Introduction
Pulmonary hypertension (PH) affects ~1% of global
population, with 80% of cases being reported in developing
countries (1). Heart and lung
diseases are the most frequent causes leading to PH (2). Connective tissue diseases (CTDs) have
also been associated with PH (1).
Pulmonary arterial hypertension (PAH) is a serious
complication and major cause of morbidity and mortality in patients
with CTDs (3). Approximately 30%
of scleroderma-related deaths are due to PAH (4). In contrast to Western countries,
epidemiological studies performed in Asian countries, including
Korea, Japan and China, have revealed that systemic lupus
erythematosus (SLE) is the main cause of PAH among patients with
CTDs instead of systemic sclerosis (5-7).
Moreover, the Chinese SLE Treatment and Research group (CSTAR)
registry revealed that the possible prevalence of PAH in patients
with SLE was 3.8% (8). Chen et
al (9) demonstrated that the
average interval from SLE diagnosis to PAH diagnosis is 3.66 years,
and that 70% of patients with SLE develop PAH within 5 years of
disease onset. However, the presence of PAH in patients with
new-onset SLE and the potential predictors remains to be
studied.
Abnormal lipid metabolism participates in the
pathogenesis of PH (10). In
patients with PH, the levels of low-density lipoprotein (LDL) and
high-density lipoprotein (HDL) are decreased (11-13).
Thus, HDL and LDL have been suggested as markers for prediction and
assessment of PH (11,14). In addition, a study suggested that
inflammation triggered by CTDs can lead to dysregulation of lipid
metabolism (15). Nonetheless, the
exact relationship between lipid biomarker and CTDs-PAH remains
unclear.
Thrombosis is one of the possible etiologies of PH,
and several coagulation indices are associated with PH. For
example, high plasma fibrinogen has been suggested as an
independent predictor of survival in patients with chronic
thromboembolic pulmonary hypertension (16). Although endogenous fibrinolysis may
be involved in the pathophysiology of PH, the role of D-dimer, a
fibrin degradation product, in PH remains controversial. A previous
study found significantly high D-dimer levels in primary pulmonary
hypertension (17). It was also
reported that D-dimmer level is not associated with pulmonary
artery pressure in patients with systemic sclerosis (18). Lipids can affect coagulation and
fibrinolytic factors (19).
However, the role of coagulation indices in the PH needs to be
further clarified, especially in CTDs-PAH. Furthermore, if
coagulation mediates the effect of lipids in CTDs-PAH is also
unclear. Therefore, in the present study, the relationship between
coagulation and lipids biomarkers in newly diagnosed patients with
SLE and PAH was investigated, and whether the coagulation
parameters showed mediating effect on the association between
lipids and PAH presence.
Materials and methods
Study design and population
Newly diagnosed, untreated patients with SLE
admitted to the Rheumatology and Immunology Department at the First
Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China)
between January 2019 and December 2020 were retrospectively
collected. All patients underwent routine echocardiography
examinations during hospitalization. The 1997 updated American
College of Rheumatology criteria for SLE was applied to diagnose
SLE. Patients with other connective tissue diseases, such as left
heart disease, antiphospholipid syndrome, congenital heart disease,
valvular heart disease, HIV and portal hypertension were excluded.
Finally, 301 patients with SLE who met these criteria were included
in the analyses (20).
The Ethics Committee of First Affiliated Hospital of
Xi'an Jiaotong University (approval no. 2022-291) approved the
study, which was conducted according to the Declaration of
Helsinki. Written informed consent were provided by all
patients.
Clinical and laboratory data
collection
Baseline characteristics (age, sex, smoking habit
and systemic blood pressure), laboratory tests, systemic lupus
erythematosus disease activity index (SLEDAI), medications and
history of thrombosis events were obtained from electronic medical
records.
Echocardiographic evaluation
Doppler echocardiogram, as a routine examination,
was performed on each admitted patient to screen for the presence
of PH. Pulmonary artery systolic pressure (sPAP) was estimated
adopting a modified Bernoulli equation (21): sPAP=4 x (tricuspid systolic
jet)2 + 10 mmHg (estimated right atrial pressure). PH
was defined as an estimated sPAH >35 mmHg using
echocardiograms.
Statistical analysis
Continuous variables are presented as mean ±
standard deviation if normally distributed proved by
Kolmogorov-Smirnov test, otherwise data are presented as median and
interquartile range (IQR). Categorical variables are presented as
counts and proportions. Demographic characteristics and relevant
risk factors were compared between PAH and no PAH groups using
independent samples t-tests or Wilcoxon rank-sum tests for
continuous variables and Chi-square or Fisher's exact tests for
categorical variables. Spearman's correlation analyses were used to
examine the relationships between coagulation and lipid index with
SLE disease activity and sPA. A multivariable logistic regression
model was built to examine potential PAH predictors.
To test if coagulation is a mediator between lipids
and PAH, package ‘mediation’ in R Studio (version 1.2.5001) was
utilized to conduct causal mediation analysis in applied empirical
research (https://cran.r-project.org/web/packages/mediation/vignettes/mediation.pdf)
(22). A mediator variable is a
variable that causes mediation in the dependent (PAH) and the
independent variables (lipids). Therefore, it explains the
relationship between the dependent variable and the independent
variable (23,24).
All analyses were performed using SAS 9.4 software
(Cary) and RStudio version 1.2.5001 (RStudio, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients' characteristics
A total of 301 newly diagnosed, untreated patients
with SLE were identified during the study period. Female patients
accounted for 88.0% of the sample, and the average age was 32 years
(range, 25-45 years). A total of 40 patients (13.3%) demonstrated
PAH after doppler echocardiogram examination. The average sPAP was
55.825±26.67 mmHg (range 35-156 mmHg).
