Introduction
The coronavirus disease 2019 (COVID-19) was first
discovered in Wuhan, China, and rapidly spread worldwide, posing a
severe threat to public health. Almost half of the patients with
COVID-19 suffer from dyspnea, with concomitant hypoxia at 1 week
following disease onset, which is primarily characterized as fever,
cough and exhaustion (1). The
functions of other organs can also be affected, in addition to
respiratory function (2-4).
In critically ill patients, complications, such as cardiac injury,
acute renal injury, acute gastrointestinal injury, coagulopathy and
liver dysfunction are highly common, and have been associated with
poor outcomes in patients with COVID-19 (5,6).
Several strains of severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) have been circulating worldwide since it
first arose. Variants of concern are those that have the potential
to evade natural or vaccine-mediated immunity. The alpha variant
(B.1.1.7), which is considered to be 40-80% more transmissible than
wild-type SARS-CoV-2, was initially discovered in November, 2020 in
a sample obtained in September in the United Kingdom, and by
mid-December, 2020, it had spread rapidly, coinciding with an
increase in the number of infections (7). The delta variant (B.1.617.2), which
was initially discovered in India, and a more virulent mutant, also
known as omicron, arose in South Africa in November, 2021
(B.1.1.529). As these variants were more transmissible than the
progenitor variants and rapidly propagated, they attracted
worldwide attention (8).
The cytokine release syndrome, which plays a key
role in the course of COVID-19 infection, is considered to be the
cause of organ damage. The stimulation of the innate and adaptive
immune systems produced by SARS-CoV-2 is responsible for the
release of excessive cytokines in patients with COVID-19. In
COVID-19, the imbalance of the immune response and excessive
inflammation is a major component of its pathogenesis (9). Several immune and inflammatory
indicators, including the neutrophil-to-lymphocyte ratio,
platelet-to-lymphocyte ratio, eosinophil-to-lymphocyte ratio,
immature granulocytes, ferritin, fibrinogen, C-reactive protein,
interleukin (IL)-6 and lactate dehydrogenase, have been shown to be
associated with disease severity and mortality in patients with
COVID-19 (10-14).
An additional index, Onodera's prognostic
nutritional index (OPNI), has been reported as a severity and
outcome indicator in patients with COVID-19(15). The OPNI is made up of serum albumin
and total lymphocyte count. The validity of OPNI to predict the
prognosis of patients undergoing gastrointestinal surgery was
initially reported by Onodera et al (16). Since then, OPNI validation has been
performed in patients with end-stage liver illness, active
tuberculosis, atypical mycobacterial infection and gastrointestinal
malignancies (17,18). The aim of the present study was to
assess and compare the clinical utility of OPNI as a prognostic
indicator in patients with COVID-19 during the periods of alpha,
delta and omicron variant predominance.
Patients and methods
Study design
The design of the present study was retrospective.
Data collection was performed at the Laiko General Hospital
(Athens, Greece) between September 20, 2020 and March 31, 2022. The
study was approved by the Institutional Board of Laiko General
Hospital and was in line with the declaration of Helsinki in 1995
(as revised in Edinburgh 2000).
Participants and data collection
In the present study, adult patients who visited or
were hospitalized in the COVID-19 Unit of Laiko General Hospital
due to SARS-CoV-2 infection were included, covering the second,
third (alpha variant), fourth (delta variant) and fifth (omicron
variant) pandemic waves. The patients were divided into three
cohorts. The first (cohort A) was comprised of unvaccinated
consecutive patients predominantly infected with the alpha
SARS-CoV-2 variant who were admitted to Laiko University Hospital
between September 20, 2020 and June 30, 2021 for COVID-19 (second
and third pandemic waves). The second cohort (cohort B) included
consecutive patients irrespective of vaccination status, who were
admitted between July 1, 2021 and December 25, 2021 (fourth
pandemic wave, predominance of the delta SARS-CoV-2 variant). The
third cohort (cohort C) was comprised of consecutive patients
irrespective of vaccination status, who were admitted between
December 26, 2021 and March 31, 2022 (fifth pandemic wave,
predominance of the omicron SARS-CoV-2 variant).
All patients were uniformly treated according to the
National Institutes of Health (NIH) protocols (19). SARS-CoV-2 infection was confirmed
by the positive detection of SARS-CoV-2 nucleic acid in examined
nasopharyngeal samples with the use of reverse
transcription-polymerase chain reaction (RT-PCR). Demographic and
clinical data were extracted retrospectively from electronic
medical records. Data on age, sex, comorbidities (cardiovascular
disease, arterial hypertension, diabetes mellitus, neurological and
hematological disorders, other malignancies, immunosuppression),
vaccination status, disease severity and outcomes (recovery,
intubation and mortality) were extracted. The patients were
classified into the following severity of illness categories:
Mild/moderate, severe and critical based on the clinical spectrum
of SARS-CoV-2 infection (19).
Laboratory investigations (complete blood count and albumin levels)
were recorded from the first electronic medical record following
hospital admission. The OPNI was calculated according to the
following formula (16): OPNI=10 x
serum albumin (g/dl) + 0.005 x peripheral lymphocyte count
(/mm3). The OPNI was associated with disease severity
and outcomes in the three studied cohorts.
Statistical analysis
Statistical analysis was performed using IBM
SPSS-Statistics version 26.0 (IBM Corp.). Categorical variables are
presented as the number and percentage, and continuous variables as
the mean ± standard deviation (SD). The normal distribution of
variables was assessed using the Kolmogorov-Smirnov test. Normally
distributed variables were compared using an independent samples
Student's t-test on factors with two groups and one-way analysis of
variance (ANOVA) with Bonferroni post hoc pairwise comparisons on
factors with three groups. Categorical variables were examined
using the Fischer's exact test or the Chi-squared test and are
shown as absolute numbers (frequency percent). Multivariate
logistic regression analysis was conducted to identify independent
variables. Associations are presented as odds ratios (OR) with
their corresponding 95% confidence intervals (95% CI). The
discriminative ability of variables was evaluated by using the area
under the receiver operating characteristic curve (ROC). Values of
P<0.05 were considered to indicate statistically significant
differences.
Results
Cohort A
Cohort A comprised of 588 patients (352 males),
predominantly infected with the alpha SARS-CoV-2 variant, with a
mean age of 64.09±16.29 years. The demographic characteristics of
cohort A are presented in Table I.
