Introduction
Ischemia-reperfusion (I/R) injury is a major cause
of acute kidney injury (AKI), which has a high morbidity and
mortality rate (1). It was found
that AKI was accompanied by free radical burst, which promoted
inflammatory response and apoptosis in the kidney (2). Herein, effective antioxidants against
reactive oxygen species (ROS) stress may be applied to protect
against I/R-induced renal injury and dysfunction.
It has been reported that AKI is accompanied by the
imbalance between oxidation and antioxidation metabolism in renal
tissue, which is manifested by the outbreak of free radicals and
the increase of oxidative stress (3). And further lead to the increase of
inflammatory response, DNA damage, and endoplasmic reticulum
stress, and finally induce renal cell apoptosis (4,5).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is considered a
key regulator for maintaining the redox balance and is involved in
the initiation of the transcriptional expression of downstream
antioxidant enzymes (6). According
to recent studies, Nrf2 activation plays a pivotal role in
preventing I/R induced multiple organ injury by upregulating
antioxidant, anti-inflammatory, and anti-apoptotic effects
(3,7-9).
Hydroxysafflor yellow A (HSYA, molecular formula,
C27H32O16; molecular weight,
612.53 g/mol), derived from Carthamus tinctorius L.
(Fig. 1A and B), is a chalcone glycoside and
predominantly used for therapy against multiple organ injury
dependent on its antioxidant, anti-inflammatory, and anti-apoptotic
properties (10). Previous studies
suggested that HSYA may protect the myocardium, cerebral, liver,
and kidney from I/R injury (11-14).
According to previous studies, administration with HSYA ameliorates
I/R induced AKI via blockade of TLR4/NF-κB pathway (15). Additionally, studies showed that
HSYA played protective effects against myocardial I/R injury by
activating the Akt/Nrf2/HO-1 pathway (16). However, whether HSYA protects
against I/R induced AKI via Nrf2-mediated exogenous antioxidant
defenses and its influence on AKI-related renal dysfunction remain
to be elucidated.
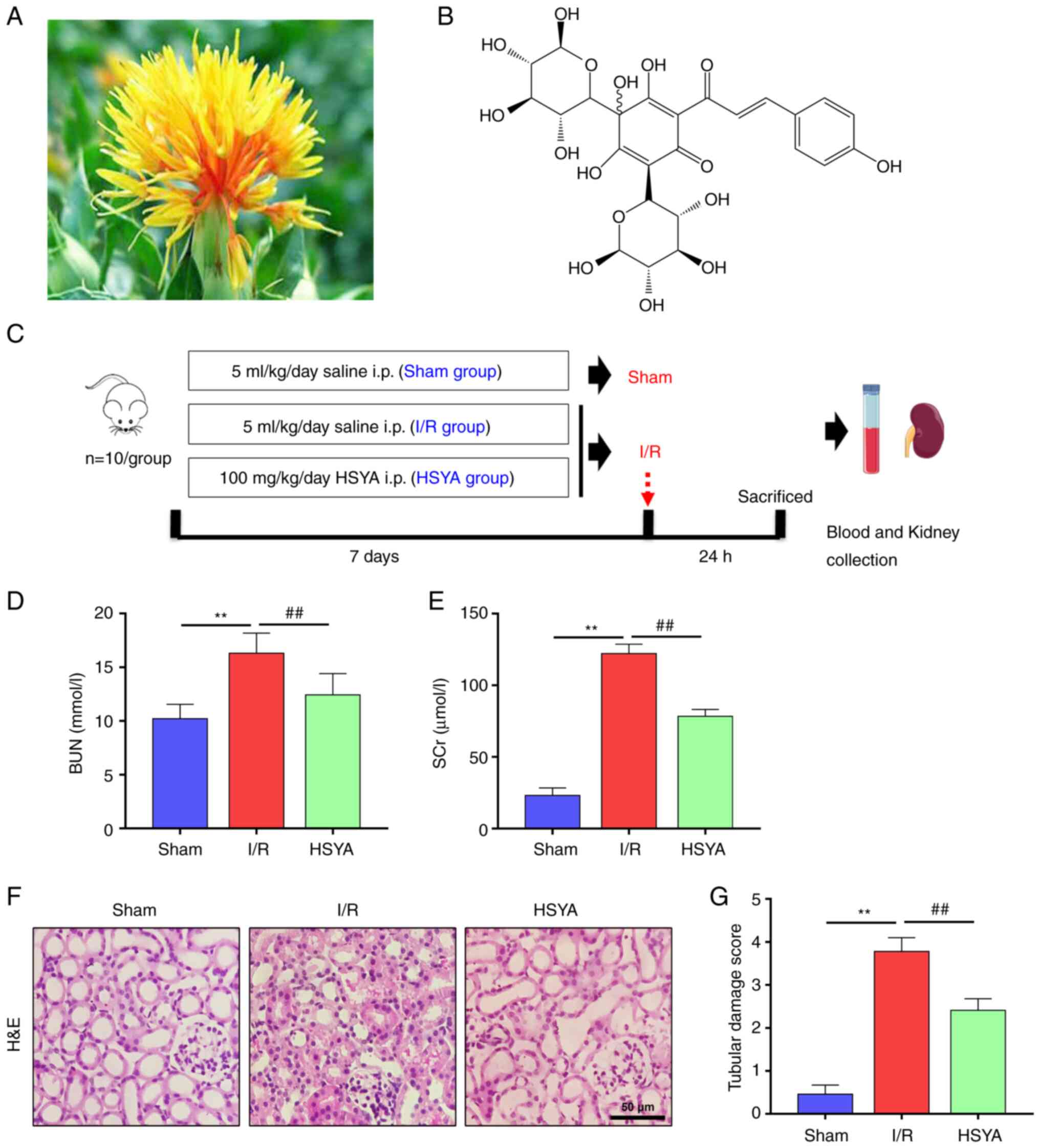 | Figure 1Effect of HSYA on renal function
parameters and histopathology in the kidney of I/R mice. (A) The
picture of Carthamus tinctorius L. (safflower). (B) The
chemical structure of HSYA (molecular formula, C27H32O16). (C) The
scheme of the experimental design. Renal functions were assessed by