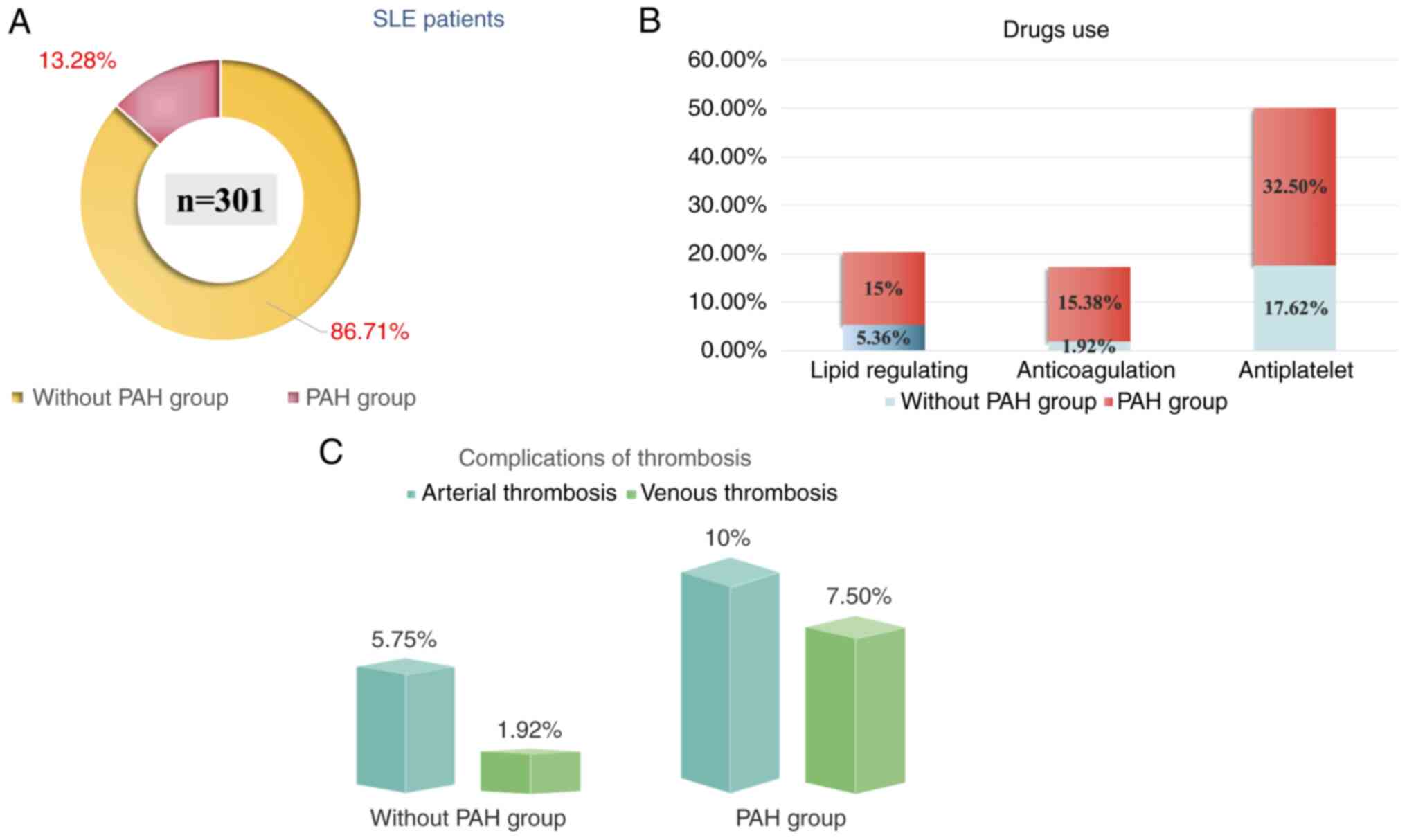
Patients were divided into two groups: Non-PAH and
PAH groups. There was no significative difference in sex, BMI,
systemic blood pressure, smoking habits, and kidney function
between the two groups (Fig. 1A;
Table I). However, patients in the
PAH group were older and had higher levels of D-dimer, FDP,
C-reactive protein (CRP), lower levels of complement 3 (C3),
complement 4 (C4) and 25-hydroxy vitamin D3
(25(OH)D3) compared with the non-PAH group. In addition,
a higher number of patients in the PAH group used lipid regulating,
anticoagulation and antiplatelet medications compared with the
non-PAH group (Fig. 1B). Moreover,
a higher number of arterial and venous thrombosis events were
recorded in the PAH group compared with the non-PAH group; however,
there was no statistical difference (Fig. 1C).
 | Table ICharacteristics of study
subjects. |
Table I
Characteristics of study
subjects.
