1. Introduction
Pachymic acid (PA), a lanostane-type triterpenoid,
was first isolated and characterized from the sclerotium of
Poria cocos (Fig. 1) in
1958(1). PA is mainly derived from
the wood-rotting fungus Poria cocos (2-4),
a saprophytic fungus that grows in diverse species of Pinus.
Its sclerotium, known as fu-ling or hoelen, has been widely used as
a health food and in traditional Chinese medicine with
pharmacological properties, including sedative, diuretic, and tonic
effects (5-7).
Overall, >160 terpenoids have been identified in Poria
cocos (8). As an important
bioactive terpenoid, PA has been reported to exhibit numerous
pharmacological effects, such as cytotoxic (9), anti-inflammatory (10), antihyperglycemic (11), antibacterial and antiviral
(12), sedative-hypnotic (13) and anti-ischemia/reperfusion
(14) effects. Thus, PA has
potential for the treatment of various diseases. However, it is
still in the preclinical development stage, possibly owing to
limited in vivo data. To provide comprehensive data for
further study, to the best of our knowledge, the current study
systematically summarized the current progress in the research and
development of PA for the first time, including its sources,
structure and properties, pharmacological activities, and
pharmacokinetics. It aimed to improve the current knowledge and
development potential of PA, and improve its development for
potential clinical applications.
PA was first discovered in the sclerotium of
Poria cocos from which its name is derived (1). Since then, it has been observed as
the active compound in several fungi, such as the sclerotium
(15-24)
and epidermis (25) of Poria
cocos and the fruiting bodies of Fomitopsis nigra
(26,27) and Fomitopsis pinicola
(28,29), which are used in traditional
medicine. In addition to fungi, PA is extracted from
Heterosmilax chinensis (30), a traditional Chinese medicine used
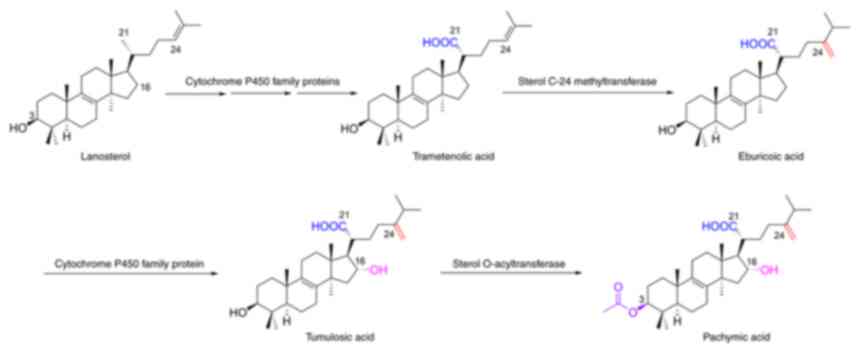
for the treatment of cancer. Zhu et al (31) reported a possible biosynthetic
pathway from lanostane to PA (Fig.
2). PA is a white powder and highly insoluble in water
(32). Therefore, the poor
solubility of PA results in its low bioavailability in vivo,
which may limit its further clinical applications. Cai et al
(32) demonstrated that
glycyrrhizin (a triterpenoid glycoside) increases the solubility of
PA in an aqueous solution, thereby improving its
bioavailability.
2. Pharmacological activities
Cytotoxic effect
At present, cancer is a major disease that seriously
affects human health (33).
Table I presents the cytotoxic
effects and underlying molecular mechanisms of PA. PA has been
reported to against various cancer cell lines, including colon
cancer (16), leukemia (34), bladder cancer (35,36),
nasopharyngeal carcinoma (37),
prostate cancer (38), primary
osteosarcoma (39), breast cancer
(40,41), lung cancer (42,43),
gastric cancer (44,45) and pancreatic cancer (46). Several molecular mechanisms are
involved in this process.
 | Table IBiological activities of PA. |
Table I
Biological activities of PA.
| Biological
effects | Details | Cell
lines/Model | Dose | Application | (Refs.) |
|---|
| Cytotoxic
effect | Inhibit
proliferation with an IC50 value of 29.1 µM and
inhibit DNA topoisomerase I and II | Colon HT-29
cells | 20-100
µM | In
vitro | (16) |
| | A novel
RXR-specific agonist and induces differentiation of HL-60 cells
with an EC50 value of 6.7±0.37 µM | Leukemia HL-60
cells | 100 µM | In
vitro | (34) |
| | Inhibit
proliferation and induce apoptosis via mitochondrial-mediated
intrinsic pathway and death receptor-mediated extrinsic
pathway | Bladder T24
cells | 5-30 µM | In
vitro | (35) |
| | Inhibit
proliferation and induce apoptosis via ROS generation,
mitochondrial- mediated intrinsic pathway, and DR5- mediated
extrinsic pathway | Bladder EJ
cells | 2.5-30
µM | In
vitro | (36) |
| | Inhibit
proliferation, induce apoptosis, and upregulate the levels of PARP,
p-ATM, p-ATR, p-Chk-1, p-Chk-2, and p-histone H2A.X | Nasopharyngeal
carcinoma CNE-1, CNE-2 cells | 10-30
µM | In
vitro | (37) |
| | Inhibit
proliferation and induce apoptosis through mitochondria dysfunction
by decreasing the phosphorylation of Bad and Bcl-2, and activating
caspases-9 and -3 | Prostate DU145
cells | 10-40
µg/ml | In
vitro | (38) |
| | Inhibit
proliferation, and induce PTEN and caspase 3/7-dependent
apoptosis | Primary
osteosarcoma cells | 10-50
µg/ml | In
vitro | (39) |
| | An activator of
PKM2 and an inhibitor of HK2, decrease glucose uptake and lactate
production, and induce mitochondrial dysfunction, ATP depletion,
and ROS generation | Breast SK-BR-3
cells | 10-100
µM | In
vitro | (40) |
| | Inhibit
proliferation with an IC50 value of 2.13±0.24
µg/ml, induce cell cycle arrest and apoptosis through
mitochondria-related and death receptor-mediated pathway | Breast MDA- MB-231
cells | 1-5 µM | In
vitro | (41) |
| | Inhibit the
proliferation, induce apoptosis, and disrupt mitochondrial membrane
potential, decrease IL-1β-induced activation of cPLA2 and COX-2 via
inhibition of MAPK and the NF-κB signaling pathway | Lung A549
cells | 1-200
µM | In
vitro | (42) |
| | Inhibit
proliferation, induce apoptosis, cell cycle arrest and ROS
generation, and suppress tumor growth | Lung NCI-H23,
NCI-H460 cells Nude mice bearing NCI-H23 xenograft tumors | 20-180 µM
10-60 mg/kg | In vitro
In vivo | (43) |
| | Inhibit
proliferation, induce cell cycle arrest, apoptosis, and ROS
generation via upregulation of Bax, cytochrome c and caspase 3, and
suppress tumor growth | Gastric SGC7901
cells Nude mice bearing | 20-80
µM | In
vitro | (44) |
| | SGC-7901 xenograft
tumors | 10-60 mg/kg | In vivo | |
| | Inhibit
proliferation, induce apoptosis via regulating the expression
levels of apoptosis-related proteins and suppressing the
mitochondrial capacity, and suppress tumor growth | Gastric SGC-7901,
MKN-49P cells | 15-240
µM | In
vitro | (45) |
| | Nude mice bearing
SGC-7901 and MKN-49P xenograft tumors | 15-60 mg/kg | In vivo | |
| | Induce apoptosis
and ER stress by increasing expression of XBP-1s, ATF4, Hsp70, CHOP
and p-eIF2α, and suppress the tumor growth | Pancreatic PANC-1,
MIA paca-2 cells Nude mice bearing MIA paca-2 xenograft tumors | 15-30
µM | In
vitro | (46) |
| | 25-50 mg/kg | In vivo | |
| | Inhibit the
promotion of skin tumor formation by TPA in DMBA-treated mice | ICR mice | 0.2
µM/mouse | In vivo | (47) |
| | Have no
cytotoxicity but enhance the cytotoxicity of vincristine | Epidermoid
carcinoma KBV200 cells | 12.5-25
µg/ml | In
vitro | (48) |
| | Sensitize cancer
cells to radiation therapy in vitro and in vivo by
upregulating Bax through HIF1α | Gastric SGC-7901,
MKN-49P cells Nude mice bearing SGC-7901 and MKN-49P xenograft
tumors | 60 µM | In
vitro | (49) |
| | 60 mg/kg | In vivo | |
| Anti-invasive
effect | Inhibit
proliferation, migration, invasion and adhesion ability, and induce
cell cycle arrest involving AKT and ERK signaling pathways | Gallbladder GBC-SD
cells | 10-50
µg/ml | In
vitro | (50) |
