Introduction
Breast cancer is one of the most frequent malignant
tumors that occurs in females worldwide, and its incidence is
increasing annually (1).
Furthermore, breast cancer is a disease with multiple subtypes
(2) and 12-17% of cases are
characterized as triple-negative breast cancer (TNBC) with the
following features: Lack of expression of estrogen receptor,
progesterone receptor and human epidermal growth factor receptor
2(3). The highly invasive nature
of TNBC is manifested in its poor clinical prognosis and rapid
relapse (3). Although the
diagnosis and treatment of breast cancer have improved, TNBC cannot
be cured using endocrine therapy or current conventional
chemotherapies (4). Therefore, it
is essential to understand the biological properties of TNBC and
discover innovative, effective and reliable drugs for TNBC
treatment to further improve patient prognosis. Normally
differentiated cells mainly rely on oxidative phosphorylation of
mitochondria to power cells, whereas most tumor cells rely on
aerobic glycolysis through the phenomenon known as the ‘Warburg
effect’ (5). In this process, most
pyruvate is reduced to lactic acid by lactate dehydrogenase rather
than entering mitochondria in cancer cells (5). TNBC cells also exhibit abnormal
glucose metabolism with a higher rate of glucose absorption and
glycolysis (6).
Hexokinase 2 (HK2) serves an important role in the
first step of glycolysis as a key enzyme (7). As an inhibitor of HK2, 3-bromopyruvic
acid (3-BrPA) has a good therapeutic effect on intrahepatic and
extrahepatic tumors, and no obvious toxic reaction is observed
(8). In addition, 2-deoxyglucose,
which is a glycolytic inhibitor, and 3-BrPA cooperate with
photodynamic therapy to inhibit the migration of TNBC cells,
demonstrating that 3-BrPA could inhibit cell proliferation by
affecting aerobic glycolysis (9).
A previous study also demonstrated that 3-BrPA inhibits human tumor
cells with high Myc expression in vivo and in vitro
but has a minimal effect on cells with low Myc expression (10). HK2 has also been demonstrated to be
a target of c-Myc (11). As
transmembrane transporters, glucose transporters (GLUTs) represent
another important factor in glycolysis for the entry of glucose
into cells (12). TNBC displays
higher levels of GLUT1, one of the most common subtypes of GLUT,
than other subtypes of breast cancer (13). The inhibition of GLUT1 expression
has been detected in lymphoma cells after treatment with 3-BrPA
(14).
Thioredoxin-interacting protein (TXNIP), a binding
partner with negative regulatory effect of thioredoxin, could
inhibit glucose uptake and aerobic glycolysis (15). TXNIP also has pro-apoptosis and
anti-proliferative activities in cancer cells (16). A negative regulatory effect of
TXNIP on tumor cells has been reported in several cancer types,
including hepatocellular carcinoma and bladder cancer (17,18).
Particularly in breast cancer, the tumor-suppressive effect of
TXNIP is well established, with one study demonstrating that higher
TXNIP expression portended an improved patient prognosis,
suggesting that TXNIP is likely to serve an important role in the
suppression of breast cancer (19,20).
Recently, another study demonstrated that TNBC cells have unique
molecular characteristics, including high levels of c-Myc and low
levels of TXNIP (21).
Furthermore, c-Myc promotes glucose consumption in TNBC to maintain
proliferation, directly competing with TXNIP (21). Another study identified the
EGFR-MYC-TXNIP axis as an important regulator of the TNBC
metabolism and demonstrated the association between the
EGFRhigh-MYChigh-TXNIPlow gene
signature with aggressive glycolytic metabolism and a poor survival
rate in TNBC (22). Based on these
findings, it can be hypothesized that 3-BrPA may inhibit c-Myc,
downregulate HK2 and GLUT1 expression, upregulate TXNIP expression,
and suppress glycolysis, subsequently inhibiting proliferation and
inducing apoptosis in TNBC cells.
To confirm this scientific hypothesis, the present
study analyzed the effects of 3-BrPA on aerobic glycolysis in TNBC
using human TNBC (HCC1143) and non-TNBC (MCF-7) cell lines. After
treatment with 3-BrPA at different concentrations and for different
durations, the effects of 3-BrPA were evaluated in terms of cell
viability using a Cell Counting Kit-8 (CCK-8) assay and apoptosis
using flow cytometry. HK activity, lactate generation, ATP
generation and the expression levels of TXNIP, GLUT1, HK2 and
c-Myc, as well as mitochondria-mediated apoptosis pathway proteins
were examined by western blotting to further clarify the internal
mechanism by which 3-BrPA may inhibit TNBC cell viability and
induce apoptosis.
Materials and methods
Cells and cell culture
TNBC (HCC1143) and non-TNBC (MCF-7) cell lines were
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. Cells were cultured in complete growth
medium consisting of DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (Beijing Solarbio
Science & Technology Co., Ltd.). Cells were incubated in an
incubator (Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2 and passaged every 3-4 days. Morphological changes
were observed under an inverted fluorescence microscope (IX73;
Olympus).
CCK-8 assay
CCK-8 (Dojindo Laboratories, Inc.) was used to
detect the viability of cells. Briefly, ~1x104
cells/well were added to 96-well plates and cultured in an
incubator with 5% CO2 at 37˚C. Subsequently, cells were
treated with 3-BrPA (Sigma-Aldrich; Merck KGaA) at 37˚C at
different concentrations (0, 20, 30, 40, 50 and 60 µM) and for
different durations (24 and 48 h). CCK-8 reagent (10 µl) was added
to each well, mixed and then incubated for an additional 2 h. The
absorbance values of the cells were immediately detected at 450 nm
using a microplate reader (Thermo Fisher Scientific, Inc.). The
inhibitory rate was calculated as
(1-Asample/Acontrol)x100%, where
Asample is the absorbance value of the sample treated
with 3-BrPA at different concentrations and time points and
Acontrol is the absorbance value of the control group
(untreated cells) recorded at the same time point. Each experiment
was performed in triplicate. The viability curves and
IC50 of 3-BrPA for each cell line were calculated by
GraphPad Prism 8.0.1 (GraphPad Software, Inc.).
Apoptosis analysis
Cells were treated with 0, 20, 40 and 60 µM 3-BrPA
for 24 h. Approximately 1x105 cells were collected after
centrifugation at 300 x g with pre-cooled phosphate buffer saline
for 10 min at 4˚C. Then cells were stained with 5 µl Annexin
V-allophycocyanin (Annexin V-APC) and 10 µl 7-amino-actinomycin D
(7-AAD) according to the manufacturer's instructions for the
Annexin V-APC/7-AAD cell apoptosis kit (Hangzhou Lianke
Biotechnology Co., Ltd.). After gentle vortexing, cells were
incubated at room temperature for 5 min in the dark. Flow
cytometric analyses were performed on a FACSCalibur (BD
Biosciences) with CellQuest Pro 5.2.1 (BD Biosciences). Each
experiment was performed in triplicate.
Measurement of HK activity
Cells were seeded into 6-well plates at a density of
2x106 cells/well and were then incubated with 3-BrPA (0,
20 and 40 µM) for both 24 and 48 h at 37˚C in 5% CO2. HK
activity detection was performed the Hexokinase Activity Detection
kit (Beijing Solarbio Science & Technology Co., Ltd.) according
to the manufacturer's instructions. The absorbance values at 340 nm
at 20 sec (A1) and 320 sec (A2) after sample addition were measured
using a spectrophotometer (Thermo Fisher Scientific, Inc.). Then,
A1 and A2 were plugged into the formula HK(U/104
cell)=[(ΔA)(Vtotal/(εxd)x109]/(500xVsample/Vsample_total)/t=2.226xΔA
(ΔA=A2-A1), where Vtotal refers to the total volume of
the reaction system (1.038x10-3 l), ε is the NADPH molar
extinction coefficient (6.22x103 l/mol/cm), d represents
the diameter of the cuvette (1 cm), Vsample is the
sample volume (0.03 ml), Vsample_total is the extract
volume (1 ml), t is the reaction time (5 min), and 500 is the total
number of cells (500x104). These factors were used to
calculate HK activity. An enzyme activity unit (1 unit) is defined
as 1 nmol of NADPH produced per min per 10,000 cells.