|
Characteristics | Non-PAH
(n=261) | PAH (n=40) | P-value |
|---|
| Sex [male;
(%)] | 31.00 (11.88%) | 5.00 (12.50%) | 1.00 |
| Median age, years
(IQR) | 31.00
(25.00-44.00) | 39
(29.00-49.00) | 0.02 |
| Median BMI,
kg/m2 (IQR) | 20.20
(17.90-22.31) | 19.67
(18.00-27.25) | 0.72 |
| Median SBP, mmHg
(IQR) | 113.00
(102.00-122.00) | 113.00
(107.00-123.50) | 0.40 |
| Median DBP, mmHg
(IQR) | 76.00
(68.00-81.00) | 75.00
(67.50-83.50) | 0.91 |
| Smoke, n (%) | 15.00 (5.75%) | 2.00 (5.00%) | 1.00 |
| Median FIB, g/l
(IQR) | 3.21
(2.62-3.91) | 2.97
(2.11-3.87) | 0.12 |
| Median DD, mg/l
(IQR) | 1.30
(0.60-2.60) | 2.00
(0.95-4.26) | 0.01 |
| Median APTT, s
(IQR) | 37.70
(34.10-41.95) | 37.15
(35.60-43.20) | 0.63 |
| Median FDP, mg/l
(IQR) | 3.60
(1.57-7.14) | 5.84
(3.20-11.40) | 0.01 |
| Median 24UTP, g
(IQR) | 0.13
(0.05-0.48) | 0.23
(0.09-1.05) | 0.13 |
| Median Cr, µmol/l
(IQR) | 49.00
(41.00-60.00) | 54.00
(40.00-60.00) | 0.66 |
| Median BUN, mmol/l
(IQR) | 4.65
(3.60-6.38) | 5.24
(3.28-6.91) | 0.47 |
| Median uric acid,
µmol/l (IQR) | 283.66 (224.50,
370.50) | 325.00
(225.00-423.00) | 0.13 |
| Median eGFR,
ml/min/1.732 (IQR) | 136.29
(109.63-170.44) | 123.45
(97.97-141.92) | 0.21 |
| Median C3, g/l
(IQR) | 0.58
(0.34-0.79) | 0.38
(0.30-0.66) | 0.04 |
| Median C4, g/l
(IQR) | 0.09
(0.06-0.16) | 0.07
(0.05-0.09) | 0.01 |
| Median dsDNA, IU/ml
(IQR) | 2.50
(1.00-25.50) | 2.00
(1.00-14.00) | 0.84 |
| Median SLEDAI
(IQR) | 9.00
(6.00-15.00) | 12.00
(8.00-16.00) | 0.10 |
| Median ESR, mm/h
(IQR) | 45.00
(23.00-77.00) | 61.00
(23.00-80.00) | 0.35 |
| Median CRP, mmol/l
(IQR) | 4.70
(3.00-11.90) | 10.00
(4.40-20.10) | 0.01 |
| Median total
cholesterol, mmol/l (IQR) | 3.28
(2.72-3.81) | 3.21
(2.53-3.95) | 0.40 |
| Median
triglyceride, mmol/l (IQR) | 1.46
(1.02-2.23) | 1.44
(1.12-2.15) | 0.97 |
| Median LDL, mmol/l
(IQR) | 1.87
(1.45-2.35) | 1.60
(1.30-2.15) | 0.12 |
| Median HDL, mmol/l
(IQR) | 0.71
(0.57-0.90) | 0.70
(0.48-0.89) | 0.36 |
| Median
25(OH)D3, ng/ml (IQR) | 10.60
(6.80-15.20) | 8.30
(6.40-11.20) | 0.02 |
Predictors of PAH in patients with
SLE
To examine potential predictors of PAH in patients
with SLE, a multivariable logistic regression model was
constructed. The age and D-dimer were independent predictor factors
for PAH (Table II).
 | Table IIMultivariable logistic regression
model for predictors of PAH presence. |
Table II
Multivariable logistic regression
model for predictors of PAH presence.
| Risk factors | OR with 95% CI | P-value |
|---|
| Age, 1 unit
increase | 1.03 (1.01,
1.06) | 0.0092 |
| DD, 1 unit
increase | 1.10 (1.02,
1.18) | 0.0132 |
Coagulation and lipid parameters in
patients with SLE-PAH
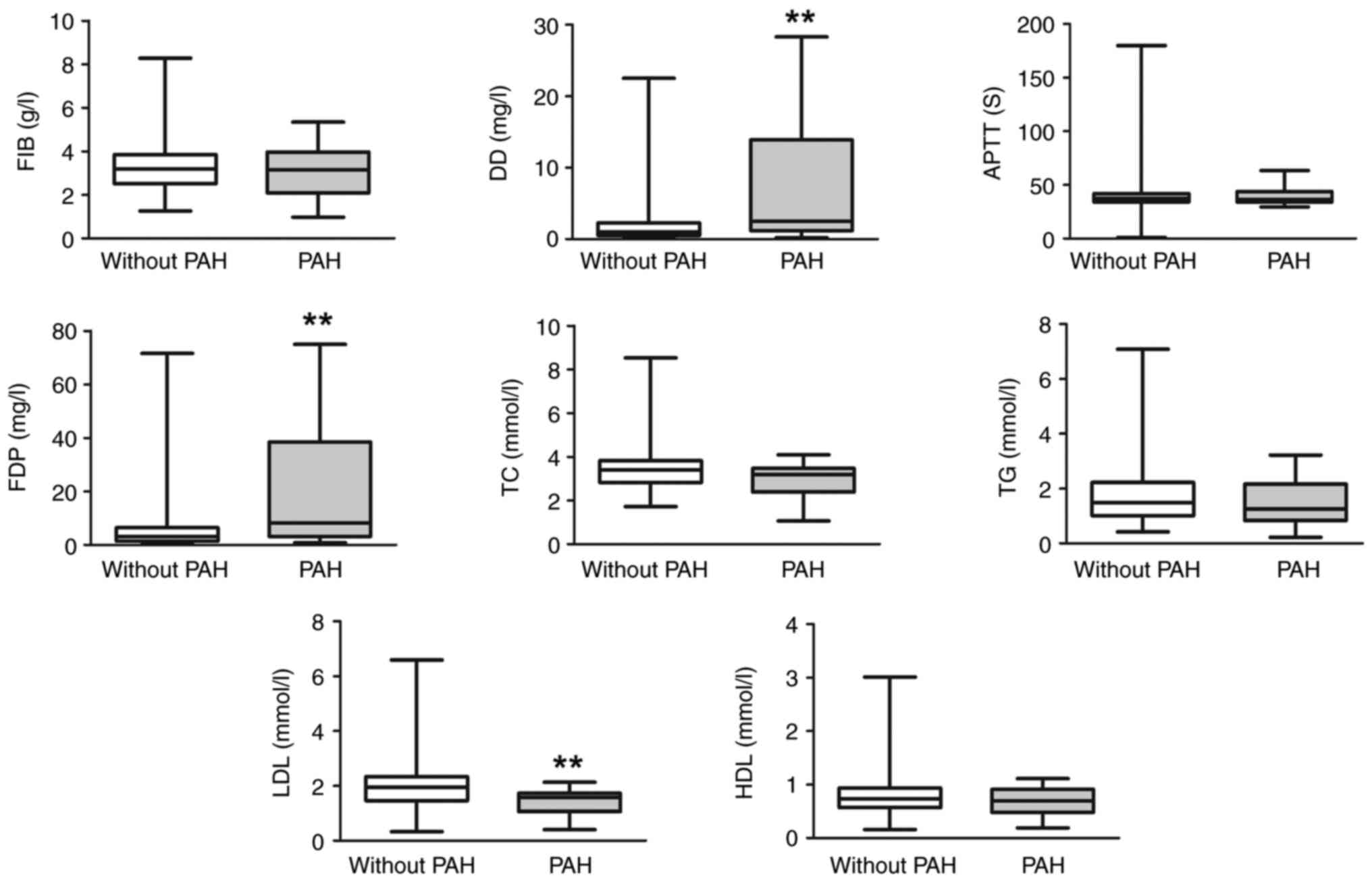
In order to further analyze coagulation and lipid
parameters in patients with SLE-PAH, medical records were examined
from 221 patients not using lipid regulating or anticoagulation
medications among the 301 subjects. A total of 19 patients
presented PAH and the average sPAP was 53.26±23.49 mmHg. Patients
in the PAH group had higher D-dimer and FDP levels, as well as
significantly lower LDL levels compared with the non-PAH group
(Fig. 2).
