Introduction
Bladder cancer (BCa) is the 10th most prevalent
malignancy worldwide, with an estimated 573,278 new cases diagnosed
and 212,536-related deaths worldwide in 2020(1). The most common type of BCa is
urothelial carcinoma of the bladder (UCB). While radical cystectomy
(RC) provides the local control of tumors for patients with muscle
invasive BCa and high-risk non-muscle invasive BCa (NMIBC), 30% of
patients experience relapse following RC (2). Moreover, the survival rate is only
12-15 months in patients with cancers of an advanced stage
undergoing classical cisplatin-based systemic chemotherapy
(3). However, the recent emergence
of novel immunotherapies, targeted agents and antibody-drug
conjugate (ADC) therapies have substantially broadened the
treatment options for UCB, with the overall survival (OS) currently
approaching 2 years (4).
Currently, based on the excellent efficacy of study EV-201
(NCT03219333), TROPHY-U-01 (NCT03547973), and NCT03507166, three
ADCs for UCB, enfortomab vedotin, sacituzumab govitecan and
disitamab vedotin, have respectively been approved for application
(5). The Cancer Genome Atlas
database provides data enabling mapping the comprehensive molecular
landscape of UCB, and accumulating evidence based on these data
demonstrate the presence of distinct molecular subtypes of UCB,
offering the potential to develop novel molecularly targeted
therapies (6,7). In this context, identifying more
specific tumor biomarkers would enable clinicians to assess cancer
risk, predict disease development, and guide treatment more
accurately in a personalized manner.
Human epidermal growth factor receptor 2 (HER2) is a
transmembrane receptor of tyrosine kinase encoded by the
HER2/neu oncogene, which participates in the processes of
cell proliferation and tumorigenesis. The oncogenic role of HER2
has been most extensively studied in breast and gastric cancer,
which has been confirmed as a poor prognostic factor; accordingly,
trastuzumab, a humanized monoclonal antibody targeting HER2, is
currently the cornerstone drug of targeted therapy for these types
of cancer (8,9). In recent years, the overexpression of
HER2 has also been detected in UCB, prompting the initiation of
several clinical trials assessing the efficacy of HER2 inhibitors,
such as trastuzumab and lapatinib, against this malignancy, with
unsatisfactory results (10).
However, the recent success of ADC therapies has brought HER2 back
into focus in clinical and basic research regarding UBC.
Notably, there is no unified standard for the
determination of the HER2 expression status in UCB, mainly due to
issues with methodology (amplification detection vs. overexpression
detection) and the diversity of available techniques [polymerase
chain reaction, in situ hybridization and
immunohistochemistry (IHC)]. A previous meta-analysis reported that
HER2 levels in BCa varied from 9 to >80% as regards protein
overexpression and from 0 to 32% in terms of gene amplification
(11). Furthermore, previous
studies investigating various BCa-associated genetic variants
obtained conflicting results for the prognostic significance of
HER2 status. Some studies revealed that upregulation of HER2 was
associated with poor prognosis (12-17),
whereas others indicated that HER2 status did not have prognostic
significance (18-20).
In addition, Gandour-Edwards et al (21) reported an increased cancer-specific
survival of the HER2 (2+/3+) population in the context of
paclitaxel-based chemotherapy. These conflicting findings mainly
result from the different patient cohorts between the studies,
particularly regarding tumor stage and histological grade, and the
methods used to evaluate HER2 status, meanwhile, it underlines the
need to gain a better understanding of the expression and potential
role of HER2 in UCB.
Based on this background, the aim of the present
study was to evaluate the expression of HER2 in patients with UCB,
as assessed using IHC, and to determine the association of HER2
status on recurrence-free survival (RFS) and OS.
Patients and methods
Patient selection
The present retrospective study was conducted
following the approval from the Institutional Ethics Committee of
The First Affiliated Hospital of Chongqing Medical University
(approval no. 2022-K21; Chongqing, China), and written informed
consent was signed by each participant prior to sample collection.
A total of 108 patients who underwent RC and bilateral regional
lymphadenectomy for UCB at the Department of Urology of The First
Affiliated Hospital of Chongqing Medical University between 2015
and 2020 were included. None of the patients had received
neoadjuvant chemotherapy prior to surgery, and all samples were
subjected to pathological examinations that led to the
identification of the tumors as urothelial carcinomas. The clinical
data of the patients were collected from the medical record system
of the hospital and included the following: sex, age, tumor size,
number of tumors, pathological stage and grade, lymph node
metastasis, lymphovascular invasion and adjuvant chemotherapy. The
pathological specimens were re-examined by two experienced
pathologists using the 2002 TNM system (22) for pathological staging and the 1973
World Health Organization system for pathological grading (23). The follow-up duration was defined
as the period from the date the patient underwent RC until the date
of recurrence of UCB, which was identified using computed
tomography imaging or the date of death of the patient.