Measurement of lactate generation
The two cell lines were seeded into 96-well plates
at a density of 1x104 cells/well. After attachment, the
cells were treated with different concentrations (0, 20 and 40 µM)
of 3-BrPA for 24 or 48 h. The culture medium was collected to
measure the level of lactate generation using the lactate assay kit
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. The optical density at 530 nm was measured using a
microplate reader (Multiskan FC; Thermo Fisher Scientific,
Inc.).
ATP generation assay
ATP generation was measured using the ATP Assay Kit
(Beijing Solarbio Science & Technology Co., Ltd.). The two cell
lines were seeded into 96-well plates at a density of
1x105 cells/well and cultured overnight at 37˚C.
Subsequently, the cells were incubated with 3-BrPA (0, 20 and 40
µM) for 4 or 8 h (based on our preliminary experiments, the ATP
generation after 24/48 h incubation with 3-BrPA is already very low
and cannot be measured accurately, therefore 4/8 h was selected).
Then, the cells were treated according to the manufacturer's
protocol. The absorbance values at 340 nm at 10 sec (A1) after
sample addition and 3 min after a 25˚C water bath (A2) were
measured using a spectrophotometer (Thermo Fisher Scientific,
Inc.). Both A1 and A2 were plugged into the formula ATP
(µmol/106
cell)=(ΔAsample)/(ΔAstandard/Cstandard)V/n(ΔA=A2-A1),
where Cstandard refers to the concentration of the
standard solution (0.625 µmol/ml), V denotes the added volume of
extraction solution (1 ml), and n indicates the number of cells
(x106). These factors were used to calculate ATP
production.
Western blotting
The two cell lines were treated with 0, 20 and 40 µM
3-BrPA for 24 or 48 h. Total protein was extracted using a Total
Protein Extraction kit (Beijing Solarbio Science & Technology
Co., Ltd.) for western blot analysis. The protein concentration was
determined using a BCA Protein Assay kit (cat. no. 23227; Thermo
Fisher Scientific, Inc.). The proteins (30 µg per lane) were
separated on a denaturing 12% SDS-PAGE gel and then transferred to
a polyvinylidene fluoride membrane. Then the membrane was cultured
in QuickBlock Blocking Buffer for Western Blot (Beyotime Institute
of Biotechnology) at room temperature for 1 h. The membrane was
incubated with antibodies against GLUT1 (1:1,000; cat. no.
TA312796; OriGene Technologies, Inc.), c-Myc (1:1,000; cat. no.
TA150121; OriGene Technologies, Inc.), TXNIP (1:1,000; cat. no.
TA349090; OriGene Technologies, Inc.), HK2 (1:1,000; cat. no.
TA500856; OriGene Technologies, Inc.), Bcl-2 (1:2,000; cat. no.
TA806591; OriGene Technologies, Inc.), Bax (1:2,000; cat. no.
TA810334; OriGene Technologies, Inc.), cytochrome c (Cyt-C;
1:5,000; cat. no. ab13575; Abcam), Caspase-3 (1:1,000; cat. no.
TA301776; OriGene Technologies, Inc.) and β-actin (1:1,000; cat.
no. TA811000; OriGene Technologies, Inc.) at room temperature
overnight. Then, the membrane was incubated with the
IRDye® 800CW goat anti-rabbit IgG secondary antibody
(1:10,000; cat. no. 926-32211; LI-COR Biosciences) and the
IRDye® 800CW goat anti-mouse IgG secondary antibody
(1:10,000; cat. no. 926-32210; LI-COR Biosciences) at room
temperature for another 2 h. Protein bands were visualized using an
Odyssey Infrared Imaging System (LI-COR Biosciences), and the
grayscale values of the bands were determined using Odyssey
Application software 3.0 (LI-COR Biosciences). Each experiment was
performed in triplicate.
Statistical analysis
All results were presented as the means ± standard
deviations and images were analyzed using SPSS 20.0 statistical
software (IBM Corp.) and GraphPad Prism 8.0.1 software (GraphPad
Software, Inc.). Statistical analysis was performed using one-way
ANOVA and Bonferroni's correction. P<0.05 was considered to
indicate a statistically significant difference. The experiments
were performed in triplicate.
Results
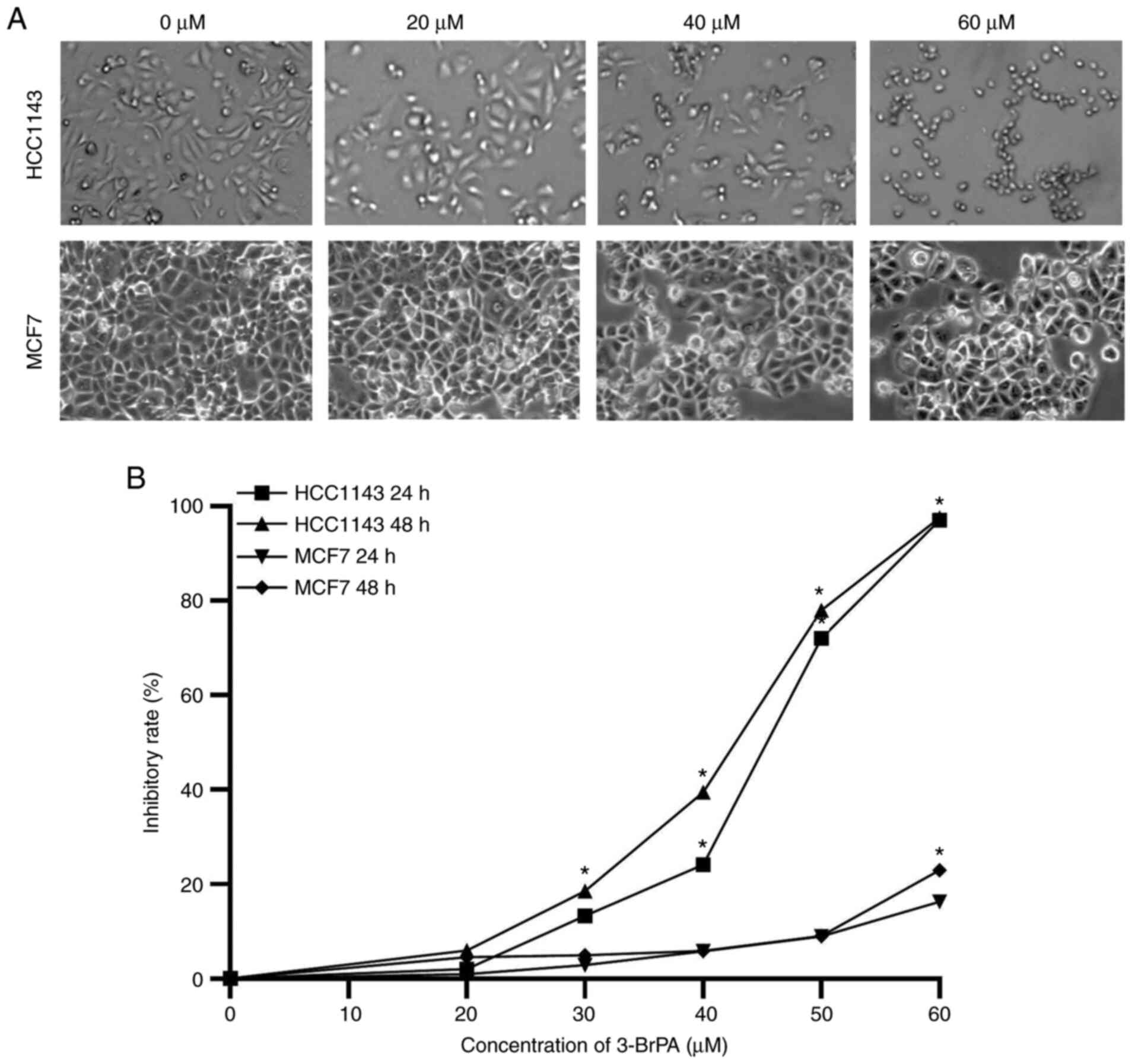
3-BrPA inhibits TNBC cell viability in
vitro
The effects of 3-BrPA on cell viability in TNBC
(HCC1143) and non-TNBC (MCF-7) cell lines were examined using a
CCK-8 assay. Morphological changes were observed under an inverted
fluorescence microscope, and numerous morphological changes
occurred in cells after treatment with 3-BrPA. Untreated cells
attached closely to one another, and were polygonal in shape
(Fig. 1A and B). However, cells treated with 3-BrPA
became round or inflated with few cellular contacts and a reduction
in the number of viable cells. Cell shrinkage, condensation of
cytoplasm and chromosomes inside the cell were observed. Compared
with the untreated control group, 3-BrPA treatment markedly reduced
the cell viability of breast cancer cells. The IC50
values of HCC1143 and MCF-7 were 44.87 µM (95% CI, 40.72-48.69 µM)
and 111.3 µM (95% CI, 104.4-117.7 µM) after 24 h and 41.26 µM (95%
CI, 36.41-45.71 µM) and 75.87 µM (95% CI, 69.83-82.08 µM) after 48
h of exposure to 3-BrPA, respectively. The inhibitory effect was
dose- and time-dependent. Notably, the inhibitory effect of 3-BrPA
on the TNBC cell line was stronger than that on the non-TNBC cell
line. The full range of results used to calculate the
IC50 of 3-BrPA in MCF-7 cells is shown in Fig. S1.
