Introduction
Mycobacterium tuberculosis (M. tb) is
the pathogen that causes tuberculosis (1). It can invade all organs of the body
and is the most common cause of tuberculosis. Globally, each year,
~10 million individuals become infected with M. tb (2), resulting in ~1.7 million mortalities,
which poses a serious threat to public health (3). Therefore, identifying a novel
theoretical and experimental basis for treating tuberculosis is
important. THP-1 cells are widely used in the study of monocyte and
macrophage-related mechanisms and signaling pathways. THP-1 cells
are easy to cultivate and expand in the laboratory, have a more
stable genetic background, and do not possess the problem of
individual differences associated with peripheral blood mononuclear
cells, which is conducive to the reproduction of experimental
results (4). Therefore, THP-1
cells are an ideal tool for studying immunity and inflammation.
Alveolar macrophages are not only the primary site
for M. tb colonization and reproduction (5), but they are also the first line of
defense against M. tb infection. Several reports indicate
that M. tb can induce necrosis (6), apoptosis (7) and autophagy in macrophages (8). Furthermore, M. tb-infected
macrophages can survive in the host cell for a substantial period
of time (9). Previous studies have
indicated that vitamin C is the most important water-soluble
antioxidant in human plasma and mammalian cells (10,11),
suggesting that vitamin C may also have important cellular
functions. It is well-established that vitamin C can prevent the
occurrence of scurvy and protect healthy cells from oxidative
damage (12). The self-protective
mechanism of M. tb in cells generates free radicals, which
increases cellular toxicity (13).
Therefore, additional research is required to determine whether
antioxidants are beneficial to patients with an M. tb
infection. As a scavenger of free radicals, it remains unclear
whether vitamin C affects THP-1 cells infected with M.
tb.
To address this, after incubating THP-1 cells with
M. tb, the apoptotic signaling and cellular inflammatory
factors of the host cells following vitamin C treatment were
further studied. Animal experiments were performed to verify the
protective effect of vitamin C after M. tb infection.
Materials and methods
Cell culture
THP-1 cells (American Type Culture Collection), a
model commonly used for studying the function of macrophages
(14), were maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
The cells were cultured in a 5% CO2 incubator at 37˚C
before seeding them in a 6-well plate at a density of
2x106 cells/well or 1x106 cells/well;
2x106 cells/well for western blotting, RT-qPCR, EdU cell
proliferation experiments and 1x106 cells/well for flow
cytometry and TUNEL assay experiments. The cells were treated with
phorbol 12-myristate 13-acetate (PMA; 100 ng/ml) (Thermo Fisher
Scientific, Inc.) at 37˚C for 24 h. PMA transformed THP-1 cells
into adherent macrophages and then the cells were incubated with
M. tb (MOI:10; Chinese Center for Disease Control and
Prevention; CCDC, Beijing, China) and vitamin C (150 µM/ml) at 37˚C
for 24 h. The concentrations and treatment durations were used for
all cell assays.
MTT assay
An MTT assay was used to assess the viability of
cells. THP-1 cells were seeded in a 96-well plate at a density of
5x103 cells/well, and PMA was used to induce cell
transformation before being pretreated with various concentrations
(0, 50, 100, 150, 200 and 300 µM/ml) of vitamin C for 24 h at 37˚C.
Subsequently, cells were treated with lipopolysaccharide (LPS; 1
µg/ml) (Thermo Fisher Scientific, Inc.) for 12 h at 37˚C, and MTT
solution (20 µl) was added to each well according to the
manufacturer's instructions. The cells were then incubated for 4 h
at 37˚C. Finally, DMSO (100 µl) was added to each well, and the
absorbance (560 nm) was measured using a microplate reader (Bio-Rad
Laboratories, Inc.).
Western blotting
Total protein was extracted from the cells in the
different treatment groups [i) Control (untreated) group; ii) M.
tb group; iii) vitamin C group; and iv) M. tb + vitamin
C group] using a M-PER mammalian protein extraction reagent kit
(Thermo Fisher Scientific, Inc.). A BCA protein assay kit was used
(Thermo Fisher Scientific, Inc.) to measure the total protein
concentration. Subsequently, 10 µg protein was mixed with 6X
loading buffer (Takara Bio, Inc.) and loaded on 10% SDS-PAGE,
followed by transfer to PVDF membranes. After blocking with
SuperBlock (Thermo Fisher Scientific, Inc.) 37˚C for 1 h, the
membrane was incubated overnight at 4˚C with the primary
antibodies, including GAPDH (cat. no. 5174), Cleaved-caspase-9
(cat. no. 20750), Cleaved-caspase-3 (cat. no. 9661), Bcl-2 (cat.
no. 15071), cytochrome c (Cyt-c; cat. no. 12963), Bax
(cat. no. 5023), IL-1β (cat. no. 12703), IL-6 (cat. no. 12912) or
NLR family pyrin domain-containing 3 (NLRP3; cat. no. 15101) (all
1:1,000; Cell Signaling Technology, Inc.). The samples were then
incubated with the secondary antibodies HRP-conjugated Affinipure
goat anti-mouse IgG (H+L) (1:10,000; cat. no. SA00001-1;
ProteinTech Group, Inc.) or HRP-conjugated Affinipure goat
anti-rabbit IgG (H+L) (1:10,000; cat. no. SA00001-2; ProteinTech
Group, Inc.) at room temperature for 1 h. A ECL chemiluminescence
kit (Analytik Jena US LLC) was used to visualize protein bands. The
intensity of each protein band was normalized to the respective
GAPDH band and analyzed using ImageJ (version 1.46; National
Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from THP-1 cells using the
MiniBEST Universal RNA extraction kit (Takara Bio, Inc.), and RNA
was reverse-transcribed to cDNA using a PrimeScript™ RT
Reagent kit (Takara Bio, Inc.). The following temperature protocol
was used for reverse transcription: 37˚C for 15 min and 85˚C for 5
sec. Subsequently, the obtained cDNA was amplified with qPCR on an
ABI 7500 Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using a TB Green Fast qPCR Mix kit (Takara
Bio, Inc.). The following thermocycling qPCR conditions were used
for amplification: 95˚C for 30 sec; followed by 40 cycles of 95˚C
for 5 sec and 65˚C for 30 sec. Relative expression levels were
evaluated using the 2-∆∆Cq method and normalized to
GAPDH as the internal control (15). qPCR reactions were performed using
the following primers: GAPDH forward, 5'-GGAGCGAGATCCCTCCAAAAT-3'
and reverse, 5'-GGCTGTTGTCATACTTCTCATGG-3'; TNF-α forward,
5'-CCTCTCTCTAATCAGCCCTCTG-3' and reverse,
5'-GAGGACCTGGGAGTAGATGAG-3'; and IL-8 forward,
5'-TTTTGCCAAGGAGTGCTAAAGA-3' and reverse,
5'-AACCCTCTGCACCCAGTTTTC-3'.