IHC
Tissues were fixed with 10% formalin at room
temperature for 24 h and were embedded in paraffin. Subsequently,
paraffin-embedded tumor sections (4 µm) were successively subjected
to dewaxing, antigen retrieval (achieved by boiling the sample in
0.01 mol/l sodium citrate buffer, pH 6.0, for 30 min), incubation
with 3% hydrogen peroxide (IHC kit; cat. no. SP-9000; OriGene
Technologies, Inc.) for 10 min at room temperature, and blocking
with 10% goat serum (cat. no. SP-9000; OriGene Technologies, Inc.)
for 15 min at room temperature. Subsequently, the slides were
incubated with anti-HER2 polyclonal antibody (cat. no. 18299-1-AP;
ProteinTech Group, Inc.; 1:50 dilution) at 4˚C overnight, washed
with phosphate-buffered saline three times, and incubated with the
biotinylated goat anti-mouse/rabbit IgG secondary antibody (cat.
no. SP-9000; OriGene Technologies, Inc.) for 20 min at room
temperature. Then, sections were incubated with horseradish
enzyme-labeled streptavidin working solution (cat. no. SP-9000;
OriGene Technologies, Inc.) for 20 min at room temperature.
Finally, the slides were stained using a 3,3'-diaminobenzidine kit
(cat. no. ZLI-9018; OriGene Technologies, Inc.) for 1 min at room
temperature, counterstained with hematoxylin (cat. no. G1080;
Beijing Solarbio Science & Technology Co., Ltd.) for 30 sec at
room temperature, dehydrated and mounted in dibutylphthalate
polystyrene xylene.
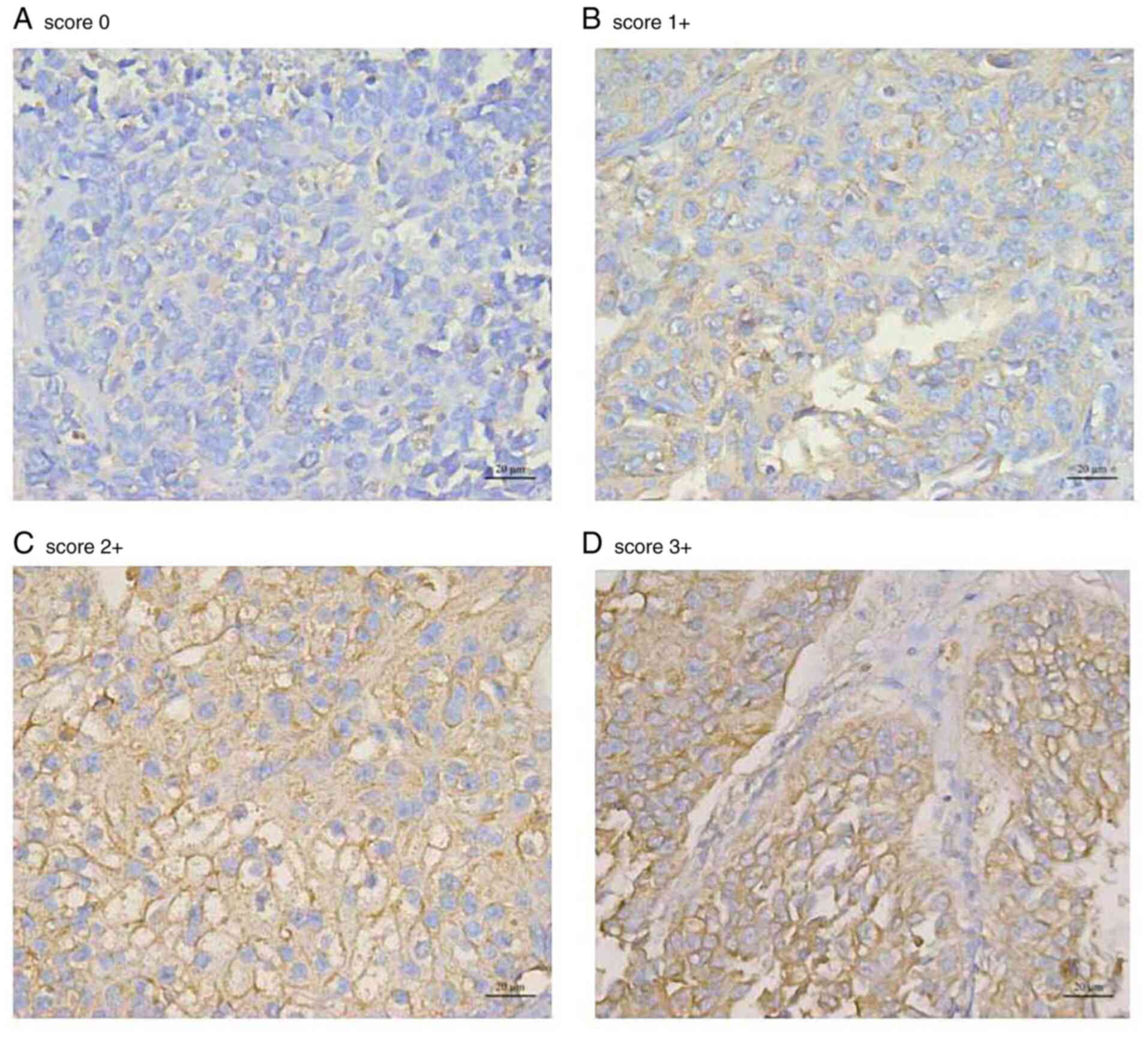
The samples were scored based on previous literature
and according to the modified 2018 American Society of Clinical
Oncology HER2 testing in breast cancer guideline (14,24).
IHC staining was scored as follows: 0, no staining or <10%
staining of tumor cells; 1, faint and partial membrane staining in
>10% of cells; 2, weak to moderate, complete membrane staining
in >10% of cells; or 3, strong, complete membrane staining in