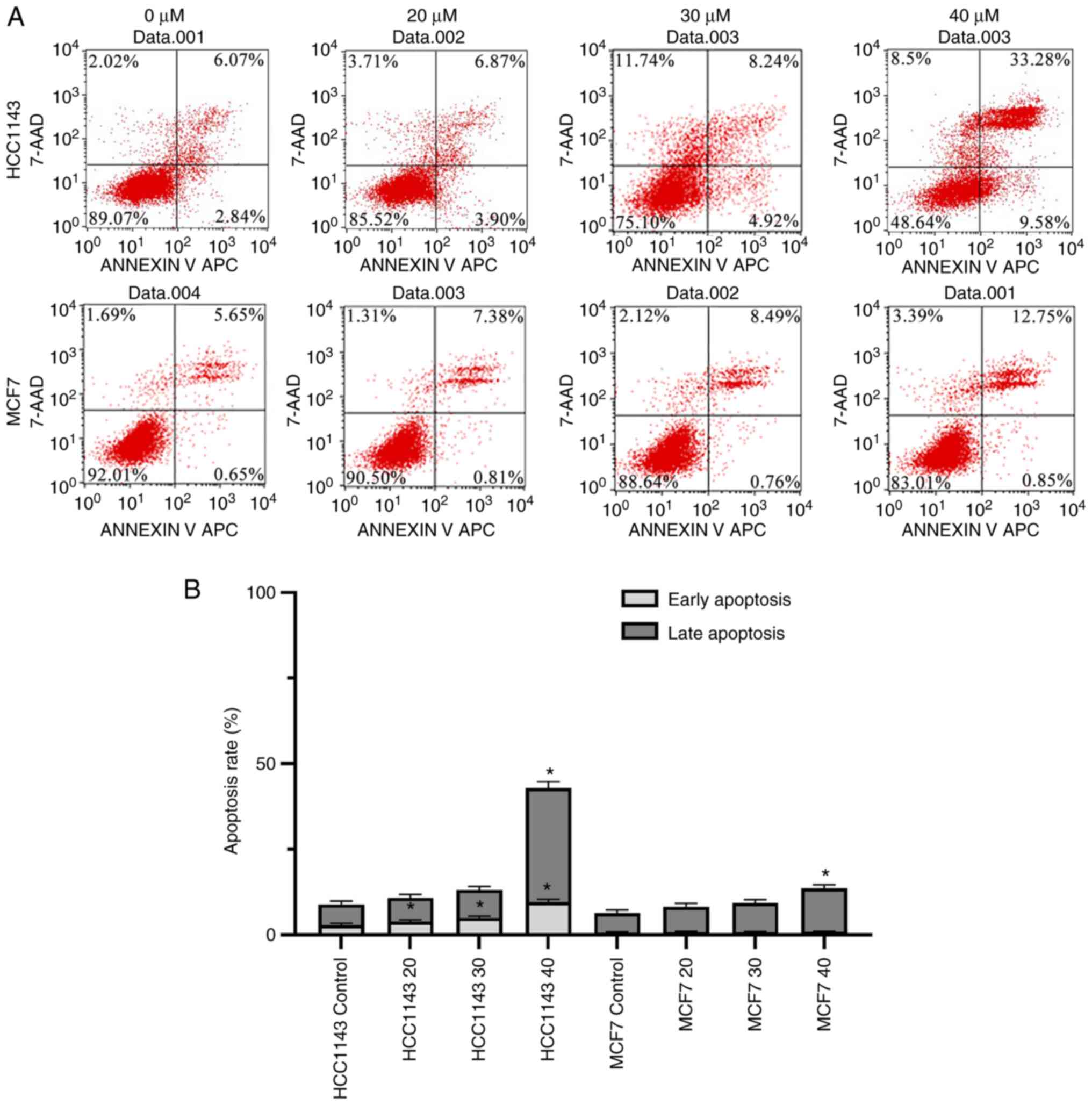
3-BrPA promotes TNBC cell apoptosis in
vitro
Flow cytometry was performed to evaluate cell
apoptosis. The results demonstrated that the early apoptosis rates
of the TNBC cell line (HCC1143) were significantly increased in a
dose-dependent manner compared with those of the corresponding
untreated control cells (Fig. 2A
and B). However, 3-BrPA did not
exert the same effect on the non-TNBC cell line (MCF-7). The late
apoptosis rates were only significantly increased at highest 40 µM
concentration in both TNBC cell line and non-TNBC cell line
(Fig. 2A and B).
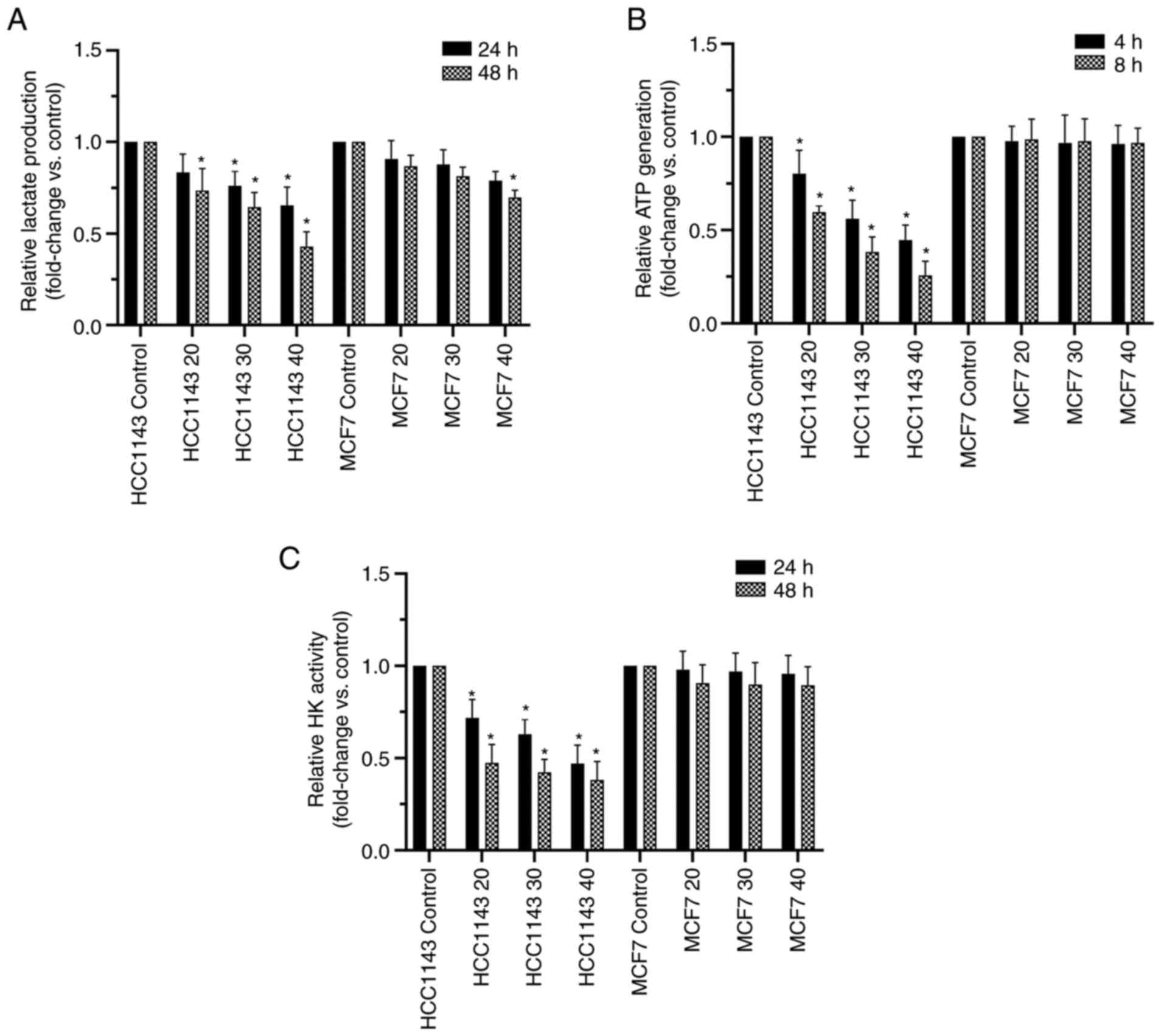
3-BrPA suppresses lactate generation
in TNBC cells
Lactate is a vital final product in glycolysis that
reflects glycolytic metabolism in cells (23). To study if 3-BrPA regulates
glycolytic metabolism in a TNBC cell line, the generation of
lactate after treatment with 3-BrPA for 24 and 48 h was measured.
