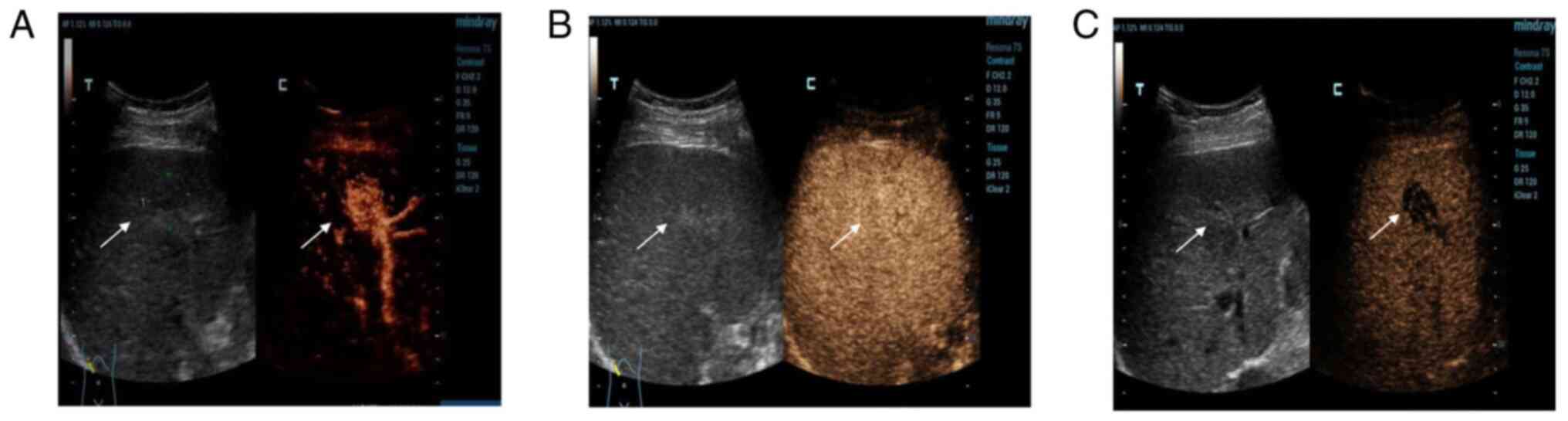
The examination showed a mass in S5 (Fig. 2A). At 13 sec, the hepatic artery
began to develop, while the hyperechoic mass developed rapidly,
reaching peak intensity at 17 sec, and then the surrounding liver
parenchyma began to develop. The development time of the
hyperechoic masses in the arterial phase was significantly earlier
than that in the hepatic parenchyma, and the range of the mass was
wider than that of conventional images. Upon further observation,
the enhancement continued to develop (Fig. 2B) and then began to decline 2 min
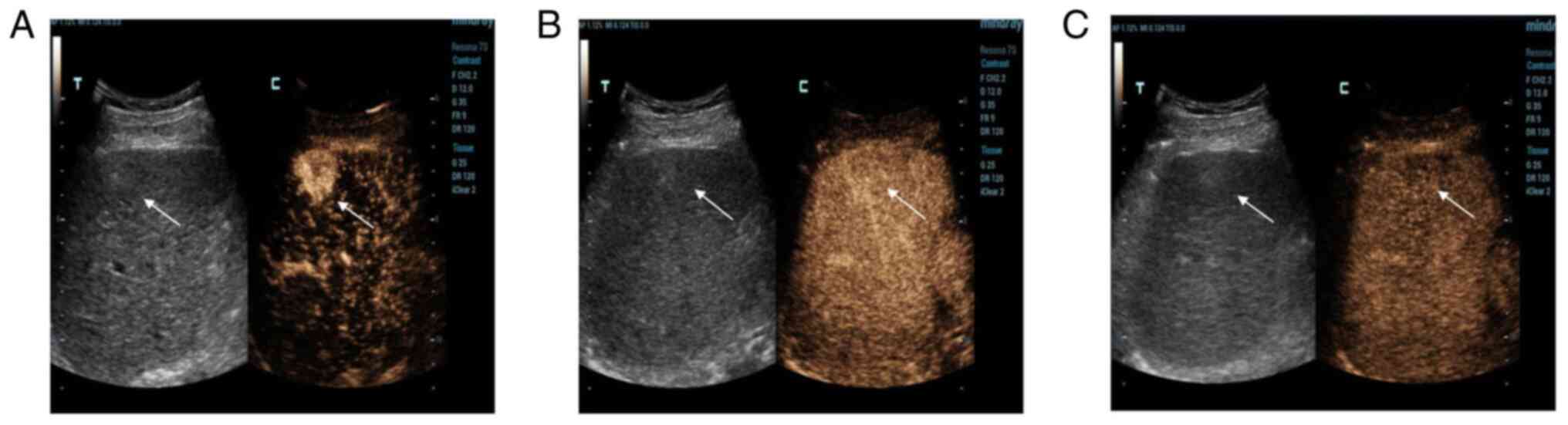
later. The mass was completely cleared after 6 min (Fig. 2C). The other mass in S6 was
developed in the same way; it developed rapidly in the arterial
phase (Fig. 3A), continuously in
the portal phase (Fig. 3B) and
slowly decreased in the delayed phase (Fig. 3C). CEUS showed a strong-equal-low
development mode, so malignant lesions were first considered.