>10% of tumor cells. An IHC score of 2+ or 3+ was defined as a
high expression or overexpression of HER2, while a score of 0 or 1+
was defined as a low expression. The scoring was performed by two
pathologists who were blinded to the clinical data.
Statistical analysis
The Pearson's and continuous calibration Chi-squared
tests were used to evaluate the association between HER2 expression
status (overexpression vs. low expression) and clinicopathological
parameters. A Kaplan-Meier curve was constructed to assess the
prognostic significance of HER2 expression on RFS and OS, and
differences between groups were statistically analyzed using the
log-rank test. A Cox regression model was used for univariate and
multivariate survival analyses. P<0.05 was considered to
indicate a statistically significant difference. All analyses were
performed using SPSS version 22.0 software (IBM Corp.).
Results
Among the 108 patients included in this study, IHC
scores of 0, 1+, 2+, and 3+ were observed in 6, 40, 47 and 15
patients, respectively. A total of 62 patients (57.4%) were found
to have a high expression of HER2 (IHC 2+/3+). HER2 overexpression
was significantly associated with a high tumor grade (P=0.006) and
an advanced stage (P<0.001); however, it was not significantly
associated with the patient's age (P=0.740), sex (P=0.839), tumor
size (P=0.147), the number of tumors (P=0.577), lymph node
metastasis (P=0.156), or lymphovascular invasion (P=0.491). The
baseline characteristics of the patients are presented in Table I and representative images of the
IHC staining intensities for the four different levels if scoring
are presented in Fig. 1.
 | Table IDescriptive characteristics for the
cohort of 108 patients with urothelial carcinoma of the bladder
treated with radical cystectomy. |
Table I
Descriptive characteristics for the
cohort of 108 patients with urothelial carcinoma of the bladder
treated with radical cystectomy.