In total, 48 patients (8.2%) had mild/moderate disease, 365
patients (62.1%) had severe disease and 175 patients (29.8%) had
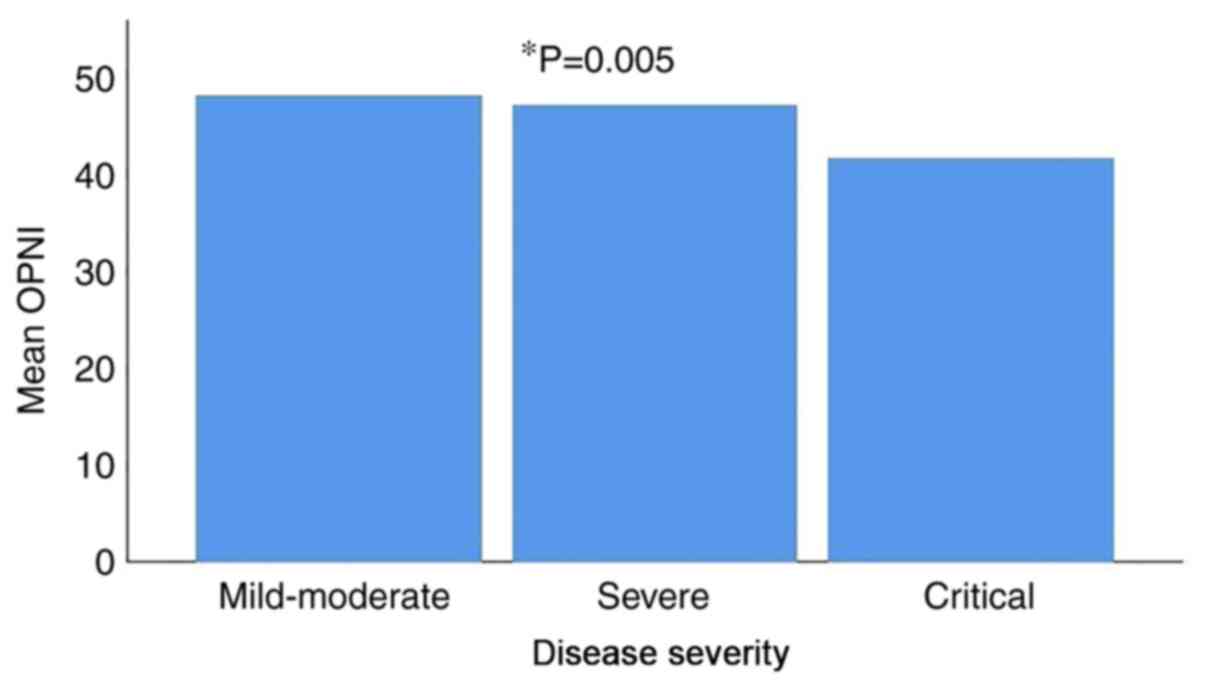
critical disease. The mean OPNI value was 47.80±6.35 in patients
with mild/moderate disease, 46.76±23.34 in patients with severe
disease and 41.35±6.32 in patients with critical disease. There was
a statistically significant difference in the mean OPNI values
between the three different disease severity groups, with the
lowest value observed in the patients with critical disease
(P=0.005) (Fig. 1 and Table II).
 | Table IDemographic characteristics of the
patients in cohorts A, B and C. |
Table I
Demographic characteristics of the
patients in cohorts A, B and C.
| Parameter | Cohort A
(n=588) | Cohort B
(n=494) | Cohort C
(n=523) |
|---|
| Age (years), mean ±
SD | 64.09±16.29 | 63.46±17.46 | 68.60±16.65 |
| Sex, n (%) | | | |
|
Female | 236 (40.1) | 208 (42.1) | 232 (44.4) |
|
Male | 352 (59.9) | 286 (57.9) | 291 (55.6) |
| Disease severity, n
(%) | | | |
|
Mild/moderate | 48 (8.2) | 94(19) | 137 (26.2) |
|
Severe | 365 (62.1) | 269 (54.5) | 223 (42.5) |
|
Critical | 175 (29.8) | 131 (26.5) | 163 (31.2) |
| Comorbidities, n
(%) | | | |
|
No | 107 (18.2) | 93 (18.8) | 44 (8.4) |
|
Yes | 481 (81.8) | 401 (81.2) | 479 (91.6) |
| Age >65 years, n
(%) | | | |
|
No | 293 (49.8) | 259 (52.3) | 199 (38.2) |
|
Yes | 295 (50.2) | 235 (47.7) | 324 (61.8) |
| Intubation, n
(%) | | | |
|
No | 528 (89.8) | 451 (91.3) | 482 (92.1) |
|
Yes | 60 (10.2) | 43 (8.7) | 41 (7.9) |
| Mortality, n
(%) | | | |
|
No | 487 (82.8) | 399 (80.8) | 403(77) |
|
Yes | 101 (17.2) | 95 (19.2) | 120(23) |
| Vaccination status,
n (%) | | | |
|
Vaccinated | 0 (0) | 167 (33.8) | 234 (44.8) |
|
Unvaccinated | 588(100) | 327 (66.2) | 289 (55.2) |
 | Table IIMean values of OPNI in the patients
in cohort A. |
Table II
Mean values of OPNI in the patients
in cohort A.
| Group | OPNI (mean ±
SD) | P-value |
|---|
| Females | 43.95±6.05 | 0.179 |
| Males | 46.09±23.97 | |
| Disease
severity | | 0.005 |
|
Mild/moderate | 47.80±6.35 | 0.759a |
|
Severe | 46.76±23.34 | 0.001b |
|
Critical | 41.35±6.32 | 0.003c |
| Comorbidities | 44.88±20.77 | 0.341 |
| No
comorbidities | 46.81±5.76 | |
| Age >65
years | 42.93±7.91 | 0.003 |
| Age ≤65 years | 47.56±25.47 | |
| Intubation | 41.32±5.96 | 0.092 |
| No intubation | 45.68±19.86 | |
| Mortality | 40.09±6.08 | 0.003 |
| Recovery | 46.3±20.49 | |
The mean OPNI value was 41.32±5.96 in patients who
were intubated and 45.68±19.86 in patients that were not (P=0.092).
The mean OPNI value was 40.09±6.08 in patients who did not survive
and 46.3±20.49 in patients who recovered. There was a statistically
significant difference in the mean OPNI values between patients who
did not survive and patients who recovered (P=0.003; Table II).
According to the univariate analysis, there was a
statistically significant association between an age >65 years
and mortality (P=0.01) and between an age >65 years and
intubation (P=0.003). Moreover, there was a statistically
significant association between the presence of comorbidities and
mortality (P=0.01), and between the presence of comorbidities and
intubation (P=0.015) (Table
III).
 | Table IIIUnivariate analysis for cohort A
(intubation and mortality). |
Table III
Univariate analysis for cohort A
(intubation and mortality).
| Parameter | Survivors | Non-survivors | P-value |
|---|
| Age >65
years | 207 | 88 | 0.01a |
| Age ≤65 years | 280 | 13 | |
| Males | 289 | 63 | 0.571 |
| Females | 198 | 38 | |
| Comorbidities | 382 | 99 | 0.01a |
| No
comorbidities | 105 | 2 | |
| Parameter | Non-intubation | Intubation | P-value |
| Age >65
years | 254 | 41 | 0.003a |
| Age ≤65 years | 274 | 19 | |
| Males | 311 | 41 | 0.158 |
| Females | 217 | 19 | |
| Comorbidities | 425 | 56 | 0.015a |
| No
comorbidities | 103 | 4 | |
Following multivariate logistic regression analysis,
including as confounders an age >65 years and the presence of
comorbidities, an independent association was found between OPNI
and intubation (OR, 0.928; 95% CI, 0.885-0.972; P= 0.002) (Table IV).
 | Table IVMultivariate logistic regression
analysis of factors independently associated with intubation and
mortality in cohort A. |
Table IV
Multivariate logistic regression
analysis of factors independently associated with intubation and
mortality in cohort A.
| Multivariate
analysis for mortality |
|---|
| Variable | P-value | Odds ratio | 95% CI |
|---|
| Age >65
years | 0.000 | 0.176 | 0.093 | 0.331 |
| Comorbidities | 0.019 | 0.174 | 0.041 | 0.746 |
| OPNI | 0.001 | 0.886 | 0.848 | 0.925 |
| Multivariate
analysis for intubation |
| Variable | P-value | Odds ratio | 95% CI |
| Age >65
years | 0.138 | 1.583 | 0.863 | 2.902 |
| Comorbidities | 0.139 | 2.252 | 0.769 | 6.599 |
| OPNI | 0.002 | 0.928 | 0.885 | 0.972 |
An independent association was also detected between
OPNI and mortality (OR, 0.886; 95% CI, 0.848-0.925; P= 0.001).