EdU cell proliferation assay
Cell proliferation was performed using an EdU cell
proliferation kit (cat. no. C0075S; Beyotime Institute of
Biotechnology). The cells (2x106 cells/well) were seeded
into 6-well plates and incubated at 37˚C for 24 h then the cells of
different treatment groups were incubated with M.tb (MOI:10)
and vitamin C (150 µM/ml) at 37˚C for 24 h. THP-1 cells were then
incubated with EdU (10 µmol/l) for 2 h at 37˚C, washed three times
with PBS, fixed with 4% paraformaldehyde for 15 min at room
temperature and permeabilized with 0.3% Triton X-100 for 15 min at
room temperature. Hoechst was used to stain the nuclei for 10 min
at room temperature after the incubation with 0.3% Triton X-100.
Cells were washed three times with PBS and the images were obtained
using a fluorescence microscope.
Flow cytometry
The cells (1x106 cells/well) were seeded
into 6-well plates and incubated at 37˚C for 24 h, then the cells
of different treatment groups were incubated with M.tb
(MOI:10) and vitamin C (150 µM/ml) at 37˚C for 24 h. After
treatment, THP-1 cells were then washed thrice in cold PBS and
pelleted by centrifugation (5 min; 650 x g; 37˚C). Next, the
supernatant was discarded, and the pellet was resuspended in 100 µl
1X Annexin V-binding buffer (Invitrogen; Thermo Fisher Scientific,
Inc.), to which 5 µl Alexa Fluor® 488 Annexin V-FITC
(Component A) and 1 µl 100 µg/ml PI working solution were added and
further incubated at room temperature for 15 min. After the
incubation period, cells were analyzed using a FACSCanto II
cytometer (BD Biosciences) and CytExpert 2.0 software (Beckman
Coulter, Inc.) to determine the rate of apoptosis in early + late
stages of cells.
TUNEL assay
The cells (1x106 cells/well) were seeded
on coverslips in 6-well plates and incubated at 37˚C for 24 h, then
the cells of different treatment groups were incubated with
M.tb (MOI:10) and vitamin C (150 µM/ml) at 37˚C for 24 h.
After treatment, THP-1 cells were then washed thrice in PBS and
fixed with 4% paraformaldehyde (at room temperature for 15 min),
followed by permeabilization using 0.2% Triton X-100 (37˚C for 10
min). Next, THP-1 cells were incubated with a TUNEL reaction
mixture at 37˚C for 60 min (Thermo Fisher Scientific, Inc.), washed
thrice with PBS, and counterstained with DAPI for 15 min at 37˚C
(Thermo Fisher Scientific, Inc.). Finally, 80 cells for each group
were observed under a confocal laser scanning microscope and
processed on Leica Confocal Software v.2.6.1 (Leica Microsystems
GmbH).
Animal experiments
A total of 16 male BALB/c mice (6-8 weeks old;
weight, 20±25 g) were purchased from Jackson ImmunoResearch
Laboratories, Inc. All animal experiments and protocols were
approved by the Ethical Committee of Ningxia University (Yinchuan,
China; specific pathogen free grade; approval no. 2020-024). All
animals were housed in a pathogen-free facility (22±2˚C; 50±5%
humidity) with a 12-h light/dark cycle, and the mice had ad
libitum access to food and water. After 1 week of acclimation,
the mice were randomly divided into four treatment groups: i)
Control group (50 µl of 0.9% normal saline administered
intragastrically); ii) M. tb group [50 µl M. tb (50
µg/ml) by intraperitoneal injection]; iii) vitamin C group [50 µl
vitamin C (0.5 µM/ml) administered intragastrically]; and iv) M.
tb + vitamin C group [50 µl vitamin C (0.5 µM/ml) administered
intragastrically and M. tb (50 µg/ml) by intraperitoneal
injection]. Treatments were intragastrically administered once a
day for 21 days. Rapid weight loss >15-20% without significant
signs of dehydration and weakness was defined as a potential humane
endpoint for the present study.
Histological evaluation
Mice were sacrificed after 21 days by cervical
dislocation. The absence of heartbeat and respiration were used as
criteria to confirm mouse death. Fresh lung tissue was isolated and
washed once with PBS. The tissue was fixed with 4% paraformaldehyde
for 20 min at 37˚C and then embedded in paraffin. The embedded
tissue was cut into 4-µm thick sections. Finally, the tissue was
stained with hematoxylin and eosin at 37˚C for 10 min. The images
of stained tissues were then scored by three independent
pathologists who were blinded prior to scoring as follows: 1,
Normal; 2, slight tissue damage; 3, mild tissue damage; and 4,
severe tissue damage (confocal laser scanning microscopy;
magnification, x100).
Immunohistochemistry
Mice were sacrificed after 21 days. Fresh lung
tissue was isolated, fixed overnight with 4% paraformaldehyde
(Solarbio, P1110) at 4˚C. Then the samples were dehydrated in
ethanol and xylene (70% ethanol 30 min; 80% ethanol 30 min; 95%
ethanol 30 min; 100% ethanol 30 min twice; xylene 30 min twice),
embedded in paraffin and cut into sections as aforementioned 4-µM
thick sections. Lung tissues was transferred to
3-aminopropyl-triethoxysilane-treated microscope slides (ZLI-9001;
Zhongshan Company) for immunofluorescence staining. In brief,
sections were deparaffinized and rehydrated (xylene 5 min twice;
100% ethanol 3 min twice; 90% ethanol 3 min; 80% ethanol 3 min; 70%
ethanol 3 min). After three washes in distilled water, antigen
retrieval was performed by microwaving for 15 min in 0.01% sodium
citrate buffer (pH 6.0). After three washes in PBS, the lung
tissues were treated with 3% H2O2 in PBS for
20 min to quench endogenous peroxidase activity. Nonspecific
binding was blocked with 5% BSA in PBS for 15 min at room
temperature. Sections were then incubated with primary antibody
(NF-κB; 1:200; cat. no. 8242; Cell Signaling Technology, Inc.)
overnight at 4˚C. After three washes in PBS, the lung tissue
sections were incubated for 20 min at room temperature with
horseradish peroxidase-labeled goat anti-rabbit IgG (Zhongshan
Company), and rinsed with PBS. The antibody complex was detected
using DAB reagent (Zhongshan Company). Finally, the sections were
incubated with hematoxylin staining solution (Zhongshan Company)
for 20 sec at room temperature and washed once with distilled
water. Images were captured using a light microscope
(magnification, x100).