Next, the patient now presented with liver
cirrhosis, which led to the consideration of HCC; however, due to
the late and slow regression, a highly differentiated HCC was
considered.
Therefore, from the imaging examinations, CEUS had
revealed a highly differentiated HCC, enhanced CT showed benign
lesions and CEUS showed malignant lesions.
Surgery
Finally, the patient chose to undergo surgery, and
during the operation, the liver exhibited small nodular cirrhosis.
The two masses were located by B-ultrasound during the operation
and were found to be located at the junction of S5 and S6. The
nodular masses were grey in pathological appearance. One was close
to the liver capsule (3.0x2.5x2.0 cm in size), and the other was
1.5 cm away from the liver capsule (3.0x2.0x2.0 cm in size). The
boundary remained clear.
Histology
The hepatobiliary surgeon removed two lesions and
sent them to the Department of Pathology for examination.
Pathologists sectioned and stained the samples, and performed
histological and immunohistochemical examinations. First, the
specimen was placed in fixative (10% formalin) overnight at a
normal atmospheric temperature. The samples were taken on the
second day (the sample size was 1.5x1.5x0.2 cm). The samples were
then dehydrated overnight in a dehydrator. On the third day, the
sample was placed in an embedding box for embedding (65˚C in
paraffin). After embedding, they were placed in a paraffin slicer
for tissue sectioning at a thickness of 3-4 µm. The cut sample
slices were placed on the slides, and the slides were baked on an
electric heating plate at 60-70˚C for 1-1.5 h. H&E staining was
then performed. The whole H&E staining process consisted of
dewaxing, dyeing, dehydration, making the section transparent and
sealing. First, wax was removed from slices using xylene three
times for 5-10 min each. The xylene was then washed away with
alcohol (anhydrous, 95, 80 and 70% sequentially for 1 min). Next,
the slices were rinsed with water for 2 min. The slices were then
stained with haematoxylin for 5 min. The cells were rinsed with
water again for 1-3 min after staining. After the sections were
rinsed with water, they were rinsed with acidic alcohol (1%) for 20
sec. The sections were rinsed in water for >15 min until the
nuclei turned blue. Eosin solution was used for dyeing for 30 sec
to 1 min, and each alcohol concentration (anhydrous, 85 and 95%,
twice) was used for 1-2 min/wash. The tissue sections were made
transparent three times (2 min/wash) with xylene to ensure the
transparency of the sections. Finally, the tablet was sealed with
