Introduction
Long-distance running has become increasingly
popular and participation in marathons and ultra-marathons (longer
than the traditional marathon, usually 50-100 km) is also
increasing. It is estimated that 349,000 individuals in Europe and
414,000 individuals in North America race in marathons annually
(1). Running is considered a
favorable exercise for the cardiovascular system, and
epidemiological research has demonstrated that 1 h of running
extends life expectancy by 7 h (2).
On the other hand, running has a considerable effect
on myocardial morphology. Running long distances as part of a daily
routine leads to cardiovascular adaptations, which are crucial both
for the conditioning of the cardiovascular system and enhancing
running performance.
Cardiovascular adaptations to exercise are relevant
to the specific sports and in the case of long-distance running,
the reported cardiovascular remodeling primarily involves the
increase in the bi-ventricular diameter, left ventricular (LV)
myocardial thickness, LV mass and the volume of both atria, while
systolic and diastolic function remain intact. The aforementioned
changes are considered to be more pronounced in highly trained
individuals (3). Recent studies
have demonstrated that at an early stage of long-distance training
and particularly during the first marathon, runners present mostly
concentric biventricular remodeling, which has been found to be
less pronounced than it was previously considered (1,4,5).
Eccentric LV hypertrophy is considered to occur later, probably
even after years of training.
At the same time, significant changes regarding
inflammatory markers are observed in marathon and ultra-marathon
runners compared with sedentary individuals and at individual
levels during the race (pre and post). Exercise is associated with
temporal muscle damage, which in turn is the origin of local
inflammation. Leucocytes are accumulated and a systemic
inflammatory response is induced (6). The systemic inflammatory response
comprises leukocytosis and an acute-phase response (4-7),
which is characterized by the increased release of cortisol,
adrenocorticotropic hormone, cytokines and acute phase proteins,
such as C-reactive protein (CRP).
Analogous to inflammatory markers, marathon runners
exhibit evidence of endothelial function alterations. Nitric oxide
(NO) handling appears to be the main topic of research in marathon
runners.
The aim of the present study was to describe the
cardiovascular adaptations of a group of ultra-marathon runners
along with the measurements of inflammatory and endothelial
function indices, and to further determine the predictive ability
of these markers as regards cardiovascular adaptation in
ultra-marathon runners.
Patients and methods
Study subjects
A total of 43 ultra-marathon runners were assessed
by echocardiography at rest (at least 2 days after a training
session or race). Runners were interviewed by the attending
physician/cardiologist regarding their training sessions, and the
kilometres run per week and their dietary habits, as well as a full
medical history were recorded. All subjects were non-smokers, had
no medical history of hypertension and were not receiving
anti-hypertensive or anti-inflammatory medication. All participants
were subjected to measurements of height and body weight (BW) and
transthoracic echocardiography. All athletes consented to undergo
an assessment of body composition and treadmill exercise
testing.
A written informed consent to participate in the
study was provided by all participants involved. The procedures
were in accordance with the Helsinki Declaration of 1975 and
approval was received by the Human Subjects Committee of the
University of Thessaly, Larissa, Greece and the General Hospital of
Giannitsa (Giannitsa, Greece), where all medical practices were
conducted (7).
In the morning and after resting in the supine
position for at least 30 min, fasting venous blood samples were
drawn from all runners enrolled in this study, centrifuged
(1,000-2,000 x g for 10 min, 4-7˚C) within 30 min from collection
and stored at -20˚C. Tumor necrosis factor-α (TNF-α), interleukin
(IL)-6) and IL-10 levels were measured using the
IMMULITE® 1000TNF-α (sensitivity, 1.7 pg/ml; upper limit
of the working range, 1,000 pg/ml; mean intra-assay variation,
3.2%), the IMMULITE® 1000IL-6 (sensitivity, 2 pg/ml;
upper limit of the working range, 1,000 pg/ml; mean intra-assay
variation, 4.65%) assays (Siemens) and the human high sensitivity
HS IL-10 solid-phase sandwich ELISA kit (analytical sensitivity,
0.05 pg/ml; assay range, 0.39-25.0 pg/ml; intra-assay variation,
6.8%) from Thermo Fischer Scientific, Inc. Asymmetric
dimethylarginine (ADMA) levels were measured using an ADMA-ELISA
kit (DLD Diagnostika GMBH) (sensitivity, 0.05 µmol/l; upper limit
of the working range, 5.0 µmol/l; mean intra-assay variation,
6.05%). CRP levels were determined using immunoturbidimetry and
creatine phosphokinase (CPK) levels using the N-acetylcysteine
(CK-NAC) method (340 nm). For the two latter assays, the COBAS
INTEGRA 400 automated system by Roche Diagnostics, Inc. and all
relevant diagnostic reagents of the same company were used
(8). Oxidative stress values have
already been previously published by the authors in the framework
of the assessment of the effects of nutrient supplementation on the
pathophysiological profile of marathon runners (7).
Echocardiography
Two experienced cardiologists-ultrasonographers
performed the transthoracic echocardiographic examination using
commercially available ultrasound systems (Vivid I; GE Medical)
with a 1.5 to 4 MHz phased-array transducer. The same echo settings
and acquisition protocols were applied. All images were
subsequently analyzed in a random order in order to avoid bias.
M-mode echocardiography or 2D images were used to calculate left
heart dimensions (9). LV mass was
calculated using the Devereux formula (9) and was then indexed to the calculated
body surface area using the Mosteller formula (10). The LV ejection fraction (LVEF) was
estimated using Simpson's biplane approach (9) Right heart dimensions were obtained as
previously described by Rudski et al (11). The frame rate for tissue Doppler
(TDI) measurements was >100/sec. The transmitral pw-Doppler
inflow at the tips of the mitral leaflets was measured to obtain E
wave velocity (12). TDI
measurements were assessed in the apical 4-chamber view. Peak early
diastolic (E΄), late diastolic (A΄) and systolic (S΄) velocities
were measured at the basal septum (13). In order to assess the global
myocardial function of each chamber, the LV and right ventricular
(RV) myocardial performance index were determined (14,15).
The tricuspid annular longitudinal velocity of excursion (RV S΄)
was assessed using pulsed-wave TDI placed in the tricuspid annulus
(11).
Treadmill exercise test
All runners were submitted to the exercise stress
test on the day of their echocardiography examination. Treadmill
exercise testing was performed until exhaustion on a treadmill with
the use of an ergometer (Ultima Series, Medgraphics, Ltd.) applying
the Bruce protocol (16). Exercise
duration, metabolic equivalents of stress test, maximum heart rate,
heart rate change at the first minute of exercise and heart rate
recovery (HRR) time were recorded. The Athens QRS score was also
calculated for each stress test (16).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). Statistical analyses were performed with SPSS
version 23 (IBM Corp.). Independent t-tests were used to compare
mean values between groups. Logistic regression analysis was
performed to investigate the association between monitored
biochemical parameters and myocardial adaptations. Differences
between categorical variables were assessed using the Chi-squared
test. A P-value <0.05 was considered to indicate a statistically
significant difference. ROC curves were constructed for the
evaluation of the predictive ability of the pro-inflammatory and
anti-inflammatory biomarkers studied in predicting runners'
myocardial adaptations to exercise.