Statistical analysis
All data collected were obtained from at least three
independent experiments for each condition. All results were
analyzed using GraphPad Prism version 6.0 (GraphPad Software, Inc.)
and are presented as the mean ± standard deviation (unless
otherwise shown). Unpaired Student's t-test was used to compare
differences between two groups, and one-way ANOVA followed by
Tukey's post hoc test was used to compare differences between >2
groups. The statistical analysis of the histological score results
was conducted using Kruskal-Wallis test followed by Dunn's post hoc
test and are presented as median + interquartile range. P<0.05
was considered to indicate a statistically significant
difference.
Results
M. tb infection induces the expression
of apoptosis-related proteins, and vitamin C pretreatment increases
the viability of THP-1 cells after LPS stimulation
After THP-1 cells were incubated with M. tb
for 24 h, the protein expression levels of the apoptosis-related
proteins cleaved caspase-9, cleaved caspase-3, Bcl-2 and
Cyt-c were analyzed using western blotting (Fig. 1A). Cleaved caspase-9, cleaved
caspase-3 and Cyt-c levels were significantly increased in
the M. tb infection group compared with the control group
(Fig. 1A and B). Furthermore, incubation with M.
tb significantly suppressed the protein expression levels of
Bcl-2 (Fig. 1A and B). An MTT assay was used to determine the
effects of LPS stimulation on the viability of THP-1 cells after
vitamin C pretreatment. THP-1 cells were pretreated with different
concentrations of vitamin C (0, 50, 100, 150, 200 and 300 µM/ml)
for 24 h and then treated with LPS (1 µg/ml) for 12 h (Fig. 1C). Experimental results
demonstrated that vitamin C pretreatment significantly increased
the viability of THP-1 cells after LPS stimulation in a
dose-dependent manner. These results suggested that M. tb
infection promoted apoptosis and that vitamin C pretreatment
increased the viability of THP-1 cells. Subsequently, it was
assessed whether vitamin C affected THP-1 cell viability infected
with M. tb.
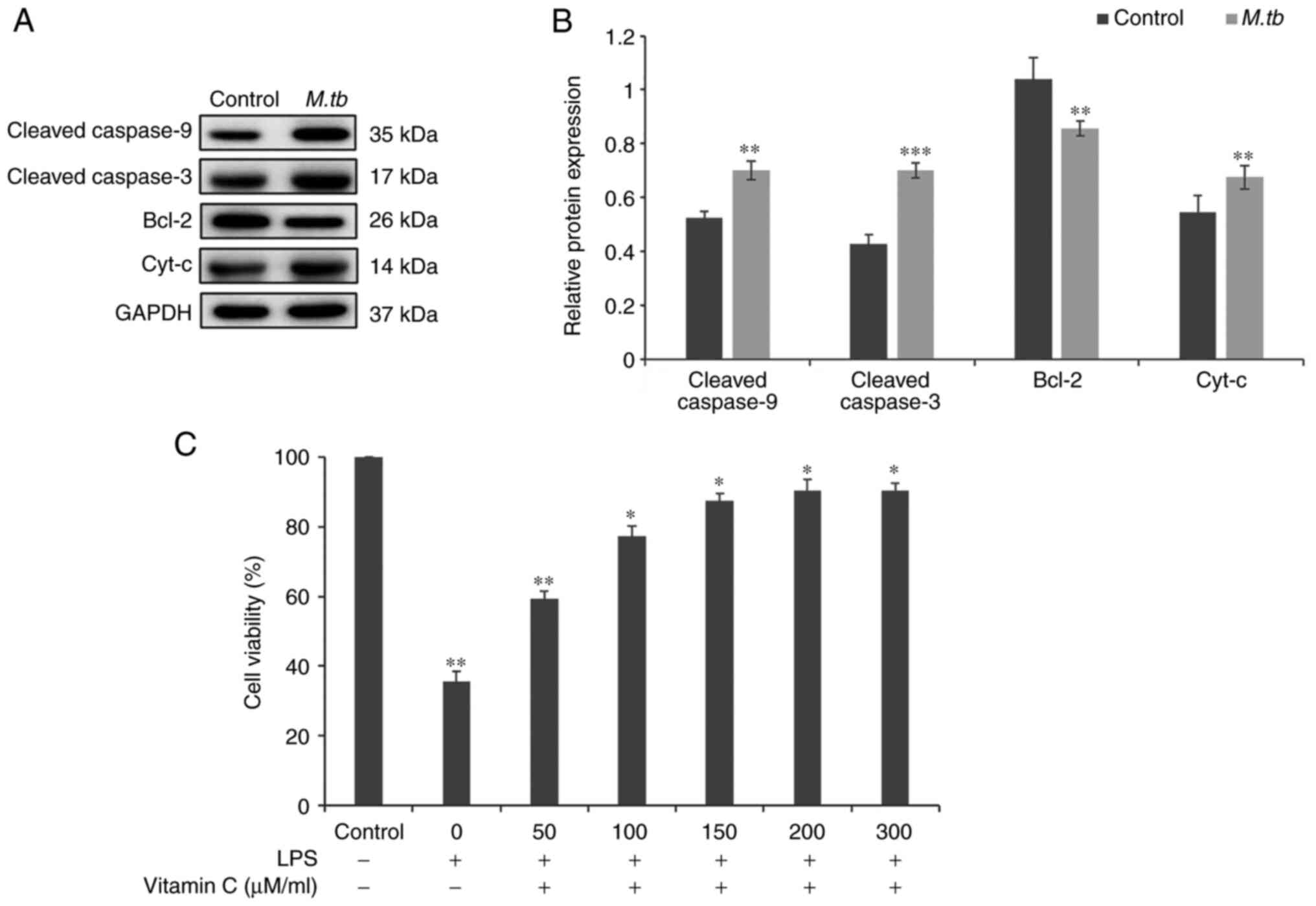 | Figure 1Effect of vitamin C on the viability