neutral gum and labelled accordingly. Images were then taken under
a light microscope (Nikon Corporation) at x200 magnification.
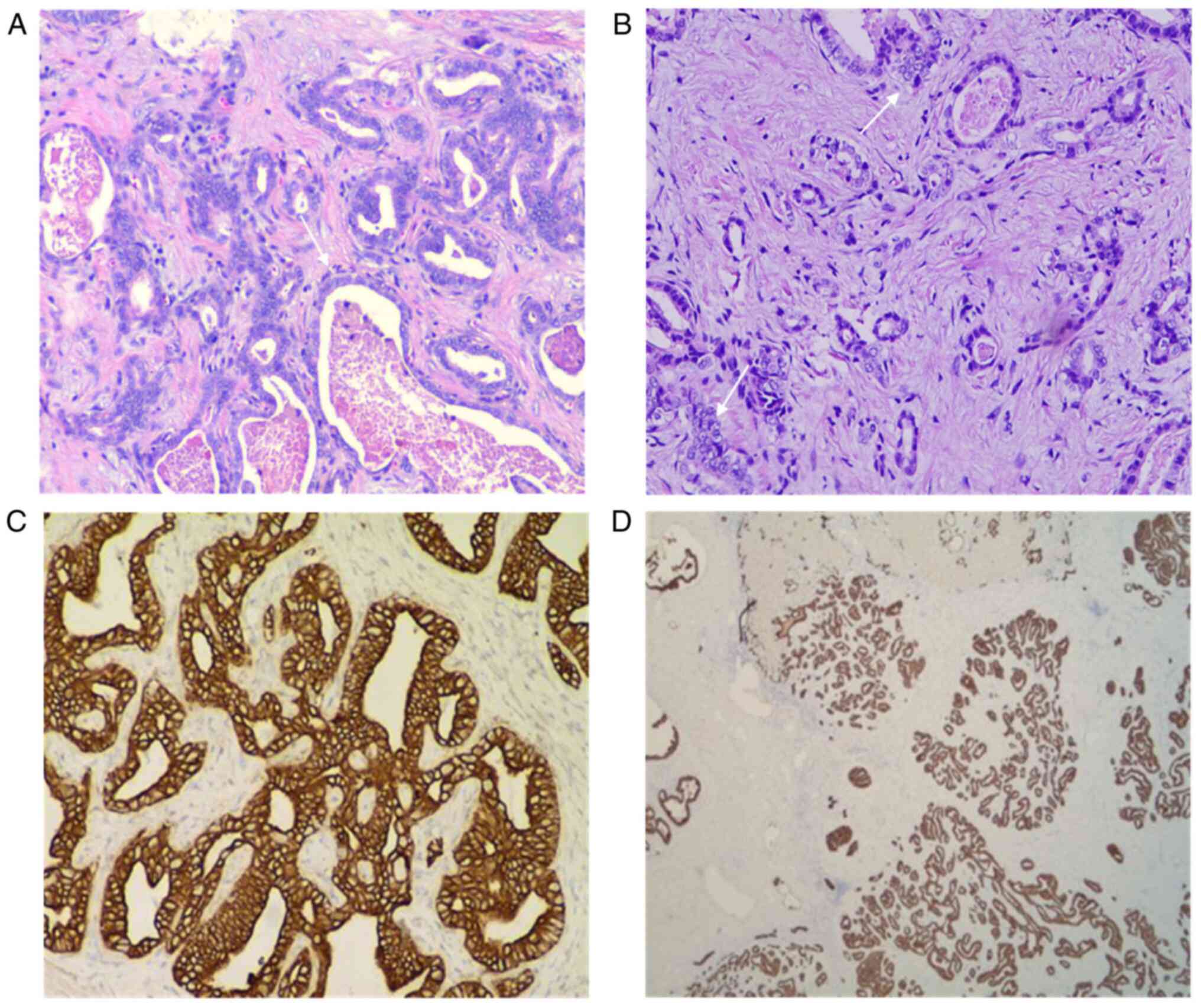
Under a light microscope, obvious hyperplasia of the
interlobular bile ducts was observed, along with lobulated and
partial cystic dilatation, suggesting LBDH (Fig. 4A). In addition, the agglomeration
of bile duct epithelial cells was observed, and there were certain
areas that had atypia and adenotubular and papillary arrangements,
with obvious nucleoli accompanied by fibrous hyperplasia (Fig. 4B).
Immunohistochemistry
Following tissue preparation as aforementioned, the
EliVision™ Super system (MXB Biotechnologies) was
applied according to the manufacturer's instructions. The system
used a two-step method in which the primary antibodies were mouse
anti-human IgG monoclonal antibodies [hepatocyte, cat. no.
MAB-0249; CD34, cat. no. KIT-0004; cytokeratin (CK) 7, cat. no.
KIT-0021; CK20, cat. no. KIT-0025; CK19, cat. no. KIT-0030;
Glypican-3, cat. no. KIT-0036; thyroid transcription factor 1
(TTF-1), cat. no. MAB-0599; and CK8, cat. no. KIT-0034; all MXB
Biotechnologies]. Then a large amount of secondary antibody
(anti-mouse/anti-rabbit) IgG polymer (cat. no. TT-0801; MXB
Biotechnologies) was indirectly linked to the antigen-bound primary
antibody in the sample by linking with reaction amplifiers.
After dewaxing and hydration, the paraffin sections
were washed with water. Pre-treated tissue sections were used
(depending on the specific instructions of primary antibody).
Peroxidase blocking reagent (3% H2O2) was
dripped onto the sections, and the sections were incubated at
normal atmospheric temperature for 10 min and rinsed with PBS (cat.
no. PBS-0060) three times (3 min/wash). After rinsing, the primary
antibody was added to the sections and incubated at normal
atmospheric temperature for 60 min. After incubation, the sections
were rinsed with PBS again three times (3 min/wash). Reaction
magnifying agent was then added to the slices, which were incubated
for 15 min at normal atmospheric temperature. After incubation, the
sections were rinsed with PBS again three times (3 min/wash). After
rinsing, high-sensitivity enzyme-conjugated anti-mouse/anti-rabbit
IgG polymer was added to the sections, which were incubated at room
temperature for 15 min. After incubation, the sections were rinsed
with PBS again. A total of 120 µl DAB chromogenic reagent (Titan
Super) was added to the slices after washing. Finally, the sealing
piece was redyed with haematoxylin. Images were then taken under a
light microscope (Nikon) (image magnification is 200 times).
