Introduction
Xenotransplantation is one of the most attractive
strategies to overcome the shortage of organ donors.
α1,3-galactosyltransferase gene-deficient-knockout (αGalTKO) pigs
have been produced to provide sufficient protection against hyper
acute rejection, which is mediated by natural antibodies to
xenogeneic antigens and complements (1-4).
The expression of complement regulatory proteins, such as C1
esterase inhibitors, decay accelerating factors, and membrane
cofactor proteins on porcine cells, downregulates
complement-mediated cytotoxicity (5-8).
However, a limited number of studies have been conducted on
cellular xenogeneic rejection (CXR). Histopathological studies have
demonstrated that the mechanisms involved in xenogeneic graft
rejection are significantly different from those associated with
allogeneic graft rejection. In allograft rejections, cytotoxic T
lymphocytes are the main infiltrating cells, while xenografts
mainly induce the infiltration of neutrophils, NK cells, and
macrophages (9-11).
CXR with innate immune cells particularly causes severe rejection
in xenotransplantation. Macrophages are activated under xenogeneic
conditions through both antibody-dependent and -independent
mechanisms. Immunocomplexes of porcine cells with xenogeneic
antigen bind to the Fc gamma receptor on macrophages and activate
macrophages (12,13). Macrophages are also activated by
interaction with neutrophils, NK cells, and Th1 cells in an
antibody-independent manner (14,15).
Furthermore, damage-associated molecular patterns (DAMPs) from dead
porcine cells activate macrophages in an antibody-independent
manner (16). Martin et al
demonstrated that the infiltration of neutrophils and macrophages
in αGalTKO islets was not different from that in wild-type islets
in a dual islet transplantation model, suggesting that
antibody-independent mechanisms are important in CXR (17). Thus, it is important to develop
strategies to suppress the action of innate immune cells, such as
macrophages and neutrophils, for successful clinical application of
xenotransplantation (18-24).
Surfactant protein-A (SP-A) and SP-D are epithelial
cell-derived immune modulators that are C-type collagen-like
lectins. SP-A and SP-D can be detected in the mucosal surfaces of
various organs, and both proteins play important roles in immune
responses (25-29).
While ligation of N-terminal collagen domains with the
calreticulin/CD91 receptor complex induces pro-inflammatory
responses, binding of the carbohydrate recognition domain (CRD) to
signal inhibitory regulatory protein α (SIRPα) prevents
inflammation (30). We have
previously reported the preparation of cDNA for the membrane-type
protein, collectin placenta 1 (CL-P1), with the CRD of SP-D
(CL-SPD) and its transfection into swine endothelial cells (SECs).
The hybrid molecule significantly suppresses macrophage-mediated
cytotoxicity in SECs (31).
Additionally, in vitro studies have indicated that the
suppressive function of CL-SPD in macrophage-mediated cytotoxicity
is more potent than that of CD47. However, the effect of CRD of
SP-A on the xenogeneic condition remains unknown.
This study aimed to examine the suppressive effect
of CRD of SP-A on macrophage-mediated xenogeneic innate immune
responses. Pro-inflammatory cytokines are key factors in innate
immune responses; hence, the activation of pro-inflammatory
cytokines and production of reactive oxygen species (ROS) in
macrophages were the focus of this study.
Materials and methods
Ethical approval
The present study was approved [approval no.
18395(T1)] by the Ethics Committee of Osaka University (Osaka,
Japan). All the participants enrolled in the study provided signed
written informed consent. The participants were over 20-year-old
healthy volunteers, and their clinical data were blinded.
Cells and generation of human
macrophages
A swine endothelial (SEC) line MYP30 was cultured in
Dulbecco's modified Eagle's medium (DMEM, Nacalai Tesque, Kyoto,
Japan) supplemented with 10% heat-inactivated fetal bovine serum
(FBS). THP-1 cells were cultured in Roswell Park Memorial Institute
(RPMI, Nacalai Tesque) 1640 medium containing 10% FBS, and
THP-1-Lucia NF-κB cells were cultured in RPMI-1640 containing 10%
FBS and 25 mM HEPES.
Human peripheral blood mononuclear cells (PBMCs)
were obtained from healthy volunteer donors using differential
density gradient centrifugation (lymphocyte isolation solution;
Nacalai Tesque). PBMCs were incubated in a 6-well plate at 37˚C for
1 h as a plastic adhesion method of isolating human monocytes. To
generate macrophages, human monocytes were cultured in the presence
of 100 ng/ml rM-CSF (Peprotech) for 6 days.
Plasmid construction
cDNA of human SPA-CRD (GenBank accession no.
M68519.1) was replaced with that of membrane-type protein CL-P1
without the cytoplasmic tail but with a flag-tag between the CRD
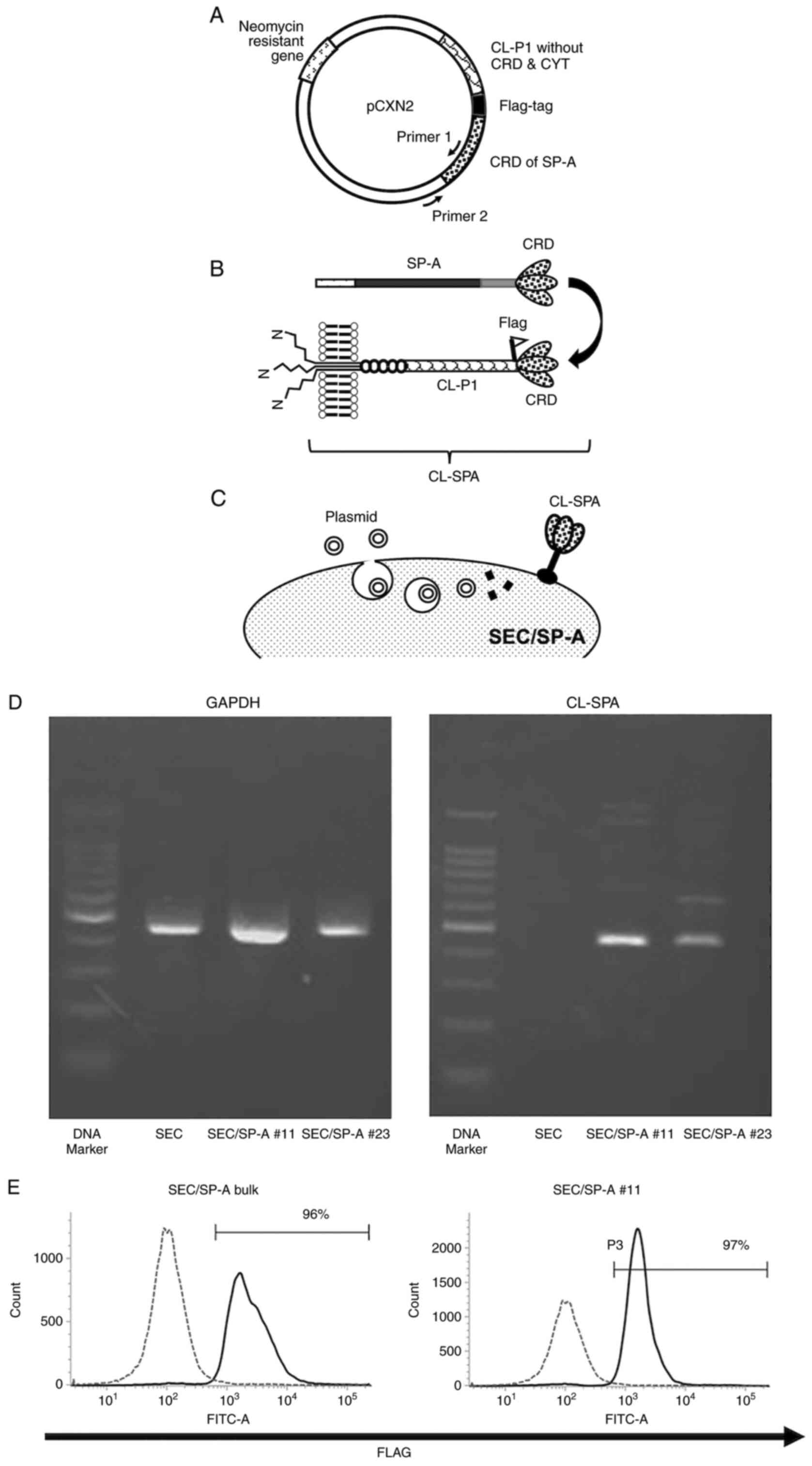
and integrin domain (Fig. 1A). The
cDNA was inserted into the expression vector pCXN2 (chick
beta-actin promoter + CMV enhancer + neomycin resistance), which
was transfected into SECs (Fig.
