Introduction
Castleman's disease (CD), which was first described
by Castleman and Towne (1) in
1954, is a rare lymphoproliferative disorder. The incidence rate of
all forms of CD is estimated at 21-25 per million person-years,
based on insurance registries in the USA (2). CD most commonly affects the
mediastinum (63%), followed by the abdomen (11%), retroperitoneum
(7%) and axilla (4%) (3). Based on
the anatomical distribution of the disease, CD may be classified
into two types: Unicentric and multicentric. Usually, ~25% of cases
are unicentric (4). Unicentric CD
(UCD) presents with isolated lymphadenopathy, usually detected in
the chest and neck and less commonly in abdominal nodes or as a
retroperitoneal mass (5).
Localization of UCD in the pelvic retroperitoneum is rare, which
accounts for 6.7% of UCD cases in the retroperitoneum (6). Diagnosis in this setting is difficult
due to the lack of characteristic features on radiographic images.
Most previous cases were diagnosed based on postoperative
pathological examination (7).
Given their proximity to major vascular structures of the pelvis,
surgeons were occasionally faced with a clinical situation in which
a large-volume blood transfusion was necessary (8). Thus, certain surgeons used
preoperative angiography and embolization of the feeding arteries
to the tumor in order to prevent or limit intraoperative bleeding
(9). The present study reported a
rare case of UCD located in the pelvic retroperitoneum, which was
completely resected by meticulous laparoscopic surgery with limited
bleeding. Although preoperative diagnosis was difficult to achieve,
CD should be included in the differential diagnoses when a pelvic
lesion is found.
Case report
CT scan
A 320-row spiral CT (uCT960+; Lianying) was used
with the following parameters: Tube voltage, 100 kV; automatic tube
current; thickness, 5.00 mm; pitch, 0.9937; rotation time, 0.8
sec/rot. The non-ionic contrast medium (Omnipaque 300 mg/ml;
Cytiva) at the dose of 1.0 ml/kg was injected with a power injector
at a rate of 3.0 ml/sec through the median cubital vein. This was
followed by flushing with 20 ml saline at a rate of 3.0 ml/sec. The
arterial and venous phases were obtained at 25 to 30 sec and 60 to
75 sec after the injection of the contrast medium.
Histopathological staining
Formalin-fixed paraffin-embedded (FFPE) sections
were cut with a Leica RM 2155 Rotary Microtome (Leica Microsystems)
and the paraffin sections were then dewaxed sequentially with
xylene, anhydrous ethanol, a decreasing concentration gradient of
ethanol (95, 90, 80 and 70%) and water. Slices were immersed in
Harris hematoxylin staining solution for 5 min and then
differentiated with 0.3% acid alcohol, followed by incubation with
0.6% ammonia. Subsequently, samples were incubated with eosin
staining solution for 1-3 min and then dehydrated with ethanol and
xylene, and finally, slides were mounted with neutral gum.
Immunohistochemistry (IHC)
For IHC staining, 5 µm-thick sections were cut from
the FFPE block. Sections were de-waxed with xylene and dehydrated
through a serial ethanol gradient, then maintained in a drying oven
at 50-54˚C for 12 h. Printed labels from the Ventana system were
pasted upon slides and each label contained a barcode comprising
all of the protocol information required. The staining was then
performed by the automated Ventana Benchmark Ultra autostainer
(Ventana Medical Systems). The brief work flow was as follows:
Antigen retrieval was performed in Tris-EDTA buffer (pH 7.8 at 95˚C
for 40 min), endogenous peroxides and protein were blocked with
Inhibitor CM included in the iVIEW DAB Detection Kit (serial no.
760-500; Roche Diagnostics) at 37˚C for 4 min. Primary antibody was
added and samples were incubated for 60 min at 37˚C. The slides
were processed with an iVIEW DAB Detection Kit (serial no. 760-500;
Roche Diagnostics), which comprised horseradish
peroxidase-conjugated rabbit secondary antibodies, DAB CM and
H2O2CM according to the manufacturer's
instructions. The slides were then washed and dehydrated in
successive baths of an ascending series of ethanol, increasing
concentrations of xylene and then mounted with coverslips.
Monoclonal primary antibodies to CD21, CD3, CD20 and CD34 were
purchased from Santa Cruz Biotechnology, Inc. (cat. nos. sc-13135,
sc-20047, sc-393894 and sc-74499 respectively; dilution,
1:200).
