1. Introduction
Pseudoexfoliation syndrome (PEXS) is an
age-associated systemic disorder characterised by abnormal
production and turnover of extracellular matrix (ECM), leading to
the progressive deposition of extracellular, fibrillary, white
flaky deposits in different tissues and organs of the body. ECM is
a 3-dimensional network of interacting macromolecular effectors
that apart from tissue support and integrity affects growth factors
availability, cell signaling and functional properties such as
oxidative stress (OS) pathways (1,2). The
most commonly affected ocular tissues reveal deposition of
pseudoexfoliation material (PEXM) in the pupillary margin of the
iris (3-5).
These alterations are responsible for pathological changes and
sequelae in the anterior part of the eye, such as cataracts,
zonular weakness, phacodonesis, lens subluxation/dislocation, iris
rigidity and synechiae, blood-aqueous barrier dysfunction, melanin
dispersion, capillary haemorrhage, poor mydriasis, radial body
complication, trabecula impairment, keratopathy, and even retinal
vein occlusion in the posterior eye segment (6,7). As
a result of the PEXM deposit on the lens capsule, a higher rate of
open-angle glaucoma cataracts and resulting blindness was observed
in most PEXS patients. In addition to the eye, deposits are also
found around the blood vessels of the connective tissue and organs
such as the lungs, heart, liver, kidneys, gallbladder, and meninges
(8-10).
Studies suggest that PEXM is associated with the development of
systemic hypertension, myocardial infarction and cerebrovascular
events.
Lindberg firstly described PEXS in 1917, when
observed the whitish-grey material deposit on the pupillary border
in glaucoma patients. However, the term PEXS was coined later in
1954 by Dvorak-Theobald, who noticed the aggregation of PEXM on the
lens capsule, ciliary body, and zonules (3-5).
Ocular deposition of PEXM can be found in all structures of the
anterior part of the eye (11,12).
PEXM deposits can be macroscopically observed during the dilated
slit-lamp examination and anterior segment optical coherence
tomography. Although the production site of PEXM has not yet been
identified, it is hypothesized to be synthesised either from the
iris, lens epithelium, ciliary body or trabecula (11,13).
Studies identified the chemical nature of the PEXM deposits, and it
is made up of a complex glycoprotein/proteoglycan matrix comprising
glycosaminoglycan, chondroitin and heparin sulfate, and
tropoelastin. Further, it also consists of fibrillin-1,
fibronectin, vitronectin, laminin, collagen, amyloid,
nidogen/entactin, microfibril-associated glycoprotein, latent TGF-b
binding proteins, residues of galactose, a-mannose,
N-acetyl-D-glucosamine, lysyl oxidase-like 1 (LOXL1), and
apolipoprotein E (14-16).
The particles of this abnormal material are insoluble, follow the
aqueous humor's (AH) natural flow, and are finally deposited in the
trabecula. Gradually, they inhibit the normal outflow of AH,
leading to an increase in the intraocular pressure (17) and the development of a severe type
of open-angle glaucoma, identified as PEXG (pseudoexfoliation
glaucoma). PEXG is the most commonly recognised cause of open-angle
glaucoma worldwide, accounting for 25% of this type of glaucoma.
The 10-year cumulative probability of PEXS patients developing PEXG
is ~15% (18). PEXG is
characterised by progressive degeneration of retinal ganglion
cells, and their axons affect peripheral vision and result in a
severe and irreversible visual loss (12,19).
Therefore, prompt diagnosis of PEXS/PEXG is crucial because the
affected patients have rapid and severe clinical course, poorer
response to medications, higher rates of surgical complications,
and worse prognosis than other forms of open-angle glaucoma
(12). Considering this and the
significant impact of PEXS and PEXG in terms of patient health and
socio-economic costs, there is a necessity for innovative
preventive and therapeutic policies. However, advances in treatment
are mainly based upon an in-depth comprehension of the underlying
molecular mechanisms, especially in the early stages of the disease
(20). Although the specific
pathogenesis of this condition remains unknown, various studies
have suggested OS, diminished cellular defence status, and ischemia
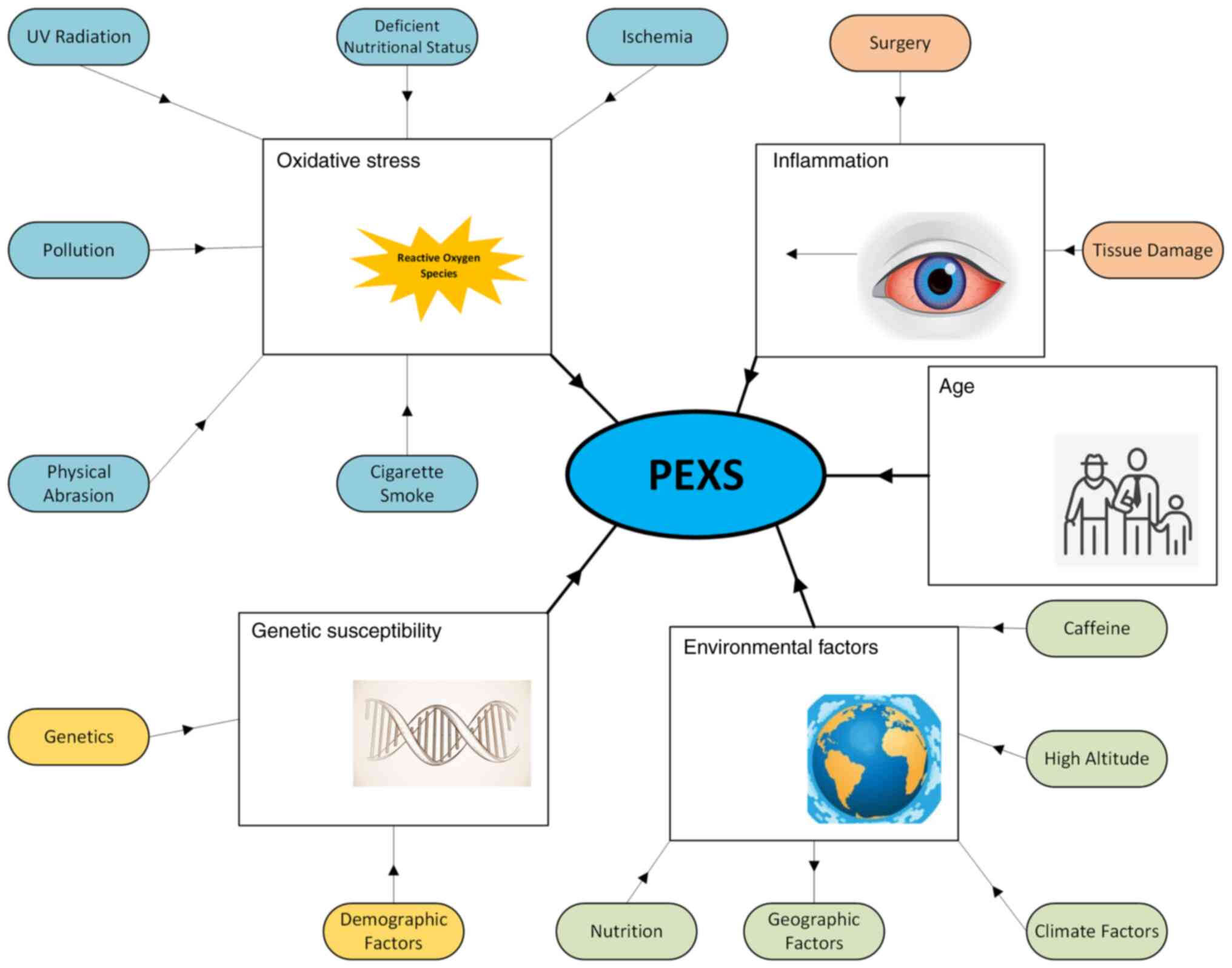
being the most frequently reported factors (Fig. 1) (21).
Although the cause of the deposition and its
resulting pathogenesis is not well understood, the role of ECM
remodelling and OS has been studied in detail. Specifically, the
link between OS and PEXS has been well established in recent years.
In this review, we discuss advances in the pathogenesis of PEXS,
especially the involvement of OS.
2. Methods
A systematic review of the literature published in
English was performed from November 2021 to March 2022 in order to
identify all published reports pertaining OS in PEXS in the eye.
Studies were identified through a search consisting of: (1) a computerized search of Cochrane,
Scopus and PubMed (National Library of Medicine) databases from
January 1952 through January 2022, (2) review of major ophthalmic textbooks
and (3) the database ClinicalTrials.gov (www.clinicaltrials.gov) was also searched for
information about clinical trials. The following keywords and MeSH
terms were used ‘pseudoexfoliation’, ‘pseudoexfoliation syndrome’,
‘pseudoexfoliation material’, ‘oxidative stress’, ‘reactive oxygen
species’, ‘eye’, ‘pathogenesis’. The searches were performed by
three independent investigators (MP, PP and KK had equal
contribution and performed the literature review and analysed the
data). We only included articles with full text available in
English. All pertinent articles were thoroughly assessed, and their
reference lists were searched to identify other potentially
relevant studies. The reviewers came to consensus on the selection
of full texts through discussion. CDG approved the final list of
included studies, finalized the work and is the academic
supervisor.
3. Incidence of PEXS
PEXS is a multifactorial disease which is widespread
worldwide. Although PEXS occurrence is negligible in the
middle-aged population, its global incidence varies considerably
across populations and countries, with the reported prevalence of
PEXS ranging from 1.5 to 40.9% worldwide (22,23).
The prevalence of PEXS varies from 3.6 to 34.2% in European
countries, from 1.5 to 22.1% in Asian countries, and from 1.5 to
40% in African countries, suggesting a general lack of consensus on
these epidemiologic studies (22,23).