(D) BUN and (E) SCr levels at the corresponding time points after
I/R. (F) Representative photomicrographs of H&E stained kidney
sections (scale bar, 50 µm). (G) Quantification of histological
renal damage. All data are expressed as the mean ± SD.
**P<0.01, Sham group vs. I/R group;
##P<0.01, I/R group vs. HSYA group. This image was
produced using images from Servier Medical Art, licensed under a
Creative Common Attribution 3.0 Generic License http://smart.servier.com. HSYA, Hydroxysafflor yellow
A; BUN, blood urea nitrogen; SCr, serum creatinine; I/R,
ischemia/reperfusion; H&E, hematoxylin and eosin. |
The purpose of the present study was to evaluate the
protective effects and underlying mechanisms of HSYA against renal
I/R injury in both in vitro and in vivo models. The
present results showed that HSYA could be used for preventing
I/R-induced renal injury via targeting the Akt/GSK-3β/Fyn-Nrf2 axis
pathway.
Materials and methods
Animals and ethics statement
Male C57BL/6 mice (8 weeks old, 20-24 g) were
obtained from the animal research center at Lvye Pharmaceutical
Co., Ltd. (Shandong, China; SYXK 2018-0028). Mice were housed in a
standard environment (40-60% humidity and 25˚C temperature) with a
regular light/dark cycle and free access to water and chow diet.
The project was approved (approval no. 2021-11) by the Ethics
Committee of Binzhou Medical University (Yantai, China).
Establishment of the renal I/R
model
The mice model of renal I/R injury was induced
according to the protocol previously described (17). Mice were fully anesthetized with
pentobarbital sodium (50 mg/kg, i.p.) and placed on a homeothermic
table to maintain core body temperature at 37˚C. A midline
laparotomy was then performed in which the abdominal cavity was
fully exposed. Next, the left kidney was subjected to 30 min of
ischemia with a non-traumatic vascular clamp followed by
reperfusion.
The mice were randomly and equally divided into 3
groups (n=10) as depicted in Fig.
1C: Sham group, the right kidney was extirpated for
sham-operated animals, but neither clamping nor infusion was
performed in the left kidney; HSYA group, mice subjected to renal
I/R injury were treated with HSYA (100 mg/kg/day) by
intraperitoneal injection for 7 consecutive days before the I/R
model established; I/R group, mice subjected to renal I/R injury
were treated with an identical volume of saline as the vehicle.
