Introduction
Osteoarthritis (OA) is a chronic degenerative
disease involving the presence of synovial lesions, which may be
indicative of an underlying inflammatory response, that precedes or
eludes identification by X-ray imaging or MRI (1,2). The
pathological process of OA involves inflammation of the synovium,
which may manifest during the initial stages of synovitis as
debilitating pain. Even if patients do not outwardly display
clinical symptoms of synovitis, the diseased joints usually exhibit
local synovitis, and this inflammatory reaction is most apparent in
the adjacent articular cartilage injury area (3,4). It
was originally suggested that obese patients were prone to OA due
to cartilage destruction caused by biomechanical stress (5). However, evidence has indicated that
the large amount of adipose tissue that accumulates in obese
patients, which stores energy and produces endocrine response
factors, can release a number of cytokines and adipokines that
participate in detrimental physiological and pathophysiological
processes, such as immune and inflammatory responses, insulin
resistance and tumorigenesis (6).
Similarly, a previous study revealed that increased calorie intake
in animal models led to both a joint and systemic inflammatory
response by promoting the release of adipokines, cytokines and
chemokines (7). These findings
highlight the importance of inflammatory responses in determining
the occurrence and outcome of OA.
Chemerin is a recently identified chemotactic
protein that guides macrophages and dendritic cells that express
its receptor, chemokine-like receptor 1 (CMKLR1) (8), to
inflammatory sites, and is involved in both adaptive and innate
immunity (9). Chemerin is
expressed in liver cells, white adipose tissue, monocyte-derived
macrophages and immature dendritic cells (10). In adipose tissues, chemerin is
widely distributed, displays endocrine activity, and has been
discovered to play a role in metabolic diseases, such as obesity,
type 2 diabetes and cardiovascular disease (11-13).
Chemerin levels in human synovial fluid have been found to
correlate with OA severity using the Kellgren-Lawrence
classification as a criterion (14). Furthermore, CMKLR1 is expressed on
articular synoviocytes (15).
Chemerin receptors in rats, called G protein-coupled receptors-DEZ,
are expressed in hepatocytes, white adipose tissue,
monocyte-derived macrophages, immature dendritic cells and
synoviocytes (16), and their
expression has been reported to be associated with the production
of a variety of inflammatory mediators that are released into
synovial lesions in OA and affect articular cartilage. Previous
evidence has suggested that the MEK/ERK signaling pathway may
mediate the inflammatory responses in OA, and MAPKs have been
evaluated as potential therapeutic targets (17,18).
However, to the best of our knowledge, the mechanisms through which
chemerin promotes inflammatory factor production in synoviocytes
have not been evaluated. Therefore, the present study aimed to
investigate whether chemerin could activate the ERK/MEK signaling
pathway, and to determine the profile of inflammatory mediators
released by joint synoviocytes in response to chemerin treatment.
The results may provide a potential novel approach for the clinical
treatment of OA via the targeting of the chemerin-associated MAPK
signaling pathway.
Materials and methods
Materials and equipment
Tanon 5200 multi automatic
chemiluminescence/fluorescence image analysis system was purchased
from Tanon Science and Technology Co., Ltd. The fluorescence
inverted microscope was obtained from Olympus Corporation.
High-glucose (95%) DMEM was acquired from Beijing Solarbio Science
& Technology Co., Ltd. Recombinant murine chemerin was
purchased from R&D Systems, Inc. Penicillin/streptomycin and
PBS solutions were acquired from Beijing Solarbio Science &
Technology Co., Ltd. High performance RIPA lysis buffer solution
and western and immunoprecipitation cell lysis solution were
purchased from Beyotime Institute of Biotechnology. BCA protein
quantification and ECL kits were obtained from MultiSciences
(Lianke) Biotech Co., Ltd. The PVDF membrane (0.45 µm) was obtained
from MilliporeSigma. Rat MMP-3 (cat. no. SEKR-0067-96T), MMP-13
(cat. no. SEKR-0035-96T), TNF-α (cat. no. SEKR-0009-96T), IL-1β
(cat. no. SEKR-0002-96T) and IL-6 (cat. no. SEKR-0005-96T) ELISA
kits were acquired from Beijing Solarbio Science & Technology
Co., Ltd.
Cell culture
Rat synoviocytes (RSC-364) were purchased from
Shanghai Zeye Biotechnology Co., Ltd. and cultured at 37˚C in 95%
glucose DMEM supplemented with 10% FBS (Beijing Solarbio Science
& Technology Co., Ltd.) and 1% penicillin/streptomycin in a 5%
CO2 incubator. The cells were sub-cultured by rinsing
twice with sterile PBS, followed by digestion using Trypsin-EDTA
solution (0.25% pancreatin +0.02% EDTA; without phenol red)
(Beijing Solarbio Science & Technology Co., Ltd.), which was
evenly applied under observation using an inverted microscope. At
the point at which 70-80% of the cells had begun to shrink and
become round and detached, 95% high-glucose DMEM was rapidly added
to the cells under gentle agitation using a sterile Papanicolaou
dropper. The cells were subsequently sub-cultured at a ratio of 1:2
or 1:3. The present study was approved by the Ethics Committee of
Guangxi Medical University (approval no. 20150303-12; Nanning,
China).
For cryopreservation, the cells were transferred
into centrifuge tubes and centrifuged to remove the supernatants.
The cell pellets were subsequently resuspended in an appropriate
amount of cryopreservation solution (containing 20% FBS, 10% DMSO
and 70% DMEM). The cells were then dispensed into 1.5 ml
cryopreservation tubes, placed in a cryopreservation box at -80˚C,
and then transferred to liquid nitrogen for long-term preservation.
To recover the cryopreserved cells, cryotubes containing the cells
were quickly placed in a 37˚C water bath for re-warming. After
thawing, the tubes were placed in a centrifuge (300 x g; 5 min;
37˚C) to remove the supernatant. The cells were then re-suspended
in DMEM and transferred to a culture flask. The rat synoviocytes
were evaluated experimentally within two to five passages.
Cell Counting Kit-8 (CCK-8) assay
After RSC-364 synoviocytes were passaged three
times, the cells were seeded into two 96-well plates at a density
of 5x103 cells/well and incubated in a 5% CO2
atmosphere at 37˚C for 24 h. The plates were subsequently divided
in half, and half of each plate was treated with a MEK inhibitor
(PD98059; 10 µM; cat. no. 9900S; Cell Signaling Technology, Inc.)
at 37˚C for 30 min. Then, 100 µl of 0, 0.25, 0.5 or 1.0 µg/ml
Reconstituted Chemerin's medium was added, and the plate was placed
back into the incubator. After 24 h of incubation, 10 µl CCK-8
solution was added to each well, according to the instructions of
the CCK-8 assay kit (Dojindo Molecular Technologies, Inc.), and
further incubated for 4 h. The absorbance was measured at a
wavelength of 450 nm using a microplate reader (Multiskan™ FC;
Thermo Fisher Scientific, Inc.). The experiment was repeated three
times. The CCk-8 control group was the group without chemerin, and
recombinant chemerin was dissolved in distillation-distillation
H2O.
