Introduction
Diabetes mellitus (DM) is a chronic metabolic
disease, characterized by hyperglycemia. Diabetic angiopathy, one
of the most common and serious complications, is also the main
cause of mortality in type 2 diabetes mellitus (T2DM) (1,2).
Long-term exposure of endothelial cells (ECs) to high glucose (HG)
contents leads to inflammation and oxidative stress in diabetics,
resulting in vascular endothelial cell injury and eventually
vascular endothelial dysfunction (3).
The mechanisms by which hyperglycemia influences
endothelial function are multiple. The vascular dysfunction in the
setting of diabetes is associated with increased vascular oxidative
stress and low-grade inflammation (4). Under hyperglycemic conditions, the
excessive production of reactive oxygen species (ROS) is one of the
key factors in the development of pathological changes in the
endothelium, causing endothelial dysfunction or cell apoptosis
(5). The endothelial pathology,
accompanied by excessive production of factors, is related to the
inflammatory process, such as TNF-α and IL-6 (6,7).
Hyperglycemia induces the production of proinflammatory cytokines
and growth factors by activating the key signal pathways that are
associated with the MAPK and NF-κB (8). Oxidative stress and inflammation are
the major mechanisms underlying the pathogenesis of DM
complications. The overproduction of ROS, which results in
oxidative damage, including lipid peroxidation, protein oxidation
and DNA damage, can lead to cell death. Furthermore, ROS can act as
the second messengers to activate transcription factors such as
NF-κB and the activation of NF-κB is involved in the multiple
aspects of diabetic pathology, including cellular differentiation,
survival, autophagy and apoptosis (9).
Autophagy serves a vital role in cellular
homeostasis by acting as a housekeeper to eliminate the damaged
organelles. The impairment of autophagy, which is observed in the
endothelial injury, is implicated especially in the DM-induced
endothelial dysfunction. In vascular pathogenesis, autophagy can
act as a survival pathway, protecting endothelial cells from
oxidative stress (10,11). Vascular endothelial dysfunction is
recognized as an initial step of diabetic vascular complications,
which might be caused by hyperglycemia-induced apoptosis (12). A complex interplay is engaged
between autophagy and apoptosis, which occurs simultaneously within
the same cell under various stresses. The interaction between
autophagy and ROS is frequently correlated with cell survival/death
in various diseases, including DM-related endothelial oxidative
damage (13,14). The regulation of autophagy,
therefore, can attenuate the endothelial oxidative damage,
eliminate the endothelial dysfunction and promote tissue repair,
especially the therapeutic angiogenesis (15). The drug 3-Methyladenine (3-MA),
which is widely used as an autophagy inhibitor in animal
experiments, has significant neurological impacts on cerebral
infarction (16,17).
Curcumin [(CUR);
1,7-bis-(4-hydroxy-3-methoxy-phenyl)-1,6-heptadiene-3,5-dione] is a
polyphenol extracted from the Curcuma longa plant, commonly
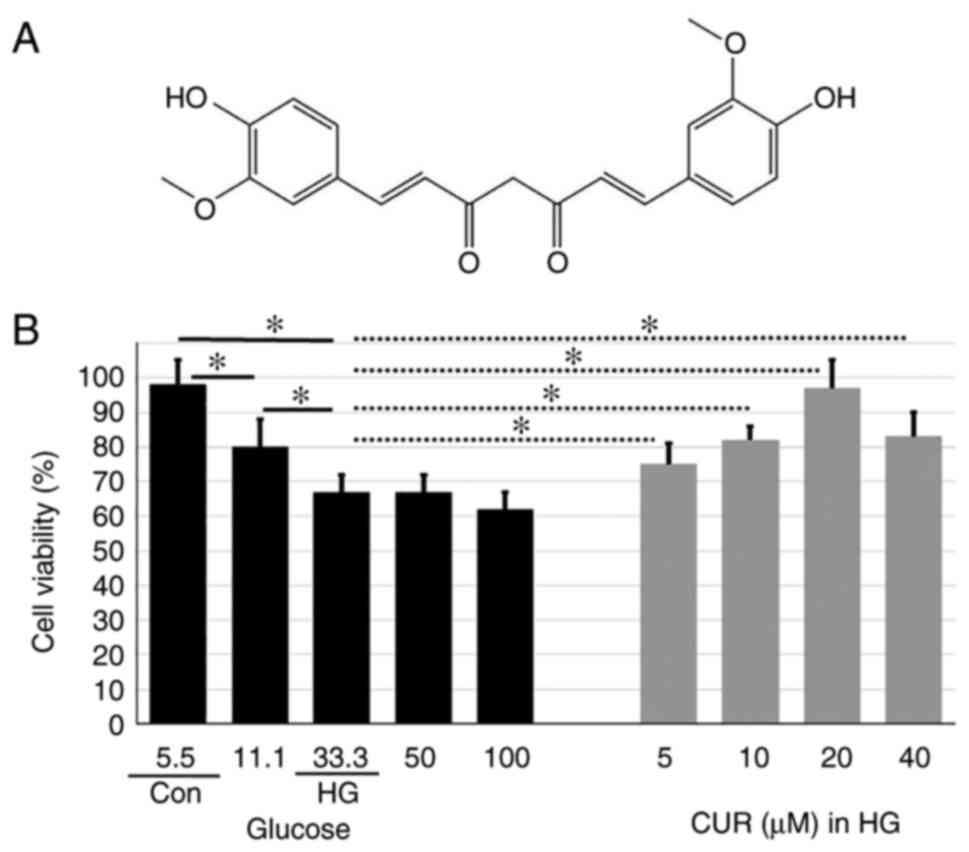
known as turmeric. Fig. 1A shows
the chemical structure of CUR. Accumulating evidence suggests that
CUR can act as an agent with anti-inflammatory, antioxidant,
anticarcinogenic and antilipidemic effects (18,19).
Not only has the natural CUR been investigated extensively for
clinical application, but also some synthetic curcumin analogs
(20,21). CUR can improve gastric emptying in
rats by blocking the production of oxidative stress and abolishing
the NF-κB signal transduction with diabetic gastroparesis (22). In addition, CUR also induces
autophagy to protect the vascular endothelial cells and reduces the
cell apoptosis from the oxidative stress damage (23-25),
suggesting a potential mechanism underlying the anti-apoptosis
effects of CUR (26,27).
Overall, previous studies have confirmed that HG
contents can promote oxidative stress and apoptosis and CUR has a
strong antioxidant and antiapoptosis effect, but the exact
mechanisms are uncertain. To understand the pharmacological
mechanisms of CUR, the current study investigated the antiapoptosis
effects of CUR in human umbilical vein endothelial cells (HUVECs).
HUVECs were pretreated with various concentrations of CUR before
the HG stimulation. Alterations in the expression of autophagy,
inflammation and apoptosis-related proteins, cell viability and
activation of the ROS were observed. The goal was to provide
evidence regarding the protective mechanism of CUR against the
HG-induced damage to HUVECs.
Materials and methods
Experimental design
The whole experiment was conducted in several parts.
To explore the dose-dependent effects of CUR on the viability of
HUVECs, the cells were pretreated for 4 h at 37˚C with different
concentrations of CUR (5, 10, 20 and 40 µM) and then incubated with
HG concentration for 24 h. Exposure of vascular ECs to a glucose
level greater than 10 mmol/l (in vitro or in vivo, as
in diabetes mellitus) is considered an HG condition. As the cell
viability is relatively high under normal glucose exposure with no
obvious glucose toxicity and excessive oxidative stress, the
effects of CUR on cell viability were examined only with CUR added
to an HG concentration. The cell viability was determined with the
use of an MTT assay. To further clarify the effect of CUR on
apoptosis and autophagy, HUVECs that were pretreated with 20 µM CUR
and then incubated in 33.3 mmol/l glucose (HG) were used. The
apoptosis was evaluated using the TUNEL assay and the expression of
the autophagy proteins was measured by western blotting. To gain an
insight into the apoptosis signaling pathway, the expression of
caspase proteins was examined by immunoblotting. To confirm whether
ROS and NF-κB would participate in the anti-apoptosis effect of
CUR, HUVECs were incubated for 4 h at 37˚C with 20 µM CUR and then
for 24 h with or without the autophagy inhibitor 3-methyladenine
(3-MA, 10 mM) in the HG condition. The ROS production was detected
with a flow cytometer while the NF-κB-related protein was detected
with the western blotting.
