Introduction
Epithelioid sarcoma (ES), a rare soft tissue
sarcoma, was first described by Enzinger (1) in 1970. It originates from primitive
mesenchymal cells with multilineage differentiation potential;
however, its epithelial differentiation is frequent. ES is
prevalent in young males aged 20-40 years. Owing to its unique
biological behavior, ES grows slowly but is prone to multifocal
disease at presentation, local recurrence and regional lymph node
spread (2). Radical tumor
resection combined with regional lymphadenectomy is an effective
treatment for patients with localized ES (3). For patients with unresectable or
advanced ES, first-line conventional systemic therapies include
anthracycline-based or gemcitabine-based regimens. Experience from
clinical practice indicates that the effect of these regimens is
limited, with an overall response rate (ORR) of 15-27% and median
overall survival (OS) of 13-19 months (4,5). The
tyrosine kinase inhibitor Pazopanib is mainly used after the
failure of standard chemotherapy. However, its ORR varied between 0
to 11.1%, and the median OS was <20 months (4,6).
Recently, a selective EZH2 inhibitor, tazemetostat, received Food
and Drug Administration approval for the treatment of advanced ES
with loss of INI1/SMARCB1. It was reported that the ORR of
tazemetostat was 15%, with a median progression-free survival of
5.5 months and OS of 19 months (7). Consequently, the prognosis after
regional recurrence or distant metastasis is alarming due to its
poor response to systemic therapies, so new therapeutic strategies
are warranted.