Reverse transcription-quantitative PCR
(RT-qPCR)
Synoviocytes passaged to the third generation were
seeded into a 6-well plate at a density of 4x104
cells/well and cultured for 24 h. The cells were subsequently
divided into two groups: The control group and the chemerin group
(0.5 µg/ml recombinant murine chemerin). After continuous culture
for 48 h, total RNA was extracted using an Cell RNA rapid
extraction kit (Beijing Solarbio Science & Technology Co.,
Ltd.), according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using a RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. qPCR was subsequently performed using a
fluorescence quantitative PCR kit (SYBR Green Master mix; Roche
Diagnostics), according to the manufacturer's instructions, on an
ABI 7500 Real-Time PCR detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primer sequences used for GAPDH,
MMP-3, MMP-13, MEK and ERK detection are listed in Table I. GAPDH was used as the internal
reference gene using the 2-ΔΔCq method (19). All experiments were repeated in
triplicate.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| ERK |
AACGGTCAGAAAGTGGCGAT |
ACGTTCTTTCGGCAGGTCAT |
| MEK |
TCTGCAGTTAACGGGACCAG |
AGCTCTAGCTCCTCCAGCTT |
| MMP-3 |
CTGGGCTATCCGAGGTCATG |
TCCGCTGAAGAAGTAAAGAAACC |
| MMP-13 |
CAAGCAGCTCCAAAGGCTAC |
TGGATGTGACCGTTTTCGGT |
| GAPDH |
AAGCCCATCACCATCTTCCAGGAG |
ATGAGCCCTTCCACAATGCCAAAG |
Western blotting
Cells passaged for three generations were divided
into the control and chemerin groups and seeded into 6-well plates
at a density of 4x104 cells/well. After 24 h of
attachment, the cells were stimulated with 0.5 µg/ml chemerin for
10 min at 37˚C, prior to the addition of RIPA lysis buffer
(containing protease inhibitors). After incubation on ice, the
cells were lysed via sonication and centrifuged to obtain the
supernatant. The protein concentration was determined using a BCA
assay and equal amounts of protein were mixed with loading buffer
in a water bath for 5 min. Proteins were separated via
electrophoresis, which was initially performed at 80 V and then at
120 V until the bromophenol blue dye had migrated to ~1 cm away
from the lower edge of the gel. The proteins were subsequently
transferred to PVDF membranes, blocked at room temperature for 1 h
under gentle agitation and washed three times with TBS-Tween 20
(TBST) for 5 min each. The membranes were then incubated with the
following primary antibodies at room temperature for 2 h or
overnight at 4˚C: Rabbit anti-ERK1/2 (cat. no. ab184699; Abcam),
rabbit anti-phosphorylated (p)-ERK1/2 (cat. no. 4377T; Cell
Signaling Technology, Inc.), rabbit anti-p38 MAPK (cat. no. 8690T;
Cell Signaling Technology, Inc.), rabbit anti-p-p38 MAPK (cat. no.
4511T; Cell Signaling Technology, Inc.) and mouse HRP-conjugated
GAPDH (cat. no. HRP-6004; ProteinTech Group, Inc.). Following the
primary antibody incubation, the membranes were washed three times
with TBST for 5 min each time and incubated with HRP-conjugated
anti-mouse (cat. no. 7076P2; Cell Signaling Technology, Inc.) or
anti-rabbit (cat. no. 7074S; Cell Signaling Technology, Inc.)
secondary antibodies for 1 h at room temperature. Protein bands
were visualized using ECL reagent and densitometric analysis was
performed using ImageJ software (National Institutes of Health).
Multiple preliminary experiments were repeated with the conclusion
that the best phosphorylation reaction time was when chemerin was
added for 10 min, and the performance was most evident at this time
point.
Animal studies
The present study was approved by the Ethics
Committee of Guangxi Medical University (approval no. 20150303-12;
Nanning, China). In total, 30 Sprague Dawley rats were divided into
three groups: Groups a, b and ch. Osteoarthritis models were
created by modified Huths anterior cruciate ligament transection in
groups b and ch (17); anesthesia
was achieved with 2% pentobarbital sodium (40 mg/kg). Following
surgery, the rats were intraperitoneally injected with 200,000
units of penicillin for 3 consecutive days and permitted to roam
freely in the cage. Subsequently, 1 week after modeling, 20 rats
were randomly divided into two groups: In group ch, each knee joint
was injected with ~0.1 ml solution containing recombinant chemerin
once every 3 days for 3 weeks, while rats in group b were injected
with the same volume of normal saline. Rats in group a did not
undergo surgical modeling, and the knee joints were injected with
the same volume of normal saline at the same time as groups b and
ch. The housing environment for each group of rats was
identical.
Modeling test
After routine disinfection and draping, a
longitudinal incision was made in the medial parapatellar region of
the knee of the rat, followed by incision of the muscle (along the
direction of the incision) to avoid opening the joint capsule.
After cutting the muscle, a scalpel was used to separate the
muscles and subcutaneous tissues on both sides of the patella and
push the patella laterally. The purpose was to separate the patella
from the knee joint, destroy their fitted binding, and dislocate
the knee joint. Subsequently, the tissue near the joint capsule was
separated with ophthalmic scissors, and a transverse incision
perpendicular to the skin incision was made at the joint capsule
after fully exposing the capsule. After finding the anterior
cruciate ligament, the anterior cruciate and medial collateral
ligaments were cut. The joint capsule was opened, the medial
meniscus of the knee was located, and the muscles and tissues
around the medial meniscus were dissected with a scalpel and
ophthalmic scissors. Then, the cruciate ligament that fixes the
medial meniscus was cut off and the medial meniscus was removed.
Subsequently, the drawer test was performed. If the drawer test was
positive, it was considered that the model was successfully
established. The wound was washed with normal saline, and finally
each layer was sutured. Because the muscle at the joint capsule is
particularly weak, muscle sutures were made above and below it. The
surgical incision was disinfected with iodophor.
H&E staining
A total of 3 weeks post final chemerin or saline
injection, the rats were injected with an overdose of 1% sodium
pentobarbital (150 mg/kg) for euthanasia. The skin was incised
along the middle of the knee joint to expose the entire knee joint.
Then, an incision was made from the upper edge of the patella to
the femur, and the soft tissue was separated from the tibia with
ophthalmic scissors along both sides of the patella. The knee joint
cavity was opened, and forceps were used to excise the patella and
surrounding tissues. The synovial tissue of the patella that
continued downwards under the patella was removed and carefully cut
with ophthalmic scissors. Subsequently, the tissues were embedded
in paraffin, deparaffinized in xylene and rehydrated in a
descending series of ethanol [xylene (I) for 5 min, xylene (II) for
5 min, 100% ethanol for 2 min, 95% ethanol for 1 min, 80% ethanol
for 1 min, 75% ethanol for 1 min and distilled water for 2 min].
Then, using a H&E staining kit (cat. no. G1120; Beijing
Solarbio Science & Technology Co., Ltd.) the sections were
stained with hematoxylin solution for 10 min and rinsed with
autoclaved water. Following hematoxylin staining, the sections were
incubated with differentiating solution for 30 sec, washed with
warm water (~50˚C) for 5 min and stained with eosin dye solution
for 2 min, prior to undergoing a final rinse with autoclaved water.
Conventional ethanol dehydration was performed step by step [95%
ethanol (I) for 1 min, 95% ethanol (II) for 1 min, 100% ethanol (I)
for 1 min, 100% ethanol (II) for 1 min, two Toluene carbolic acid
(3:1) for 1 min, xylene (I) for 1 min and xylene (II) for 1 min],
and the sections were subsequently sealed with neutral resin,
covered with a coverslip and observed under a microscope. The above
method refers to relevant kits.
ELISA
Sprague Dawley rat synoviocytes were cultured for
three passages and then seeded into a 6-well plate at a density of
4x104 cells/well, which were cultured in a 37˚C constant
temperature incubator for 24 h. Following the incubation, the cells
were divided into three groups: i) Control group (group N); ii)
chemerin group (0.5 µg/ml chemerin; group C); and iii) inhibitor
group (pretreatment with 10 µM PD98059 for 1 h, then treatment with
0.5 µg/ml chemerin; group P). Following 48 h of treatment, the
supernatant was collected (300 x g; 5 min; 37˚C) to determine the
levels of MMP-3, MMP-13, TNF-α, IL-1β and IL-6 secreted by the
synoviocytes in each group using the corresponding ELISA kits,
according to the manufacturer's protocols.
In addition, the synovial tissue was excised from
rats according to the aforementioned method, added to sterile PBS
(1 g tissue per 10 ml PBS) and mashed. The suspension was
centrifuged at 1,000 x g for 10 min to obtain the supernatant,
which was used to measure the levels of MMP-3, MMP-13, TNF-α, IL-1β
and IL-6 according to the instructions of the respective ELISA
kits.