As of yet, it is unclear whether incidence of PEXS varies across
populations or whether the reported variation could be because of
study parameters such as study design, location, age of the
population and target sample size. Nevertheless, older age,
Scandinavian and Mediterranean race, genetic mutations, and
solar/cosmic radiation are considered major risk factors for PEXS
(24,25). Especially, PEXS have a high
incidence among Scandinavians, and half of open-angle glaucoma
cases are caused by pseudoexfoliation in this population (7). Population studies have shown that
PEXS is rarely observed before the age of 40, and its incidence
increases with age (Fig. 1).
Several studies on the PEXS estimated a 5 to 20% prevalence in an
aged population regardless of geographical features (26). Specifically, PEXS is common in
individuals >50 years, with its incidence increasing with age.
Notably, the prevalence rates of PEXS are 25% in Icelanders over
the age of 60, 20% in Finlanders, 0 in the Inuit population, 4.7%
in Germans, and 4% in English individuals (27-29).
Notably, the prevalence of PEXS was 5.0% over the age of 40 in
Turkey. Further the population-based studies suggest the prevalence
of PEXS in India (1.5%), Pakistan (6.4%), England (4.0%), Saudi
Arabia (9.3%), China (0.4%), Germany (4.7%), Saudi Arabia (9.3%),
Greece (11.5-17%) and Norway (6.3%). In general, there was no
gender preference in PEXS occurrence (22,27-30).
Besides, although PEXS is an age-related disorder and most affected
individuals are over 50 years old, there are also reported cases at
younger ages (31). In these
cases, it was remarkable that all patients had previously undergone
one or more intraocular procedures, and so it was suggested that
might be a causative association (31).
4. Genetic susceptibility of PEXS
Genetic studies conducted in populations worldwide
clearly suggest a significant role of genetics in the pathogenesis
of PEXS (Fig. 1). Initially, the
genetic basis of PEXS was uncovered through a genome-wide
association study (GWAS) conducted on northern Europeans. Two
single nucleotide polymorphisms (SNPs), rs1048661, and rs3825942,
located in the coding region of the lysyl oxidase-like one gene
(LOXL1), were linked to the development of PEXS in Scandinavians
(32). LOXL1, a gene that encodes
a lysyl oxidase, catalyses elastin and collagen crosslinking,
located on chromosome region 15q24.1, is essential for elastin
fibre formation and homeostasis. After this initial observation,
the association between these LOXL1 variants and PEXS has been
extensively studied worldwide. Subsequent genetic studies have
demonstrated that SNPs in exon 1 of the LOXL1 gene indicate the
critical genetic risk factor for PEXS and PEXG in different
individuals (9,33). These two SNPs of the LOXL1 gene
have been identified across the globe (80-100%) in PEXS/PEXG
patients. Especially the association of LOXL1 SNPs with PEXS have
been found in several human populations, including Europe, North
America, Asia, Africa, and Australia.
In addition to LOXL1 polymorphisms, loss of
heterozygosity (LOH) was observed in 94.11% of PEXS patients, with
the highest incidence being observed in the markers D13S175, D7S478
and D7S479. The authors concluded that LOH possibly suggests a
genetic role in PEXS pathogenesis (34,35).
Its correlation with the altitude at which these patients lived
could indicate an increased vulnerability to ultraviolet radiation
(UVR) in the examined chromosomal regions (34,35).
Similarly, genetic variants in Calcium Voltage-Gated Channel
Subunit Alpha1 A (CACNA1A) are linked with susceptibility to PEXS.
Studies indicate that CACNA1A could change calcium levels at the
cell surface leading to PEXM deposition (9,33,36).
Besides, PEXS pathogenesis has been linked to fibulin-5 (FBLN5), an
extracellular framework protein that activates LOXL1, in PEXS
progression. The authors reported that two novel noncoding
polymorphisms within the FBLN5 gene were significantly associated
with PEXS as risk factors (37).
Interestingly, the mRNA and protein expression levels of FBLN5 are
reduced in PEXS affected lens capsules, and this downregulation is
associated with ECM remodelling (37).
5. Environmental factors affecting PEXS
onset and progression
Nutrition plays an essential role in the progression
and pathogenesis of PEXS (Fig. 1).
A diet containing nutrients such as selenium can regulate the PEXS
onset and progression. OS has also been linked to PEXS pathogenesis
in the presence of nutrient deficiences (38). Specifically, regular consumption of
dietary fibre-rich vegetables and fruits, particularly when started
from a young age, has been related to a lower risk of PEXS
occurrence, signifying an antioxidative and protective role against
this condition. Similarly, mild to moderate caffeine consumers were
less likely to present PEXS than those who consume a lot of coffee
(39,40). It has been proposed that caffeine
consumption on a long-term basis may contribute to a continuous
PEXM accumulation in the eye.
Moreover, caffeine consumption has been shown to
increase plasma homocysteine (Hcy) concentrations (41,42).
Since Hcy has also been found elevated in AH, tear fluid and serum
of PEXS patients (43), it could
be suggested that the Hcy-increasing effect of caffeine may signify
a good association between coffee consumption and PEXS (20). It is also known that Hcy has
pro-oxidant action. Its high concentrations may participate in the
abnormal ECM repair detected in PEXS and other tissues, thus
explaining the high vascular risk observed in PEXS patients
(11,12). Therefore, it would be rational for
such patients, especially those with bilateral eye involvement, to
have total serum Hcy levels screened.
In a study by Kozobolis et al (44) about the epidemiology of
pseudoexfoliation on the island of Crete, Greece, the authors
reported that the prevalence of PEXS was higher in men and
increased with age. They also found a possible correlation with
high altitude and that PEXS was a risk factor for early intraocular
presure (IOP) disturbances, especially in women. In two large
studies from the USA, demographic, geographic and climatic factors
were associated with PEXS occurrence (45,46).
The authors examined mainly Caucasian individuals of different
European ancestry and could not identify differences in PEXS
incidence among the various descents. They found that participants
who lived in the middle and southern regions of the country
exhibited a reduced risk of PEXS, and they concluded that ambient
temperature and sun exposure might be significant environmental
factors involved in PEXS pathogenesis, as recent studies also
confirmed (47,48).
6. Pathogenesis of PEXS
The pathogenesis of PEXS manifested mainly through
the generation and deposition of insoluble fibrillary extracellular
material on connective tissues and tissues close to the
bloodstream. Other pathological changes that contribute to the PEXS
include dysregulated degradation and ECM production, increased
inflammation, and enhanced OS. Since PEXM is insoluble, it
aggregates at the trabecular meshwork and blocks the normal flow of
AH and thus, increasing the intraocular pressure in the eye.
Although the primary cause is not yet understood, it is
hypothesized that the PEXM deposition is one of the reasons for
complications, including cataracts, zonular weakness, and lens
dislocation.
LOXL1
Defects in the functions of LOXL1 are one of the
major contributors to abnormal deposits of PXEM in ocular tissues.
LOXL1 essentially maintains the homeostasis of fibrillar ECM via
regulating the generation, maintenance and repair of the elastic
connective tissue (49). LOXL1
essentially acts as a framework element ensuring spatially defined
elastin deposition. Particularly, LOXL1 is involved in the
crosslinking of elastin and collagen through its pro-peptide, which
binds to both fibulin-5 and tropoelastin to target elastic
microfibrils at elastogenesis sites (49). It has been demonstrated that LOXL1
is a vital factor in preventing age-related elastic fibre damage
and loss of elasticity (50).
Furthermore, LOXL1 expression is essential for standard IOP
control, while deficiency causes modified conventional outflow
physiology and ECM repair and homeostasis (51). Specifically, the fibrillar material
deposits found in PEXS patients contain elastin and tropoelastin,
suggesting the link between defects of LOXL1 and the pathogenesis
of PEXS. Changes in LOXL1 activation can result in an excessive
aggregation of elastic fibre fragments into PEXS eyes.
Further, LOXL1 deficiency was found in the eye, and
its deficiency increases susceptibility to optic nerve damage
(52). Since the dysregulation in
elastic fibre production and crosslinking is hypothesized to be the
major contributor to the pathogenesis of PEXS, LOXL1 expression and
polymorphisms have also been linked to the pathogenesis of this
syndrome. Besides this, OXL1-AS1, a long non-coding RNA (lncRNA)
synthesised from the LOXL1 gene, also has been linked to the PEXS.
The nuclear LOXL1-AS1 selectively bind to the mRNA processing
protein, the heterogeneous nuclear ribonucleoprotein-L (hnRNPL).
Both have a vital role in regulating total gene expression in eye
cells. Interestingly, SNPs regulating the expression of LOXL1-AS1
have been found in the patients of PEXS, suggesting the vital role
of the LOXL1 gene in the pathogenesis of PEXS (53).
TGF-β1
Another critical protein involved in the ECM
remodelling and the pathogenesis of PEXS is tumor growth factor-β1
(TGF-β1), a fibrosis-associated growth factor found in high levels,
specifically in fibrotic diseases and experimental fibrosis models
(54). Increased TGF-β1 levels
were noted in the AH of PEXS patients, and it has been associated
with the production of several elastic fibrillary elements, like
fibrillin-1, that comprise the PEXM (55). Notably, TGF-b1 is one of the most
vital factors that triggers the expression of both LOXL1 and
fibrillin-1, which is the critical element of PEXS fibrils.