| | HER2 expression, n
(%) | |
|---|
| Variable | Patients, n (%) | Low | High | P-value |
|---|
| Total | 108(100) | 46 (42.6) | 62 (57.4) | |
| Age, years | | | | 0.740 |
|
≤65 | 52 (48.1) | 23 (44.2) | 29 (55.8) | |
|
>65 | 56 (51.9) | 23 (41.1) | 33 (58.9) | |
| Sex | | | | 0.839 |
|
Female | 11 (10.2) | 5 (45.5) | 6 (54.5) | |
|
Male | 97 (89.8) | 41 (42.3) | 56 (57.7) | |
| Tumor size | | | | 0.147 |
|
<3
cm | 57 (52.8) | 28 (49.1) | 29 (50.9) | |
|
≥3 cm | 51 (47.2) | 18 (35.3) | 33 (64.7) | |
| No. of tumors | | | | 0.577 |
|
1 | 19 (17.6) | 7 (36.8) | 12 (63.2) | |
|
>1 | 89 (82.4) | 39 (43.8) | 50 (56.2) | |
| T stage | | | | 0.001 |
|
Ta, T1 | 38 (35.2) | 27 (71.1) | 11 (28.9) | |
|
T2 | 37 (34.3) | 11 (29.7) | 26 (70.3) | |
|
T3 | 20 (18.5) | 6 (30.0) | 14 (70.0) | |
|
T4 | 13 (12.0) | 2 (15.4) | 11 (84.6) | |
| G grade | | | | 0.006 |
|
G1/2 | 20 (18.5) | 14 (70.0) | 6 (30.0) | |
|
G3 | 88 (81.5) | 32 (36.4) | 56 (63.6) | |
| Lymph node
metastasis | | | | 0.156 |
|
Negative | 100 (92.6) | 45 (45.0) | 55 (55.0) | |
|
Positive | 8 (7.4) | 1 (12.5) | 7 (87.5) | |
| Lymphovascular
invasion | | | | 0.491 |
|
Negative | 96 (88.9) | 42 (43.8) | 54 (56.3) | |
|
Positive | 12 (11.1) | 4 (33.3) | 8 (66.7) | |
| Adjuvant
chemotherapy | | | | 0.643 |
|
Negative | 89 (82.4) | 37 (41.6) | 52 (58.4) | |
|
Positive | 19 (17.6) | 9 (47.4) | 10 (52.6) | |
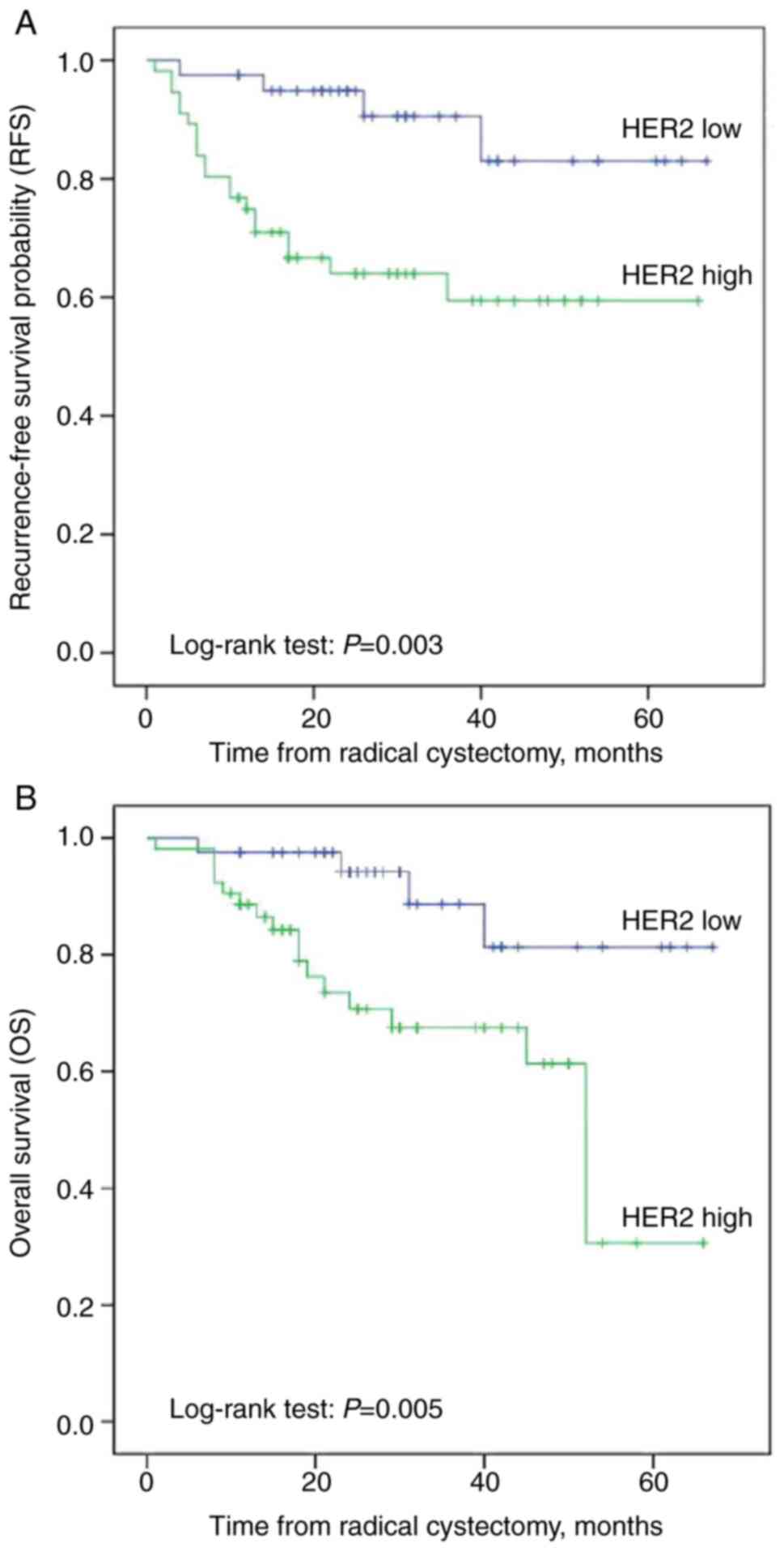
At the time of data analysis and with a median
follow-up of 31.5 months [95% confidence interval (CI), 28-40)], 24
patients (22.2%) experienced relapse and 22 succumbed to the
disease (20.4%). Kaplan-Meier analysis revealed that patients with
HER2 overexpression had a shorter OS (P=0.005) and RFS (P=0.003)
than those with low levels of HER2 expression (Fig. 2). Univariate Cox regression
analysis also revealed that HER2 overexpression was significantly
associated with a poor RFS [hazard ratio (HR), 4.37; 95% CI,
1.49-12.79; P=0.007) and OS (HR, 4.12; 95% CI, 1.39-12.21; P=0.011)
(Table II). In multivariate Cox
regression analyses controlling for the effects of standard
clinicopathologic variables, such as HER2 expression, pathological