Statistical analysis
Statistical analyses were performed, and data are
presented as the mean ± SD. Statistical differences between more
than two groups were determined using one-way ANOVA with Tukey's
post hoc test, while differences between two groups were determined
using unpaired Student's t-test. SPSS software v25.0 (IBM Corp.)
was used for statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Chemerin regulates the viability of
synoviocytes in a dose-dependent manner
To determine the effect of chemerin on synoviocytes,
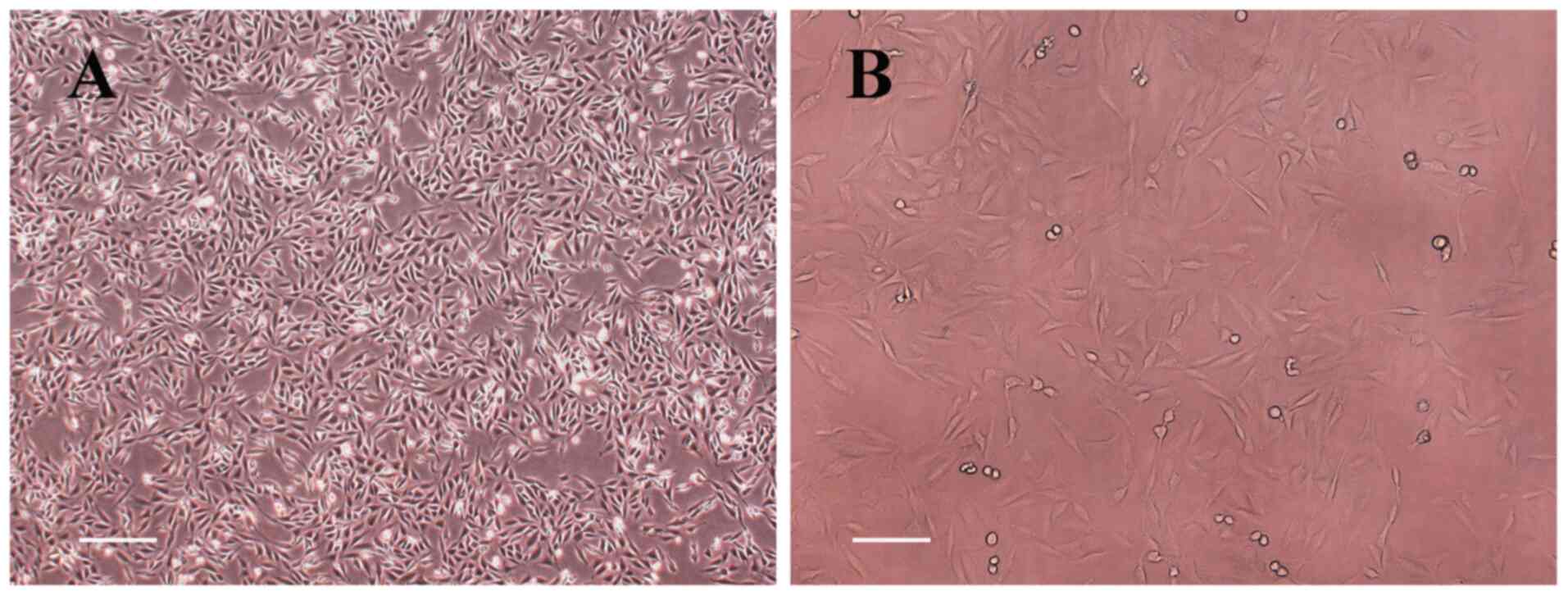
RSC-364 cells were used. The morphology of the cells was observed
and as expected, the cells were found to be elongated and polygonal
in shape (Fig. 1). Subsequently, a
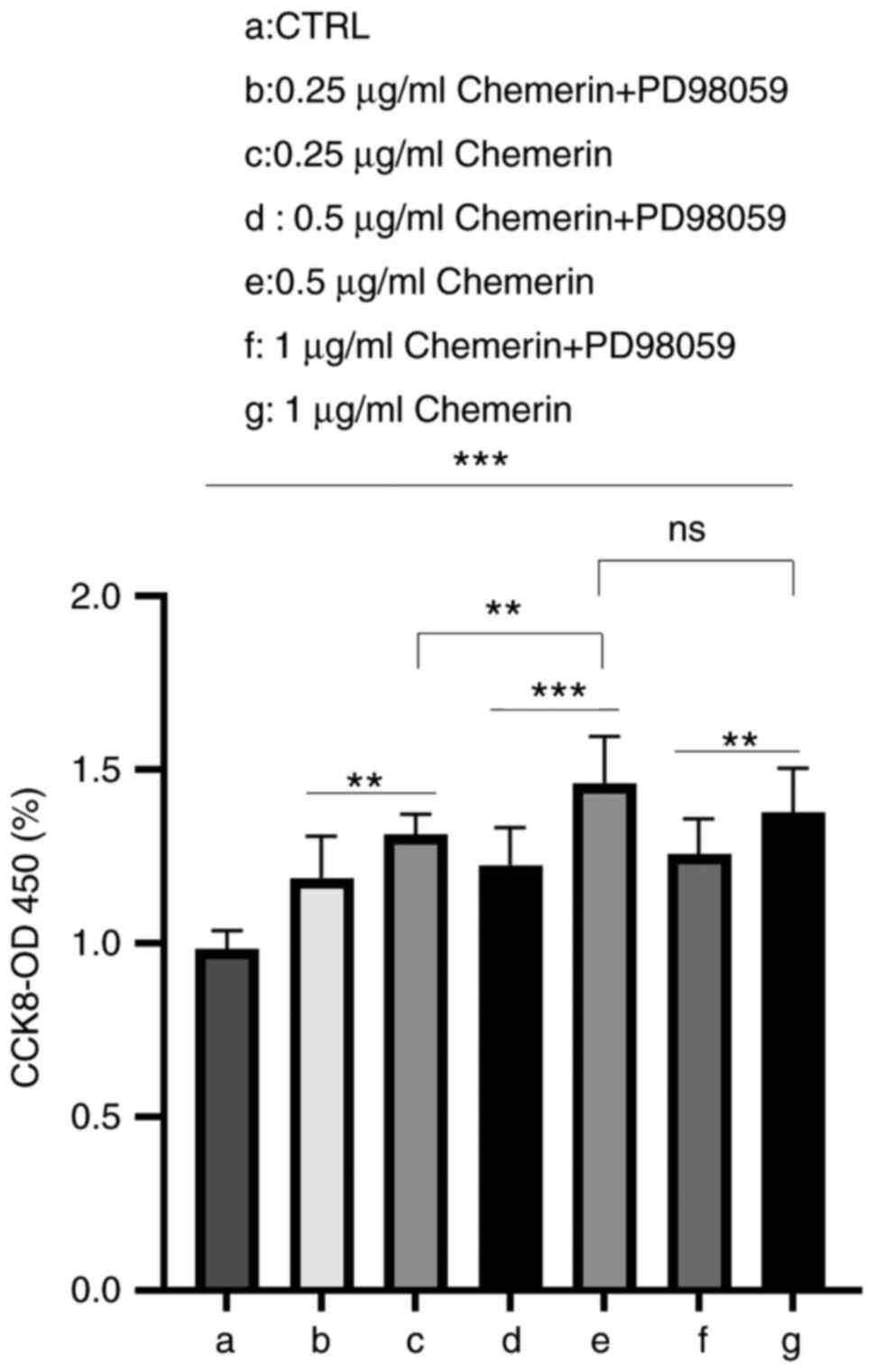
CCK-8 assay was performed following 24 h of treatment with
different concentrations of recombinant mouse chemerin. The results
revealed that after 24 h, chemerin increased viability, which could
be prevented by pretreating the cells with the MEK/ERK inhibitor,
PD98059 (Fig. 2). The difference
in the effects of chemerin at 24 h suggested that the effects of
chemerin may be time- and dose-dependent. In addition, the results
supported the use of 0.5 µg/ml chemerin as the optimal dose to
affect synoviocytes in a 24-h period to use in subsequent
experiments, and further indicated that the effect of chemerin may
be related to the MEK/ERK signaling pathway.