Intracellular lactate generation was significantly suppressed at
30/40 µM at 24 h and 20/30/40 µM at 48 h in 3-BrPA-treated TNBC
cells compared with control TNBC cells (HCC1143; Fig. 3A) and the results were dose- and
time-dependent. In the non-TNBC group (MCF-7), 3-BrPA showed a
similar significant effect only at highest concentration at 48 h,
showing this effect was not as strong as that in the TNBC
group.
3-BrPA reduces intracellular ATP
generation in TNBC cells
The ATP generation level was considered to be a
favorable indicator of the glycolysis rate in cells because it is
the main ultimate product in glycolysis (24). Treatment with 3-BrPA reduced
intracellular ATP generation TNBC cells (HCC1143) in a time- and
dose-dependent manner (P<0.05; Fig.
3B). In the non-TNBC cell line (MCF-7), the ATP level was not
significantly affected (P>0.05).
3-BrPA decreases HK activity in TNBC
cells
As a key enzyme in aerobic glycolysis, HK activity
reflects the strength of glycolytic metabolism in tumor cells
(25). The effect of 3-BrPA on HK
in TNBC (HCC1143) and non-TNBC (MCF-7) cell lines was investigated.
The present results demonstrated that HK activity was decreased in
a dose-dependent manner in the TNBC cell line after incubation with
3-BrPA (P<0.05; Fig. 3C). The
effect was prominent when the cells were treated with 30 and 40 µM
3-BrPA. However, no significant differences in the non-TNBC cell
line group were noted compared with the control group.
Protein expression in TNBC cells is
altered by 3-BrPA
To confirm the effect of 3-BrPA on c-Myc, TXNIP, HK2
and GLUT1 expression, TNBC (HCC1143) and non-TNBC (MCF-7) cells
were treated with 3-BrPA at different doses for different
durations. Subsequently, protein expression levels were detected
through western blotting. The protein expression levels of c-Myc
and HK2 in the TNBC group were dose and time-dependently decreased
following treatment with 3-BrPA (Fig.
4A-C). Meanwhile, TXNIP protein expression was enhanced upon
treatment with 3-BrPA. In the non-TNBC group, 3-BrPA did not
exhibit a similar regulatory effect on c-Myc, HK2 and TXNIP. No
evident changes were observed in the protein expression levels of
GLUT1 in both the TNBC and non-TNBC groups.
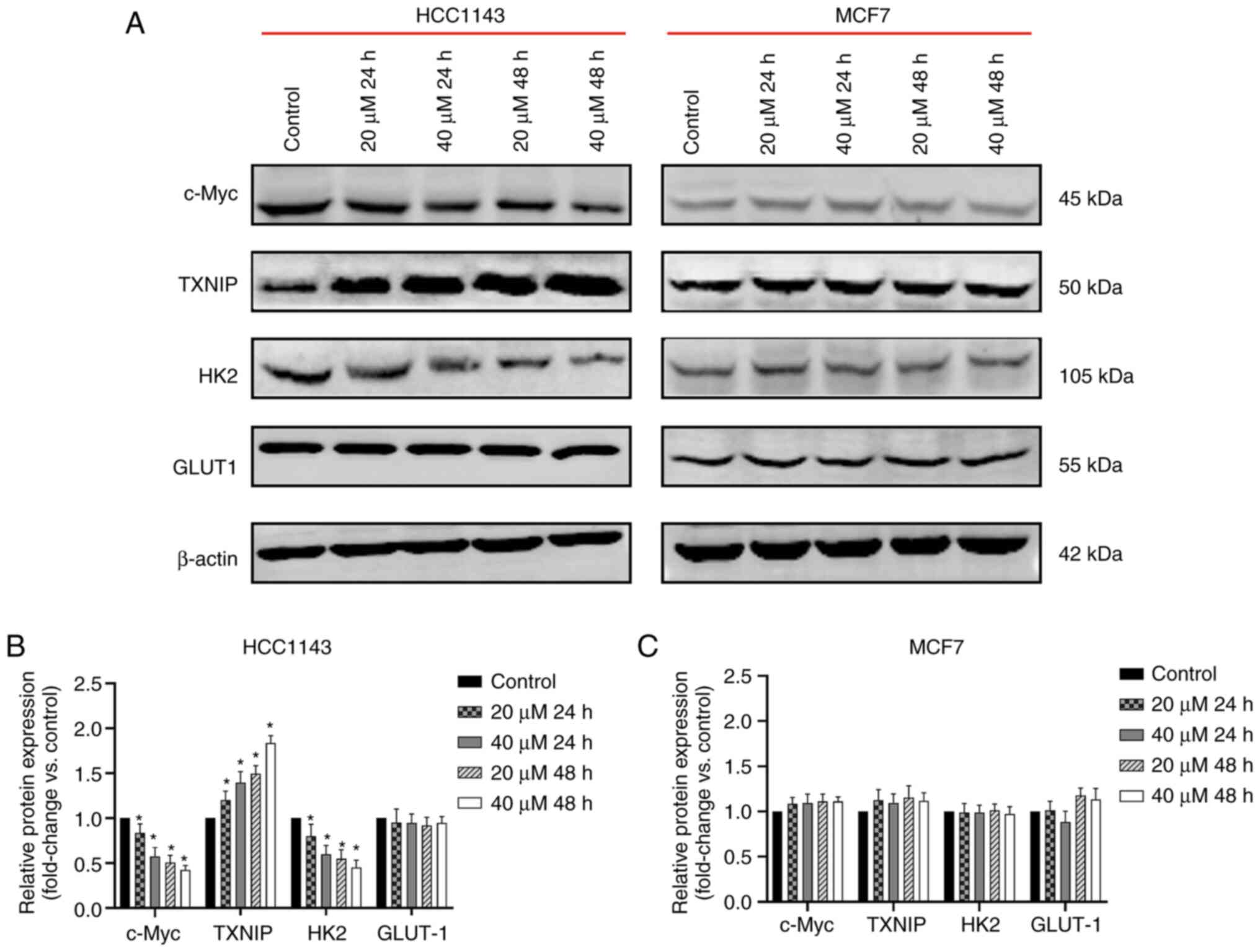 | Figure 43-BrPA regulates expression of c-Myc,
TXNIP and HK2. (A) Expression levels of c-Myc, TXNIP, HK2 and GLUT1
were detected through western blot analysis. Grayscale values of
the bands were determined using software. (B) 3-BrPA downregulated
c-Myc and HK2 protein expression, whereas it upregulated TXNIP
protein expression in TNBC cells (*P<0.05 vs. control
group, the same cell line with 0 µM 3-BrPA). (C) However, in
non-TNBC cells, the expression levels of c-Myc, TXNIP, HK2 and
GLUT1 were not significantly affected. The expression levels of
GLUT1 were not significantly affected in both TNBC and non-TNBC
cells. TXNIP, thioredoxin-interacting protein; HK2, hexokinase 2;
GLUT1, glucose transporter 1; 3-BrPA, 3-bromopyruvic acid; TNBC,
triple-negative breast cancer. |
Expression of mitochondria-regulated
apoptosis pathway proteins is altered by 3-BrPA
To examine the effect of 3-BrPA on the
mitochondria-mediated apoptosis pathway, the relative expression
levels of anti-apoptotic Bcl-2 and pro-apoptotic Bax, Cyt-C and
Caspase-3 were analyzed. Both TNBC and non-TNBC cells were exposed
to 3-BrPA at different doses and for different durations.