stage, pathological grade, lymph node metastasis and lymphovascular
invasion, HER2 expression (HR, 3.61; 95% CI, 1.07-12.18; P=0.039),
pathological stage (P=0.003) and adjuvant chemotherapy (HR, 0.09;
95% CI, 0.01-0.79; P=0.029) remained independent predictors of UCB
recurrence. However, HER2 expression was not significantly
associated with OS (HR, 3.03; 95% CI, 0.95-9.74; P=0.062) (Table III).
 | Table IIUnivariate Cox regression model of
pathological features for the prediction of RFS and OS in 108
patients with urothelial carcinoma of the bladder treated with
radical cystectomy. |
Table II
Univariate Cox regression model of
pathological features for the prediction of RFS and OS in 108
patients with urothelial carcinoma of the bladder treated with
radical cystectomy.
| | RFS | OS |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 2.15
(0.92-5.04) | 0.078 | 2.27
(0.93-5.50) | 0.071 |
| Sex | 0.92
(0.27-3.09) | 0.893 | 0.86
(0.25-2.93) | 0.809 |
| Tumor size | 2.19
(0.96-5.01) | 0.064 | 2.21
(0.94-5.19) | 0.068 |
| No. of tumors | 0.77
(0.29-2.06) | 0.602 | 0.58
(0.21-1.57) | 0.281 |
| G grade | 1.89
(0.56-6.32) | 0.304 | 6.86
(0.92-51.10) | 0.060 |
| T stage trend | |
<0.001a | | 0.001a |
|
T2 vs. Ta,
T1, Tis | 0.94
(0.24-3.76) | 0.929 | 1.27
(0.36-4.57) | 0.711 |
|
T3 vs. Ta,
T1, Tis | 11.45
(3.59-36.51) |
<0.001a | 7.88
(2.35-26.45) | 0.001a |
|
T4 vs. Ta,
T1, Tis | 5.00
(1.33-18.76) | 0.017a | 5.55
(1.36-22.68) | 0.017a |
| Lymph node
metastasis | 13.49
(5.04-36.12) |
<0.001a | 13.76
(4.77-39.65) |
<0.001a |
| Lymphovascular
invasion | 2.70
(1.01-7.23) | 0.049a | 3.30
(1.28-8.51) | 0.013a |
| Adjuvant
chemotherapy | 0.15
(0.02-1.13) | 0.066 | 0.34
(0.09-1.21) | 0.096 |
| HER2
expression | 4.37
(1.49-12.79) | 0.007a | 4.12
(1.39-12.21) | 0.011a |
 | Table IIIMultivariate Cox regression model of
pathological features for prediction of RFS and OS in 108 patients
with urothelial carcinoma of the bladder treated with radical
cystectomy. |
Table III
Multivariate Cox regression model of
pathological features for prediction of RFS and OS in 108 patients
with urothelial carcinoma of the bladder treated with radical
cystectomy.