Chemerin promotes the activation of
the MAPK signaling pathways in synoviocytes
Based on the aforementioned findings and given the
important role of the MAPK signaling pathways in the pathogenesis
of OA (17,18), the present study next sought to
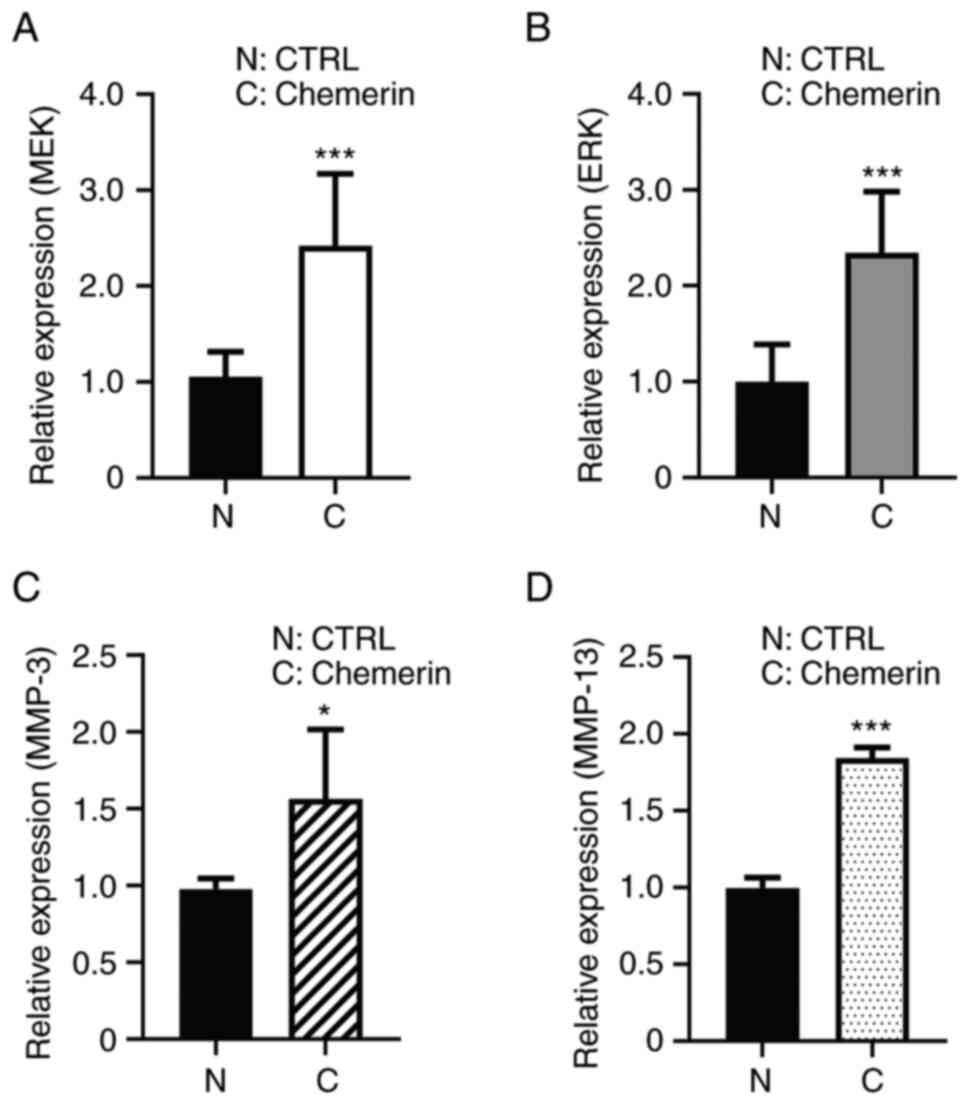
determine whether chemerin may regulate the expression of MAPKs.
After 48 h of incubation, the mRNA expression levels of MEK and ERK
were significantly upregulated in synoviocytes that were cultured
in the presence of 0.5 µg/ml recombinant mouse chemerin compared
with those observed in the control group (Fig. 3A and B). Furthermore, the mRNA expression
levels of MMP-3 and MMP-13 were also upregulated (Fig. 3C and D). MMP-3 and MMP-13 were examined by
RT-qPCR assays to investigate whether inflammatory factors were
expressed at the gene level and provide a corresponding basis for
subsequent ELISA assays.
MAPK activity is regulated by phosphorylation
(18). Therefore, the current
study also evaluated the levels of total and p-MAPK proteins after
exposing the synoviocytes to chemerin for 10 min. Western blotting
analysis demonstrated that the expression levels of ERK1/2,
p-ERK1/2, MEK, p-MEK and p-p38 MAPK were upregulated following
chemerin treatment, while the expression levels of total p38 MAPK
were unaltered (Fig. 4). These
findings are consistent with the mRNA expression levels observed
via RT-qPCR analysis and indicated that chemerin may regulate cell
viability by inducing ERK1/2 expression and p38 MAPK
phosphorylation.
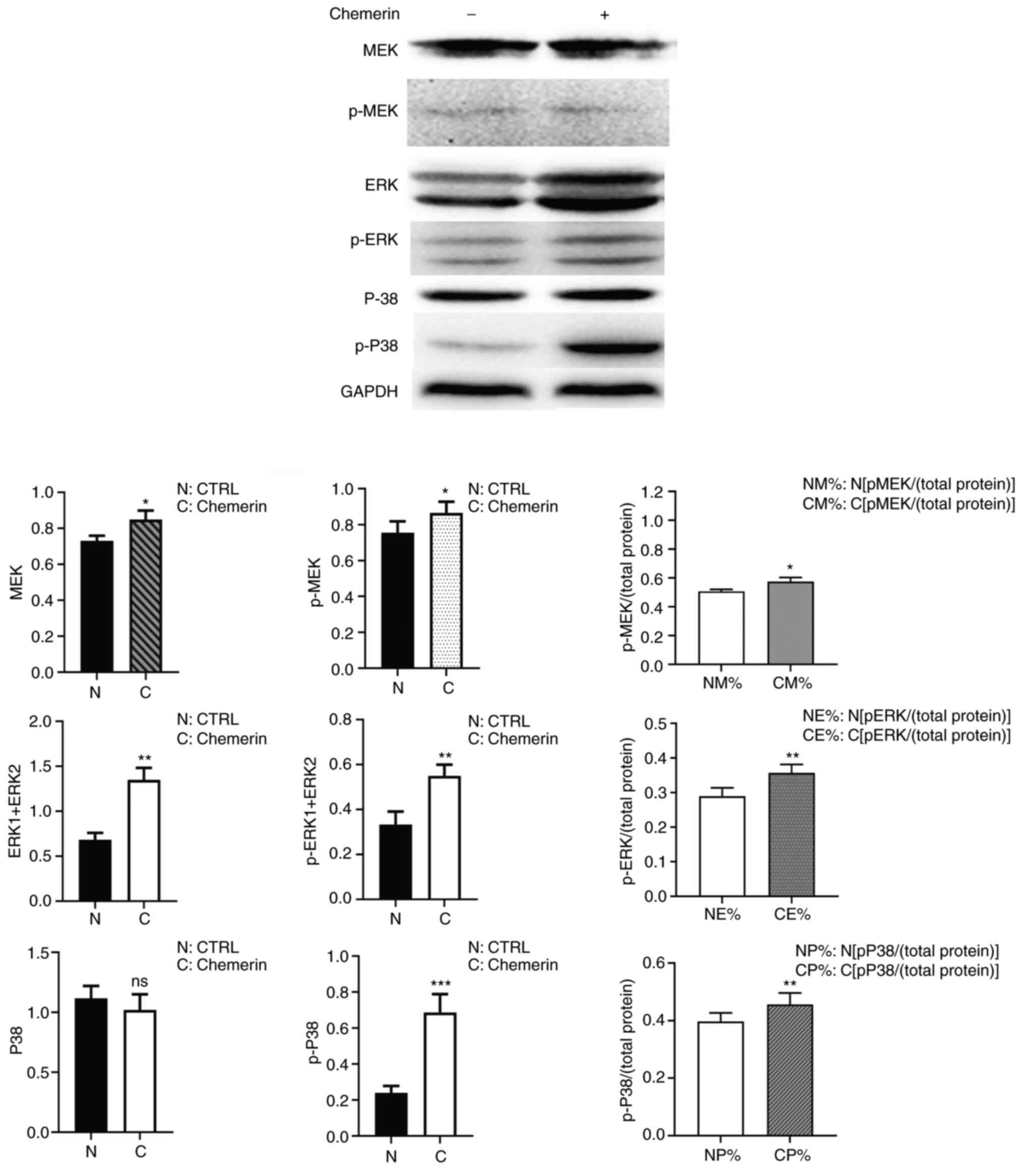 | Figure 4Chemerin upregulates the expression
and phosphorylation of MAPK signaling pathway-related proteins in
RSC-364 synoviocytes. Western blotting analysis was performed using
synoviocytes that were untreated or stimulated with 0.5 µg/ml
chemerin for 10 min (indicated by - and + symbols).
Semi-quantification of the expression levels is shown.