ES is divided into two subtypes according to the
location, i.e., distal type (also known as the classical type,
which is the most frequent and mainly located at the end of the
limbs) and proximal type (occurring in proximal limbs, trunk or
solid organs) (8). The present
study reported a rare case of proximal adrenal ES in a Chinese male
adult. To the best of our knowledge, only 3 adult cases of this
disease have been reported (9-11)
and this is the first report of effective treatment with immune
checkpoint inhibitors (ICIs) in advanced adrenal ES.
Case report
A 28-year-old male complained of persistent
bilateral lumbar backache, cough and frailty lasting for 8 months
prior to admission to the Oncology Department of Huadong Hospital
(Shanghai, China; October, 2018). The patient had a history of
hypertension for 2 years. Ultrasonography demonstrated giant masses
(>100 mm) in the bilateral adrenal regions, suggesting malignant
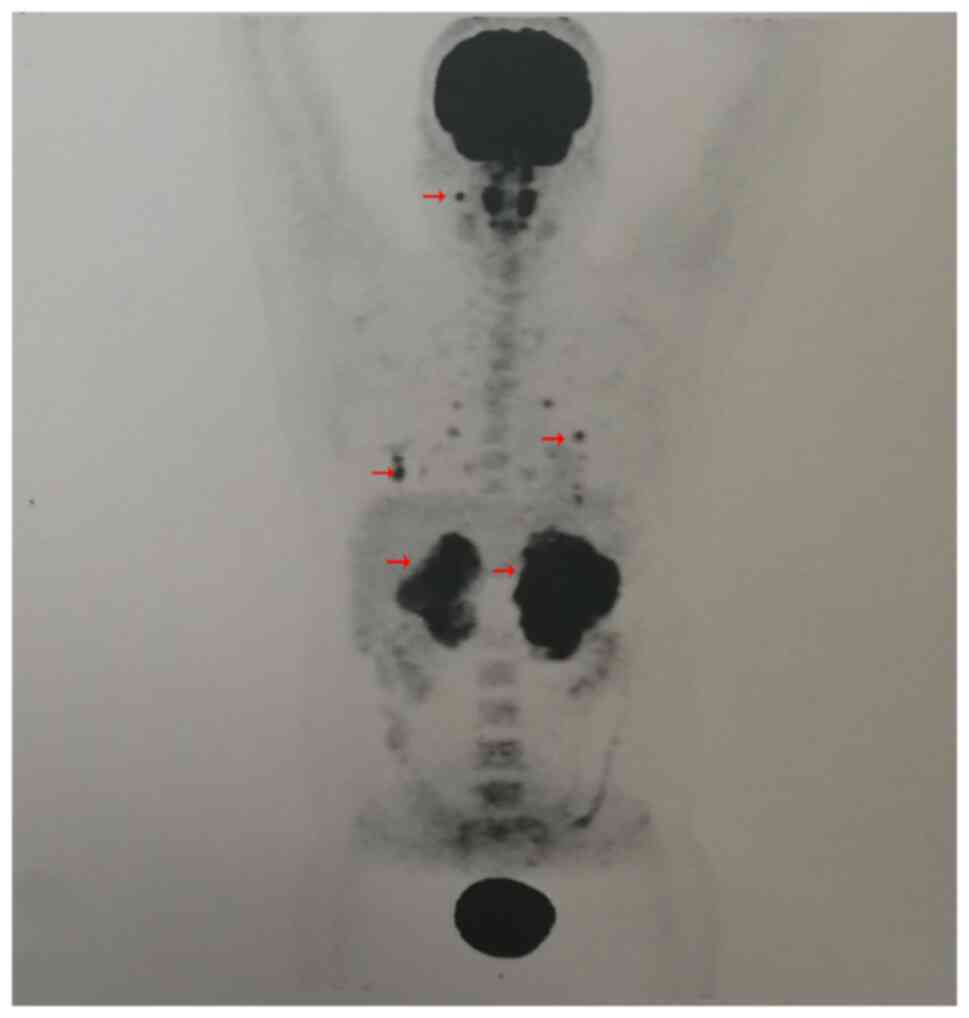
disease. Positron emission tomography-CT revealed that in addition
to the masses in the adrenal glands, multiple bilateral pulmonary
lesions were present, and an enlarged lymph node was detected in
the right neck with increased
2-deoxy-2-18F-fluoro-D-glucose metabolism (Fig. 1). A comprehensive metabolic workup
was performed, including plasma concentrations of angiotensin,
aldosterone, cortisol, adrenocorticotropic hormones and plasma
renin activity. All these adrenal hormone levels were in the normal
ranges. Therefore, the laboratory test results were indicative of a
non-functional adrenal tumor. The patient was referred for a