Other factors independently associated with mortality in this
cohort were an age >65 years and the presence of comorbidities
(Table IV).
Cohort B
Cohort B comprised of 494 patients (286 males),
predominantly infected with the delta SARS-CoV-2 variant, with a
mean age of 63.46±17.46 years. The demographic characteristics of
the patients in cohort B are presented in Table I. A total of 94 (19%) patients had
mild/moderate disease, 269 patients (54.5%) had severe disease and
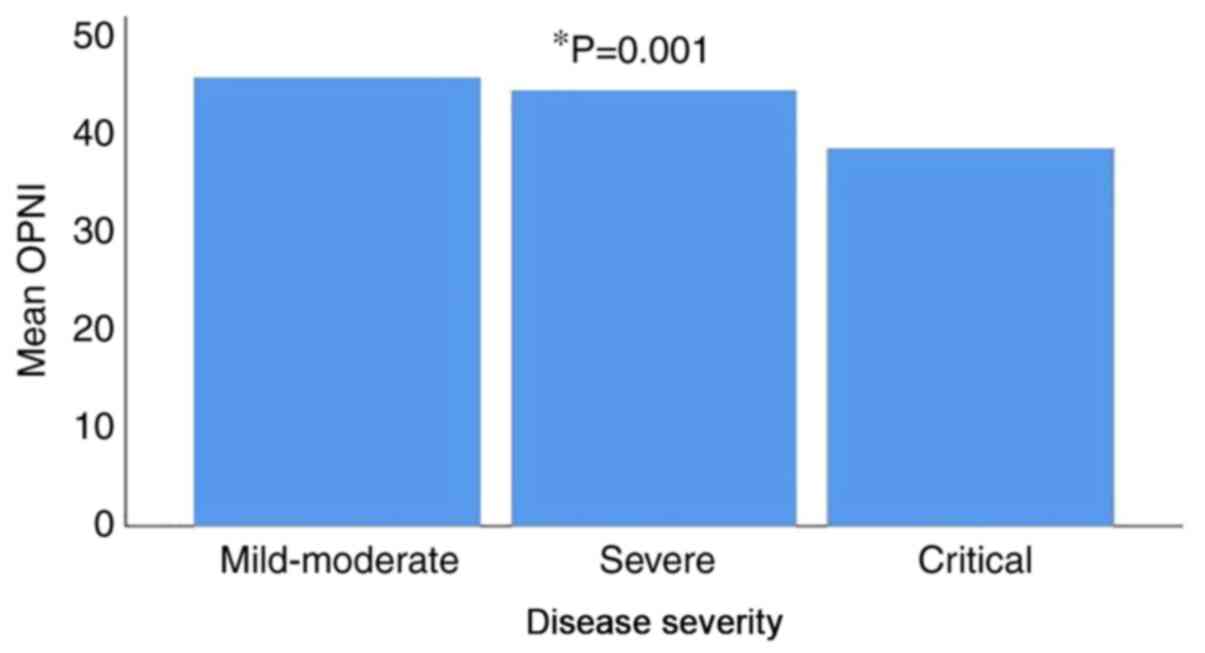
131 patients (26.5%) had critical disease. The mean OPNI value was
45.80±5.92 in patients with mild/moderate disease, 44.42±6.04 in
patients with severe disease and 38.58±6.37 in patients with
critical disease. There was a statistically significant difference
in the mean OPNI values between the three different disease
severity groups, with the lowest value observed in patients with
critical disease (P=0.001) (Fig. 2
and Table V).
 | Table VMean OPNI values in the patients in
cohort B. |
Table V
Mean OPNI values in the patients in
cohort B.
| Group | OPNI (mean ±
SD) | P-value |
|---|
| Females | 42.67±7.01 | 0.193 |
| Males | 43.48±6.44 | |
| Disease
severity | | 0.001 |
|
Mild/moderate | 45.80±5.92 | 0.067a |
|
Severe | 44.42±6.04 | 0.001b |
|
Critical | 38.58±6.37 | 0.001c |
| Comorbidities | 44.88±20.77 | 0.341 |
| No
comorbidities | 46.81±5.76 | |
| Age >65
years | 40.92±6.53 | 0.001 |
| Age ≤65 years | 45.21±6.17 | |
| Vaccinated | 42.52±6.75 | 0.153 |
| Unvaccinated | 43.45±6.66 | |
| Intubation | 38.55±5.88 | 0.001 |
| No intubation | 43.59±6.61 | |
| Death | 37.35±6.20 | 0.001 |
| Recovery | 44.50±6.05 | |
The mean OPNI value was 38.55±5.88 in patients who
were intubated and 43.59±6.61 in patients that were not. The mean
OPNI value was 37.35±6.20 in patients who did not survive and
44.50±6.05 in patients who recovered. There was a statistically
significant difference in the mean OPNI values between patients who
were intubated and those who were not intubated and between
patients who did not survive and patients who recovered (P=0.001;
Table V).
According to the univariate analysis, there was a
statistically significant association between an age >65 years
and mortality (P=0.01) and between an age >65 years and
intubation (P=0.01). Moreover, there was a statistically
significant association between the presence of comorbidities and
mortality (P=0.01), and between the presence of comorbidities and
intubation (P=0.014). In addition, there was a statistically
significant association between the male sex and intubation
(P=0.048) (Table VI).
 | Table VIUnivariate analysis for cohort B
(intubation and mortality). |
Table VI
Univariate analysis for cohort B
(intubation and mortality).
| Parameter | Survivors | Non-survivors | P-value |
|---|
| Age >65
years | 153 | 82 | 0.01a |
| Age ≤65 years | 246 | 13 | |
| Males | 232 | 54 | 0.817 |
| Females | 167 | 41 | |
| Comorbidities | 308 | 93 | 0.01a |
| No
comorbidities | 91 | 2 | |
| Parameter | Non-intubation | Intubation | P-value |
| Age >65
years | 204 | 31 | 0.01a |
| Age ≤65 years | 247 | 12 | |
| Males | 255 | 31 | 0.048a |
| Females | 196 | 12 | |
| Comorbidities | 359 | 42 | 0.014a |
| No
comorbidities | 92 | 1 | |
Following multivariate logistic regression analysis,
including as confounders an age >65 years, the male sex and the
presence of comorbidities, an independent association was found
between OPNI and intubation (OR, 0.903; 95% CI, 0.856-0.953; P=
0.001). Other factors independently associated with intubation in
this cohort were an age >65 years and the male sex (Table VII).