Results
The demographic and specific training
characteristics of the study population along with the inflammatory
indices, biochemical markers and oxidative stress levels are
presented in Table I. The
echocardiographic parameters and the main indices of the treadmill
exercise tests the marathon runners underwent are presented in
Table II.
 | Table IDemographics, specific training
characteristics and biochemical/oxidative stress indices of the
study population. |
Table I
Demographics, specific training
characteristics and biochemical/oxidative stress indices of the
study population.
| Parameter | No. of subjects or
average ± standard deviation value |
|---|
| Sex | |
|
Male | 39 |
|
Female | 4 |
| Age,
yearsa | 44.9±11.3 |
| Body surface area
(BSA, m2)a | 1.89±1.7 |
| Weight
(kg)a | 74.7±10.1 |
| Height
(cm)a | 176.3±8 |
| Training experience
(years)a | 7.2±5.3 |
| Training
(/week)a | 61.9±26 |
| Training
sessions/weeka | 4.4±1.3 |
| Smoking | |
|
No | 40 |
|
Yes | 3 |
| IL-6
(pg/ml)a | 1.05±0.15 |
| TNF-a
(pg/ml)a | 15.5±1.38 |
| IL-10
(pg/ml)a | 2.06±0.22 |
| CRP
(mg/l)a | 1.77±0.35 |
| CPK
(mg/dl)a | 212.1±34.93 |
| ADMA
(µmol/ml)a | 1.01±0.09 |
| GSH
(µmol/l)a,b | 30.6±11.5 |
| Carbonyls (nmol/g
protein)a,b | 0.68±0.18 |
| TBARS
(µmol/l)a,b | 6.95±1.36 |
| TAC (mmol
DPPH/l)a,b | 0.97±0.12 |
 | Table IIEchocardiographic parameters and the
main indices of the treadmill exercise tests of the marathon
runners of the study population. |
Table II
Echocardiographic parameters and the
main indices of the treadmill exercise tests of the marathon
runners of the study population.
| Parameter | Value |
|---|
| Echocardiographic
measurementsa | |
|
LV end
diastolic diameter (mm) | 52.9±4.83 |
|
LV Septal
thickness (mm) | 8.93±1.84 |
|
Posterior LV
wall diameter (mm) | 10.7±1.93 |
|
RWT | 0.380±0.100 |
|
LV EDV
(ml) | 97.5±32.3 |
|
LV EDV
indexed (ml/m2) | 51.4±15.1 |
|
LV mass
(g) | 244±56.3 |
|
LV mass
indexed (g/m²) | 128±25.3 |
|
Male | 132±21.9 |
|
Female | 91.9±32.2 |
|
LA volume
(ml) | 49.8±16.9 |
|
Left atrial
volume index (LAVI) (ml/m2) | 26.2±8.94 |
|
RV end
diastolic mid diameter (mm) | 36.3±5.24 |
|
RA volume
(ml) | 48.6±19.8 |
|
LV EF
(%) | 74.7±8.33 |
|
LV MPI | 0.370±0.0600 |
|
RV MPI | 0.390±0.110 |
|
Early mitral
inflow velocity (E) (cm/sec) | 0.890±0.250 |
|
Lateral
mitral e΄(cm/sec) | 13.6±2.63 |
|
E/a
ratio | 2.07±0.500 |
|
E/e΄
ratio | 6.63±2.14 |
|
RV TDI S
wave (cm/sec) | 15.8±2.24 |
| Treadmill exercise
test measurements1 | |
|
Exercise
stress test duration (sec) | 19.5±4.13 |
|
Heart rate
at rest (beats/min) | 71.1±12.3 |
|
Maximum
heart rate (beats/min) | 175±14.6 |
|
Heart rate
increase in the 1st minute of exercise (beats) | 19.7±7.81 |
|
Heart rate
recovery (beats) | 47.3±16.4 |
|
Metabolic
equivalents (METS) | 18.1±5.62 |
|
Athens QRS
score | 10.9±6.32 |
Ultra-marathon runners, who presented with augmented
LV end-diastolic diameters (9)
>55 mm, had higher ADMA values (1.07±0.07 vs. 0.99±0.08 µmol/ml,
P<0.01) and lower CPK values (192.5±21.3 vs. 219.1±37.3 mg/dl,
P<0.05) compared with those with normal LV diameters. In
addition, runners with increased absolute LV mass values >225 g
presented with higher TNF-α values compared with runners with a
normal LV mass (15.9±1.40 vs. 14.7±1.02 pg/ml, P<0.05). Table III presents the levels of
inflammatory and endothelial dysfunction markers in ultra-marathon
runners according to the presence of abnormal LV diameter or mass.
Runners with ‘abnormal’ LV diastolic volumes >155 ml exhibited
lower CPK values compared with runners with normal LV diastolic
volumes (197±10.1 vs. 222±35.4 mg/dl, P<0.05). As regards the LV
volume, runners with increased volumes followed more training
sessions per week and covered more kilometers on a weekly basis,
compared with runners with normal LV volumes (5.3±1.1 vs. 3.8±1.2
sessions/week, P<0.05 and 78.8±22.5 vs. 57.2±27.2 km/week,
P<0.05, respectively). Finally, runners with augmented left
atrium (LA) volumes >58 ml presented lower IL-10 values compared
with runners with normal left atrial volumes (1.97±0.17 vs.
2.14±0.23 pg/ml, P<0.05).
 | Table IIIInflammatory and endothelial
dysfunction markers (presented as the average ± standard deviation)
in ultra-marathon runners with abnormal LV diameter or mass. |
Table III
Inflammatory and endothelial
dysfunction markers (presented as the average ± standard deviation)
in ultra-marathon runners with abnormal LV diameter or mass.
| | Abnormal LV
diameter (>55 mm) | Abnormal LV mass
(>225 g) |
|---|
| Parameter | Yes | No | P-value | Yes | No | P-value |
|---|
| No. of
subjects | 11 | 28 | | 27 | 12 | |
| Sex (n) | | | | | | |
|
Male | 11 | 24 | 0.186a | 27 | 8 |
0.002a |
|
Female | 0 | 4 | | 0 | 4 | |
| IL-6 (pg/ml) | 1.06±0.12 | 1.02±0.16 | 0.47b | 1.02±0.10 | 1.07±0.23 | 0.33b |
| IL-10 (pg/ml) | 2.11±0.14 | 2.05±0.25 | 0.40b | 2.05±0.19 | 2.10±0.30 | 0.54b |
| TNF-α (pg/ml) | 15.6±1.61 | 15.6±1.33 | 0.87b | 15.9±1.40 | 14.7±1.02 |
0.02b |
| ADMA (µmol/ml) | 1.07±0.07 | 0.99±0.08 |
0.005b | 1.02±0.08 | 1.00±0.09 | 0.49b |
| CRP (mg/l) | 1.80±0.31 | 1.77±0.38 | 0.83b | 1.71±0.35 | 1.94±0.32 | 0.07b |
| CPK (mg/dl) | 192±21.3 | 219±37.2 |
0.033b | 209 ±30.6 | 217±45.6 | 0.6b |
| Age (years) | 47.3±7.4 | 41.9±10.4 | 0.12b | 46.0±9.32 | 37.7±9.00 |
0.01b |
In the present study, runners with an abnormal RV
diameter (17) (mid RV segment)
>34 mm were those who had been training for a greater number of
years, covering more kilometers per week and following more
training sessions per week (9.5±7.4 vs. 4.8±3.3 training years,
P<0.05; 69.7±29.6 vs. 52.3±15.55 km/week, P<0.05; 4.7±1.5 vs.
3.7±0.7 training sessions/week, P<0.01, respectively). The
runners' diet habits, characteristics of exercise stress test (such
as exercise duration, heart rate change in the first minute of
exercise or heart rate recovery time in the first minute of
recovery-HRR), were not associated with alterations in RV
dimensions. Specifically, bread daily consumption did not differ in
marathon runners with an abnormal RV diameter compared to runners
with normal values (2.7±1.5 portions vs. 2.5±1.6 portions, P=0.49),
nor carbohydrates consumption (4.8±4.4 vs. 5.2±6.1 portions per
week, P=0.82), nor meat consumption (2.6±1.5 vs. 2.3±0.8 portions
per week, P=0.46). Exercise test duration did not differ between
athletes with an abnormal RV diameter compared to runners with
normal values (20.5±4.4 vs. 18.3±2.2 min, P=0.1) nor did heart rate
change in the first minute of exercise (18.9±7.7 vs. 21.1±7.1beats,
P=0.41), nor did HRR (46.2±15.1 vs. 52.8±18.8 beats, P=0.24).
