Introduction
Excessive oxidative stress (OS) is a common
complication in patients with chronic kidney disease and end-stage
renal disease (ESRD) (1,2). The imbalance between oxidation and
antioxidation induced by ESRD is the main cause of OS, which may be
further exacerbated during hemodialysis (HD) treatment (3,4).
Cell components of patients with ESRD undergoing HD, such as
proteins, lipids and nucleic acids, are vulnerable to oxidative
damage after continuous exposure to free radicals, potentially
leading to an increased risk of cardiovascular disease (5). Therefore, either treatment with
antioxidant supplements or modification of the dialyzer (e.g.
coated with vitamin E) that can effectively alleviate the
disordered redox metabolism has become a promising therapeutic
strategy for the treatment of OS in patients with ESRD undergoing
HD (6,7).
Renal dysfunction, chronic inflammation and other
complications are considered to be the main source of free radicals
in patients with ESRD, which results in increased levels of OS
biomarkers, including malondialdehyde (MDA), oxidized low-density
lipoprotein and deoxyguanosine in different tissues and/or plasma
(8). In addition, the interaction
of blood with materials of the dialyzer and hemolysis has been
considered to be the main source of free radicals in patients with
ESRD undergoing hemodialysis (HD) (4,9).
During this process, the antioxidant resources of red blood cells
(RBCs) that are permanently exposed to high-level OS will be
exhausted, resulting in a decrease in membrane lipid fluidity and
oxidative damage of membrane proteins, which further leads to an
abnormal deformability of the RBCs and rheological properties
(10,11).
Vitamin E is a powerful hydrophobic antioxidant that
protects RBCs from lipid peroxidation (12). It has been reported that serum
levels of vitamin E in patients undergoing HD were significantly
decreased, and this decrease was accompanied by a decrease in
antioxidant capacity and an increase in lipid peroxidation
(13,14). According to previous reports,
treatment with vitamin E-coated dialyzer membranes (VEMs) may
restore the blood antioxidant capacity and suppress the
lipoperoxidation process in vitro and in vivo
(15,16). Bargnoux et al (17) reported that VEMs were able to
markedly increase superoxide dismutase (SOD) activity and reduce OS
injury in RBCs of patients undergoing HD. However, whether VEM
treatment is able to ameliorate abnormal deformability and
rheological properties in RBCs of patients with ESRD undergoing HD
via regulating the redox metabolism remained to be clarified.
Therefore, the present study aimed to explore the
protective effects and underlying mechanisms of VEM treatment on
the redox metabolism and deformability of RBCs in patients with
ESRD undergoing HD. The results of the present study are expected
to provide a theoretical basis for the formulation of a therapeutic
schedule for patients with ESRD undergoing HD with erythrocyte
dysfunction and associated cardiovascular disease.
Materials and methods
Subjects
Patients with ESRD undergoing maintenance HD three
times per week for at least 12 months were recruited to the present
study between January and June 2021 from the Department of
Nephrology, Yantai Hospital of Traditional Chinese Medicine
(Yantai, China). The patients were randomly assigned to two groups:
HD with VEMs (Excebrane E15; Terumo Medical Corporation) (VEM
group, n=24, 10 male and 14 female patients, aged 50±14 years) and
HD with polysulfone dialyzer membranes (PMs) [F60; Fresenius SE
& Co.) (PM group, n=24, 13 male and 11 female patients, aged
55±15 years). The inclusion criteria were as follows: i) The
patient was on chronic HD; ii) the treatment was received three
times weekly for ≥12 months; iii) the age of the patient was >18
years; and iv) the patient was able to give their informed consent.
The exclusion criteria were as follows: The patient i) was
pregnant; ii) was already involved in another study, iii) had
active/chronic inflammation or a malignancy; and iv) was receiving
antioxidant and anti-inflammatory therapy. In addition, 24 healthy
volunteers (10 male and 14 female individuals, aged 54±12 years)
were recruited as the control group. The study population
characteristics are summarized in Table I.
 | Table IClinical parameters and causes of
ESRD in patients and control subjects. |
Table I
Clinical parameters and causes of
ESRD in patients and control subjects.
| A, Clinical
parameters |
|---|
| Variable | Control (n=24) | PM (n=24) | VEM (n=24) |
|---|
| Age (year) | 54±12 | 55±15 | 50±14 |
| Sex (M/F) | 11/13 | 13/11 | 10/14 |
| Weight (kg) | 62±8 | 64±7 | 60±10 |
| HD treatment
(months) | - | 26±16 | 33±18 |
| B, Cause of
ESRD |
| Variable | Control (n=24) | PM (n=24) | VEM (n=24) |
| Polycystic kidney
disease | - | 5 | 6 |
| Chronic kidney
failure | - | 6 | 6 |
| Chronic
glomerulonephritis | - | 4 | 3 |
|
Nephrosclerosis | - | 1 | 2 |
| Others | - | 8 | 7 |
None of the patients with ESRD or healthy volunteers
took drugs with a potential oxidizing effect or undertook any
exhaustive exercise; nor did they take antioxidants, such as
vitamin C or E, during the 8-week experimental period. All
experimental procedures were conducted according to the Guidelines
of the Declaration of Helsinki, and approved by the Ethics
Committee of Yantai Hospital of Traditional Chinese Medicine
(approval no. 2020-06). Patients and healthy volunteers were
informed of the procedures in detail, and provided written consent
before the examination.
HD
For all patients with ESRD undergoing HD, the blood
flow rate was set to 250-300 ml/min, the dialysate flow rate was
500 ml/min, and during the HD therapy, there were three sessions
per week (each session lasting for 4 h). The HD treatment was
delivered by a low-flux dialyzer (ARTIS CN; Gambro Lundia AB). The
transmembrane pressure of the dialyzer was 0-500 mmHg and the
system was maintained at 36˚C. No changes were made to the
dialysate composition (HCO3-, 35.0 mmol/l;
Mg2+, 0.50 mmol/l; Na+, 139.8 mmol/l;
Cl-, 106.8 mmol/l; K+, 2.0 mmol/l and
CH3COO-, 4.0 mmol/l) used by patients
undergoing HD during this study period.
Preparation of the blood samples and
detection of the hematological parameters
Fresh blood was collected by venipuncture from both
the patients with ESRD undergoing HD and the healthy volunteers.