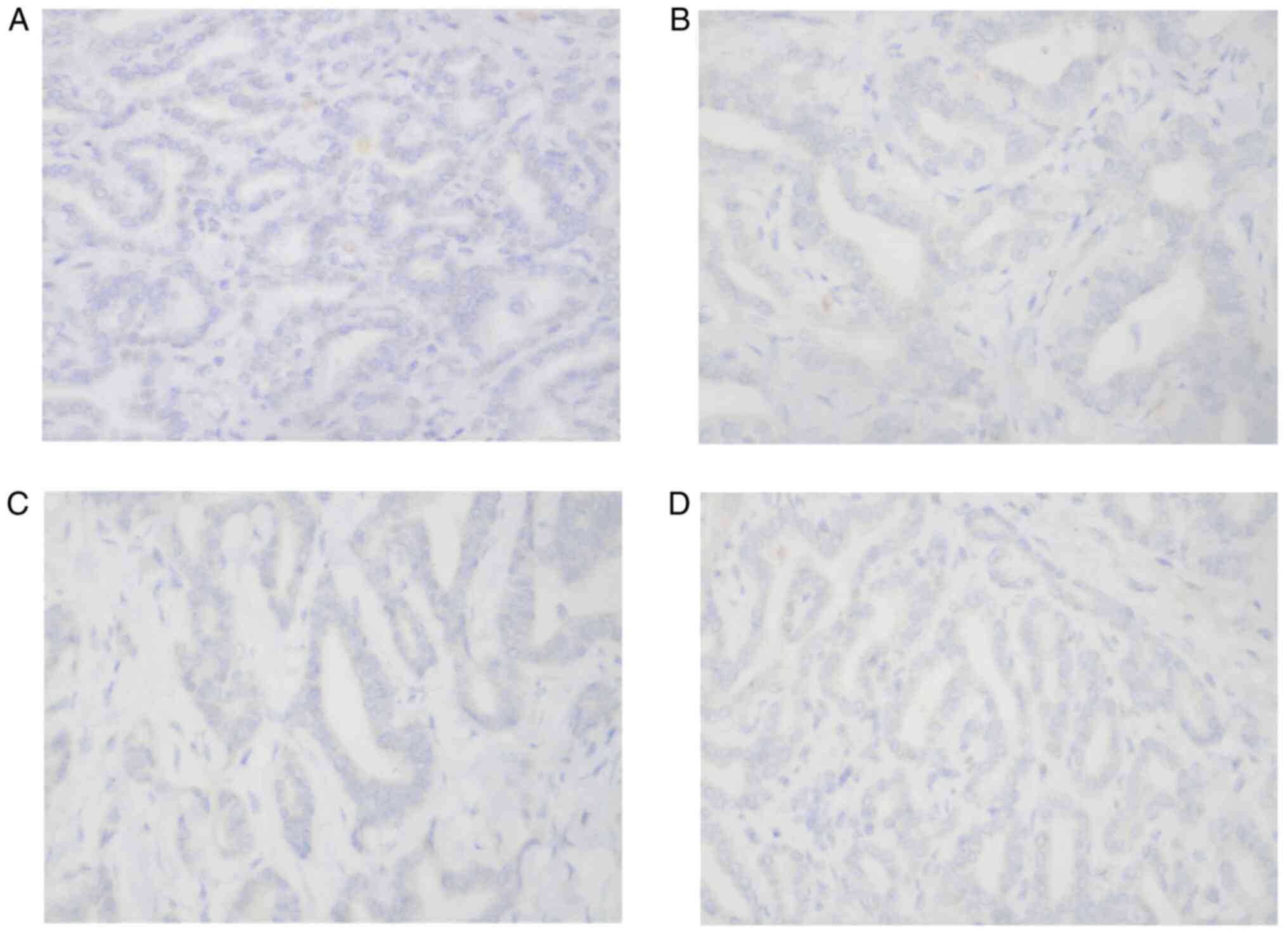
The immunohistochemical staining results of the
tumour cells from the patient showed the following results:
Hepatocyte(-) (Fig. 5A), CD34(-),
CK7(+) and CK20(-) (Fig. 5B),
CDX2(-), CK19(+), TTF1(-) (Fig.
5C), Glypican-3(-) (Fig. 5D),
CK8(+) and S100(-). There was no hepatocyte origin due to the
hepatocyte(-) results. CK8 (Fig.
4C) is mainly used to label non-squamous epithelium and can be
used for the diagnosis of adenocarcinoma and ductal carcinoma. CK19
(Fig. 4D) is mainly used to label
different types of monolayer epithelia, including the glandular
epithelium, and is mainly used for the diagnosis of adenocarcinoma.
CK19 has no staining for liver cells, and has specific staining for
bile duct epithelium and intrahepatic cholangiocarcinoma (ICC),
with a positive rate of 77-100%. CK19 is the best
immunohistochemical marker for the diagnosis of ICC at present
(7). A previous case study
(8) reported a diagnosis of bile
duct adenoma, with the immunohistochemical analysis revealing
CK7(+), CK19(+), CEA(-) and AFP(+) results. In another case report
on multicystic biliary hamartoma (9), immunohistochemical staining revealed
that dilated ducts were positive for CK19. As indicated,
hepatocytes do not express CK19, whereas the present case was
CK19(+), suggesting that the tumour originated from the bile duct
epithelium, and the final histological diagnosis was of an LBDH
malignant transformation into a well-differentiated ICC. The final
pathological results of this case were different from those
suggested by enhanced CT and CEUS. According to the pathological
mechanism of LDBH, the enhanced CT and CEUS manifestations of this
rare case were analysed and explained by comparing the pathological
principles and imaging principles.
Literature review
Search strategy
The PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/) and Web of Science
(http://webofscience.com) databases were
systematically searched up to June 2021. The following key words
were used: (‘bile duct hamartomas in liver’ OR ‘malignant
transformation’) AND (‘contrast-enhanced ultrasonography’ OR
‘enhanced CT’ OR ‘imaging analysis’). Only studies published in
English or Chinese and full-text journal articles of original
studies were included. All other studies were excluded.
Furthermore, the references cited in the relevant studies were
reviewed for additional eligible publications. In the process of
the literature review, >30 case reports and literature reviews
were searched. The majority of studies were for individual cases,
and most of the diagnostic methods focused on imaging examinations.