Subsequently, the expression levels of mitochondria-mediated
apoptosis pathway proteins were detected through western blotting.
Treatment with 3-BrPA significantly decreased the protein
expression levels of anti-apoptotic Bcl-2 at 40 µM at 24 h, and
20/40 µM at 48 h; while it increased the protein expression levels
of pro-apoptotic Bax and Caspase-3 at both 20/40 µM at 24/48 h; and
Cyt-C at 40 µM at 24 h and 20/40 µM at 48 h in TNBC cells (Fig. 5A and B). The regulatory effect of 3-BrPA was
dose-dependent. However, the same effect of 3-BrPA on these
apoptosis-related proteins was not observed in non-TNBC cells
(Fig. 5A-C).
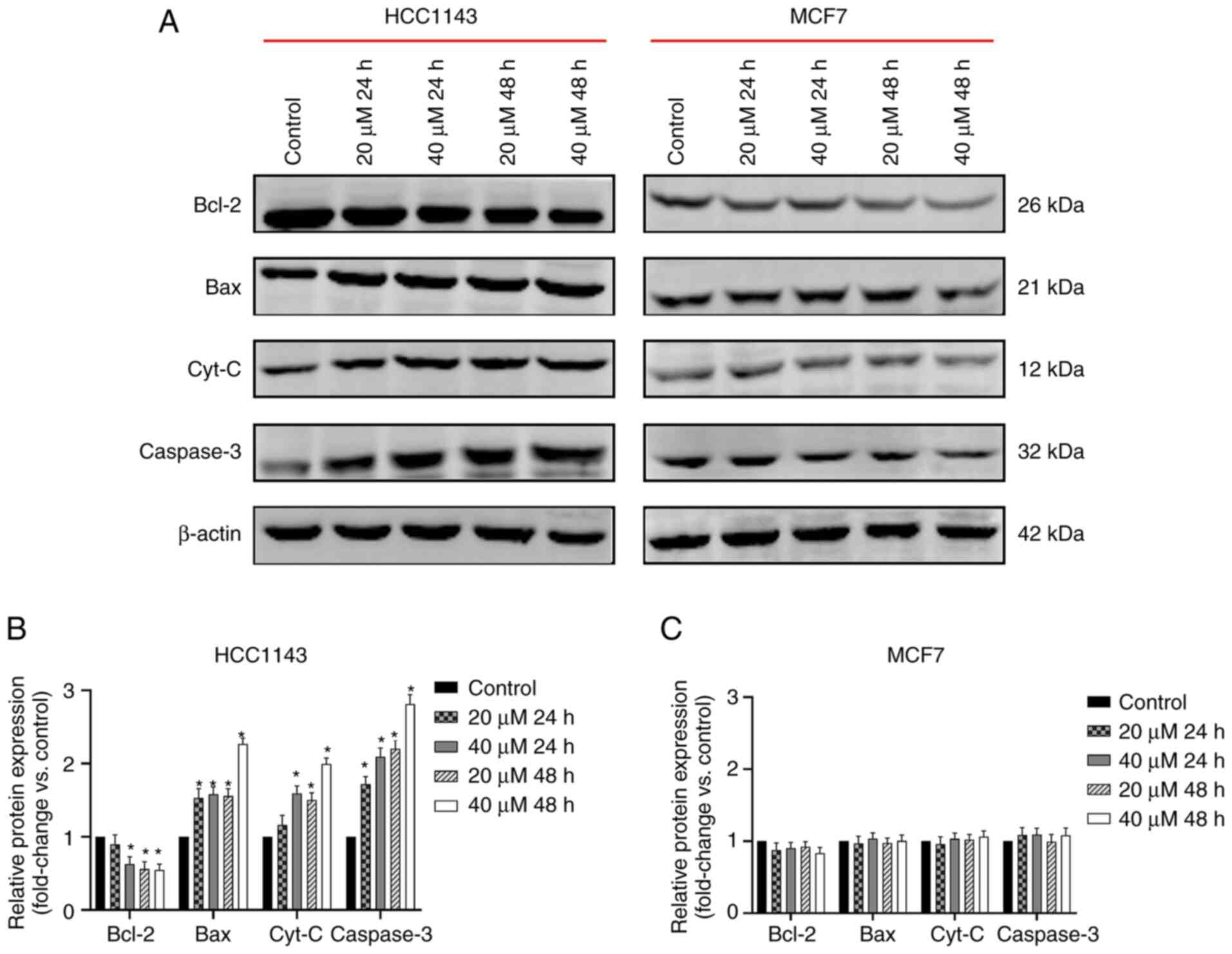 | Figure 53-BrPA regulates expression of
mitochondria-regulated apoptosis pathway proteins. (A) Expression
levels of mitochondria-regulated apoptosis pathway proteins,
including Bcl-2, Bax, Cyt-C and Caspase-3 were detected through
western blot analysis. (B) Bcl-2 protein expression was decreased,
while Bax, Cyt-C and Caspase-3 protein expression was increased
following 3-BrPA treatment in TNBC cells (*P<0.05 vs.
control group, the same cell line with 0 µM 3-BrPA). (C) However,
in non-TNBC cells, the expression levels of Bcl-2, Bax, Cyt-C and
Caspase-3 were not significantly affected. 3-BrPA, 3-bromopyruvic
acid; Cyt-C, cytochrome c; TNBC, triple-negative breast
cancer. |
Discussion
TNBC is a notable type of breast cancer because of
its unique molecular type. The 5-year survival rate of patients
with TNBC is poorer than that of patients with other types of
breast cancer (26), and TNBC most
commonly arises in premenopausal patients with a high recurrence
rate (27). TNBC is extremely
invasive and has a higher incidence of remote metastasis than
non-TNBC subtypes; <30% of patients with metastatic TNBC survive
for >5 years (27). Targeted
therapies for TNBC are lacking. Compared with non-TNBC cells, TNBC
cells possess unique metabolic characteristics: Higher glucose
uptake, increased lactate production and low mitochondrial
respiration levels (4). These
characteristics suggest that suppressing breast cancer cell
proliferation by inhibiting the glycolytic pathway may represent a
novel therapeutic mechanism for antitumor drugs.
3-BrPA is receiving increasing attention given the
increasing interest in research on antitumor drugs. 3-BrPA is an
analog of pyruvate with high tumor selectivity (28). This small alkylating compound can
induce cell death through two mechanisms: One involves inhibiting
HK2 to prevent the glycolysis process (29), and the other involves activating
the mitochondrial pathway of apoptosis (30). 3-BrPA functions in a variety of
tumor cells, including multiple myeloma cells (31) and hepatocellular carcinoma cells
(32). However, normal cells avoid
the damage induced by this drug (33). Therefore, 3-BrPA represents a
promising antitumor drug that may be useful for clinical treatment
in the future. The present study revealed that 3-BrPA inhibited the
viability of TNBC cells and promoted their apoptosis; however, this
effect was not significant in non-TNBC cells, suggesting a unique
mechanism.