After centrifugation at 900 x g at 4˚C for 10 min, the plasma and
buffy coat were removed, and RBCs were isolated and washed three
times in isotonic Hepes buffer. Subsequently, RBCs were resuspended
in a hematocrit (Hct) of 50% in Krebs buffer containing 2 g/l
glucose (pH 7.4) for further experiments. The hematological
parameters of the participants were determined using an electronic
hematology analyzer (ABX Micros 60; Horiba, Ltd.). Biochemical
parameters of the patients were obtained from the patients'
records.
Preparation and detection of
erythrocytes by scanning electron microscopy
The RBCs were fixed with 2% glutaraldehyde for 24 h
at 4˚C. Subsequently, the cells were washed in phosphate buffer for
30 min, and then the material was dehydrated in an ascending series
of ethanol concentrations (50, 70, 80, 95 and 100%) (15 min in each
concentration); finally, the material was allowed to remain in pure
ethanol for 30 min. The RBCs were subsequently dried for 12 h at
room temperature. Then, the samples were coated with gold using a
sputter coater (cat. no. Q150RS; Quorum). Under high vacuum and
accelerating voltage [acceleration voltage (EHT), 10 kV] the
ultrastructure of the RBCs from patients with ESRD undergoing HD
and the healthy volunteers were detected using a scanning
microscope (ZEISS EVO LS15 SEM; Zeiss GmbH) with an SE1 detector.
Individual forms of the erythrocytes were ascribed morphological
indices according to the Bessis scale (18).
Detection of erythrocyte
deformability
By using an ektacytometer (LBY-BX; Beijing Precil
Instrument Co., Ltd), the deformability of RBCs from patients with
ESRD undergoing HD and healthy volunteers was analyzed by laser
diffraction analyses (19). The
elongation index (EI) was calculated under shear stresses of 3 and
30 Pa, as based on the geometry of the elliptical diffraction
pattern (where ‘L’ and ‘W’ are the length and width of the
diffraction pattern): EI=(L-W)/(L+W).
Detection of OS
The intracellular production of reactive oxygen
species (ROS) in RBCs from patients with ESRD undergoing HD and
healthy volunteers was assessed using 2',7'-dichlorofluorescein
diacetate (H2DCF-DA; MilliporeSigma). The RBCs were collected,
washed with PBS solution, and then H2DCF-DA (10 µM) was added to
the cells and incubated at 37˚C for 30 min. After incubation, cells
were washed and analyzed using a flow cytometer (FACSCanto II; BD
Biosciences, Inc.) and analyzed using FlowJo software 8.7.1 (FlowJo
LLC). The levels of MDA (cat. no. A003-1-2) and methemoglobin
(MetHb; cat. no. A102-1-1) were assessed by colorimetry using
commercial kits (all from Nanjing Jiancheng Bioengineering
Institute). The manufacturer's instructions for the corresponding
assay kit were precisely followed (20).
Detection of antioxidant capacity
The ferric-reducing ability of plasma (FRAP) values
were assessed according to the method of Benzie and Strain
(21). The activities of catalase
(CAT; cat. no. A007-1-1) and SOD (cat. no. A001-3-2) were assessed
by colorimetry using commercial kits (all from Nanjing Jiancheng
Bioengineering Institute). The manufacturer's instructions for the
corresponding assay kit were preceisely followed.
Detection of vitamin E
The serum concentration of vitamin E was detected by
colorimetry using a commercial kit (cat. no. BC1420; Beijing
Solarbio Science & Technology Co., Ltd). The manufacturer's
instructions for the corresponding assay kit were precisely
followed.
Western blot analysis
Western blot analyses were performed as described
previously (22). Total protein
from the RBCs was obtained using a protein extraction kit (cat. no.
BC3711; Beijing Solarbio Science & Technology Co., Ltd)
according to the manufacturer's instructions. The protein content
of the membranes was quantified using a BCA protein assay kit (cat.
no. PC0020; Beijing Solarbio Science & Technology Co., Ltd).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was
performed by heating the samples for 8 min at 100˚C and loading 10
µg membrane proteins on to a 5-15% linear acrylamide gradient gel
(10 µg protein/lane).
After SDS-PAGE, proteins were electrotransferred to
PVDF membranes and blocked in 5% BSA (cat. no. SW3015; Beijing
Solarbio Science & Technology Co., Ltd) dissolved in
TBS-Tween-20 (20%) for 2 h at room temperature. After incubating
the PVDF membranes with primary antibody overnight at 4˚C, the
membranes were incubated with secondary antibody for 2 h at room
temperature. The primary antibodies used were anti-Band 3 (1:3,000
dilution; cat. no. ab108414), anti-phosphotyrosine (1:3,000; cat.
no. ab190824), and anti-β-actin (1:3,000; cat. no. ab8227; all
antibodies were purchased from Abcam). The horseradish
peroxidase-conjugated secondary antibodies were purchased from
Santa Cruz Biotechnology, Inc. (1:5,000; cat. no. sc-2357). The
protein bands were visualized using an ECL kit (cat. no. 32209;
Thermo Fisher Scientific, Inc.), and semi-quantitative densitometry
was performed on the identified bands using Image Quant 5.2
software (Molecular Dynamics, Inc.). The levels of the proteins of
interest were normalized against those of β-actin.
Immunofluorescence and image
analysis
RBCs from patients with ESRD undergoing HD and
healthy volunteers were fixed with 4% paraformaldehyde and 0.05%
glutaraldehyde at room temperature for 30 min, before being
permeabilized in the same solution containing 0.05% Triton X-100 at
4˚C for 10 min. RBCs were treated with primary antibodies against
Band 3 (1:1,000; cat. no. ab108414; Abcam) for 1 h at room
temperature after being blocked with 3% BSA and 0.1% Tween-20 at
4˚C for 1 h. After being washed three times in PBS, RBCs were
incubated at room temperature for 1 h with goat anti-rabbit IgG
H&L secondary antibody (1:200; cat. no. ab150077; Abcam). After
being washed three times in PBS, fluorescence images were captured
using an Olympus IX71 fluorescence microscope (Olympus Corporation)
(23).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 22.0; IBM Corp.). Data are presented as the mean
± standard deviation of three independent experiments. Differences
between and within groups were determined using mixed-design ANOVA
followed by Bonferroni's post hoc tests. Spearman's correlation
analysis was used for correlation analysis of two independent
samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographic, clinical and
hematological features in healthy donors and patients with ESRD
undergoing HD
As shown in Tables
I and II, the patients with
ESRD undergoing HD were characterized by a significantly reduced
RBC count and markedly lower hemoglobin (Hb), Hct and mean
corpuscular volume (MCV) values, whereas the parameters RBC
distribution width (RDW), blood urea nitrogen (BUN), serum
creatinine (SCr), uric acid (UA) and C-reactive protein (CRP) were
markedly increased compared with the healthy volunteers 8 weeks
before the examination. After 8 weeks, the levels of the RBC count,
Hb, Hct and MCV were notably improved, whereas the values of RDW,
BUN, SCr, UA and CRP were markedly decreased, in the VEM group
compared with the PM group.