| | RFS | OS |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| HER2
expression | 3.61
(1.07-12.18) | 0.039a | 3.03
(0.95-9.74) | 0.062 |
| G grade | 0.68
(0.18-2.60) | 0.569 | 4.85
(0.56-41.66) | 0.150 |
| T stage trend | | 0.003a | | 0.108 |
|
T2 vs. Ta,
T1, Tis | 0.74
(0.17-3.14) | 0.683 | 1.54
(0.40-5.96) | 0.533 |
|
T3 vs. Ta,
T1, Tis | 7.28
(1.83-29.00) | 0.005 | 4.96
(1.08-22.82) | 0.040 |
|
T4 vs. Ta,
T1, Tis | 4.20
(0.94-18.80) | 0.062 | 5.24
(1.01-27.11) | 0.048 |
| Lymph node
metastasis | 2.91
(0.70-12.01) | 0.140 | 2.13
(0.46-9.82) | 0.332 |
| Lymphovascular
invasion | 1.65
(0.40-6.84) | 0.489 | 3.49
(0.97-12.51) | 0.055 |
| Adjuvant
chemotherapy | 0.09
(0.01-0.79) | 0.029a | 0.13
(0.03-0.59) | 0.008a |
Discussion
In the present study, it was found that HER2 was
upregulated in 57.4% of patients with UCB, which is within the
range of 27.8 to 85.2% reported previously (11). The wide range and discrepancies in
the reported frequency of HER2 overexpression in UCB may be related
to the combination of cases, varying definitions of ‘positive’, and
the application of different evaluation techniques among studies
(25). Although the present study
did not perform fluorescence in situ hybridization (FISH) to
confirm the expression levels in the cases showing an IHC score of
2+, the main aim of the study was to assess the expression of HER2
in UBC as a preliminary analysis of an evaluation method for
selecting potential ADC candidates and to compare the findings with
similar reports on this topic, which have largely used IHC.
Based on the overexpression of HER2, two HER2-ADC
drugs, T-DM1, and DS-8201a, were successively approved by the
United States Food and Drug Administration (FDA) mainly for the
treatment of advanced HER2-positive (IHC 3+, and/or FISH positive)
breast cancer, and the efficacy of these treatments was
demonstrated in the pivotal trials, EMILIA, TH3RESA and
DESTINY-Breast01 (26-28).
In contrast to research on breast and gastric cancer, the current
clinical trials for UCB mainly highlight the assessment of HER2
expression using IHC, rather than FISH, which is attributed to the
particular pharmacological characteristics of HER2-ADC. A phase 1b
trial (NCT03523572) combining DS-8201a with nivolumab for the
treatment of advanced breast or urothelial cancer is currently open
for enrollment, and the main inclusion criterion is patients
exhibiting any degree of expression of HER2 based on IHC (scores of
1+, 2+, 3+) or FISH positivity (5). Another novel ADC drug, RC48-ADC, has
exhibited outstanding efficacy and survival benefits when used in
the treatment of patients with locally advanced and metastatic
urothelial carcinoma with HER2 overexpression (IHC 2+/3+) who had
failed systematic chemotherapy (29). This drug was then given accelerated
approval by the National Medical Products Administration of China
and FDA in June, 2020 and is currently used in routine clinical
applications.
The association between clinical outcomes and HER2
expression in UCB has been reported in several single-center
studies; however, the overall result remains controversial.
Recently, Gan et al (30)
comprehensively analyzed 14 studies that included a total of 1,398
patients with BCa with regard to the HER2 status determined using
IHC. They found that the overexpression of HER2 was strongly
related to an advanced pathological stage, high tumor grade and
tumor recurrence, but not to OS, disease-specific survival, or
progression-free survival. By contrast, a previous meta-analysis
indicated that HER2 expression was associated with a poor
disease-specific and disease-free survival (11). Consistent with the results of the
study by Gan et al (30),
the present study also found that the overexpression of HER2 was
significantly associated with an advanced tumor stage and a high
grade. In addition, the Kaplan-Meier curves and Cox regression
analysis suggested a significant association between HER2
overexpression and disease recurrence. Although HER2 overexpression
was found to be associated with a higher risk of mortality, this
effect was not statistically significant, which may be attributable
to the insufficient sample size and the inclusion of patients with
NMIBC, who generally have improved survival rates.
HER2 can be activated through hetero- or
homodimerization. The formation of heterodimers and subsequent HER2
activation are temporally and spatially controlled in normal cells
and tissues, but the associated pathway is dysregulated in cancer
cells, where upregulated expression of HER2 or HER1 offers a growth
advantage (31). HER2 regulates
the expression of multiple genes, such as those related to
proliferation, differentiation and angiogenesis, mainly through the
PI3K/AKT and MAPK/ERK signaling pathways (31). HER2 has also been identified as a
metastasis-promoting factor; HER family members have been reported
to play an essential role in promoting the metastatic potential of
tumors, owing to their ability to enhance the release of matrix
metalloproteases (32). These
results may explain why UCB with HER2 overexpression tends to show
malignant phenotypic characteristics and is associated with a
poorer prognosis.