| | Inhibit
proliferation, induce cell cycle arrest and suppress migration and
invasion via decreasing β-catenin and COX-2 expression and
increasing E-cadherin expression | Ovarian HO-8910
cells | 0.5-2
µM | In
vitro | (51) |
| | Inhibit
proliferation and cell invasion by suppressing NF-κB-dependent
MMP-9 expression | Breast MDA-MB-231
and MCF-7 cells | 2.5-40
µM | In
vitro | (52) |
| | Inhibit migration
and invasion via suppressing the phosphorylation of PITPNM3 | Breast MDA-MB-231
cells | 10-40
µg/ml | In
vitro | (53) |
| | Inhibit
proliferation with an IC50 value of 0.26 µM and
suppress invasion by downregulating MMP-7 expression | Pancreatic bxpc-3
cells | 0.125-25
µM | In
vitro | (54) |
| Anti-inflammatory
effect | Restore AH
Plus-damaged cell viability and ALP activity, suppress secretion of
NO, TNF-a and IL-1β, reduce ROS formation and NF-κB
translocation | MC-3T3 E1
cells | 15 µM | In
vitro | (26) |
| | Inhibit
inflammatory effect, induce odontoblast differentiation through
increasing HO-1 expression, show cytoprotection and mineralization,
suppress NF-κB translocation and induce Nrf2 translocation | Human dental pulp
cells | 15 µM | In
vitro | (27) |
| | Inhibit the
activity of phospholipase A2 with an IC50 value of 2.897
mM | Phospholipase
A2 | 0.5-4 mM | In
vitro | (56) |
| | Inhibit leukotriene
B4 release | Human
leukocytes | 100 µM | In
vitro | (57) |
| | Reduce LPS-induced
apoptosis, attenuate LPS-induced increased mRNA expression levels
of IL1, IL6 and TNFα, and inhibit LPS-induced apoptosis via ERK1/2
and p38 pathways | H9c2 cells | 0.125-5
µM | In
vitro | (58) |
| | Inhibit TPA-induced
mouse ear edema with an ID50 value of
4.7x10-3 µM/ear | Mouse |
1x10-2-1x10-4
mg/ear | In vivo | (59) |
| | Inhibit TPA-induced
mouse ear edema with an ID50 value of 0.044 mg/ear | Mouse | - | In vivo | (60) |
| | Inhibit
serotonin-induced mouse paw edema, TPA-induced mouse ear swelling
and PLA2-induced mouse paw edema | Swiss mice | 0.5 mg/ear and 50
mg/kg | In vivo | (61) |
| | Reduce intravesical
IL-1, IL-6 and LDH levels, downregulate TNF-α and upregulate TP53
proteins | ICR mice | 20-40 mg/kg | In vivo | (62) |
| | Improve the
survival of septic rats and attenuate CLP-induced acute lung
injury, downregulate the serum levels of TNF-a, IL-1β and IL-6,
decrease malondialdehyde and myeloperoxidase contents and increase
SOD level | Wistar rats | 1-10 mg/kg | In vivo | (63) |
| | Alleviate
LPS-induced lung injury, relieve LPS-inflammation such as TNF-α,
IL-6, MCP-1 and IL-1β, reduce cell numbers in the BALF, inhibit
LPS-induced cell apoptosis and suppress NF-κB and MAPK signaling
pathways | Rats | 10-20 mg/kg | In vivo | (64) |
| | Decrease the kidney
index, drop the contents of Cre and BUN, inhibit the renal
inflammation via reducing the levels of TNF-α, IL-6 and iNOS and
enhance the expression of Nrf2 and HO-1 | SD rats | 5-5 mg/kg | In vivo | (65) |
| | Ameliorate renal
injury markers, improve renal inflammation, restore renal klotho
levels and ameliorate renal Wnt/β-catenin signaling, improve renal
tissue structure, and ameliorate renal fibrosis in
doxorubicin-induced nephropathy | Wistar albino
rats | 10 mg/kg | In vivo | (66) |
| Antihyperglycemic
effect | Induce GLUT4
expression, stimulate GLUT4 redistribution from intracellular
vesicles to the plasma membrane, increase the phosphorylation of
IRS-1, AKT and AMPK, induce triglyceride accumulation and inhibit
lipolysis | 3T3-L1 cells | 0.01-1
µM | In
vitro | (67) |
| | Increase glucose
uptake by 50% | 3T3-L1 cells | 5 µM | In
vitro | (68) |
| | Decrease blood
glucose levels in streptozocin-treated mice via enhanced insulin
sensitivity irrespective of PPAR-γ | Db/db mice | 1-10 mg/kg | In vivo | (69) |
| Antibacterial and
antiviral effects | Exhibit inhibitory
effecton the SARS- CoV-2 3CL hydrolytic enzyme with an
IC50 value of 18.607 µM | Mpro protease | 10-50
µM | In
vitro | (12) |
| | Inhibit EBV-EA
activation induced by TPA | Raji cells | - | In
vitro | (17) |
| | Inhibit the biofilm
formation of E. coli | E. coli | 32-256
µg/ml | In
vitro | (70) |
| Sedative-hypnotic
effect | Suppress locomotion
activity, prolong sleeping time, and enhance hypnotic effect in
pentobarbital-treated mice via chloride channel activation and
GABA- ergic mechanisms | ICR mice | 1-5 mg/kg | In vivo | (13) |
| | Increase total
sleep time and non-rapid eye movement sleep and reduce numbers of
sleep/wake cycles | SD rats | 5 mg/kg | In vivo | (72) |
| Anti-ischemia/
reperfusion injury | Increase cerebral
blood flow, reduce infarct volume and brain water content and
decrease neuronal damage and neuronal apoptosis via upregulation of
p-PTEN, p-PDK1, p-Akt and p-BAD, and downregulation of cleaved
caspase protein expression | SD rats | 12.5-100 mg/kg | In vivo | (14) |
| | Exhibit protective
effect on ischemia-reperfusion induced acute kidney injury through
inhibition of ferroptosis, activation of NRF2, and upregulation of
the expression of the downstream ferroptosis related proteins,
GPX4, SLC7A11 and HO1 | C57BL/6 mice | 5-20 mg/kg | In vivo | (74) |
| Other
pharmacological effects | Suppress
5-HT-stimulated inward current and inhibit I5-HT in Xenopus oocytes
expressing human 5-HT3A receptor with an IC50 value of
5.5±0.6 µM | Xenopus
oocytes | 0.1-100
µM | In
vitro | (75) |
| | Inhibit
IACh in oocytes expressing nicotinic type α3β4
acetylcholine channel receptors with an IC50 value of
24.9 µM, via a concentration dependent and reversible
manner | Xenopus
oocytes | 3-300
µM | In
vitro | (76) |
| | Decrease allograft
rejection, protect PBLs from apoptosis involving stabilization of
the mitochondrial transmembrane potential, and reduce the
percentage of CD8+ lymphocyte | SD rats | 1-10 mg/kg | In vivo | (77) |
| | Induce autophagy
via upregulation of the LC3-II, Beclin 1 and Atg7 expression
levels, and negative modulation of IGF-1 signaling pathway | WI-38 cells | 1-4 µM | In
vitro | (78) |
| | Reduce the
cytotoxicity of root canal sealers | L929 cells | 300 mg/ml | In
vitro | (79) |
| | Maintain the
physiochemical properties of AH Plus sealer, reduce the flow, film
thickness and setting time of AH Plus and improve the sealing
ability of the modified sealer with time | Ah plus | 0.5% | In
vitro | (80) |
| | Inhibit KLK5
protease activity with an IC50 value of 5.9
µM | Human kallikrein
5 | 1-100
µM | In
vitro | (81) |
| | Decrease free fatty
acid-induced increase in intracellular triglyceride levels and
induce the phosphorylation of AMPK | Hepatoma hepg2
cells | 0.63-1.25
µM | In
vitro | (82) |
| | Improve the
abnormal metabolism, increase the potential of GV oocytes, reduce
the number of abnormal MII oocytes and damaged embryos,
downregulate the expression ofovarian- related genes in ovarian
tissue and pro- inflammatory cytokines in adipose tissue | ICR mice | 50 mg/kg | In vivo | (83) |
| | Reverse right
ventricular hypertrophy and pulmonary vascular remodeling, suppress
proliferation and induce apoptosis in hypoxia-induced pulmonary