HRR in an exercise maximum stress test is a reliable
marker of the balance between parasympathetic and sympathetic
nervous system and is associated with years of physical exercise
and good cardiorespiratory fitness. Runners usually tend to have
better HRR values compared with individuals who lead a sedentary
lifestyle (18). In the present
study, ‘normal’ HRR values were considered as >35 beats
according to the findings of Mann et al (19). The present study found that runners
with lower than usual HRR values (<35 beats) had lower exercise
duration at the exercise stress test compared with runners with
usual HRR (17.6±2.2 min vs. 20.5±4.6 min, P<0.05), while they
did not differ in years of training, kilometers run or training
sessions per week.
The majority (72%) of ultra-marathon runners of the
present study presented an abnormal LV mass indexed to BSA >115
g/m2 (9). Runners with
abnormal LV mass indexed did not present statistically significant
differences in IL-10, IL-6, TNF-A, ADMA, CPK, CRP levels compared
with runners with normal LV mass indexed. However, runners with
moderate and severe abnormal indexed LV mass >131
g/m2 had statistically significant higher TNF-α values
compared with runners with mildly elevated and normal LV mass
indexed (16.2±1.42 vs. 14.0+1.16 pg/ml, P<0.05).
Runners with an abnormal left atrial volume index
(9) (LAVI) >29 ml/m2
had higher IL-6 values compared with runners with a normal LAVI
(1.09+0.19 vs. 0.99±0.08 pg/ml, P<0.05).
Elite marathon runners of the present study, as
defined according to their training status (i.e., kilometers
covered per week >55) (20),
presented specific differences from the remaining marathon runners
with a less intense training program (<40 km/week). Elite
runners were characterized by higher RV end-diastolic diameters
(37.8±5.6 vs. 33.2±3.4 mm, P=0.009), right atrium volume (52±23.3
vs. 39.6±9.7 ml, P=0.045) and a lower maximum heart rate achieved
at the treadmill stress test (171.3±11.9 vs. 190.2±12.5 beats/min,
P<0.001). However, they did not present any significant
differences in the biochemical or oxidative stress indices measured
in the present study.
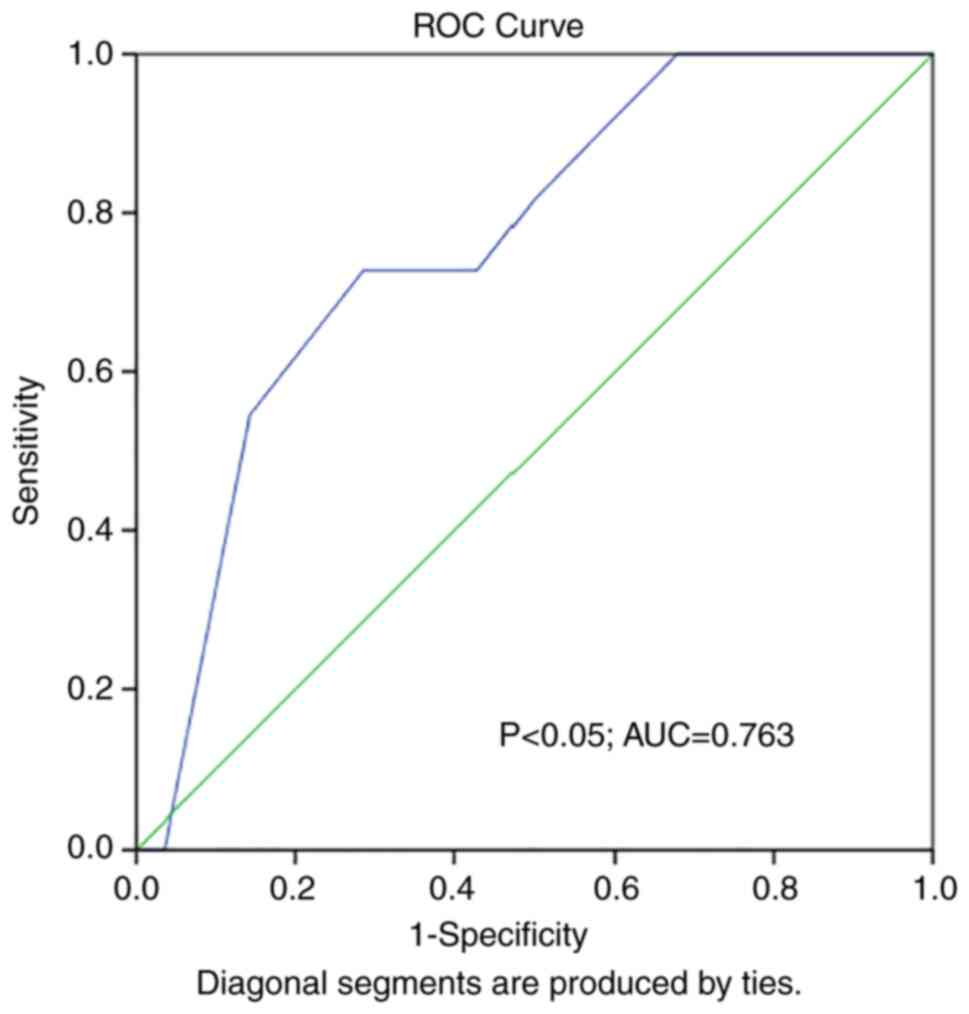
ROC curves were constructed to evaluate the ability
of ADMA to predict the presence of abnormal LV diameter in marathon
runners. ROC curve analysis revealed statistical significance
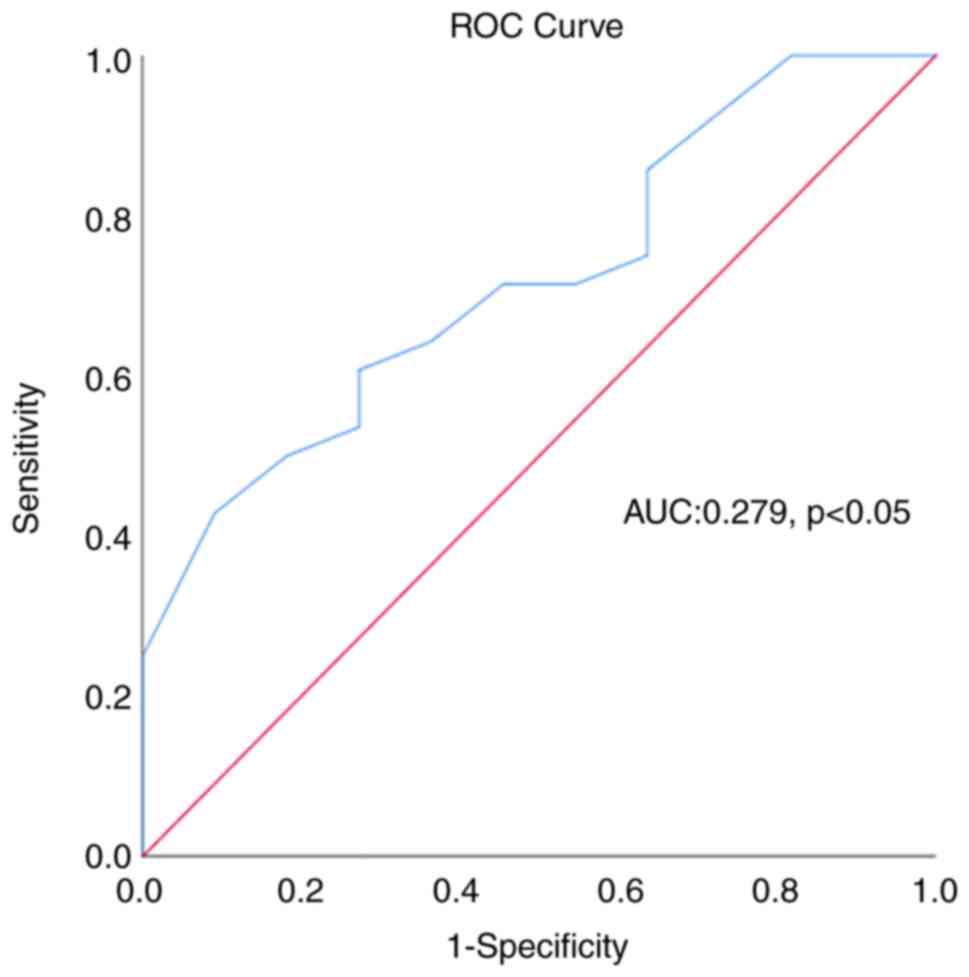
[P<0.05; area under the curve (AUC), 0.763] (Fig. 1). At the same time, ROC curve
analysis for the predictive ability of CPK regarding the presence
of an augmented LV diameter in marathon runners also yielded
statistical significance (P<0.05), although with a low
predictive ability (AUC: 0.28) (Fig.