of THP-1 cells during incubation with M. tb and stimulation
by LPS. (A) After incubating THP-1 cells with M. tb for 24
h, the protein expression levels of apoptosis-related proteins,
including Cleaved caspase-9, Cleaved caspase-3, Bcl-2 and
Cyt-c, were analyzed using western blotting. (B) Cleaved
caspase-9, Cleaved caspase-3, Bcl-2 and Cyt-c protein
semi-quantitative expression levels. GAPDH was used as a loading
control. (C) THP-1 cells were pretreated with different
concentrations of vitamin C (0, 50, 100, 150, 200 or 300 µM/ml) for
24 h and then treated with LPS for 12 h. Cell viability was
measured using an MTT assay. *P<0.05,
**P<0.01 and ***P<0.001 vs. control;.
M. tb, Mycobacterium tuberculosis; Cyt-c,
cytochrome c; LPS, lipopolysaccharide. |
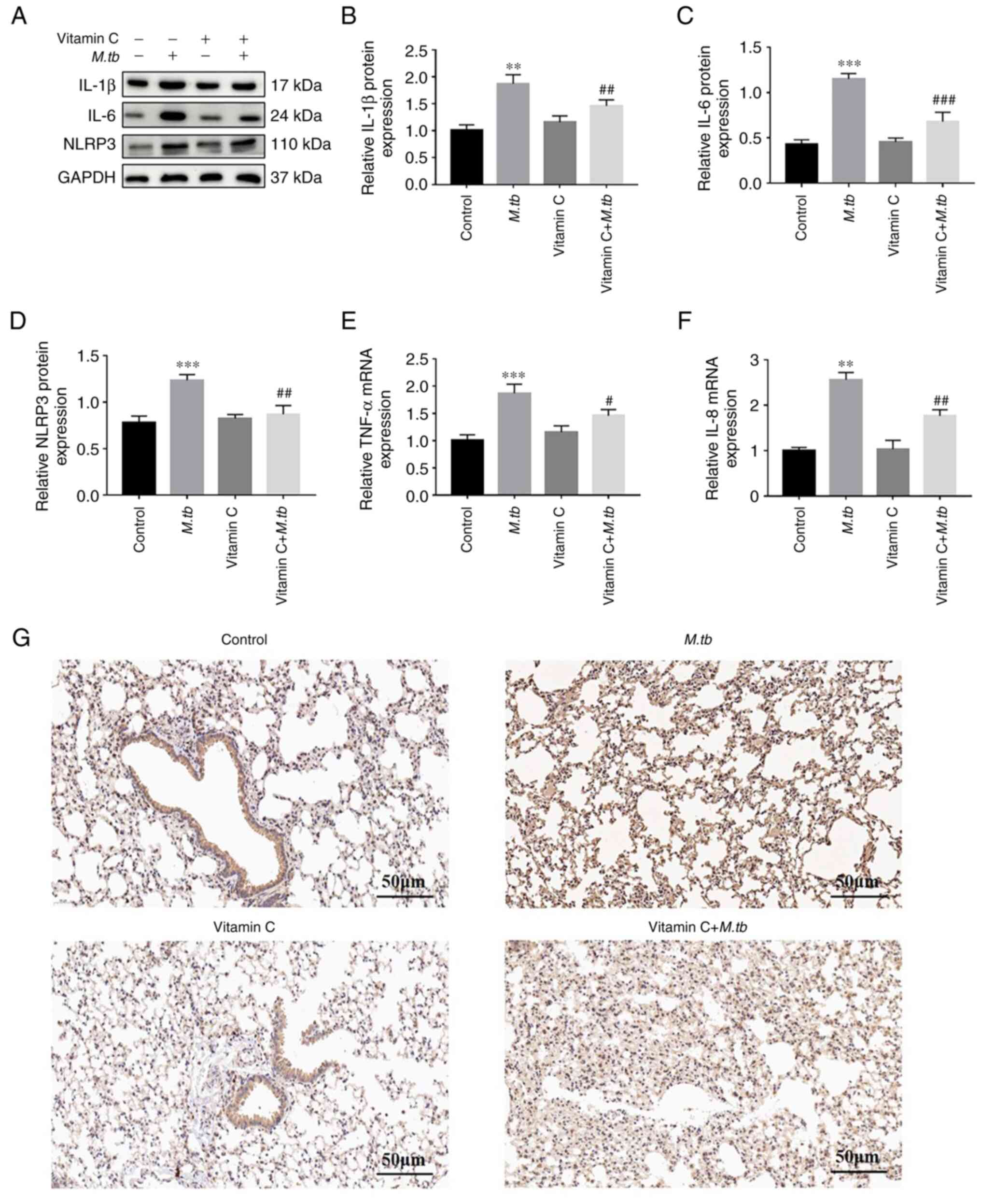
Vitamin C increases the viability and
reduces the levels of inflammatory factors released by THP-1 cells
following M. tb infection
Vitamin C exerted a protective effect on the
viability of THP-1 cells after LPS stimulation. Thus, whether
vitamin C could inhibit the levels of inflammatory factors in THP-1
cells after M. tb infection was assessed. To determine
whether vitamin C regulated the inflammatory response of THP-1
cells after M. tb infection, the protein levels of
inflammatory factors IL-1β, IL-6 and NLRP3 were assessed using
western blotting (Fig. 2A). The
results indicated that the expression levels of IL-1β, IL-6 and
NLRP3 were significantly higher in the M. tb group compared
with the control group and that vitamin C treatment significantly
decreased their expression levels compared with the M. tb
group (Fig. 2A-D). Furthermore,
TNF-α and IL-8 mRNA expression levels were significantly lower in
the vitamin C + M. tb group compared with the M. tb
group (Fig. 2E and F). Therefore, western blotting and
RT-qPCR analysis demonstrated that vitamin C exerted a significant
anti-inflammatory effect. However, the anti-inflammatory effect of
vitamin C should be studied further in animal models. IL-1β and
IL-6 ultimately regulate inflammatory gene expression by activation
of NF-κB (16). Given the
important role of NF-κB in host defense and its role in regulating
inflammation, the expression of NF-κB in lung tissues was examined
using immunohistochemistry. As presented in Fig. 2G, compared with the control group,
the M. tb group exhibited higher expression levels of NF-κB,
and the expression of NF-κB in the vitamin C + M. tb was
markedly lower. These results suggested that vitamin C may
attenuate M. tb infection by inhibiting the inflammatory
response.