Additionally, these factors also seemed to activate the
construction of a specific elastic microfibrillar network into
PEXS-like fibrils, suggesting the contribution of TGF-β1 in the
PEXM deposition (56). Further,
TGF-β1 expression is correlated with decreased degradation of ECM
via regulation of the activities of matrix metalloproteases (MMPs)
and their tissue inhibitors (TIMPs). Of note, TGF-β1 reduces the
expression of MMP1 and MMP3 while increasing the expression of
MMP2, TIMP1, and TIMP3, leading to reduced degradation of the newly
synthesised matrix material. In patients with PEXS, inactive forms
of MMP-2, MMP-3, and the active forms of TIMP1 and TIMP2 are higher
than other MMPs. The aberrant expression of these tissue
remodelling enzymes leads to insufficient degradation of excess
matrix material leading to the accumulation of PEXM (57).
Clusterin
Studies suggest that TGF-β1 activation causes
downregulation of clusterin (CLU), a molecular chaperone essential
for folding denatured and misfolded proteins in the AH during the
PEXM generation (58,59). CLU is a glycoprotein component of
biological fluids and is found at higher levels in ocular cells.
CLU isoforms act as an extracellular chaperone that reduces
abnormal aggregation of proteins by favouring their unfolded state
for proper refolding. Notably, the expression levels of CLU have
been correlated with both physiological and pathological processes,
including regulation of lipid transport, apoptosis, cell-cell and
cell-matrix interactions, and OS. Interestingly, elevated levels of
CLU are found in PEXM deposits, which are colocalised with
exfoliation fibrils and LOXL1 (59,60).
Furthermore, the expression of CLU decreases in PEX individuals,
which could be responsible for reduced chaperone function and
deposition of PEXM in the anterior segment of the eye (61).
Fibulin-5
Studies have suggested that LOXL1 activity is
tightly regulated by fibulin-5 (FBLN5), an extracellular scaffold
protein. FBLN5 plays a crucial role in the activation of LOXL1,
thereby controlling the deposition of elastin in the ECM. Notably,
FBLN5 activates LOXL1 via binding to the N-terminus of LOXL1.
Studies have suggested that two polymorphisms that have been found
in the noncoding part of the FBLN5 gene could be a risk factor for
PEXS (37). Further, studies have
shown that low mRNA and protein levels of FBLN5 in the lens of PEXS
patients promote deposition of PEXM by affecting ECM dynamics.
Remarkably, FBLN5 deficiency and the loss of interaction between
FBLN5 and LOXL1 could cause accumulation of the inactive form of
LOXL1, leading to the pathogenesis of PEXS.
7. Involvement of OS in PEXS
OS
OS is defined as excess reactive oxygen species
(ROS) production in cells, mainly due to the imbalance in the
generation and clearance of free radicals and reactive metabolites.
The presence of active oxygen radicals in biological materials was
first established in 1954 by Gerschman et al (62). Specifically, the toxic nature of
oxygen was related to its partially reduced forms (63). Two years later, a hypothesis that
oxygen radicals are produced as by-products of biological reactions
were responsible for mutations, cellular damage, cancer, and ageing
(64). The discovery of the enzyme
peroxidase dismutase was the beginning of a new era for exploring
the effects of ROS on living organisations (65). In the subsequent decade, extensive
investigations revealed that ROS is capable of causing oxidative
damage in DNA, lipids, proteins, and other cellular targets
(66). Nowadays, it has become
clear that living organisms have adapted to moderately increased
levels of ROS and have also developed mechanisms for using them in
numerous physiological functions. Free radicals are now products of
normal cellular metabolism and play a dual role: either beneficial
to cells and organisms or harmful, depending on the amount
generated at a particular time (67).
In biological systems, OS typically occurs when ROS
are overproduced or the antioxidant defense mechanisms are
insufficient. The delicate balance between ROS's beneficial and
harmful effects is critical to living organisms and is maintained
by ‘redox regulation’. The redox regulation maintains homeostasis
and protects living organisms from OS (68). Importantly, OS is essentially a
disorder in the redox regulation (69), OS plays a significant role in
biology and has been implicated in numerous pathophysiological
processes (70). Depending on OS,
a wide range of disorders may occur involving cellular
dysregulation or altered processes such as inflammatory responses
dysfunction, accelerated ageing, abnormal proliferation,
carcinogenesis and even cell death (71).
OS in the eye
Recently we have reviewed the critical role of ECM
in pathogenesis and treatment and particularly the roles of ECM
effectors and biochemical pathways involved in the development and
the progression of the PEXS (Fig.
1) (8). Here we further
focused on the emerging roles of OS in PEXS. The eye is a highly
metabolic organ devouring large amounts of energy. OS can affect
the eye due to its anatomical and functional features.
Specifically, the structural characteristics of the anterior eye
segment tissues render them susceptible to a number of risk factors
that can lead to an oxidative status (20). Notably, the eye is one of the
organs constantly exposed to environmental factors that induce ROS
production. Its anterior part and mainly the cornea is directly
exposed to harmful atmospheric oxygen, toxins, radiation, physical
abrasion, air pollution, artificial light, cigarette smoke, fumes
and gases from household cleaning products, toxic chemicals and
some drugs (72,73). Further, the solar UVR consists of
UVA (315-400 nm), UVB (280-315 nm) and UVC (100-280 nm) is the
primary source of ROS in the eye. The cornea is exposed directly to
UVR and absorbs all UVC, 80% UVB and 34% UVA. Besides this, AH
absorbs some of the UVB, the lens absorbs 66% of UVA and 20% of
UVB, and the retina absorbs only a minimal percentage of UVA
(<1%), but no UVB or UVC (72).
Absorption of UVR by ocular tissues, especially UVC and UVB,
eventually leads to photochemical production of ROS [e.g. singlet
oxygen (1O2), superoxide
(O2•-), hydroxyl radical (OH•),
peroxyl radical (ROO•)] (73,74)
causing UVR-induced molecular modifications (e.g. chain breaking,
pyrimidine and thymine dimers and protein crosslinks) associated
with pathological ophthalmic conditions such as cataract, glaucoma
and age-related macular degeneration (AMD) (75,76).
In addition to UVR, some chemotherapeutic,
phototoxic or even herbal origin drugs and diagnostic dyes can
induce the generation of ROS in the eye and thus cause early
cataracts or transient vision loss (77). For example, a drug widely used in
photodynamic tumour therapy, such as γ-cyclodextrin bicapped
C60 [(γCyD)2/C60, CDF0],
can effectively produce 1O2 (78). Apart from its anatomical
characteristics, the eye can also be affected by OS by virtue of
its physical and metabolic characteristics. Notably, the
mitochondria are a significant endogenous source of ROS in the eye,
as it is a metabolically active organ that consumes large amounts
of O2. Additionally, the eye's transparent structures,
such as the cornea, AH, lens, vitreous and retina, allow continuous
photochemical production of ROS (79). The biomolecular effectors of OS are
summarised in Table I.
 | Table IBiomolecular effectors of OS in
PEXS/PEXG. |
Table I
Biomolecular effectors of OS in
PEXS/PEXG.