artery smooth muscle cells, downregulate the peroxy- related factor
expression | SD rats | 5 mg/kg | In vivo | (84) |
| | Demonstrates
improvements in weight and kidney damage, and lower fasting blood
glucose, Scr, BUN, U-Pro, p-AKT, p-PI3K levels and higher SOD
activity | C57BL/6J mice | 50 mg/kg | In vivo | (85) |
| | PA alone or as an
adjuvant therapy with losartan lower serum BNP and improve systolic
function and cardiac fiber diameter via suppressing miR-24 and
preserving cardiac junctophilin-2 | Albino rats | 10 mg/kg | In vivo | (86) |
| | Ameliorates
doxorubicin-induced renal injury via regulation of serum
cystatin-C, and urine albumin/creatinine ratio, renal content of
podocin and klotho, TNF-α, IL-6 and IL-1β | Wistar albino
rats | 10 mg/kg | In vivo | (66) |
Notably, PA has been demonstrated to be a novel
retinoid X receptor (RXR)-specific agonist that induces the
differentiation of leukemia HL-60 cells (34). Moreover, it displays
antiproliferative activity against SK-BR-3 cells by specifically
activating pyruvate kinase muscle isozyme M2 (PKM2), inhibiting
hexokinase 2 (HK2), and decreasing glucose uptake and lactate
production (40). PA can inhibit
the proliferation of several cancer cell lines by downregulating
the expression of CDK1, CDK2, CDK4 and cyclin E to block cell cycle
arrest, including in MDA-MB-231, SGC-7901 and MKN-49P cells
(41,44,45).
Apoptosis also plays an important role in PA's cytotoxicity. PA
induces apoptosis in various cancer cells, including bladder EJ
cells, breast SK-BR-3 cells, pancreatic PANC-1 cells, MIA paca-2
cells and prostate DU145 cells, through the generation of reactive
oxygen species (ROS) and endoplasmic reticulum (ER) stress,
upregulation of cleaved caspase-3,-8 and -9, cleaved poly
(ADP-ribose) polymerases, Bax and Bad, phosphorylated (p)-ataxia
telangiectasia mutated protein, p-ataxia telangiectasia-mutated and
Rad3-related kinase, p-checkpoint kinase 1, p-histone H2A.X,
X-box-binding protein-1s, activating transcription factor 4 (ATF4),
heat shock protein 70, C/EBP homologous protein and p-eukaryotic
initiation factor-2α and downregulation of Bcl-2 and B-cell
lymphoma-extra large (36,37,38,44,46).
Studies on animal models have further demonstrated
the in vivo anticancer activities of PA. For example,
Kaminaga et al (47)
revealed that PA suppresses the promotion of skin tumor formation
by 12-O-tetradecanoylphorbol-13-acetate (TPA) in
7,12-dimethylbenz[a]anthracene-treated mice. Ma et al
(43) demonstrated that PA
significantly inhibits the growth of NCI-H23 xenograft tumors by
inhibiting cell proliferation and inducing apoptosis without
causing toxicity. Sun and Xia (44) reported that PA significantly
suppresses the growth of SGC-7901 tumors in nude mice. In addition,
PA pretreatment before animal xenograft model construction resulted
in a significant decrease in tumor volume and weight (45). In addition, PA pretreatment
increased xenograft survival and median times in vivo. This
suggested that cancer cells pretreated with PA possessed weaker
tumorigenicity and lethality. In a study by Cheng et al
(46), PA was demonstrated to
significantly inhibit MIA PaCa-2 tumor growth via the induction of
apoptosis and the expression of ER stress-related proteins in tumor
tissues; in addition, no major toxicity is observed in animals
treated with 25 mg/kg PA. However, some toxic effects have been
observed in mice treated with 50 mg/kg PA, including obvious
pathological changes in the liver, kidney and spleen. Moreover, PA
also demonstrates a sensitization effect. For example, Shan et
al (48) reported that PA has
no cytotoxicity at a concentration of 12.5 µg/ml, but does
significantly enhance vincristine-induced cytotoxicity in
drug-resistant KBV200 cells. PA was revealed to sensitize SGC-7901
and MKN-49P cells to radiation therapy by upregulating Bax under
hypoxic conditions in vitro and in vivo (49).
Anti-invasion effect
In addition to its cytotoxic effect, PA also has an
anti-invasion effect (Table I). It
was reported to inhibit the invasion and adhesion ability of GBC-SD
cells by affecting the ERK and AKT signaling pathways (50), HO-8910 cells by decreasing
β-catenin and COX-2 expression and increasing E-cadherin expression
(51), MDA-MB-231 cells via
suppression of NF-κB-dependent MMP-9 expression and phosphorylation
of membrane-associated phosphatidylinositol transfer protein 3
(52,53), and BxPc-3 cells via downregulation
of MMP-7 expression (54).
Anti-inflammatory effect
Inflammation underlies numerous physiological and
pathological processes involved in numerous diseases such as
cancer, obesity and cardiovascular diseases (55). A number of studies have reported
that PA has anti-inflammatory effects, and its mechanisms of action
have been partially identified (Table
I). Cuéllar et al (56)
demonstrated that PA exhibits inhibitory activity against
phospholipase A2 in vitro. PA has been observed to inhibit
leukotriene B4 release, which is involved in chronic inflammation
of skin pathologies (57). In
addition, PA reduces lipopolysaccharide (LPS)-induced apoptosis,
attenuates LPS-induced increases in mRNA expression levels of IL-1,
IL-6 and TNF-α and inhibits LPS-induced apoptosis via the ERK1/2
and p38 pathways in H9c2 cells (58). Moreover, PA has been reported to
inhibit inflammation in oral cells. For example, Kim et al
(26) demonstrated that PA
restores resin sealer AH Plus-damaged cell viability and alkaline
phosphatase activity, suppresses the secretion of nitric oxide,
TNF-α and IL-1β and reduces ROS formation and NF-κB translocation
in MC-3T3 E1 cells. In addition, this study demonstrated that PA
inhibits inflammation and induces odontoblast differentiation by
increasing heme oxygenase 1 (HO-1) expression, suppressing NF-κB
translocation and inducing nuclear factor erythroid 2-related
factor 2 (Nrf2) translocation in human dental pulp cells (27). Furthermore, PA has been observed to
inhibit TPA-induced mouse ear edema and phospholipase A2-induced
mouse paw edema in vivo (59-61).
Feng et al (62) revealed
that, in animal models, PA reduces intravesical IL-1, IL-6 and
lactate dehydrogenase levels, downregulates TNF-α and upregulates
TP53 proteins in bladder samples. In cecal ligation and puncture
(CLP)-induced septic rats, PA increases the survival of rats and
attenuates CLP-induced acute lung injury by downregulating the
serum levels of TNF-α, IL-1β and IL-6, decreasing malondialdehyde
and myeloperoxidase levels and increasing superoxide dismutase
levels (63). Gui et al
(64) reported that PA alleviates
LPS-induced lung injury by relieving LPS-inflammation, such as
TNF-α, IL-6, monocyte chemoattractant protein 1 and IL-1β, reducing
cell numbers in bronchoalveolar lavage fluid, and inhibiting
LPS-induced apoptosis. Additionally, PA ameliorates renal injury
in vivo by inhibiting renal inflammation, reducing the
levels of TNF-α, IL-6 and inducible nitric oxide synthase,
enhancing the expression of Nrf2 and HO-1, and preventing renal
Wnt/β-catenin signaling (65,66).