Case report
A 38-year-old male patient was referred to The First
Affiliated Hospital of Shandong First Medical University and
Shandong Provincial Qianfoshan Hospital (Jinan, China) in April
2021 due to a retroperitoneal tumor found in the left side of the
pelvis without any symptoms. The patient had no previous illnesses
or family history. No enlarged lymph nodes were palpated in the
cervical, clavicular or inguinal zones. Laboratory parameters were
all normal. Preoperative CT scans of the chest, abdomen and pelvis
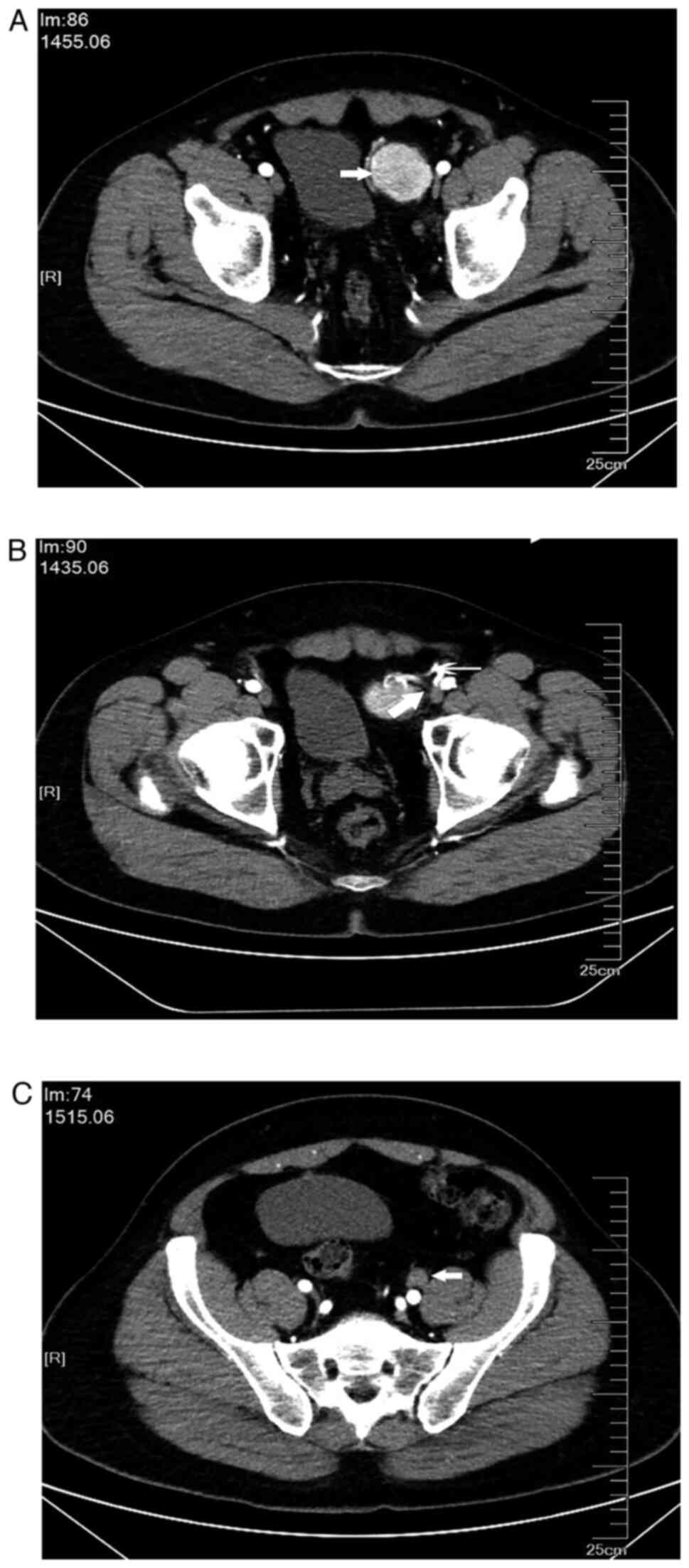
revealed a retroperitoneal tumor on the left of the bladder
measuring 42x38 mm, with a marked contrast effect (Fig. 1A). The arterial supply to the tumor
arose from branches of the left external iliac artery, with venous
drainage entering the left external iliac vein (Fig. 1B). An enlarged lymph node (21x18
mm) was also detected lateral to the left external iliac artery
with no enhancement on CT imaging (Fig. 1C).