*P<0.05, **P<0.01 and
***P<0.001 indicate that the control group (N, NM%,
NE% and NP%) was compared with the respective treated group (C,
CM%, CE% and CP%). CTRL, control; p, phosphorylated; ns, not
significant; N, control synoviocytes; C, synoviocytes exposed to
0.5 µg/ml chemerin for 10 min. |
Chemerin upregulates the expression of
inflammatory cytokines in a MAPK-dependent manner
Given the role of synovial tissue inflammation in OA
progression (3,4), whether chemerin could modulate the
production of inflammatory mediators in synoviocytes and whether
MAPKs may be involved in this process was further investigated.
Cells were stimulated with chemerin for 48 h in the presence or
absence of the MEK/ERK pathway inhibitor, PD98059, and ELISAs were
performed to evaluate the concentrations of MMP-3, MMP-13, TNF-α,
IL-1β and IL-6 in the cell culture supernatants. The results
demonstrated that chemerin significantly increased the levels of
each of these inflammatory modulators (Fig. 5A-E), and that the increase in MMP-3
and MMP-13 was reduced in synoviocytes pretreated with PD98059
(Fig. 5D and E). The levels of TNF-α, IL-1β and IL-6
were also significantly reduced by PD98059 compared with the
chemerin group (Fig. 5A-C). These
results suggested the potential role of the MEK/ERK signaling
pathway in mediating the activation of MMPs, and potentially other
inflammatory mediators, in synoviocytes.
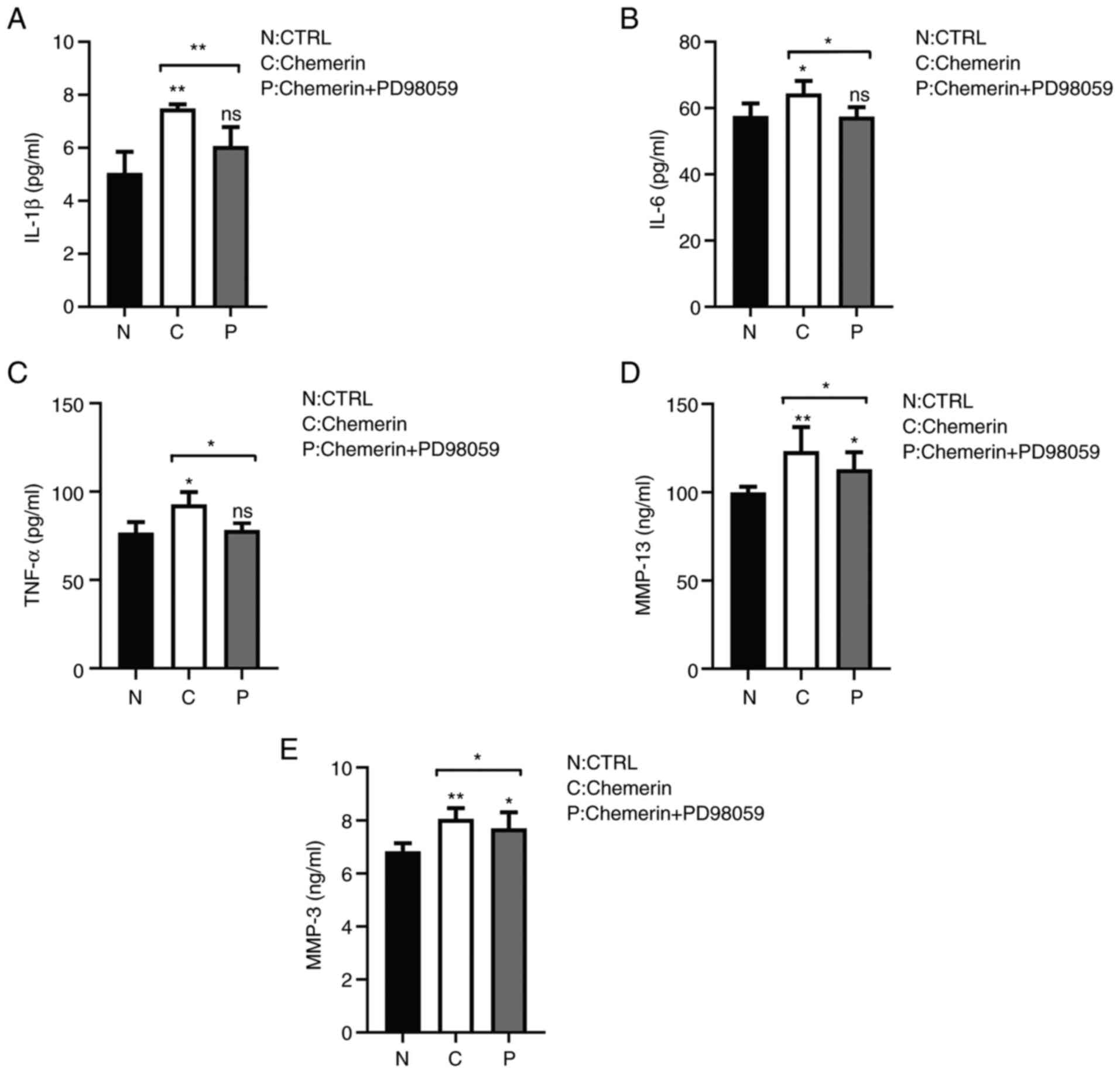 | Figure 5Chemerin enhances the secretion of
inflammatory factors in RSC-364 synoviocytes in a MAPK-dependent
manner. The levels of MMP-3, MMP-13, TNF-α, IL-1β and IL-6 in
synoviocyte supernatants were determined using ELISA after 48 h of
culture in the presence or absence of chemerin and the MEK
inhibitor, PD98059. Secretory levels of (A) IL-1β, (B) IL-6, (C)
TNF-α, (D) MMP-13 and (E) MMP-3 were increased compared with the N
group, and decreased following the addition of the inhibitor.
Semi-quantification of the expression levels is shown.
*P<0.05 and **P<0.001. CTRL, control;
ns, not significant; N, blank control group; C, chemerin group; P,
chemerin + inhibitor PD98059 group. |
Chemerin promotes synoviocyte
inflammatory hyperplasia and the release of inflammatory factors in
rats
To determine whether the effects of chemerin could