Antihyperglycemic effect
Previously, several studies reported that PA has a
significant antihyperglycaemic effect (67-69)
(Table I). For example, Huang
et al (67) revealed that
PA induces glucose transporter type 4 (GLUT4) expression,
stimulates GLUT4 redistribution from intracellular vesicles to the
plasma membrane by upregulating the insulin-independent AMPK and
insulin receptor substrate-1-PI3K-AKT pathways and induces
triglyceride accumulation. Similarly, Chen et al (68) revealed that PA increases the
glucose uptake by 50% in 3T3-L1 cells. PA has been further observed
to decrease blood glucose levels in streptozocin-treated mice by
enhancing insulin sensitivity irrespective of peroxisome
proliferator-activated receptor-γ (69).
Antiviral and antibacterial
effects
As presented in Table
I, PA displays inhibitory effects on the SARS-CoV-2 3CL
hydrolytic enzyme in vitro, with an IC50 value of
18.607 µM (12). Akihisa
et al (17) demonstrated
that PA exhibits antiviral activity against Epstein-Barr virus
early antigen-induced TPA in Raji cells, with an IC50
value of 286 mol ratio/32 pmol TPA. In addition, PA at
concentrations ranging from 32 to 256 µg/ml demonstrate
antibacterial activity against Escherichia coli by
inhibiting biofilm formation (70).
Sedative-hypnotic effect
Poria cocos is used in traditional Chinese
medicine to treat various sleep disorders, such as insomnia
(71). As presented in Table I, PA at a dose of 5 mg/kg
suppresses locomotor activity, prolongs sleep time and enhances
hypnotic effects in pentobarbital-treated mice via chloride channel
activation and GABA-ergic mechanisms (13). Their subsequent study further
indicated that PA increases total sleep time and non-rapid eye
movement sleep, and reduces the number of sleep/wake cycles and
wakefulness (72). To the best of
our knowledge, no lanostane-type triterpenoids have been reported
to show sedative-hypnotic effects, except for PA.
Anti-ischemia/reperfusion injury
Ischemia/reperfusion injury is a serious clinical
problem that causes several diseases, including myocardial
hibernation and cerebral dysfunction (73). Pang et al (14) reported that PA exhibits
neuroprotective effects on brain ischemia/reperfusion injury in
vivo by increasing cerebral blood flow, reducing infarct volume
and brain water content and decreasing neuronal damage and
apoptosis. Jiang et al (74) revealed that PA exhibits a
dose-dependent protective effect on ischemia-reperfusion-induced
acute kidney injury in vivo through the inhibition of
ferroptosis in the kidneys, activation of Nrf2 and upregulation of
the expression of the downstream ferroptosis-related proteins,
glutathione peroxidase 4, solute carrier family 7 member 11 and
HO1. These studies indicate that PA has the potential to treat
ischemia/reperfusion injury (Table
I).
Other pharmacological effects
As presented in Table
I, PA has been revealed to have several other pharmacological
effects. Specifically, it suppresses 5-hydroxytryptamine
(5-HT)-stimulated inward currents, and inhibits 5-HT-stimulated
inward current in Xenopus oocytes expressing the human
5-HT3A receptor. It also inhibits the acetylcholine-induced inward
current in oocytes expressing nicotinic type α3β4 acetylcholine
channel receptors (75,76). PA decreases allograft rejection in
rats, protects peripheral blood lymphocytes from apoptosis by
stabilizing the mitochondrial transmembrane potential and reduces
the percentage of CD8+ lymphocytes (77). Lee and Kim (78) reported that PA could induce WI-38
cell autophagy to delay the aging process. Furthermore, PA can
reduce the cytotoxicity of root canal sealers (79), maintain the physiochemical
properties of AH Plus sealer, reduce the flow, film thickness and
setting time of AH Plus, and improve the sealing ability of the
modified sealer over time (80).
PA has been revealed to affect the skin barrier function via
inhibition of KLK5 protease activity (81) and to ameliorate hepatic steatosis
by decreasing the free fatty acid-induced increase in intracellular
triglyceride levels (82). Fu
et al (83) reported that
PA can protect oocytes by improving abnormal metabolism, increasing
the potential of GV oocytes and reducing the number of abnormal
metaphase II oocytes and damaged embryos in mice with polycystic
ovary syndrome. Recently, He et al (84) demonstrated that PA significantly
reverses right ventricular hypertrophy and pulmonary vascular
remodeling, suppresses proliferation, induces apoptosis in
hypoxia-induced pulmonary artery smooth muscle cells, downregulates
the expression of peroxy-related factors and upregulates the
expression levels of antioxidant-related factors. PA can protect
against kidney injury in mice with diabetic nephropathy by
inhibiting the PI3K/AKT pathway (85). PA alone or as adjuvant therapy with
losartan can lower serum B-type natriuretic peptide and improve
systolic function and cardiac fiber diameter to attenuate
doxorubicin-induced heart failure in vivo (86). Moreover, PA ameliorates
doxorubicin-induced renal injury by regulating of serum cystatin-C,
urine albumin/creatinine ratio, renal podocin and klotho content,
TNF-α, IL-6 and IL-1β (66).
3. Pharmacokinetics
Several analytical methods have been reported for
the determination of PA in vitro and in vivo. The
pharmacokinetic properties of PA, including its absorption,
distribution, metabolism and excretion are summarized in Table II. Using the LC-ESI-MSn method,
Ling et al (87) reported
that seven out of 34 compounds in the extract of Poria cocos
can be detected in rat urine after oral administration, whereas
only PA can be detected in rat urine and plasma. In vitro
determination of PA, using Caco-2 cell monolayers as an intestinal
epithelial cell model with reversed-phase-high performance liquid
chromatography, revealed that PA is transported through the Caco-2
cell monolayer in a concentration-dependent manner, and that the
Papp values of PA are (9.50±2.20)10-7 cm/sec from the
apical (AP) side to the basolateral (BL) side, and
(11.30±5.90)10-7 cm/sec from the BL side to the AP side,
respectively.
 | Table IIPharmacokinetic information of
PA. |
Table II
Pharmacokinetic information of
PA.