Association of coagulation and lipid
index with SLE disease activity and PAH
The relationship of coagulation and lipid index with
SLE disease activity and PAH was analyzed in patients with no
records of lipid regulating and anticoagulation medications
(Fig. 3). No association was found
among anti-double-stranded (ds)-DNA, coagulation (FIB, D-dimer and
FDP) and lipid index (TG, TC, LDL and HDL). SLEDAI was positively
associated with D-dimer and FDP and negatively associated with
total cholesterol (TC) and HDL. C3 and C4 were negatively
associated with D-dimer and FDP, and positively associated with
HDL. The coagulation index was positively associated with
inflammatory markers erythrocyte sedimentation rate (ESR) and CRP.
Moreover, no significant association was found among lipid index,
ESR and CRP. For PAH, there was a positive association between sPAP
and D-dimer and FDP, and a negative association among sPAP, TC and
LDL.
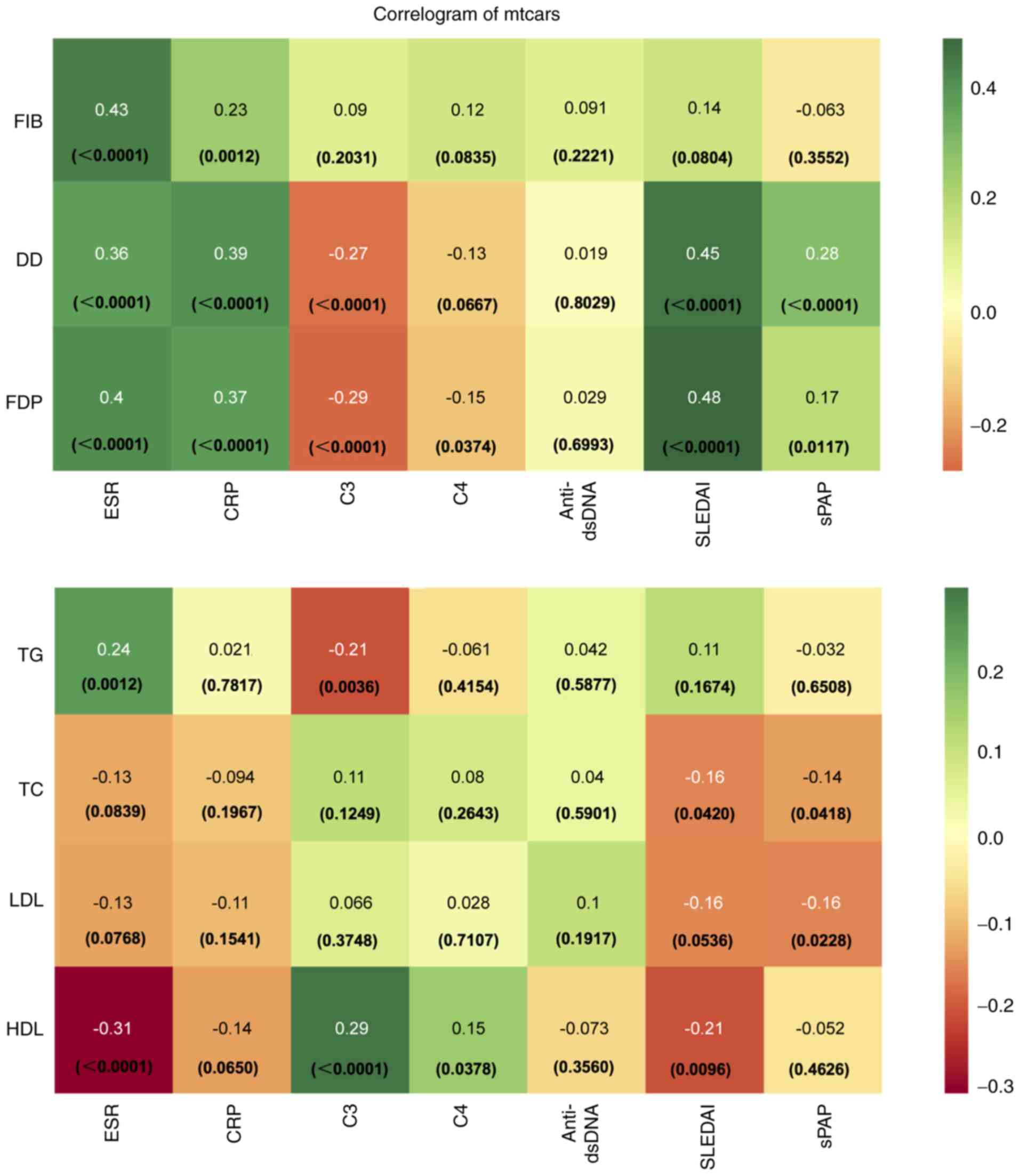 | Figure 3Correlogram of Mtcars dataset of this
study. Presents the degree of correlation between coagulation/lipid
index and SLE disease activity/sPAP. No correlation is shown in
white, positive correlation in green, and negative correlation in
orange. Darker color indicates higher absolute value of the
correlation. SLE, systemic lupus erythematosus; sPAP, pulmonary
artery systolic pressure; FIB, fibrinogen; DD, D-dimer blood test;
FDP, fibrin degradation products; ESR, erythrocyte sedimentation
rate; CRP, C-reactive protein; C3, complement 3; C4, complement 4;
dsDNA, double stranded DNA; SLEDAI, systemic lupus erythematosus
disease activity index; LDL, low-density lipoprotein; HDL,
high-density lipoprotein; TC, total cholesterol; TG,
triglyceride. |
Mediation effects of D-dimer for the
association between LDL and PAH
As aforementioned, patients with PAH had higher
levels of D-dimer and FDP and a lower LDL level compared with the
non-PAH group, and sPAP had a positive association with D-dimer and
FDP and a negative association with TC and LDL. Therefore,
mediation analyses were performed with D-dimer and FDP as potential
mediators for the associations of TC, LDL with the presence of PAH
(Table III). In the present
model, PAH presence was the dependent variable, the TC and LDL
levels were the independent variable, and D-dimer and FDP were the
mediator variables. For patients with no records of lipid
regulating and anticoagulation medications, data indicated that the
relationship between lower LDL and PAH presence was partially
mediated by D-dimer. Mediation analysis indicated that 25.61% of
the effect of low LDL on the presence of PAH in SLE was mediated by
D-dimer. No other mediation effects were found.
 | Table IIIMediation analysis of DD and FDP for
the association of lipid index with PAH. |
Table III
Mediation analysis of DD and FDP for
the association of lipid index with PAH.
| | PAH | |
|---|
| Mediator | Item | Coefficient β | P-value |
|---|
| DD | Total effect of
TC | -1.600 | 0.024 |
| | Effect mediated by
DD | -0.296 | 0.128 |
| | Effect no mediated
by DD | -1.303 | 0.044 |