1B).
Preparation of SEC transfectants via
lipofection
Five micrograms of a plasmid containing SPA-CRD were
mixed with 40 µl Lipofectamine™ LTX with Plus™ Reagent (Thermo
Fisher Scientific, Tokyo, Japan) and incubated with SECs at 37˚C
for 3 h (Fig. 1C). Transfected
cells were selected by culturing in DMEM containing 400 µg/ml G418
(Thermo Fisher Scientific, Inc.). The CL-SPA-transfected SEC
(SEC/SP-A) was either bulk type (SEC/SP-A bulk) or a clonal line
(SEC/SP-A #11 and #23) obtained via limiting dilution. The SEC/SP-A
bulk, #11 or #23 was used for the assay after 2 months of
transfection. SEC without lipofection was used as a control because
we have confirmed that there is no difference between SEC without
lipofection and SEC with lipofection by an empty vector in the
cytotoxicity assay.
Semi-quantitative RT-PCR of SP-A
Total RNA was extracted from SEC and SEC/SP-A #11
and #23 with the TRIZOL reagent (Invitrogen). cDNA was synthesized
from total RNA using Prime Script TM II 1st strand cDNA synthesis
kit (Takara Bio Inc.). The specific PCR products for sus scrofa
GAPDH and human SP-A were amplified with KOD FX Neo (Toyobo) using
Veriti 96 well Thermal Cycler (Applied Biosystems, Waltham, MA).
PCR products were loaded on 1% agarose gel. After electrophoresis,
the agarose gel was visualized under ultraviolet light. The
sequences of the primers were as follows: Sus Scrofa GAPDH F,
5'-ACCACGGTCCATGCCATCACTGC-3'; Sus Scrofa GAPDH R,
5'-TCCACCACCCTGTTGCTGTAGCC-3'; Primer1:
5'-GAGAAGGTGTTCAGCAGCAACG-3', Primer2:
5'-GCCACACCAGCCACCACCTTCTG-3'. The sites of Primer1 and 2 in the
transfecting plasmids were shown in Fig. 1A.
Cytotoxicity assay
Naive SEC and SEC/SP-A were plated at a
concentration of 1.0x104 or 1.3x104
cells/well in a flat-bottom gelatin-coated 96-well plate at 37˚C on
Day 0. On Day 1, THP-1 or THP-1-Lucia cells (5.0x104
cells/well) were added to each well, and the cells were co-cultured
with 200 nM phorbol-12-myristate-13-acetate (PMA). On Day 2, 10 µl
of WST-8 reagent solution was added to each well, and the
absorbance was measured at 450 nm using a microplate reader (Thermo
Fischer Scientific, Inc.).
Phagocytosis assay
Human PBMCs were plated at a concentration of
3x106 cells/well in a flat-bottomed 6-well plate on Day
0 and cultured in the presence of 100 ng/ml rM-CSF for 6 days. SECs
or SEC/SP-As (3x105) were labeled with calcein-AM
(Nakalai Tesque) by incubating with 2 µl/ml calcein-AM at 37˚C for
10 min. Calcein-AM-stained SEC or SEC/SP-A was added to plates, and
the cells were co-cultured for 24 h. The cells were then harvested
and stained with APC-labeled anti-human CD14 antibody (1:40; cat.
no. 982506; BioLegend). The stained cells were analyzed using a
fluorescence-activated cell sorter (FACS) FACSVerse™ flow cytometer
(BD Bioscience) using the BD FAC Suite software (ver. 10.6). The
percentage of phagocytosis was calculated as (CD14+ and
calcein-AM+ cells)/(CD14+ cells) x100(%).
Detection of ROS in macrophages
ROS levels were quantified after staining with
CellROX™ Green Reagent (Life Technologies). CellROX™ reagent was
added to cells at a final concentration of 5 µM, and this was
followed by incubation in the dark at 37˚C for 30 min. The stained
cells were then resuspended in phosphate-buffered saline, and
fluorescence images were evaluated using a FACSVerse™ flow
cytometer (BD Biosciences).