HK is a key rate-limiting enzyme in the first step
of glycolysis in tumor tissues, and its expression and activity
increase in tumor tissues to promote effective anaerobic glycolysis
even under anaerobic conditions (7). In mammals, four HK subtypes, HK1,
HK2, HK3 and HK4, are encoded by separate genes (34). HK2 is absent or expressed at low
levels in the majority of adult normal cells but is widely
upregulated in several cancer cells (35). Patra et al (36) found that HK2 expression is markedly
elevated in tumors derived from mouse models of lung and breast
cancer. Marini et al (37)
revealed that HK1 and HK2 inhibition caused by metformin could
modify glucose metabolism in TNBC both in vitro and in
xenografted mice models. These results suggest that HK2 may
represent an alternative target for cancer therapy. The present
data demonstrated that 3-BrPA reduced the protein expression levels
of HK2 in a dose-dependent manner in TNBC cells. However, the same
inhibitory effect on HK2 protein expression was not found in
non-TNBC cells.
An increasing number of researchers have recognized
the prognostic and predictive power of TXNIP, which is a potent
negative regulator of glucose uptake, in human breast cancer. Shen
et al (21) demonstrated
the unique molecular mechanism of TNBC cells: Increased c-Myc
expression and reduced TXNIP and c-Myc expression promote glucose
uptake and use in tumor cells, which subsequently accelerates
cancer cell proliferation. Park et al (38) demonstrated that TXNIP operates to
suppress the high proliferative activity and estrogen-dependent
cell proliferation in breast cancer. Qu et al (39) demonstrated that ectopic TXNIP
expression decreased glucose uptake induced by c-Myc and led to
suppression of a broad range of glycolytic target genes in prostate
cancer. The present data demonstrated that 3-BrPA upregulated TXNIP
protein expression and that this upregulating effect was stronger
in TNBC cells than in non-TNBC cells. Therefore, it was concluded
that 3-BrPA inhibited TNBC cell proliferation by assisting TXNIP
and inhibiting glycolytic enzymes, such as HK2.
Among various oncogenes, c-Myc, which is a strong
transcription factor participating in several aspects of the
oncogenic process, acts as a vital member of the Myc family
(40). The idea that c-Myc serves
an important role in cell proliferation and differentiation has
been proposed and confirmed (41).
The relevant protein encoded by c-Myc affects cell proliferation
(42). c-Myc functions to
determine the entry of cells from G1 into S phase and
contributes to progression to the G2 phase (43). Evidence also indicates that both
low expression and overexpression of c-Myc lead to cell apoptosis
(44). More than half of human
cancers exhibit loss of the c-Myc oncogene, which results in a poor
prognosis and survival rate (40).
Additionally, other studies demonstrated that c-Myc can mediate
other pathways to regulate cell metabolism. For example, the
activation of the serine biosynthesis pathway mediated by c-Myc
serves a critical role in cancer progression under
nutrient-deficient conditions (45). Furthermore, enzymes in glucose
metabolism, such as GLUT1, phosphofructokinase, M2 isoform of
pyruvate kinase and HK2, are all targets of c-Myc (46-49).
Based on these findings, it is reasonable to hypothesize that
3-BrPA affects the metabolism of TNBC cells mainly by targeting the
c-Myc/TXNIP axis, downregulating c-Myc and upregulating TXNIP, and
this effect can only be fully displayed in cells with high
c-Myc/low TXNIP. In the present study, the HCC1143 cell line was
selected because of its high c-Myc/low TXNIP expression based on
the dataset from Cancer Cell Line Encyclopedia (CCLE, portals.broadinstitute.org/ccle). The
MCF-7 cell line does not have this feature. In addition, although
most TNBC cells have this feature, not all cells do, for example,
MDA-MB-231 cells do not (10). In
preliminary experiments performed by this research group, 3-BrPA
was tested on different TNBC cells not having the same high
c-Myc/low TXNIP characteristic, and it was revealed that the
inhibitory effect was lower than that of TNBC cells with high
c-Myc/low TXNIP expression (data not shown). The reason is that the
upregulation of c-Myc is closely related to an increased tumor
invasiveness, increased drug resistance and reduced survival of
patients with TNBC (50). In terms
of glucose metabolism, c-Myc can indirectly promote the expression
of monocarboxylate transporter (MCT) 1 and MCT-2(21). MCT-1 is ubiquitously expressed and
involved in the uptake or efflux of lactate (51) and, due to the Warburg effect of
tumor cells, it can reprogram the energy metabolism, including
inducing high glucose consumption and increased lactate production.
The channels that transport lactate play an important role in
regulating the balance of lactate inside and outside of cancer
cells (51). In response to a lack
of glucose in cancer cells, lactic acid can be used as ‘fuel’ to be
taken into cells by MCT-1 for energy production. As a lactate
analog, 3-BrPA can be taken up by cancer cells through this
channel, thereby entering the cells and binding to related targets
(51,52). In addition, high c-Myc expression
can promote and encode key enzymes of glycolysis and GLUTs to
increase glucose tumor cell uptake and utilization for
proliferation (53).
The following mechanisms may be involved in the
inhibition of TXNIP caused by c-Myc. Myc, which is a member of the
basic region helix-loop-helix leucine zipper (bHLHZip) family of
transcription factors, operates by binding to another member of the
same family, MYC associated factor X (MAX) (54). TXNIP is a direct target of the
MAX-like protein (Mlx):Mlx-interacting protein (MondoA) complex,
another member of the bHLHZip family, which controls uptake and
utilization of glucose and has an activation efficacy on the TXNIP
promoter stronger than that of Myc:MAX complexes (17,55-57). Thus, the Myc:MAX complex competes
with the MondoA:Mlx complex to bind and displace TXNIP, resulting
in repression of TXNIP.
Apoptosis is a form of programmed cell death that
occurs in multicellular organisms and is induced by a variety of
physical and chemical factors. Although there are three predominant
signaling pathways in apoptosis, apoptotic signaling usually occurs
at the mitochondrial-mediated pathway level, which is mainly
regulated by Bcl-2 family genes (58). A previous study has demonstrated
that Bcl-2 is located at the mitochondrial outer membrane where it
serves an important role in promoting cellular survival and
inhibiting the actions of pro-apoptotic proteins (59). Furthermore, Bax alters
mitochondrial outer membrane permeabilization antagonizing the
function of Bcl-2, leading to the release of bioactive substances
such as Cyt-C from the intermembrane space into the cytosol
(59). Thereby, Cyt-C activates
caspase-3/caspase-9 and initiates a caspase signaling cascade and
induces apoptosis (59,60). The relationship between glycolysis
and apoptosis was studied by a team (61), but the conclusions had some
limitations, showing that related research needs to be further
carried out. In the present study, apoptosis was closely related to
glycolytic enzyme inhibition. By interacting with the outer
membrane protein voltage dependent anion channel (VDAC), HK
protects the normal function of the mitochondrial outer membrane
and maintains the balance between pro-apoptotic proteins and
anti-apoptotic proteins, prevents the release of Cyt-C, and
enhances cell proliferation and inhibits apoptosis (62). Based on this, it is reasonable to
hypothesize that 3-BrPA inhibited HK activity and dissociated it
from the mitochondria, destroying the integrity of the
mitochondrial outer membrane and breaking the balance between
pro-apoptotic proteins and anti-apoptotic proteins, thereby
enabling VDAC to open and release Cyt-C and ultimately inducing
caspase-mediated apoptosis. Thus, there may be a link between
glycolysis and the mitochondrial apoptotic pathway that can be
targeted by 3-BrPA.
The results obtained by different teams were
dissimilar despite the same drug and the same cell line being used.
For example, Kwiatkowska et al (63) used 3-BrPA to act on MCF-7 for 24 h,
and the IC50 value was 101±28 µM. However, Wu et
al (64) reported that if the
cells were treated with 3-BrPA (100 µM) for 24 h, the inhibition
rate of MCF-7 cells was as high as 95%. We hypothesized that the
results of the experiment are affected by multiple factors, such as
drug batch, cell state, culture conditions, laboratory environment
and other comprehensive factors, which lead different research
groups to obtain different results. Although the present results
indicated that the HK activity was not significantly affected by
3-BrPA in MCF-7 cells within the existing concentration range,
which disagrees with the results from Wu et al (64), the HK activity in MCF-7 cells
continued to decline while increasing the 3-BrPA concentration in
our preliminary experiments (data not shown). However, in terms of
a future clinical application of 3-BrPA, a high drug concentration
could produce more toxic and side effects.