2).
Logistic regression analysis was performed for the
prediction of the presence of an abnormal LV diameter in
echocardiography in ultra-marathon runners. The model presented
statistical significance (P<0.001, Chi-squared=25.8) and could
explain 69.6% of the variance of the abnormal LV diameter presence
(Nagelkerke R Square) and correctly classify 87% of the athletes.
As shown in Table IV, IL-6, ADMA,
height and age, but not IL-10, significantly contributed to the
model.
 | Table IVLogistic regression analysis for the
prediction of the presence of abnormal LV diameter as detected by
echocardiography in ultra-marathon runners. |
Table IV
Logistic regression analysis for the
prediction of the presence of abnormal LV diameter as detected by
echocardiography in ultra-marathon runners.
| | 95% CI OR |
|---|
| Parameter | B | SE | Wald
χ2 | df | P-value | OR | Ll | UL |
|---|
| IL-6 (pg/ml) | -9.61 | 4.71 | 4.16 | 1 | 0.041 | 0.00 | 0.00 | 0.68 |
| IL-10 (pg/ml) | -5.96 | 3.68 | 2.62 | 1 | 0.105 | 0.01 | 0.00 | 3.50 |
| Height (cm) | -0.40 | 0.16 | 6.27 | 1 | 0.012 | 0.66 | 0.49 | 0.91 |
| Age (years) | -0.14 | 0.07 | 3.91 | 1 | 0.048 | 0.86 | 0.75 | 0.99 |
| ADMA (µmol/ml) | -25.55 | 11.05 | 5.34 | 1 | 0.021 | 0.00 | 0.00 | 0.02 |
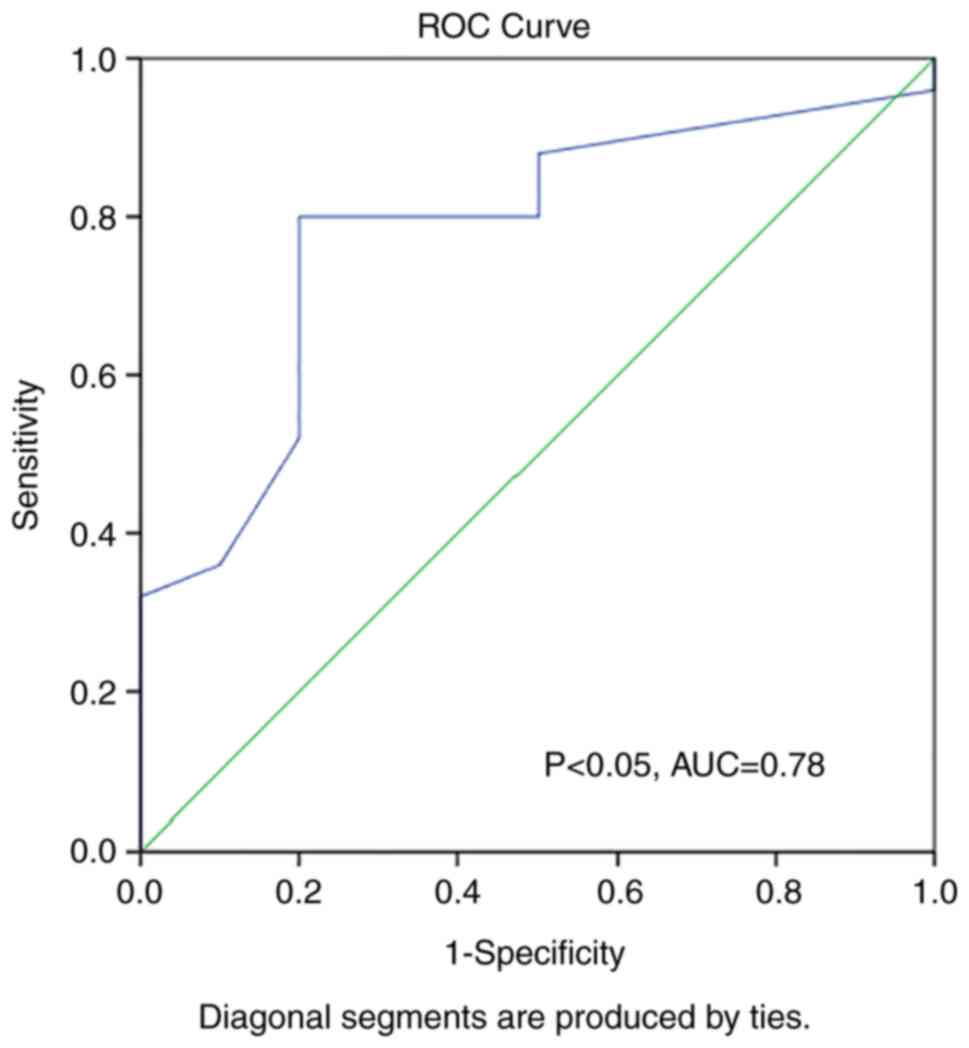
ROC curve analysis was also performed to evaluate
whether TNF-α can predict abnormal LV mass in runners and
statistically significant findings were obtained (P<0.05, AUC:
0.78) (Fig. 3).