| | Proportion of
mediation | 0.185 | |
| | Total effect of
LDL | -2.311 | <0.001 |
| | Effect mediated by
DD | -0.592 | 0.024 |
| | Effect no mediated
by DD | -1.719 | 0.032 |
| | Proportion of
mediation | 0.256 | |
| FDP | Total effect of
TC | -1.600 | 0.008 |
| | Effect mediated by
FDP | -0.251 | 0.160 |
| | Effect no mediated
by FDP | -1.349 | 0.024 |
| | Proportion of
mediation | 0.157 | |
| | Total effect of
LDL | -2.312 | <0.001 |
| | Effect mediated by
FDP | -0.480 | 0.088 |
| | Effect no mediated
by FDP | -1.832 | 0.004 |
| | Proportion of
mediation | 0.207 | |
Discussion
To the best of our knowledge, the present study is
the first to report the association between coagulation index and
lipids with PAH. The novel finding of the present study is that the
effect of low LDL on SLE-PAH could mediated by D-dimer, which
mediated 25.61% of this effect.
Mortality remains relatively high in patients with
SLE and PAH (25). Cohort studies
have reported that the prevalence of PAH ranges from 0.5 to 43% in
SLE (8). However, a previous study
estimated a lower prevalence of PAH (2.54%) (26). Johnson et al (27) found a prevalence of 14% in patients
with systemic lupus who underwent transthoracic echocardiogram. In
the present study, the prevalence of PAH in newly diagnosed
drug-naive patients with SLE was 13.3%. PAH can be an initial
presentation of SLE, including severe PAH (28). Moreover, in the present study sPAP
could reach 156 mmHg. Since the presence of PAH indicates a worse
prognosis in patients with SLE, prompt recognition and early
initiation of PAH treatment are extremely important.
Previous studies showed that Raynaud's phenomenon,
pleuritis, pericarditis, disease duration, interstitial lung
disease, anti-RNP antibodies, anti-SSA/Ro antibodies and
anticardiolipin antibodies are independent predictors of PAH in
systemic lupus erythematosus (29). In the present study, age and
D-dimer were independently associated with the development of PAH
in SLE. In the Registry to Evaluate Early and Long-term Pulmonary
Arterial Hypertension Disease Management with right heart
catheterizations (RHC) confirmed SLE-PAH, the mean age at the time
of the PAH diagnosis was ~45 years (45.5±11.9 years) (30). In the present study, PAH presented
at a younger age (~39 years; range 28.5-49 years), and the risk of
PAH increased with the patient's age. The Chinese CSTAR-PAH
registry study revealed a case of SLE-PAH at a younger age
(35.3±10.3 years) (31). Thus, it
is recommended to screen for PAH soon after SLE diagnosis.
A previous study found an increased level of D-dimer
in patients with active SLE (32).
Moreover, the hypercoagulable state is also considered a
contributor in the development of PAH in SLE (33). High fibrinogen and D-dimer levels
are found in SLE-PAH patients (33). In the present study, patients with
SLE-PAH had high D-dimer and FDP levels, and D-dimer was an
independent predictor for SLE-PAH. The changes in thrombogenic risk
factors are thought to be the rationale for using oral
anticoagulation in patients with SLE-PAH as a management
strategy.