Imaging examinations mainly included ultrasound, enhanced CT, MR
and CEUS. Among them, the use of CEUS was relatively rare, and
mostly reports were on multiple benign lesions. No case report on
CEUS manifestations of the malignant transformation of LBDH was
found.
Discussion
LBDH, also known as VMC, was first described by von
Meyenburg (10) in 1918. The
lesions are usually small (<5 mm) and appear as multiple
scattered lesions throughout the liver. The aetiology is not yet
clear. Some scholars believe that LBDH is caused by the abnormal
development of the bile duct plate or the abnormal remodelling of
the bile duct plate during embryonic development (11-14).
From the 6 to 8th weeks of embryonic development, the hepatocytes
in contact with the mesenchyme around the portal vein express bile
duct keratin, which induces the hepatocytes near the portal vein
branch to differentiate into bile duct epithelial cells. These
cells form a double-layer cuff-like bile duct plate around the
portal vein branch that contains the capillary plexus, which forms
the hepatic sinus system. After the 12th week of embryonic
development, a more mature bile duct is formed around the hepatic
portal vein, the excess bile duct becomes apoptotic, and finally, a
bile duct network is formed around the portal vein. The order of
bile duct reconstruction is from the hilar to the peripheral part
of the liver, first forming the bold duct, then the segmental bile
duct, the interlobular bile duct and, finally, the smallest
capillary bile duct. Under normal circumstances, any excess bile
duct will be degenerated and absorbed. When the transformation from
the bile duct plate to the bile duct in late embryos is blocked and
the absorption is insufficient, a labyrinthine bile duct is formed,
resulting in the retention of secretory epithelial cells and fluid
in the surrounding tissue, which develops into cystic lesions
(15). Moreover, due to its high
incidence with visceral polycystic lesions, it has been inferred
that this cystic formation has a certain genetic tendency (16), and some scholars have speculated
that it is the result of liver inflammation, ischaemia or genetic
abnormalities (17).
LBDHs have a variety of ultrasonic imaging features,
manifesting as multiple divergent cystic echoes in the liver, and
diffuse echo changes in the liver parenchyma, with patchy high echo
and low echo, and intrahepatic multiple comet tail signs. The
different manifestations on ultrasound images are closely related
to the size of the dilated bile duct structure (18). A cystic dilated bile duct wall is
composed of bile duct epithelial cells, ductal glands and fibrous
connective tissue surrounded by a fibrous matrix tissue (19). When the dilated bile duct is
visible, it shows a cystic echo pattern. There is a high
concentration of cholestasis in the dilated bile duct lumen, so
there is no enhancement effect behind some of the cystic lesions.
When the dilated bile duct is small, it is difficult for ultrasound
to depict the internal anechoic part, and only the thick capsule
wall interface can be observed, so ultrasound shows only
hyperechoic or hypoechoic nodules. The appearance of comet tail
signs is often caused by multiple reflections off the dilated bile
duct. If carefully observed, the front of the comet tail is often
accompanied by hyperechoic or hypoechoic lesions. Occasionally,
aggregated bile duct hamartomas can also appear as large solitary
lesions on imaging (20).
A small number of patients experience malignant
progression of multiple LBDH to ICC (more common) or HCC. The risk
factors for malignant transformation include chemical or mechanical
stimulation, cholestasis or chronic inflammation caused by calculi
(21-23).
Therefore, long-term follow-up of patients with clearly diagnosed
multiple LBDHs is important. If there is a definite malignant
manifestation, the lesion can be removed in time, and the prognosis
of the patient can be greatly improved.
In a number of cases of LBDH reported in the past,
ultrasound, CT, magnetic resonance imaging (MRI), digital
subtraction angiography (DSA) and magnetic resonance
cholangiography (MRC) have been used to help diagnose the disease
(24,25). There are few reports in the
existing literature on CEUS manifestations of isolated or sporadic
LBDH, and all of these reports are of benign disease, while CEUS
manifestations of malignant LBDH are almost completely absent from
the literature. CEUS and enhanced CT are certainly first-line tests
for such focal liver lesions, and in most cases, they provide
sufficient diagnostic information. If the results of ultrasound and
CT are unclear or the possibility of a rare disease is considered,
the clinician may recommend subsequent MRI. LBDH is a benign
hepatic cystic lesion that may undergo cystic enlargement and
internal haemorrhage. Complicated giant-LBDH coexists with smaller
LBDH and the MRI features of giant-LBDH are characteristic
(26). MRI is considered to be the
gold standard for the diagnosis of LBDH due to its higher
sensitivity and specificity than CT. However, as the current case
was very rare, presenting two space-occupying lesions and not the
typical multiple and variously sized cystic lesions, the clinician
did not consider MRI first when selecting the type of imaging
examination. It is also necessary to consider whether the physical
condition of the patient is suitable for MRI examination and the
economic burden on the patient, as an MRI examination is relatively
expensive. In addition, through a review of the literature, it was
found that such isolated or sporadic bile duct hamartomas reported
in numerous studies were mostly examined only by ultrasound and CT
before diagnosis, and rarely by MRI (27). Moreover, as an invasive
interventional examination, DSA has great advantages for the
diagnosis of liver space-occupying lesions. However, with the
continuous promotion of minimally invasive examinations and even
non-invasive examinations, its current application is mainly
focused on the heart, brain and other organs, while its application
in liver tumours is gradually decreasing. Therefore, DSA
examination is not the first choice in this case. MRC is mainly
applied in patients with bile duct dilation and suspected biliary
tract lesions (28). No obvious
intrahepatic biliary tract changes were observed in the present
patient case, and the disease mainly manifested as focal
space-occupying lesions. Therefore, CEUS and enhanced CT, which can
be performed in primary hospitals, were selected for imaging
comparison.