Cell culture
Immortalized HUVECs (Lonza Group Ltd.) were cultured
in Dulbecco's modified Eagle's medium (DMEM; MilliporeSigma),
supplemented with 10% FBS and 1% penicillin-streptomycin in a
humidified 5% CO2, 37˚C incubator (Thermo Fisher
Scientific, Inc.). All parts of the experiment on HUVECs were
conducted at the ~3-7 passages. Those HUVECs that were cultured in
a medium of 33.3 mmol/l glucose for 24 h served as the HG group
(8), while those HUVECs that were
cultured in a medium of 5.5 mmol/l glucose for an equivalent time
served as the control (Con) group.
MTT cell viability assay
The colorimetric MTT assay was used to detect the
cell viability in the 96-well plates. The MTT substrate was
prepared in a physiologically balanced solution, added to HUVECs in
culture and incubated for 4 h at 37˚C. Viable cells with active
metabolism converted MTT into purple formazan. The optical density
of formazan (directly proportional to the number of viable cells)
after dissolution in DMSO was measured at 450 nm with a microplate
reader (Molecular Devices, LLC.). The mean optical density of 6
wells was used to calculate the cell viability percentage relative
to the cell viability of control wells.
Caspase-3 activity
The caspase-3 activity was determined with a
Caspase-3 Assay kit (Colorimetric) ab39401 (Abcam) according to the
manufacturer's protocol. Briefly, the cells were lysed with the
cell lysis buffer, incubated on ice for 10 min at 37˚C and
centrifuged at 10,000 g for 1 min. The supernatant was collected to
measure the protein concentration with the bicinchoninic acid (BCA)
assay (Thermo Fisher Scientific, Inc.). To measure the caspase-3
activity, the sample was mixed with an equal volume of 2X reaction
buffer (containing 10 mM DTT) and 200 µM of DEVD-p-NA substrate.
The mixture was incubated at 37˚C for 2 h. The optical density was
measured at 400 nm with a microplate reader (BioTek Instruments,
Inc.).
Assay of intracellular ROS
The cells were seeded in a 6-well plate at a density
of 5x104 cells/well. The levels of intracellular ROS
were measured with the fluorescent probe
dichloro-dihydro-fluorescein diacetate (DCFH-DA; Beijing
Baiaosentai Biotechnology). Following treatment with stimuli, the
cells were incubated with DCFH-DA (10 µM) at 37˚C for 30 min. The
cells and probe were mixed thoroughly for 30 min by inverting the
flask once every 3-5 min. The cell suspension was centrifuged at
12,000 x g for 5 min at 4˚C, and the supernatant was discarded. The
cells were resuspended and washed 3 times with 1 ml serum-free
medium to remove the DCFH-DA that did not enter the cells. The
cells were then centrifuged again at 12,000 x g for 5 min at 4˚C,
and the supernatant was discarded, followed by the addition of 500
ml phosphate-buffered saline (PBS) to resuspend the cells. After 30
min, the cells were subjected to flow cytometry (using the
parameters set for FITC) at an excitation wavelength of 535 nm and
an emission wavelength of 610 nm to detect the fluorescence
intensity before and after stimulation. The fluorescence images
were obtained with fluorescence microscopy (BX41F, Olympus
Corporation).
Western blotting analysis
HUVECs were homogenized in RIPA lysis (Wuhan
Servicebio Technology Co., Ltd.) buffer to obtain total proteins.
According to the previous studies (28,29),
the protein concentrations were measured by a BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.) and denatured at 72˚C for 10 min.
Protein (~50 µg ) was separated by 10% SDS-PAGE and then
transferred onto a PVDF membranes (MilliporeSigma). After blocking
for 1 h at room temperature with 5 % skimmed milk in TBS buffer (10
mM Tris, 150 mM NaCl), the membranes were probed with primary
antibodies, including rabbit polyclonal antibodies (Cell Signaling
Technology, Inc.): Anti-LC3 (1:500; cat. no. 4108), anti-Beclin1
(1:500; cat. no. 3738), anti-cleaved-caspase3 (1:500; cat. no.
9661), anti-Bcl-2 (1:1,000; cat. no. 3498), anti-Bax (1:1,000; cat.
no. 2774) and a mouse monoclonal antibody against β-actin (1:1,000;
cat. no. 8457) at 4˚C overnight. Next, the membranes were washed
four times, 15 min each, with TBST buffer (10 mM Tris, 150 mM NaCl,
and 0.1% Tween-20) and incubated for 30 min at 37˚C with
appropriate HRP-conjugated secondary antibodies (anti-rabbit IgG,
HRP-linked antibody cat. no. 7074; Cell Signaling Technology,
Inc.). The protein bands were visualized with chemiluminescent
reagents following the manufacturer's instructions and exposed to
Hyperfilm-ECL (Amersham; Cytiva). Densitometry analysis of band
intensity was performed using ImageJ software v 1.8.0 (National
Institutes of Health).
Apoptosis assay
Cell apoptosis was determined with an Annexin V-FITC
Apoptosis kit (cat. no. C1062s; Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. In total,
1x106 cells were collected and the cells from each
sample were suspended in 195 µl of 1X Annexin V-FITC binding buffer
and 5 µl Annexin V-FITC. The cells were incubated at room
temperature for 10 min. Then, each sample was centrifuged at 12,000
x g for 5 min, suspended again in 190 µl of binding buffer, to
which was added 10 µl of propidium iodide (PI) working solution.
The suspension was mixed and incubated in the dark at room
temperature for 15 min. Finally, cell apoptotic rates (early +
late) were determined using a flow cytometer (FACSCanto II; BD
Biosciences). FlowJo version 7.6.1 software (FlowJo LLC) was used
to analyze the data.
Statistical analysis
Data analysis was conducted with the GraphPad Prism
8 software. All results were presented as mean ± standard deviation
(SD). To compare measurements obtained in different test
conditions, a one-way analysis of variances (ANOVA) was used to
examine the effect of test condition on the dependent variable. The
post-hoc analysis was conducted with Tukey's test for pairwise
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
CUR enhances HUVEC viability in
HG
The first part of the experiment was to measure the
cell viability in different cell groups with the purpose to show
the protective ability of CUR against the damage induced by HG
conditions (Fig. 1B). The cell
variability for cells that were treated only with different glucose
concentrations (5.5-100 mmol/l) for 24 h was also included in
Fig. 1B. The cell viability
decreased in a dose-dependent manner from the Con group (5.5 mmol/l
glucose) to the HG group (33.3 mmol/l glucose). In addition, the
cell viability for the HG group was similar to the cell viability
for cells treated with higher glucose concentrations (50 and 100
mmol/l). The cell viability for the HG group was significantly
lower than the cell viability for the Con group, P<0.01
(Fig. 1B), indicating HG
conditions reduced the number of healthy cells in the sample.
Meanwhile, for those cells that were pretreated with 5, 10, 20
and40 µM CUR for 4 h and then incubated in 33.3 mmol/l HG for 24 h,
the cell activity increased in a dose-dependent manner that was
peaked at 20 µM CUR. Compared to the cell viability (67%) for the
HG group, the cell viability (96%) for cells treated with 20 µM CUR
in the HG condition was significantly higher, P<0.05 (Fig. 1B), suggesting the 20 µM CUR
treatment could significantly enhance the cell viability in HG
conditions. Thus, the 20 µM CUR concentration provided the optimal
cell viability enhancement in HG conditions and the cells treated
with 20 µM CUR in HG were labeled as the HG+CUR group for the
subsequent parts of the experiment.