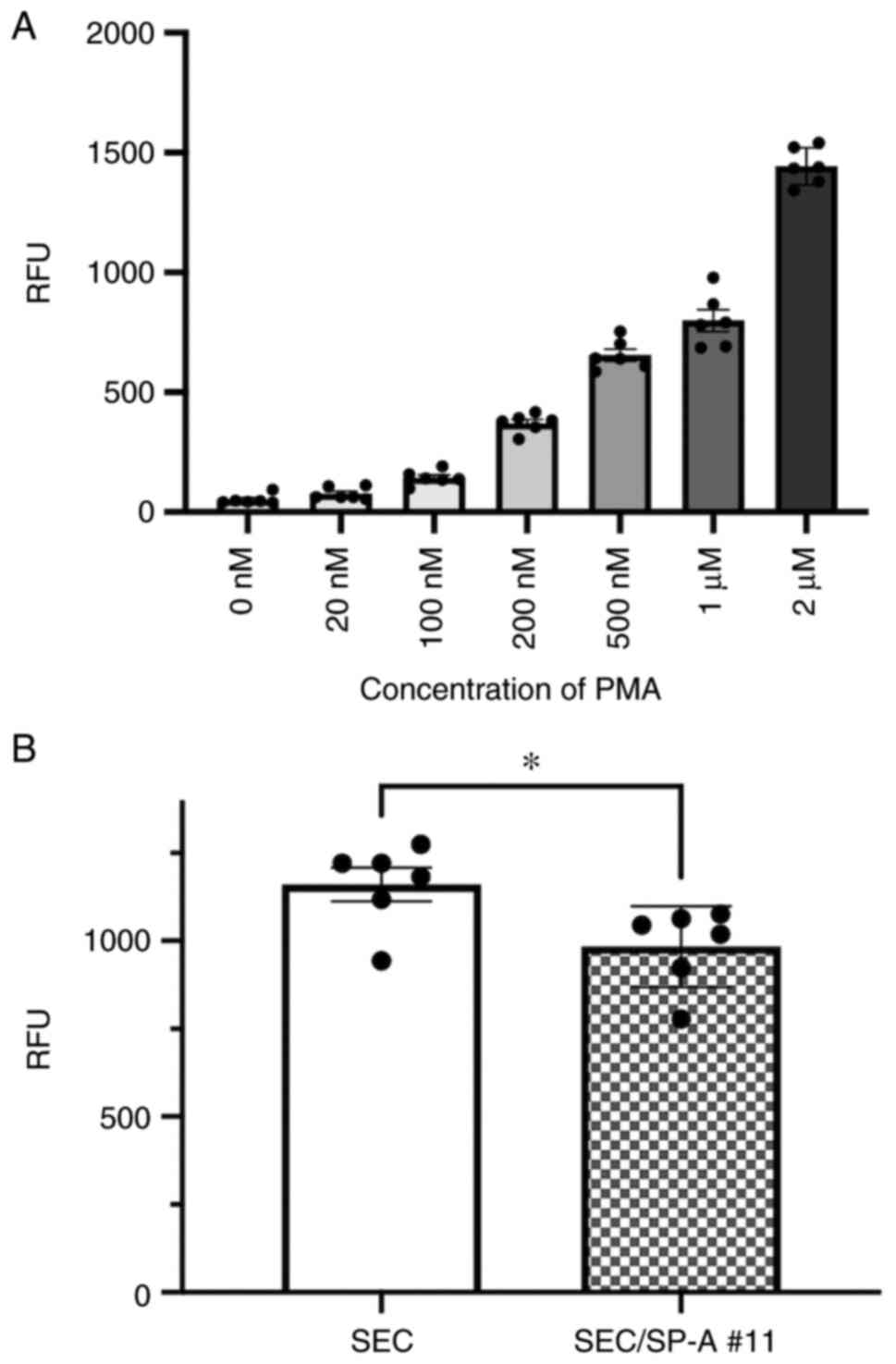
Luciferase assay and PMA-induced
activation of THP-1-Lucia NF-κB cells
To evaluate the PMA-induced activation of
THP-1-Lucia NF-κB cells, PMA was added to the cell culture at
concentrations ranging between 20 nM and 2 µM. After 24 h, a 10 µl
aliquot of supernatant from each well was placed into a 96-well
white plate. Following this, 50 µl of QUANTI-Luc™ assay solution,
the substrate for luciferase reaction, was added to each well, and
light signals were measured using a luminometer (Centro XS3 LB960,
Berthold Technologies). The obtained values are expressed as
relative light units.
Naive SECs or SEC/SP-As were plated in a 96-well
plate as in the cytotoxicity assay on Day 0. Following this,
THP-1-Lucia NF-κB cells (5.0x104) were added to each
well on Day 1. After co-culturing, a 10 µl aliquot of supernatant
in each well was measured using 50 µl of the QUANTI-Luc™ assay
solution and a luminometer.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen) and used for RT-qPCR
experiments. mRNA was amplified using a One-Step SYBR®
Prime Script® RT-PCR kit (Takara Bio Inc.), and it was
quantified using a Light Cycler 96 (Roche). A total of 20 µl of PCR
mixture was prepared containing 50 ng RNA, 2 U TaKaRa Ex Taq HS,
0.4 µl PrimerScript RT enzyme Mix II, and the corresponding paired
primers at a concentration of 0.2 µmol/l of each primer. The
thermal cycling conditions were as follows: 42˚C for 5 min,
followed by 95˚C for 10 s and then 40 cycles at 95˚C for 5 s and
60˚C for 20 s. The amount of mRNA was normalized to that of GAPDH
mRNA and analyzed (32). The
primer sequences for each gene were the same as previously reported
(33): iNOS-Fwd,
ATTCTGCTGCTTGCTGAGGT; iNOS-Rev, TTCAAGACCAAATTCCACCAC; IL-1β-Fwd,
ACAGATGAAGTGCTCCTTCCA; IL-1β-Rev, GTCGGAGATTCGTAGCTGGAT; TNF-α-Fwd,
CCCAGGGACCTCTCTCTAATC; TNF-α-Rev, ATGGGCTACAGGCTTGTCACT; ARG-1-Fwd,
GTTTCTCAAGCAGACCAGCC; ARG-1-Rev, GCTCAAGTGCAGCAAAGAGA; IL-10-Fwd,
GGCGCTGTCATCGATTTCTT; IL-10-Rev, GGCTTTGTAGATGCCTTTCTCTTG;
GAPDH-Fwd, TTAAAAGCAGCCCTGGTGAC, GAPDH-Rev,
CTCTGCTCCTCCTGTTCGAC.
Statistical analysis
GraphPad Prism9 software [GraphPad Prism version
9.0.0 for Mac; GraphPad Software, Inc.] was used to perform
statistical tests and to generate graphs. Comparisons between two
groups were performed using a two-tailed Welch's t-test or unpaired
Student's t-test. Comparisons between multiple groups were
evaluated using a two-way ANOVA multiple comparisons test and
Tukey's test. Statistical significance was set at P<0.05. Data
in the figures are presented as mean ± standard deviation. P-values
are shown as; *P<0.05; **P≤0.01; and
***P≤0.001.
Results
CL-SPA expression on SEC/SP-A
To investigate the effect of CL-SPA on
macrophage-mediated xenogeneic rejection, a plasmid containing cDNA
for CL-SPA was transfected into SECs. The RNA expression of CL-SPA
in SEC/SP-A was confirmed by semi-quantitative RT-PCR (Fig. 1D). The expression of a flag on
SEC/SP-A itself was measured using flow cytometry because CL-SPA is
a membranous protein (Fig. 1E). We
used SEC/SP-A bulk cells and a clonal line that expressed more than
95% of CL-SPA.