| Model | Dose | Administration
method | Quantitative
method | Details | (Refs.) |
|---|
| SD rats | 200 mg/kg (Poria
cocos extract) | Oral gavage | LC-ESI-MSn
method | PA is the only one
detected both in rat urine and plasma | (87) |
| Caco-2 cells | 10-50
µM | Mixed system | RP-HPLC method | PA is transported
through the Caco-2 cell monolayer in a concentration-dependent
manner and the Papp values of PA are (9.50±2.20)10-7
cm/sec from AP side to BL side, and (11.30±5.90)10-7
cm/sec from BL side to AP side, respectively | (88) |
| SD rats | 30 mg/kg | Intravenous
administration | HPLC method |
t1/2
=8.79±6.80 h; CL=0.53±0.28 l/h; AUC0-∞=18.90±9.39
µg h/ml; MRT0-∞=12.58±9.95 h | (89) |
| Wistar rats | 10 mg/kg | Oral
administration | LC-MS/MS
method |
t1/2β=4.96±1.33
h; AUC0-∞=1466.9±361.7 ng h/ml; CL=6.82±1.73 l/h | (90) |
| SD rats | 12.3 mg/kg | Oral
administration | UPLC-Q- Orbi-trap
MS method |
t1/2z=11.51±9.90
h; AUC0-∞=336.29±161.99 ng h/ml; CL=45.07±73.64 l/h | (91) |
| Human liver
microsomes | 100 µM | Mixed system | HPLC method | Inhibit CYP3A4,
2E1, and 2C9 in a concentration-dependent manner, with
IC50 values of 15.04, 27.95 and 24.22 µM,
respectively | (92) |
| SD rats | 5 mg/kg | Oral
administration | LC-MS/MS
method | Increase AUC and
t1/2, decrease CL, enhance
metabolic stability, and inhibit transport of bavachin via the
inhibition of CYP2C9 and P-gp | (93) |
In addition to the passive diffusion of PA, ATP
partially participates in its transport (88). After sublingual vein injection of
PA at a dose of 30 mg/kg, the pharmacokinetic parameters in rat
plasma were obtained using HPLC with half-life
(t1/2) at 8.79±6.80 h, clearance
(CL) at 0.53±0.28 l/h, area under the curve (AUC)0-∞ at
18.90±9.39 µg h/ml and mean residence time0-∞ at
12.58±9.95 h (89). Following oral
administration of PA (10 mg/kg), the main pharmacokinetic
parameters in rat plasma using liquid chromatography tandem mass
spectrometry (MS) were elimination half-life at 4.96±1.33 h,
AUC0-∞ at 1466.9±361.7 ng h/ml and CL at 6.82±1.73 l/h
(90). Determination of PA (12.3
mg/kg) in rats after oral administration using UPLC-Q-Orbi-trap MS
demonstrated that the pharmacokinetic parameters were terminal
half-life at 11.51±9.90 h, AUC0-∞ at 336.29±161.99 ng
h/ml and CL at 45.07±73.64 L/h. PA is mainly distributed in the
intestine and stomach and is considered to be further converted
into other molecules in vivo (91). In addition, treatment of human
liver microsomes with PA (100 µM) demonstrated that PA
inhibits cytochrome P450 (CYP)3A4, 2E1 and 2C9, with
IC50 values of 15.04, 27.95 and 24.22 µM,
respectively, indicating potential drug-drug interactions (92). Moreover, after oral administration
of PA (5 mg/kg) in rats, Zhang et al (93) indicated that PA increases the AUC
and t1/2, decreases CL, enhances
metabolic stability and further inhibits transport of natural
flavonoid bavachin via regulation of CYP2C9 and P-glycoprotein.
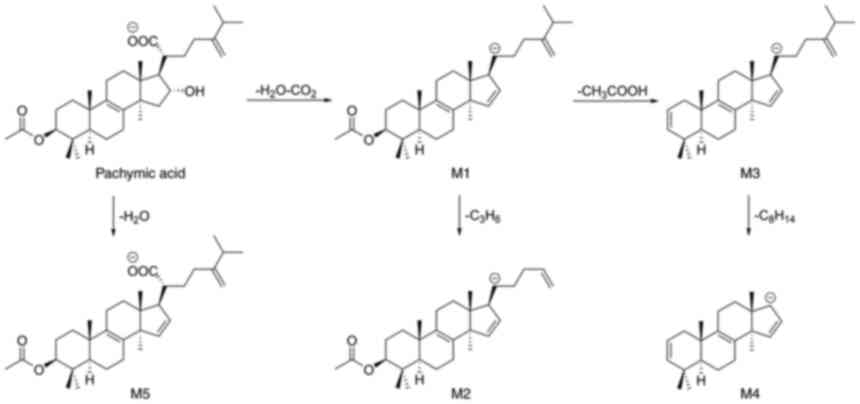
As presented in Fig.
3, three metabolic pathways are proposed for PA (87). It may undergo loss of
H2O + CO2 to generate fragment M1, which is
converted to fragment M2 by the loss of C3H6,
and forms fragment M3 via the loss of CH3COOH (87). A very low-abundance ion had been
detected in the fragment M4 spectra via the loss of
C8H14 from M3(87). Finally, PA is converted to fragment
M5 via dehydration (87).
4. Conclusions
PA can be obtained from a number of natural sources,
and has attracted great attention owing to its various
pharmacological activities in the potential treatment of several
diseases. In addition, progress has been made in exploring the
pharmacological profiles and underlying molecular mechanisms of PA.
In particular, PA has been demonstrated to be a novel RXR-specific
agonist and can induce the differentiation of leukaemia HL-60 cells
(34). In addition, PA has been
demonstrated to be an activator of PKM2 and an inhibitor of HK2,
and can inhibit the proliferation of breast cancer SK-BR-3 cells
(40). It demonstrates significant
anti-inflammatory effects by inhibiting the activity of
PLA2(56). To the best of our
knowledge, no lanostane-type triterpenoids have been reported to
show sedative-hypnotic effects, except PA (72).
Although considerable progress has been made in
previous years regarding PA, tremendous challenges still lie ahead
owing to the shortcomings of its low content in nature, complex
structure, physical and chemical properties and pharmacokinetic
profiles. Its low content in nature hinders further investigation
and clinical applications. For example, the percentage of PA in
Poria cocos crude extract was 0.053‰ (69). The bioavailability of PA is
relatively low due to its poor water solubility after oral
administration. Regarding intravenous administration, the
dissolution of PA is complex (89). Mixed solubilizers, including DMSO,
PEG-400 and 1, 2-propylene glycol are used to solubilize PA in
physiological saline, which may increase the risk of side effects
and adverse reactions (89).
A limited amount of research on the pharmacological
activity, underlying molecular mechanisms and other new biological
effects of PA warrants further investigation (8). Investigators use different methods
including experimental animals (in vivo), tissue and cell
cultures (in vitro) in order to investigate novel therapies
of human diseases (94). The
mentioned procedures have their own advantages and disadvantages.
In vitro models are used in biomedical fields for the
advantages of low cost, efficiency and ease of quantification
(94). The disadvantage of in
vitro methods is they are usually conducted on cell lines.
Although animal models (in vivo) provide some drawbacks such
as high cost, inefficiency and a difference in biokinetics
parameters in comparison with humans, they are more credible
compared with in vitro tests (95). The majority of studies are focused
on in vitro experiments (Table
I); hence, it is necessary to validate the in vivo
effectiveness and efficacy of PA, such as its anticancer and
anti-inflammatory effects in different animal models. In addition
to the above challenges, another critical concern is the safety
assessment of PA (46). The main
natural source of PA is Poria cocos, which is homologous to
medicine and food (22). Evidence
suggests that PA may be toxic in vivo (46). However, at present, the toxicity
and relationship between dose and toxicity remains unaddressed.
To address these challenges, five strategies and
suggestions aimed at enhancing the development and clinical
application of PA are proposed in the present review. First, it is
necessary to improve the efficiency of PA separation or efficiently
prepare PA via chemistry and biocatalytic technologies. Second, the
adoption of appropriate pharmaceutical or chemical methods would
improve bioavailability. For example, some suitable formulation
technologies (solid dispersion and micronization), chemical
modifications with water-solubilizing groups, and PA derivatives
designed as prodrugs or prepared in the form of sodium salts could
be adopted to improve solubility and bioavailability. Third,
regarding the stage of current research on the pharmacology and
molecular mechanism of PA, further investigation of the in
vivo effectiveness and efficiency of PA should be performed.
Fourth, special pharmacological profiles of PA, such as its
sedative-hypnotic effect, should be given more attention, as no
other lanostane-type triterpenoids have been reported to exhibit a
sedative-hypnotic effect (13,72).
Fifth, novel PA derivatives library for exploring more promising
candidates with higher pharmacological activities and improved
drug-like properties should be performed. PA has a 33-carbon
skeleton with five available sites at the C-3, C-8, C-16, C-21, and
C-24 positions for modification (Fig.
1), which might promote the synthesis of novel molecules with
higher potency and selectivity, fewer side effects and gradual
expansion of the scope of patent protection. At present, to the
best of our knowledge, only one study has reported the synthesis of
novel PA derivatives with in vitro pharmacological
evaluation (96). Finally, more
attention should be paid to investigating the toxicity and
underlying mechanism of PA in animals, and drug-drug interactions,
to elucidate the relationship between dosage and toxic effects and
decrease or avoid side effects. Although the awareness of PA has
grown in recent years, it is necessary to further investigate its
safety, efficacy, mechanism and pharmacokinetics.