Lipid metabolism is important during cellular
metabolism and effective immune responses. Dyslipidemia contributes
to disease pathogenesis and associated comorbidities in autoimmune
rheumatic diseases including SLE (34). Increased cardiovascular disease
(CVD) risk in autoimmune diseases is related to dyslipidemia
(35). Previous data showed that
dyslipidemia is a major factor in the progression of CVD in SLE
(36), but whether dyslipidemia is
associated with PAH in patients with SLE remains unclear. Lipid
homeostasis is dysregulated in patients with PH in which low HDL
and LDL levels have been found (11,37,38);
therefore, their anti-inflammatory properties may be relevant for
predicting disease severity and prognosis in patients with PH, and
the decreased HDL and LDL levels is also associated with the right
heart function in patients with idiopathic PAH (39). Wang et al (13) found that the optimal cutoff value
of the serum HDL concentration for predicting PAH was 1.32 mmol/l.
Moreover, it remains unclear if cholesterol levels are altered in
patients with SLE-PAH. Nonetheless, the results of the present
study demonstrated decreased LDL level in the development of PAH in
patients with SLE. In the present study, LDL was negatively
associated with pulmonary artery pressure. Reduced LDL levels have
also been represented in other chronic diseases such as rheumatoid
arthritis (40), cancer diseases
(41), end-stage renal failure
(42) or chronic heart failure
(43). However, the exact
mechanisms accounting for the association between low LDL and PAH
have not been fully understood. To the best of our knowledge, the
present study is the first reporting changes in LDL levels in
patients with SLE-PAH. This may be due to chronic inflammatory
condition (44), malnutrition
(45) and altered liver metabolism
(46) in patients with SLE-PAH.
The present study showed that the relationship between low LDL and
occurrence of PAH in SLE is partially mediated by D-dimer.
Nevertheless, the potential molecular mechanisms need to be further
investigated.
To the best of our knowledge, the potential
mechanisms by which low LDL affects PH, including SLE-PAH, have not
been clarified. Limited basic research data suggest that LDL
receptor is downregulated in the lung tissue of patients with PH,
while LDL receptor knockout mice can develop PH; in addition, LDL
receptor knockdown significantly increases proliferation of human
pulmonary artery smooth muscle cells in vitro (47). However, the potential molecular
mechanisms have not been clarified, which need more related basic
researches.
The strength of the present study is that the
intermediary variable D-dimer is first used to connect the
relationship between dyslipidemia and PAH in SLE. The present
cohort study included newly diagnosed drug-naïve patients, whereas
patients using lipid-lowering/anticoagulant were excluded to
minimize the effect of possible confounding factors. To the best of
our knowledge, the present results demonstrated for the first time
that LDL could affect the PAH severity via D-dimer in patients with
SLE. The present study also has several limitations. First of all,
no measurements through RHC were made to quantify pulmonary artery
pressure. Nonetheless, considering the invasive nature of RHC,
indirect echocardiography was used to estimate pulmonary artery
systolic pressure. Although Doppler echocardiography is widely
accepted as a screening tool in PH, it has certain limitations. A
higher discrepancy between echocardiographic and RHC data has been
reported (48). Secondly, the
present study is cross-sectional, therefore it is difficult to
identify the causal or temporal relationship between LDL levels and
the presence of PAH in SLE. Follow-up is needed to clarify whether
LDL level is associated with PH progression and patients'
prognosis. Thirdly, the present study had a relatively small sample
size. Lastly, the present study did not explore the exact molecular
mechanisms of the interplay between coagulation index and
lipids.
Early recognition of PAH in patients with SLE is of
utmost importance for patient' prognosis. The present data
indicated that the effect of low LDL on SLE-PAH may be mediated by
D-dimer, which mediated 25.61% of this effect. Dynamic monitoring
and regulation of lipids would be important in the PAH early
recognition, treatment and prognosis prediction in patients with
SLE, while lipid modulation may achieve additional benefits to the
management of patients with SLE-PAH. Further studies are necessary
to explore the underlying mechanisms of this apparent association
and the mediation effect.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Natural Science
Foundation of Shaanxi Province (grant no. 2022JQ-761 and
2022JQ-940), Chinese Postdoctoral Science Foundation (grant no.
2021M702610) and Fundamental Research Funds for the Central
Universities (grant no. xzy012020060).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW, LH and JH conceived the study design. JH, QA and
CZ collected the medical records, and analyzed and interpreted
data. QA and CZ confirm the authenticity of all the raw data. LW
was a major contributor in writing the manuscript. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by Ethics Committee of First
Affiliated Hospital of Xi'an Jiaotong University (approval no.
2022-291) and was performed in accordance with the Declaration of
Helsinki. The study protocol and data collection instruments were
submitted and approved by the Data Protection Commission of Xi'an
Jiaotong University. All patients provided their written informed
consent prior to inclusion in the study.s
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoeper MM, Humbert M, Souza R, Idrees M,
Kawut SM, Sliwa-Hahnle K, Jing ZC and Gibbs JS: A global view of
pulmonary hypertension. Lancet Respir Med. 4:306–322.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Raza T and Dilawar M: Pulmonary
hypertension: A comprehensive review. Heart Views. 8:90–99.