| Author | Biomarkers | Sample source | Expression levels
increase/decrease | (Refs.) |
|---|
| Koliakos et
al |
8-Iso-PGF2a | ΑH | ↑ | (127) |
| Saxena et
al | AGEs | ΑH, serum | ↑ |
(92,110,111,112) |
| Aydın Yaz et
al | | | | |
|
Schlötzer-Schrehardt U, Shirakami et
al | | | | |
| Botling Taube et
al | Antothrombin
III | ΑH | ↑ | (128) |
| Strzalka- Mrozik
et al | ALDH1A1
(expression) | Anterior lens
capsule | ↑ | (114) |
| Dursun et
al | ARE | ΑH, serum | ↓ | (129) |
| Dmuchowska et
al | Arginine and
homo-arginine | AH | ↓ | (130) |
| Koliakos et
al | Ascorbic acid | ΑH | ↑ | (130-132) |
| Ferreira et
al | | | | |
| Dmuchowska et
al | | | | |
| Yimaz et
al | Ascorbic acid | Serum | ↓ | (108) |
| Botling Taube et
al | C3 | ΑH | ↑ | (128) |
| Dmuchowska et
al | Carnitine
(Hydroxybutyryl- and decatrienoyl- ) | AH | ↓ | (130) |
| Dairou et
al | CAT | serum | ↓ | (97,99,110) |
| Hosler et
al | | | | |
| Aydın Yaz et
al | | | | |
| Botling Taube et
al | CLSTN1 | ΑH | ↑ | (128) |
| Doudevski et
al | Clusterin | ΑH | ↑ | (59) |
| Zenkel et
al | Clusterin | Lens epithelial
cells | ↓ | (61) |
| Botling Taube et
al | CPE | ΑH | ↓ | (128) |
| Sorkhabi et
al | CRP | Serum | ↑ | (133) |
| Browne et
al | CTGF | ΑH | ↑ | (117) |
| Botling Taube et
al | DBP | ΑH | ↑ | (128) |
| Tetikoğlu et
al | Disulphide | Serum | ↑ | (134) |
| Koliakos et
al | ET-1 | ΑH | ↑ | (135,136) |
| Koukoula et
al | | | | |
| Park et
al | Flt3 ligand | AH | ↑ | (137) |
| Park et
al | Fractalkine | AH | ↓ | (137) |
| Botling Taube et
al | GPX3 | ΑH | ↓ | (128) |
| Gartaganis et
al | GSH | Lens epithelial
cells | ↓ | (115) |
| Aydın Yaz et
al | GSH | Serum | ↑ | (110) |
| Gartaganis et
al | GSSG | ΑH | ↓ | (116) |
| Gartaganis et
al | GSH/GSSG | ΑH | ↓ | (116) |
| Reddan et
al |
H2O2 | ΑH, serum | ↑ | (98,105) |
| Megaw Puustjärvi
et al | Hcy | ΑH, serum | ↑ | (43) |
| Dmuchowska et
al | Hydroxyanthranilic
acid | AH | ↓ | (130) |
| Zenkel et
al | IL-6 | ΑH | ↑ | (138) |
| Park et
al | IL-8 | ΑH | ↑ | (137,138) |
| Zenkel et
al | | | | |
| Dmuchowska et
al |
Indoleacetaldehyde | AH | ↑ | (130) |
| Botling Taube et
al | KNG-1 | ΑH | ↑ | (128) |
| Gartaganis et
al | MDA | Lens epithelial
cells | ↑ | (115) |
| Yağci et
al | MDA | Serum | ↑ | (107,108,110) |
| Yimaz et
al | | | | |
| Aydın Yaz et
al | | | | |
| Strzalka-Mrozik
et al | MGST1
(expression) | Anterior lens
capsule | ↑ | (114) |
| Stafiej et
al | MGST1
(expression) | Lens epithelial
cells | ↓ | (139) |
| Park et
al | MIP-1α | AH | ↑ | (137) |
|
Schlötzer-Schrehardt et al | MMP-2 | ΑH | ↑ | (120) |
| Strzalka-Mrozik
et al | mRNA of SOD2,
ALDH1A1, MGST1 | Lens tissues | ↑ | (114) |
| Vulovic et
al | NO• | ΑH | ↑ | (140) |
| Yağci et
al |
NO2- (levels) | Serum | ↑ | (107) |
| Turan G and Turan
M | PCNA | Lens epithelial
cells | ↓ | (141) |
| Dursun et
al | PON (activity
levels) | ΑH | ↓ | (129) |
| Yağci et
al | PON (activity
levels) | Serum | ↓ | (107) |
| Dursun et
al | | | ↓ | (129) |
| Yağci et
al | Protein
carbonyls | ΑH | ↑ | (107,142) |
| Papadopoulou et
al | | | | |
| Papadopoulou et
al | Protein
carbonyls | Lens epithelial
cells and Anterior lens capsule | ↑ | (142) |
| Yağci et
al | Protein
carbonyls | Serum | ↑ | (107) |
| Botling Taube et
al | RBP3 | ΑH | ↓ | (128) |
| Dmuchowska et
al |
S-adenosylmethionine | AH | ↓ | (130) |
| Yimaz et
al | Selenium | ΑH, serum | ↓ | (38) |
| Ferreira et
al | SOD (activity
levels) | ΑH | ↑ | (132) |
| Uçakhan et
al | SOD (activity
levels) | Lens capsule | ↑ | (143) |
| Yağci et
al | SOD (activity
levels) | Serum | ↑ | (107,110) |
| Aydın Yaz et
al | | | | |
| Strzalka-Mrozik
et al | SOD2 (MnSOD)
(expression) | Anterior lens
capsule | ↑ | (114) |
| Tetikoğlu et
al | SPA | Serum | ↓ | (144) |
| Dursun et
al | TAC | ΑH | ↓ | (129) |
| Dursun et
al | TAC | Serum | ↓ | (129) |
| Faschinger et
al | TBARS | ΑH | ↑ | (109,116) |
| Gartaganis et
al | | | | |
|
Schlötzer-Schrehardt et al | TGF-b1 | ΑH | ↑ | (56,137) |
| Park et
al | | | | |
|
Schlötzer-Schrehardt et al | TIMP-2 | ΑH | ↑ | (120) |
| Fountoulakis et
al | TIMP-4 | AH | ↑ | (145) |
| Vulovic et
al | TNF-α | ΑH | ↑ | (140) |
| Sorkhabi et
al | TNF-α | serum | ↑ | (133) |
| Dursun et
al | TOS | ΑH | ↑ | (129) |
| Dursun et
al | TOS | Serum | ↑ | (129) |
| Tetikoğlu et
al | Total thiol and
native thiol | Serum | ↓ | (134) |
| Tetikoğlu et
al |
Thiol/disulfide | Serum | ↓ | (134) |
| Turan G and Turan
M | TUNEL | Lens epithelial
cells | ↑ | (141) |
| Simavli et
al | XO (activity
levels) | ΑH | ↑ | (146) |
| Yağci et
al | XO (activity
levels) | Serum | ↑ | (107) |
| Yildirim et
al | Zinc | Lens | ↓ | (22) |
OS in the crystalline lens
The lens is particularly vulnerable to OS due to its
continuous exposure to solar UVR and oxidants throughout its life.
Since the lens has mostly fibrous cells, it does not regenerate
after damage. Moreover, the reduced levels of antioxidant molecules
in the lens nucleus and the absence of protein turnover lead to an
impaired ability to repair. Thus, damages accumulate over time
(80). Except for solar UVR, other
sources of OS in the lens include smoke and oxidants from AH or
those produced by the lens cells themselves. The lens acts as a
filter and absorbs over 60% of UVA and 20% of UVB, thus preventing
much of the radiation to reach the retina (73). As a result, the photooxidation of
the thiol groups of the lens's crystallins forms disulfide bridges
between the molecules, leading to protein aggregation and cataract
formation (81) ROS in the lens
[e.g., O2•-, hydrogen peroxide
(H2O2), OH•] can also be created
endogenously through cellular metabolism in different cell
compartments, such as mitochondria, peroxisomes and cytoplasm.
For example, O2•-can be
produced by the typical electron transport system and the activity
of cytochrome P450. The nicotinamide adenine dinucleotide phosphate
(NADPH) oxidase complex with Rac GTPases, associated with
activating plasma membrane receptors by external signals like
growth factors, can also produce O2•-. In
addition, growth factor receptors can be activated by the UVR and
produce ROS. Intracellular H2O2 can be
derived from superoxide dismutase (SOD) activity or created from
ascorbate and O2 in the presence of Fe+3 or
even generated from the AH. Finally, the reaction of
H2O2 with metal ions (M+) can lead to
OH• H O2•- via the Fenton reaction
(82). However, the endogenous
production of ROS in the lens does not necessarily lead to OS since
low concentrations of H2O2 could play a role
as a signal transduction factor in the differentiation of lens
epithelial cells and also be a significant regulatory molecule of
numerous vital enzymes, such as phosphatases and kinases (83). Under OS conditions, the selective
oxidation of specific amino acids in the lens results in
aggregation and degradation of proteins, reduction of water
solubility, crosslinking, charge changes, etc. (84,85)
can significantly contribute to cataract development (86).
Additionally, ROS causes increased oxidation of
amino acid residues of methionine, cysteine, tryptophan and
phenylalanine in the lens, as evidenced by the formation of protein
disulfides kynurenine, o-Tyr and Met-SO (87,88).
Specifically, the associated ageing increase in Met-SO formation is
related to the loss of a number of protein activities that affect
various functions of the lens (89,90).
To protect against oxidation, the lens has evolved as an anaerobic
system containing high concentrations of ascorbic acid and
glutathione (GSH), an endogenous antioxidant molecule part of the
antioxidant defence system (91).
However, these defensive antioxidant molecules decrease with age,
with GSH declining significantly in the lens nucleus. Subsequently,
ascorbic acid is increasingly oxidised, leading to the accumulation
of crystalline-bound advanced glycation end products (AGEs) that
contribute to the cataractogenesis (92).
OS in the epithelium of the
crystalline lens
The metabolically active lens epithelial cells are
primarily responsible for their defence against OS (93). UVA is the main responsible for ROS
production in the lens epithelial cells. About 70% of UVA passes
through the cornea towards lens epithelial cells reacting with
NADPH and nicotinamide adenine dinucleotide (NADH), which are
present in high concentrations in these cells producing ROS (e.g.,
1O2, O2•-,
H2O2) (94).
Exposure of lens epithelial cells to UVA results in increased lipid
peroxidation, decreased antioxidant enzymes e.g. catalase (CAT),
reduced viability and cell death (95). UVB is estimated to be only 3% of
the total UVR reaching the lens and can trigger apoptotic
mechanisms in the lens epithelial cells and inactivate enzymes
(96,97).
In addition to UV-induced photooxidation, oxidative
damage to the macromolecules of lens epithelial cells is also
caused by elevated levels of cellular oxidants produced by exposure
to toxic chemicals and failure of antioxidant defences (97). Moreover, lens epithelial cells can
be affected by the high levels of H2O2 in the
AH (98).
H2O2 and peroxynitrite (ONOO-) are
thought to be the essential oxidants of acute or chronic exposure
of lens epithelial cells (97) and
their elevated levels can cause oxidation-dependent inactivation of
crucial enzymes such as CAT, proteasome, and arylamine
N-acetyltransferases (NATs) (97,99).
Oxidative damage to lens epithelial cells can also cause osmotic
swelling of the lens and loss of its transparency (100).
OS in the AH
ROS production in AH is mainly due to UVR and
inflammatory processes in adjacent structures, e.g., surgery
(101,102). The AH contains ascorbic acid,
proteins, certain amino acids (e.g. tyrosine, phenylalanine,
cysteine, tryptophan) and uric acid, which act as filters absorbing
the majority of UVR, leaving only a tiny part of it reaching the
structures of the eye (103).
Despite the critical role of endogenous UV filters like ascorbic
acid, their photooxidation can produce potent oxidising molecules,
such as 1O2 and H2O2
(104). The increased
concentration of H2O2 in the AH has been
found to reduce GSH metabolism (105). It may cause damage to the corneal
endothelium, the lens and the radial body, particularly the
trabecular meshwork. In vitro studies have associated
increased H2O2 levels with reduced AH
drainage, resulting in glaucoma (79).
In contrast to the findings mentioned earlier, there
is a view that OS does not cause permanent damage to the AH. Any
alterations of vital components are considered to be part of its
antioxidant defence and are relatively reversible after restoring
optimal conditions. However, long-term exposure to OS results in
loss of antioxidants and tissue damage (89).