Suppression of macrophage-mediated
cytotoxicity via CL-SPA
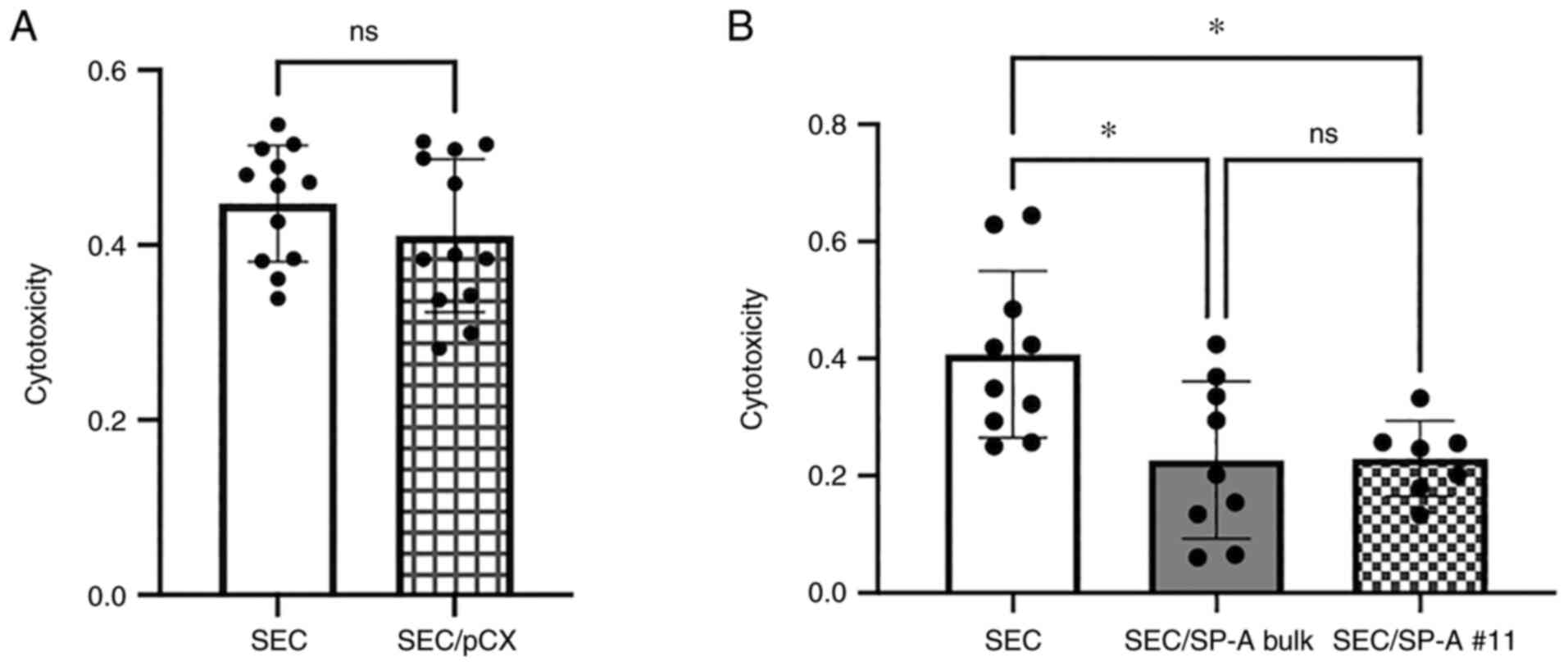
We confirmed that SEC without lipofection and SEC
with lipofection by empty vector (SEC/pCX) had no difference in the
THP-1-induced cytotoxicity assay (SEC: 0.447 vs. SEC/pCX: 0.410,
P=0.371, Fig. 2A). SECs without
lipofection were used as a control.
To evaluate the cytotoxicity of macrophages against
SEC cells, THP-1 cells were used as effector cells (Fig. 2B). After co-culturing SEC or
SEC/SP-A with THP-1 cells, we evaluated the cytotoxicity of
effector cells using a WST-8 assay. CL-SPA significantly suppressed
THP-1-induced cytotoxicity (SEC: 0.407 vs. SEC/SP-A bulk: 0.2265 or
SEC/SP-A #11: 0.2293, P=0.0131, P=0.0252, respectively).
Suppression of macrophage-mediated
phagocytosis via CL-SPA
To evaluate macrophage-mediated phagocytosis against
SECs, macrophages were generated by culturing monocytes with 100
ng/ml rM-CSF, and then co-cultured with SEC or SEC/SP-A.
Significant phagocytosis was induced in SECs (75.9%), and CL-SPA
significantly suppressed phagocytosis by macrophages (SEC/SP-A
bulk: 27.1%, SEC/SP-A #11:33.0%, P=0.0085, P=0.0010, respectively,
Fig. 3).
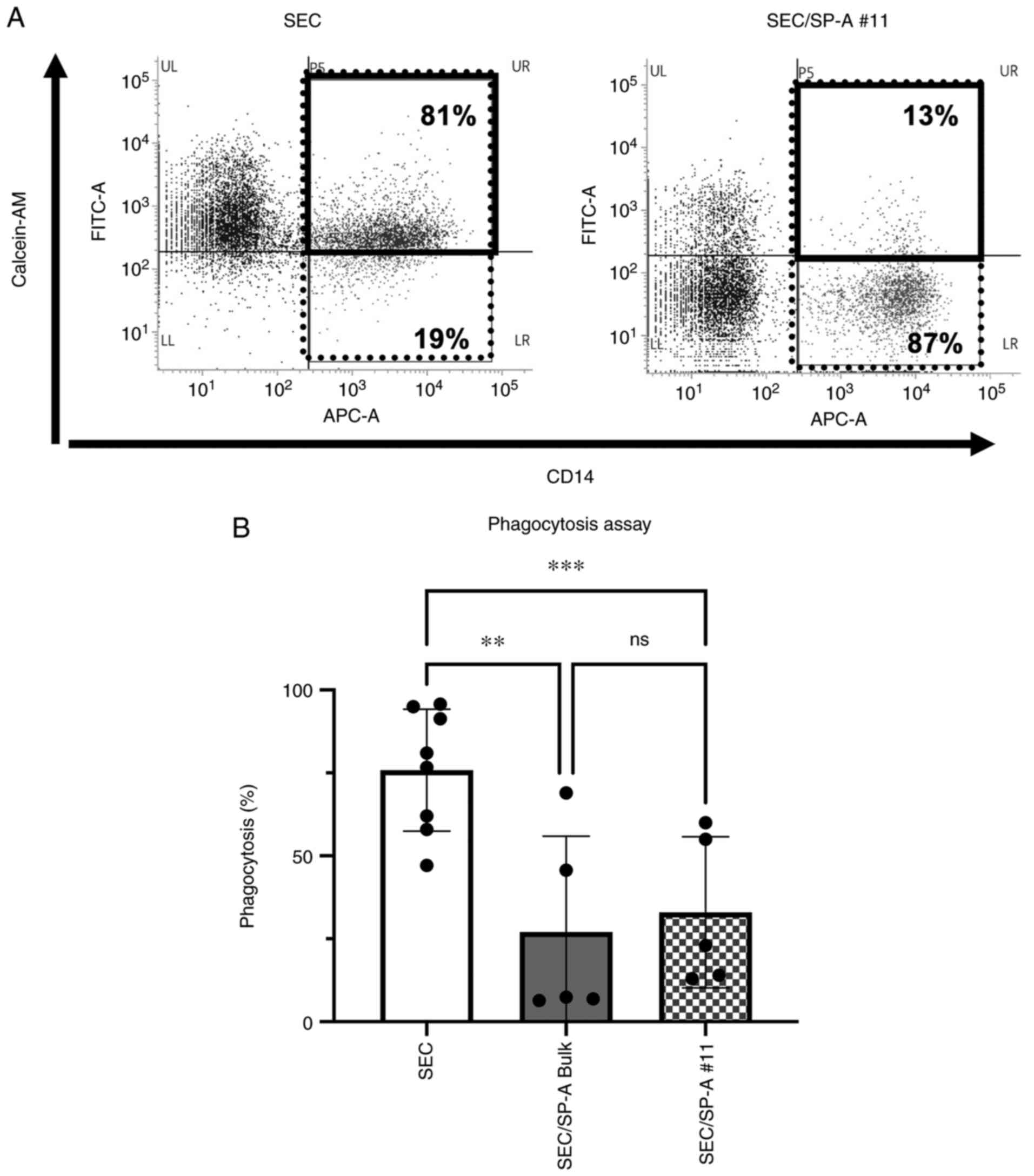 | Figure 3Suppression of macrophage-mediated
phagocytosis with CL-SPA. Human macrophages were generated by
culturing monocytes with M-CSF (100 ng/ml), and subsequently, the
cells were co-cultured with SEC or SEC/SP-A for 24 h. (A)
Phagocytosis of SEC or SEC/SP-A #11 was assessed via flow
cytometry. Data are representative of five different experiments.