be observed in an in vivo model, rats were divided into
three groups: Group a (the blank group) did not receive surgery and
received injections of saline; group b (the control group)
underwent knee surgery to induce arthritis, followed by saline
injections; and group ch (the chemerin group) received knee
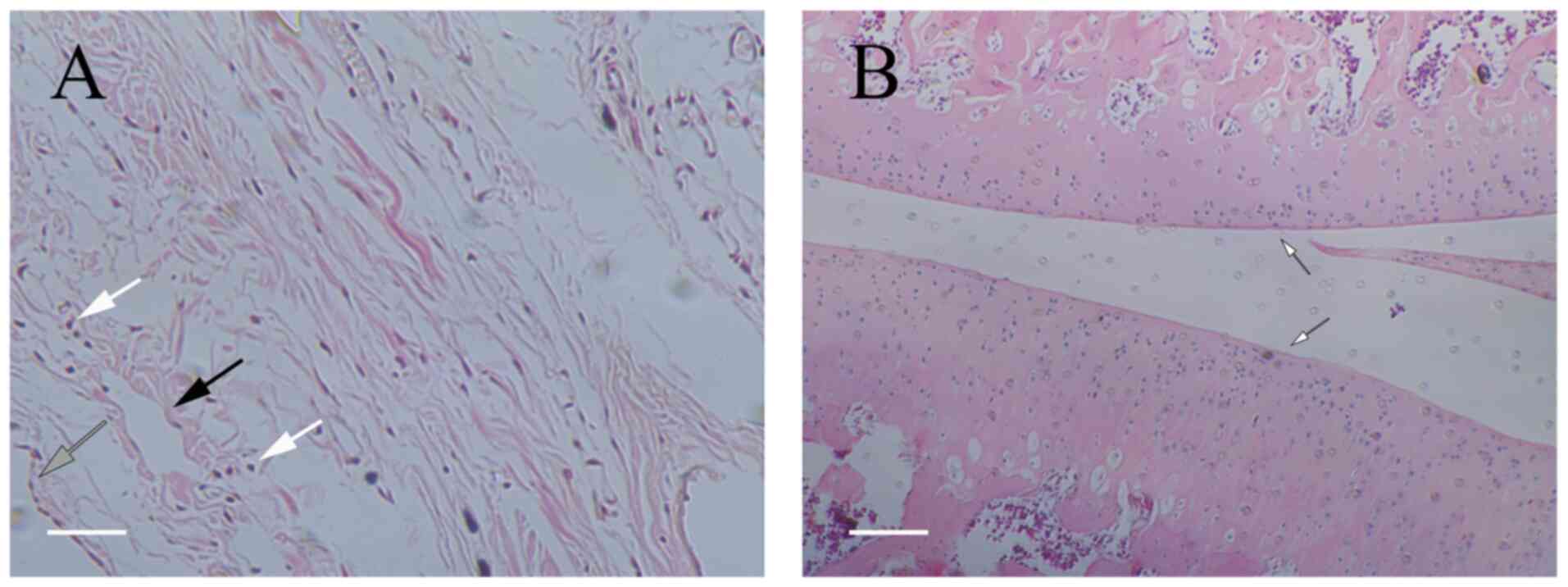
surgery, followed by chemerin injections. Observation of
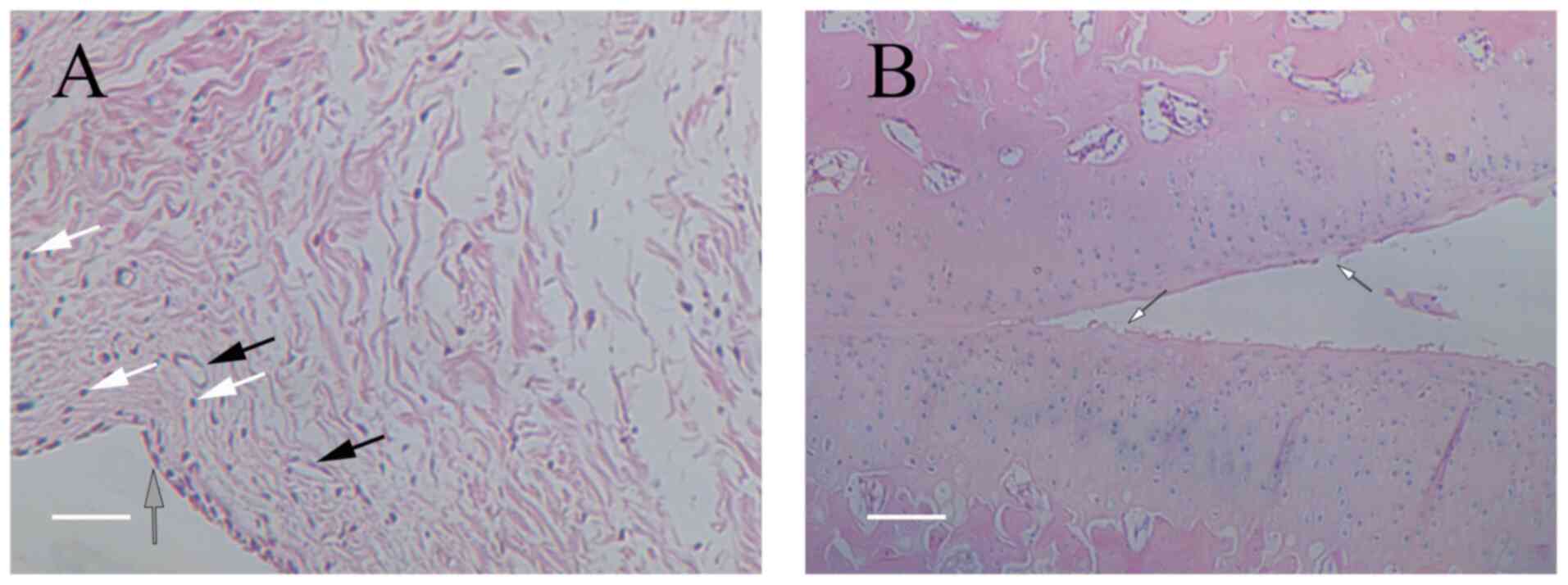
H&E-stained synovial tissue under a microscope revealed that
the tissue from the blank group contained one to two layers of
synoviocytes arranged regularly, which were mostly flat in shape
(Fig. 6). The tissue was
relatively loose, with a small number of capillaries and no evident
inflammatory cell infiltration; the cartilage surface was smooth
and flat, without damage or bone destruction, and no inflammatory
cell infiltration could be observed under the microscope. For the
control group (Fig. 7), the
synovial tissue was more hyperplasic compared with that of the
blank group. A small amount of synovial fibroblast proliferation
was observed, and inflammatory cell infiltration was identified in
the intercellular space. There was also slight cartilage
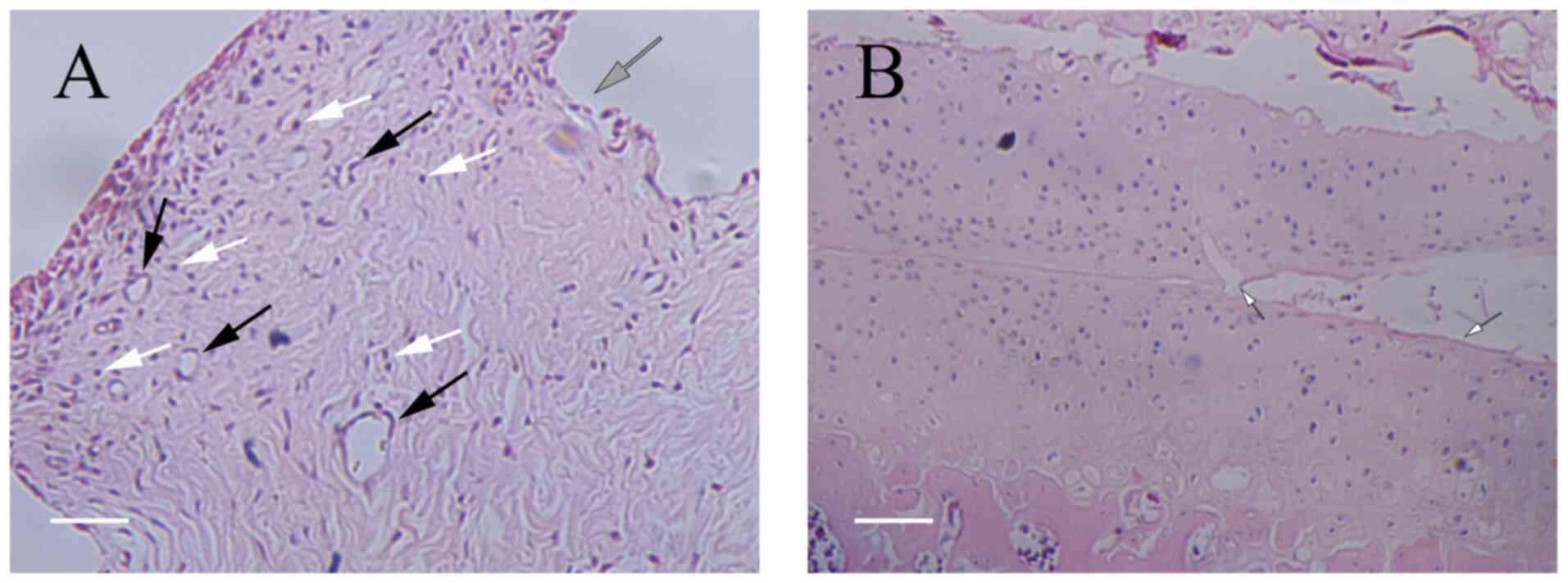
destruction, but no evident bone destruction. Finally, in the
chemerin group (Fig. 8), the
synovial tissue showed obvious signs of proliferation and an
increased infiltration of inflammatory cells (mostly lymphocytes)
compared with that of the control group. There was also obvious
vascular proliferation, and the continuity of the articular
cartilage surface was interrupted, with obvious cartilage
defects.
The results of the ELISAs revealed that recombinant
mouse chemerin also promoted the production of inflammatory
mediators in the synovial tissue. Compared with the control group,
each of the assayed inflammatory factors in the chemerin group was
significantly increased. In addition, when the blank and control
groups were compared, synovial inflammation was observed in the
control group; however, the degree of lesions and the levels of
inflammatory factors were decreased compared with the chemerin
group (Fig. 9). These results
suggested that chemerin may activate MMPs and potentially other
inflammatory mediators in synoviocytes.