nasopharyngeal biopsy in November 2018. Biopsy pathology revealed
chronic nasopharyngeal inflammation. Sequentially, the patient
underwent an adrenal biopsy and wedge resection of the lung.
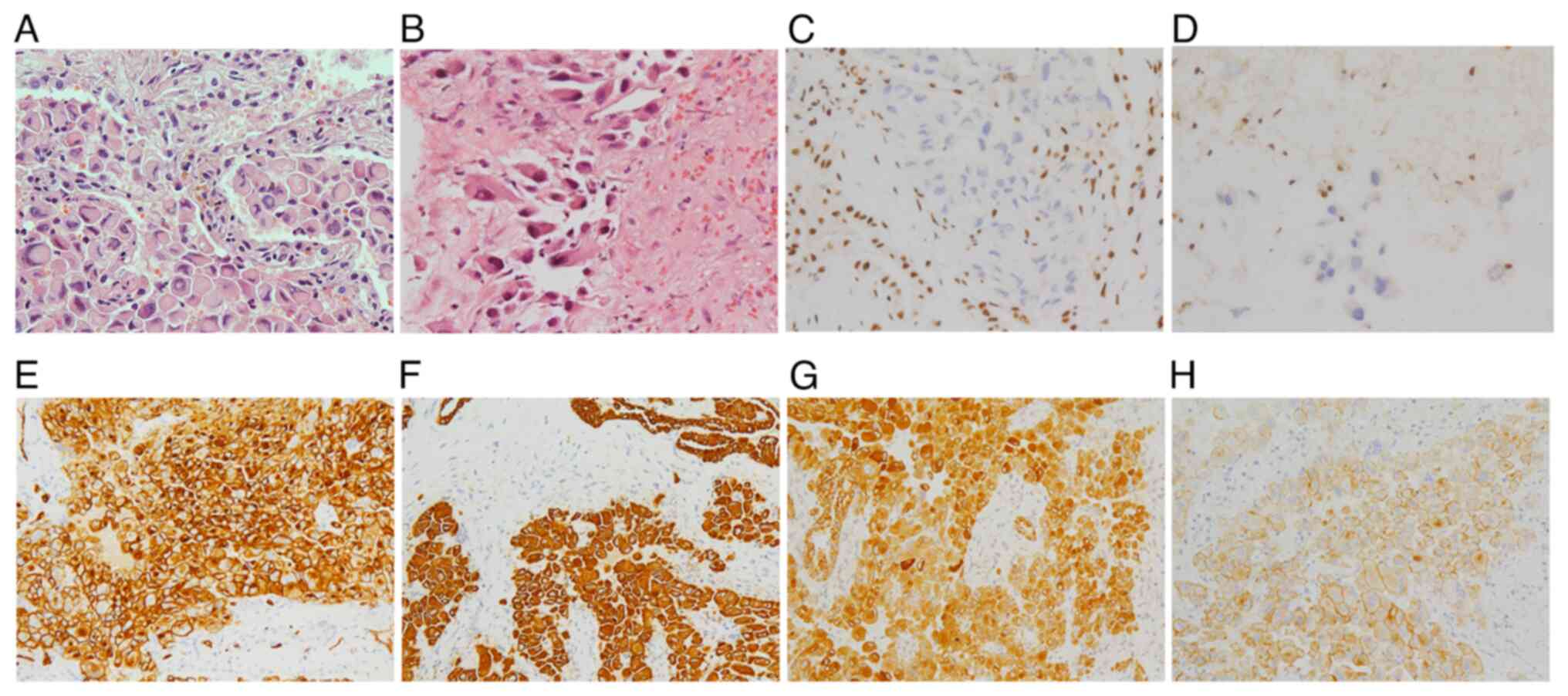
Microscopically, epithelioid cells with abundant eosinophilic
cytoplasm were detected in both tissue samples. Typically, these
cells had heteromorphic vesicular nuclei and prominent nucleoli.
Immunohistochemistry (IHC) was performed using antibodies listed in
Table I and indicated that the
tumor cells were strongly positive for CD34, cytokeratin (CK),
epithelial membrane antigen (EMA), (Fig. 2) and CK8 (data not shown). The
cells exhibited negative staining for integrase interactor 1
(INI-1; Fig. 2), cytokeratin
(CK)7, myoblast determination protein 1 (MYOD1), myogenin, desmin,
anaplastic lymphoma kinase (ALK), cluster of differentiation
(CD)31, Avian v-ets erythroblastosis virus E26 oncogene homolog
(ERG), thyroid transcription factor 1(TTF-1), synaptophysin (SYN),
chromogranin (CHG), protein 63 (P63), Melan-A (A103) and inhibin
(data not shown). Therefore, the patient was diagnosed with
proximal adrenal ES of stage IV.
 | Table ITest kits and instruments used for
immunohistochemistry and next-generation sequencing. |
Table I
Test kits and instruments used for
immunohistochemistry and next-generation sequencing.
| A, Kits |
|---|
| Procedure/step/test
kit | Catalogue number | Supplier |
|---|
|
Immunohistochemistry | | |
|
Hematoxylin | 118604 | Dako; Agilent
Technologies, Inc. |
|
INI-1 | MAB-0696 | MXB
Biotechnologies |
|
CD 34 | kit-0004 | MXB