 | Table IIHematological parameters of the
patients and control subjects. |
Table II
Hematological parameters of the
patients and control subjects.
| | Control (n=24) | PM (n=24) | VEM (n=24) |
|---|
| Variable | Before | After 8 weeks | Before | After 8 weeks | Before | After 8 weeks |
|---|
| RBC, mil/µl | 5.21±0.14 | 5.32±0.16 |
3.88±0.18a |
3.45±0.16a |
3.79±0.34a |
4.25±0.2b,c |
| Hb, g/dl | 14.11±1.43 | 14.42±1.55 |
11.51±1.64a |
10.32±1.26a |
11.34±1.71a |
12.70±1.52b,c |
| RDW, % | 12.51±3.71 | 12.92±4.13 |
16.52±5.41a |
17.34±6.43a |
15.81±4.63a |
14.55±3.74b,c |
| Hct, % | 43.54±4.43 | 44.24±4.65 |
34.58±5.45a |
32.72±6.51a |
33.82±5.21a |
37.52±4.93b,c |
| MCHC, g/dl | 31.51±1.94 | 30.81±1.74 | 34.55±1.71 | 36.21±1.93 | 35.61±1.85 | 32.61±1.53 |
| MCH, pg | 32.83±1.31 | 31.83±1.51 | 30.11±1.53 | 28.91±1.74 | 30.73±2.65 | 31.51±2.34 |
| MCV, fl | 98.51±3.43 | 99.23±3.61 |
89.41±5.13a |
87.15±5.91a |
88.71±4.73a |
94.71±4.43b,c |
| HDL, mg/dl | 38.63 ± 9.74 | 37.81±9.55 | 39.41± 9.56 | 40.15± 9.66 | 38.91± 8.26 | 39.91± 10.51 |
| BUN, mg/dl | 14.82±4.61 | 14.53±4.41 |
52.11±8.12a |
58.71±8.83a |
52.51±9.70a |
42.51±9.12b,c |
| SCr, mg/dl | 0.68±0.14 | 0.69±0.15 |
7.69±2.47a |
8.89±3.42a |
7.78±1.35a |
6.78±1.44b,c |
| UA, mg/dl | 3.68±1.15 | 3.57±1.05 |
5.57±3.35a |
5.89±3.87a |
5.44±2.92a |
4.44±2.61b,c |
| CRP, mg/dl | 2.98±0.97 | 3.02±0.86 |
14.55±9.45a |
15.75±9.88a |
14.78±7.73a |
9.78±6.83b,c |
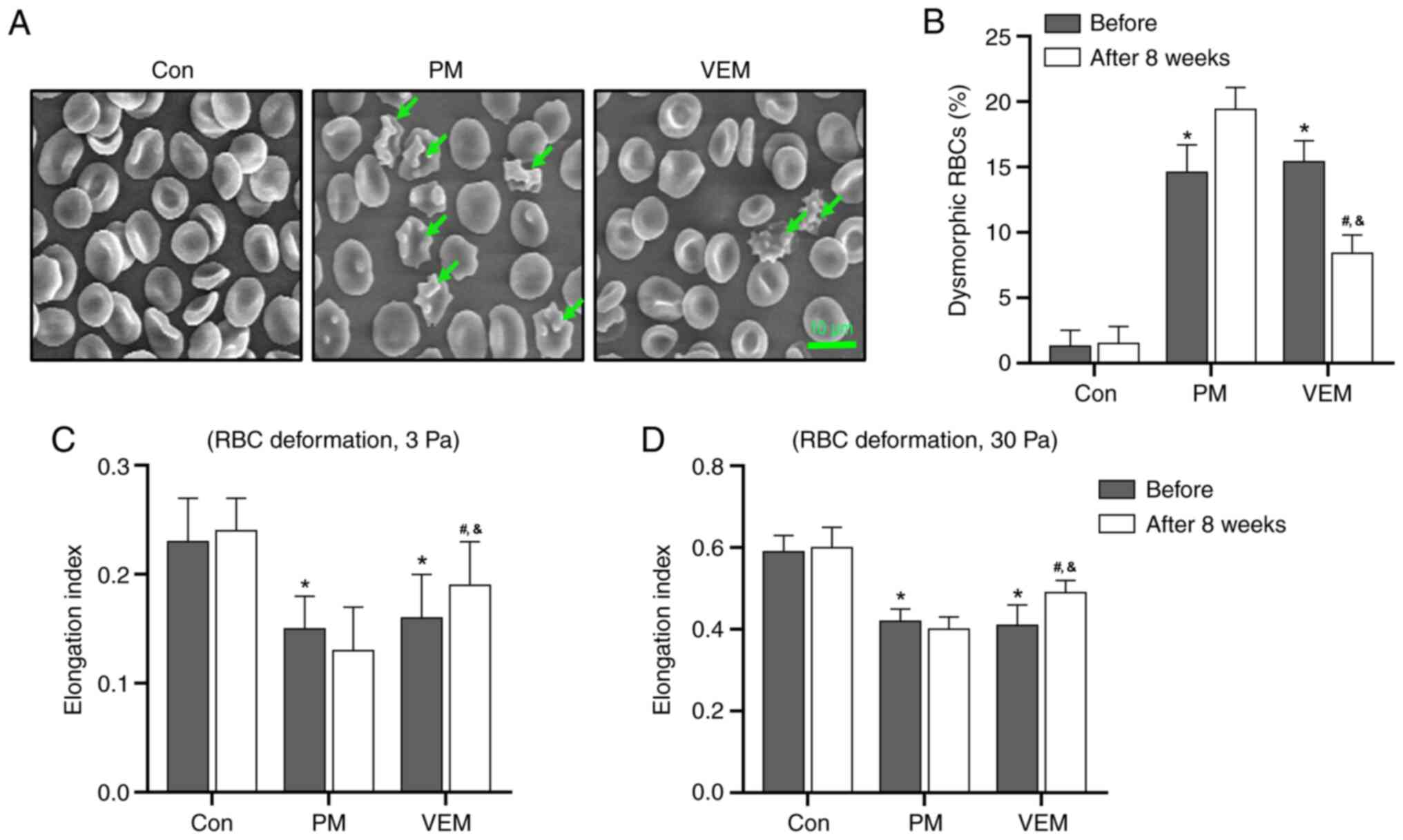
VEM alleviates the abnormal morphology
and deformability in RBCs of patients with ESRD undergoing HD
The flattened biconcave disc morphology of RBCs was
found to be damaged in patients with ESRD undergoing HD, and this
was accompanied by the presence of dysmorphic RBCs (which featured,
e.g., surface blebbing typical of acanthocytes). However, the
counts of dysmorphic RBCs were markedly decreased in the VEM group
compared with the PM group after 8 weeks (Fig. 1A and B).