These histograms showed the percentage of calcein-AM-positive cells
within CD14-positive cells. The percentage of phagocytosis was
calculated as (CD14+ and calcein-AM+ cells,
thick-bordered box)/(CD14+ cells, thin box) x100(%). (B)
CL-SPA induced a significant suppression of phagocytosis. Bars
represent SD. n=5-8, **P≤0.01, ***P≤0.001.
SEC, swine endothelial cell; SP-A, surfactant protein-A; CL-SPA,
collectin placenta 1 with carbohydrate recognition domain of SP-A;
SEC/SP-A, CL-SPA-transfected SEC. |
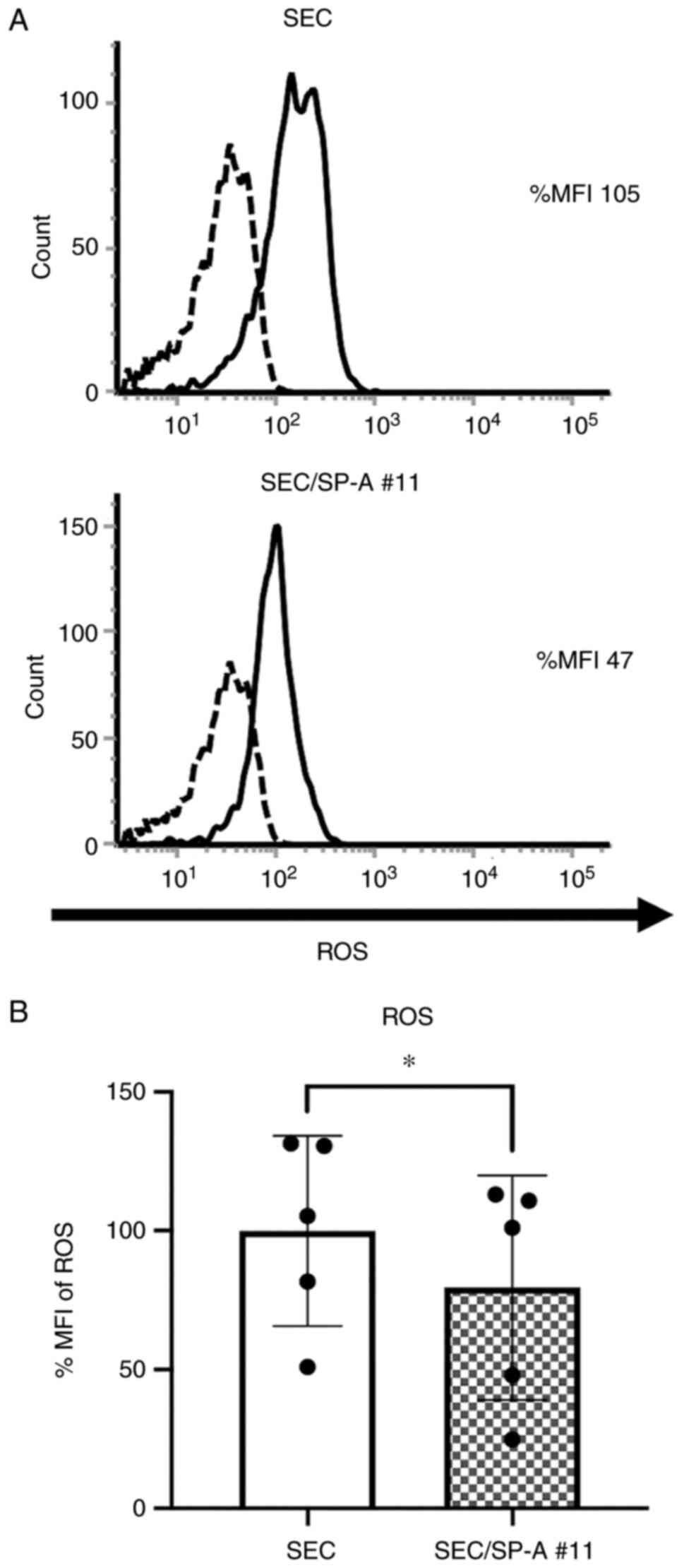
Suppression of innate immune responses
via CL-SPA
To evaluate the suppressive effect of CL-SPA on
innate immune responses, macrophages were cultured with SEC or
SEC/SP-A. The production of ROS in macrophages under xenogeneic
conditions was significantly suppressed by CL-SPA (SEC: 99.9 vs.
SEC-SP-A #11:79.5, P=0.0138, Fig.