2007.
|
|
3
|
McGoon MD and Miller DP: REVEAL: A
contemporary US pulmonary arterial hypertension registry. Eur
Respir Rev. 21:8–18. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Athanasiou KA, Sahni S, Rana A and Talwar
A: Diagnosing and managing scleroderma-related pulmonary arterial
hypertension. JAAPA. 30:11–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shirai Y, Yasuoka H, Okano Y, Takeuchi T,
Satoh T and Kuwana M: Clinical characteristics and survival of
Japanese patients with connective tissue disease and pulmonary
arterial hypertension: A single-centre cohort. Rheumatology
(Oxford). 51:1846–1854. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kang KY, Jeon CH, Choi SJ, Yoon BY, Choi
CB, Lee CH, Suh CH, Lee CW, Cho CS, Nam EJ, et al: Survival and
prognostic factors in patients with connective tissue
disease-associated pulmonary hypertension diagnosed by
echocardiography: Results from a Korean nationwide registry. Int J
Rheum Dis. 20:1227–1236. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jing ZC, Xu XQ, Han ZY, Wu Y, Deng KW,
Wang H, Wang ZW, Cheng XS, Xu B, Hu SS, et al: Registry and
survival study in Chinese patients with idiopathic and familial
pulmonary arterial hypertension. Chest. 132:373–379.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li M, Wang Q, Zhao J, Li Z, Ye Z, Li C, Li
X, Zhu P, Wang Z, Zheng Y, et al: Chinese SLE treatment and
research group (CSTAR) registry: II. Prevalence and risk factors of
pulmonary arterial hypertension in Chinese patients with systemic
lupus erythematosus. Lupus. 23:1085–1091. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen HA, Hsu TC, Yang SC, Weng CT, Wu CH,
Sun CY and Lin CY: Incidence and survival impact of pulmonary
arterial hypertension among patients with systemic lupus
erythematosus: A nationwide cohort study. Arthritis Res Ther.
21(82)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jankov RP, Luo X, Cabacungan J, Belcastro
R, Frndova H, Lye SJ and Tanswell AK: Endothelin-1 and O2-mediated
pulmonary hypertension in neonatal rats: A role for products of
lipid peroxidation. Pediatr Res. 48:289–298. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kopeć G, Waligóra M, Tyrka A, Jonas K,
Pencina MJ, Zdrojewski T, Moertl D, Stokwiszewski J, Zagożdżon P
and Podolec P: Low-density lipoprotein cholesterol and survival in
pulmonary arterial hypertension. Sci Rep. 7(41650)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Al-Naamani N, Palevsky HI, Lederer DJ,
Horn EM, Mathai SC, Roberts KE, Tracy RP, Hassoun PM, Girgis RE,
Shimbo D, et al: Prognostic significance of biomarkers in pulmonary
arterial hypertension. Ann Am Thorac Soc. 13:25–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang GF, Guan LH, Zhou DX, Chen DD, Zhang
XC and Ge JB: Serum high-density lipoprotein cholesterol is
significantly associated with the presence and severity of
pulmonary arterial hypertension: A retrospective cross-sectional
study. Adv Ther. 37:2199–2209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jonas K, Magoń W, Waligóra M, Seweryn M,
Podolec P and Kopeć G: High-density lipoprotein cholesterol levels
and pulmonary artery vasoreactivity in patients with idiopathic
pulmonary arterial hypertension. Pol Arch Intern Med. 128:440–446.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pašková U: Lipid profile and risks of
cardiovascular diseases in conditions of rheumatoid arthritis.
Ceska Slov Farm. 68:219–228. 2019.PubMed/NCBI
|
|
16
|
Kato F, Tanabe N, Urushibara T, Kasai H,
Takeuchi T, Sekine A, Suda R, Nishimura R, Jujo T, Sugiura T, et
al: Association of plasma fibrinogen and plasminogen with prognosis
of inoperable chronic thromboembolic pulmonary hypertension. Circ
J. 78:1754–1761. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shitrit D, Bendayan D, Rudensky B, Izbicki
G, Huerta M, Fink G and Kramer MR: Elevation of ELISA D-dimer
levels in patients with primary pulmonary hypertension.
Respiration. 69:327–329. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kiatchoosakun S, Ungkasekvinai W,
Wonvipaporn C, Tatsanavivat P, Foocharoen C, Suwannaroj S and
Nanagara R: D-dimer and pulmonary arterial hypertension in systemic
sclerosis. J Med Assoc Thai. 90:2024–2029. 2007.PubMed/NCBI
|
|
19
|
Tracy RP: Diet and hemostatic factors.
Curr Atheroscler Rep. 1:243–248. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hochberg MC: Updating the American college
of rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40(1725)1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pyxaras SA, Pinamonti B, Barbati G,
Santangelo S, Valentincic M, Cettolo F, Secoli G, Magnani S, Merlo
M, Lo Giudice F, et al: Echocardiographic evaluation of systolic
and mean pulmonary artery pressure in the follow-up of patients
with pulmonary hypertension. Eur J Echocardiogr. 12:696–701.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Crouse WL, Keele GR, Gastonguay MS,
Churchill GA and Valdar W: A bayesian model selection approach to
mediation analysis. PLoS Genet. 18(e1010184)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Baron RM and Kenny DA: The
moderator-mediator variable distinction in social psychological
research: Conceptual, strategic, and statistical considerations. J
Pers Soc Psychol. 51:1173–1182. 1986.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shrout PE and Bolger N: Mediation in
experimental and nonexperimental studies: New procedures and
recommendations. Psychol Methods. 7:422–445. 2002.PubMed/NCBI
|
|
25
|
Guo X, Lai J, Wang H, Tian Z, Wang Q, Zhao
J, Li M, Fang Q, Fang L, Liu Y and Zeng X: Predictive value of
non-invasive right ventricle to pulmonary circulation coupling in
systemic lupus erythematosus patients with pulmonary arterial
hypertension. Eur Heart J Cardiovasc Imaging. 22:111–118.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qu J, Li M, Wang Y, Duan X, Luo H, Zhao C,
Zhan F, Wu Z, Li H, Yang M, et al: Predicting the risk of pulmonary
arterial hypertension in systemic lupus erythematosus: A chinese
systemic lupus erythematosus treatment and research group cohort
study. Arthritis Rheumatol. 73:1847–1855. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Johnson SR, Gladman DD, Urowitz MB, Ibanez
D and Granton JT: Pulmonary hypertension in systemic lupus. Lupus.