The present study has several limitations. The first
is the technical restrictions of IHC, including the lack of
standardization, semi-quantitative output and subjective scoring
system. The present study opted to use IHC, as it is the
first-level technique for HER2 detection in clinical practice.
Nevertheless, since UCB presents unique characteristics of HER2
expression, the evaluation of HER2 alterations at the DNA, RNA and
protein expression levels may provide a more exhaustive analysis
and new insight into the relevance of HER2 as a tumor driver and
potential therapeutic target in UCB. The second limitation refers
to the assessment of lymph nodes. Previous studies have confirmed a
strong association between HER2 overexpression and lymph node
metastasis, which has also been found to be stronger than that of
the matched primary tumors (17,33).
The present study was not able to verify this association owing to
the small number of cases involving lymph node metastasis and the
limitations of the available specimens. Despite these limitations,
it is considered that the findings of the present study add to the
mounting evidence demonstrating worse clinical outcomes for
patients with UCB exhibiting HER2 overexpression. Additionally,
these findings may prove to be useful in identifying patients who
are at an increased risk of disease recurrence and would likely
benefit from HER2-targeted therapies.
In conclusion, the overexpression of HER2 is related
to the pathological malignancy of UCB and may serve as an
independent prognostic factor for recurrence in patients with UCB
following RC. The present study provides a reference for the
pre-treatment evaluation of HER2 as a therapeutic target for UCB;
however, further prospective, large-scale, multi-detection studies
are warranted to confirm these findings.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG, WH, HYi, ZW and XB designed the study. XB, HYi,
XZ, HYu and XL performed the research and analyzed the data. XG, WH
and XL wrote the manuscript. XZ, ZW and HYu revised the manuscript.
XB and HYi confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study were
in accordance with the ethical standards of the Ethics Committee of
the Institutional Ethical Review Board of The First Affiliated
Hospital of Chongqing Medical University (approval no. 2022-K21;
Chongqing, China). Written informed consent forms were signed by
each participant prior to sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077.
2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lenis AT, Lec PM, Chamie K and Mshs MD:
Bladder cancer: A review. JAMA. 324:1980–1991. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sarfaty M and Rosenberg JE: Antibody-drug
conjugates in urothelial carcinomas. Curr Oncol Rep.
22(13)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cancer Genome Atlas Research Network.
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kamoun A, de Reyniès A, Allory Y, Sjödahl
G, Robertson AG, Seiler R, Hoadley KA, Groeneveld CS, Al-Ahmadie H,
Choi W, et al: A consensus molecular classification of
muscle-invasive bladder cancer. Eur Urol. 77:420–433.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yarden Y: Biology of HER2 and its
importance in breast cancer. Oncology. 61 (Suppl 2):1–13.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hindy JR, Souaid T, Kourie HR and Kattan
J: Targeted therapies in urothelial bladder cancer: A disappointing
past preceding a bright future? Future Oncol. 15:1505–1524.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao J, Xu W, Zhang Z, Song R, Zeng S, Sun
Y and Xu C: Prognostic role of HER2 expression in bladder cancer: A
systematic review and meta-analysis. Int Urol Nephrol. 47:87–94.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen PC, Yu HJ, Chang YH and Pan CC: Her2
amplification distinguishes a subset of non-muscle-invasive bladder
cancers with a high risk of progression. J Clin Pathol. 66:113–119.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim D, Kim JM, Kim JS, Kim S and Kim KH:
Differential expression and clinicopathological significance of
HER2, indoleamine 2,3-dioxygenase and PD-L1 in urothelial carcinoma
of the bladder. J Clin Med. 9(1265)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Krüger S, Weitsch G, Büttner H,
Matthiensen A, Böhmer T, Marquardt T, Sayk F, Feller AC and Böhle
A: HER2 overexpression in muscle-invasive urothelial carcinoma of
the bladder: Prognostic implications. Int J Cancer. 102:514–518.
2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Abdelrahman AE, Rashed HE, Elkady E,
Elsebai EA, El-Azony A and Matar I: Fatty acid synthase, Her2/neu,
and E2F1 as prognostic markers of progression in non-muscle
invasive bladder cancer. Ann Diagn Pathol. 39:42–52.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kolla SB, Seth A, Singh MK, Gupta NP,
Hemal AK, Dogra PN and Kumar R: Prognostic significance of Her2/neu
overexpression in patients with muscle invasive urinary bladder
cancer treated with radical cystectomy. Int Urol Nephrol.