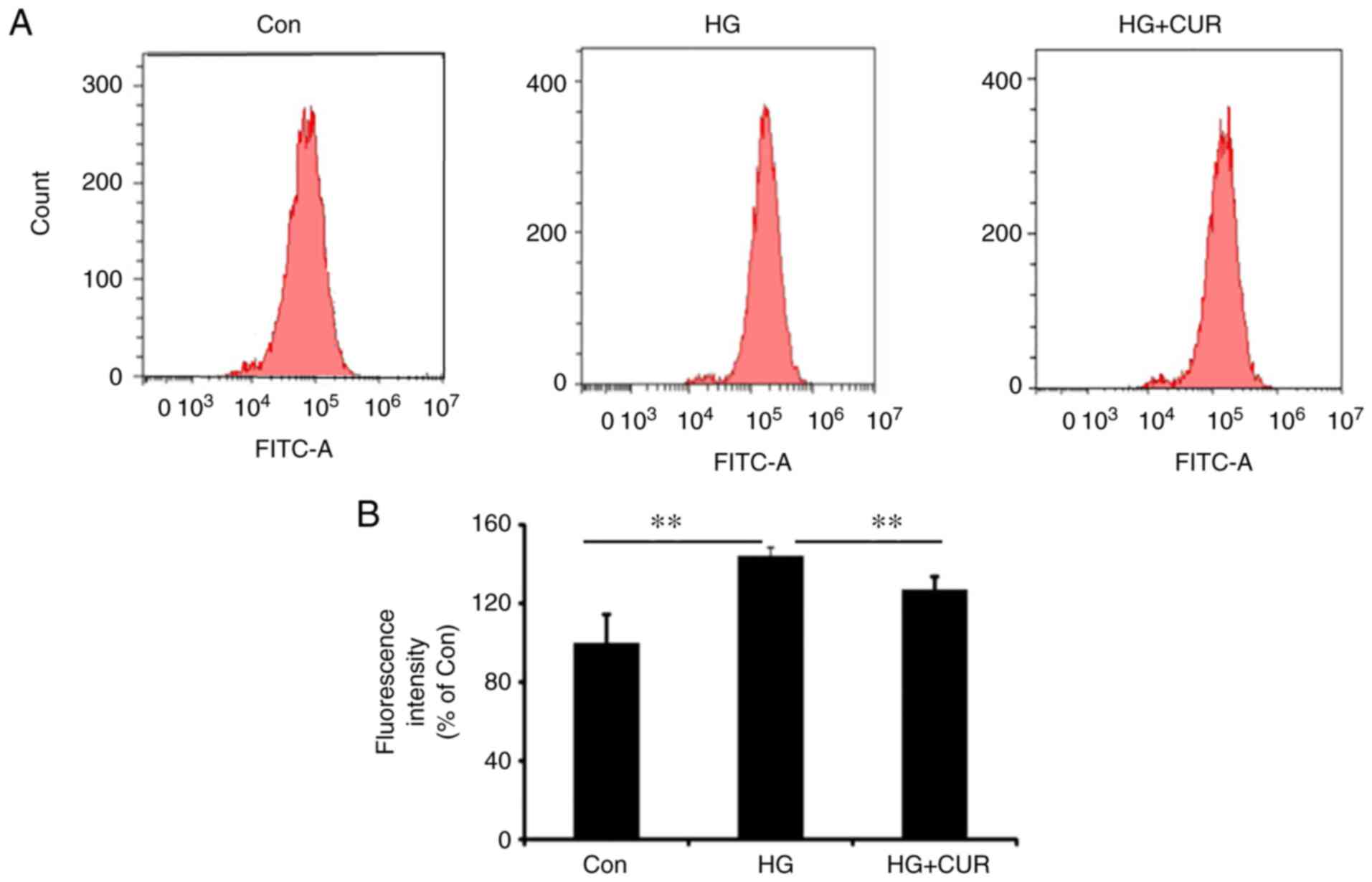
CUR reduces ROS generation in HG
The second part of the experiment was conducted to
measure the ROS content for the three groups (Con, HG and HG+CUR)
to show the effect of CUR on ROS generation in a HG condition.
Fig. 2A shows the histogram of
counting the ROS content using a flow cytometer for the three
groups. The fluorescence intensity of the ROS content (%) among the
three groups is presented in Fig.
2B. The ROS content in the HG group was significantly higher
than the ROS content in the Con group, P<0.01; and the ROS
content in the HG+CUR group was significantly lower than the ROS
content in the HG group, P<0.01 (Fig. 2B). Therefore, HG conditions could
induce ROS production in HUVECs, while the CUR treatment
significantly decreased the intracellular ROS generation in the HG
condition.
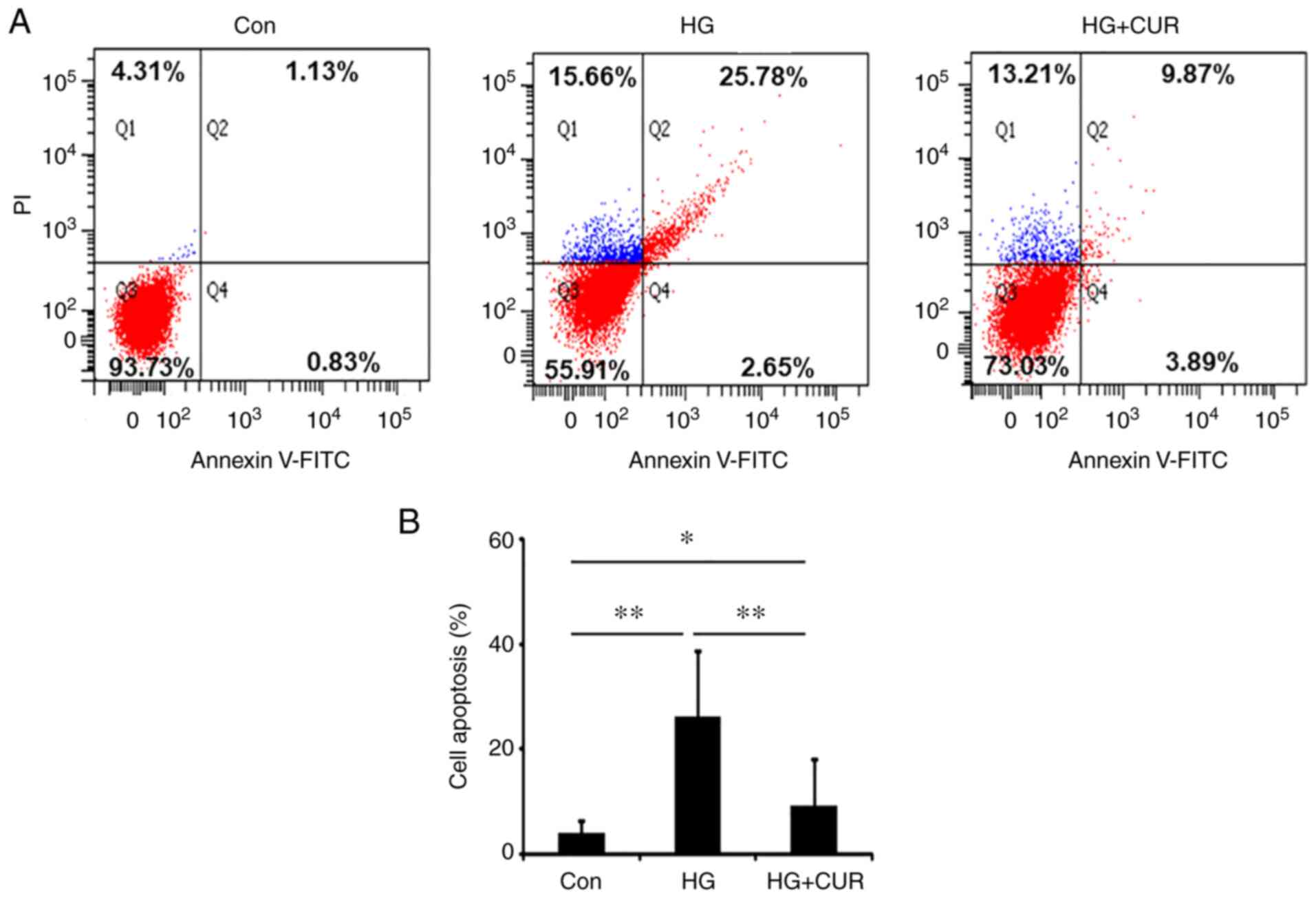
CUR reduces HG-induced HUVEC
apoptosis
The third part of the experiment determined whether
CUR could reduce cell apoptosis in the HG-induced injury model.