Biotechnologies |
|
Cytokeratin | kit-0009 | MXB
Biotechnologies |
|
EMA | kit-0011 | MXB
Biotechnologies |
|
PD-L1 | RMA-0732 | MXB
Biotechnologies |
| Preparation of
DNA/RNA | | |
|
Construction | | |
|
NEBNext
Ultra II DNA Library Prep Kit for Illumina | E7645L | New England
Biolabs |
|
KAPA
HiFi HotStart Ready Mix | 07958935001 | Roche
Diagnostics |
| Capture | | |
|
SeqCap EZ
Capture Beads | 06977952001 | Roche
Diagnostics |
|
SeqCap EZ
Accessory Kit v2 | 07145594001 | Roche
Diagnostics |
|
KAPA HiFi
HotStart PCR Kit | KK2502 | Roche
Diagnostics |
|
SeqCap
Hybridization and Wash Kit | 05634253001 | Roche
Diagnostics |
| Sequencing | | |
|
NovaSeq 6000
S1 Reagent Kit (300 cycles) | 20025960 | Illumina, Inc. |
|
Paired-end
sequencing (151+8+8+151) | | |
| B, Instruments for
quantification of samples |
| Procedure/step/test
kit | Catalogue number | Supplier |
| DNA | | |
|
Nanodrop | SMA4000 | Amoydox |
|
Quantus | E2670 | Promega
Corporation |
| Library | | |
|
Agilent 2100
Electrophoresis Bioanalyzer Instrument; Loading concentration:
271.7 nM; Concentration measured: 59 (ng/µl, fluorescence
concentration) x1,000,000/329 (capture library fragment)/660 | | |
After being diagnosed, the patient underwent two
cycles of the VIDE regimen (vindesine 2 mg/m2 d1,
ifosfamide 1.5 g/m2 d1-4, doxorubicin 25
mg/m2 d1-2 and etoposide 80 mg/m2 d1-4, Q3W)
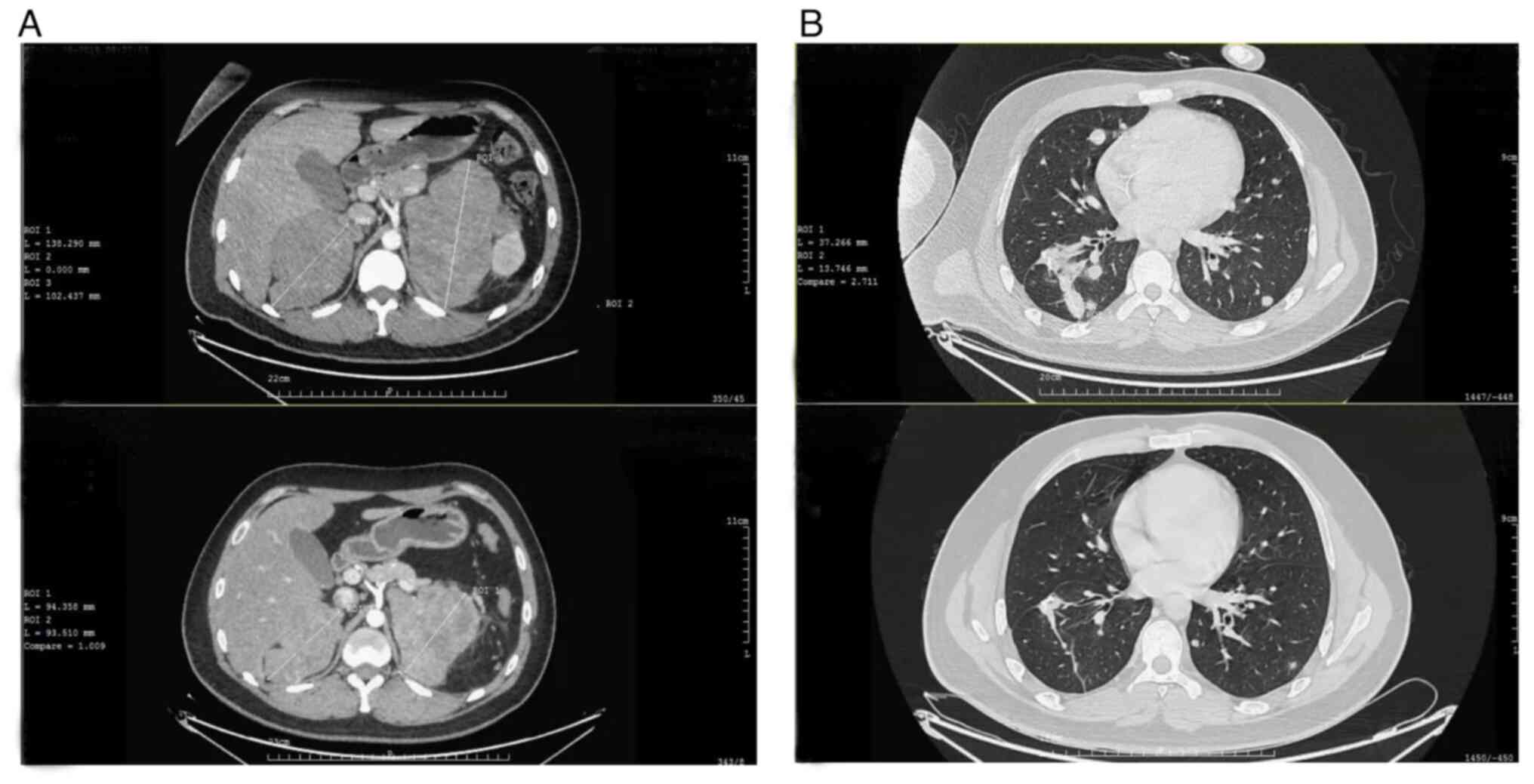
in January 2019. Subsequently, a CT scan revealed new lesions
erupting in the bilateral lung while the adrenal lesions remained
unchanged (longest diameter: Left, 137 mm; right, 102 mm).