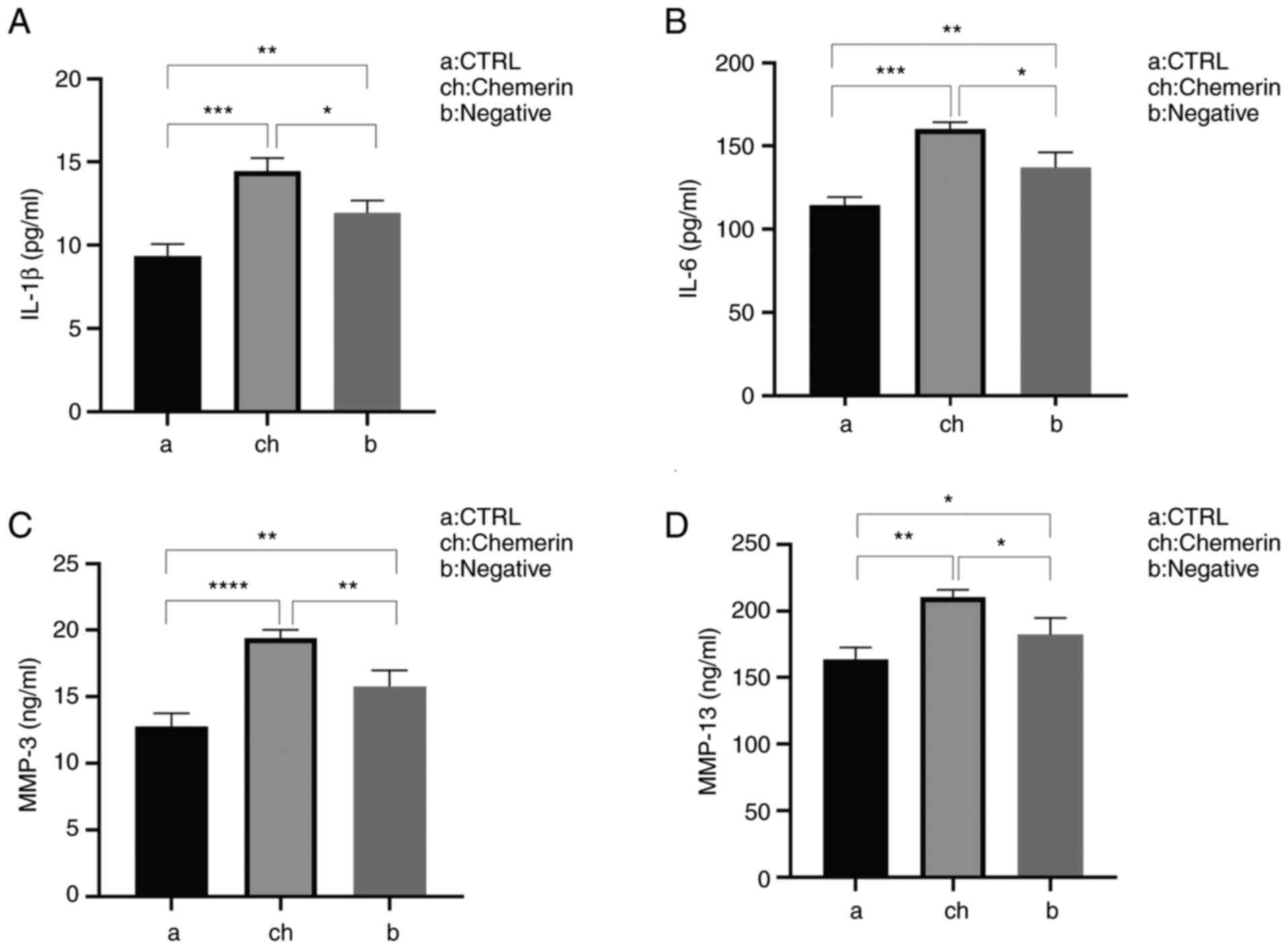 | Figure 9Inflammatory factor levels in rats
after knee surgery and/or chemerin injection, determined using
ELISA. Secretory levels of (A) IL-1β, (B) IL-6, (C) MMP-3 and (D)
MMP-13 were significantly increased in the ch group compared with
the a and b groups, but to the greatest extent compared with the a
group. *P<0.05, **P<0.01,
***P<0.001 and ****P < 0.001. CTRL,
control; a, blank (saline) group; ch, model (surgery + chemerin)
group; b, control (surgery + saline) group. |
Discussion
It has been demonstrated that chemerin is an
adipokine with chemotactic activity and its levels, as well as
those of other inflammatory factors, have been found to be higher
in obese individuals (20).
Chemerin was discovered to promote the secretion of TNF, IL-1β,
IL-6, MMP-1 and MMP-8 by chondrocytes, and these factors are known
to play an important role in the development of OA (21). Furthermore, chemerin has been
detected in the synovial fluid of patients with OA, and its
expression was found to be associated with the levels of
inflammatory factors (22).
Therefore, we hypothesized that chemerin may regulate the secretion
of inflammatory factors in synoviocytes.
The results of the present study provided direct
evidence that chemerin may promote the production of inflammatory
mediators in synoviocytes using ELISAs to determine the secretory
levels of a variety of inflammatory cytokines and chemokines,
including MMP-3, MMP-13, TNF-α, IL-1β and IL-6. A previous study
suggested that systemic MMP-13 may play a role in knee OA and may
be regulated by inflammatory signaling (23), thus supporting the relevance of the
findings of the present study. Furthermore, TNF-α, IL-1β and IL-6
have each been shown to increase the expression levels of the
receptor for chemerin, CMKLR1(24), which suggested the existence of a
positive feedback loop that may exacerbate inflammation in OA and
other inflammatory conditions. In addition, the current in
vivo experimental results demonstrated that chemerin promoted
synovial inflammatory hyperplasia and cartilage damage, and also
increased the number of inflammatory cells and inflammatory factors
secreted. While the pathogenesis of OA involves the degeneration of
fragments and particles of cartilage, inflammatory responses in the
synovium, including the release of inflammatory mediators by
synovial lesions, are known to aggravate articular cartilage
destruction, contributing to a vicious cycle that leads to disease
progression (25). Therefore, the
results of the present study provided evidence supporting a model
in which chemerin functionally interacts with inflammatory factors
and amplifies their ability to mediate cartilage degeneration.
MAPK signaling is also known to play an important
role in the pathogenesis of OA (25). In the present study, the results
indicated that chemerin activated MAPK expression in synoviocytes
at both the transcriptional and post-transcriptional level. In
addition, pretreatment with a MAPK pathway-specific inhibitor
attenuated the induction of MMP-3, MMP-13, TNF-α, IL-1β and IL-6 by
chemerin. These results indicated that chemerin may induce or
exacerbate the development of OA by regulating the MEK/ERK
signaling pathway, thereby affecting the secretion of inflammatory
factors by synoviocytes. Furthermore, animal osteoarthritis models
were established via the modified Huths anterior cruciate ligament
transection modeling method, which has been traditionally used for
the modeling of large animals and subsequently, small mammals,
including rabbits, rats and mice, which are now considered to be
the most widely used models (26,27).
This method has been found to induce events associated with OA in
the knee joint of animals, including synovial inflammation, changes
in chondrocyte physiology and destruction of the cartilage and
osteophyte formation. Using this model, the anterior cruciate
ligament is completely transected after direct observation through
arthrotomy (medial or lateral) or arthroscopic surgical methods,
causing joint instability, thereby leading to the occurrence of OA
(28).
In conclusion, the findings of the present study
suggested that chemerin may play an important role in
obesity-induced OA, and that it may exert its effect via the
activation of the MAPK signaling pathway in synoviocytes, which
subsequently leads to the production of inflammatory mediators that
cause cartilage degeneration. These results support the potential
of chemerin to serve as a biomarker of disease severity. In animal
experiments, preliminary experiments were repeated several times,
and it was finally concluded that chemerin injection should be
optimally performed every 3 days, while the third injection
response was the most pronounced. Since synovitis is most apparent
in the early stages of osteoarthritis, if the injection time is
prolonged and the joint damage is severe, synovitis will be
relatively attenuated. Furthermore, in the modified Huths model, in
order to make the joints wear and tear normally, the mice are
usually allowed to move after the model is completed. This is done
to model the wear and tear of joints in humans due to factors
associated with walking in daily life.
However, the current study has certain limitations.