Fig. 3A shows the detection of
apoptosis in two-dimension using FITC Annexin V and PI for the
three groups: Con, HG and HG+CUR. Fig.
3B shows the apoptosis rate detected with the TUNEL assay. The
apoptosis rate in the HG group was significantly higher than the
apoptosis rate in the Con group, P<0.01 (Fig. 3B), suggesting the HG treatment for
24 h could sufficiently induce HUVEC apoptosis. In addition, the
cell apoptosis rate in the HG+CUR group was reduced significantly
when compared with the apoptosis rate in the HG group, P<0.01
(Fig. 3B), demonstrating that the
CUR treatment could reduce the apoptosis of HUVECs in the HG
condition. These findings suggested that CUR might have a
protective effect on the endothelial cell apoptosis that was
induced by HG conditions.
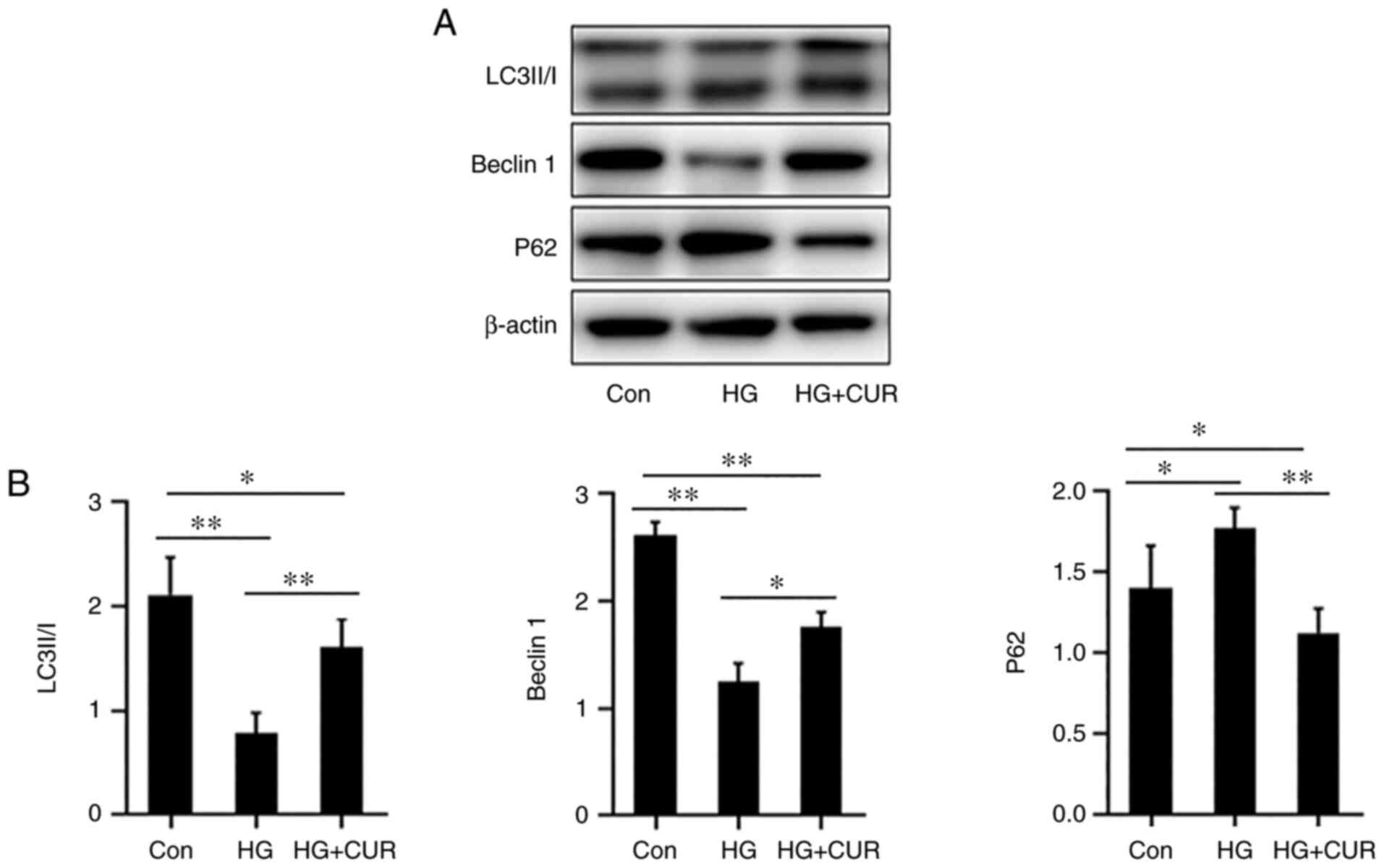
CUR enhances autophagy in HG-cultured
HUVECs
The fourth part of the experiment was to determine
whether the CUR treatment could enhance the cell autophagy of
endothelial cells in HG stress. The autophagy-related protein
expressions of p62, Beclin1 and LC3-II/I were detected with the
western blotting (Fig. 4A). In
Fig. 4B, when compared with the
Con group, the stress in the HG group could significantly increase
the expression of p62 (P<0.05) while decreasing the expression
of Beclin1 and LC3-I/II (P<0.01). However, when compared with
the protein expressions in the HG group, the expression of p62 in
the HG+CUR group was significantly lower (P<0.01) and the
expressions of Beclin1 and LC3-II/I were significantly higher
(P<0.05). These results indicated that CUR enhanced autophagy
for HUVECs that were incubated in the HG condition.