In the imaging analysis of this case, enhanced CT
showed a strong-equal-equal enhancement mode, suggesting a benign
hepatic haemangioma. However, CEUS showed a strong-equal-low
development mode, so malignant lesions were first considered. Next,
the patient presented with liver cirrhosis, which led to the
consideration of HCC first, but due to the late and slow
regression, a less well-differentiated HCC was then considered.
Therefore, enhanced CT and CEUS were performed, but one suggested a
benign lesion, and the other suggested a malignant lesion. The
question thus becomes why such discrepant findings were observed.
Ultrasound contrast agent is a blood pool contrast agent that is
injected into the human body through a peripheral vein and enters
the hepatic mass through the pulmonary circulation to achieve
enhancement and development (29).
Due to the obvious differences in the vascular distribution and
haemodynamic characteristics between benign and malignant liver
tumours, contrast agents show different development patterns in the
liver, which has become an important basis for differentiating
between benign and malignant tumours. As malignant liver tumours
are rich in blood vessels and as their blood supply is from the
hepatic artery, their arteries dilate, have circuity, and are
around and at the centre of an abnormal proliferation of tumour
blood vessels and arteriovenous anastomosis, with ~75% of the
normal liver parenchyma being supplied by the portal vein (30). The contrast agent enters the tumour
early but fades fast, so the enhancement time for this is short,
while it enters the liver parenchyma late, so the enhancement time
for this is long. Therefore, the vast majority of enhancement modes
for enhanced CT and CEUS of malignant liver tumours are similar,
showing rapid enhancement and rapid decline (fast in and fast out)
(31). However, contrast-enhanced
CT uses an ionic contrast agent that can penetrate into the mass
organization before clearance, so for some non-hepatocellular
carcinoma tumours, such as intrahepatic bile duct carcinoma (ICC),
the contrast agent will gradually penetrate into the tumour; thus,
these tumours are characterized by rapid enhancement without
decreasing the enhanced mode (fast in and slow out). This
enhancement model is a double-edged sword that can be used to
distinguish HCC from ICC, but can easily lead to a misdiagnosis of
a benign liver tumour such as hepatic haemangioma. This occurs
since hepatic haemangioma is composed of sinuses of different
sizes, and its blood flow velocity is relatively slow compared with
that of HCC. Therefore, the contrast agent does not easily enter
and exit, thus presenting a typical image of slow enhancement and
slow decline (slow in and slow out). The tumour in the present case
was an LBDH that turned into a highly differentiated ICC, which
showed a pattern of continuous enhancement without regression in
the delayed stage, so it could be easily misdiagnosed as a benign
haemangioma. The ultrasound contrast agent is a real blood pool
contrast agent that will not enter the tissue space, so the
enhancement mode will be fast enhancement and fast decline (fast in
and fast out) for the vast majority of malignant tumours. For the
differentiation between benign and malignant tumours, CEUS has
obvious advantages over CT, as it can accurately reflect the
characteristics of blood flow distribution and the haemodynamic
changes in liver tumours, which is why CEUS could be used to
diagnose the malignant tumours in the present case. However, for
the differentiation of different pathological types of malignant
lesions, such as HCC and ICC, enhanced CT may be more intuitive
than CEUS imaging, as the contrast agent can better penetrate into
the tissue space.
In addition, enhanced CT has time constraints. For
some masses with late regression, imaging technicians are likely to
fail to capture the point of regression during the delay period,
resulting in the illusion that the mass does not fade during the
delay period and thus causing the mass to be misdiagnosed as
benign. Moreover, enhanced CT exposes patients to a certain level
of radiation, so it cannot be applied for a long time. CEUS, by
contrast, is full-course, real-time and dynamic, and can be applied
for as long as desired, providing a clearer indication of whether
the delay has subsided. In conclusion, enhanced CT and CEUS both
have their advantages. CEUS is more advantageous in differentiating
benign from malignant masses, while enhanced CT is more intuitive
and accurate in differentiating malignant tumours of different
pathological types. In the present case, when a benign haemangioma
was indicated by enhanced CT due to the strong-equal-equal
enhancement mode, CEUS assisted in the diagnosis of malignancy due
to the strong-equal-low enhancement mode, both of which provided
different diagnostic ideas and surgical bases for the clinic,
helped clinicians remove the lesion early, and greatly improved the
prognosis of the patient. The advantages of CEUS in differentiating
benign from malignant lesions should be emphasized.