The deformability of the RBCs in patients with ESRD
undergoing HD and healthy volunteers was evaluated by measuring the
value of EI under shear stresses of 3 and 30 Pa. The EI was
markedly reduced in RBCs of patients with ESRD undergoing HD
compared with the healthy volunteers under both 3 and 30 Pa shear
stress. In addition, a notable amelioration of the RBCs'
deformability was found in the VEM group compared with the PM group
after 8 weeks (Fig. 1C and
D). These results demonstrated
that HD with VEMs alleviated the abnormalities of RBC morphology
and deformability in patients with ESRD undergoing HD.
VEM restores the imbalance of redox
metabolism in RBCs of patients with ESRD undergoing HD
To investigate the reason for the abnormal
morphology and function of the RBCs of patients with ESRD
undergoing HD, the ROS and antioxidant capacity indexes of the RBCs
were detected. The levels of ROS, MetHb and MDA in RBCs were
markedly increased in patients with ESRD undergoing HD compared
with the healthy volunteers. However, a notable decline in the ROS,
MetHb and MDA values in RBCs were found in the VEM group compared
with the PM group after 8 weeks (Fig.
2A-C).
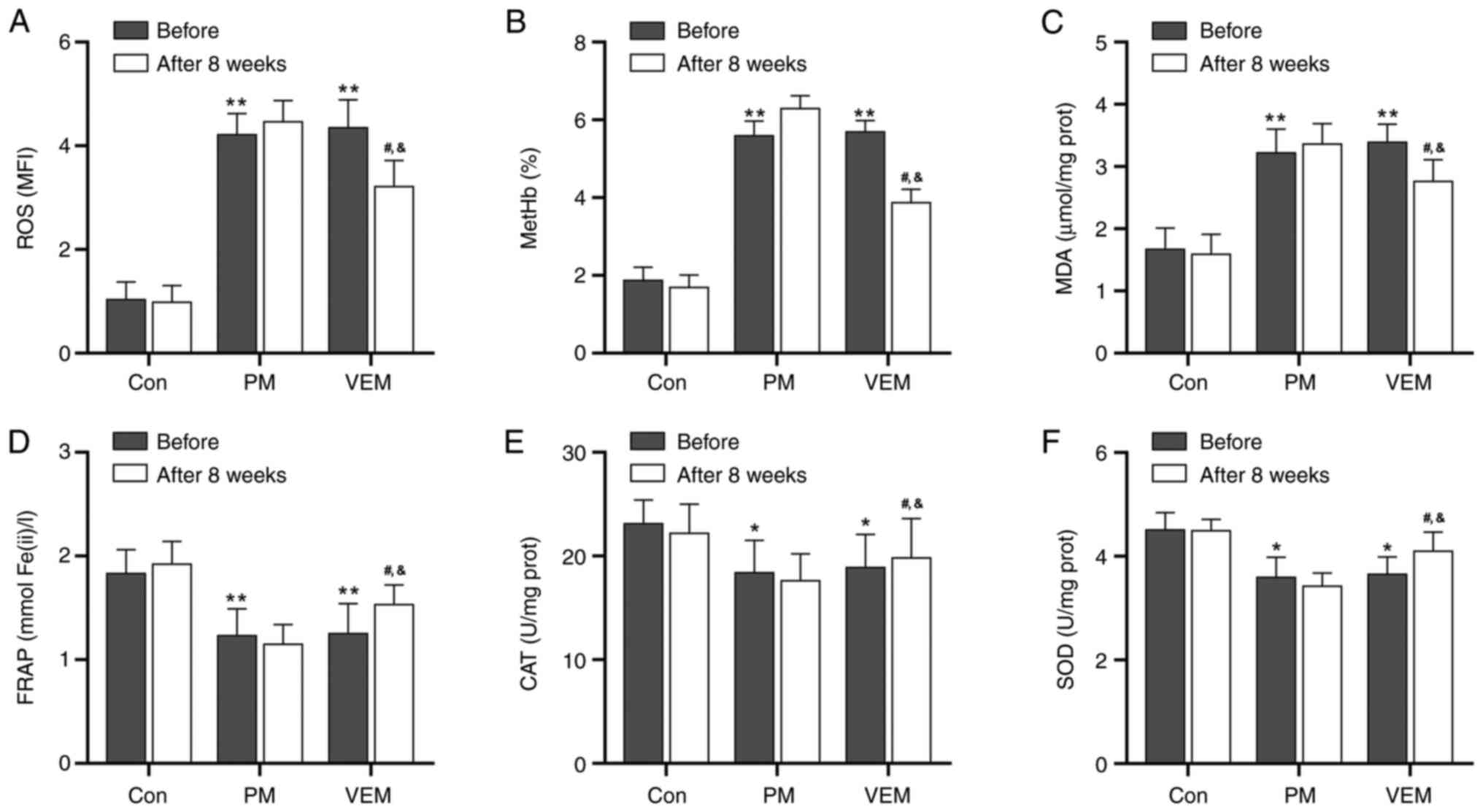 | Figure 2RBC redox metabolism parameters in
patients with ESRD undergoing HD and healthy volunteers. The
oxidative stress parameters of (A) ROS, (B) MetHb and (C) MDA in
RBCs of healthy donors and patients with ESRD undergoing HD. The
antioxidant parameters of (D) FRAP, (E) CAT and (F) SOD in RBCs of
healthy donors and patients with ESRD undergoing HD. All data are
expressed as the mean ± SD. *P<0.05,
**P<0.01, PM or VEM group vs. control group before
examination; #P<0.05, VEM group vs. control group 8
weeks after examination; &P<0.05, VEM group vs.