The mass was considered a vascular-derived tumor
after consulting vascular and interventional doctors prior to
surgery. Primary venous leiomyosarcoma was suspected, although this
is rare. In the differential diagnosis of a malignant
retroperitoneal tumor, four other diseases were also considered:
Mesenchymal soft-tissue sarcomas, tumors of neurogenic origin, germ
cell tumors and lymphoproliferative disorders. Thus, the patient
was advised to undergo surgical removal for appropriate diagnosis
and treatment. As the tumor was well vascularized and adjacent to
the great vessels, laparoscopy was able to provide magnified images
with the capacity to facilitate and secure dissection. Thus,
laparoscopic surgery was performed via the transperitoneal approach
and the tumor was completely removed along with the enlarged lymph
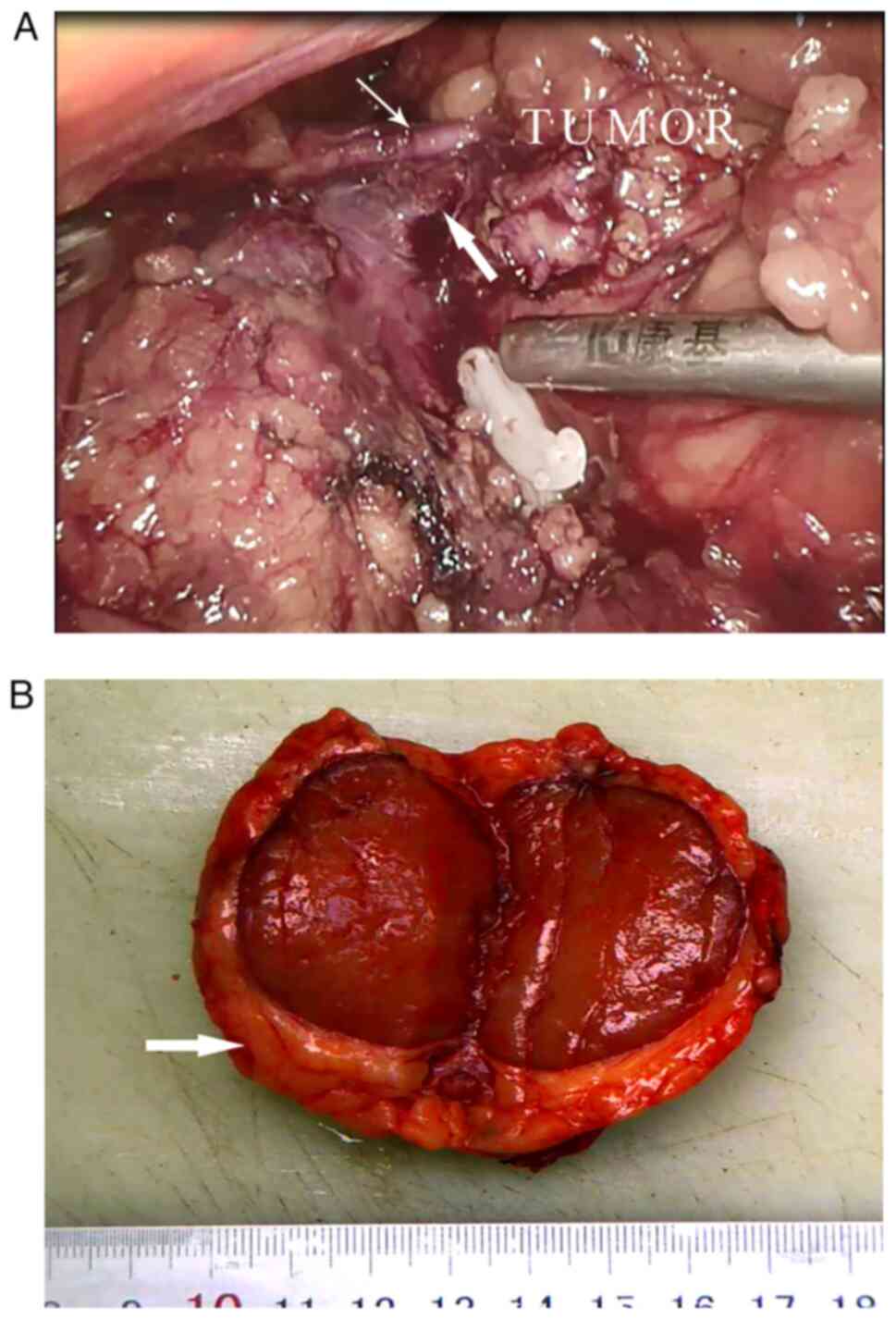
node. The feeding artery of the tumor was confirmed to be the pubic
branch of the left inferior epigastric artery, which originated
from the distal external iliac artery (Fig. 2A). Meticulous dissection was
performed and the feeding artery was carefully isolated, ligated
and divided. The vein of the tumor entered the left external iliac
vein and was secured subsequently. Care had to be taken to avoid
rupture of venous drainage of the tumor, as the vein was short and
likely to tear easily. The mass was then completely resected,
measuring 45x35x30 mm.