Logistic regression analysis was performed for the
prediction of the presence of abnormal LV mass in echocardiography
in ultra-marathon runners. The model presented statistical
significance (P<0.001, Chi-squared=28.4) and could explain 79.6%
of the variance of the abnormal LV mass presence (Nagelkerke R
Square) and correctly classify 91.4% of the athletes. As presented
in Table V, TNF-α, and not age or
weight significantly contributed to the model.
 | Table VLogistic regression analysis for the
prediction of the presence of abnormal LV mass as detected by
echocardiography in ultra-marathon runners. |
Table V
Logistic regression analysis for the
prediction of the presence of abnormal LV mass as detected by
echocardiography in ultra-marathon runners.
| | 95% CI OR |
|---|
| Parameter | B | SE | Wald
χ2 | df | P-value | OR | LL | UL |
|---|
| Αge (years) | -0.28 | 0.16 | 3.20 | 1 | 0.073 | 0.75 | 0.54 | 1.02 |
| TNF-α (pg/ml) | -2.06 | 1.02 | 4.01 | 1 | 0.045 | 0.13 | 0.02 | 0.95 |
| Weight (kg) | -0.28 | 0.15 | 3.30 | 1 | 0.069 | 0.75 | 0.55 | 1.02 |
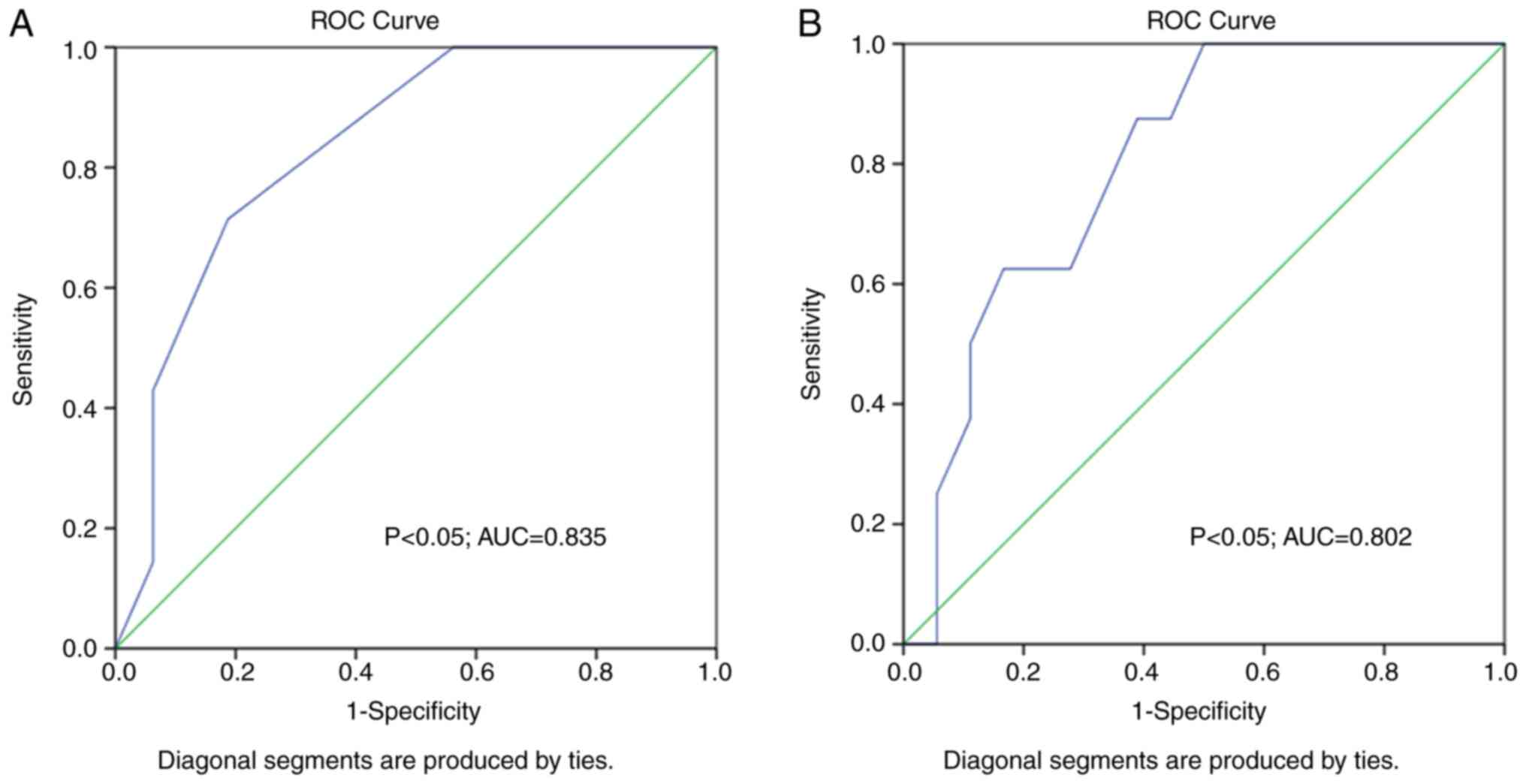
ROC curve analysis for the predictive ability of
training sessions and kilometers covered per week on runners'
abnormal RV dimensions produced nearly significant results (P=0.05,
AUC: 0.702; P=0.502, AUC:0.691, respectively). However, the number
of training sessions and kilometers run per week appear to be
important for runners' LV adaptations, as ROC curve analysis of the
said parameters on predicting abnormal LV volumes produced
significant results (P<0.05, AUC: 0.835; and P<0.05, AUC:
0.802, respectively) (Fig. 4).
Logistic regression analysis was performed for the
prediction of the presence of an abnormal LV volume in
echocardiography in ultra-marathon runners. The model presented
statistical significance (P<0.001, Chi-squared=9.52) and could
explain 47.9% of the variance of the abnormal LV volume presence
(Nagelkerke R Square) and correctly classify 82.6% of the athletes.
As shown in Table VI, the number
of training sessions per week, and not the kilometers ran per week
or weight significantly contributed to the model.
 | Table VILogistic regression analysis for the
prediction of the presence of abnormal LV volume as detected by
echocardiography in ultra-marathon runners. |
Table VI
Logistic regression analysis for the
prediction of the presence of abnormal LV volume as detected by
echocardiography in ultra-marathon runners.
| | 95% CI OR |
|---|
| Parameter | B | SE | Wald
χ2 | df | P-value | OR | LL | UL |
|---|
| Training sessions
per week | -2.36 | 1.18 | 3.99 | 1 | 0.046 | 0.09 | 0.01 | 0.95 |
| KM per week | 0.05 | 0.04 | 1.48 | 1 | 0.223 | 1.05 | 0.97 | 1.15 |
| Weight (kg) | -0.08 | 0.07 | 1.17 | 1 | 0.278 | 0.92 | 0.81 | 1.06 |
Discussion
Muscle damage, glycogen deficiency and oxidative
stress are all characteristics of strenuous exercise. In addition
to these, the release of endotoxins, and the increase of plasma
cortisol and catecholamine levels are observed. The subsequent
increase in the levels of pro-inflammatory cytokines is considered
to be both a trigger and a consequence of the said phenomena
(6,21). Exercise intensity and the
availability of energy sources are the main determinants of the
tight regulation of IL-6 levels in response to exercise (22).
Circulating monocytes or fatigued contracting muscle
express TNF-α, although its main source is macrophages (23). IL-6 has both pro- and
anti-inflammatory properties and is induced by exercise. IL-6
induction along with the cytokine inhibition (IL-1ra) by exercise
partly counteracts the increase in TNF-α levels. However, strenuous
exercise triggers oxidative stress, which in turn impairs
intracellular signaling and induces inflammation with an increase
in pro-inflammatory cytokine expression and the disruption of
anti-inflammatory cytokine production (24).
Previous studies have shown that strenuous exercise
and specifically marathon running is associated with an increase in
blood levels of specific inflammatory markers, namely IL-6,
high-sensitivity CRP and TNF-α (23,25).
ADMA is an analogue of L-arginine and is produced
via methylation from L-arginine (L-Arg) by protein arginine
methyltransferase type I. ADMA is a marker and also a determinant
of endothelial system dysfunction, as it directly inhibits
endothelial NO synthase (eNOS) and reduces the bioavailability of
NO through the activation of the vascular renin-angiotensin system,
and as a consequence of an increased production of reactive oxygen
species (ROS). NO is one of the major endothelium-derived
vaso-active substances; thus, increased ADMA levels lead to an
impairment of the NO regulation of vascular tone (26). Emerging evidence suggests that NO
plays a role in heart muscle response to mechanical stimulus in the
form of chronic volume or pressure overload. NO deficiency is
considered to induce myocardial hypertrophy and remodeling, as in
the case of hypertension or in animal models of hemodynamically
overloaded circulation (27). A
recent study on patients undergoing coronary artery bypass graft
also demonstrated that ADMA levels measured in pericardial fluid
were positively associated with end-diastolic and end-systolic LV
diameters, and negatively with LV ejection fraction (28). In the present study, ultra-marathon
runners with increased LV end diastolic diameters, had elevated
ADMA levels, while ROC curve analysis revealed that ADMA values
could predict the presence of abnormal LV diameter at
echocardiography.