Overall, the present review focused on
comprehensive research on the biological properties and therapeutic
potential of PA, and will be beneficial for the development and
utilization of PA in the future.
Acknowledgements
Not applicable.
Funding
Funding: This work was financially supported by the National
Natural Science Foundation of China (grant no. 81860622),
Department of Science and Technology of Zunyi City [grant no.
(2020)293] and Education and Teaching Reform Program of Zunyi
Medical University (grant no. ZYK38).
Availability of data and materials
Not applicable.
Authors' contributions
CW, HW, XS and JW carried out the literature
search, summarized data and wrote the paper. GB, YX and LZ reviewed
and edited the manuscript and analyzed the manuscript contents. QY
contributed to manuscript reviewing and design of tables and
figures. LZ and ZB revised the manuscript. All authors read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li J, Wang Y, Xue J, Wang P and Shi S:
Dietary exposure risk assessment of flonicamid and its effect on
constituents after application in lonicerae japonicae flos. Chem
Pharm Bull (Tokyo). 66:608–611. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shingu T, Tai T and Akahori A: A lanostane
triterpenoid from Poria cocos. Phytochemistry. 31:2548–2549.
1992.
|
|
3
|
Zheng Y and Yang XW: Two new lanostane
triterpenoids from Poria cocos. J Asian Nat Prod Res.
10:323–328. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dong HJ, Xue ZZ, Geng YL, Wang X and Yang
B: Lanostane triterpenes isolated from epidermis of Poria
cocos. Phytochem Lett. 22:102–106. 2017.
|
|
5
|
Ríos JL: Chemical constituents and
pharmacological properties of Poria cocos. Planta Med.
77:681–691. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao YY, Feng YL, Du X, Xi ZH, Cheng XL
and Wei F: Diuretic activity of the ethanol and aqueous extracts of
the surface layer of Poria cocos in rat. J Ethnopharmacol.
144:775–778. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nie A, Chao Y, Zhang X, Jia W, Zhou Z and
Zhu C: Phytochemistry and pharmacological activities of
WolfiPoria cocos (FA Wolf) Ryvarden & Gilb. Front
Pharmacol. 11(505249)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu J, Tian J, Zhou L, Meng L, Chen S, Ma
C, Wang J, Liu Z, Li C and Kang W: Phytochemistry and Biological
Activities of Poria. J Chem. 2021(6659775)2021.
|
|
9
|
Lai KH, Lu MC, Du YC, El-Shazly M, Wu TY,
Hsu YM, Henz A, Yang JC, Backlund A, Chang FR and Wu YC: Cytotoxic
Lanostanoids from Poria cocos. J Nat Prod. 79:2805–2813.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Taofiq O, Martins A, Barreiro MF and
Ferreira ICFR: Anti-inflammatory potential of mushroom extracts and
isolated metabolites. Trends Food Sci Tech. 50:193–210. 2016.
|
|
11
|
Tang X, Chen J and Shen X: The signaling
pathway for regulation of glucose transporter 4 and its application
in drug devel-opment. Sci Sin Chim. 42:1760–1773. 2012.
|
|
12
|
Wu Z, Chen X, Ni W, Zhou D, Chai S, Ye W,
Zhang Z, Guo Y, Ren L and Zeng Y: The inhibition of Mpro, the
primary protease of COVID-19, by Poria cocos and its active
compounds: A network pharmacology and molecular docking study. RSC
Adv. 11:11821–11843. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shah VK, Choi JJ, Han JY, Lee MK, Hong JT
and Oh KW: Pachymic acid enhances pentobarbital-induced sleeping
be-haviors via GABAA-ergic systems in mice. Biomol Ther (Seoul).
22:314–320. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pang Y, Zhu S and Pei H: Pachymic acid
protects against cerebral ischemia/reperfusion injury by the
PI3K/Akt signaling pathway. Metab Brain Dis. 35:673–680.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tai T, Shingu T, Kikuchi T, Tezuka Y and
Akahori A: Isolation of lanostane-type triterpene acids having an
acetoxyl group from sclerotia of Poria cocos.
Phytochemistry. 40:225–231. 1995.
|
|
16
|
Li G, Xu ML, Lee CS, Woo MH, Chang HW and
Son JK: Cytotoxicity and DNA topoisomerases inhibitory activity of
constituents from the sclerotium of Poria cocos. Arch Pharm
Res. 27:829–833. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Akihisa T, Nakamura Y, Tokuda H, Uchiyama
E, Suzuki T, Kimura Y, Uchikura K and Nishino H: Triterpene acids
from Poria cocos and their anti-tumor-promoting effects. J
Nat Prod. 70:948–953. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou L, Zhang Y, Gapter LA, Ling H,
Agarwal R and Ng KY: Cytotoxic and anti-oxidant activities of
lanostane-type triterpenes isolated from Poria cocos. Chem
Pharm Bull (Tokyo). 56:1459–1462. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cai TG and Cai Y: Triterpenes from the
fungus Poria cocos and their inhibitory activity on nitric
oxide production in mouse macrophages via blockade of activating
protein-1 pathway. Chem Biodivers. 8:2135–2143. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang H, Shen Y, Chen B, Jia X and Cai B:
RP-HPLC-DAD determination of six triterpenes in a herbal tonic
hoelen. J Liq Chromatogr Relat Technol. 34:1772–1782. 2011.
|
|
21
|
Li S, Wang Z, Gu R, Zhao Y, Huang W, Wang
Z and Xiao W: A new epidioxy-tetracyclic triterpenoid from Poria
cocos Wolf. Nat Prod Res. 30:1712–1717. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li M, Wang GZ, Nie L and Shen JY: Study on
the content comparison of pachymic acid from different medicinal
parts of Poria cocos (Schw.) Wolf. Lishizhen Med Mater Med
Res. 26:2858–2860. 2015.
|
|
23
|
Fu M, Wang L, Wang X, Deng B, Hu X and Zou
J: Determination of the five main terpenoids in different tissues
of WolfiPoria cocos. Molecules. 23(1839)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang PF, Hua T, Wang D, Zhao ZW, Xi GL and
Chen ZF: Phytochemical and chemotaxonomic study of Poria
cocos (Schw.) Wolf. Biochem Syst Ecol. 83:54–56. 2019.
|
|
25
|
Jiang TT, Ding LF, Nie W, Wang LY, Lei T,
Wu XD and Zhao QS: Tetranorlanostane and lanostane triterpenoids
with cytotoxic activity from the epidermis of Poria cocos.
Chem Biodivers. 18(e2100196)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim TG, Lee YH, Lee NH, Bhattarai G, Lee
IK, Yun BS and Yi HK: The antioxidant property of pachymic acid
improves bone disturbance against AH Plus-induced inflammation in
MC-3T3 E1 cells. J Endod. 39:461–466. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee YH, Lee NH, Bhattarai G, Kim GE, Lee
IK, Yun BS, Hwang PH and Yi HK: Anti-inflammatory effect of
pachymic acid promotes odontoblastic differentiation via HO-1 in
dental pulp cells. Oral Dis. 19:193–199. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Keller AC, Maillard MP and Hostettmann K:
Antimicrobial steroids from the fungus Fomitopsis pinicola.
Phytochemistry. 41:1041–1046. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kuo PC, Tai SH, Hung CC, Hwang TL, Kuo LM,
Lam SH, Cheng KC, Kuo DH, Hung HY and Wu TS: Anti-inflammatory
triterpenoids from the fruiting bodies of Fomitopsis
pinicola. Bioorg Chem. 108(104562)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma J, Liu J, Lu CW and Cai DF: Pachymic
acid modified carbon nanoparticles reduced angiogenesis via
inhibition of MMP-3. Int J Clin Exp Pathol. 8:5464–5470.