Vitamin C reduces apoptosis of THP-1
cells after M. tb infection
To determine whether vitamin C regulated apoptosis
of THP-1 cells after M. tb infection, western blotting was
used to analyze the protein levels of apoptosis-related factors,
including cleaved caspase-3, Bax, cleaved caspase-9 and
Cyt-c (Fig. 3A). Compared
with the M. tb group, treatment with vitamin C significantly
decreased the protein expression levels of cleaved caspase-3, Bax,
cleaved caspase-9 and Cyt-c following M. tb infection
(Fig. 3C). An EdU cell
proliferation assay was used to investigate the effect of vitamin C
on THP-1 cell proliferation after M. tb infection. As
presented in Fig. 3B, red
fluorescence, indicative of proliferation, was markedly reduced by
M. tb treatment and increased by vitamin C. Moreover,
apoptosis was evaluated using flow cytometry and TUNEL staining
assays. Flow cytometry results demonstrated that M. tb
infection significantly promoted apoptosis of THP-1 cells compared
with the control, and the apoptotic rate was ~74% in the M.
tb group. By contrast, vitamin C treatment following M.
tb treatment decreased the apoptotic rate as compared with the
M. tb group and the apoptotic rate was only ~48% (Fig. 3D and E). TUNEL staining assay results
demonstrated that the number of TUNEL-positive cells was increased
in the M. tb group. By contrast, in the vitamin C and
vitamin C + M. tb groups, the number of TUNEL-positive cells
was markedly reduced (Fig. 3F).
These results suggested that treatment with vitamin C may attenuate
M. tb infection by inhibiting apoptosis in THP-1 cells.
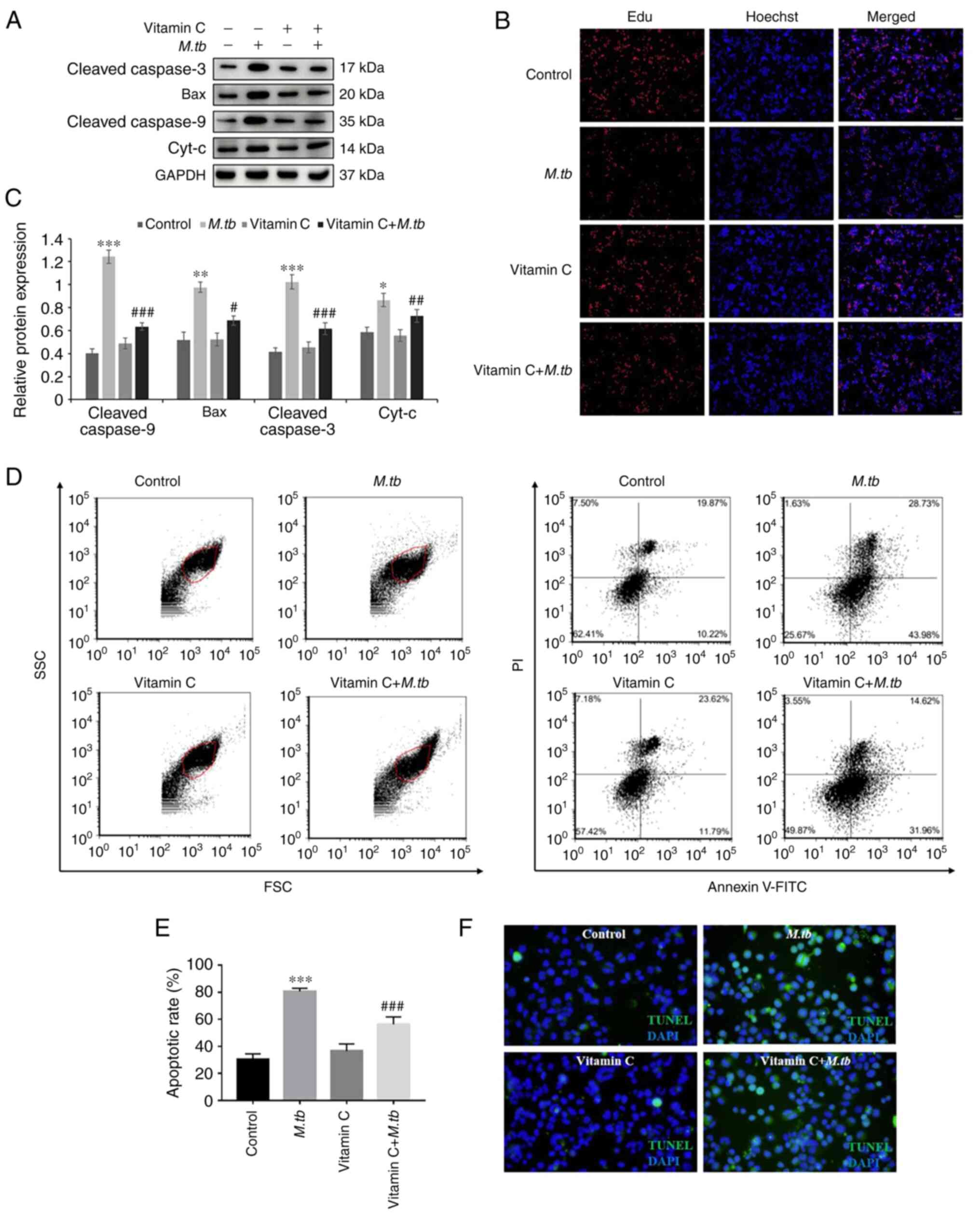 | Figure 3Effect of M. tb, vitamin C
(100 µM/ml) and the combination of both on THP-1 cell apoptosis.
(A) After M. tb, vitamin C and vitamin C + M. tb
treatment for 24 h, the protein expression levels of the apoptosis
factor markers Cleaved caspase-3, Bax, Cleaved caspase-9 and
Cyt-c were analyzed using western blotting. (B)
Representative images of EdU staining in the control, M. tb,
vitamin C and vitamin C + M. tb groups. EdU labelling is
shown in red, and nuclei are labelled by Hoechst staining in blue
(magnification, x100). (C) Cleaved caspase-3, Bax, Cleaved
caspase-9 and Cyt-c protein semi-quantitative expression
levels. GAPDH was used as a loading control. (D) Cell apoptosis was
detected by Alexa® Fluor 488 Annexin V and PI assay. (E)
Apoptotic rate (%) in the control, M. tb, vitamin C and
vitamin C + M. tb groups. (F) Representative images of TUNEL
staining in the control, M. tb, vitamin C and vitamin C +
M. tb groups. TUNEL labelling is shown in green, and nuclei
are labelled using DAPI (blue). Scale bar, 50 µM.