 | Table VIIMultivariate logistic regression
analysis of factors independently associated with intubation and
mortality in cohort B. |
Table VII
Multivariate logistic regression
analysis of factors independently associated with intubation and
mortality in cohort B.
| Multivariate
analysis for intubation |
|---|
| Variable | P-value | Odds ratio | 95% CI |
|---|
| Age >65
years | 0.020 | 2.489 | 1.155 | 5.366 |
| Male sex | 0.010 | 2.634 | 0.856 | 0.953 |
| Comorbidities | 0.622 | 1.108 | 0.738 | 1.662 |
| OPNI | 0.001 | 0.903 | 0.856 | 0.953 |
| Multivariate
analysis for mortality |
| Variable | P-value | Odds ratio | 95% CI |
| Age >65
years | 0.001 | 6.793 | 3.430 | 13.450 |
| Comorbidities | 0.736 | 1.068 | 0.727 | 1.571 |
| OPNI | 0.001 | 0.840 | 0.798 | 0.884 |
An independent association was also detected between
OPNI and mortality (OR, 8.40; 95% CI, 0.798-0.884; P= 0.001).
Another factor independently associated with mortality in this
cohort was an age >65 years (Table VII).
Cohort C
Cohort C comprised of 523 patients (291 males),
predominantly infected with the omicron SARS-CoV-2 variant, with a
mean age of 68.60±16.65 years. The demographic characteristics of
the cohort are presented in Table
I. In total, 137 patients (26.2%) had mild/moderate disease,
223 patients (42.5%) had severe disease and 163 patients (31.2%)
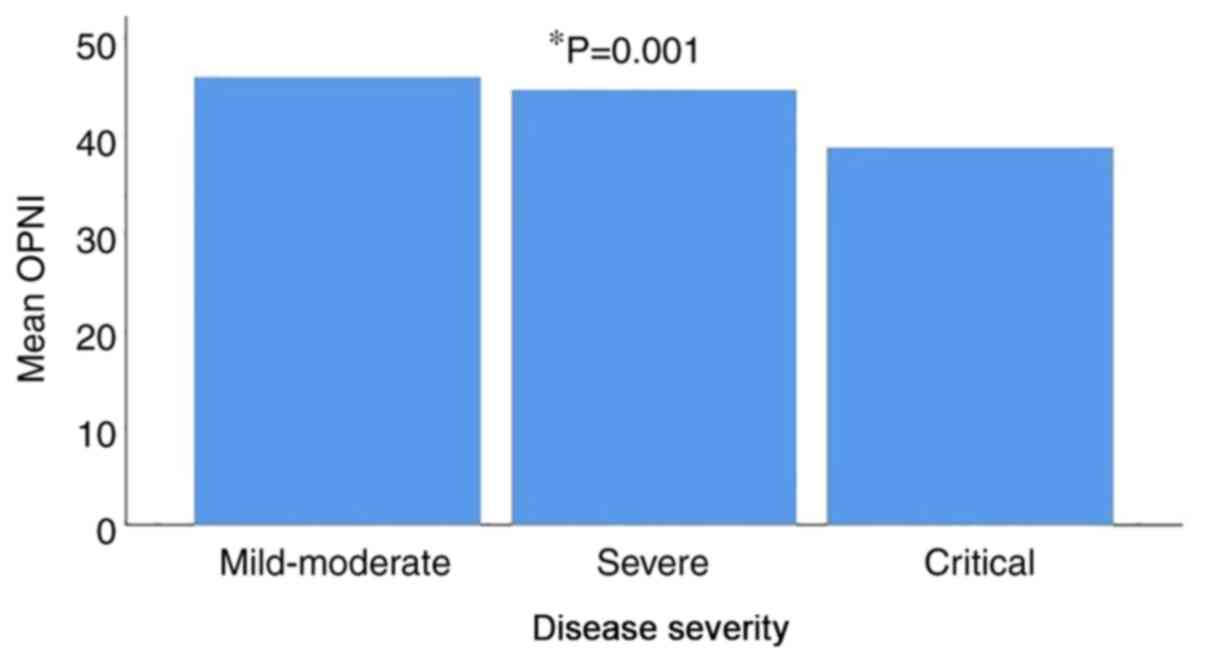
had critical disease. The mean OPNI value was 44.61±7.56 in
patients with mild/moderate disease, 42.94±6.95 in patients with
severe disease and 38.36±6.57 in patients with critical disease.
There was a statistically significant difference in the mean OPNI
values between the three different disease severity groups, with
the lowest value observed in patients with critical disease
(P=0.001) (Fig. 3 and Table VIII).
 | Table VIIIMean OPNI values in the patients in
cohort C. |
Table VIII
Mean OPNI values in the patients in
cohort C.
| Group | OPNI (mean ±
SD) | P-value |
|---|
| Females | 41.83±7.36 | 0.758 |
| Males | 42.04±7.48 | |
| Disease
severity | | 0.001 |
|
Mild/moderate | 44.61±7.56 | 0.036a |
|
Severe | 42.94±6.95 | 0.001b |
|
Critical | 38.36±6.57 | 0.001c |
| Comorbidities | 41.74±7.51 | 0.035 |
| No
comorbidities | 44.25±5.91 | |
| Age >65
years | 40.55±6.92 | 0.001 |
| Age ≤65 years | 44.25±7.63 | |
| Vaccinated | 42.82±7.91 | 0.018 |
| Unvaccinated | 41.23±6.93 | |
| Intubation | 39.21±6.04 | 0.001 |
| No intubation | 42.17±7.49 | |
| Mortality | 37.42±6.52 | 0.014 |
| Recovery | 43.29±7.14 | |
The mean OPNI value was 39.21±6.04 in patients who
were intubated and 42.17±7.49 in patients that were not. The mean
OPNI value was 37.42±6.52 in patients who did not survive and
43.29±7.14 in patients who recovered. There was a statistically
significant difference in the mean OPNI values between patients who
were intubated and those who were not intubated, and between
patients who did not survive and patients who recovered (P=0.001
and P=0.014 respectively; Table
VIII).