Thus, renin-angiotensin system (RAS) activation in
the arterial wall could be induced by increased ADMA values.
Angiotensin II plays a central role in RAS. Angiotensin II has
growth hormone properties. Reduced NO levels and local RAS
activation can synergistically lead to cardiac hypertrophy
(28). The majority of
ultra-marathon runners monitored in the present study indeed
presented an abnormal LV mass indexed to BSA >115
g/m2, which was not associated with ADMA levels.
However, runners with moderate and severe abnormal indexed LV mass
>131 g/m2 had statistically significant higher TNF-α
values and ROC curve analysis revealed that TNF-α values could
predict the presence of abnormal LV mass in marathon runners.
Animal models of experimental hypertension using aortic banding for
the induction of pressure overload showed that TNF-α/TNFR1
signaling are crucial for the development of myocardial
hypertrophy, as there is an association between hypertrophy and
myocardial TNF-α levels (29,30).
TNF-α myocardial signaling is considered to be
concentration-dependent (31) and
at an early stage of hypertrophy, there is recent evidence to
suggest that it is cardioprotective (32).
There are limited reports on the mechanistic insight
of a possible link between inflammation and cardiovascular
adaptations, and no solid evidence is provided thus far (6,33).
Running in humans is associated with increased cardiovascular
activity and increased ventricular pressure. B-type natriuretic
peptide (BNP) and its cleaved inactive NH2-terminal fragment
(NTproBNP) are secreted by ventricles in response to cardiomyocyte
stress produced by volume or pressure overload (34). Although BNP elevations in healthy
individuals, in the context of increased ventricular pressure, were
reported >20 years ago (35),
there is a long debate whether elevations in the levels of this
biomarker after running is an epiphenomenon or a warning sign of
possible cardiac damage. BNP and NT-proBNP levels at rest in
endurance athletes are similar to their untrained and age-matched
peers, but increase 5- to 10-fold after exercise in subjects
participating in endurance exercise events (34). Exercise duration and not intensity
affect BNP and NT-proBNP release. BNP and NT-proBNP levels increase
the most with exercise in the least trained athletes, suggesting
that the acute increase may help initiate a training response.
Although the role of the primary transcriptional
response factor for hypoxic adaptation of the skeletal muscle, the
hypoxia inducible factor (HIF)-1α, during endurance training, has
been widely studied (36,37) relevant reports on cardiac muscle
were not found. Since HIFs are key oxygen sensors that mediate the
ability of the cell to cope with decreased oxygen tension, a
persistently activated hypoxic response in the heart as occurs
during pressure overload or tachypacing may stabilize HIF-1α levels
(36). In addition, mitochondria
are implicated in multiple HIF-dependent and -independent pathways
through the production of mitochondrial ROS and HIF-1-mediated
adaptations influence lactate production, transport and metabolism.
In that sense, similar studies on heart muscle may shed light on
training adaptations and energy demands of the heart during
exercise.
Cardiac volume overload characterizes endurance
sports. It is associated with LV and left atrium dilation, and an
increase in relative wall thickness and LV mass. No systolic and
diastolic dysfunction has been found in this specific remodeling
observed in aerobic dynamic exercise (38,39).
Previous studies have revealed an association between left atrial
size and IL-6 levels (40,41), mainly in the setting of atrial
fibrillation. Atrial myocardial stretch is believed to induce IL-6
expression (42). In the present
study, runners with an abnormal left atrial volume index had higher
IL-6 values compared with runners with a normal LAVI.
On the other hand, RV volume augmentation and
dysfunction have been reported following a marathon race in
previous studies on (ultra-)endurance athletes (43-45).
RV dilation is considered to be associated with bradycardia and an
increased venous return post-exercise in marathon runners, also
connected with an increased ventricular systolic function (46,47).
Ultra-marathon runners have been found to have an increased RV
end-diastolic area and RV fractional area changing compared with
marathon runners (48). In the
present study, runners with an abnormal RV diameter were those who
had been training more years and with a more demanding training
program, indicating an elite running status.
A well-known theory advocates that at exercise, the
pulmonary circulation presents a lower rate of decrease in vascular
resistance in comparison to the systemic circulation and as a
result, the stroke work at aerobic exercise required by the right
ventricle to be achieved in order to sustain adequate flow is
higher than the left ventricle, thus explaining both the more
pronounced acute and chronic effects of marathon running on RV
structure and function (44). The
right ventricle is considered more vulnerable to fatigue after
prolonged exercise and the hemodynamic theory attributes the said
fatigue to the increased ventricular load imposed on the right
ventricle, which is additionally increased with higher exercise
volumes and intensity (43).
In amateur athletes training and those participating
in marathon or half-marathon runs, an increased sympathetic drive
has been found which outlasts the period of exercise, and this
cardiac sympathetic modulation potentially is linked to adverse
cardiovascular prognosis (49).
Runners of the present study with lower than usual HRR values
(<35 beats) had lower exercise duration at the exercise stress
test.
Volume overload observed in marathon athletes is the
cause of the augmented LV and RV chambers. High dynamic exercise
also leads to an increase in LV thickness in relation to volume,
leading to LV eccentric hypertrophy (50).
The study by Arbab-Zadeh et al (4) demonstrated that in previously
sedentary individuals who began training in order to participate in
a marathon, the RV volume increased according to training intensity
from the beginning of the training, while the LV volume increased
only after 6 months. The LV and RV mass responded with hypertrophy
to marathon training. In the first 6 months, concentric hypertrophy
was noted, and after this time point, higher intensity and
prolonged running training led to eccentric hypertrophy as the LV
dilated (4). In the present study,
in agreement with the study by Arbab-Zadeh et al (4), ultra-marathon runners presented mild
eccentric myocardial hypertrophy. It was found that in middle-aged
marathon runners trained with a mild program covering 40k m per
week, less pronounced myocardial adaptations were observed despite
the fact that peak oxygen consumption during cardiopulmonary
exercise test was increased at the end of the 18-week training
period (5).
In young athletes, criteria have been developed to
distinguish a physiological adaptation of cardiac morphology and
function to exercise (‘athlete's heart’) from early cardiovascular
disorders (51). In older-aged
adults characterized by a higher prevalence of cardiovascular risk
factors and possible subclinical cardiac disease, the
differentiation of athlete's heart from early cardiac disease may
be more challenging. Increases in LV mass and LV volume may not
only represent a response to exercise, but are also dependent on
age and blood pressure. In addition, a left ventricular hypertrophy
without an increase in volume may be an indicator for early
subclinical cardiac alterations in response to risk factor exposure
(52). The findings of the present
study may help to distinguish physiological adaptation to exercise
from alterations in response to cardiovascular risk factors and
aging and protect athletes from sudden cardiovascular events
(53). In this regard, other
imaging techniques may be very helpful in distinguishing
cardiovascular adaptations, such as myocardial magnetic resonance
imaging, which is a valuable tool to classify myocardial
hypertrophy, as a manifestation of cardiovascular remodeling due to
exercise or a sign of underlying pathology (54,55).