*P<0.05, **P<0.01 and
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. M.
tb . M. tb, Mycobacterium tuberculosis;
Cyt-c, cytochrome c. |
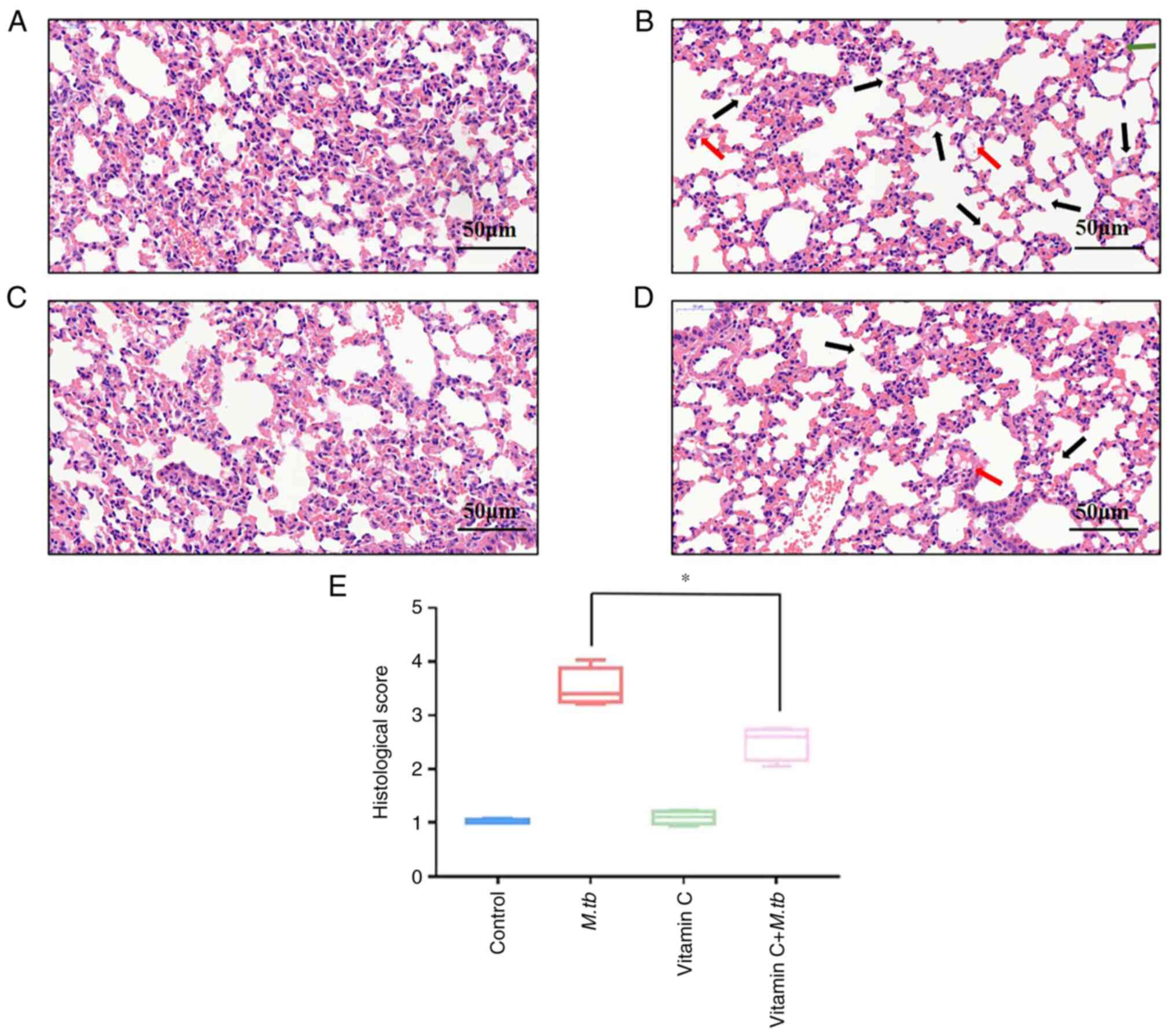
Vitamin C reduces lung damage after M.
tb infection
Finally, to confirm the immunosuppressive function
of M. tb and the protective effect of vitamin C, mice were
treated with vitamin C and M. tb intragastrically,
hematoxylin and eosin staining was performed and the pathological
changes in the lung tissue were observed under a microscope. The
lung tissue sections from the control group (Fig. 4A) and the vitamin C group (Fig. 4C) exhibited a clear alveolar
structure with no edema, congestion or inflammatory cell
infiltration in the interstitial space. In the M. tb group
(Fig. 4B), lung tissue sections
were notably damaged, with interstitial edema, congestion and
inflammatory cell infiltration, indicating a pathological
appearance. Compared with the M. tb group, the degree of
alveolar wall damage and infiltrating inflammatory cells in the
vitamin C + M. tb group were also significantly decreased
(Fig. 4D and E).
Discussion
The pathogenic mechanism of M. tb has always
been the focus of tuberculosis research (17). According to WHO statistics, the
death rate of pulmonary tuberculosis is 1/10,000. Due to the
relatively effective anti-tuberculosis drug treatment currently
available, the mortality rate of pulmonary tuberculosis is
significantly lower than that before drug treatment (18). Although the rate of mortalities
caused by tuberculosis has declined, it remains one of the most
serious threats to global public health (19). Therefore, exploring a novel method
to study the immunomodulatory effect of M. tb in the process
of alveolar macrophage infection and the immune escape mechanism of
pathogens has become a subject of current research.
Vitamin C is an important element in the body and
plays a notable role in the immune system. Previous studies have
revealed that vitamin C can improve immunity by generating
(20) and activating immune cells
(21) and resisting cell damage
caused by pathogens and free radicals (22). The possible mechanism of action of
vitamin C as an antioxidant is by inhibiting the production of
reactive oxygen species (ROS) (23), and it is also a powerful regulator
of Ca2+ signaling (24). Therefore, vitamin C may alter
intracellular Ca2+ homeostasis and affect apoptosis.
However, the ability of vitamin C to protect THP-1 cells infected
by M. tb may help to understand the pathological mechanism
of tuberculosis.
Inflammation is a basic pathological process,
corresponding to the body's defense response to the stimulation of
various pro-inflammatory factors (25). THP-1 cells can secrete cytokines,
such as TNF-α, IL-2, IL-3, granulocyte macrophage
colony-stimulating factor and NLRP3, amongst others. IL-8, also
known as C-X-C motif chemokine ligand 8, is a cytokine secreted by
macrophages and epithelial cells (26). Chemokines/inflammatory factors play
an important role in the cellular immune response (27). Pro-inflammatory cytokines are
needed for the coordination of cell-mediated immune responses
(28), often regulating the
growth, activation, differentiation and homing of immune cells to
the site of infection, aiming to control and eradicate
intracellular pathogens (29).