13:506–509. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kiani R, Siddiqui MD and Tantoush H:
Severe pulmonary hypertension as initial presentation of SLE: A
case report and literature review. Case Rep Rheumatol.
2020(6014572)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lian F, Chen D, Wang Y, Ye Y, Wang X, Zhan
Z, Xu H, Liang L and Yang X: Clinical features and independent
predictors of pulmonary arterial hypertension in systemic lupus
erythematosus. Rheumatol Int. 32:1727–1731. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chung L, Liu J, Parsons L, Hassoun PM,
McGoon M, Badesch DB, Miller DP, Nicolls MR and Zamanian RT:
Characterization of connective tissue disease-associated pulmonary
arterial hypertension from REVEAL: Identifying systemic sclerosis
as a unique phenotype. Chest. 138:1383–1394. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu X, Zhou F, Yang Y, Wang W, Niu L, Zuo
D, Li X, Hua H, Zhang B, Kou Y, et al: MiR-409-3p and MiR-1896
co-operatively participate in IL-17-induced inflammatory cytokine
production in astrocytes and pathogenesis of EAE mice via targeting
SOCS3/STAT3 signaling. Glia. 67:101–112. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ferreira KS, Cicarini WB, Alves LCV,
Loures CMG, Campos FMF, Santos LID, da Silva MVF, Guimarães TMD,
Toledo VPCP, Reis EA, et al: Correlation between active disease and
hypercoagulability state in patients with systemic lupus
erythematosus. Clin Chim Acta. 490:107–112. 2019.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
33
|
Xu SZ, Liang Y, Li XP, Li XM, Shuai ZW,
Leng RX, Pan HF and Ye DQ: Features associated with pulmonary
arterial hypertension in Chinese hospitalized systemic lupus
erythematosus patients. Clin Rheumatol. 37:1547–1553.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Y, Yu H and He J: Role of
dyslipidemia in accelerating inflammation, autoimmunity, and
atherosclerosis in systemic lupus erythematosus and other
autoimmune diseases. Discov Med. 30:49–56. 2020.PubMed/NCBI
|
|
35
|
Robinson G, Pineda-Torra I, Ciurtin C and
Jury EC: Lipid metabolism in autoimmune rheumatic disease:
Implications for modern and conventional therapies. J Clin Invest.
132(e148552)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou B, Xia Y and She J: Dysregulated
serum lipid profile and its correlation to disease activity in
young female adults diagnosed with systemic lupus erythematosus: A
cross-sectional study. Lipids Health Dis. 19(40)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Heresi GA, Aytekin M, Newman J, DiDonato J
and Dweik RA: Plasma levels of high-density lipoprotein cholesterol
and outcomes in pulmonary arterial hypertension. Am J Respir Crit
Care Med. 182:661–668. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhao QH, Peng FH, Wei H, He J, Chen FD, Di
RM, Jiang X, Jiang R, Chen YJ, Heresi GA and Jing ZC: Serum
high-density lipoprotein cholesterol levels as a prognostic
indicator in patients with idiopathic pulmonary arterial
hypertension. Am J Cardiol. 110:433–439. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen J, Rathinasabapathy A, Luo J, Yang X,
Luo P, Chen Y, Li Z and Li J: Differential serum lipid distribution
in IPAH and CHD-PAH patients. Respir Med.
191(106711)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Choy E and Sattar N: Interpreting lipid
levels in the context of high-grade inflammatory states with a
focus on rheumatoid arthritis: A challenge to conventional
cardiovascular risk actions. Ann Rheum Dis. 68:460–469.
2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Alexopoulos CG, Pournaras S, Vaslamatzis
M, Avgerinos A and Raptis S: Changes in serum lipids and
lipoproteins in cancer patients during chemotherapy. Cancer
Chemother Pharmacol. 30:412–416. 1992.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Peev V, Nayer A and Contreras G:
Dyslipidemia, malnutrition, inflammation, cardiovascular disease
and mortality in chronic kidney disease. Curr Opin Lipidol.
25:54–60. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gombos T, Förhécz Z, Pozsonyi Z, Jánoskuti
L, Prohászka Z and Karádi I: Long-term survival and apolipoprotein
A1 level in chronic heart failure: Interaction with tumor necrosis
factor α-308 G/A polymorphism. J Card Fail. 23:113–120.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu Y, Coresh J, Eustace JA, Longenecker
JC, Jaar B, Fink NE, Tracy RP, Powe NR and Klag MJ: Association
between cholesterol level and mortality in dialysis patients: Role
of inflammation and malnutrition. JAMA. 291:451–459.
2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Araújo JP, Friões F, Azevedo A, Lourenço
P, Rocha-Gonçalves F, Ferreira A and Bettencourt P: Cholesterol-a
marker of nutritional status in mild to moderate heart failure. Int
J Cardiol. 129:65–68. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Møller S and Bernardi M: Interactions of
the heart and the liver. Eur Heart J. 34:2804–2811. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Umar S, Ruffenach G, Moazeni S,
Vaillancourt M, Hong J, Cunningham C, Cao N, Navab S, Sarji S, Li
M, et al: Involvement of low-density lipoprotein receptor in the
pathogenesis of pulmonary hypertension. J Am Heart Assoc.
9(e012063)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tehrani DM, Grinstein J, Kalantari S, Kim
G, Sarswat N, Adatya S, Sayer G and Uriel N: Cardiac output
assessment in patients supported with left ventricular assist
device: Discordance between thermodilution and indirect fick
cardiac output measurements. ASAIO J. 63:433–437. 2017.PubMed/NCBI View Article : Google Scholar
|