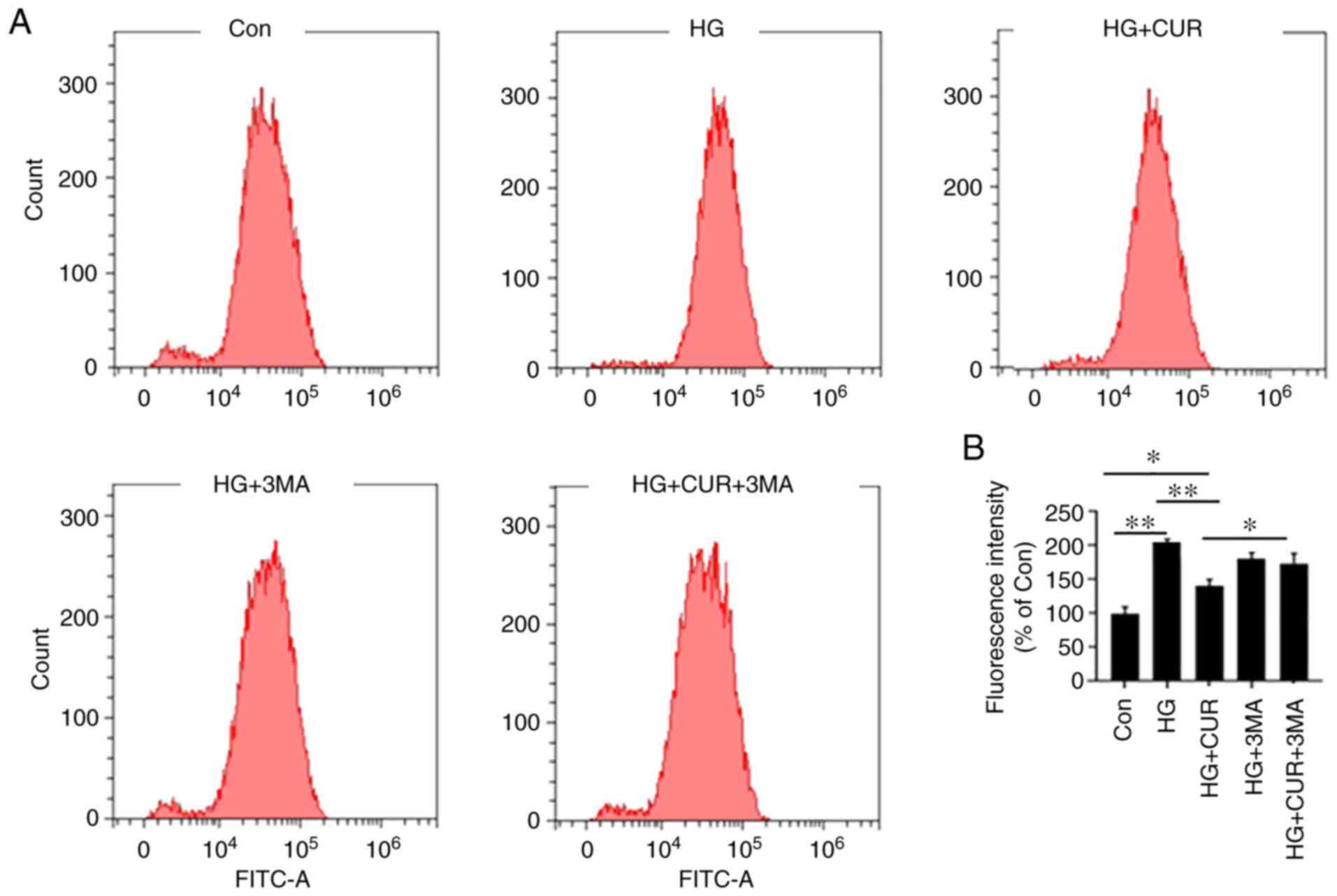
Autophagy inhibition attenuates the
antioxidant effect of CUR
The fifth part of the experiment was to investigate
the interaction between autophagy and ROS in the HG+CUR group
(Fig. 5). The amount of ROS was
measured with the flow cytometer (fluorescence intensity). In
Fig. 5B, by comparing the results
in the Con group, the intracellular ROS in the HG group were
significantly increased, P<0.01. However, compared with the ROS
in the HG group, the CUR treatment in the HG+CUR group
significantly decreased the overproduced ROS that was triggered by
HG, P<0.01. Furthermore, the inhibition of the HG-induced ROS in
the HG+CUR group was significantly attenuated by the autophagy
inhibitor 3-MA treatment as observed in the HG+CUR+3MA group,
P<0.05. Therefore, the CUR treatment could decrease ROS
production in the HG condition, whereas the 3-MA treatment
significantly reversed this effect. These findings suggested that
the CUR supplementation might have increased the autophagy effect
by inhibiting the HG-induced ROS.
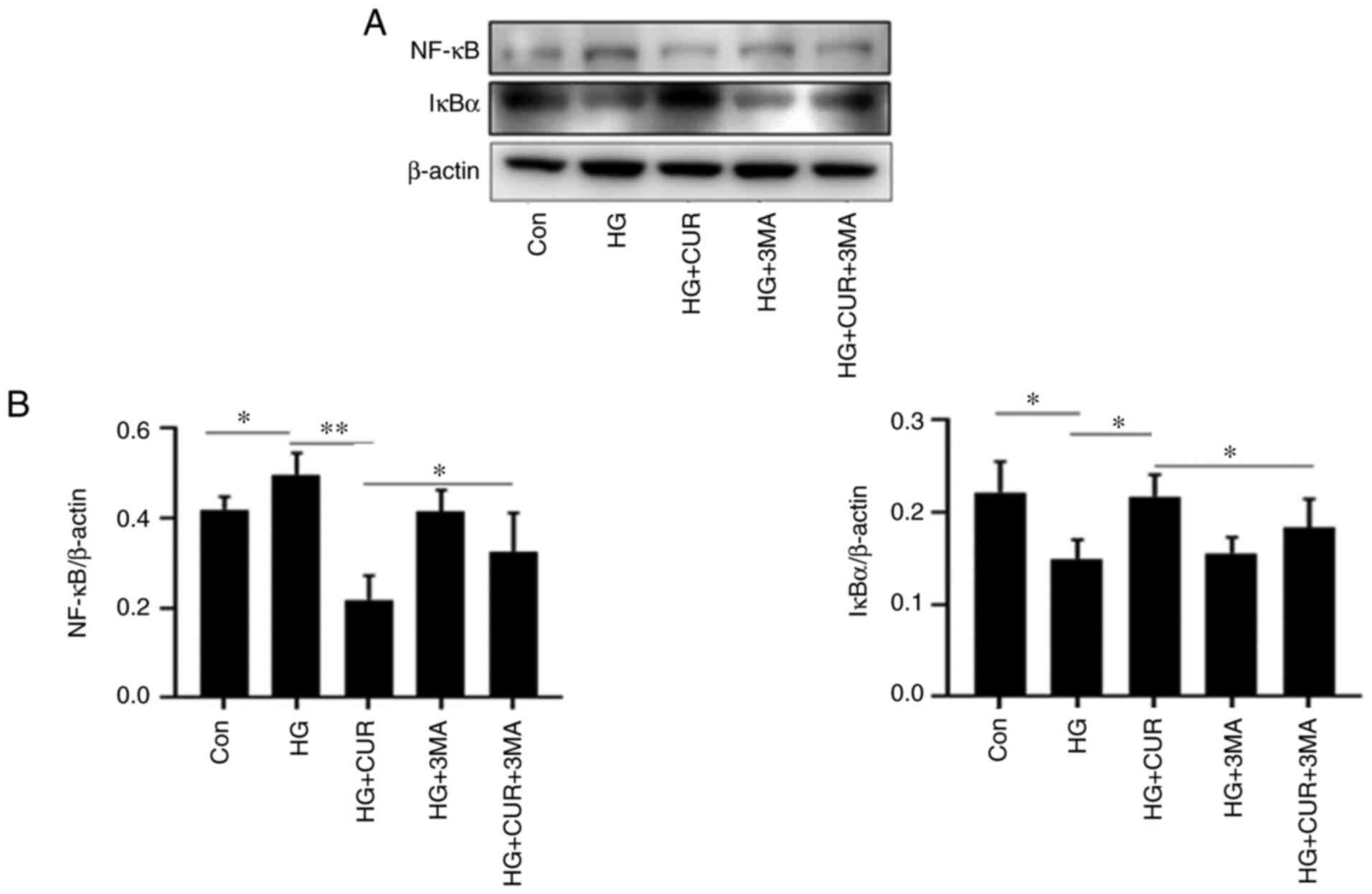
Autophagy inhibition attenuates the
anti-inflammatory effect of CUR
To investigate the interaction between cell
autophagy and inflammation in the HG+CUR group, the sixth part of
the experiment measured the expressions of NF-κB (mediator of
inflammatory responses) and IκBα (inhibitor of NF-κB) in different
cell groups (Fig. 6). By comparing
the results in the Con group (Fig.
6B), the NF-κB level in the HG group was significantly
increased and the IκBα level was downregulated when HUVECs were
exposed to HG, P<0.05. The CUR treatment, however, significantly
decreased the overproduction of HG-triggered NF-κB (P<0.01) and
increased the IκBα level (P<0.05). Furthermore, this
anti-inflammatory effect of CUR was significantly attenuated by the
3-MA treatment as observed in the HG+CUR+3MA group, P<0.05.