Firstly, the study used surgical modeling to establish a rat OA
model, which to a certain extent differs from the pathogenesis of
human OA, therefore the effect of surgery-induced trauma cannot be
excluded. Secondly, the present study used Sprague Dawley rats as
the animal model, whose joints are small; thus, biomechanical
factors, such as body weight, will cause varying extents of joint
destruction, thereby leaving the potential for certain errors to
occur in the experimental results. Thirdly, regarding the
experimental design, the current study only focused on the effect
of MEK/ERK in the MAPK signaling pathway and the inflammatory
response in synoviocytes; therefore, the findings may only relate
to part of the mechanism of action of chemerin on the bone and
joints. The inflammatory factors measured were the main
inflammatory factors associated with synovitis, which have certain
hallmarks. However, these inflammatory factors are not
comprehensive, and subsequent related studies are required. In
addition, the upstream and downstream mechanisms affecting chemerin
expression were not investigated. Therefore, future studies will
aim to explore the upstream and downstream mechanisms affecting
chemerin and whether chemerin exerts an effect in OA through other
signaling pathways. Moreover, cartilage differentiation of stem
cells has been studied in regenerative medicine; however, whether
chemerin has an effect on this process currently remains unknown,
which is another direction of further research. Finally, following
the expansion of research into adipokines, it was found that they
may play an important role in obesity-induced OA; therefore,
adipokines may have the potential to be used as a biomarker to
reflect the severity of the disease, as well as help the clinical
monitoring and intervention of early OA in obese patients.
Altogether, adipokines may play an active role in improving the
current diagnosis and treatment of OA, which provides a novel
insight into the potential treatment strategies to render the early
prevention of OA more achievable, thereby prolonging the mechanics
of human autologous joints.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81660372), the Natural
Science Foundation of Guangxi Zhuang Autonomous Region (grant no.
2017GXNSFAA198159) and the Scientific Research Project of Guangxi
Zhuang Autonomous Region (grant no. AB19110030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL conceived the study. CW and SZ designed the
study. CW, SZ, LH and JL performed the data analysis. SL acquired
the funding, and SL and GD supervised the study. CW and SZ confirm
the authenticity of all the raw data. SL, CW, SZ, LH, JL, QZ and GD
helped to interpret the data, write and review the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of Guangxi Medical University (approval no.
20150303-12; Nanning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crema MD, Roemer FW, Marra MD and Guermazi
A: MR imaging of intra- and periarticular soft tissues and
subchondral bone in knee osteoarthritis. Radiol Clin North Am.
47:687–701. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Loeuille D, Rat AC, Goebel JC,
Champigneulle J, Blum A, Netter P, Gillet P and Chary-Valckenaere
I: Magnetic resonance imaging in osteoarthritis: Which method best
reflects synovial membrane inflammation? Correlations with
clinical, macroscopic and microscopic features. Osteoarthritis
Cartilage. 17:1186–1192. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ayral X, Pickering EH, Woodworth TG,
Mackillop N and Dougados M: Synovitis: A potential predictive
factor of structural progression of medial tibiofemoral knee
osteoarthritis-results of a 1 year longitudinal arthroscopic study
in 422 patients. Osteoarthritis Cartilage. 13:361–367.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Benito MJ, Veale DJ, FitzGerald O, van den
Berg WB and Bresnihan B: Synovial tissue inflammation in early and
late osteoarthritis. Ann Rheum Dis. 64:1263–1267. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Samad F, Badeanlou L, Shah C and Yang G:
Adipose tissue and ceramide biosynthesis in the pathogenesis of
obesity. Adv Exp Med Biol. 721:67–86. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Deng Y and Scherer PE: Adipokines as novel
biomarkers and regulators of the metabolic syndrome. Ann N Y Acad
Sci. 1212:E1–E19. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pita J, Panadero A, Soriano-Guillén L,
Rodríguez E and Rovira A: The insulin sensitizing effects of PPAR-γ
agonist are associated to changes in adiponectin index and
adiponectin receptors in Zucker fatty rats. Regul Pept. 174:18–25.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mariani F and Roncucci L: Chemerin/chemR23
axis in inflammation onset and resolution. Inflammation Res.
64:85–95. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wittamer V, Franssen JD, Vulcano M,
Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R,
Blanpain C, Detheux M, et al: Specific recruitment of
antigen-presenting cells by chemerin, a novel processed ligand from
human inflammatory fluids. J Exp Med. 198:977–985. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bluher M, Rudich A, Klöting N, Golan R,
Henkin Y, Rubin E, Schwarzfuchs D, Gepner Y, Stampfer MJ, Fiedler
M, et al: Two patterns of adipokine and other biomarker dynamics in
a long-term weight loss intervention. Diabetes Care. 35:342–349.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Stojek M: The role of chemerin in human
disease. Postepy Hig Med Dosw (Online). 71:110–117. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sitar-Taut AV, Coste SC, Tarmure S, Orasan
OH, Fodor A, Negrean V, Pop D, Zdrenghea D, Login C, Tiperciuc B
and Cozma A: Diabetes and obesity-cumulative or complementary
effects on adipokines, inflammation, and insulin resistance. J Clin
Med. 9(2767)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kaur J, Mattu HS, Chatha K and Randeva HS:
Chemerin in human cardiovascular disease. Vascul Pharmacol.
110:1–6. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang K, Du G, Li L, Liang H and Zhang B:
Association of chemerin levels in synovial fluid with the severity
of knee osteoarthritis. Biomarkers. 17:16–20. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kaneko K, Miyabe Y, Takayasu A, Fukuda S,
Miyabe C, Ebisawa M, Yokoyama W, Watanabe K, Imai T, Muramoto K, et
al: Chemerin activates fibroblast-like synoviocytes in patients
with rheumatoid arthritis. Arthritis Res Ther.
13(R158)2011.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Blüher M, Rudich A, Klöting N, Golan R,
Henkin Y, Rubin E, Schwarzfuchs D, Gepner Y, Stampfer MJ, Fiedler
M, et al: Two patterns of adipokine and other biomarker dynamics in
a long-term weight loss intervention. Diabetes Care. 35:342–349.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Saklatvala J: Inflammatory signaling in
cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use
of inhibitors for research into pathogenesis and therapy of
osteoarthritis. Curr Drug Targets. 8:305–313. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Loeser RF, Erickson EA and Long DL:
Mitogen-activated protein kinases as therapeutic targets in
osteoarthritis. Curr Opin Rheumatol. 20:581–586. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Buechler C, Feder S, Haberl EM and
Aslanidis C: Chemerin isoforms and activity in obesity. Int J Mol
Sci. 20(1128)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huss RS, Huddleston JI, Goodman SB,
Butcher EC and Zabel BA: Synovial tissue-infiltrating natural
killer cells in osteoarthritis and periprosthetic inflammation.
Arthritis Rheum. 62:3799–3805. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Valcamonica E, Chighizola CB, Comi D, De
Lucia O, Pisoni L, Murgo A, Salvi V, Sozzani S and Meroni PL:
Levels of chemerin and interleukin 8 in the synovial fluid of
patients with inflammatory arthritides and osteoarthritis. Clin Exp
Rheumatol. 32:243–250. 2014.PubMed/NCBI
|
|
23
|
Ruan G, Xu J, Wang K, Wu J, Zhu Q, Ren J,
Bian F, Chang B, Bai X, Han W and Ding C: Associations between knee
structural measures, circulating inflammatory factors and MMP13 in
patients with knee osteoarthritis. Osteoarthr Cartil. 26:1063–1069.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mengshol JA, Vincenti MP, Coon CI,
Barchowsky A and Brinckerhoff CE: Interleukin-1 induction of
collagenase 3 (matrix metalloproteinase 13) gene expression in
chondrocytes requires p38, c-jun N-terminal kinase, and nuclear
factor κB: Differential regulation of collagenase 1 and collagenase
3. Arthritis Rheum. 43:801–811. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rogart JN, Barrach HJ and Chichester CO:
Articular collagen degradation in the Hulth-Telhag model of
osteoarthritis. Osteoarthritis Cartilage. 7(539)1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kuyinu EL, Narayanan G, Nair LS and
Laurencin CT: Animal models of osteoarthritis: Classification,
update, and measurement of outcomes. J Orthop Surg Res.
11(19)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kamekura S, Hoshi K, Shimoaka T, Chung U,
Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K and
Kawaguchi H: Osteoarthritis development in novel experimental mouse
models induced by knee joint instability. Osteoarthritis Cartilage.
13:632–641. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
McCoy AM: Animal models of osteoarthritis:
Comparisons and key considerations. Vet Pathol. 52:803–818.
2015.PubMed/NCBI View Article : Google Scholar
|