In conclusion, the present study demonstrated that,
in ultra-marathon runners, cardiovascular adaptations to running
developed in combination with specific patterns of inflammatory and
endothelial alterations. The levels of such biochemical markers
may, in their turn, be used to predict the occurrence of the said
cardiovascular adaptations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KT, AS and CT organized and performed the research,
collected relevant information, wrote the manuscript and performed
overall project management. CT performed the statistical analysis,
data assessment and manuscript preparation. GK, AS, DK and DAS
performed the statistical analysis and the evaluation of the
results, and were involved in the preparation and writing of the
research article. FB, DK and CS reviewed the manuscript and
comprehensively assessed the study design and the data analysis,
prepared and wrote the manuscript, organized the references and
reviewed the current study. KT, CS and GK confirm the authenticity
of all the raw data. All authors have read and approved the final
version of this manuscript.
Ethics approval and consent to
participate
Written informed consent to participate in the study
was provided by all participants involved. The procedures were in
accordance with the Helsinki declaration of 1975 and approval was
received by the Human Subjects Committee of the University of
Thessaly, Larissa, and the General Hospital of Giannitsa (city of
Giannitsa), where all medical practices were conducted.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
D'Silva A, Bhuva AN, van Zalen J,
Bastiaenen R, Abdel-Gadir A, Jones S, Nadarajan N, Medina KD, Ye Y,
Augusto J, et al: Cardiovascular remodeling experienced by
real-world, unsupervised, young novice marathon runners. Front
Physiol. 11(232)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lee DC, Pate RR, Lavie CJ, Sui X, Church
TS and Blair SN: Leisure-time running reduces all-cause and
cardiovascular mortality risk. J Am Coll Cardiol. 64:472–481.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gabrielli L, Sitges M, Chiong M, Jalil J,
Ocaranza M, Llevaneras S, Herrera S, Fernandez R, Saavedra R, Yañez
F, et al: Potential adverse cardiac remodelling in highly trained
athletes: Still unknown clinical significance. Eur J Sport Sci.
18:1288–1297. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arbab-Zadeh A, Perhonen M, Howden E,
Peshock RM, Zhang R, Adams-Huet B, Haykowsky MJ and Levine BD:
Cardiac remodeling in response to 1 year of intensive endurance
training. Circulation. 130:2152–2161. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zilinski JL, Contursi ME, Isaacs SK,
Deluca JR, Lewis GD, Weiner RB, Hutter AM Jr, d'Hemecourt PA,
Troyanos C, Dyer KS and Baggish AL: Myocardial adaptations to
recreational marathon training among middle-aged men. Circ
Cardiovasc Imaging. 8(e002487)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Krzeminski K, Buraczewska M, Miskiewicz Z,
Dąbrowski J, Steczkowska M, Kozacz A and Ziemba A: Effect of
ultra-endurance exercise on left ventricular performance and plasma
cytokines in healthy trained men. Biol Sport. 33:63–69.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Samaras A, Tsarouhas K, Paschalidis E,
Giamouzis G, Triposkiadis F, Tsitsimpikou C, Becker AT,
Goutzourelas N and Kouretas D: Effect of a special
carbohydrate-protein bar and tomato juice supplementation on
oxidative stress markers and vascular endothelial dynamics in
ultra-marathon runners. Food Chem Toxicol. 69:231–236.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Domke I, Cremer P and Huchtemann M:
Therapeutic drug monitoring on COBAS INTEGRA 400-evaluation
results. Clin Lab. 46:509–515. 2000.PubMed/NCBI
|
|
9
|
Lang RM, Bierig M, Devereux RB,
Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward
J, Shanewise JS, et al: Recommendations for chamber quantification:
A report from the American society of echocardiography's guidelines
and standards committee and the chamber quantification writing
group, developed in conjunction with the European association of
echocardiography, a branch of the European society of cardiology. J
Am Soc Echocardiogr. 18:1440–1463. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mosteller RD: Simplified calculation of
body-surface area. N Engl J Med. 317(1098)1987.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rudski LG, Lai WW, Afilalo J, Hua L,
Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and
Schiller NB: Guidelines for the echocardiographic assessment of the
right heart in adults: A report from the American society of
Echocardiography endorsed by the European association of
echocardiography, a registered branch of the European Society of
cardiology, and the canadian society of echocardiography. J Am Soc
Echocardiogr. 23:685–713. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mantero A, Gentile F, Azzollini M, Barbier
P, Beretta L, Casazza F, Corno R, Faletra F, Giagnoni E,
Gualtierotti C, et al: Effect of sample volume location on
Doppler-derived transmitral inflow velocity values in 288 normal
subjects 20 to 80 years old: An echocardiographic, two-dimensional
color Doppler cooperative study. J Am Soc Echocardiogr. 11:280–288.
1998.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim YJ and Sohn DW: Mitral annulus
velocity in the estimation of left ventricular filling pressure:
Prospective study in 200 patients. J Am Soc Echocardiogr.
13:980–985. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bruch C, Schmermund A, Marin D, Katz M,
Bartel T, Schaar J and Erbel R: Tei-index in patients with
mild-to-moderate congestive heart failure. Eur Heart J.
21:1888–1895. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tei C, Dujardin KS, Hodge DO, Bailey KR,
McGoon MD, Tajik AJ and Seward SB: Doppler echocardiographic index
for assessment of global right ventricular function. J Am Soc
Echocardiogr. 9:838–847. 1996.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alvi R, Sklyar E, Gorski R, Atoui M,
Afshar M and Bella JN: Athens QRS score as a predictor of coronary
artery disease in patients with chest pain and normal exercise
stress test. J Am Heart Assoc. 5(e002832)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Foale R, Nihoyannopoulos P, McKenna W,
Kleinebenne A, Nadazdin A, Rowland E and Smith G: Echocardiographic
measurement of the normal adult right ventricle. Br Heart J.
56:33–44. 1986.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Du N, Bai S, Oguri K, Kato Y, Matsumoto I,
Kawase H and Matsuoka T: Heart rate recovery after exercise and
neural regulation of heart rate variability in 30-40 year old
female marathon runners. J Sports Sci Med. 4:9–17. 2005.PubMed/NCBI
|
|
19
|
Mann TN, Webster C, Lamberts RP and
Lambert MI: Effect of exercise intensity on post-exercise oxygen
consumption and heart rate recovery. Eur J Appl Physiol.
114:1809–1820. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Clauss S, Wakili R, Hildebrand B, Kääb S,
Hoster E, Klier I, Martens E, Hanley A, Hanssen H, Halle M and
Nickel T: MicroRNAs as biomarkers for acute atrial remodeling in
marathon runners (The miRathon Study-A Sub-Study of the Munich
Marathon Study). PLoS One. 11(e0148599)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Suzuki K, Nakaji S, Yamada M, Totsuka M,
Sato K and Sugawara K: Systemic inflammatory response to exhaustive
exercise. Cytokine kinetics. Exerc Immunol Rev. 8:6–48.
2002.PubMed/NCBI
|
|
22
|
Wallberg L, Mattsson CM, Enqvist JK and
Ekblom B: Plasma IL-6 concentration during ultra-endurance
exercise. Eur J Appl Physiol. 111:1081–1088. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bernecker C, Scherr J, Schinner S, Braun
S, Scherbaum WA and Halle M: Evidence for an exercise induced
increase of TNF-alpha and IL-6 in marathon runners. Scand J Med Sci
Sports. 23:207–214. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mohan S and Gupta D: Crosstalk of
toll-like receptors signaling and Nrf2 pathway for regulation of
inflammation. Biomed Pharmacother. 108:1866–1878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ostrowski K, Rohde T, Asp S, Schjerling P
and Pedersen BK: Pro- and anti-inflammatory cytokine balance in
strenuous exercise in humans. J Physiol. 515:287–291.