Next-generation sequencing technology (tested by AmoyDx Medical
Institute; Table I) was performed
with peripheral blood, revealing a low tumor mutation burden (TMB)
of 4.11 Muts/Mb and somatic mutations in the SMARCB1
(10.31%), SDHC (0.85%), XPO1 (0.6%), RANBP2
(0.98%), FANCD2 (0.66%) and SCN8A (0.86%) genes. IHC
of tumor samples suggested that the tumor proportion score of
programmed cell death protein 1 ligand (PD-L1) was >50%. In
February 2019, anti-programmed death receptor 1 (anti-PD-1) therapy
was initiated with pembrolizumab (200 mg, Q3W) for seven cycles.
The patient was closely followed up by CT at 2-3-month intervals.
Follow-up CT scans indicated that masses began to reduce after two
cycles of pembrolizumab and immune partial response [assessed by
iRECIST (12)] was achieved at the
6th cycle (Fig. 3). Furthermore,
the patient's blood pressure returned to normal; however, a decline
in heart function was observed since the 5th cycle of
pembrolizumab. The left ventricular ejective fraction (LVEF) was
66% at baseline but declined to 52% after anti-PD-1 therapy. The
dynamic monitoring results of myocardium enzyme, brain natriuretic
peptide and electrocardiogram were normal. Methylprednisolone did
not improve cardiac function. Therefore, the treatment was
terminated in November 2019. From December 2019 to March 2020, the
patient was treated with anlotinib (12 mg po d1-14, Q3W). The
follow-up CT scan revealed an increased left adrenal mass (longest
diameter, 127 mm) and multiple bone metastases emerged. Palliative
radiotherapy was given for bone metastases and adrenal lesions, but
it failed to control the disease. When LVEF returned to the normal
range, the patient was given anti-PD-L1 therapy (atezolizumab 1,200
mg Q3W) in May 2020 to avoid a cardiac adverse reaction. After
three cycles, immune stable disease was observed during subsequent
imaging that was performed in July. In October 2020, the patient
developed cachexia and succumbed to community acquired respiratory
infection.
Discussion
Currently, most of the available studies on ES are
case reports. Reports on the involvement of the adrenal gland in ES
are rare. In 2017, Alikhan et al (9) first described a case of primary
adrenal ES in an elderly patient. The disease relapsed 24 months
after the patient underwent laparoscopic adrenalectomy, but a
second open resection prolonged survival (9). The second adrenal ES case was
reported by Huang et al (10) in 2019. A 31-year-old female was
diagnosed with stage III by CT-guided core needle biopsy, after
which the patient was given three cycles of neoadjuvant
chemotherapy with ifosfamide and anthracycline. During cycle 3, the
disease progressed, and in spite of the treatment with
tazemetostat, an activating enhancer of zeste homolog 2 (EZH2)
inhibitor for ES, the patient died 2 months later after the
therapy. Recently, Martinez et al (11) reported the third adrenal ES case in
an 82-year-old female. The patient underwent extensive surgical
excision. However, the case report does not provide any information
regarding prognosis due to the short follow-up period. In the
current case, sufficient tissue was successfully obtained from both
adrenal gland masses and lung lesions. A summary of the clinical
characteristics and treatment outcomes of these four cases is
provided in Table II. PubMed
databases were searched from 1970 to 2022, combining terms
describing epithelioid sarcoma and adrenal gland. The inclusion
criterion was adult primary epithelioid sarcoma of adrenal gland
confirmed by pathology. IHC staining suggested that the patient had
an IHC pattern similar to those in the previous reports (9-11,13).