According to the univariate analysis, there was a
statistically significant association between the presence of
comorbidities and mortality (P=0.022) and between an age >65
years and mortality (P=0.01). No statistically significant
association was found between the presence of comorbidities and
intubation, between age an >65 years and intubation, between the
vaccination status and intubation, and between sex and intubation
(Table IX).
 | Table IXUnivariate analysis for cohort C
(intubation and mortality). |
Table IX
Univariate analysis for cohort C
(intubation and mortality).
| Parameter | Survivors | Non-survivors | P-value |
|---|
| Age >65
years | 218 | 106 | 0.01a |
| Age ≤65 years | 185 | 14 | |
| Males | 222 | 69 | 0.625 |
| Females | 181 | 51 | |
| Comorbidities | 363 | 116 | 0.022a |
| No
comorbidities | 40 | 4 | |
| Vaccinated | 189 | 45 | 0.66 |
| Unvaccinated | 214 | 75 | |
| Parameter | Non-intubation | Intubation | P-value |
| Age >65
years | 294 | 30 | 0.292 |
| Age ≤65 years | 188 | 11 | |
| Males | 266 | 25 | 0.453 |
| Females | 216 | 16 | |
| Comorbidities | 440 | 37 | 0.765 |
| No
comorbidities | 40 | 4 | |
| Vaccinated | 219 | 15 | 0.281 |
| Unvaccinated | 263 | 26 | |
An independent association was detected between the
OPNI and death according to the multivariate logistic regression
analysis (OR, 0.886; 95% CI 0.848-0.925; P= 0.001). Other factors
independently associated with mortality in this cohort were an age
>65 years and the presence of comorbidities (Table X).
 | Table XMultivariate logistic regression
analysis of factors independently associated with mortality in
cohort C. |
Table X
Multivariate logistic regression
analysis of factors independently associated with mortality in
cohort C.
| Multivariate
analysis for mortality |
|---|
| Variable | P-value | Odds ratio | 95% CI |
|---|
| Age >65
years | 0.001 | 0.176 | 0.093 | 0.925 |
| Comorbidities | 0.019 | 0.174 | 0.041 | 0.746 |
| OPNI | 0.001 | 0.886 | 0.848 | 0.925 |
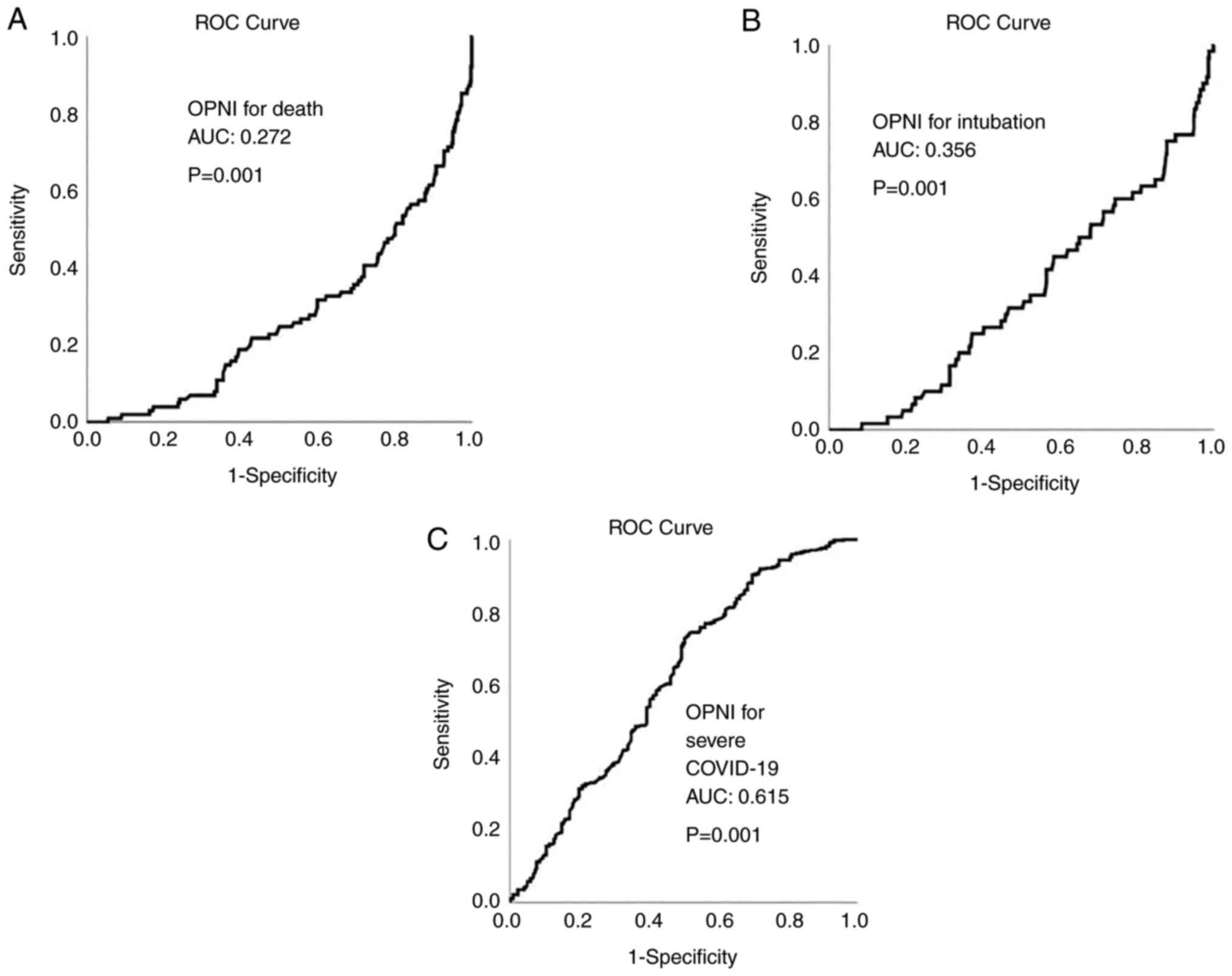
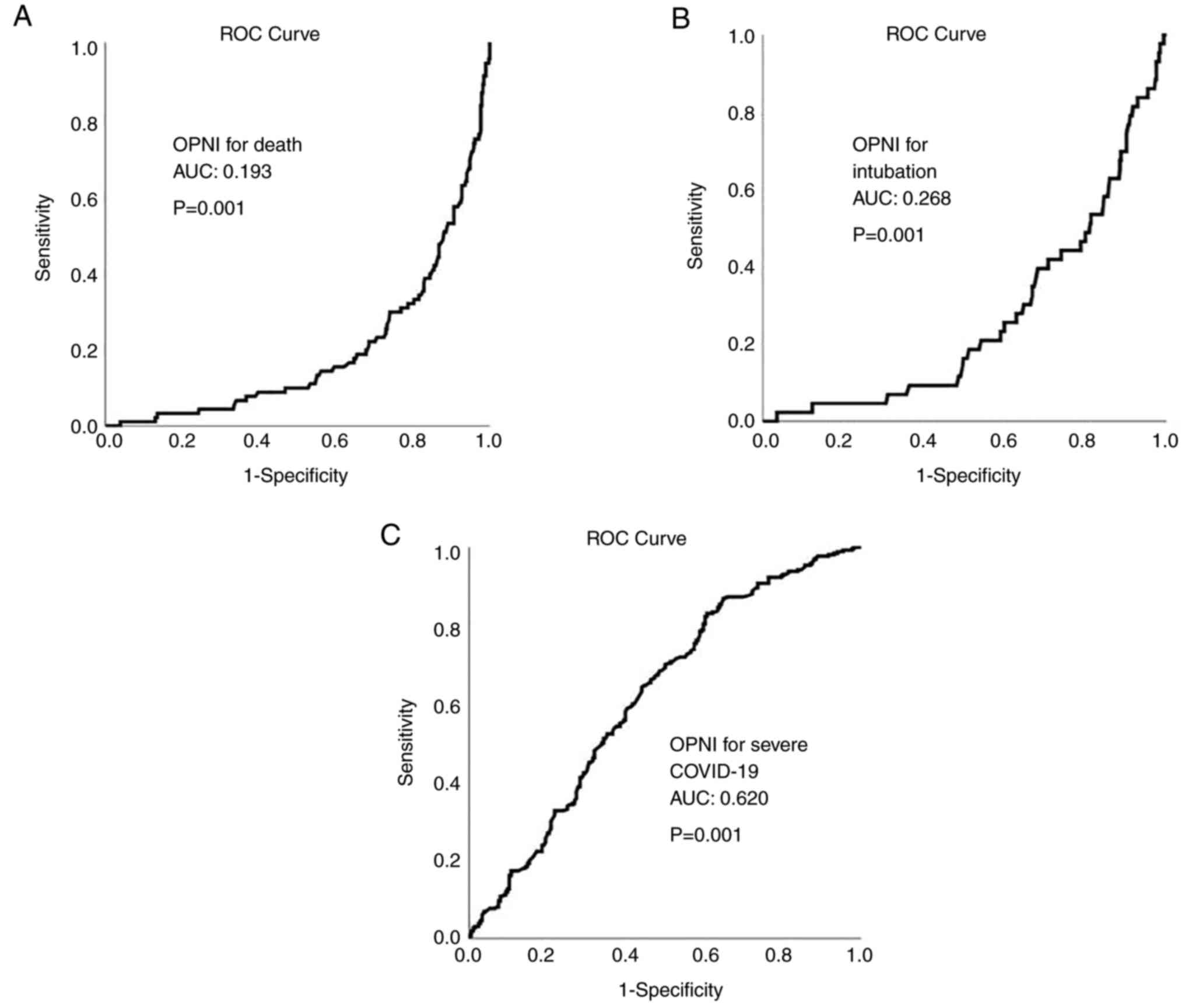
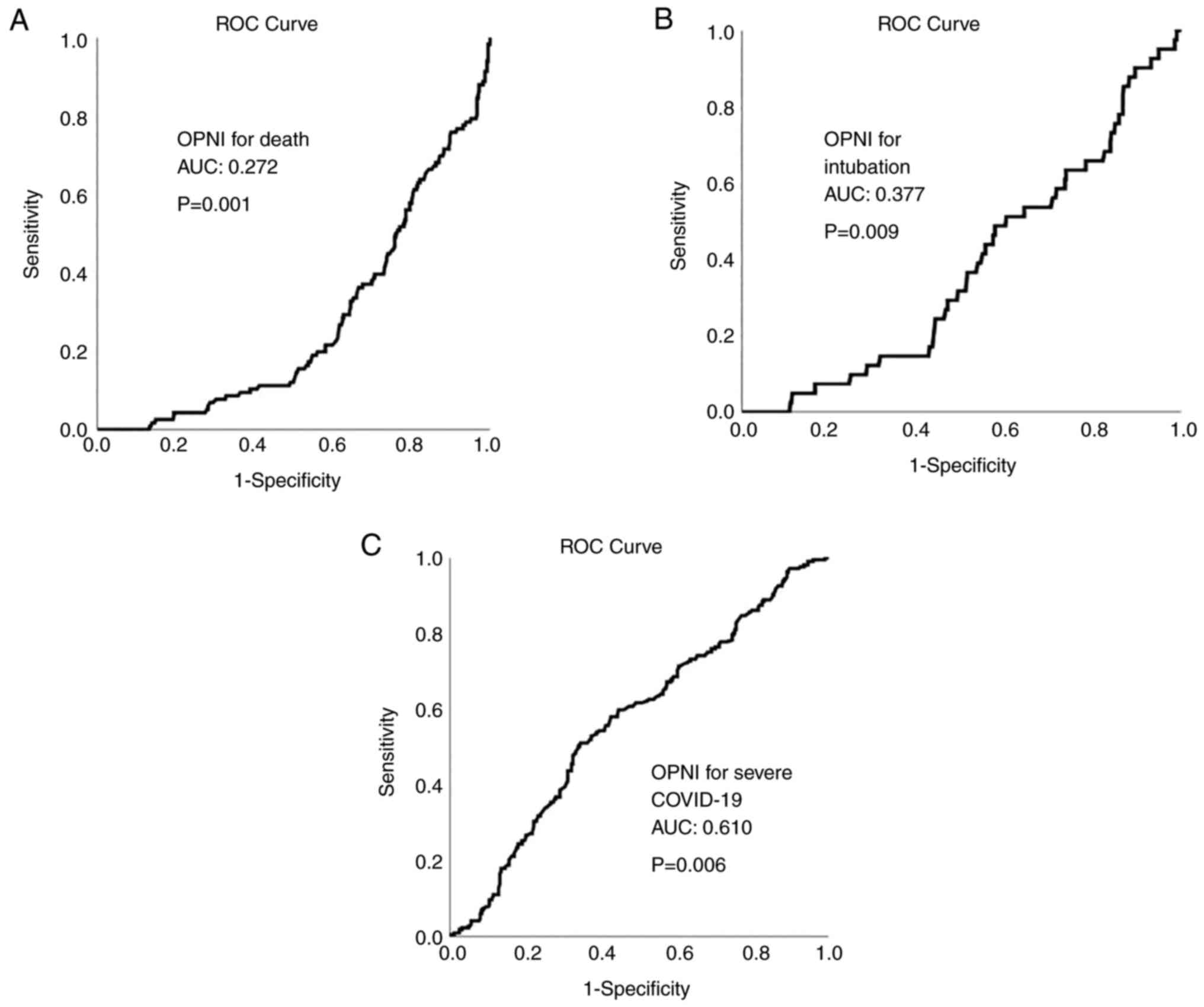
ROC analysis revealed that OPNI had no