PM group 8 weeks after examination. PM, polysulfone dialyzer
membrane; VEM, vitamin E-coated dialyzer membrane; RBC, red blood
cell; SOD, superoxide dismutase; FRAP, ferric-reducing ability of
plasma; CAT, catalase; ROS, reactive oxygen species; MDA,
malondialdehyde; MetHb, methemoglobin; ESRD, end-stage renal
disease. |
In addition, the activities of FRAP, CAT and SOD in
RBCs were markedly decreased in patients with ESRD undergoing HD
compared with the healthy volunteers (Fig. 2D-F). Moreover, after 8 weeks of VEM
treatment, the activities of FRAP, CAT and SOD in the RBCs were
markedly improved compared with the PM treatment group (Fig. 2D-F). These results indicated that
HD with VEMs alleviated the imbalance of redox metabolites of RBCs
in patients with ESRD undergoing HD.
VEM participates in the regulation of
redox metabolism in RBCs of patients with ESRD undergoing HD by
transferring vitamin E
The effect of VEM treatment on the serum
concentration of vitamin E and its association with the change of
redox metabolism was further evaluated in patients with ESRD
undergoing HD. Patients with ESRD undergoing HD were characterized
by a markedly lower concentration of serum vitamin E compared with
the healthy volunteers (Fig. 3A).
In addition, the level of serum vitamin E was markedly improved in
the VEM group compared with the PM group after 8 weeks.
Additionally, Pearson's correlation analysis suggested a notable
positive correlation between the concentration of serum vitamin E
and the FRAP level in patients with ESRD undergoing HD
(r=0.762, P<0.001) (Fig.
3B). Conversely, a notable negative correlation between the
concentration of serum vitamin E and the ROS level was found in
patients with ESRD undergoing HD (r=-0.795, P<0.001)
(Fig. 3C). These data revealed
that HD with VEMs restored the imbalance of redox metabolism of
RBCs in patients with ESRD undergoing HD by transferring vitamin
E.
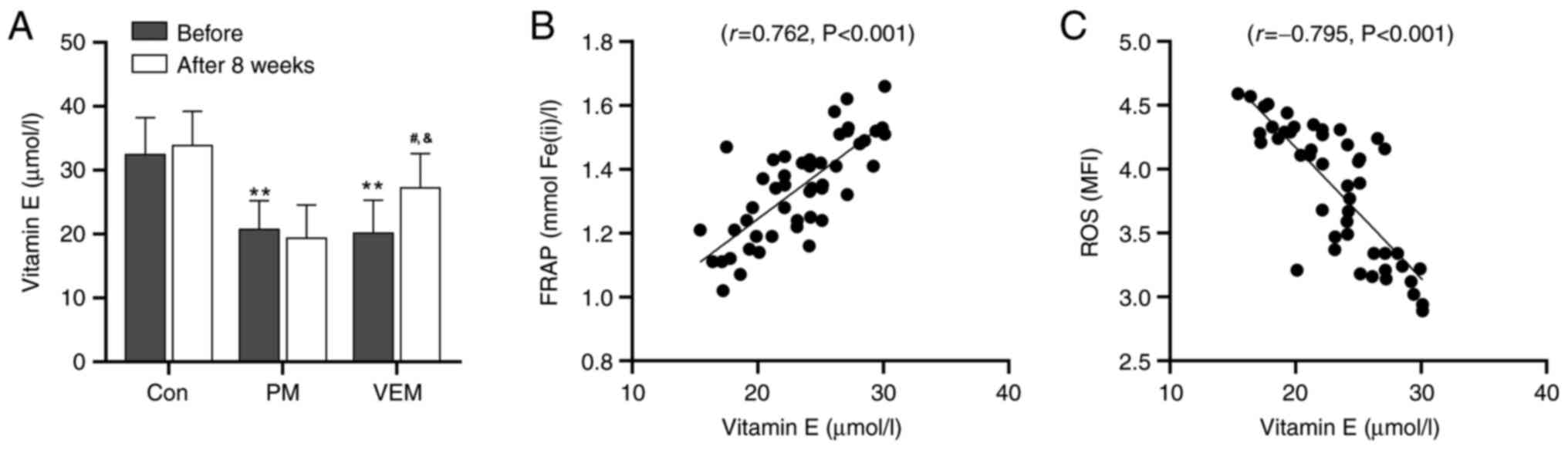 | Figure 3Red blood cell serum vitamin E
concentration in patients with ESRD undergoing HD and healthy
volunteers. (A) Concentration of serum vitamin E in healthy donors
and patients with ESRD undergoing HD. All data are expressed as the
mean ± SD. **P<0.01, PM or VEM group vs. control
group before examination; #P<0.05, VEM group vs.
control group 8 weeks after examination; &P<0.05,
VEM group vs. PM group 8 weeks after examination. (B) Pearson
correlation analysis between the concentration of serum vitamin E
and the FRAP level in patients with ESRD undergoing HD
(r=0.762, P<0.001). (C) Pearson correlation analysis
between the concentration of serum vitamin E and the ROS level in
patients with ESRD undergoing HD (r=0.795, P<0.001).