Tumor cells were positive for epithelial markers (CK8, CK and EMA)
and vascular marker (CD34) and negative for nuclear INI1
expression. Characteristically, the somatic mutation of
SMARCB1 leads to INI1 expression loss (13). Therefore, the pathological
diagnosis of ES was established, indicating huge masses in the
bilateral adrenal glands as primary sites.
 | Table IIClinical characteristics and treatment
outcomes of adrenal epithelioid sarcoma cases. |
Table II
Clinical characteristics and treatment
outcomes of adrenal epithelioid sarcoma cases.
| Author (year) | Age, years | Sex | Symptoms | Relapse/metastatic
sites | Treatment | Outcome | Overall survival | (Refs.) |
|---|
| Alikhan (2017) | 72 | Male | Abdominal pain,
nausea | Local relapse | Adrenalectomy and
radiotherapy | No evidence of
disease after re-excision | >2.5 years | (9) |
| Huang (2019) | 31 | Female | Nausea, rectal
bleeding | Retroperitoneal lymph
node | Neoadjuvant
chemotherapy, tazemetostat as first-line therapy after PD | PD after 3 cycles of
neoadjuvant chemotherapy and 4 cycles of tazemetostat therapy | N/A | (10) |
| Martinez (2020) | 82 | Female | Flank pain | No | Nephrectomy,
adrenalectomy and mass excision | No evidence of
disease | N/A | (11) |
| Present case | 28 | Male | Lumbar backache,
cough, frailty | Lung, bone | Palliative
chemotherapy and radiotherapy, ICIs, anlotinib | PD occurred after
chemotherapy, radiotherapy and anlotinib; the disease was
controlled during ICI treatment | 25 months | / |
Due to the absence of large-scale prospective
clinical studies, there is no standard systemic therapy regimen for
advanced ES. Anthracycline-based therapy is one of the regimens
with a wide application. In retrospective studies, palliative
chemotherapy with anthracyclines was combined with ifosfamide,
where median overall survival (OS) ranged from 9.8 to 16.8 months
(6,14,15).
Considering the performance status of the patient, the VIDE regimen
was selected as the first-line chemotherapy. However, severe
myelosuppression arose from the combined regimen without any
improvement of prognosis. The effectiveness of tazemetostat
monotherapy in patients with advanced ES characterized by loss of
INI1/SMARCB1 has been confirmed in a basket study, reporting an ORR
of 15% and median OS of 19.0 months. The safety and tolerability
were excellent, as most toxicities were grade 1-2(7). With the expectation of providing
better survival data, a clinical trial of tazemetostat combined
with doxorubicin is ongoing (NCT04204941). Unfortunately,
tazemetostat is still unavailable in China.
Various studies reported that ICIs of PD-1 and its
ligand PD-L1 have promising activity in multiple tumor types.
However, PD1 or PD-L1 antagonist monotherapy has limited efficacy
in unselected soft tissue sarcomas, with an ORR ranging from 5 to
18% (16-18).
Most subtypes of soft tissue sarcoma (STS) are resistant to ICIs
due to the insufficiency of CD8-positive T cells in the tumor
microenvironment (19). The TMB is
a controversial predictor of the treatment efficacy of ICIs.
According to data from The Cancer Genome Atlas research network,
STS is a category of diseases with a low somatic TMB (average, 1.06
Muts/Mb) (20). In addition, in
certain STS, no correlation was established between TMB, the
expression of PD-L1 by tumor cells and CD8+ tumor-infiltrating
lymphocytes (21). Thus, TMB alone
may not predict the likelihood of a response to ICIs. In the
current case, the patient who had a low TMB but a high level of
PD-L1 expression exhibited tumor shrinkage. Therefore, it may be
presumed that PD-L1 expression is a stronger predictor of ICI
treatment efficacy than TMB. Although patients with PD-L1-positive
tumors gained survival benefits from ICI treatment, responses were
also recorded in PD-L1-negative cases (16). The role of PD-L1 expression as a
predictive biomarker remains unclear.
Due to the limited number of reports, it remains
elusive whether ES is sensitive to ICI treatment. In a
retrospective study of patients with relapsed
metastatic/unresectable sarcomas, 3 patients with ES were enrolled.