OS in the pathogenesis of PEXS
OS plays a crucial role in the pathogenesis of
several eye diseases, including PEXS. Disturbances in the delicate
balance between ROS and antioxidant defense mechanisms of the eye
may contribute to the development of PEXS. Evidence suggests that
high malondialdehyde (MDA) levels, which are the end-product of
polyunsaturated fatty acid peroxidation reaction and a marker of
free radical-mediated lipid peroxidation, are found in the patients
of PEXS (106-108).
Similarly, high levels of thiobarbituric acid reactive substances
(TBARS), the major breakdown products of lipid peroxides, were
significantly higher in the AH samples collected from primary
open-angle glaucoma patients (109). Besides, increased levels of AGEs
are also observed in the AH and serum of PEXS patients (110,111).
Additionally, these specific oxidation and glycation
products could trigger the glaucoma formation associated with PEXS
(112). Specifically, these end
products can induce ROS generation, thus resulting in the
deterioration of trabecular meshwork cells. Therefore, correcting
the OS-induced damage using antioxidants could be considered a
therapeutic strategy to prevent glaucoma in PEXS patients (113). According to these findings, OS
and its end products might be a vital factor in PEXS pathogenesis.
Further, the levels of superoxide dismutase 2 (SOD2), aldehyde
dehydrogenase 1a1 (ALDH1A1), and microsomal glutathione transferase
1 (MGST1) which are part of the essential antioxidant defense
system, are high in the anterior lens capsule of PEXS patients
(114). However, antioxidant
enzymes, SOD and CAT were significantly lower in PEXS patients
(110). Similarly, the levels of
GSH decrease in the lens epithelial cells of PXES patients
(115,116). The antioxidant system defence
failure could result in inadequate OS response and
pseudoexfoliation development.
OS elevates the levels of free radicals, TGF-b1 and
other growth factors in the eye. It is a critical factor in
developing fibrosis in the PEXS-affected eyes (117). Also, OS disrupts the balance
between MMPs and TIMPs, leading to dysregulated ECM in the eye of
PEXS patients (118). TIMPs
imbalance has been implicated in various abnormal fibroblastic
disorders (119), including the
development of PEXM and PEXG (120). Studies have found that TGF-b1 may
up-regulate OS and have a synergic external role in the PEXS
development (121,122). Especially, TGF-b1-mediated
upregulation of LOXL1 could promote fibrosis in the eye of PEXS
patients. Further, studies have indicated the synergy between
TGF-β1 and OS in the activation of the LOXL1 (20,122). Therefore it could be suggested
that the abnormal ECM deposition can be triggered by OS, and TGF-b1
under the high-risk LOXL1 haplotype may contribute to the PEXS
aggregates in the ocular tissues (20).
OS is also known to modify glutamine synthase and
thus influence glutamate/glutamine metabolism, leading to increased
neurotoxic concentrations of glutamate (123). Further, OS can also damage the
mitochondria present in the cells of optic nerves resulting in a
reduced energy supply. Considering the critical role of
mitochondrial disfunction in glaucoma evolution, therapies
targeting mitochondria with specific antioxidants may improve the
survival of retina ganglion cells to protect them from glaucomatous
degeneration (124). Indirectly,
OS can also cause vascular changes resulting in impaired blood flow
to the optic nerve, injury to trabecular meshwork that decreases AH
outflow and elevated IOP and glial cell dysfunction (110,125,126). Overall these studies point to the
regulation of OS to regulate the pathogenesis of PEXS.
8. Conclusions and future directions
Numerous studies have shown that OS plays a crucial
role in pseudoexfoliation and is critical for determining the onset
and evolution of either the PEXS or PEXG. With human longevity
increasing, PEXS and PEXG will become severe clinical problems as
they cause several severe complications, including vision loss.
Hence, a prompt diagnosis is essential to avoid a challenging
clinical course with poor response to treatment, timely surgery and
an overall good prognosis. Diagnostics have greatly improved, and
standardised treatment protocols are currently available. Current
disease management focuses primarily on increasing antioxidants
concentrations to compensate for OS. Specifically, the diet
modifications and various antioxidant supplements have been used in
patients with PEXS, however, with limited success. Therefore, we
need to develop novel tools to reduce OS in the eye of the patient
with PEXS.
Over the past decade, several studies have been
undertaken to elucidate the molecular basis of this disease. Still,
the exact mechanism of triggering the PEXM and its deposition and
associated pathologies remain unclear. Efforts to unearth the
causes for this devastating disease should be a priority. At
present, we better comprehend the genetic and environmental factors
involved in the PEXS. However, the involvement of genetic and
environmental factors in making one population susceptible has to
be elucidated. Recent studies have uncovered several genes involved
in the pathogenesis of PEXS/PEXG. Still, we do not have clear
reasons for all the pathological processes. Thus, we may need to
expand the studies on both population and molecular levels to get
insights into the pathogenesis of PEXS. Also, future studies are
required to uncover the reasons for varying degrees of
susceptibility between human populations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SM conceived, designed and wrote the review and is
the corresponding author. MP, PP and KK had equal contribution,
performed the literature review and analyzed the data. CDG reviewed
and edited the manuscript, and supervised and administered the
project. All authors read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karamanos NK, Theocharis AD, Piperigkou Z,
Manou D, Passi A, Skandalis SS, Vynios DH, Orian-Rousseau V,
Ricard-Blum S, Schmelzer CEH, et al: A guide to the composition and
functions of the extracellular matrix. FEBS J. 288:6850–6912.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iozzo RV, Theocharis AD, Neill T and
Karamanos NK: Complexity of matrix phenotypes. Matrix Biol Plus.
6-7(100038)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dvorak-Theobald G: Pseudo-exfoliation of
the lens capsule: Relation to true exfoliation of the lens capsule
as reported in the literature and role in the production of
glaucoma capsulocuticulare. Am J Ophthalmol. 37:1–12.
1954.PubMed/NCBI
|
|
4
|
Roche J: Pseudo-exfoliation of the lens
capsule. Br J Ophthalmol. 52:265–269. 1968.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shakib M, Ashton N and Blach R: Electron
microscopic study of pseudo-exfoliation of the lens capsule. Ii.
Iris and ciliary body. Invest Ophthalmol. 4:154–161.
1965.PubMed/NCBI
|
|
6
|
Conway RM, Schlötzer-Schrehardt U, Küchle
M and Naumann GO: Pseudoexfoliation syndrome: Pathological
manifestations of relevance to intraocular surgery. Clin Exp
Ophthalmol. 32:199–210. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tekin K, Inanc M and Elgin U: Monitoring
and management of the patient with pseudoexfoliation syndrome:
Current perspectives. Clin Ophthalmol. 13:453–464. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mastronikolis S, Pagkalou M, Baroutas G,
Kyriakopoulou K, Makri OE and Georgakopoulos CD: Pseudoexfoliation
syndrome: The critical role of the extracellular matrix in
pathogenesis and treatment. IUBMB Life: Feb 24, 2022 (Epub ahead of
print).
|
|
9
|
Challa P: Genetics of pseudoexfoliation
syndrome. Curr Opin Ophthalmol. 20:88–91. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ariga M, Nivean M and Utkarsha P:
Pseudoexfoliation syndrome. J Curr Glaucoma Pract. 7:118–120.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Elhawy E, Kamthan G, Dong CQ and Danias J:
Pseudoexfoliation syndrome, a systemic disorder with ocular
manifestations. Hum Genomics. 6(22)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schlötzer-Schrehardt U and Naumann GO:
Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol.
141:921–937. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ritch R: Ocular and systemic
manifestations of exfoliation syndrome. J Glaucoma. 23 (8 Suppl
1):S1–S8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ovodenko B, Rostagno A, Neubert TA, Shetty
V, Thomas S, Yang A, Liebmann J, Ghiso J and Ritch R: Proteomic
analysis of exfoliation deposits. Invest Ophthalmol Vis Sci.
48:1447–1457. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gartaganis SP, Georgakopoulos CD, Assouti
M, Mela EK, Exarchou A, Giannelou I, Gotsis SS, Ziouti N, Vynios
DH, Tripathi BJ and Tripathi RC: Changes in HNK-1 epitope and
collagen type IX in the aqueous humour of patients with
pseudoexfoliation syndrome. Curr Eye Res. 28:5–10. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sharma S, Chataway T, Burdon KP,
Jonavicius L, Klebe S, Hewitt AW, Mills RA and Craig JE:
Identification of LOXL1 protein and apolipoprotein E as components
of surgically isolated pseudoexfoliation material by direct mass
spectrometry. Exp Eye Res. 89:479–485. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tran VT: Washout of pseudoexfoliation
material combined with cataract surgery: A new surgical approach to
lower intraocular pressure in pseudoexfoliation syndrome. Int
Ophthalmol. 35:209–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ritch R and Schlötzer-Schrehardt U:
Exfoliation syndrome. Surv Ophthalmol. 45:265–315. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schlötzer-Schrehardt UM, Koca MR, Naumann
GO and Volkholz H: Pseudoexfoliation syndrome. Ocular manifestation
of a systemic disorder? Arch Ophthalmol. 110:1752–1756.