The resected tumor was rubbery, firm and well
circumscribed with a thick capsule (Fig. 2B). The cut surface was orange-red
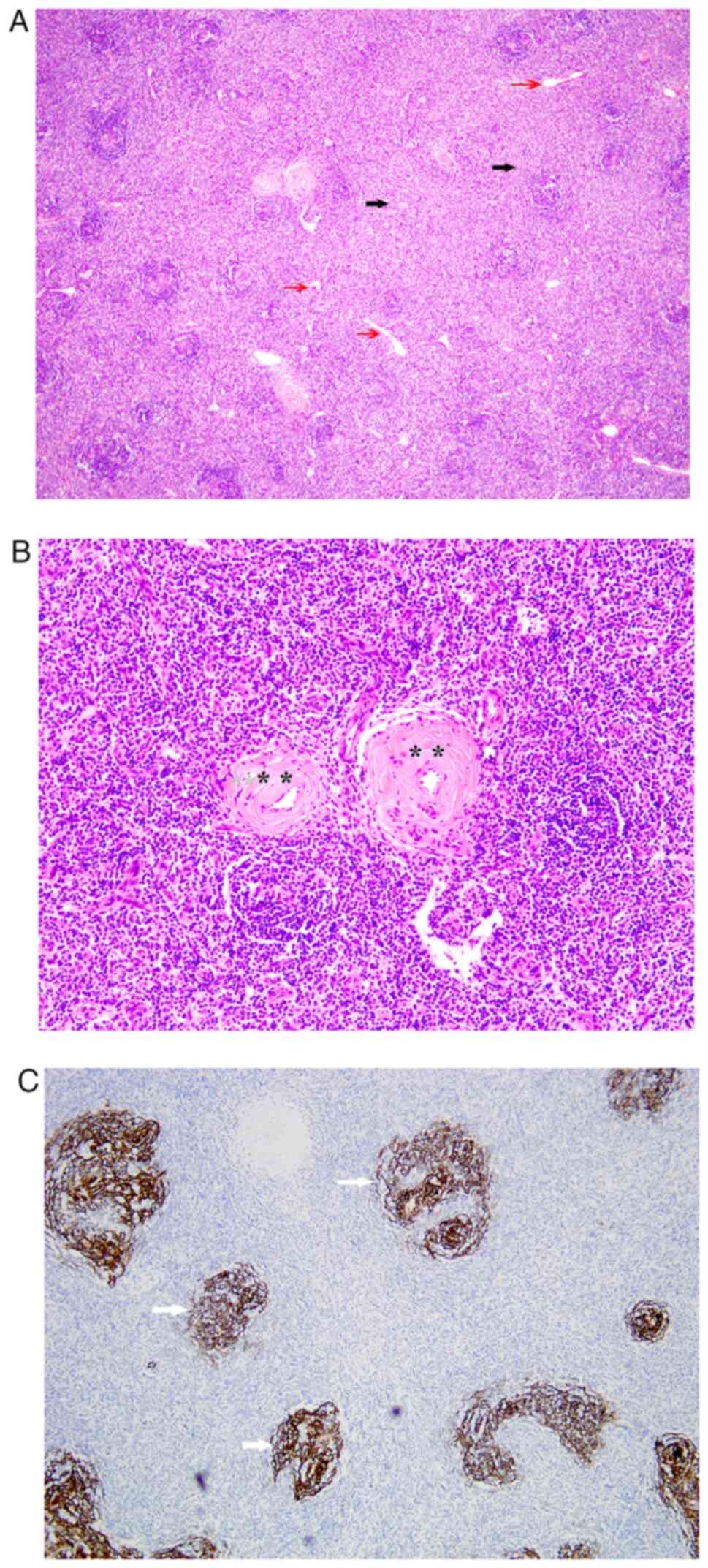
in color. On histopathology, hyperplastic lymphoid follicles around
the degenerative germinal centers were observed (Fig. 3A), with prominent vascular
proliferation and hyalinization of the vessel walls (Fig. 3B). The specimen was positive for a
T cell marker (CD3), a B cell marker (CD20) and a vascular
endothelial cell marker (CD34), and had increased meshworks of
follicular dendritic cells marked with CD21 immunostain, as
assessed by IHC (Fig. 3C). These
findings are diagnostic for UCD, hyaline vascular type. The test
for human herpesvirus 8 (HHV-8) was negative. The resected enlarged
lymph node was proved to be reactive hyperplasia on microscopy.