Therefore, these pro-inflammatory cytokines have been identified as
molecular targets for inflammation control (30). The present study demonstrated that
treatment with M. tb significantly increased the levels of
inflammatory factors in cells. It was concluded that vitamin C may
attenuate M. tb infection by inhibiting inflammation.
Apoptosis is a cellular mechanism of programmed cell
death that is tightly regulated by a family of proteases called
caspases (31), which are normally
present in healthy cells as inactive precursors, but become active
during apoptosis (32). Apoptosis
plays a notable role in cell survival under certain stress
conditions by scavenging proteins and damaged organelles to
maintain cell homeostasis and integrity (33). The major effector caspases, caspase
3 and 9(34), are the key
molecules in the intrinsic pathway of apoptosis (35). Caspase-3 is present in the cell as
an inactive dimer, which is cleaved and activated by
Caspase-9(36). Different
proteases cleave the caspase-3 zymogen to activate it (Cleaved
Caspase-3). Cleaved Caspase-3 further cleaves different substrates,
leading to the expansion of the protease cascade and eventually
cell death (37). The cleaved
substrates lead to alterations in protein function and thus
cellular changes associated with apoptosis. Therefore, caspase-3
activation leads to induction of cellular apoptosis (38).
Bcl-2 is the founding member of the Bcl-2 family of
apoptosis regulatory proteins that either induce (pro-apoptotic) or
inhibit (anti-apoptotic) apoptosis (39). The anti-apoptotic Bcl-2 is
classified as an oncogene, as damage to the Bcl-2 gene has been
shown to cause a number of types of cancer, including lymphoma
(40). Bcl-2 inhibits apoptosis by
preventing the release of Cyt-c from the mitochondria to the
cytoplasm (41). Cyt-c
activated in response to apoptotic stimuli and alter the
permeability of the mitochondrial outer membrane, which is
considered a key step in apoptosis (42).
Cyt-c possesses multiple functions, including
the generation and scavenging of ROS, and it plays an important
role in the mitochondrial electron transport chain (43). In addition, Cyt-c is a key
regulator of apoptosis. In the current study, THP-1 cells were
incubated with M. tb for 24 h with vitamin C or vitamin C +
M. tb. First, it was demonstrated that M. tb
treatment significantly decreased expression of the anti-apoptotic
protein Bcl-2. The protein expression levels of cleaved caspase-3,
Bax, cleaved caspase-9 and Cyt-c in the vitamin C + M.
tb groups were significantly decreased compared with the M.
tb group. Next, an EdU cell proliferation assay was used to
assess cell proliferation, and the results indicated that red
fluorescence representing proliferation was inhibited by M.
tb treatment and was promoted by vitamin C. These results
supported the hypothesis that vitamin C decreased apoptosis and
promoted cell proliferation in THP-1 cells infected with M.
tb.
In conclusion, the present study demonstrated that
the molecular mechanism of vitamin C promoted apoptosis in THP-1
cells infected by M. tb. However, the precise mechanism by
which vitamin C promotes the expression of apoptosis-related
proteins needs further research and it may also be necessary to
repeat the experiments in a different type of macrophage cell line
to further test the hypothesis. Vitamin C may reduce the damage to
mitochondrial function caused by M. tb infection by
inhibiting the release of apoptosis-related proteins and
inflammatory factors. The results indicated that the mechanism of
action of vitamin C may enhances the signal transduction in the
host cell to inhibit phagosome and lysosome fusion, eventually
inhibiting apoptotic signaling pathways present in macrophages to
decrease the occurrence of apoptosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Key Project of
Research and Development of Ningxia Hui Autonomous Region of China
(grant nos. 2020BEG03019 and 2022BEG03126), Natural Science
Foundation of Ningxia (grant nos. 2022AAC03029, 2021AAC03109,
2022AAC03548 and 2022AAC03470) and Key Project of Research and
Development of Ningxia Hui Autonomous Region of China (grant no.
2021BEG03090).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YuW designed the project, revised the article and
provided technical guidance. DX designed the project, revised the
article and coordinated all aspects of the present work. FS and YiW
participated in all experiments, performed data analysis, created
the figures and wrote the article. XL was responsible for sample
preparation and documentation. YiW and FS confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments and experimental protocols
were approved by the Ethics Committee of Ningxia University
(Yinchuan, China; approval no. 2020-024).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu H, Zhu T, Li Q, Xiong X, Wang J, Zhu
X, Zhou X, Zhang L, Zhu Y, Peng Y, et al: TRIM25 upregulation by
Mycobacterium tuberculosis infection promotes intracellular
survival of M. tb in RAW264.7 cells. Microb Pathog.
148(104456)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rajaram MV, Ni B, Dodd CE and Schlesinger
LS: Macrophage immunoregulatory pathways in tuberculosis. Semin
Immunol. 26:471–485. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang H, Lu Q, Zhang J, Qu W, Xie S, Huang
L, Yuan Z and Pan Y: Discovery of novel nitrogenous
heterocyclic-containing quinoxaline-1,4-di-N-oxides as potent
activator of autophagy in M. tb-infected macrophages. Eur J Med
Chem. 223(113657)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Qin Z: The use of THP-1 cells as a model
for mimicking the function and regulation of monocytes and
macrophages in the vasculature. Atherosclerosis. 221:2–11.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ali S, Ehtram A, Arora N, Manjunath P, Roy
D, Ehtesham NZ and Hasnain SE: The M. tuberculosis Rv1523
methyltransferase promotes drug resistance through
methylation-mediated cell wall remodeling and modulates macrophages
immune responses. Front Cell Infect Microbiol.
11(622487)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dallenga T, Repnik U, Corleis B, Eich J,
Reimer R, Griffiths GW and Schaible UE: M, M. tuberculosis-induced
necrosis of infected neutrophils promotes bacterial growth
following phagocytosis by macrophages. Cell Host Microbe.
22:519–530.e3. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang H, Chen J, Chen Y, Jiang Y, Ge B and
Hong L: Sirtuin inhibits M. tuberculosis-induced apoptosis in
macrophage through glycogen synthase kinase-3β. Arch Biochem
Biophys. 694(108612)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fol M, Druszczynska M, Wlodarczyk M,
Ograczyk E and Rudnicka W: Immune response gene polymorphisms in
tuberculosis. Acta Biochim Pol. 62:633–640. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Adikesavalu H, Gopalaswamy R, Kumar A,
Ranganathan UD and Shanmugam S: Autophagy induction as a
host-directed therapeutic strategy against Mycobacterium
tuberculosis infection. Medicina (Kaunas).