In conclusion, in the present study, 3-BrPA reduced
the HK2 expression and promoted the TXNIP protein expression in
TNBC cells by downregulating the expression of c-Myc, thereby
inhibiting glycolysis including suppressing lactate generation,
intracellular ATP generation and HK activity, inducing
mitochondria-regulated apoptosis, and eventually limiting TNBC cell
proliferation. These findings contribute to the field of breast
cancer therapy, and 3-BrPA is expected to become an effective drug
in breast cancer therapy in the future. The concept of using
glycolysis inhibitors combined with chemotherapeutic drugs has
already been proposed (24,65).
However, whether targeting c-Myc and the glycolysis pathway could
increase the sensitivity of cancer cells to chemotherapy and
radiotherapy requires further studies. To verify the proposed
mechanism, transfection and in vivo experiments will be
performed in future research. Further research is required to
provide basic data on novel therapeutic targets to improve the
clinical treatment of TNBC.
Supplementary Material
Cell Counting Kit-8 cytotoxicity
curves used to calculate the IC50 of 3-BrPA in MCF-7
cells at 24 and 48 h. 3-BrPA, 3-bromopyruvic acid.
Acknowledgements
Not applicable.
Funding
Funding: This research was sponsored by the National Natural
Science Foundation of China (grant no. 81760476).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and JP participated in the preliminary
experimental design, preliminary experiment, main experiment
operation, data analysis, manuscript writing and revision. YL, XL,
CY, WX and QL participated in data analysis, manuscript writing and
revision. LY and XZ participated in early experimental design,
including selecting drugs and designing possible signal pathways.
JL and JP confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Woolston C: Breast cancer. Nature.
527(S101)2015.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pelicano H, Zhang W, Liu J, Hammoudi N,
Dai J, Xu RH, Pusztai L and Huang P: Mitochondrial dysfunction in
some triple-negative breast cancer cell lines: Role of mTOR pathway
and therapeutic potential. Breast Cancer Res.
16(434)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Long JP, Li XN and Zhang F: Targeting
metabolism in breast cancer: How far we can go? World J Clin Oncol.
7:122–130. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tan VP and Miyamoto S: HK2/hexokinase-II
integrates glycolysis and autophagy to confer cellular protection.
Autophagy. 11:963–964. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oronsky BT, Reid T, Knox SJ and Scicinski
JJ: The scarlet letter of alkylation: A mini review of selective
alkylating agents. Transl Oncol. 5:226–229. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Feng X, Wang P, Liu Q, Zhang T, Mai B and
Wang X: Glycolytic inhibitors 2-deoxyglucose and 3-bromopyruvate
synergize with photodynamic therapy respectively to inhibit cell
migration. J Bioenerg Biomembr. 47:189–197. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gan L, Xiu R, Ren P, Yue M, Su H, Guo G,
Xiao D, Yu J, Jiang H, Liu H, et al: Metabolic targeting of
oncogene MYC by selective activation of the proton-coupled
monocarboxylate family of transporters. Oncogene. 35:3037–3048.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Penny HL, Sieow JL, Adriani G, Yeap WH,
See Chi Ee P, San Luis B, Lee T, Mak SY, Ho YS, Lam KP, et al:
Warburg metabolism in tumor-conditioned macrophages promotes
metastasis in human pancreatic ductal adenocarcinoma.
Oncoimmunology. 5(e1191731)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thorens B and Mueckler M: Glucose
transporters in the 21st Century. Am J Physiol Endocrinol Metab.
298:E141–E145. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Choi J, Jung WH and Koo JS:
Metabolism-related proteins are differentially expressed according
to the molecular subtype of invasive breast cancer defined by
surrogate immunohistochemistry. Pathobiology. 80:41–52.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yadav S, Pandey SK, Kumar A, Kujur PK,
Singh RP and Singh SM: Antitumor and chemosensitizing action of
3-bromopyruvate: Implication of deregulated metabolism. Chem Biol
Interact. 270:73–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu N, Zheng B, Shaywitz A, Dagon Y, Tower
C, Bellinger G, Shen CH, Wen J, Asara J, McGraw TE, et al:
AMPK-dependent degradation of TXNIP upon energy stress leads to
enhanced glucose uptake via GLUT1. Mol Cell. 49:1167–1175.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alhawiti NM, Al Mahri S, Aziz MA, Malik SS
and Mohammad S: TXNIP in metabolic regulation: Physiological role
and therapeutic outlook. Curr Drug Targets. 18:1095–1103.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
O'Shea JM and Ayer DE: Coordination of
nutrient availability and utilization by MAX- and MLX-centered
transcription networks. Cold Spring Harb Perspect Med.
3(a014258)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou J, Yu Q and Chng WJ: TXNIP (VDUP-1,
TBP-2): A major redox regulator commonly suppressed in cancer by
epigenetic mechanisms. Int J Biochem Cell Biol. 43:1668–1673.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cadenas C, Franckenstein D, Schmidt M,
Gehrmann M, Hermes M, Geppert B, Schormann W, Maccoux LJ, Schug M,
Schumann A, et al: Role of thioredoxin reductase 1 and thioredoxin
interacting protein in prognosis of breast cancer. Breast Cancer
Res. 12(R44)2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen JL, Merl D, Peterson CW, Wu J, Liu
PY, Yin H, Muoio DM, Ayer DE, West M and Chi JT: Lactic acidosis
triggers starvation response with paradoxical induction of TXNIP
through MondoA. PLoS Genet. 6(e1001093)2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shen L, O'Shea JM, Kaadige MR, Cunha S,
Wilde BR, Cohen AL, Welm AL and Ayer DE: Metabolic reprogramming in
triple-negative breast cancer through Myc suppression of TXNIP.
Proc Natl Acad Sci USA. 112:5425–5430. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iqbal MA, Chattopadhyay S, Siddiqui FA, Ur
Rehman A, Siddiqui S, Prakasam G, Khan A, Sultana S and Bamezai RN:
Silibinin induces metabolic crisis in triple-negative breast cancer
cells by modulating EGFR-MYC-TXNIP axis: Potential therapeutic
implications. FEBS J. 288:471–485. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rabinowitz JD and Enerbäck S: Lactate: The
ugly duckling of energy metabolism. Nat Metab. 2:566–571.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: Progress and
prospects. Mol Cancer. 12(152)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang M, Liu S, Lin J, Hao W, Wei B, Gao
Y, Kong C, Yu M and Zhu Y: A pan-cancer analysis of molecular
characteristics and oncogenic role of hexokinase family genes in
human tumors. Life Sci. 264(118669)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Howlader N, Cronin KA, Kurian AW and
Andridge R: Differences in breast cancer survival by molecular
subtypes in the United States. Cancer Epidemiol Biomarkers Prev.
27:619–626. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jiao L, Zhang HL, Li DD, Yang KL, Tang J,
Li X, Ji J, Yu Y, Wu RY, Ravichandran S, et al: Regulation of
glycolytic metabolism by autophagy in liver cancer involves
selective autophagic degradation of HK2 (hexokinase 2). Autophagy.
14:671–684. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fan T, Sun G, Sun X, Zhao L, Zhong R and
Peng Y: Tumor energy metabolism and Potential of 3-bromopyruvate as
an inhibitor of aerobic glycolysis: Implications in tumor
treatment. Cancers (Basel). 11(317)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nikravesh H, Khodayar MJ, Behmanesh B,
Mahdavinia M, Teimoori A, Alboghobeish S and Zeidooni L: The
combined effect of dichloroacetate and 3-bromopyruvate on glucose
metabolism in colorectal cancer cell line, HT-29; the mitochondrial
pathway apoptosis. BMC Cancer. 21(903)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Niedźwiecka K, Dyląg M, Augustyniak D,
Majkowska-Skrobek G, Cal-Bąkowska M, Ko YH, Pedersen PL, Goffeau A
and Ułaszewski S: Glutathione may have implications in the design
of 3-bromopyruvate treatment protocols for both fungal and algal
infections as well as multiple myeloma. Oncotarget. 7:65614–65626.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ko YH, Verhoeven HA, Lee MJ, Corbin DJ,
Vogl TJ and Pedersen PL: A translational study ‘case report’ on the
small molecule ‘energy blocker’ 3-bromopyruvate (3BP) as a potent
anticancer agent: From bench side to bedside. J Bioenerg Biomembr.