The patient recovered well and was discharged 8 days
after surgery, and no local recurrence was found during 1 year of
follow-up. Written informed consent was obtained from the patient
for the publication of this study.
Discussion
CD describes a rare group of lymphoproliferative
disorders with characteristic histopathologic appearances (10), the etiology of which remains to be
fully elucidated. This disease includes unicentric and multicentric
forms, which are thought to represent distinct clinical entities
with different patient characteristics, presentation, treatment
responses and long-term outcomes (4). UCD presents with isolated
lymphadenopathy, usually diagnosed in the fourth decade of life. It
is frequently found incidentally due to a lack of symptoms and
benign course (11). By contrast,
multicentric CD (MCD) presents with the enlargement of multiple
lymph nodes, usually accompanied by mild to life-threatening
symptoms such as fatigue, fever, weight loss, anemia, sweat,
splenomegaly, dyspnea and pulmonary fibrosis (12,13).
The presentation of MCD may occasionally be associated with HIV
infection, Kaposi's sarcoma and POEMS symptoms (peripheral
sensorimotor neuropathy, organomegaly, endocrinopathy, monoclonal
gammopathy and skin lesions) (14). MCD usually develops later in the
fifth and sixth decades of life, with an estimated 5-year overall
survival of ~65% (11).
Although the underlying etiology of CD remains to be
established, excessive secretion of the cytokine interleukin-6
(IL-6) is thought to contribute to numerous symptoms associated
with MCD (15). Certain cases of
MCD have been attributed to HHV-8 infection in immunosuppressed
patients. Viral IL-6, human IL-6 and several other proinflammatory
proteins are thought to be involved in the pathogenesis of
HHV-8-associated MCD (16). In
patients with HHV-8-negative idiopathic MCD, a separate malignant
disease may be found to co-exist, suggesting a common genetic
mutation may be a contributor (17).
A total of three histological types of CD have been
identified: Hyaline vascular, plasma cell and the mixed variant.
The hyaline vascular type is most commonly seen in UCD,
characterized by an increased number of lymphoid follicles with
degenerative germinal centers and broad mantle zones composed of
concentric rings of small lymphoid cells (‘onion skin pattern’).
Germinal centers are predominantly composed of follicular dendritic
cells, penetrated by sclerotic hyalinized vessels (‘lollipop
lesions’). By contrast, the plasma cell type most commonly occurs
in MCD, characterized by the presence of sheets of plasma cells in
the interfollicular zone and hyperplastic germinal centers
(18). In the present case,
prominent vascular proliferation and hyalinization of the vessel
walls were obvious with a lack of an ‘onion skin pattern’
appearance. Germinal centers were degenerative, consisting of
follicular dendritic cells marked with CD21 immunostain.