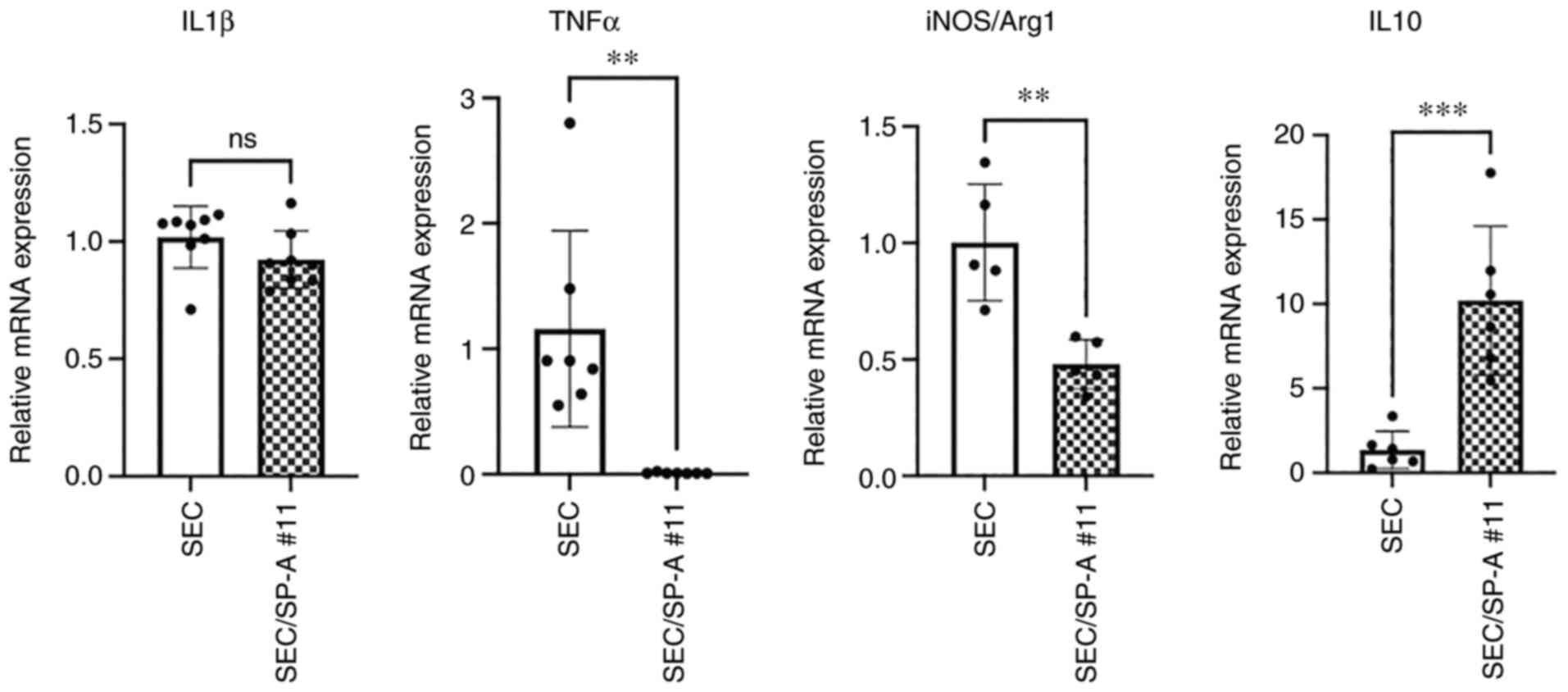
4). The expression of pro- and anti-inflammatory cytokines in
macrophages was analyzed using RT-qPCR. Significant suppression of
TNF-α expression (SEC: 1.160 vs. SEC/SP-A #11:0.009, P=0.0022) and
upregulation of IL-10 expression (SEC: 1.357 vs. SEC/SP-A
#11:10.20, P=0.0007) were observed in macrophages cultured with
SEC/SP-A as compared to that in those cultured with SECs (Fig. 5). The balance between iNOS and
Arg-1 was significantly suppressed via CL-SPA (SEC: 1.002 vs.
SEC/SP-A #11:0.4792, P=0.0027), indicating that CL-SPA on porcine
cells inhibited the differentiation of peripheral blood monocytes
into inflammatory M1 macrophages. Additionally, NF-κB activation in
macrophages was evaluated with a luciferase assay using THP-1 Luc
NF-κB cells. CL-SPA significantly suppressed PMA-induced NF-κB
activation as compared to THP-1 cells co-cultured with SEC (SEC:
1160 vs. SEC/SP-A #11:983.8, P=0.0258, Fig. 6).
Discussion
The study findings indicated that SP-A induced a
significant suppression of macrophage-mediated phagocytosis. This
is the first report evaluating the suppressive function of
SPA-CRD.
Xenotransplantation using a porcine with transgenic
modifications has been reported recently (34). The genetically engineered porcine
harbored ten genetic modifications, including the targeted
insertion of two human complement regulatory genes (hDAF, hCD46),
two human anti-coagulant genes (hTBM, hEPCR), and two
immunomodulatory genes (hCD47, hHO1) as well as deletion (knockout)
of 3 pig carbohydrate antigens and the pig growth hormone receptor
gene. Our study suggested that the control of macrophage-mediated
xenogeneic reaction by SPA-CRD may potentially be a new approach to
genetic modification of xenografts.
SP-A has been reported to suppress macrophage
phagocytosis by binding to SIRPα in macrophages (30). As expected, SP-A significantly
suppressed phagocytosis. In RT-qPCR analysis, SP-A caused
suppression of the pro-inflammatory cytokine, TNF-α, and
upregulation of the anti-inflammatory cytokine, IL-10, similar to
our previous report describing the effect of SP-D on macrophages
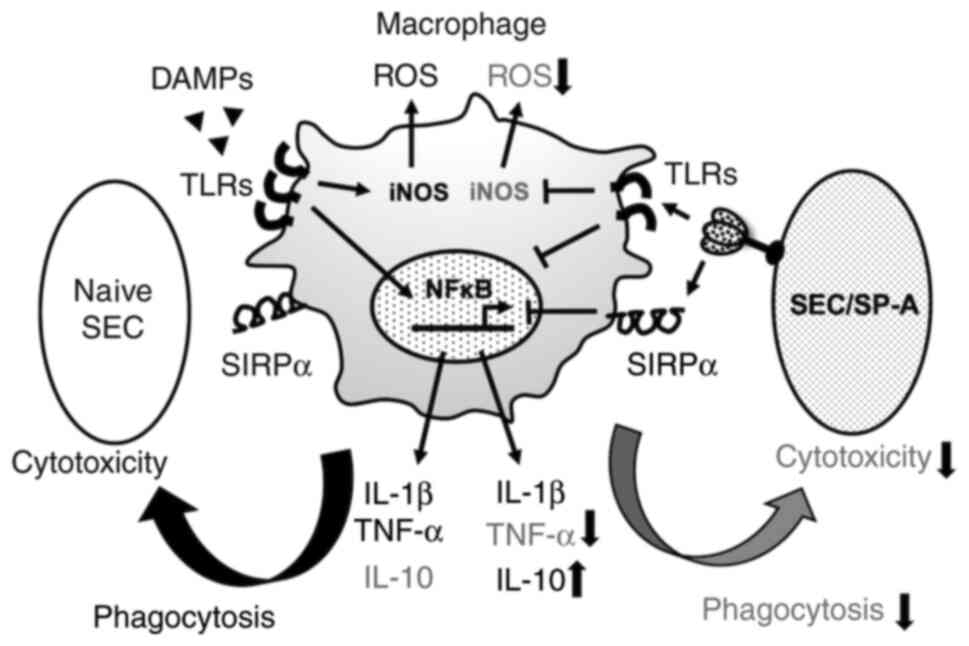
(31). The graphical abstract is
shown in Fig. 7. The CRD of SP-A
has been reported to bind to toll-like receptor (TLR)-2,4 and
suppress TLR-mediated inflammation (35-37).