1992.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chiras D, Kitsos G, Petersen MB,
Skalidakis I and Kroupis C: Oxidative stress in dry age-related
macular degeneration and exfoliation syndrome. Crit Rev Clin Lab
Sci. 52:12–27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yüksel N, Karabaş VL, Arslan A, Demirci A
and Cağlar Y: Ocular hemodynamics in pseudoexfoliation syndrome and
pseudoexfoliation glaucoma. Ophthalmology. 108:1043–1049.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yildirim N, Yasar E, Gursoy H and Colak E:
Prevalence of pseudoexfoliation syndrome and its association with
ocular and systemic diseases in Eskisehir, Turkey. Int J
Ophthalmol. 10:128–134. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Topouzis F and Anastasopoulos E: Incidence
of pseudoexfoliation syndrome. Am J Ophthalmol. 148:181–182.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chan TCW, Bala C, Siu A, Wan F and White
A: Risk factors for rapid glaucoma disease progression. Am J
Ophthalmol. 180:151–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aboobakar IF, Johnson WM, Stamer WD,
Hauser MA and Allingham RR: Major review: Exfoliation syndrome;
advances in disease genetics, molecular biology, and epidemiology.
Exp Eye Res. 154:88–103. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mansour AM, Konstas AGP, Mansour HA,
Charbaji AR and Jawhari KM: A case-cohort study of exfoliation risk
factors and literature review. Middle East Afr J Ophthalmol.
28:36–50. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Whigham BT and Allingham RR: Review: The
role of LOXL1 in exfoliation syndrome/glaucoma. Saudi J Ophthalmol.
25:347–352. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Konstas AGP and Ringvold A: Epidemiology
of exfoliation syndrome. J Glaucoma. 27 (Suppl 1):S4–S11.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Forsius H: Exfoliation syndrome in various
ethnic populations. Acta Ophthalmol. Suppl (1985) 184:71–85.
1988.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Miglior S and Bertuzzi F: Exfoliative
glaucoma: New evidence in the pathogenesis and treatment. Prog
Brain Res. 221:233–241. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Amini H, Daneshvar R, Eslami Y, Moghimi S
and Amini N: Early-onset pseudoexfoliation syndrome following
multiple intraocular procedures. J Ophthalmic Vis Res. 7:190–196.
2012.PubMed/NCBI
|
|
32
|
Thorleifsson G, Magnusson KP, Sulem P,
Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, JonRasdottir
A, Jonasdottir A, Stefansdottir G, et al: Common sequence variants
in the LOXL1 gene confer susceptibility to exfoliation glaucoma.
Science. 317:1397–1400. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schlötzer-Schrehardt U: Genetics and
genomics of pseudoexfoliation syndrome/glaucoma. Middle East Afr J
Ophthalmol. 18:30–36. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zalewska R, Pepinski W, Smolenska-Janica
D, Mariak Z, Proniewska-Skretek E, Skawronska M and Janica J: Loss
of heterozygosity in patients with pseudoexfoliation syndrome. Mol
Vis. 9:257–261. 2003.PubMed/NCBI
|
|
35
|
Kozobolis VP, Detorakis ET, Sourvinos G,
Pallikaris IG and Spandidos DA: Loss of heterozygosity in
pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci.
40:1255–1260. 1999.PubMed/NCBI
|
|
36
|
Aung T, Ozaki M, Mizoguchi T, Allingham
RR, Li Z, Haripriya A, Nakano S, Uebe S, Harder JM, Chan AS, et al:
A common variant mapping to CACNA1A is associated with
susceptibility to exfoliation syndrome. Nat Genet. 47:387–392.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Padhy B, Kapuganti RS, Hayat B, Pranjya
Paramita Mohanty PP and Alone DP: De novo variants in an
extracellular matrix protein coding gene, fibulin-5 (FBLN5) are
associated with pseudoexfoliation. Eur J Hum Genet. 27:1858–1866.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yimaz A, Ayaz L and Tamer L: Selenium and
pseudoexfoliation syndrome. Am J Ophthalmol. 151:272–276.e1.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Arnarsson A, Sasaki H and Jonasson F:
Twelve-year incidence of exfoliation syndrome in the Reykjavik eye
study. Acta Ophthalmol. 91:157–162. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Arnarsson AM: Epidemiology of exfoliation
syndrome in the Reykjavik eye study. Acta Ophthalmol 87 Thesis.
3:1–17. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pasquale LR, Wiggs JL, Willett WC and Kang
JH: The relationship between caffeine and coffee consumption and
exfoliation glaucoma or glaucoma suspect: A prospective study in
two cohorts. Invest Ophthalmol Vis Sci. 53:6427–6433.
2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Christensen B, Mosdol A, Retterstol L,
Landaas S and Thelle DS: Abstention from filtered coffee reduces
the concentrations of plasma homocysteine and serum cholesterol-a
randomized controlled trial. Am J Clin Nutr. 74:302–307.
2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Puustjärvi T, Blomster H, Kontkanen M,
Punnonen K and Teräsvirta M: Plasma and aqueous humour levels of
homocysteine in exfoliation syndrome. Graefes Arch Clin Exp
Ophthalmol. 242:749–754. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kozobolis VP, Papatzanaki M, Vlachonikolis
IG, Pallikaris IG and Tsambarlakis IG: Epidemiology of
pseudoexfoliation in the island of Crete (Greece). Acta Ophthalmol
Scand. 75:726–729. 1997.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kang JH, Loomis S, Wiggs JL, Stein JD and
Pasquale LR: Demographic and geographic features of exfoliation
glaucoma in 2 United States-based prospective cohorts.
Ophthalmology. 119:27–35. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Stein JD, Pasquale LR, Talwar N, Kim DS,
Reed DM, Nan B, Kang JH, Wiggs JL and Richards JE: Geographic and
climatic factors associated with exfoliation syndrome. Arch
Ophthalmol. 129:1053–1060. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Pasquale LR, Jiwani AZ, Zehavi-Dorin T,
Majd A, Rhee DJ, Chen T, Turalba A, Shen L, Brauner S, Grosskreutz
C, et al: Solar exposure and residential geographic history in
relation to exfoliation syndrome in the United States and Israel.
Jama Ophthalmol. 132:1439–1445. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pasquale LR, Kang JH, Fan B,
Levkovitch-Verbin H and Wiggs JL: LOXL1 polymorphisms: Genetic
biomarkers that presage environmental determinants of exfoliation
syndrome. J Glaucoma. 27 (Suppl 1):S20–S23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu X, Zhao Y, Gao J, Pawlyk B, Starcher
B, Spencer JA, Yanagisawa H, Zuo J and Li T: Elastic fiber
homeostasis requires lysyl oxidase-like 1 protein. Nat Genet.
36:178–182. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
50
|
Oleggini R, Gastaldo N and Di Donato A:
Regulation of elastin promoter by lysyl oxidase and growth factors:
Cross control of lysyl oxidase on TGF-beta1 effects. Matrix Biol.
26:494–505. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li G, Schmitt H, Johnson WM, Lee C,
Navarro I, Cui J, Fleming T, Gomez-Caraballo M, Elliott MH,
Sherwood JM, et al: Integral role for lysyl oxidase-like-1 in
conventional outflow tissue function and behavior. FASEB J.
34:10762–10777. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Schlötzer-Schrehardt U, Hammer CM, Krysta
AW, Hofmann-Rummelt C, Pasutto F, Sasaki T, Kruse FE and Zenkel M:
LOXL1 deficiency in the lamina cribrosa as candidate susceptibility
factor for a pseudoexfoliation-specific risk of glaucoma.
Ophthalmology. 119:1832–1843. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Schmitt HM, Johnson WM, Aboobakar IF,
Strickland S, Gomez-Caraballo M, Parker M, Finnegan L, Corcoran DL,
Skiba NP, Allingham RR, et al: Identification and activity of the
functional complex between hnRNPL and the pseudoexfoliation
syndrome-associated lncRNA, LOXL1-AS1. Hum Mol Genet. 29:1986–1995.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Liu RM and Gaston Pravia KA: Oxidative
stress and glutathione in TGF-beta-mediated fibrogenesis. Free
Radic Biol Med. 48:1–15. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Takai Y, Tanito M and Ohira A: Multiplex
cytokine analysis of aqueous humor in eyes with primary open-angle
glaucoma, exfoliation glaucoma, and cataract. Invest Ophthalmol Vis
Sci. 53:241–247. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zenkel M, Krysta A, Pasutto F, Juenemann
A, Kruse FE and Schlötzer-Schrehardt U: Regulation of lysyl
oxidase-like 1 (LOXL1) and elastin-related genes by pathogenic
factors associated with pseudoexfoliation syndrome. Invest
Ophthalmol Vis Sci. 52:8488–8495. 2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Djordjević-Jocić J, Zlatanović G,
Veselinović D, Jovanović P, Djordjević V, Zvezdanović L,
Stanković-Babić G, Vujanović M, Cekić S, Zenkel M and
Schlotzer-Schrehardt U: Transforming growth factor beta1,
matrix-metalloproteinase-2 and its tissue inhibitor in patients
with pseudoexfoliation glaucoma/syndrome. Vojnosanit Pregl.
69:231–236. 2012.PubMed/NCBI
|
|
58
|
Schlötzer-Schrehardt U, Zenkel M, Küchle
M, Sakai LY and Naumann GO: Role of transforming growth
factor-beta1 and its latent form binding protein in
pseudoexfoliation syndrome. Exp Eye Res. 73:765–780.
2001.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Doudevski I, Rostagno A, Cowman M,
Liebmann J, Ritch R and Ghiso J: Clusterin and complement
activation in exfoliation glaucoma. Invest Ophthalmol Vis Sci.
55:2491–2499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Morris J, Myer C, Cornet T, Junk AK, Lee
RK and Bhattacharya SK: Proteomics of pseudoexfoliation materials
in the anterior eye segment. Adv Protein Chem Struct Biol.