UCD in the pelvic retroperitoneum is rare and the
preoperative diagnosis is difficult due to its low frequency and
lack of characteristic features on radiographic images (7). Similar to lymphoma, CT scans display
homogenous enhancement of the lesions if they are smaller than 5
cm. Calcification may also be seen in up to 31% of cases (19). In the present case, the tumor was
homogenously enhanced without calcification. However, it is
important to consider the possibility of pelvic CD in the
differential diagnosis of a calcified pelvic tumor. Positron
emission tomography (PET)/CT is thought to be helpful for
distinguishing CD from lymphoma. When difficulties are encountered,
biopsy of the site with the highest standardized uptake value (SUV)
is recommended (5).
The differential diagnosis of UCD includes lymphoma,
sarcoma, lymph node metastasis, gastrointestinal stromal tumor,
lipomas, leiomyomas, neurofibromas, paraganglioma and infectious
diseases (20). It is challenging
to differentiate UCD from lymphoma preoperatively. The
lymphadenopathy of UCD is unifocal and the swollen node is commonly
larger than the size of lymphoma lymph nodes (4). Furthermore, when PET/CT is performed,
the median maximum SUV is typically ~3-8, whereas higher values
would suggest lymphoma (5). As
mentioned above, core biopsy of the tumor may be helpful to
distinguish the two entities.
In the present case, given the highly vascular
nature of the tumor, biopsy was not performed, as the mass was
thought to be derived from great vessels, which may have possibly
led to bleeding and increased the difficulty of subsequent surgical
procedures. Furthermore, the diagnosis of CD requires a
pathological review of the affected lymph node, which should
ideally be performed from an excisional biopsy, as the definitive
diagnosis is mainly based on cell architecture (21).
MCD requires systemic treatment and individualized
strategies on the basis of different subtypes (14). Surgery is reserved to obtain tissue
for a full histopathologic diagnosis (4). By contrast, complete surgical
resection has been considered the gold standard treatment in
patients with UCD (4). Challenges
may be encountered during resection of UCD in the pelvis due to its
proximity to major vessels and adhesions. Hypervascularity is
frequently associated with UCD at excision, increasing the risk of
vascular injury and blood transfusion (8). To avoid massive hemorrhage, as
observed in the present case, meticulous dissection around the
tumor and slight traction were required to expose the feeding
vessels. A detailed review of CT scans served a paramount role in
the scheduled step-by-step procedures during laparoscopic surgery.
Although no evidence of local recurrence or systemic disease was
detected during 1 year of follow-up, long-term follow-up is
required, as a rare recurrence has been reported in the literature
(22).
In summary, the present study reported a rare case
of UCD in the pelvic retroperitoneum in a male patient, which was
treated successfully by complete resection of the tumor. A
definitive diagnosis was not accomplished until histological
analysis was performed. Meticulous dissection should be warranted
to minimize hemorrhage during laparoscopic surgery. Surgical
resection is the gold standard treatment strategy for this neoplasm
and long-term follow-up is needed to detect any recurrence.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Shandong Medical and
Health Science and Technology Development Plan Project (grant no.
2019WS510).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SH was the principal responsible person for the
study and contributed to the conception and the design of the
study. QZ and XZ obtained and analyzed the patient's information
and contributed to manuscript drafting and critical revisions of
the intellectual content. XY performed the histological examination
of the tumor and lymph node. QZ, XZ, XY and SH confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the ethics committee
of the First Affiliated Hospital of Shandong First Medical
University (Jinan, China; approval no. 2022S478).
Patient consent for publication
The publication of the article was with the written
informed consent of the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Castleman B and Towne VW: Case records of
the massachusetts general hospital; weekly clinicopathological
exercises; founded by Richard C. Cabot. N Engl J Med. 251:396–400.
1954.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Munshi N, Mehra M, van de Velde H, Desai
A, Potluri R and Vermeulen J: Use of a claims database to
characterize and estimate the incidence rate for Castleman disease.
Leuk Lymphoma. 56:1252–1260. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bucher P, Chassot G, Zufferey G, Ris F,
Huber O and Morel P: Surgical management of abdominal and
retroperitoneal Castleman's disease. World J Surg Oncol.