Suppression of NF-κB by SP-A (Fig.
6) might be a result of CL-SPA binding to TLRs. Since our
result may involve TLR-mediated inhibitory effects, further
investigation of these mechanisms is necessary. Regarding the
control of CXR, suppression of pro-inflammatory cytokines from
macrophages may also contribute to the suppression of
neutrophil-induced rejection. Pro-inflammatory cytokines from
macrophages have been reported to contribute to nuclear
extracellular trap (NET)osis in neutrophils (38), and ectopic expression of SP-A in
SEC is an effective strategy for suppressing the innate immunity
associated with xenotransplantation. Neutrophils also contribute to
CXR and induce tissue damage under xenogeneic conditions in
antibody-dependent and -independent manners (18,39,40).
NETs produced by neutrophils cause extensive endothelial cell
damage in response to xenogeneic antigen (41-43).
However, neutrophil apoptosis contributes to the resolution of
inflammation. The phagocytosis of apoptotic cells by macrophages is
central to the successful resolution of an inflammatory response,
and it is increasingly apparent that the dying neutrophils
themselves exert anti-inflammatory effects by modulating
surrounding cell responses, particularly the release of
inflammatory cytokines from macrophages (44,45).
These findings indicate that the ectopic expression of CRD in SP-A
can suppress macrophage-mediated cytotoxicity as well as
neutrophil-mediated tissue damage. Furthermore, increasing
neutrophil apoptosis and decreasing NETosis by NF-κB suppression
would lead to anti-inflammatory conditions and resolution of
inflammation.
In conclusion, we reported that the CRD of SP-A
suppressed macrophage-mediated cytotoxicity and phagocytosis.
Moreover, SP-A suppressed NF-κB activation and reduced the
expression of pro-inflammatory cytokines in macrophages. To further
investigate the effects of SP-A on innate immunity in more detail,
in vivo studies should be performed in the future.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by Grants-in-Aid for Scientific
Research and Health and Labor Sciences Research Grants, Japan
(grant no. 19K09092).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CT performed the research and wrote the manuscript.
AM and KM participated in study design and contributed materials.
SK, RY and KM performed the research. MK, TU, YT, HE and HO
participated in data analysis. SM participated in the study design
and wrote the manuscript. CT, AM, SK, RY and SM confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Osaka
University ethics committee [approval no. 18395(T1)]. All the
participants enrolled in the study provided signed written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Niu D, Wei HJ, Lin L, George H, Wang T,
Lee IH, Zhao HY, Wang Y, Kan Y, Shrock E, et al: Inactivation of
porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science.
357:1303–1307. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Servick K: Xenotransplant advances may
prompt human trials. Science. 357(1338)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cooper DKC, Ezzelarab M, Iwase H and Hara
H: Perspectives on the optimal genetically engineered pig in 2018
for initial clinical trials of kidney or heart xenotransplantation.
Transplantation. 102:1974–1982. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cooper DK, Gaston R, Eckhoff D, Ladowski
J, Yamamoto T, Wang L, Iwase H, Hara H, Tector M and Tector AJ:
Xenotransplantation-The current status and prospects. Br Med Bull.
125:5–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fukuta D, Miyagawa S, Yamada M, Matsunami
K, Kurihara T, Shirasu A, Hattori H and Shirakura R: Effect of
various forms of the C1 esterase inhibitor (C1-INH) and DAF on
complement-mediated xenogeneic cell lysis. Xenotransplantation.
10:132–141. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miyagawa S, Kubo T, Matsunami K, Kusama T,
Beppu K, Nozaki H, Morita T, Ahn C, Kim JY, Fukuta D and Shirakura
R: Delta-short consensus repeat 4-decay accelerating factor (DAF:
CD55) inhibits complement-mediated cytolysis but not NK
cell-mediated cytolysis. J Immunol. 173:3945–3952. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Miyagawa S, Fukuta D, Kitano E, Kobayashi
C, Fumimoto Y, Shirasu A, Hattori H, Shirakura R and Fukuzawa M:
Effect of tandem forms of DAF(CD55) on complement-mediated
xenogeneic cell lysis. Xenotransplantation. 13:433–439.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cardone J, Le Friec G and Kemper C: CD46
in innate and adaptive immunity: An update. Clin Exp Immunol.
164:301–311. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cadili A and Kneteman N: The role of
macrophages in xenograft rejection. Transplant Proc. 40:3289–3293.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang H and Yang YG: Innate cellular
immunity and xenotransplantation. Curr Opin Organ Transplant.
17:162–167. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vadori M and Cozzi E: The immunological
barriers to xenotransplantation. Tissue Antigens. 86:239–253.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Candinas D, Belliveau S, Koyamada N,
Miyatake T, Hechenleitner P, Mark W, Bach FH and Hancock WW: T cell
independence of macrophage and natural killer cell infiltration,
cytokine production, and endothelial activation during delayed
xenograft rejection. Transplantation. 62:1920–1927. 1996.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fox A, Mountford J, Braakhuis A and
Harrison LC: Innate and adaptive immune responses to nonvascular
xenografts: Evidence that macrophages are direct effectors of
xenograft rejection. J Immunol. 166:2133–2140. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu XC, Goodman J, Sasaki H, Lowell J and
Mohanakumar T: Activation of natural killer cells and macrophages
by porcine endothelial cells augments specific T-cell xenoresponse.
Am J Transplant. 2:314–322. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Adams S, van der Laan LJ, Vernon-Wilson E,
Renardel de Lavalette C, Döpp EA, Dijkstra CD, Simmons DL and van
den Berg TK: Signal-regulatory protein is selectively expressed by
myeloid and neuronal cells. J Immunol. 161:1853–1859.
1998.PubMed/NCBI
|
|
16
|
Itoh T, Hata Y, Nishinakamura H, Kumano K,
Takahashi H and Kodama S: Islet-derived damage-associated molecular
pattern molecule contributes to immune responses following
microencapsulated neonatal porcine islet xenotransplantation in
mice. Xenotransplantation. 23:393–404. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Martin BM, Samy KP, Lowe MC, Thompson PW,
Cano J, Farris AB, Song M, Dove CR, Leopardi FV, Strobert EA, et
al: Dual islet transplantation modeling of the instant
blood-mediated inflammatory reaction. Am J Transplant.
15:1241–1252. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matsunami K, Miyagawa S, Nakai R, Yamada M
and Shirakura R: Modulation of the leader peptide sequence of the
HLA-E gene up-regulates its expression and down-regulates natural
killer cell-mediated swine endothelial cell lysis. Transplantation.
73:1582–1589. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Matsunami K, Kusama T, Okura E, Shirakura
R, Fukuzawa M and Miyagawa S: Involvement of position-147 for HLA-E
expression. Biochem Biophys Res Commun. 347:692–697.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Maeda A, Kawamura T, Ueno T, Usui N,
Eguchi H and Miyagawa S: The suppression of inflammatory
macrophage-mediated cytotoxicity and proinflammatory cytokine
production by transgenic expression of HLA-E. Transpl Immunol.