127:271–290. 2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zenkel M, Kruse FE, Jünemann AG, Naumann
GO and Schlötzer-Schrehardt U: Clusterin deficiency in eyes with
pseudoexfoliation syndrome may be implicated in the aggregation and
deposition of pseudoexfoliative material. Invest Ophthalmol Vis
Sci. 47:1982–1990. 2006.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gerschman R, Gilbert DL, Nye SW, Dwyer P
and Fenn WO: Oxygen poisoning and x-irradiation: A mechanism in
common. Science. 119:623–626. 1954.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Commoner B, Townsend J and Pake GE: Free
radicals in biological materials. Nature. 174:689–691.
1954.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Harman D: Aging: A theory based on free
radical and radiation chemistry. J Gerontol. 11:298–300.
1956.PubMed/NCBI View Article : Google Scholar
|
|
65
|
McCord JM and Fridovich I: Superoxide
dismutase. An enzymic function for erythrocuprein (hemocuprein). J
Biol Chem. 244:6049–6055. 1969.PubMed/NCBI
|
|
66
|
Beckman KB and Ames BN: The free radical
theory of aging matures. Physiol Rev. 78:547–581. 1998.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40.
2006.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Jones DP: Redefining oxidative stress.
Antioxid Redox Signal. 8:1865–1879. 2006.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017(8416763)2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Li R, Jia Z and Trush MA: Defining ROS in
biology and medicine. React Oxyg Species (Apex). 1:9–21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shoham A, Hadziahmetovic M, Dunaief JL,
Mydlarski MB and Schipper HM: Oxidative stress in diseases of the
human cornea. Free Radic Biol Med. 45:1047–1055. 2008.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Cejka C and Cejkova J: Oxidative stress to
the cornea, changes in corneal optical properties, and advances in
treatment of corneal oxidative injuries. Oxid Med Cell Longev.
2015(591530)2015.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Chen Y, Mehta G and Vasiliou V:
Antioxidant defenses in the ocular surface. Ocul Surf. 7:176–185.
2009.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Cai CX, Birk DE and Linsenmayer TF:
Nuclear ferritin protects DNA from UV damage in corneal epithelial
cells. Mol Biol Cell. 9:1037–1051. 1998.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Sacca SC, Bolognesi C, Battistella A,
Bagnis A and Izzotti A: Gene-environment interactions in ocular
diseases. Mutat Res. 667:98–117. 2009.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Roberts JE: Screening for ocular
phototoxicity. Int J Toxicol. 21:491–500. 2002.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zhao B, He YY, Chignell CF, Yin JJ, Andley
U and Roberts JE: Difference in phototoxicity of cyclodextrin
complexed fullerene [(gamma-CyD)2/C60] and its aggregated
derivatives toward human lens epithelial cells. Chem Res Toxicol.
22:660–667. 2009.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Cabrera MP and Chihuailaf RH: Antioxidants
and the integrity of ocular tissues. Vet Med Int.
2011(905153)2011.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Beebe DC, Holekamp NM and Shui YB:
Oxidative damage and the prevention of age-related cataracts.
Ophthalmic Res. 44:155–165. 2010.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ozaki Y, Mizuno A, Itoh K and Iriyama K:
Inter- and intramolecular disulfide bond formation and related
structural changes in the lens proteins. A Raman spectroscopic
study in vivo of lens aging. J Biol Chem. 262:15545–15551.
1987.PubMed/NCBI
|
|
82
|
Berthoud VM and Beyer EC: Oxidative
stress, lens gap junctions, and cataracts. Antioxid Redox Signal.
11:339–353. 2009.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ohguro N, Fukuda M, Sasabe T and Tano Y:
Concentration dependent effects of hydrogen peroxide on lens
epithelial cells. Br J Ophthalmol. 83:1064–1068. 1999.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Bodaness RS, Leclair M and Zigler JS Jr:
An analysis of the H2O2-mediated crosslinking of lens crystallins
catalyzed by the heme-undecapeptide from cytochrome c. Arch Biochem
Biophys. 231:461–469. 1984.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Zigler JS Jr, Huang QL and Du XY:
Oxidative modification of lens crystallins by H2O2 and chelated
iron. Free Radic Biol Med. 7:499–505. 1989.PubMed/NCBI View Article : Google Scholar
|
|
86
|
McNamara M and Augusteyn RC: The effects
of hydrogen peroxide on lens proteins: A possible model for nuclear
cataract. Exp Eye Res. 38:45–56. 1984.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Garner MH and Spector A: Selective
oxidation of cysteine and methionine in normal and senile
cataractous lenses. Proc Natl Acad Sci USA. 77:1274–1277.
1980.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Fu S, Dean R, Southan M and Truscott R:
The hydroxyl radical in lens nuclear cataractogenesis. J Biol Chem.
273:28603–28609. 1998.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Vogt W: Oxidation of methionyl residues in
proteins: Tools, targets, and reversal. Free Radic Biol Med.
18:93–105. 1995.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Truscott RJ and Augusteyn RC: Oxidative
changes in human lens proteins during senile nuclear cataract
formation. Biochim Biophys Acta. 492:43–52. 1977.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Rose RC, Richer SP and Bode AM: Ocular
oxidants and antioxidant protection. Proc Soc Exp Biol Med.
217:397–407. 1998.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Saxena P, Saxena AK, Cui XL, Obrenovich M,
Gudipaty K and Monnier VM: Transition metal-catalyzed oxidation of
ascorbate in human cataract extracts: Possible role of advanced
glycation end products. Invest Ophthalmol Vis Sci. 41:1473–1481.
2000.PubMed/NCBI
|
|
93
|
Spector A: Oxidative stress-induced
cataract: Mechanism of action. FASEB J. 9:1173–1182.
1995.PubMed/NCBI
|
|
94
|
Dillon J, Zheng L, Merriam JC and Gaillard
ER: The optical properties of the anterior segment of the eye:
Implications for cortical cataract. Exp Eye Res. 68:785–795.
1999.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Rogers CS, Chan LM, Sims YS, Byrd KD,
Hinton DL and Twining SS: The effects of sub-solar levels of UV-A
and UV-B on rabbit corneal and lens epithelial cells. Exp Eye Res.
78:1007–1014. 2004.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Long AC, Colitz CM and Bomser JA:
Apoptotic and necrotic mechanisms of stress-induced human lens
epithelial cell death. Exp Biol Med (Maywood). 229:1072–1080.
2004.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Dairou J, Malecaze F, Dupret JM and
Rodrigues-Lima F: The xenobiotic-metabolizing enzymes arylamine
N-acetyltransferases in human lens epithelial cells: Inactivation
by cellular oxidants and UVB-induced oxidative stress. Mol
Pharmacol. 67:1299–1306. 2005.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Reddan JR, Steiger CA, Dziedzic DC and
Gordon SR: Regional differences in the distribution of catalase in
the epithelium of the ocular lens. Cell Mol Biol (Noisy-le-grand).
42:209–219. 1996.PubMed/NCBI
|
|
99
|
Hosler MR, Wang-Su ST and Wagner BJ:
Targeted disruption of specific steps of the ubiquitin-proteasome
pathway by oxidation in lens epithelial cells. Int J Biochem Cell
Biol. 35:685–697. 2003.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Giblin FJ, McCready JP, Schrimscher L and
Reddy VN: Peroxide-induced effects on lens cation transport
following inhibition of glutathione reductase activity in vitro.
Exp Eye Res. 45:77–91. 1987.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Cejková J, Stípek S, Crkovská J, Ardan T,
Pláteník J, Cejka C and Midelfart A: UV Rays, the
prooxidant/antioxidant imbalance in the cornea and oxidative eye
damage. Physiol Res. 53:1–10. 2004.PubMed/NCBI
|
|
102
|
Barros PS, Padovani CF, Silva VV, L
Queiroz L and Barros SBM: Antioxidant status of dog aqueous humor
after extracapsular lens extraction. Braz J Med Biol Res.
36:1491–1494. 2003.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Ringvold A, Anderssen E, Jellum E, Bjerkås
E, Sonerud GA, Haaland PJ, Devor TP and Kjønniksen I: UV-Absorbing
compounds in the aqueous humor from aquatic mammals and various
non-mammalian vertebrates. Ophthalmic Res. 35:208–216.
2003.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Wielgus AR and Sarna T: Ascorbate enhances
photogeneration of hydrogen peroxide mediated by the iris melanin.
Photochem Photobiol. 84:683–691. 2008.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Megaw JM: Glutathione and ocular
photobiology. Curr Eye Res. 3:83–87. 1984.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Benoist d'Azy C, Pereira B, Chiambaretta F
and Dutheil F: Oxidative and anti-oxidative stress markers in
chronic glaucoma: A systematic review and meta-analysis. PLoS One.
11(e0166915)2016.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Yağci R, Gürel A, Ersöz I, Keskin UC,
Hepşen IF, Duman S and Yiğitoğlu R: Oxidative stress and protein
oxidation in pseudoexfoliation syndrome. Curr Eye Res.
31:1029–1032. 2006.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Yimaz A, Adigüzel U, Tamer L, Yildirim O,
Oz O, Vatansever H, Ercan B, Değirmenci US and Atik U: Serum
oxidant/antioxidant balance in exfoliation syndrome. Clin Exp
Ophthalmol. 33:63–66. 2005.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Faschinger C, Schmut O, Wachswender C and
Mossböck G: Glaucoma and oxidative stress. Determination of
malondialdehyde-a product of lipid peroxidation. Ophthalmologe.
103:953–959. 2006.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
110
|
Aydın Yaz Y, Yildirim N, Yaz Y, Tekin N,
İnal M and Şahin FM: Role of oxidative stress in pseudoexfoliation
syndrome and pseudoexfoliation glaucoma. Turk J Ophthalmol.
49:61–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Schlötzer-Schrehardt U: Oxidative stress
and pseudoexfoliation glaucoma. Klin Monbl Augenheilkd.