40:321–327. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bolenz C, Shariat SF, Karakiewicz PI,
Ashfaq R, Ho R, Sagalowsky AI and Lotan Y: Human epidermal growth
factor receptor 2 expression status provides independent prognostic
information in patients with urothelial carcinoma of the urinary
bladder. BJU Int. 106:1216–1222. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jimenez RE, Hussain M, Bianco FJ Jr,
Vaishampayan U, Tabazcka P, Sakr WA, Pontes JE, Wood DP Jr and
Grignon DJ: Her-2/neu overexpression in muscle-invasive urothelial
carcinoma of the bladder: Prognostic significance and comparative
analysis in primary and metastatic tumors. Clin Cancer Res.
7:2440–2447. 2001.PubMed/NCBI
|
|
19
|
Kassouf W, Black PC, Tuziak T, Bondaruk J,
Lee S, Brown GA, Adam L, Wei C, Baggerly K, Bar-Eli M, et al:
Distinctive expression pattern of ErbB family receptors signifies
an aggressive variant of bladder cancer. J Urol. 179:353–358.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Soria F, Moschini M, Haitel A, Wirth GJ,
Gust KM, Briganti A, Rouprêt M, Klatte T, Hassler MR, Karakiewicz
PI and Shariat SF: The effect of HER2 status on oncological
outcomes of patients with invasive bladder cancer. Urol Oncol.
34:533.e1–533.e10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gandour-Edwards R, Lara PN Jr, Folkins AK,
LaSalle JM, Beckett L, Li Y, Meyers FJ and DeVere-White R: Does
HER2/neu expression provide prognostic information in patients with
advanced urothelial carcinoma? Cancer. 95:1009–1015.
2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: AJCC cancer staging manual (ed
6). New York, NY, Springer-Verlag, 2002.
|
|
23
|
Mostofi FK, Sobin LH and Torloni H: World
Health Organization (WHO): Histological typing of urinary bladder
tumours / F. K. Mostofi, in collaboration with L. H. Sobin, H.
Torloni and pathologists in fourteen countries. WHO, Geneva,
1973.
|
|
24
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sanguedolce F, Russo D, Mancini V,
Selvaggio O, Calò B, Carrieri G and Cormio L: Human epidermal
growth factor receptor 2 in non-muscle invasive bladder cancer:
Issues in assessment methods and its role as prognostic/predictive
marker and putative therapeutic target: A comprehensive review.
Urol Int. 102:249–261. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Diéras V, Miles D, Verma S, Pegram M,
Welslau M, Baselga J, Krop IE, Blackwell K, Hoersch S, Xu J, et al:
Trastuzumab emtansine versus capecitabine plus lapatinib in
patients with previously treated HER2-positive advanced breast
cancer (EMILIA): A descriptive analysis of final overall survival
results from a randomised, open-label, phase 3 trial. Lancet Oncol.
18:732–742. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Krop IE, Kim SB, Martin AG, LoRusso PM,
Ferrero JM, Badovinac-Crnjevic T, Hoersch S, Smitt M and Wildiers
H: Trastuzumab emtansine versus treatment of physician's choice in
patients with previously treated HER2-positive metastatic breast
cancer (TH3RESA): Final overall survival results from a randomised
open-label phase 3 trial. Lancet Oncol. 18:743–754. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive breast
cancer. N Engl J Med. 382:610–621. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo
H, Shi B, Liu J, He Z, Yu G, et al: Open-label, multicenter, phase
II study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in
patients with locally advanced or metastatic urothelial carcinoma.
Clin Cancer Res. 27:43–51. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gan K, Gao Y, Liu K, Xu B and Qin W: The
clinical significance and prognostic value of HER2 expression in
bladder cancer: A meta-analysis and a bioinformatic analysis. Front
Oncol. 11(653491)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gutierrez C and Schiff R: HER2: biology,
detection, and clinical implications. Arch Pathol Lab Med.
135:55–62. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ménard S, Pupa SM, Campiglio M and
Tagliabue E: Biologic and therapeutic role of HER2 in cancer.
Oncogene. 22:6570–6578. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fleischmann A, Rotzer D, Seiler R, Studer
UE and Thalmann GN: Her2 amplification is significantly more
frequent in lymph node metastases from urothelial bladder cancer
than in the primary tumours. Eur Urol. 60:350–357. 2011.PubMed/NCBI View Article : Google Scholar
|