1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Veresh Z, Racz A, Lotz G and Koller A:
ADMA impairs nitric oxide-mediated arteriolar function due to
increased superoxide production by angiotensin II-NAD(P)H oxidase
pathway. Hypertension. 52:960–966. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Simko F and Simko J: The potential role of
nitric oxide in the hypertrophic growth of the left ventricle.
Physiol Res. 49:37–46. 2000.PubMed/NCBI
|
|
28
|
Nemeth Z, Cziraki A, Szabados S, Biri B,
Keki S and Koller A: Elevated levels of asymmetric dimethylarginine
(ADMA) in the pericardial fluid of cardiac patients correlate with
cardiac hypertrophy. PLoS One. 10(e0135498)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rolski F and Blyszczuk P: Complexity of
TNF-α signaling in heart disease. J Clin Med.
9(3267)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun M, Chen M, Dawood F, Zurawska U, Li
JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R and Liu
PP: Tumor necrosis factor-alpha mediates cardiac remodeling and
ventricular dysfunction after pressure overload state. Circulation.
115:1398–1407. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sack MN, Smith RM and Opie LH: Tumor
necrosis factor in myocardial hypertrophy and ischaemia-an
anti-apoptotic perspective. Cardiovasc Res. 45:688–695.
2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Besse S, Nadaud S, Balse E and Pavoine C:
Early protective role of inflammation in cardiac remodeling and
heart failure: Focus on TNFα and resident macrophages. Cells.
11(1249)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
La Gerche A, Inder WJ, Roberts TJ, Brosnan
MJ, Heidbuchel H and Prior DL: Relationship between inflammatory
cytokines and indices of cardiac dysfunction following intense
endurance exercise. PLoS One. 10(e0130031)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Eijsvogels TM, Fernandez AB and Thompson
PD: Are there deleterious cardiac effects of acute and chronic
endurance exercise? Physiol Rev. 96:99–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vilela EM, Bettencourt-Silva R, Nunes JP
and Ribeiro VG: BNP and NT-proBNP elevation after running-a
systematic review. Acta Cardiol. 70:501–509. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kumar H and Choi DK: Hypoxia inducible
factor pathway and physiological adaptation: A cell survival
pathway? Mediators Inflamm. 2015(584758)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mounier R, Pialoux V, Roels B, Thomas C,
Millet G, Mercier J, Coudert J, Fellmann N and Clottes E: Effect of
intermittent hypoxic training on HIF gene expression in human
skeletal muscle and leukocytes. Eur J Appl Physiol. 105:515–524.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pluim BM, Zwinderman AH, van der Laarse A
and van der Wall EE: The athlete's heart. A meta-analysis of
cardiac structure and function. Circulation. 101:336–344.
2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pelliccia A, Maron BJ, Di Paolo FM, Biffi
A, Quattrini FM, Pisicchio C, Roselli A, Caselli S and Culasso F:
Prevalence and clinical significance of left atrial remodeling in
competitive athletes. J Am Coll Cardiol. 46:690–696.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rafaqat S, Sharif S, Majeed M, Naz S,
Manzoor F and Rafaqat S: Biomarkers of metabolic syndrome: Role in
pathogenesis and pathophysiology of atrial fibrillation. J Atr
Fibrillation. 14(20200495)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Psychari SN, Apostolou TS, Sinos L,
Hamodraka E, Liakos G and Kremastinos DT: Relation of elevated
C-reactive protein and interleukin-6 levels to left atrial size and
duration of episodes in patients with atrial fibrillation. Am J
Cardiol. 95:764–767. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kazanski V, Mitrokhin VM, Mladenov MI and
Kamkin AG: Cytokine effects on mechano-induced electrical activity
in atrial myocardium. Immunol Invest. 46:22–37. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
La Gerche A, Burns AT, Mooney DJ, Inder
WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbüchel H and Prior DL:
Exercise-induced right ventricular dysfunction and structural
remodelling in endurance athletes. Eur Heart J. 33:998–1006.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Oxborough D, Shave R, Warburton D,
Williams K, Oxborough A, Charlesworth S, Foulds H, Hoffman MD,
Birch K and George K: Dilatation and dysfunction of the right
ventricle immediately after ultraendurance exercise: Exploratory
insights from conventional two-dimensional and speckle tracking
echocardiography. Circ Cardiovasc Imaging. 4:253–263.
2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Neilan TG, Januzzi JL, Lee-Lewandrowski E,
Ton-Nu TT, Yoerger DM, Jassal DS, Lewandrowski KB, Siegel AJ,
Marshall JE, Douglas PS, et al: Myocardial injury and ventricular
dysfunction related to training levels among nonelite participants
in the Boston marathon. Circulation. 114:2325–2333. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Erol MK and Karakelleoglu S: Assessment of
right heart function in the athlete's heart. Heart Vessels.
16:175–180. 2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
D'Andrea A, Riegler L, Golia E, Cocchia R,
Scarafile R, Salerno G, Pezzullo E, Nunziata L, Citro R, Cuomo S,
et al: Range of right heart measurements in top-level athletes: The
training impact. Int J Cardiol. 164:48–57. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ujka K, Bastiani L, D'Angelo G, Catuzzo B,
Tonacci A, Mrakic-Sposta S, Vezzoli A, Giardini G and Pratali L:
Enhanced right-chamber remodeling in endurance ultra-trail athletes
compared to marathon runners detected by standard and
speckle-tracking echocardiography. Front Physiol.
8(527)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
De Maria B, de Oliveira Gois M, Catai AM,
Marra C, Lucini D, Porta A, Pagani M and Vecchia LAD: Ten-year
follow-up of cardiac function and neural regulation in a group of
amateur half-marathon runners. Open Heart.
8(e001561)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Vitarelli A, Capotosto L, Placanica G,
Caranci F, Pergolini M, Zardo F, Martino F, Chiara SD and Vitarelli
M: Comprehensive assessment of biventricular function and aortic
stiffness in athletes with different forms of training by
three-dimensional echocardiography and strain imaging. Eur Heart J
Cardiovasc Imaging. 14:1010–1020. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Maron BJ, Douglas PS, Graham TP, Nishimura
RA and Thompson PD: Task Force 1: Preparticipation screening and
diagnosis of cardiovascular disease in athletes. J Am Coll Cardiol.
45:1322–1326. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nassenstein K, Breuckmann F, Lehmann N,
Schmermund A, Hunold P, Broecker-Preuss M, Sandner TA, Halle M,
Mann K, Jöckel KH, et al: Left ventricular volumes and mass in
marathon runners and their association with cardiovascular risk
factors. Int J Cardiovasc Imaging. 25:71–79. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Stamatopoulos PG, Dangas G, Tsarouhas K,
Ziogas G, Tsitsimpikou C, Stamatopoulos G and Chrousos G: Subtotal
occlusion of left anterior coronary artery in a professional
athlete. Cardiology. 140:71–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mavrogeni SI, Tsarouhas K, Spandidos DA,
Kanaka-Gantenbein C and Bacopoulou F: Sudden cardiac death in
football players: Towards a new pre-participation algorithm. Exp
Ther Med. 17:1143–1148. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Markousis-Mavrogenis G, Giannakopoulou A,
Andreou N, Papadopoulos G, Vartela V, Kolovou G, Bacopoulou F,
Tsarouhas K, Kanaka-Gantenbein C, Spandidos DA and Mavrogeni SI:
Cardiovascular magnetic resonance clarifies arrhythmogenicity in
asymptomatic young athletes with ventricular arrhythmias undergoing
pre-participation evaluation. Exp Ther Med. 20:561–571.
2020.PubMed/NCBI View Article : Google Scholar
|