227:108–113. 2010.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
112
|
Shirakami T, Yamanaka M, Fujihara J,
Matsuoka Y, Gohto Y, Obana A and Tanito M: Advanced glycation end
product accumulation in subjects with open-angle glaucoma with and
without exfoliation. Antioxidants (Basel). 9(755)2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Park CH and Kim JW: Effect of advanced
glycation end products on oxidative stress and senescence of
trabecular meshwork cells. Korean J Ophthalmol. 26:123–131.
2012.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Strzalka-Mrozik B, Prudlo L, Kimsa MW,
Kimsa MC, Kapral M, Nita M and Mazurek U: Quantitative analysis of
SOD2, ALDH1A1 and MGST1 messenger ribonucleic acid in anterior lens
epithelium of patients with pseudoexfoliation syndrome. Mol Vis.
19:1341–1349. 2013.PubMed/NCBI
|
|
115
|
Gartaganis SP, Patsoukis NE, Nikolopoulos
DK and Georgiou CD: Evidence for oxidative stress in lens
epithelial cells in pseudoexfoliation syndrome. Eye (Lond).
21:1406–1411. 2007.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Gartaganis SP, Georgakopoulos CD,
Patsoukis NE, Gotsis SS, Gartaganis VS and Georgiou CD: Glutathione
and lipid peroxide changes in pseudoexfoliation syndrome. Curr Eye
Res. 30:647–651. 2005.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Browne JG, Ho SL, Kane R, Oliver N, Clark
AF, O'Brien CJ and Crean JK: Connective tissue growth factor is
increased in pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci.
52:3660–3666. 2011.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Wang HJ and Kochevar IE: Involvement of
UVB-induced reactive oxygen species in TGF-beta biosynthesis and
activation in keratinocytes. Free Radical Bio Med. 38:890–897.
2005.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Galli A, Svegliati-Baroni G, Ceni E,
Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini
G, Benedetti A, et al: Oxidative stress stimulates proliferation
and invasiveness of hepatic stellate cells via a MMP2-mediated
mechanism. Hepatology. 41:1074–1084. 2005.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Schlötzer-Schrehardt U, Lommatzsch J,
Küchle M, Konstas AG and Naumann GO: Matrix metalloproteinases and
their inhibitors in aqueous humor of patients with
pseudoexfoliation syndrome/glaucoma and primary open-angle
glaucoma. Invest Ophthalmol Vis Sci. 44:1117–1125. 2003.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Majora M, Wittkampf T, Schuermann B,
Schneider M, Franke S, Grether-Beck S, Wilichowski E, Bernerd F,
Schroeder P and Krutmann J: Functional consequences of
mitochondrial DNA deletions in human skin fibroblasts: Increased
contractile strength in collagen lattices is due to oxidative
stress-induced lysyl oxidase activity. Am J Pathol. 175:1019–1029.
2009.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Voloshenyuk TG, Hart AD, Khoutorova E and
Gardner JD: TNF-α increases cardiac fibroblast lysyl oxidase
expression through TGF-β and PI3Kinase signaling pathways. Biochem
Biophys Res Commun. 413:370–375. 2011.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Tezel G, Yang X and Cai J: Proteomic
identification of oxidatively modified retinal proteins in a
chronic pressure-induced rat model of glaucoma. Invest Ophthalmol
Vis Sci. 46:3177–3187. 2005.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Chrysostomou V, Rezania F, Trounce IA and
Crowston JG: Oxidative stress and mitochondrial dysfunction in
glaucoma. Curr Opin Pharmacol. 13:12–15. 2013.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Fan Gaskin JC, Shah MH and Chan EC:
Oxidative stress and the role of NADPH oxidase in glaucoma.
Antioxidants (Basel). 10(238)2021.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Izzotti A, Bagnis A and Saccà SC: The role
of oxidative stress in glaucoma. Mutat Res. 612:105–114.
2006.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Koliakos GG, Konstas AG,
Schlötzer-Schrehardt U, Hollo G, Katsimbris IE, Georgiadis N and
Ritch R: 8-Isoprostaglandin F2a and ascorbic acid concentration in
the aqueous humour of patients with exfoliation syndrome. Br J
Ophthalmol. 87:353–356. 2003.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Botling Taube A, Konzer A, Alm A and
Bergquist J: Proteomic analysis of the aqueous humour in eyes with
pseudoexfoliation syndrome. Br J Ophthalmol. 103:1190–1194.
2019.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Dursun F, Vural Ozec A, Aydin H, Topalkara
A, Dursun A, Toker MI, Erdogan H and Arici MK: Total oxidative
stress, paraoxonase and arylesterase levels at patients with
pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Int J
Ophthalmol. 8:985–990. 2015.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Dmuchowska DA, Pietrowska K, Krasnicki P,
Kowalczyk T, Misiura M, Grochowski ET, Mariak Z, Kretowski A and
Ciborowski M: Metabolomics reveals differences in aqueous humor
composition in patients with and without pseudoexfoliation
syndrome. Front Mol Biosci. 8(682600)2021.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Koliakos GG, Konstas AG,
Schlötzer-Schrehardt U, Bufidis T, Georgiadis N and Ringvold A:
Ascorbic acid concentration is reduced in the aqueous humor of
patients with exfoliation syndrome. Am J Ophthalmol. 134:879–883.
2002.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Ferreira SM, Lerner SF, Brunzini R,
Evelson PA and Llesuy SF: Antioxidant status in the aqueous humour
of patients with glaucoma associated with exfoliation syndrome. Eye
(Lond). 23:1691–1697. 2009.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Sorkhabi R, Ghorbanihaghjo A, Ahoor M,
Nahaei M and Rashtchizadeh N: High-sensitivity C-reactive protein
and tumor necrosis factor alpha in pseudoexfoliation syndrome. Oman
Med J. 28:16–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Tetikoğlu M, Aktas S, Sağdik HM, Özcura F,
Uçar F, Koçak H, Neşelioğlu S and Erel Ö: Thiol disulfide
homeostasis in pseudoexfoliation syndrome. Curr Eye Res.
42:876–879. 2017.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Koliakos GG, Konstas AG,
Schlötzer-Schrehardt U, Hollo G, Mitova D, Kovatchev D, Maloutas S
and Georgiadis N: Endothelin-1 concentration is increased in the
aqueous humour of patients with exfoliation syndrome. Br J
Ophthalmol. 88:523–527. 2004.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Koukoula SC, Katsanos AK, Tentes IK,
Labiris G and Kozobolis VP: Retrobulbar hemodynamics and aqueous
humor levels of endothelin-1 in exfoliation syndrome and
exfoliation glaucoma. Clin Ophthalmol. 12:1199–1204.
2018.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Park DY, Kim M and Cha SC: Cytokine and
growth factor analysis in exfoliation syndrome and glaucoma. Invest
Ophthalmol Vis Sci. 62(6)2021.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Zenkel M, Lewczuk P, Jünemann A, Kruse FE,
Naumann GO and Schlötzer-Schrehardt U: Proinflammatory cytokines
are involved in the initiation of the abnormal matrix process in
pseudoexfoliation syndrome/glaucoma. Am J Pathol. 176:2868–2879.
2010.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Stafiej J, Hałas-Wiśniewska M, Izdebska M,
Gagat M, Grzanka D, Grzanka A and Malukiewicz G:
Immunohistochemical analysis of microsomal glutathione
S-transferase 1 and clusterin expression in lens epithelial cells
of patients with pseudoexfoliation syndrome. Exp Ther Med.
13:1057–1063. 2017.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Sarenac Vulovic TS, Pavlovic SM,
Jakovljevic VLj, Janicijevic KB and Zdravkovic NS: Nitric oxide and
tumour necrosis factor alpha in the process of pseudoexfoliation
glaucoma. Int J Ophthalmol. 9:1138–1142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Turan G and Turan M: The evaluation of
TUNEL, PCNA and SOX2 expressions in lens epithelial cells of
cataract patients with pseudoexfoliation syndrome. Curr Eye Res.
45:12–16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Papadopoulou G, Zisimopoulos D,
Kalaitzopoulou E, Makri OE, Tsapardoni FN, Georgakopoulos CD and
Georgiou CD: Age-related aqueous humor (AH) and lens epithelial
cell/capsule protein carbonylation and AH protein concentration in
cataract patients who have pseudoexfoliative diseases. Mol Vis.
24:890–901. 2018.PubMed/NCBI
|
|
143
|
Uçakhan OO, Karel F, Kanpolat A, Devrim E
and Durak I: Superoxide dismutase activity in the lens capsule of
patients with pseudoexfoliation syndrome and cataract. J Cataract
Refract Surg. 32:618–622. 2006.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Tetikoğlu M, Sağdik HM, Aktas S, Uçar F
and Özcura F: Serum prolidase activity and oxidative stress in
patients with pseudoexfoliation syndrome. Graefes Arch Clin Exp
Ophthalmol. 254:1339–1343. 2016.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Fountoulakis N, Labiris G, Aristeidou A,
Katsanos A, Tentes I, Kortsaris A and Kozobolis VP: Tissue
inhibitor of metalloproteinase 4 in aqueous humor of patients with
primary open angle glaucoma, pseudoexfoliation syndrome and
pseudoexfoliative glaucoma and its role in proteolysis imbalance.
BMC Ophthalmol. 13(69)2013.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Simavli H, Tosun M, Bucak YY, Erdurmus M,
Ocak Z, Onder HI and Acar M: Serum and aqueous xanthine oxidase
levels, and mRNA expression in anterior lens epithelial cells in
pseudoexfoliation. Graefes Arch Clin Exp Ophthalmol. 253:1161–1167.
2015.PubMed/NCBI View Article : Google Scholar
|