FRAP, ferric-reducing ability of plasma; ROS, reactive oxygen
species; ESRD, end-stage renal disease; PM, polysulfone dialyzer
membrane; VEM, vitamin E-coated dialyzer membrane. |
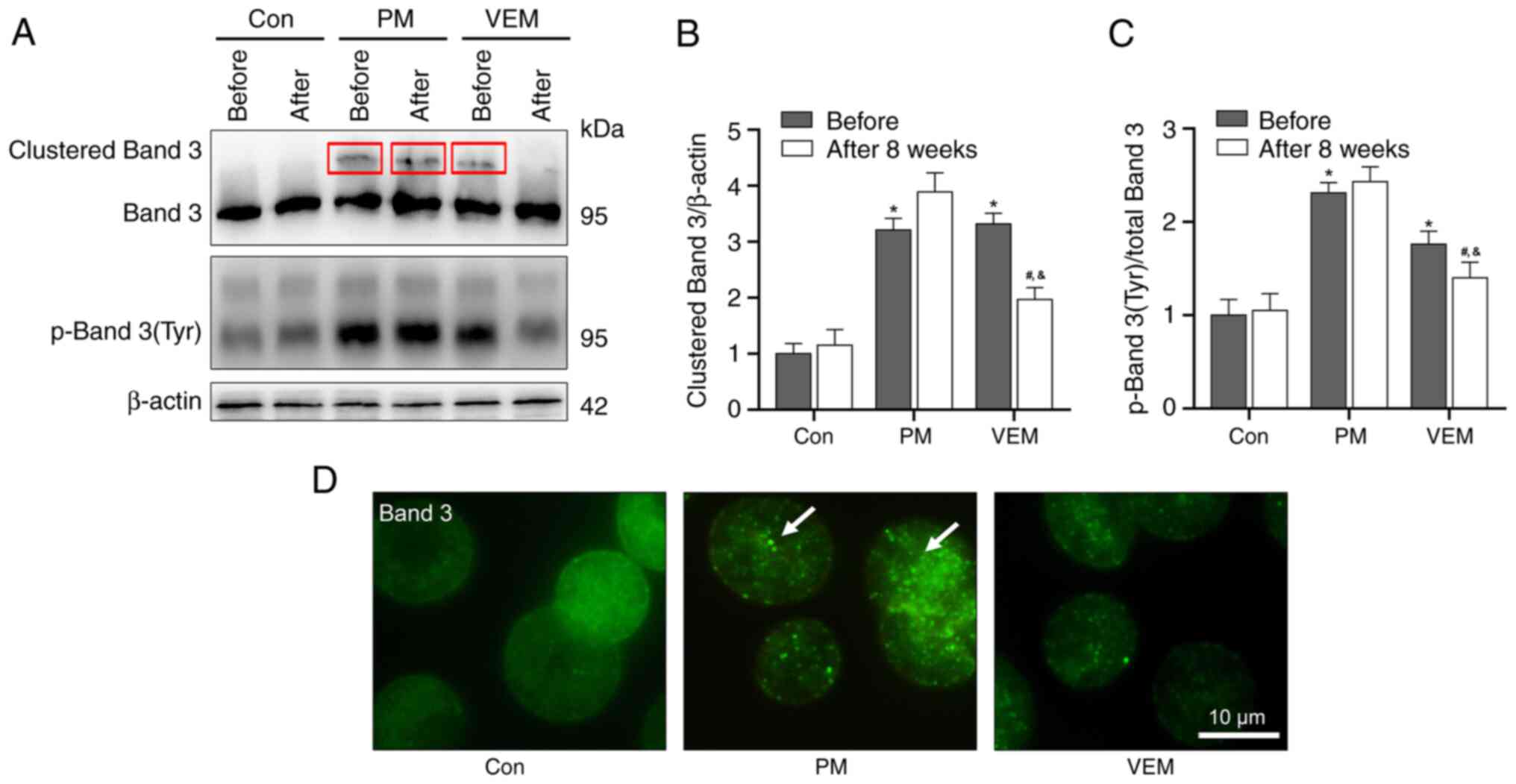
VEM attenuates the oxidative
phosphorylation of Band 3 in RBCs of patients with ESRD undergoing
HD
To further clarify the potential molecular mechanism
of VEM treatment in alleviating the abnormal morphology and
deformability of RBCs in patients with ESRD undergoing HD, the
degree of oxidative phosphorylation damage of the membrane skeleton
protein Band 3 in RBCs was detected. Densitometric analyses
revealed markedly higher levels of clustered and
tyrosine-phosphorylated (p)-Band 3 in patients with ESRD undergoing
HD compared with the healthy volunteers. In addition, after 8 weeks
of VEM treatment, the levels of clustered and p-Band 3 were
markedly decreased compared with the PM group (Fig. 4A-C). Subsequent microscopic
analysis of Band 3 verified the presence and differences of Band 3
aggregates in RBCs of patients with ESRD undergoing HD (Fig. 4D).
Discussion
RBCs are vulnerable to oxidative damage and abnormal
deformability under OS induced by a variety of physiological and
pathological conditions; this is considered a potential triggering
factor of various cardiovascular and cerebrovascular diseases. It
is now accepted that the RBCs of patients with ESRD undergoing HD
are susceptible to OS and chronic inflammation due to the primary
diseases and HD process. Therefore, effective therapy to restore
the imbalances of redox metabolism in RBCs of patients with ESRD
undergoing HD is desirable for improving the curability of the
condition and the survival rate. The present study has shown that
HD with VEM may alleviate the abnormalities of RBC morphology and
deformability in patients with ESRD undergoing HD by restoring the
equilibrium of redox metabolism.
Accumulating evidence has suggested that patients
with ESRD are subject to enhanced OS, which triggers oxidative
damage to nucleic acids, lipids and proteins (24,25).
Furthermore, patients with ESRD undergoing HD usually present a
higher level of OS due to the accumulation of oxidative products
and the loss of antioxidants during HD procedures (3,26).
Consistent with previous studies, the present study also found
markedly higher OS levels (ROS, MetHb and MDA) and lower
antioxidant capacity (FRAP, CAT and SOD) in patients with ESRD
undergoing HD compared with healthy volunteers. It was evidenced
that patients undergoing HD suffer from serum vitamin E deficiency,
as vitamin E is the most important lipophilic antioxidant in cell
membranes, and this is considered to be the potential reason for
the decrease of antioxidant capacity in patients undergoing HD
(13). Based on this, a
therapeutic schedule of vitamin E supplementation may be beneficial
to these patients. Maccarrone et al (12) showed that vitamin E intake could
alleviate the membrane lipid peroxidation of blood cells in
patients undergoing HD. In addition, in vivo and in
vitro studies have reported that VEM administration can
alleviate vitamin E deficiency in patients undergoing HD, improve
the antioxidant capacity and reduce the extent of OS injury
(16,27-29).
Similarly, the present study detected markedly increased levels of
antioxidant capacity and decreased OS in patients with ESRD
undergoing HD under VEM treatment compared with the PM treatment
group. In addition, VEM treatment led to a marked improvement in
the values of serum vitamin E in patients with ESRD undergoing HD,
and this was notably correlated with the antioxidant capacity of
RBCs. These results indicated that VEM treatment alleviated the
imbalance of redox metabolism of RBCs by transferring vitamin E in
patients with ESRD undergoing HD.
Severe OS injury may lead to lipid peroxidation and
cross-linking of membrane skeleton proteins, resulting in an
abnormal deformability and increased incidence rate of
cardiovascular disease in patients with ESRD. Previously, it has
been shown that patients with ESRD undergoing peritoneal HD
presented with abnormal morphology and hemorheology of RBCs
(30-32).
The present study also found the occurrence of abnormal morphology
and deformability of RBCs in patients with ESRD undergoing dialysis
compared with healthy controls. Furthermore, VEM treatment
alleviated the dysfunction of RBCs in terms of morphology and
deformability in patients with ESRD undergoing HD compared with the
PM treatment group. Therefore, the use of VEMs in HD may provide a
means of reducing the risk of cardiovascular disease in patients
with ESRD by repairing the abnormal rheological properties of
RBCs.