The time it takes for the contrast agent to regress
in the delayed stage of HCC can reflect the blood supply ratio of
arteries and portal veins, which can be used to judge the level of
differentiation of HCC. As the degree of malignancy of HCC
increases, the contrast agent regression time decreases (32,33).
The question remains as to whether the development pattern is
similar for cases of ICC. The pathology results of the present case
revealed that the lesion was a highly differentiated ICC, which was
illustrated by late regression on CEUS, consistent with the
angiographic pattern of HCC. However, due to the rarity of this
disease, a large amount of data and case accumulation are still
needed to prove this link.
There are few reports in the existing literature on
CEUS manifestations of isolated or sporadic LBDH, and all of these
reports are of benign disease. Meanwhile, CEUS manifestations of
malignant LBDH are almost completely absent from the literature
(34). In the present case, the
manifestations of malignant LBDH on CEUS were significantly
different from those of benign LBDH.
The differences between enhanced CT and CEUS in the
current rare case were analysed, and the imaging principles and
pathophysiological basis of the two examinations in the diagnosis
of LBDH malignant transformation were analysed, providing new
diagnostic ideas and examination methods for subsequent clinical
work.
The present study reveals that the use of a
combination of multiple imaging methods in the diagnosis of this
disease can greatly improve the rate of clinical diagnosis and
reduce the rate of misdiagnosis, and reveal any malignant trend in
the lesions in a timely manner, thus helping clinicians remove the
lesions as early as possible and greatly improving the prognosis of
the patient.
In conclusion, LBDH is a rare lesion with malignant
potential that lacks specific clinical and imaging manifestations.
By analysing the characteristic imaging features of intrahepatic
bile duct hamartomas that correspond to its pathological features,
combined with the imaging rules of enhanced CT and CEUS, the
diagnostic accuracy of imaging can be improved to a great extent.
Malignant lesions can be found early, the lesion can be removed in
a timely manner and the prognosis of these patients can be greatly
improved.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
JF designed the study. YY was responsible for the
evaluation and analysis of the data and contributed to writing the
manuscript. GF was responsible for the clinical management of the
patient. FW and XLC were responsible for the preparation of the
data analysis and the revision of the manuscript for important
intellectual content. YY and JF confirm the authenticity of all the
raw data. The final version of the manuscript was read and approved
by all authors.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of the Second Hospital of Wuxi Affiliated to Nanjing
Medical University (Wuxi, China), and the patient provided written
informed consent.
Patient consent for publication
Written informed consent was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fernández-Carrión MJ, Robles Campos R,
López Conesa A, Brusadín R and Parrilla Paricio P: Intrahepatic
multicystic biliary hamartoma: Presentation of a case report. Cir
Esp. 93:e103–e105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yoh T, Okamura R, Nakayama H, Lin X,
Nakamura Y and Kato T: Multicystic biliary hamartoma mimicking
intrahepatic cholangiocarcinoma: Report of a case. Clin J
Gastroenterol. 7:418–421. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Min JK, Kim JM, Li S, Lee JW, Yoon H, Ryu
CJ, Jeon SH, Lee JH, Kim JY, Yoon HK, et al: L1 cell adhesion
molecule is a novel therapeutic target in intrahepatic
cholangiocarcinoma. Clin Cancer Res. 16:3571–3580. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Karahan OI, Kahriman G, Soyuer I and Ok E:
Hepatic von Meyenburg complex simulating biliary
cystadenocarcinoma. Clin Imaging. 31:50–53. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sahani DV, Kadavigere R, Saokar A,
Fernandez-del Castillo C, Brugge WR and Hahn PF: Cystic pancreatic
lesions: A simple imaging-based classification system for guiding
management. Radiographics. 25:1471–1484. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yingzi S, Yanhong M, Liping W, Shiqin J,
et al: Analysis on ultrasonic diagnosis and misdiagnostic causes of
bile duct hamartoma in liver. J Med Imaging. 27:1509–1511.
2017.
|
|
7
|
Jain R, Fischer S, Serra S and Chetty R:
The use of cytokeratin 19 (CK19) immunohistochemistry in lesions of
the pancreas, gastrointestinal tract, and liver. Appl
Immunohistochem Mol Morphol. 18:9–15. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen L, Xu MY and Chen F: Bile duct
adenoma: A case report and literature review. World J Surg Oncol.
12(125)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mu W, Su P and Ning S: Case report:
Incidentally discovered a rare cystic lesion of liver: Multicystic
biliary hamartoma. Pathol Oncol Res. 27(628323)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jáquez-Quintana JO, Reyes-Cabello EA and
Bosques-Padilla FJ: Multiple biliary hamartomas, the ‘von Meyenburg
complexes’. Ann Hepatol. 16:812–813. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Venkatanarasimha N, Thomas R, Armstrong
EM, Shirley JF, Fox BM and Jackson SA: Imaging features of ductal
plate malformations in adults. Clin Radiol. 66:1086–1093.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Veigel MC, Prescott-Focht J, Rodriguez MG,
Zinati R, Shao L, Moore CA and Lowe LH: Fibropolycystic liver
disease in children. Pediatr Radiol. 39:317–327, 420-421.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Levy AD and Rohrmann CA Jr: Biliary cystic
disease. Curr Probl Diagn Radiol. 32:233–263. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lefere M, Thijs M, De Hertogh G, Verslype
C, Laleman W, Vanbeckevoort D, Van Steenbergen W and Claus F:
Caroli disease: Review of eight cases with emphasis on magnetic
resonance imaging features. Eur J Gastroenterol Hepatol.