Thus, the CUR treatment decreased inflammation in the HG condition,
whereas the 3-MA treatment significantly reversed the effect. These
findings suggested that the CUR supplementation might have
increased the autophagy effect as protection by inhibiting the
HG-induced inflammation.
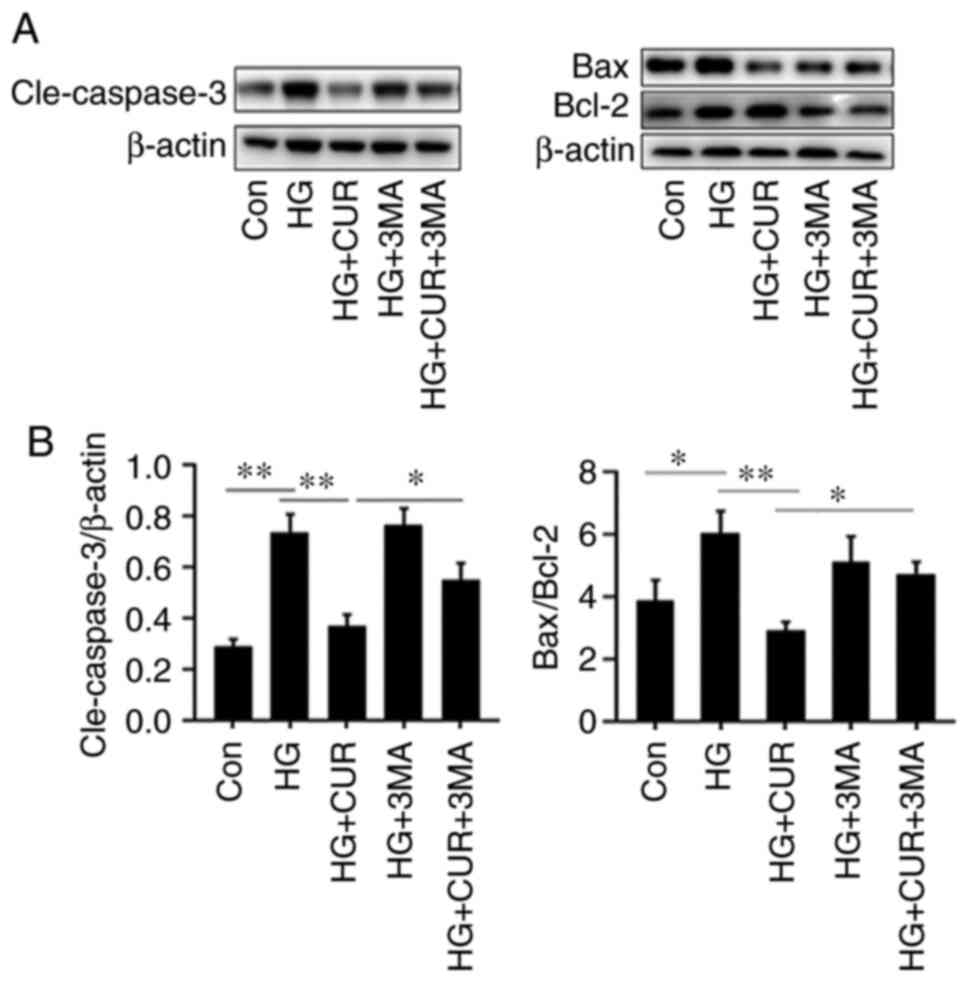
Autophagy inhibition attenuates the
anti-apoptosis effect of CUR
The last part of the experiment investigated the
interaction between apoptosis and autophagy by measuring the levels
of apoptosis-related cleaved (Cle-) caspase-3, Bcl-2 and Bax in
different cell groups (Fig. 7).
Compared with the results in the HG group (Fig. 7B), the CUR treatment in the HG+CUR
group could significantly lower the apoptotic levels of
Cle-caspase-3/β-actin and Bax/Bcl-2 that were induced by HG,
P<0.01. By contrast, compared with the results in the HG+CUR
group, the Cle-caspase-3/β-actin and Bax/Bcl-2 expressions in the
CUR+HG+3MA group were significantly increased after the 3-MA
treatment, P<0.05. Taken together, these findings suggested that
CUR could protect HUVECs from HG-induced apoptosis through
autophagy.
Discussion
The current study investigated the effects of CUR on
apoptosis and autography in HUVECs under HG conditions. It also
explored the molecular mechanism of CUR and the ROS/NF-κB pathway.
The current results have shown that CUR can promote autophagy and
decrease apoptosis in HUVECs by inhibiting ROS and NF-κB. The
present study further supported the putative role of CUR on
autophagy induction, revealing the underlying mechanisms that may
account for the beneficial effects on endothelial cell
apoptosis.
CUR, a hydrophobic polyphenol compound extracted
from the spice turmeric, has different pharmacological effects on
both in vitro and in vivo models. A number of studies
have reported that CUR is capable of triggering autophagy in
several types of cells. For example, CUR is known for its potential
anti-inflammatory and antioxidant properties (30). Increasing evidence suggests that
CUR may take a protective effect against diabetic complications
(31). CUR, therefore, protects
against diabetic cardiomyopathy by modulating the crosstalk between
autophagic and apoptotic machinery. The modulation of autophagy may
be an effective strategy for the treatment of cardiovascular
diseases associated with diabetes (32-35).
Focusing on how CUR can target autophagy in different cellular
settings may extend our knowledge of the new pharmacological agents
in overcoming the relevant diseases.
In T2DM, elevated oxidative stress can cause injury
to the vascular endothelial cells during the process of diabetic
vascular complications (36).
Evidence has revealed that HG conditions induce the production of
ROS, causing endothelial cell apoptosis (37). Constant oxidative stress eventually
leads to apoptosis or cell death. The current findings demonstrated
that the HG group promoted more ROS generation. However, the CUR
treatment markedly restored the HG-reduced ROS protein levels,
while the HG-reduced Bax/Bcl-2 ratio was significantly reverted and
the Cle-caspase-3 under HG stimulation was also downregulated.
Autophagy is usually an adaptive mechanism that
regulates the cell response to stress and enhances the resistance
to apoptosis, and defective autophagy has been linked to increased
apoptosis (38). Abundant evidence
indicates that HG decreases autophagy in different cell types
(39). The current study
demonstrated that HG decreased both the protein expressions of
LC3II/I and Beclin-1 and increased the level of p62. Thus,
insufficient autophagy has been observed in HUVECs treated with HG
(40). In addition, compared to
the HG group, the CUR treatment markedly upregulated the
expressions of LC3II/I and Beclin-1 and downregulated p62 in
HUVECs. The present study also has found that the Cle-caspase-3 and
Bax/Bcl-2 levels were downregulated when autophagy was activated
and that treatment with autophagy inhibitor 3-MA abolished the
autophagy upregulation triggered by CUR in the HG condition with
aggravated apoptosis. In summary, autophagy had a protective effect
on the HG-induced apoptosis in HUVECs. Previous studies have
confirmed the current findings and have shown that CUR protects
against diabetic cardiomyopathy and nephropathy by promoting
autophagy and alleviating apoptosis (16,32).
Inflammation is probably another key factor for the
onset and progression of endothelial dysfunction. Inflammatory
processes can enhance vascular ROS generation and endothelial cell
apoptosis. The present study found that the CUR treatment inhibited
the HG-induced inflammatory response, as evidenced by a decrease in
IκBα protein level and an induced NF-κB activity. The enhanced
generation of ROS and NF-κB was markedly suppressed by CUR in cells
subjected to the HG-induced injury. Furthermore, although the
current study investigated ROS and NF-κB separately after autophagy
inhibition, the ROS/NF-κB pathway is speculative and generally
accepted. Based on these findings, it is hypothesized that ROS
could be an important cellular mediator that triggers the
NF-κB-dependent pathway after the administration of CUR in HUVECs.