discriminative ability for mortality and intubation in all cohorts
[cohort A: area under the curve (AUC, 0.272 for mortality and 0.356
for intubation; cohort B: AUC, 0.193 for mortality and 0.268 for
intubation; cohort C: AUC, 0.272 for mortality and 0.377 for
intubation]. OPNI exhibited an acceptable discriminative ability
for severe disease in all cohorts (cohort A: AUC, 0.615; cohort B;
AUC, 0.620; and cohort C: AUC, 0.610). The ROC curves for OPNI in
cohorts A, B and C are presented in Figs. 4, 5 and 6,
respectively.
Discussion
According to the results of the present study, OPNI
was an independent indicator of disease severity and mortality in
all the pandemic waves. OPNI, which is derived using albumin and
lymphocyte levels, is an objective measure of inflammation and
nutritional status. Previous research has indicated that albumin
levels were inversely associated with disease progression and a
poor prognosis in patients with COVID-19 (20,21).
Low albumin levels in non-survivors may be attributed to
intubation-induced insufficient intake, impaired synthesis due to
liver failure and increased consumption due to organ injury.
Several mechanisms can mediate the link between poor outcomes and
low albumin levels. The albumin level is an indication of liver
function as it is produced by hepatocytes (22). Inflammatory cytokines, such as IL-6
and tumor necrosis factor α (TNF-α) can suppress hepatocyte
synthesis, leading to a decline in albumin levels in the blood. As
already aforementioned, severe organ damage in patients with
COVID-19 is caused by a cytokine storm characterized by a large
release of cytokines, such as IL-1, IL-6, TNF-α, monocyte
chemotactic protein (MCP)-1, inducible protein-10 (IP-10),
interferon-γ and granulocyte colony-stimulating factor (G-CSF)
(23). Cytokines, such as IL-1ra,
IL-2R, IL-6, IL-10, TNF-a, IP-10 and MCP-3 have been found to be
associated with disease severity and progression in patients with
COVID-19. As a result, low albumin levels in patients with COVID-19
may signal a severe cytokine storm and organ damage, including
liver impairment (24-26).