3(33)2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Talat N, Belgaumkar AP and Schulte KM:
Surgery in castleman's disease a systematic review of 404 published
cases. Ann Surg. 255:677–684. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oksenhendler E, Boutboul D, Fajgenbaum D,
Mirouse A, Fieschi C, Malphettes M, Vercellino L, Meignin V, Gérard
L and Galicier L: The full spectrum of castleman disease: 273
patients studied over 20 years. Br J Haematol. 180:206–216.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gopi P, Potty VS, Kaurav RS and Govindan
K: Unicentric Castleman's disease as a localized retroperitoneal
mass: A case report and review of literature. Int J Appl Basic Med
Res. 8:259–262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nakata K, Iwahashi N, Matsukawa H, Noguchi
T, Yahata T, Ota N, Mabuchi Y and Ino K: Laparoscopically resected
castleman's disease in the pelvic retroperitoneum: A case report.
Mol Clin Oncol. 12:169–173. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nepal SP, Shichijo T, Ogawa Y, Naoe M,
Oshinomi K and Morita J: Surgical challenges of Castleman's disease
of the pelvis. Urol Case Rep. 34(101518)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kitakaze M, Miyoshi N, Fujino S, Ogino T,
Takahashi H, Uemura M, Mizushima T, Doki Y and Eguchi H: Surgical
resection for pelvic retroperitoneal Castleman's disease: A case
report and review literature. Biomed Rep. 14(29)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Szalat R and Munshi NC: Diagnosis of
castleman disease. Hematol Oncol Clin North Am. 32:53–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dispenzieri A, Armitage JO, Loe MJ, Geyer
SM, Allred J, Camoriano JK, Menke DM, Weisenburger DD, Ristow K,
Dogan A and Habermann TM: The clinical spectrum of Castleman's
disease. Am J Hematol. 87:997–1002. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu LI, Tu M, Cortes J, Xu-Monette ZY,
Miranda RN, Zhang J, Orlowski RZ, Neelapu S, Boddu PC, Akosile MA,
et al: Clinical and pathological characteristics of HIV- and
HHV-8-negative Castleman disease. Blood. 129:1658–1668.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Murakami M, Johkoh T, Hayashi S, Ohshima
S, Mizuki M, Nakatsuka SI, Tomobe M, Kuroyanagi K, Nakasone A and
Nishimoto N: Clinicopathologic characteristics of 342 patients with
multicentric Castleman disease in Japan. Mod Rheumatol. 30:843–851.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lomas OC, Streetly M, Pratt G, Cavet J,
Royston D, Schey S and Ramasamy K: British Society for Haematology
(BSH) Committee. The management of Castleman disease. Br J
Haematol. 195:328–337. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yoshizaki K, Matsuda T, Nishimoto N,
Kuritani T, Taeho L, Aozasa K, Nakahata T, Kawai H, Tagoh H, Komori
T, et al: Pathogenic signifificance of interleukin-6 (IL-6/BSF-2)
in Castleman's disease. Blood. 74:1360–1367. 1989.PubMed/NCBI
|
|
16
|
Suthaus J, Stuhlmann-Laeisz C, Tompkins
VS, Rosean TR, Klapper W, Tosato G, Janz S, Scheller J and
Rose-John S: HHV-8-encoded viral IL-6 collaborates with mouse IL-6
in the development of multicentric Castleman disease in mice.
Blood. 119:5173–5181. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu AY, Nabel CS, Finkelman BS, Ruth JR,
Kurzrock R, van Rhee F, Krymskaya VP, Kelleher D, Rubenstein AH and
Fajgenbaum DC: Idiopathic multicentric Castleman's disease: A
systematic literature review. Lancet Haematol. 3:e163–e175.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dispenzieri A and Fajgenbaum DC: Overview
of Castleman disease. Blood. 135:1353–1364. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Meador TL and McLarney JK: CT features of
Castleman's disease of the abdomen and pelvis. AJR Am J Roentgenol.
175:115–118. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jiang Y, Hou G, Zhu Z, Huo L, Li F and
Cheng W: The value of multiparameter 18F-FDG PET/CT
imaging in differentiating retroperitoneal paragangliomas from
unicentric Castleman disease. Sci Rep. 10(12887)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Carbone A, Borok M, Damania B, Gloghini A,
Polizzotto MN, Jayanthan RK, Fajgenbaum DC and Bower M: Castleman
disease. Nat Rev Dis Primers. 7(84)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ren N, Ding L, Jia E and Xue J: Recurrence
in unicentric Castleman's disease postoperatively: A case report
and literature review. BMC Surg. 18(1)2018.PubMed/NCBI View Article : Google Scholar
|