Previous studies have confirmed that Band 3 has a
vital role in maintaining the structural stability and mechanical
properties of RBCs by linking phospholipid bilayer and
spectral-based skeletal networks (23,33).
Band 3 cluster caused by OS is usually accompanied by a decrease in
the deformability of RBCs (19,34).
Indeed, the present study showed notably increased levels of
oxidative phosphorylation and clustering of Band 3 in patients with
ESRD undergoing HD. In addition, VEM treatment could effectively
reduce the oxidative phosphorylation and clustering of Band 3 in
patients with ESRD undergoing HD compared with PM treatment group.
This may potentially be a molecular mechanism for VEM treatment to
alleviate the abnormal morphological and rheological properties of
RBCs in patients with ESRD undergoing HD.
Although a number of studies have shown that
adequate vitamin E supplementation can alleviate the oxidative
damage of tissues and organs in a variety of physiological and
pathological environments by improving antioxidant capacity
(35,36), there is some evidence to show that
an excessive vitamin E intake is also associated with side effects
(37,38). A dose-response analysis showed that
high-dosage vitamin E supplements may increase all-cause mortality
risk (37). A meta-analysis study
showed that high-dose vitamin E supplements may interact with
prescription drugs, such as aspirin, warfarin, tamoxifen and
cyclosporine A, and this was suggested as a possible underlying
mechanism of the side effects of high-dose vitamin E supplements
(38). In addition, it has been
reported that an excessive vitamin E intake, when combined with
salmon oil in the diet, lowers the activities of antioxidant
enzymes in erythrocytes without affecting in vivo hemolysis
(39). The present study, however,
did not find that VEM treatment caused significant side effects in
patients with ESRD.
The present study was associated with a couple of
important limitations. First, there was a lack of information on
the time- and dose-dependence of VEM treatment. Another limitation
was that the numbers of patients and control individuals were
relatively small.
In conclusion, the present study has presented
evidence that patients with ESRD undergoing HD suffered from severe
redox metabolic disorder, which led to the abnormal morphology and
deformability of RBCs, thereby becoming a potential pathogenic
factor for ESRD-associated cardiovascular disease. In patients with
ESRD undergoing HD, VEM treatment effectively improved the
antioxidant capacity, repaired the oxidative phosphorylation damage
and resolved the problem of clustering of Band 3 in RBCs by
delivering vitamin E. This process was demonstrated to alleviate
the abnormal morphological and mechanical properties of RBCs, and
it is anticipated that this will lead to improvements in the
nursing care and cure rates of patients with ESRD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL conceived and designed the experiments. YZ and WG
performed the experiments. YZ and XL analyzed the data. XL wrote
the paper. All authors read and approved the final version of the
manuscript. YZ and XL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This study protocol was reviewed and approved by the
Ethics Committee of Yantai Hospital of Traditional Chinese Medicine
(approval no. 2020-06). Patients and healthy volunteers were
informed in detail and written consent was obtained before the
examination.
Patient consent for publication
Written informed consent was obtained from the
patients prior to publication at the time of admission.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Daenen K, Andries A, Mekahli D, Van
Schepdael A, Jouret F and Bammens B: Oxidative stress in chronic
kidney disease. Pediatr Nephrol. 34:975–991. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ruiz S, Pergola PE, Zager RA and Vaziri
ND: Targeting the transcription factor Nrf2 to ameliorate oxidative
stress and inflammation in chronic kidney disease. Kidney Int.
83:1029–1041. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liakopoulos V, Roumeliotis S, Gorny X,
Dounousi E and Mertens PR: Oxidative stress in hemodialysis
patients: A review of the literature. Oxid Med Cell Longev.
2017(3081856)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zouridakis A, Simos YV, Verginadis II,
Charalabopoulos K, Ragos V, Dounousi E, Boudouris G, Karkabounas S,
Evangelou A and Peschos D: Correlation of bioelectrical impedance
analysis phase angle with changes in oxidative stress on end-stage
renal disease patients, before, during, and after dialysis. Ren
Fail. 38:738–743. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ahmadmehrabi S and Tang WHW:
Hemodialysis-induced cardiovascular disease. Semin Dial.
31:258–267. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Roumeliotis S, Roumeliotis A, Dounousi E,
Eleftheriadis T and Liakopoulos V: Dietary antioxidant supplements
and uric acid in chronic kidney disease: A review. Nutrients.
11(1911)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang CC, Hsu SP, Wu MS, Hsu SM and Chien
CT: Effects of vitamin C infusion and vitamin E-coated membrane on
hemodialysis-induced oxidative stress. Kidney Int. 69:706–714.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kitabayashi C, Naruko T, Sugioka K, Yunoki
K, Nakagawa M, Inaba M, Ohsawa M, Konishi Y, Imanishi M, Inoue T,
et al: Positive association between plasma levels of oxidized
low-density lipoprotein and myeloperoxidase after hemodialysis in
patients with diabetic end-stage renal disease. Hemodial Int.
17:557–567. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Eiselt J, Racek J and Opatrný K Jr: Free
radicals and extracorporeal renal replacement therapy. Vnitr Lek.
45:319–324. 1999.PubMed/NCBI(In Czech).
|
|
10
|
Georgatzakou HT, Antonelou MH, Papassideri
IS and Kriebardis AG: Red blood cell abnormalities and the
pathogenesis of anemia in end-stage renal disease. Proteomics Clin
Appl. 10:778–790. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brimble KS, McFarlane A, Winegard N,
Crowther M and Churchill DN: Effect of chronic kidney disease on
red blood cell rheology. Clin Hemorheol Microcirc. 34:411–420.
2006.PubMed/NCBI
|
|
12
|
Maccarrone M, Taccone-Gallucci M, Meloni
C, Cococcetta N, Di Villahermosa SM, Casciani CU and Finazzi-Agrò
A: Activation of 5-lipoxygenase and related cell membrane
lipoperoxidation in hemodialysis patients. J Am Soc Nephrol.
10:1991–1996. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bayes B, Pastor MC, Bonal J, Junca J and
Romero R: Homocysteine and lipid peroxidation in haemodialysis:
Role of folinic acid and vitamin E. Nephrol Dial Transplant.