29:76–81. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Maeda A, Kawamura T, Nakahata K, Ueno T,
Usui N, Eguchi H and Miyagawa S: Regulation of macrophage-mediated
xenocytotoxicity by overexpression of alpha-2,6-sialyltransferase
in swine endothelial cells. Transplant Proc. 46:1256–1258.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Esquivel EL, Maeda A, Eguchi H, Asada M,
Sugiyama M, Manabe C, Sakai R, Matsuura R, Nakahata K, Okuyama H
and Miyagawa S: Suppression of human macrophage-mediated
cytotoxicity by transgenic swine endothelial cell expression of
HLA-G. Transpl Immunol. 32:109–115. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Maeda A, Eguchi H, Nakahata K, Lo PC,
Yamanaka K, Kawamura T, Matsuura R, Sakai R, Asada M, Okuyama H and
Miyagawa S: Monocytic MDSCs regulate macrophage-mediated xenogenic
cytotoxicity. Transpl Immunol. 33:140–145. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sakai R, Maeda A, Choi TV, Lo PC,
Jiaravuthisan P, Shabri AM, Wang HT, Matsuura R, Kodama T, Eguchi
H, et al: Human CD200 suppresses macrophage-mediated xenogeneic
cytotoxicity and phagocytosis. Surg Today. 48:119–126.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bridges JP, Davis HW, Damodarasamy M,
Kuroki Y, Howles G, Hui DY and McCormack FX: Pulmonary surfactant
proteins A and D are potent endogenous inhibitors of lipid
peroxidation and oxidative cellular injury. J Biol Chem.
275:38848–38855. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
LeVine AM and Whitsett JA: Pulmonary
collectins and innate host defense of the lung. Microbes Infect.
3:161–166. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Borron P, McIntosh JC, Korfhagen TR,
Whitsett JA, Taylor J and Wright JR: Surfactant-associated protein
A inhibits LPS-induced cytokine and nitric oxide production in
vivo. Am J Physiol Lung Cell Mol Physiol. 278:L840–L847.
2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kremlev SG and Phelps DS: Surfactant
protein A stimulation of inflammatory cytokine and immunoglobulin
production. Am J Physiol. 267 (6 Pt 1):L712–L719. 1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gardai SJ, Xiao YQ, Dickinson M, Nick JA,
Voelker DR, Greene KE and Henson PM: By binding SIRPalpha or
calreticulin/CD91, lung collectins act as dual function
surveillance molecules to suppress or enhance inflammation. Cell.
115:13–23. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Janssen WJ, McPhillips KA, Dickinson MG,
Linderman DJ, Morimoto K, Xiao YQ, Oldham KM, Vandivier RW, Henson
PM and Gardai SJ: Surfactant proteins A and D suppress alveolar
macrophage phagocytosis via interaction with SIRPalpha. Am J Respir
Crit Care Med. 178:158–167. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jiaravuthisan P, Maeda A, Takakura C, Wang
HT, Sakai R, Shabri AM, Lo PC, Matsuura R, Kodama T, Eguchi H, et
al: A membrane-type surfactant protein D (SP-D) suppresses
macrophage-mediated cytotoxicity in swine endothelial cells.
Transpl Immunol. 47:44–48. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittengen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Noguchi Y, Maeda A, Lo PC, Takakura C,
Haneda T, Kodama T, Yoneyama T, Toyama C, Tazuke Y, Okuyama H and
Miyagawa S: Human TIGIT on porcine aortic endothelial cells
suppresses xenogeneic macrophage-mediated cytotoxicity.
Immunobiology. 224:605–613. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Porrett PM, Orandi BJ, Kumar V, Houp J,
Anderson D, Cozette Killian A, Hauptfeld-Dolejsek V, Martin DE,
Macedon S, Budd N, et al: First clinical-grade porcine kidney
xenotransplant using a human decedent model. Am J Transplant.
22:1037–1053. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ohya M, Nishitani C, Sano H, Yamada C,
Mitsuzawa H, Shimizu T, Saito T, Smith K, Crouch E and Kuroki Y:
Human pulmonary surfactant protein D binds the extracellular
domains of Toll-like receptors 2 and 4 through the carbohydrate
recognition domain by a mechanism different from its binding to
phosphatidylinositol and lipopolysaccharide. Biochemistry.
45:8657–8664. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Henning LN, Azad AK, Parsa KV, Crowther
JE, Tridandapani S and Schlesinger LS: Pulmonary surfactant protein
A regulates TLR expression and activity in human macrophages. J
Immunol. 180:7847–7858. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Agrawal V, Smart K, Jilling T and Hirsch
E: Surfactant protein (SP)-A suppresses preterm delivery and
inflammation via TLR2. PLoS One. 8(e63990)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nakazawa D, Shida H, Kusunoki Y, Miyoshi
A, Nishio S, Tomaru U, Atsumi T and Ishizu A: The responses of
macrophages in interaction with neutrophils that undergo NETosis. J
Autoimmun. 67:19–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
al-Mohanna F, Collison K, Parhar R, Kwaasi
A, Meyer B, Saleh S, Allen S, al-Sedairy S, Stern D and Yacoub M:
Activation of naive xenogeneic but not allogeneic endothelial cells
by human naive neutrophils: A potential occult barrier to
xenotransplantation. Am J Pathol. 151:111–120. 1997.PubMed/NCBI
|
|
40
|
Sachs UJ, Andrei-Selmer CL, Maniar A,
Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT,
Chavakis T and Santoso S: The neutrophil-specific antigen CD177 is
a counter-receptor for platelet endothelial cell adhesion
molecule-1 (CD31). J Biol Chem. 282:23603–23612. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sayah DM, Mallavia B, Liu F, Ortiz-Muñoz
G, Caudrillier A, DerHovanessian A, Ross DJ, Lynch JP III, Saggar
R, Ardehali A, et al: Neutrophil extracellular traps are pathogenic
in primary graft dysfunction after lung transplantation. Am J
Respir Crit Care Med. 191:455–463. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang H, Tohme S, Al-Khafaji AB, Tai S,
Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y and Tsung A:
Damage-associated molecular pattern-activated neutrophil
extracellular trap exacerbates sterile inflammatory liver injury.
Hepatology. 62:600–614. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu FC, Chuang YH, Tsai YF and Yu HP: Role
of neutrophil extracellular traps following injury. Shock.
41:491–498. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kennedy AD and Deleo FR: Neutrophil
apoptosis and the resolution of infection. Immunol Res. 43:25–61.
2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fox S, Leitch AE, Duffin R, Haslett C and
Rossi AG: Neutrophil apoptosis: Relevance to the innate immune
response and inflammatory disease. J Innate Immun. 2:216–227.
2010.PubMed/NCBI View Article : Google Scholar
|