23:578–585. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li J, Nie HF and Xiao R: Findings of
intrahepatic bile duct hamartoma imaging. Mod Diagn Treat.
24:2819–2820. 2013.
|
|
16
|
Zhong HB, Quan GM and Yuan T: Evaluation
of CT and MRI in the bile duct malformation. Radiol Pract.
27:1293–1297. 2012.
|
|
17
|
Aishima S, Tanaka Y, Kubo Y, Shirabe K,
Maehara Y and Oda Y: Bile duct adenoma and von Meyenburg
complex-like duct arising in hepatitis and cirrhosis: Pathogenesis
and histological characteristics. Pathol Int. 64:551–559.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ogura T, Kurisu Y, Miyano A and Higuchi K:
A huge rapidly-enlarging multicystic biliary hamartoma. Dig Liver
Dis. 50(723)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nakanuma Y, Hoso M, Sanzen T and Sasaki M:
Microstructure and development of the normal and pathologic biliary
tract in humans, including blood supply. Microsc Res Tech.
38:552–570. 1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zheng RQ, Zhang B, Kudo M, Onda H and
Inoue T: Imaging findings of biliary hamartomas. World J
Gastroenterol. 11:6354–6359. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Röcken C, Pross M, Brucks U, Ridwelski K
and Roessner A: Cholangiocarcinoma occurring in a liver with
multiple bile duct hamartomas (von Meyenburg complexes). Arch
Pathol Lab Med. 124:1704–1706. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Holzinger F, Z'graggen K and Büchler MW:
Mechanisms of biliary carcinogenesis: A pathogenetic multi-stage
cascade towards cholangiocarcinoma. Ann Oncol. 10 (Suppl
4):S122–S126. 1999.PubMed/NCBI
|
|
23
|
Nakanuma Y, Hoso M and Terada T: Clinical
and pathological features of cholangiocarcinoma. In: Okuda K, Tabor
E (eds). Liver Cancer. Churchill Livingstone, New York, pp279-290,
1997.
|
|
24
|
Martinoli C, Cittadini G Jr, Rollandi GA
and Conzi R: Case report: Imaging of bile duct hamartomas. Clin
Radiol. 45:203–205. 1992.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nagano Y, Matsuo K, Gorai K, Sugimori K,
Kunisaki C, Ike H, Tanaka K, Imada T and Shimada H: Bile duct
hamartomas (von Mayenburg complexes) mimicking liver metastases
from bile duct cancer: MRC findings. World J Gastroenterol.
12:1321–1323. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Martin DR, Kalb B, Sarmiento JM, Heffron
TG, Coban I and Adsay NV: Giant and complicated variants of cystic
bile duct hamartomas of the liver: MRI findings and pathological
correlations. J Magn Reson Imaging. 31:903–911. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu AM, Xian ZH, Zhang SH and Chen XF:
Intrahepatic cholangiocarcinoma arising in multiple bile duct
hamartomas: Report of two cases and review of the literature. Eur J
Gastroenterol Hepatol. 21:580–584. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mortelé B, Mortelé K, Seynaeve P,
Vandevelde D, Kunnen M and Ros PR: Hepatic bile duct hamartomas
(von Meyenburg complexes): MR and MR cholangiography findings. J
Comput Assist Tomogr. 26:438–443. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qing Q, Wenping W, Zhizhang X, et al:
Hemodynamics study of enhanced color flow images with ultrasonic
contrast agent levovist in hepatic focal lesions. Chin J Ultrason.
4:216–218. 2001.(In Chinese).
|
|
30
|
Zanrui S, Dingbiao M, Long Z, et al:
Clinical analysis of the blood supply of middle and advanced
primary liver cancer. Youjiang Med J. 4:471–472. 2008.(In
Chinese).
|
|
31
|
Lixue W: Characteristics and diagnostic
value of spiral CT dual phase enhanced scanning in hepatocellular
carcinoma. Youjiang Med J. 4:378–379. 2005.(In Chinese).
|
|
32
|
Jung EM, Clevert DA, Schreyer AG, Schmitt
S, Rennert J, Kubale R, Feuerbach S and Jung F: Evaluation of
quantitative contrast harmonic imaging to assess malignancy of
liver tumors: A prospective controlled two-center study. World J
Gastroenterol. 13:6356–6364. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jianhua Z, Feng H, Anhua L, et al: The
value of quantitative analysis of blood perfusion in the arterial
phase of contrast-enhanced ultrasound in the differential diagnosis
for focal nodular hyperplasia and hepatocellular carcinoma. Chin J
Ultrasound. 6:19–21. 2009.(In Chinese).
|
|
34
|
Shi QS, Xing LX, Jin LF, Wang H, Lv XH and
Du LF: Imaging findings of bile duct hamartomas: A case report and
literature review. Int J Clin Exp Med. 8:13145–13153.
2015.PubMed/NCBI
|