2015.PubMed/NCBI
|
|
31
|
Zhu W, Liu Y, Tang J, Liu H, Jing N, Li F,
Xu R and Shu S: Functional analysis of sterol O-Acyltransferase
involved in the biosynthetic pathway of pachymic acid in
WolfiPoria cocos. Molecules. 27(143)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cai SY, Lv SW, Wang YH, Guo YY and Li YJ:
Solubility and Apparent Oil/Water Partition Coefficient of
Glycyrrhizin and Pachymic Acid. Inf Tradit Chin Med. 29:118–121.
2012.(In Chinese).
|
|
33
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu H, Wang Y, Zhao J, Jurutka PW, Huang D,
Liu L, Zhang L, Wang S, Chen Y and Cheng S: Triterpenes from
Poria cocos are revealed as potential retinoid X receptor
selective agonists based on cell and in silico evidence. Chem Biol
Drug Des. 95:493–502. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jeong JW, Baek JY, Kim KD, Choi YH and Lee
JD: Induction of apoptosis by pachymic acid in T24 human bladder
cancer cells. J Life Sci. 25:93–100. 2015.
|
|
36
|
Jeong JW, Lee WS, Go SI, Nagappan A, Baek
JY, Lee JD, Park C, Kim GY, Kim HJ, Kim GS, et al: Pachymic acid
induces apoptosis of EJ bladder cancer cells by DR5 up-regulation,
ROS generation, modulation of Bcl-2 and IAP family members.
Phytother Res. 29:1516–1524. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang YH, Zhang Y, Li XY, Feng XD, Jian W
and Li RQ: Antitumor activity of the pachymic acid in
nasopharyngeal carcinoma cells. Ultrastruct Pathol. 41:245–251.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gapter L, Wang Z, Glinski J and Ng KY:
Induction of apoptosis in prostate cancer cells by pachymic acid
from Poria cocos. Biochem Biophys Res Commun. 332:1153–1161.
2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wen H, Wu Z, Hu H, Wu Y, Yang G, Lu J,
Yang G, Guo G and Dong Q: The anti-tumor effect of pachymic acid on
osteosarcoma cells by inducing PTEN and Caspase 3/7-dependent
apoptosis. J Nat Med. 72:57–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Miao G, Han J, Zhang J, Wu Y and Tong G:
Targeting pyruvate kinase M2 and Hexokinase II, pachymic acid
impairs glucose metabolism and induces mitochondrial apoptosis.
Biol Pharm Bull. 42:123–129. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jiang Y and Fan L: Evaluation of
anticancer activities of Poria cocos ethanol extract in
breast cancer: In vivo and in vitro, identification and mechanism.
J Ethnopharmacol. 257(112851)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ling H, Jia X, Zhang Y, Gapter LA, Lim YS,
Agarwal R and Ng KY: Pachymic acid inhibits cell growth and
modulates arachidonic acid metabolism in nonsmall cell lung cancer
A549 cells. Mol Carcinog. 49:271–282. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ma J, Liu J, Lu C and Cai D: Pachymic acid
induces apoptosis via activating ROS-dependent JNK and ER stress
pathways in lung cancer cells. Cancer Cell Int.
15(78)2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sun KX and Xia HW: Pachymic acid inhibits
growth and induces cell cycle arrest and apoptosis in gastric
cancer SGC-7901 cells. Oncol Lett. 16:2517–2524. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lu C, Ma J and Cai D: Pachymic acid
inhibits the tumorigenicity of gastric cancer cells by the
mitochondrial pathway. Anticancer Drugs. 28:170–179.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cheng S, Swanson K, Eliaz I, McClintick
JN, Sandusky GE and Sliva D: Pachymic acid inhibits growth and
induces apoptosis of pancreatic cancer in vitro and in vivo by
targeting ER stress. PLoS One. 10(e0122270)2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kaminaga T, Yasukawa K, Kanno H, Tai T,
Nunoura Y and Takido M: Inhibitory effects of lanostane-type
triterpene acids, the components of Poria cocos, on tumor
promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage
carcinogenesis in mouse skin. Oncology. 53:382–385. 1996.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shan H, Qinglin Z, Fengjun X, Yuxin L,
Xiaochen C and Yuan H: Reversal of multidrug resistance of KBV200
cells by triterpenoids isolated from Poria cocos. Planta
Med. 78:428–433. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lu C, Cai D and Ma J: Pachymic acid
sensitizes gastric cancer cells to radiation therapy by
upregulating bax through hypoxia. Am J Chin Med. 46:875–890.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chen Y, Lian P, Liu Y and Xu K: Pachymic
acid inhibits tumorigenesis in gallbladder carcinoma cells. Int J
Clin Exp Med. 8:17781–17788. 2015.PubMed/NCBI
|
|
51
|
Gao AH, Zhang L, Chen X, Chen Y, Xu ZZ,
Liu YN and Zhang H: Inhibition of ovarian cancer proliferation and
invasion by pachymic acid. Int J Clin Exp Pathol. 8:2235–2241.
2015.PubMed/NCBI
|
|
52
|
Ling H, Zhang Y, Ng KY and Chew EH:
Pachymic acid impairs breast cancer cell invasion by suppressing
nuclear factor-κB-dependent matrix metalloproteinase-9 expression.
Breast Cancer Res Treat. 126:609–620. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hong R, Shen MH, Xie XH and Ruan SM:
Inhibition of breast cancer metastasis via PITPNM3 by pachymic
acid. Asian Pac J Cancer Prev. 13:1877–1880. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cheng S, Eliaz I, Lin J, Thyagarajan-Sahu
A and Sliva D: Triterpenes from Poria cocos suppress growth
and invasiveness of pancreatic cancer cells through the
downregulation of MMP-7. Int J Oncol. 42:1869–1874. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cuéllar MJ, Giner RM, Recio MC, Just MJ,
Máñez S and Ríos JL: Two fungal lanostane derivatives as
phospholipase A2 inhibitors. J Nat Prod. 59:977–979.
1996.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Prieto JM, Recio MC, Giner RM, Máñez S,
Giner-Larza EM and Ríos JL: Influence of traditional Chinese
anti-inflammatory medicinal plants on leukocyte and platelet
functions. J Pharm Pharmacol. 55:1275–1282. 2003.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Li FF, Yuan Y, Liu Y, Wu QQ, Jiao R, Yang
Z, Zhou MQ and Tang QZ: Pachymic acid protects H9c2 cardiomyocytes
from lipopolysaccharide-induced inflammation and apoptosis by
inhibiting the extracellular signal-regulated kinase 1/2 and p38
pathways. Mol Med Rep. 12:2807–2813. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Cuélla MJ, Giner RM, Recio MC, Just MJ,
Mañez S and Rios JL: Effect of the basidiomycete Poria cocos
on experimental dermatitis and other inflammatory conditions. Chem
Pharm Bull (Tokyo). 45:492–494. 1997.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yasukawa K, Kaminaga T, Kitanaka S, Tai T,
Nunoura Y, Natori S and Takido M:
3beta-p-Hydroxybenzoyldehydrotumulosic acid from Poria
cocos, and its anti-inflammatory effect. Phytochemistry.
48:1357–1360. 1998.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Giner EM, Máñez S, Recio MC, Giner RM,
Cerdá-Nicolás M and Ríos JL: In vivo studies on the
anti-inflammatory activity of pachymic and dehydrotumulosic acids.
Planta Med. 66:221–227. 2000.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Feng Z, Shi H, Liang B, Ge T, Cai M, Liu
F, Huang K, Wen J, Chen Q and Ge B: Bioinformatics and experimental
findings reveal the therapeutic actions and targets of pachymic
acid against cystitis glandularis. Biofactors. 47:665–673.
2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li JY, Wu HX and Yang G: Pachymic acid
improves survival and attenuates acute lung injury in septic rats
induced by cecal ligation and puncture. Eur Rev Med Pharmacol Sci.