16:2172–2175. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Galli F, Varga Z, Balla J, Ferraro B,
Canestrari F, Floridi A, Kakuk G and Buoncristiani U: Vitamin E,
lipid profile, and peroxidation in hemodialysis patients. Kidney
Int Suppl. 78:S148–S154. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yamadera S, Nakamura Y, Inagaki M, Ohsawa
I, Gotoh H, Goto Y, Sato N, Oguchi T, Gomi Y, Tsuji M, et al:
Vitamin E-coated dialyzer inhibits oxidative stress. Blood Purif.
44:288–293. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rodríguez-Ribera L, Corredor Z, Silva I,
Díaz JM, Ballarín J, Marcos R, Pastor S and Coll E: Vitamin
E-coated dialysis membranes reduce the levels of oxidative genetic
damage in hemodialysis patients. Mutat Res Genet Toxicol Environ
Mutagen. 815:16–21. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bargnoux AS, Cristol JP, Jaussent I,
Chalabi L, Bories P, Dion JJ, Henri P, Delage M, Dupuy AM, Badiou
S, et al: Vitamin E-coated polysulfone membrane improved red blood
cell antioxidant status in hemodialysis patients. J Nephrol.
26:556–563. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bessis M: Erythrocyte form and
deformability for normal blood and some hereditary hemolytic
anemias (author's transl). Nouv Rev Fr Hematol Blood Cells.
18:75–94. 1977.PubMed/NCBI(In French).
|
|
19
|
Xiong Y, Li Y, Xiong Y, Zhao Y, Tang F and
Wang X: Cluster of erythrocyte Band 3: A potential molecular target
of exhaustive exercise-induced dysfunction of erythrocyte
deformability. Can J Physiol Pharmacol. 91:1127–1134.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y and Xiong Y, Zhang A, Zhao N, Zhang
J, Zhao D, Yu Z, Xu N, Yin Y, Luan X and Xiong Y: Oligosaccharide
attenuates aging-related liver dysfunction by activating Nrf2
antioxidant signaling. Food Sci Nutr. 8:3872–3881. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Benzie IF and Strain JJ: The ferric
reducing ability of plasma (FRAP) as a measure of ‘antioxidant
power’: The FRAP assay. Anal Biochem. 239:70–76. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xiong Y, Xiong Y, Zhang H, Zhao Y, Han K,
Zhang J, Zhao D, Yu Z, Geng Z, Wang L, et al: hPMSCs-derived
exosomal miRNA-21 protects against aging-related oxidative damage
of CD4(+) T cells by targeting the PTEN/PI3K-Nrf2 axis. Front
Immunol. 12(780897)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Y, Zhao N and Xiong Y, Zhang J, Zhao
D, Yin Y, Song L, Yin Y, Wang J, Luan X and Xiong Y: Downregulated
recycling process but not de novo synthesis of glutathione limits
antioxidant capacity of erythrocytes in hypoxia. Oxid Med Cell
Longev. 2020(7834252)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Locatelli F, Canaud B, Eckardt KU,
Stenvinkel P, Wanner C and Zoccali C: Oxidative stress in end-stage
renal disease: an emerging threat to patient outcome. Nephrol Dial
Transplant. 18:1272–1280. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Duni A, Liakopoulos V, Roumeliotis S,
Peschos D and Dounousi E: Oxidative stress in the pathogenesis and
evolution of chronic kidney disease: Untangling Ariadne's thread.
Int J Mol Sci. 20(3711)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liakopoulos V, Roumeliotis S, Zarogiannis
S, Eleftheriadis T and Mertens PR: Oxidative stress in
hemodialysis: Causative mechanisms, clinical implications, and
possible therapeutic interventions. Semin Dial. 32:58–71.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Galli F, Rovidati S, Chiarantini L, Campus
G, Canestrari F and Buoncristiani U: Bioreactivity and
biocompatibility of a vitamin E-modified multi-layer hemodialysis
filter. Kidney Int. 54:580–589. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
D'Arrigo G, Baggetta R, Tripepi G, Galli F
and Bolignano D: Effects of vitamin E-coated versus conventional
membranes in chronic hemodialysis patients: A systematic review and
meta-analysis. Blood Purif. 43:101–122. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mydlík M, Derzsiová K, Rácz O, Sipulová A,
Lovásová E, Molcányiová A and Petrovicová J: Vitamin E-coated
dialyzer and antioxidant defense parameters: Three-month study.
Semin Nephrol. 24:525–531. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ertan NZ, Bozfakioglu S, Ugurel E, Sinan M
and Yalcin O: Alterations of erythrocyte rheology and cellular
susceptibility in end stage renal disease: Effects of peritoneal
dialysis. PLoS One. 12(e0171371)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sotirakopoulos N, Tsitsios T, Stambolidou
M, Athanasiou G, Peiou M, Kokkinou V and Mavromatidis K: The red
blood cell deformability in patients suffering from end stage renal
failure on hemodialysis or continuous ambulatory peritoneal
dialysis. Ren Fail. 26:179–183. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Georgatzakou HT, Tzounakas VL, Kriebardis
AG, Velentzas AD, Kokkalis AC, Antonelou MH and Papassideri IS:
Short-term effects of hemodiafiltration versus conventional
hemodialysis on erythrocyte performance. Can J Physiol Pharmacol.
96:249–257. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bruce LJ, Beckmann R, Ribeiro ML, Peters
LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ and Tanner MJ: A
Band 3-based macrocomplex of integral and peripheral proteins in
the RBC membrane. Blood. 101:4180–4188. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Remigante A, Morabito R and Marino A: Band
3 protein function and oxidative stress in erythrocytes. J Cell
Physiol. 236:6225–6234. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Higgins MR, Izadi A and Kaviani M:
Antioxidants and exercise performance: With a focus on vitamin E
and C supplementation. Int J Environ Res Public Health.
17(8452)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jiang Q: Natural forms of vitamin E:
Metabolism, antioxidant, and anti-inflammatory activities and their
role in disease prevention and therapy. Free Radic Biol Med.
72:76–90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Miller ER III, Pastor-Barriuso R, Dalal D,
Riemersma RA, Appel LJ and Guallar E: Meta-analysis: High-dosage
vitamin E supplementation may increase all-cause mortality. Ann
Intern Med. 142:37–46. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Podszun M and Frank J: Vitamin E-drug
interactions: Molecular basis and clinical relevance. Nutr Res Rev.
27:215–231. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Eder K, Flader D, Hirche F and Brandsch C:
Excess dietary vitamin E lowers the activities of antioxidative
enzymes in erythrocytes of rats fed salmon oil. J Nutr.
132:3400–3404. 2002.PubMed/NCBI View Article : Google Scholar
|