Among them, 1 patient had partial remission after four cycles of
nivolumab combined with pazopanib, but progressive disease after
four additional cycles. The other 2 patients suffered from
progressive disease (22). In the
case report by Pecora et al (23), the combination between anti-CTLA-4
and anti-PD-1 checkpoint inhibition therapy led to a durable
complete response in a 19-year-old male with stage IV ES. The
patient of the present study experienced partial remission after
original anti-PD-1 therapy and stable disease (no change in volume)
after sequential anti-PD-L1 therapy. The patient's OS reached 25
months. The main dose-limiting toxicity of pembrolizumab induced
cardiotoxicity characterized by asymptomatic heart failure but was
not observed in atezolizumab, which may be attributed to the
differences in antibody-binding sites. ICI-associated
cardiotoxicity is a rare occurrence that may be fatal. Although
data from a large population-based epidemiology study indicated no
difference between PD-1 and PD-L1 inhibitors in terms of risk of
cardiotoxicity, patients initiated on pembrolizumab were vulnerable
to developing cardiotoxicity (24).
In conclusion, the long survival of the present case
demonstrated the effectiveness of ICI treatment in patients with
ES. The IHC-based PD-L1 expression predicted the response to ICIs.
Therefore, irrespective of the degree of TMB, ICI monotherapy may
be a feasible treatment for patients with ES with a strong
expression of PD-L1.
Acknowledgements
The authors thank Dr Li Xiao (Department of
Pathology, Huadong Hospital affiliated to Fudan University,
Shanghai, China) for her assistance with the pathological
diagnosis.
Funding
Funding: This research was supported by the Talents Training
Plan of Huadong Hospital affiliated to Fudan University (grant no.
HDGG2017021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT performed the therapeutic regimen. CL performed
pathological diagnosis by immunohistochemistry. JW acquired the
clinical data and drafted the manuscript. XT and JW confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All procedures performed in this study were in
accordance with the ethical standards of the institutional and
national research committees and with the Helsinki Declaration (as
revised in 2013). This study was approved by the Ethics Committee
at Huadong Hospital, Affiliated to Fudan University (Shanghai,
China; approval no. 2021K111).
Patient consent for publication
Written informed consent was obtained from the
patient's father for the publication of this case report including
medical data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Enzinger FM: Epitheloid sarcoma. A sarcoma
simulating a granuloma or a carcinoma. Cancer. 26:1029–1041.
1970.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Spillane AJ, Thomas JM and Fisher C:
Epithelioid sarcoma: The clinicopathological complexities of this
rare soft tissue sarcoma. Ann Surg Oncol. 7:218–225.
2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Visscher SA, van Ginkel RJ, Wobbes T,
Veth RP, Ten Heuvel SE, Suurmeijer AJ and Hoekstra HJ: Epithelioid
sarcoma: Still an only surgically curable disease. Cancer.
107:606–612. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Frezza AM, Jones RL, Lo Vullo S, Asano N,
Lucibello F, Ben-Ami E, Ratan R, Teterycz P, Boye K, Brahmi M, et
al: Anthracycline, gemcitabine, and pazopanib in epithelioid
sarcoma: A multi-institutional case series. JAMA Oncol.
4(e180219)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gounder MM, Merriam P, Ratan R, Patel SR,
Chugh R, Villalobos VM, Thornton M, Van Tine BA, Abdelhamid AH,
Whalen J, et al: Real-world outcomes of patients with locally
advanced or metastatic epithelioid sarcoma. Cancer. 127:1311–1317.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Touati N, Schöffski P, Litière S, Judson
I, Sleijfer S, van der Graaf WT, Italiano A, Isambert N, Gil T,
Blay JY, et al: European organisation for research and treatment of
cancer soft tissue and bone sarcoma group experience with
advanced/metastatic epithelioid sarcoma patients treated in
prospective trials: Clinical profile and response to systemic
therapy. Clin Oncol (R Coll Radiol). 30:448–454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gounder M, Schöffski P, Jones RL, Agulnik
M, Cote GM, Villalobos VM, Attia S, Chugh R, Chen TW, Jahan T, et
al: Tazemetostat in advanced epithelioid sarcoma with loss of
INI1/SMARCB1: An international, open-label, phase 2 basket study.