44:163–170. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lis P, Dyląg M, Niedźwiecka K, Ko YH,
Pedersen PL, Goffeau A and Ułaszewski S: The HK2 dependent ‘Warburg
Effect’ and mitochondrial oxidative phosphorylation in cancer:
Targets for effective therapy with 3-bromopyruvate. Molecules.
21(1730)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wilson JE: Isozymes of mammalian
hexokinase: Structure, subcellular localization and metabolic
function. J Exp Biol. 206 (Pt 12):2049–2057. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Patra KC and Hay N: Hexokinase 2 as
oncotarget. Oncotarget. 4:1862–1863. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Patra KC, Wang Q, Bhaskar PT, Miller L,
Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, et al:
Hexokinase 2 is required for tumor initiation and maintenance and
its systemic deletion is therapeutic in mouse models of cancer.
Cancer Cell. 24:213–228. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Marini C, Salani B, Massollo M, Amaro A,
Esposito AI, Orengo AM, Capitanio S, Emionite L, Riondato M,
Bottoni G, et al: Direct inhibition of hexokinase activity by
metformin at least partially impairs glucose metabolism and tumor
growth in experimental breast cancer. Cell Cycle. 12:3490–3499.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Park JW, Lee SH, Woo GH, Kwon HJ and Kim
DY: Downregulation of TXNIP leads to high proliferative activity
and estrogen-dependent cell growth in breast cancer. Biochem
Biophys Res Commun. 498:566–572. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Qu X, Sun J, Zhang Y, Li J, Hu J, Li K,
Gao L and Shen L: c-Myc-driven glycolysis via TXNIP suppression is
dependent on glutaminase-MondoA axis in prostate cancer. Biochem
Biophys Res Commun. 504:415–421. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen H, Liu H and Qing G: Targeting
oncogenic Myc as a strategy for cancer treatment. Signal Transduct
Target Ther. 3(5)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Stasevich EM, Murashko MM, Zinevich LS,
Demin DE and Schwartz AM: The Role of Non-Coding RNAs in the
regulation of the proto-oncogene MYC in different types of cancer.
Biomedicines. 9(921)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chauhan A, Paul R, Debnath M, Bessi I,
Mandal S, Schwalbe H and Dash J: Synthesis of fluorescent
binaphthyl amines that Bind c-MYC G-Quadruplex DNA and Repress
c-MYC expression. J Med Chem. 59:7275–7281. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gao Y, Miles SL, Dasgupta P, Rankin GO,
Cutler S and Chen YC: Trichodermin Induces G0/G1 cell cycle arrest
by inhibiting c-Myc in ovarian cancer cells and tumor
xenograft-bearing mice. Int J Mol Sci. 22(5022)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
McMahon SB: MYC and the control of
apoptosis. Cold Spring Harb Perspect Med. 4(a014407)2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sun L, Song L, Wan Q, Wu G, Li X, Wang Y,
Wang J, Liu Z, Zhong X, He X, et al: cMyc-mediated activation of
serine biosynthesis pathway is critical for cancer progression
under nutrient deprivation conditions. Cell Res. 25:429–444.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu Y, Xiang F, Huang Y, Shi L, Hu C, Yang
Y, Wang D, He N, Tao K, Wu K and Wang G: Interleukin-22 promotes
aerobic glycolysis associated with tumor progression via targeting
hexokinase-2 in human colon cancer cells. Oncotarget.
8:25372–25383. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yu P, Li AX, Chen XS, Tian M, Wang HY,
Wang XL, Zhang Y, Wang KS and Cheng Y: PKM2-c-Myc-survivin cascade
regulates the cell proliferation, migration, and tamoxifen
resistance in breast cancer. Front Pharmacol.
11(550469)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo
F, Lyssiotis CA, Aldape K, Cantley LC and Lu Z: ERK1/2-dependent
phosphorylation and nuclear translocation of PKM2 promotes the
Warburg effect. Nat Cell Biol. 14:1295–1304. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai
W and Guo C: Emerging roles and the regulation of aerobic
glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res.
39(126)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dhanasekaran R, Deutzmann A,
Mahauad-Fernandez WD, Hansen AS, Gouw AM and Felsher DW: The MYC
oncogene - the grand orchestrator of cancer growth and immune
evasion. Nat Rev Clin Oncol. 19:23–36. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Skaripa-Koukelli I, Hauton D,
Walsby-Tickle J, Thomas E, Owen J, Lakshminarayanan A, Able S,
McCullagh J, Carlisle RC and Vallis KA: 3-Bromopyruvate-mediated
MCT1-dependent metabolic perturbation sensitizes triple negative
breast cancer cells to ionizing radiation. Cancer Metab.
9(37)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pereira-Vieira J, Azevedo-Silva J, Preto
A, Casal M and Queirós O: MCT1, MCT4 and CD147 expression and
3-bromopyruvate toxicity in colorectal cancer cells are modulated
by the extracellular conditions. Biol Chem. 400:787–799.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shen S, Yao T, Xu Y, Zhang D, Fan S and Ma
J: CircECE1 activates energy metabolism in osteosarcoma by
stabilizing c-Myc. Mol Cancer. 19(151)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Meyer N and Penn LZ: Reflecting on 25
years with MYC. Nat Rev Cancer. 8:976–990. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kaadige MR, Yang J, Wilde BR and Ayer DE:
MondoA-Mlx transcriptional activity is limited by mTOR-MondoA
interaction. Mol Cell Biol. 35:101–110. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Peterson CW, Stoltzman CA, Sighinolfi MP,
Han KS and Ayer DE: Glucose controls nuclear accumulation, promoter
binding, and transcriptional activity of the MondoA-Mlx
heterodimer. Mol Cell Biol. 30:2887–2895. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang B, Lyu J, Liu Y, Wu C, Yang EJ,
Pardeshi L, Tan K, Wong KH, Chen Q, Xu X, et al: BRCA1 deficiency
sensitizes breast cancer cells to bromodomain and extra-terminal
domain (BET) inhibition. Oncogene. 37:6341–6356. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Gao YH, Zhang HP, Yang SM, Yang Y, Ma YY,
Zhang XY and Yang YM: Inactivation of Akt by arsenic trioxide
induces cell death via mitochondrial-mediated apoptotic signaling
in SGC-7901 human gastric cancer cells. Oncol Rep. 31:1645–1652.
2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging.(Albany NY). 8:603–619. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Yu C, Du F, Zhang C, Li Y, Liao C, He L,
Cheng X and Zhang X: Salmonella enterica serovar Typhimurium sseK3
induces apoptosis and enhances glycolysis in macrophages. BMC
Microbiol. 20(151)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Krasnov GS, Dmitriev AA, Lakunina VA,
Kirpiy AA and Kudryavtseva AV: Targeting VDAC-bound hexokinase II:
A promising approach for concomitant anti-cancer therapy. Expert
Opin Ther Targets. 17:1221–1233. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kwiatkowska E, Wojtala M, Gajewska A,
Soszyński M, Bartosz G and Sadowska-Bartosz I: Effect of
3-bromopyruvate acid on the redox equilibrium in non-invasive MCF-7
and invasive MDA-MB-231 breast cancer cells. J Bioenerg Biomembr.
48:23–32. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wu L, Xu J, Yuan W, Wu B, Wang H, Liu G,
Wang X, Du J and Cai S: The reversal effects of 3-bromopyruvate on
multidrug resistance in vitro and in vivo derived from human breast
MCF-7/ADR cells. PLoS One. 9(e112132)2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Akins NS, Nielson TC and Le HV: Inhibition
of glycolysis and glutaminolysis: An emerging drug discovery
approach to combat cancer. Curr Top Med Chem. 18:494–504.
2018.PubMed/NCBI View Article : Google Scholar
|