57(522)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kuhn SO, Meissner K, Mayes LM and Bartels
K: Vitamin C in sepsis. Curr Opin Anaesthesiol. 31:55–60.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aumailley L and Lebel M: The impact of
vitamin C on different system models of Werner syndrome. Antioxid
Redox Signal. 34:856–874. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van Gorkom GNY, Lookermans EL, Van Elssen
CHMJ and Bos GMJ: The effect of vitamin C (ascorbic acid) in the
treatment of patients with cancer: A systematic review. Nutrients.
11(977)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Varghese GM, Turaka VP, Janardhanan J,
Yadav S, Lakshmi KM, S VT and Cherayil B: Serum siderocalin levels
in patients with tuberculosis and HIV infection. Int J Infect Dis.
85:132–134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chanput W, Mes JJ and Wichers HJ: THP-1
cell line: An in vitro cell model for immune modulation approach.
Int Immunopharmacol. 23:37–45. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu H, Zhang M, Li W, Zhu S and Zhang D:
Stachydrine attenuates IL-1β-induced inflammatory response in
osteoarthritis chondrocytes through the NF-κB signaling pathway.
Chem Biol Interact. 326(109136)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wawrocki S and Druszczynska M:
Inflammasomes in Mycobacterium tuberculosis-driven immunity.
Can J Infect Dis Med Microbiol. 2017(2309478)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Silva DR, Menegotto DM, Schulz LF, Gazzana
MB and Dalcin Pde T: Factors associated with mortality in
hospitalized patients with newly diagnosed tuberculosis. Lung.
188:33–41. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Connor SR: Palliative care for
tuberculosis. J Pain Symptom Manage. 55:S178–S180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Carr AC and Maggini S: Vitamin C and
immune function. Nutrients. 9(1211)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ang A, Pullar JM, Currie MJ and Vissers
MCM: Vitamin C and immune cell function in inflammation and cancer.
Biochem Soc Trans. 46:1147–1159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ferrada L, Barahona MJ, Salazar K,
Vandenabeele P and Nualart F: Vitamin C controls neuronal
necroptosis under oxidative stress. Redox Biol.
29(101408)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Carr AC, Spencer E, Dixon L and Chambers
ST: Patients with community acquired pneumonia exhibit depleted
vitamin C status and elevated oxidative stress. Nutrients.
12(1318)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Herb M and Schramm M: Functions of ROS in
macrophages and antimicrobial immunity. Antioxidants (Basel).
10(313)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fu C, Chen J, Lu J, Yi L, Tong X, Kang L,
Pei S, Ouyang Y, Jiang L, Ding Y, et al: Roles of inflammation
factors in melanogenesis (review). Mol Med Rep. 21:1421–1430.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME,
Teijeira Á, Oñate C, González Á, Ponz M, Schalper KA, Pérez-Gracia
JL and Melero I: Interleukin-8 in cancer pathogenesis, treatment
and follow-up. Cancer Treat Rev. 60:24–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim YG, Kim SM, Kim KP, Lee SH and Moon
JY: The role of inflammasome-dependent and inflammasome-independent
NLRP3 in the kidney. Cells. 8(1389)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6(a016295)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shao BZ, Xu ZQ, Han BZ, Su DF and Liu C:
NLRP3 inflammasome and its inhibitors: A review. Front Pharmacol.
6(262)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Y, Liu X, Shi H, Yu Y, Yu Y, Li M and
Chen R: NLRP3 inflammasome, an immune-inflammatory target in
pathogenesis and treatment of cardiovascular diseases. Clin Transl
Med. 10:91–106. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cui L, Bu W, Song J, Feng L, Xu T, Liu D,
Ding W, Wang J, Li C, Ma B, et al: Apoptosis induction by
alantolactone in breast cancer MDA-MB-231 cells through reactive
oxygen species-mediated mitochondrion-dependent pathway. Arch Pharm
Res. 41:299–313. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Prasad S, Gupta SC and Tyagi AK: Reactive
oxygen species (ROS) and cancer: Role of antioxidative
nutraceuticals. Cancer Lett. 387:95–105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lv X, Zhou X, Yan J, Jiang J and Jiang H:
Propofol inhibits LPS-induced apoptosis in lung epithelial cell
line, BEAS-2B. Biomed Pharmacother. 87:180–187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang H, Zhu J, Jiang L, Shan B, Xiao P, Ai
J, Li N, Qi F and Niu S: Mechanism of Heshouwuyin inhibiting the
Cyt c/Apaf-1/Caspase-9/Caspase-3 pathway in spermatogenic cell
apoptosis. BMC Complement Med Ther. 20(180)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
An HK, Chung KM, Park H, Hong J, Gim JE,
Choi H, Lee YW, Choi J, Mun JY and Yu SW: CASP9 (caspase 9) is
essential for autophagosome maturation through regulation of
mitochondrial homeostasis. Autophagy. 16:1598–1617. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
D'Amelio M, Cavallucci V and Cecconi F:
Neuronal caspase-3 signaling: not only cell death. Cell Death
Differ. 17:1104–1114. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wall DM and McCormick BA: Bacterial
secreted effectors and caspase-3 interactions. Cell Microbiol.
16:1746–1756. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tawa P, Hell K, Giroux A, Grimm E, Han Y,
Nicholson DW and Xanthoudakis S: Catalytic activity of caspase-3 is
required for its degradation: stabilization of the active complex
by synthetic inhibitors. Cell Death Differ. 11:439–447.
2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ebrahim AS, Sabbagh H, Liddane A, Raufi A,
Kandouz M and Al-Katib A: Hematologic malignancies: Newer
strategies to counter the BCL-2 protein. J Cancer Res Clin Oncol.
142:2013–2022. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pattingre S and Levine B: Bcl-2 inhibition
of autophagy: A new route to cancer? Cancer Res. 66:2885–2888.
2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Willis S, Day CL, Hinds MG and Huang DC:
The Bcl-2-regulated apoptotic pathway. J Cell Sci. 116:4053–4056.
2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shakeri R, Kheirollahi A and Davoodi J:
Apaf-1: Regulation and function in cell death. Biochimie.
135:111–125. 2017.PubMed/NCBI View Article : Google Scholar
|