21:1904–1910. 2017.PubMed/NCBI
|
|
64
|
Gui Y, Sun L, Liu R and Luo J: Pachymic
acid inhibits inflammation and cell apoptosis in lipopolysaccharide
(LPS)-induced rat model with pneumonia by regulating NF-κB and MAPK
pathways. Allergol Immunopathol (Madr). 49:87–93. 2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Cai ZY, Sheng ZX and Yao H: Pachymic acid
ameliorates sepsis-induced acute kidney injury by suppressing
inflammation and activating the Nrf2/HO-1 pathway in rats. Eur Rev
Med Pharmacol Sci. 21:1924–1931. 2017.PubMed/NCBI
|
|
66
|
Younis NN, Mohamed HE, Shaheen MA,
Abdelghafour AM and Hammad SK: Potential therapeutic efficacy of
pachymic acid in chronic kidney disease induced in rats: Role of
Wnt/β-catenin/renin-angiotensin axis. J Pharm Pharmacol.
74:112–123. 2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Huang YC, Chang WL, Huang SF, Lin CY, Lin
HC and Chang TC: Pachymic acid stimulates glucose uptake through
enhanced GLUT4 expression and translocation. Eur J Pharmacol.
648:39–49. 2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Chen B, Zhang J, Han J, Zhao R, Bao L,
Huang Y and Liu H: Lanostane triterpenoids with
glucose-uptake-stimulatory activity from peels of the cultivated
edible mushroom Wolfi Poria cocos. J Agric Food Chem.
67:7348–7364. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Li TH, Hou CC, Chang CL and Yang WC:
Anti-Hyperglycemic properties of crude extract and triterpenes from
Poria cocos. Evid. Based Complement. Alternat Med.
2011(128402)2011.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yang Z, Du R, Chang A, Zhang J and Li Q:
The in vitro anti-biofilm activity of the etoac extract of Poria
cocos against Escherichia coli. Asian J Pharma Res Deve.
1:152–156. 2013.
|
|
71
|
Singh A and Zhao K: Treatment of insomnia
with traditional Chinese herbal medicine. Int Rev Neurobiol.
135:97–115. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shah VK, Na SS, Chong MS, Woo JH, Kwon YO,
Lee MK and Oh KW: Poria cocos ethanol extract and its active
constituent, pachymic acid, modulate sleep architectures via
activation of GABAA-ergic transmission in rats. J Biomed Res.
16:84–92. 2015.
|
|
73
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Jiang GP, Liao YJ, Huang LL, Zeng XJ and
Liao XH: Effects and molecular mechanism of pachymic acid on
ferroptosis in renal ischemia reperfusion injury. Mol Med Rep.
23(63)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lee JH, Lee YJ, Shin JK, Nam JW, Nah SY,
Kim SH, Jeong JH, Kim Y, Shin M, Hong M, et al: Effects of
triterpenoids from Poria cocos Wolf on the serotonin type 3A
receptor-mediated ion current in Xenopus oocytes. Eur J
Pharmacol. 615:27–32. 2009.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Eom S, Kim YS, Lee SB, Noh S, Yeom HD, Bae
H and Lee JH: Molecular determinants of α3β4 nicotinic
acetylcholine receptors inhibition by triterpenoids. Biol Pharm
Bull. 41:65–72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zhang F, Zhang XF, Wang BC, Liu HY, Li CY,
Liu ZH, Zhang GW, Lü H, Chi C and Wang F: Pachymic acid, a novel
compound for anti-rejection: Effect in rats following cardiac
allograft transplantation. Chin Med J (Engl). 122:2898–2902.
2009.PubMed/NCBI
|
|
78
|
Lee SG and Kim MM: Pachymic acid promotes
induction of autophagy related to IGF-1 signaling pathway in WI-38
cells. Phytomedicine. 36:82–87. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Arun S, Sampath V, Mahalaxmi S and
Rajkumar K: A comparative evaluation of the effect of the addition
of pachymic acid on the cytotoxicity of 4 different root canal
sealers-an in vitro study. J Endod. 43:96–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kamalakannan Preethi O, Sampath V,
Ravikumar N and Mahalaxmi S: Comparative evaluation of
physicochemical properties and apical sealing ability of a resin
sealer modified with Pachymic Acid. Eur Endod J. 5:23–27.
2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Matsubara Y, Matsumoto T, Koseki J, Kaneko
A, Aiba S and Yamasaki K: Inhibition of human kallikrein 5 protease
by triterpenoids from natural sources. Molecules.
22(1829)2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kim JH, Sim HA, Jung DY, Lim EY, Kim YT,
Kim BJ and Jung MH: Poria cocus Wolf extract ameliorates
hepatic steatosis through regulation of lipid metabolism,
inhibition of ER stress, and activation of autophagy via AMPK
activation. Int J Mol Sci. 20(4801)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Fu XP, Xu L, Fu BB, Wei KN, Liu Y, Liao
BQ, He SW, Wang YL, Chen MH, Lin YH, et al: Pachymic acid protects
oocyte by improving the ovarian microenvironment in polycystic
ovary syndrome mice. Biol Reprod. 103:1085–1098. 2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
He Y, Zhong JH, Wei XD, Huang CY, Peng PL,
Yao J, Song XS, Fan WL and Li GC: Pachymic acid ameliorates
pulmonary hypertension by regulating Nrf2-Keap1-ARE pathway. Curr
Med Sci. 42:56–67. 2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Li W, Wang C, Zhang M, Yuan Y, Zhang Z,
Liu X, Zhang F and Wu Y: Pachymic acid protects against kidney
injury in mice with diabetic nephropathy by inhibiting the PI3K/AKT
pathway. Trop J Pharm Res. 20:2539–2544. 2021.
|
|
86
|
Younis NN, Salama A, Shaheen MA and Eissa
RG: Pachymic acid attenuated doxorubicin-induced heart failure by
suppressing miR-24 and preserving cardiac junctophilin-2 in rats.
Int J Mol Sci. 22(10710)2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Ling Y, Chen M, Wang K, Sun Z, Li Z, Wu B
and Huang C: Systematic screening and characterization of the major
bioactive components of Poria cocos and their metabolites in
rats by LC-ESI-MS(n). Biomed Chromatogr. 26:1109–1117.
2012.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Zheng Y and Yang XW: Absorption and
transport of pachymic acid in the human intestinal cell line Caco-2
monolayers. Zhong Xi Yi Jie He Xue Bao. 6:704–710. 2008.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Alatengqimuge Y, Yang XW, Zheng Y, Ma L
and Lu W: LC analysis and pharmacokinetic study of pachymic acid
after intravenous administration to rats. Chroma. 67:807–811.
2008.
|
|
90
|
Wang FY, Lv WS and Han L: Determination
and pharmacokinetic study of pachymic acid by LC-MS/MS. Biol Pharm
Bull. 38:1337–1344. 2015.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Zhang J, Guo H, Yan F, Yuan S, Li S, Zhu
P, Chen W, Peng C and Peng D: An UPLC-Q-Orbitrap method for
pharmacokinetics and tissue distribution of four triterpenoids in
rats after oral administration of Poria cocos ethanol
extracts. J Pharm Biomed Anal. 203(114237)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ding B, Ji X, Sun X, Zhang T and Mu S: In
vitro effect of pachymic acid on the activity of Cytochrome P450
enzymes. Xenobiotica. 50:913–918. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Zhang J, Liu L, Li H and Zhang B:
Pharmacokinetic study on the interaction between pachymic acid and
bavachin and its potential mechanism. Pharm Biol. 59:1256–1259.
2021.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Staton CA, Reed MW and Brown NJ: A
critical analysis of current in vitro and in vivo angiogenesis
assays. Int J Exp Path. 90:195–221. 2009.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Saeidnia S, Manayi A and Abdollahi M: From
in vitro experiments to in vivo and clinical studies; pros and
cons. Curr Drug Discov Technol. 12:218–224. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Wang P, She G, Yang Y, Li Q, Zhang H, Liu
J, Cao Y, Xu X and Lei H: Synthesis and biological evaluation of
new ligustrazine derivatives as anti-tumor agents. Molecules.
17:4972–4985. 2012.PubMed/NCBI View Article : Google Scholar
|