It appears that ROS/NF-κB activation is involved in the
pathogenesis of the HG-induced cell dysfunction and apoptosis,
suggesting that CUR, which can block the ROS/NF-κB signaling, may
be effective in protecting the cell activity. A previous study
shows that the stimulation of autophagy can reduce oxidative status
and rapamycin can protect HUVECs from the damages caused by HG
(41). The present study found
that HG could increase ROS generation and activate the downstream
NF-κB pathway in endothelial cells, which indicated that HG induced
an oxidative stress reaction in endothelial cells. This result is
consistent with previous studies that demonstrated that oxidative
stress may be a potential mechanism for HG-related cell
degenerative changes (42,43).
Overall, the present study indicated that CUR could
promote autography and decrease apoptosis in HUVECs under HG
conditions and that CUR treatment markedly restored the HG-reduced
ROS protein levels and NF-kB expression. The ROS/NF-κB signaling
pathway was, therefore, considered as the potential mechanism
involved in the autophagy activation by curcumin. Previous reports
have shown that there are a number of different potential
intracellular sources of ROS which are capable of influencing or
being influenced by NF-κB (44,45).
ROS can activate NF-κB through IκB kinase (IKK)-dependent pathway
(46). In other words, ROS can
modulate an NF-κB response and NF-κB target genes can decrease ROS
to promote survival in a number of ways. Depending on the setting,
ROS can both act as promoters or inhibitors of NF-κB signaling
(47). For example, NF-κB activity
is able to influence ROS levels via increased expression of
antioxidant proteins and NF-κB would also induce inflammatory
enzymes that could promote the production of ROS (46). Therefore, the ROS/NF-κB signaling
pathway in autophagy activated by CUR may serve a role in the
potential anti-inflammatory and antioxidant properties of CUR.
To confirm that the enhanced autophagy due to CUR
has a potential role in reducing the generation of NF-κB, caspase-3
and Bax/Bcl-2 in the HG-treated HUVECs, the present study adopted
the autophagy inhibitor 3-MA. The results showed that 3-MA induced
an additional increase in the Cle-caspase-3, Bax/Bcl-2 and NF-κB
levels when compared to the levels in HG treated with CUR. This
finding suggested that autophagy served an essential role in
reducing caspase-3, Bax/Bcl-2 and NF-κB generation in HG and that
CUR could promote autophagy and reduce apoptosis and inflammation.
Therefore, the effect of inhibition on autophagy not only
exacerbates the HG-induced apoptosis but also diminishes the
suppression effects of CUR in NF-κB, providing a scientific basis
for further research and clinical application of CUR.
The present study had several limitations. Since it
was an in vitro simulation of the diabetic endothelial cells
exposed to HG, it may not be equivalent to a T2DM model. It is also
agreed that the mechanisms by which CUR activates autophagy should
be the focus of research. The current study may be considered a
pilot study in understanding some fundamental effects of CUR on
cells exposed to HG, the protective mechanisms of CUR need to be
examine further in another study.
The activation of autophagy is to protect the
HG-induced HUVECs. Meanwhile, curcumin protects the HG-induced
HUVECs by restoring autophagy, an effect attributed to the
inhibition of the ROS/NF-B pathway. Therefore, it was hypothesized
that enhancing autophagy by inhibiting ROS/NF-B expression may be a
potential therapeutic strategy for treating vascular complications
in diabetes. Furthermore, the regulation of endothelial cell
autophagy may be another key point of control in regulating
vascular function under disease conditions associated with
oxidative stress.
Acknowledgements
The authors would like to thank Dr Chi C Lau, an
independent scholar, for his invaluable advice in preparing the
manuscript.
Funding
Funding: The present study was supported by a grant from the
Administration of Traditional Chinese Medicine of Zhejiang Province
(grant no. 2017ZA087).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was designed by QHJ, data were collected
by HYZ, data analysis and interpretation were performed by XJH and
QHJ, and the manuscript was written by QHJ and XJH. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng Y, Ley SH and Hu FB: Global
aetiology and epidemiology of type 2 diabetes mellitus and its
complications. Nat Rev Endocrinol. 14:88–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fowler MJ: Microvascular and macrovascular
complications of diabetes. Clin Diabetes. 26:77–82. 2008.
|
|
3
|
Popov D: Endothelial cell dysfunction in
hyperglycemia: Phenotypic change, intracellular signaling
modification, ultrastructural alteration, and potential clinical
outcomes. Int J Diabetes Mellitus. 2:189–195. 2010.
|
|
4
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang X, He N, Xing Y and Lu Y: Knockdown
of GCN2 inhibits high glucose-induced oxidative stress and
apoptosis in retinal pigment epithelial cells. Clin Exp Pharmacol
Physiol. 47:591–598. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang J, Li G, Wang Z, Zhang X, Yao L, Wang
F, Liu S, Yin J, Ling EA, Wang L and Hao A: High glucose-induced
expression of inflammatory cytokines and reactive oxygen species in
cultured astrocytes. Neuroscience. 202:58–68. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu T, Gong J, Chen Y and Jiang S:
Periodic vs constant high glucose in inducing pro-inflammatory
cytokine expression in human coronary artery endothelial cells.
Inflamm Res. 62:697–701. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sweet IR, Gilbert M, Maloney E, Hockenbery
DM, Schwartz MW and Kim F: Endothelial inflammation induced by
excess glucose is associated with cytosolic glucose 6-phosphate but
not increased mitochondrial respiration. Diabetologia. 52:921–931.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Verma N and Manna SK: Advanced glycation
end products (AGE) potently induce autophagy through activation of
RAF protein kinase and nuclear factor κB (NF-κB). J Biol Chem.
291:1481–1491. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xie Y, You SJ, Zhang YL, Han Q, Cao YJ, Xu
XS, Yang YP, Li J and Liu CF: Protective role of autophagy in
AGE-induced early injury of human vascular endothelial cells. Mol
Med Rep. 4:459–464. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ge D, Jing Q, Meng N, Su L, Zhang Y, Zhang
S, Miao J and Zhao J: Regulation of apoptosis and autophagy by
sphingosylphosphorylcholine in vascular endothelial cells. J Cell
Physiol. 226:2827–2833. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Allen DA, Yaqoob MM and Harwood SM:
Mechanisms of high glucose-induced apoptosis and its relationship
to diabetic complications. J Nutr Biochem. 16:705–713.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ao H, Li H, Zhao X, Liu B and Lu L: TXNIP
positively regulates the autophagy and apoptosis in the rat müller
cell of diabetic retinopathy. Life Sci. 267(118988)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Q, Yin Y, Zheng Y, Chen F and Jin P:
Inhibition of autophagy promoted high glucose/ROS-mediated
apoptosis in ADSCs. Stem Cell Res Ther. 9(289)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu H, Yu S, Zhang H and Xu J:
Angiogenesis impairment in diabetes: Role of methylglyoxal-induced
receptor for advanced glycation endproducts, autophagy and vascular
endothelial growth factor receptor 2. PLoS One.
7(e46720)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tu Q, Li Y, Jin J, Jiang X, Ren Y and He
Q: Curcumin alleviates diabetic nephropathy via inhibiting podocyte
mesenchymal transdifferentiation and inducing autophagy in rats and
MPC5 cells. Pharm Biol. 57:778–786. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang L, Xiong X, Zhang X, Ye Y, Jian Z,
Gao W and Gu L: Sodium Tanshinone IIA sulfonate protects against
cerebral ischemia-reperfusion injury by inhibiting autophagy and
inflammation. Neuroscience. 441:46–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kelany ME, Hakami TM and Omar AH: Curcumin
improves the metabolic syndrome in high-fructose-diet-fed rats:
Role of TNF-α, NF-κB, and oxidative stress. Can J Physiol
Pharmacol. 95:140–150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Z and Li K: Curcumin attenuates high
glucose-induced inflammatory injury through the reactive oxygen
species-phosphoinositide 3-kinase/protein kinase B-nuclear
factor-κB signaling pathway in rat thoracic aorta endothelial
cells. J Diabetes Investig. 9:731–740. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang J, Fu J, Liu B, Wang R and You T: A
synthetic curcuminoid analog,
(2E,6E)-2,6-bis(2-(trifluoromethyl)benzylidene)cyclohexanone,
ameliorates impaired wound healing in streptozotocin-induced
diabetic mice by increasing miR-146a. Molecules.