Lancet Oncol. 21:1423–1432. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guillou L, Wadden C, Coindre JM, Krausz T
and Fletcher CD: ‘Proximal-type’ epithelioid sarcoma, a distinctive
aggressive neoplasm showing rhabdoid features. Clinicopathologic,
immunohistochemical, and ultrastructural study of a series. Am J
Surg Pathol. 21:130–146. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alikhan MB, Pease G, Watkin W, Grogan R,
Krausz T and Antic T: Primary epithelioid sarcoma of the kidney and
adrenal gland: Report of 2 cases with immunohistochemical and
molecular cytogenetic studies. Hum Pathol. 61:158–163.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang X, Nayar R and Zhou H: Primary
adrenal gland epithelioid sarcoma: A case report and literature
review. Diagn Cytopathol. 47:918–921. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Martinez VP, Nicholson M and Patel T:
Abnormal Adrenal mass presents as proximal epithelioid sarcoma.
Case Rep Urol. 2020(8864218)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hornick JL, Dal Cin P and Fletcher CD:
Loss of INI1 expression is characteristic of both conventional and
proximal-type epithelioid sarcoma. Am J Surg Pathol. 33:542–550.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jones RL, Constantinidou A, Olmos D, Thway
K, Fisher C, Al-Muderis O, Scurr M and Judson IR: Role of
palliative chemotherapy in advanced epithelioid sarcoma. Am J Clin
Oncol. 35:351–357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim C, Yoo KH, Kim MH, Chon HJ, Lee SI,
Lee HJ, Koh S, Lee HY, Lee HR, Kim KS, et al: Different subtypes of
epithelioid sarcoma and their clinical implication: Long-term
multi-institutional experience with a rare sarcoma. APMIS.
125:223–229. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Italiano A, Bellera C and D'Angelo S:
PD1/PD-L1 targeting in advanced soft-tissue sarcomas: A pooled
analysis of phase II trials. J Hematol Oncol. 13(55)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
D'Angelo SP, Mahoney MR, Van Tine BA,
Atkins J, Milhem MM, Jahagirdar BN, Antonescu CR, Horvath E, Tap
WD, Schwartz GK and Streicher H: Nivolumab with or without
ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two
open-label, non-comparative, randomised, phase 2 trials. Lancet
Oncol. 19:416–426. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tawbi HA, Burgess M, Bolejack V, Van Tine
BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA,
et al: Pembrolizumab in advanced soft-tissue sarcoma and bone
sarcoma (SARC028): A multicentre, two-cohort, single-arm,
open-label, phase 2 trial. Lancet Oncol. 18:1493–1501.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Toulmonde M, Penel N, Adam J, Chevreau C,
Blay JY, Le Cesne A, Bompas E, Piperno-Neumann S, Cousin S,
Grellety T, et al: Use of PD-1, targeting macrophage infiltration,
and IDO pathway activation in sarcomas: A phase 2 clinical trial.
JAMA Oncol. 4:93–97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cancer Genome Atlas Research Network.
Electronic address: simpleelizabeth.demicco@sinaihealthsystem.ca;
Cancer Genome Atlas Research Network. Comprehensive and integrated
genomic characterization of adult soft tissue sarcomas. Cell.
171:950–965.e28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
He M, Abro B, Kaushal M, Chen L, Chen T,
Gondim M, Yan W, Neidich J, Dehner LP and Pfeifer JD: Tumor
mutation burden and checkpoint immunotherapy markers in primary and
metastatic synovial sarcoma. Hum Pathol. 100:15–23. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Paoluzzi L, Cacavio A, Ghesani M,
Karambelkar A, Rapkiewicz A, Weber J and Rosen G: Response to
anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin
Sarcoma Res. 6(24)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pecora A, Halpern S, Weber M, Paleoudis
EG, Panush D, Patterson F and Toretsky J: Rapid and Complete
response to combination anti-CTLA-4 and Anti-PD-1 checkpoint
inhibitor therapy in a patient with stage IV refractory end-stage
epithelioid sarcoma: A case report. J Immunother. 43:286–290.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li C, Bhatti SA and Ying J: Immune
checkpoint inhibitors-associated cardiotoxicity. Cancers (Basel).
14(1145)2022.PubMed/NCBI View Article : Google Scholar
|