Moreover, a low albumin level may result in the
exudation of intravascular fluid, exacerbating the severity of
pulmonary edema. In patients with COVID-19, serum albumin levels
have been found to be inversely associated with the development of
acute respiratory distress syndrome (ARDS). The onset of ARDS is
unquestionably a risk factor for poor outcomes of patients with
COVID-19. As a result, low albumin levels do not contribute to
favorable outcomes of patients with COVID-19 by decreasing
pulmonary function (27).
As a typical metric of nutritional status, low
albumin levels in critically ill patients may reflect a high
consumption status induced by tissue injury and hypermetabolism. A
poor nutritional status, as reflected by albumin levels, is not
conducive to tissue regeneration and recovery in patients with
COVID-19.
The lymphocyte count is another essential component
of OPNI. Decreased peripheral lymphocyte numbers in patients with
COVID-19 are due to a reduction in the numbers of T-cells, namely
CD3+, CD4+ and CD8+ T-cells
(28). The reduction in the
numbers of CD4+ and CD8+ T-cells, as well as
their increased activation, are critical features of
immunocompromise and are associated with an unfavorable disease
progression in patients with COVID-19(29). The numbers of T-cells may be
reduced as a result of direct viral assault on lymphocytes, antigen
presentation cell malfunction, and apoptosis induced by excessive
cytokine production (26,30,31).
In patients with COVID-19, lymphopenia has been identified as an
independent risk factor for disease severity and poor outcomes. The
lower lymphocyte count may be interpreted as a sign of poor
immunological function and rapidly increasing cytokine levels
(32). Thus, taken together, as a
combination of both serum albumin levels and peripheral lymphocyte
count, OPNI may more accurately indicate the nutritional and
inflammatory state in patients with COVID-19.
OPNI has been reported as an independent indicator
of COVID-19 severity (33-35).
Moreover, OPNI has been found to be independently associated with
the mortality of patients with SARS-CoV-2 infection (15,35-37).
To the best of our knowledge, the present study is the first to
demonstrate that OPNI is an independent marker of COVID-19 severity
and outcomes in all three different pandemic waves examined. More
specifically, OPNI exhibited an independent association with
disease severity and mortality, even in the period of omicron
variant predominance. It has been well established that the omicron
variant contains a markedly higher number of novel mutations than
other variants in its spike protein, the majority of which are in
its receptor binding region, which increases transmissibility,
while decreasing antibody and vaccination responses (38).
The key strengths of the present study are the large
number of participants, and the availability of detailed
information on the characteristics and outcomes of patients with
COVID-19. The limitations of the study are that it was a
single-center study, it was conducted retrospectively, and that
there was no healthy control group. Moreover, no other inflammatory
indices, potentially relevant in COVID-19 related outcomes, were
evaluated.
In conclusion, the results of the present study, to
the best of our knowledge, provide the first direct evidence that a
lower OPNI value is associated with greater disease severity in
patients with COVID-19. OPNI values upon admission were independent
predictors of intubation and mortality in patients with COVID-19 in
all pandemic waves examined.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VEG and NM conceptualized the study. VEG, NM, MZ,
CD, MT, AK, SS, GK, SP, PS, GF, NT, KT and PP advised on patient
treatments, obtained data, wrote and prepared the draft of the
manuscript. DAS, VEG and NVS were involved in the study design, and
analyzed the data and provided critical revisions. VEG and NVS
confirm the authenticity of all the data. All authors contributed
to manuscript revision, and have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Ethical approval for the study was obtained from the
Research Ethics Committee of Laiko General Hospital (Athens,
Greece). The study was in line with the declaration of Helsinki in
1995 (as revised in Edinburgh 2000). Due to the retrospective
design of the study, a waiver for informed consent was granted by
the Institutional Review Board.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Rodriguez-Morales AJ, Cardona-Ospina JA,
Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y,
Escalera-Antezana JP, Alvarado-Arnez LE, Bonilla-Aldana DK,
Franco-Paredes C, Henao-Martinez AF, et al: Clinical, laboratory
and imaging features of COVID-19: A systematic review and
meta-analysis. Travel Med Infect Dis. 34(101623)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zaim S, Chong JH, Sankaranarayanan V and
Harky A: COVID-19 and multiorgan response. Curr Probl Cardiol.
45(100618)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Georgakopoulou VE, Avramopoulos P,
Papalexis P, Bitsani A, Damaskos C, Garmpi A, Venetikou MS,
Paramythiotis D, Karlafti E, Sklapani P, et al: COVID-19 induced
hypoparathyroidism: A case report. Exp Ther Med.
23(346)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Georgakopoulou VE, Gkoufa A, Damaskos C,
Papalexis P, Pierrakou A, Makrodimitri S, Sypsa G, Apostolou A,
Asimakopoulou S, Chlapoutakis S, et al: COVID-19-associated acute
appendicitis in adults. A report of five cases and a review of the
literature. Exp Ther Med. 24(482)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gabarre P, Dumas G, Dupont T, Darmon M,
Azoulay E and Zafrani L: Acute kidney injury in critically ill
patients with COVID-19. Intensive Care Med. 46:1339–1348.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cholongitas E, Bali T, Georgakopoulou VE,
Giannakodimos A, Gyftopoulos A, Georgilaki V, Gerogiannis D,
Basoulis D, Eliadi I, Karamanakos G, et al: Prevalence of abnormal
liver biochemistry and its impact on COVID-19 patients' outcomes: A
single-center Greek study. Ann Gastroenterol. 35:290–296.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Martínez-García L, Espinel MA, Abreu M,
González-Alba JM, Gijón D, McGee A, Cantón R, Galán JC and Aranaz
J: Emergence and spread of B.1.1.7 lineage in primary care and
clinical impact in the morbi-mortality among hospitalized patients
in Madrid, Spain. Microorganisms. 9(1517)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
El-Shabasy RM, Nayel MA, Taher MM,
Abdelmonem R, Shoueir KR and Kenawy ER: Three waves changes, new
variant strains, and vaccination effect against COVID-19 pandemic.
Int J Biol Macromol. 204:161–168. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Darif D, Hammi I, Kihel A, El Idrissi Saik
I, Guessous F and Akarid K: The pro-inflammatory cytokines in
COVID-19 pathogenesis: What goes wrong? Microb Pathog.
153(104799)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Regolo M, Vaccaro M, Sorce A, Stancanelli
B, Colaci M, Natoli G, Russo M, Alessandria I, Motta M, Santangelo
N, et al: Neutrophil-to-lymphocyte ratio (NLR) is a promising
predictor of mortality and admission to intensive care unit of
COVID-19 patients. J Clin Med. 11(2235)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sayah W, Berkane I, Guermache I, Sabri M,
Lakhal FZ, Yasmine Rahali S, Djidjeli A, Lamara Mahammed L, Merah
F, Belaid B, et al: Interleukin-6, procalcitonin and
neutrophil-to-lymphocyte ratio: Potential immune-inflammatory
parameters to identify severe and fatal forms of COVID-19.