25(920)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shimizu K, Sunagawa Y, Funamoto M,
Wakabayashi H, Genpei M, Miyazaki Y, Katanasaka Y, Sari N, Shimizu
S, Katayama A, et al: The synthetic curcumin analogue GO-Y030
effectively suppresses the development of pressure overload-induced
heart failure in mice. Sci Rep. 10(7172)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jin QH, Shen HX, Wang H, Shou QY and Liu
Q: Curcumin improves expression of SCF/c-kit through attenuating
oxidative stress and NF-κB activation in gastric tissues of
diabetic gastroparesis rats. Diabetol Metab Syndr.
5(12)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han J, Pan XY, Xu Y, Xiao Y, An Y, Tie L,
Pan Y and Li XJ: Curcumin induces autophagy to protect vascular
endothelial cell survival from oxidative stress damage. Autophagy.
8:812–825. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu W, Zha W, Ke Z, Min Q, Li C, Sun H and
Liu C: Curcumin protects neonatal rat cardiomyocytes against high
glucose-induced apoptosis via PI3K/Akt signaling pathway. J
Diabetes Res. 2016(4158591)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tu Y, Guo C, Song F, Huo Y, Geng Y, Guo M,
Bao H, Wu X and Fan W: Mild hypothermia alleviates diabetes
aggravated cerebral ischemic injury via activating autophagy and
inhibiting pyroptosis. Brain Res Bull. 150:1–12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wei Y, Gao J, Qin L, Xu Y, Shi H, Qu L,
Liu Y, Xu T and Liu T: Curcumin suppresses AGEs induced apoptosis
in tubular epithelial cells via protective autophagy. Exp Ther Med.
14:6052–6058. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guo S, Long M, Li X, Zhu S, Zhang M and
Yang Z: Curcumin activates autophagy and attenuates oxidative
damage in EA.hy926 cells via the Akt/mTOR pathway. Mol Med Rep.
13:2187–2193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Y, Gao A, Xu X, Dang B, You W, Li H,
Yu Z and Chen G: The neuroprotection of lysosomotropic agents in
experimental subarachnoid hemorrhage probably involving the
apoptosis pathway triggering by cathepsins via chelating
intralysosomal iron. Mol Neurobiol. 52:64–77. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yan H, Ma Y, Li Y, Zheng X, Lv P, Zhang Y,
Li J, Ma M, Zhang L, Li C, et al: Insulin inhibits inflammation and
promotes atherosclerotic plaque stability via PI3K-Akt pathway
activation. Immunol Lett. 170:7–14. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Arshad L, Haque MA, Abbas Bukhari SN and
Jantan I: An overview of structure-activity relationship studies of
curcumin analogs as antioxidant and anti-inflammatory agents.
Future Med Chem. 9:605–626. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Parsamanesh N, Moossavi M, Bahrami A,
Butler AF and Sahebkar A: Therapeutic potential of Curcumin in
diabetic complications. Pharmacol Res. 136:181–193. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yao Q, Ke ZQ, Guo S, Yang XS, Zhang FX,
Liu XF, Chen X, Chen HG, Ke HY and Liu C: Curcumin protects against
diabetic cardiomyopathy by promoting autophagy and alleviating
apoptosis. J Mol Cell Cardiol. 124:26–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang X, Li ZL, Crane JA, Jordan KL, Pawar
AS, Textor SC, Lerman A and Lerman LO: Valsartan regulates
myocardial autophagy and mitochondrial turnover in experimental
hypertension. Hypertension. 64:87–93. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P,
Mao X, Huang K, Xie Z and Zou MH: Hyperglycemia-driven inhibition
of AMP-activated protein kinase α2 induces diabetic cardiomyopathy
by promoting mitochondria-associated endoplasmic reticulum
membranes in vivo. Circulation. 139:1913–1936. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yu W, Gao B, Li N, Wang J, Qiu C, Zhang G,
Liu M, Zhang R, Li C, Ji G and Zhang Y: Sirt3 deficiency
exacerbates diabetic cardiac dysfunction: Role of
Foxo3A-Parkin-mediated mitophagy. Biochim Biophys Acta Mol Basis
Dis. 1863:1973–1983. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ihnat MA, Thorpe JE, Kamat CD, Szabó C,
Green DE, Warnke LA, Lacza Z, Cselenyák A, Ross K, Shakir S, et al:
Reactive oxygen species mediate a cellular ‘memory’ of high glucose
stress signalling. Diabetologia. 50:1523–1531. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Quagliaro L, Piconi L, Assaloni R,
Martinelli L, Motz E and Ceriello A: Intermittent high glucose
enhances apoptosis related to oxidative stress in human umbilical
vein endothelial cells: The role of protein kinase C and
NAD(P)H-oxidase activation. Diabetes. 52:2795–2804. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ortiz-Cordero C, Bincoletto C, Dhoke NR,
Selvaraj S, Magli A, Zhou H, Kim DH, Bang AG and Perlingeiro RC:
Defective autophagy and increased apoptosis contribute toward the
pathogenesis of FKRP-associated muscular dystrophies. Stem Cell
Reports. 16:2752–2767. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xu J, Kitada M, Ogura Y, Liu H and Koya D:
Dapagliflozin restores impaired autophagy and suppresses
inflammation in high glucose-treated HK-2 cells. Cells.
10(1457)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang Z, Zhang S, Wang Y, Yang M, Zhang N,
Jin Z, Ding L, Jiang W, Yang J, Sun Z, et al: Autophagy inhibits
high glucose induced cardiac microvascular endothelial cells
apoptosis by mTOR signal pathway. Apoptosis. 22:1510–1523.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rezabakhsh A, Ahmadi M, Khaksar M,
Montaseri A, Malekinejad H, Rahbarghazi R and Garjani A: Rapamycin
inhibits oxidative/nitrosative stress and enhances angiogenesis in
high glucose-treated human umbilical vein endothelial cells: Role
of autophagy. Biomed Pharmacother. 93:885–894. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hou G, Zhao H, Teng H, Li P, Xu W, Zhang
J, Lv L, Guo Z, Wei L, Yao H and Xu Y: N-Cadherin attenuates high
glucose-induced nucleus pulposus cell senescence through regulation
of the ROS/NF-κB pathway. Cell Physio Biochem. 47:257–265.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cao G, Fan J, Yu H and Chen Z: Resveratrol
attenuates high glucose-induced cardiomyocytes injury via
interfering ROS-MAPK-NF-κB signaling pathway. Int J Clin Exp
Pathol. 11:48–57. 2018.PubMed/NCBI
|
|
44
|
Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R,
Qiu WQ, Pan R, Law BY, Wong VK, Yu CL, et al: Polyphyllin VI
induces Caspase-1-mediated pyroptosis via the Induction of
ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer.
Cancers (Basel). 12(193)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ma B, Wang X, Zhang R, Niu S, Rong Z, Ni
L, Di X, Han Q and Liu C: Cigarette smoke extract stimulates PCSK9
production in HepG2 cells via ROS/NF-κB signaling. Mol Med Rep.
23(331)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Morgan MJ and Liu Z: Crosstalk of reactive
oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xu X, Huang X, Zhang L, Huang X, Qin Z and
Hua F: Adiponectin protects obesity-related glomerulopathy by
inhibiting ROS/NF-κB/NLRP3 inflammation pathway. BMC Nephrol.
22(218)2021.PubMed/NCBI View Article : Google Scholar
|