Cytokine. 141(155428)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Georgakopoulou VE, Vlachogiannis NI,
Basoulis D, Eliadi I, Georgiopoulos G, Karamanakos G, Makrodimitri
S, Samara S, Triantafyllou M, Voutsinas PM, et al: A simple
prognostic score for critical COVID-19 Derived from patients
without comorbidities performs well in unselected patients. J Clin
Med. 11(1810)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Georgakopoulou VE, Makrodimitri S,
Triantafyllou M, Samara S, Voutsinas PM, Anastasopoulou A,
Papageorgiou CV, Spandidos DA, Gkoufa A, Papalexis P, et al:
Immature granulocytes: Innovative biomarker for SARS-CoV-2
infection. Mol Med Rep. 26(217)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Georgakopoulou VE, Garmpis N, Damaskos C,
Valsami S, Dimitroulis D, Diamantis E, Farmaki P, Papageorgiou CV,
Makrodimitri S, Gravvanis N, et al: The impact of peripheral
eosinophil counts and eosinophil to lymphocyte ratio (ELR) in the
clinical course of COVID-19 patients: A retrospective study. In
Vivo. 35:641–648. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang R, He M, Yin W, Liao X, Wang B, Jin
X, Ma Y, Yue J, Bai L, Liu D, et al: The Prognostic nutritional
index is associated with mortality of COVID-19 patients in Wuhan,
China. J Clin Lab Anal. 34(e23566)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.PubMed/NCBI(In Japanese).
|
|
17
|
Kang SH, Cho KH, Park JW, Yoon KW and Do
JY: . Onodera's prognostic nutritional index as a risk factor for
mortality in peritoneal dialysis patients. J Korean Med Sci.
27:1354–1358. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moon SW, Lee EH, Choi JS, Leem AY, Lee SH,
Lee SH, Kim SY, Chung KS, Jung JY, Park MS, et al: Impact of
prognostic nutritional index on outcomes in patients with
Mycobacterium avium complex pulmonary disease. PLoS One.
15(e0232714)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
National Institutes of Health (NIH):
Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. NIH,
Bethesda, MD, 2021. https://www.covid19treatmentguidelines.nih.gov/.
Accessed October 20, 2021.
|
|
20
|
Zhang J, Wang X, Jia X, Li J, Hu K, Chen
G, Wei J, Gong Z, Zhou C, Yu H, et al: Risk factors for disease
severity, unimprovement, and mortality in COVID-19 patients in
Wuhan, China. Clin Microbiol Infect. 26:767–772. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan
L, Cao J, Tan M, Xu W, Zheng F, et al: A tool for early prediction
of severe coronavirus disease 2019 (COVID-19): A multicenter study
using the risk nomogram in Wuhan and Guangdong, China. Clin Infect
Dis. 71:833–840. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Peters SJ, Vanhaecke T, Papeleu P, Rogiers
V, Haagsman HP and van Norren K: Co-culture of primary rat
hepatocytes with rat liver epithelial cells enhances
interleukin-6-induced acute-phase protein response. Cell Tissue
Res. 340:451–457. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi
Y, Sun R, Tian Z, Xu X and Wei H: Pathogenic T-cells and
inflammatory monocytes incite inflammatory storms in severe
COVID-19 patients. Natl Sci Rev. 7:998–1002. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y,
Shi J, Zhou M, Wu B, Yang Z, et al: Risk factors for severity and
mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin
Immunol. 146:110–118. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang Y, Shen C, Li J, Yuan J, Wei J, Huang
F, Wang F, Li G, Li Y, Xing L, et al: Plasma IP-10 and MCP-3 levels
are highly associated with disease severity and predict the
progression of COVID-19. J Allergy Clin Immunol. 146:119–127.e4.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tan L, Wang Q, Zhang D, Ding J, Huang Q,
Tang YQ, Wang Q and Miao H: Lymphopenia predicts disease severity
of COVID-19: A descriptive and predictive study. Signal Transduct
Target Ther. 5(33)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S,
Huang H, Zhang L, Zhou X, Du C, et al: Risk factors associated with
acute respiratory distress syndrome and death in patients with
coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern
Med. 180:934–943. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Han Y, Zhang H, Mu S, Wei W, Jin C, Xue Y,
Tong C, Zha Y, Song Z and Gu G: Lactate dehydrogenase, a risk
factor of severe COVID-19 patients. medRxiv: doi: https://doi.org/10.1101/2020.03.24.20040162.
|
|
29
|
Xu Z, Shi L, Wang Y, Zhang J, Huang L,
Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al: Pathological findings
of COVID-19 associated with acute respiratory distress syndrome.
Lancet Respir Med. 8:420–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhao J, Zhao J, Van Rooijen N and Perlman
S: Evasion by stealth: Inefficient immune activation underlies poor
T cell response and severe disease in SARS-CoV-infected mice. PLoS
Pathog. 5(e1000636)2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Channappanavar R and Perlman S: Pathogenic
human coronavirus infections: Causes and consequences of cytokine
storm and immunopathology. Semin Immunopathol. 39:529–539.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Georgakopoulou VE, Lembessis P, Skarlis C,
Gkoufa A, Sipsas NV and Mavragani CP: Hematological abnormalities
in COVID-19 disease: Association with type I interferon pathway
activation and disease outcomes. Front Med (Lausanne).
9(850472)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang ZH, Lin YW, Wei XB, Li F, Liao XL,
Yuan HQ, Huang DZ, Qin TH, Geng H and Wang SH: Predictive value of
prognostic nutritional index on COVID-19 severity. Front Nutr.
7(582736)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nalbant A, Demirci T, Kaya T, Aydın A,
Altındiş M and Güçlü E: Can prognostic nutritional index and
systemic immune-inflammatory index predict disease severity in
COVID-19? Int J Clin Pract. 75(e14544)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rashedi S, Keykhaei M, Pazoki M, Ashraf H,
Najafi A, Kafan S, Peirovi N, Najmeddin F, Jazayeri SA, Kashani M,
et al: Clinical significance of prognostic nutrition index in
hospitalized patients with COVID-19: Results from single-center
experience with systematic review and meta-analysis. Nutr Clin
Pract. 36:970–983. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wei W, Wu X, Jin C, Mu T, Gu G, Min M, Mu
S and Han Y: Predictive significance of the prognostic nutritional
index (PNI) in patients with severe COVID-19. J Immunol Res.
2021(9917302)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Çınar T, Hayıroğlu Mİ, Çiçek V, Kılıç Ş,
Asal S, Yavuz S, Selçuk M, Yalçınkaya E, Keser N and Orhan AL: Is
prognostic nutritional index a predictive marker for estimating
all-cause in-hospital mortality in COVID-19 patients with
cardiovascular risk factors? Heart Lung. 50:307–312.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bazargan M, Elahi R and Esmaeilzadeh A:
OMICRON: Virology, immunopathogenesis, and laboratory diagnosis. J
Gene Med. 24(e3435)2022.PubMed/NCBI View Article : Google Scholar
|