HSYA (purity >98%) was purchased from MedChemExpress (cat. no.
HY-N0567). Analgesics (carprofen, 5 mg/kg, s.c.) and anaesthetics
(propofol, 26 mg/kg, i.v.) were used to minimize the suffering of
mice during the surgery. A total of 24 h after the I/R procedure,
mice were anesthetized with pentobarbital sodium (40 mg/kg, i.p.)
and blood was collected from the eye socket, then sacrificed by
cervical dislocation. After careful evaluation, an animal would be
sacrificed if it began to exhibit symptoms of hypothermia (colonic
temperature of 34˚C) or weight loss >20% (the maximum percentage
of body weight loss observed in the present study was 21.3%),
including hunched posture, immobility, ruffled fur, failure to eat,
and failure to drink. The animal would be sacrificed right away to
minimize pain if it was unable to stand, had trouble breathing, had
significant muscle tightness, severe ulceration, or bled heavily.
Twice daily, special crew monitored and recorded the health and
behavior of the animals (once in the morning and once in the
evening). C57BL/6 mice (n=30) were euthanized after the experiment.
Mice were confirmed dead when there was no autonomous respiration
and no reflex activity, and no heart activity. The kidneys were
harvested, some of them were fixed in 4% paraformaldehyde for
histological evaluation, and the others were snap-frozen in liquid
nitrogen and stored at -80˚C for subsequent protein and mRNA
analysis.
Assessment of kidney functions
Blood was received immediately after the mice were
sacrificed. The blood was centrifuged at 3,000 x g for 15 min at
4˚C to collect serum. Concentrations of blood urea nitrogen (BUN;
cat. no. C013-2-1) and serum creatinine (SCr; cat. no. C011-2-1)
were measured using assay kits (Nanjing Jiancheng Bioengineering
Institute).
Histology and
immunohistochemistry
Kidney tissues were fixed with 4% formaldehyde for
24 h at 4˚C and embedded in paraffin, and then cut into 4-µm
sections and taken on slides. The prepared slides were
deparaffinized twice in xylene and rehydrated in gradient ethanol,
and then stained separately with Hematoxylin and Eosin (H&E) (5
min each, room temperature). Tissue damage was assessed in a
blinded manner and scored using a tubular damage score as
previously described (18), these
changes included tubular epithelial cell swelling, vacuolization,
cast formation, and desquamation. The specimens were analyzed by
confocal microscopy (FV1000; Olympus Corp.).
Detection of oxidative stress
RIPA lysis buffer (1 ml; cat. no. R0010; Beijing
Solarbio Science & Technology Co., Ltd.) was added to 100 mg of
renal tissue and homogenized with glass homogenizer. It was then
placed on ice for 30 min to render it fully homogenized, and then
centrifuged at 13,000 x g for 15 min at 4˚C, and finally
supernatants were collected. ELISA was used to detect
8-hydroxyguanosine (8-OH-dG; cat. no. STA-320-T; Cell BioLabs,
Inc.) in renal tissue, and the colorimetric method was used to
detect malondialdehyde (MDA; cat. no. S0131S) and ROS (cat. no.
S0033S; both from Beyotime Institute of Biotechnology) levels in
renal tissue by using Multiskan FC Microplate Reader (Thermo Fisher
Scientific, Inc.) and Orion AquaMate UV-Vis spectrophotometer
(Thermo Fisher Scientific, Inc.) referring to the product manual
for specific determination methods. The manufacturer's instructions
for the corresponding assay kit were followed.
Detection of antioxidant capacity
The homogenized renal tissue was collected according
to the aforementioned method, and the amine oxidase copper
containing (T-AOC; cat. no. A015-1-2), superoxide dismutase (SOD;
cat. no. A001-3-2), and catalase (CAT; cat. no. A007-1-1)
activities of kidney tissue were measured by the Orion AquaMate
UV-Vis spectrophotometer (Thermo Fisher Scientific, Inc.) with
commercial kits (all from Nanjing Jiancheng Bioengineering
Institute). The manufacturer's instructions for the corresponding
assay kit were followed. Briefly, the protein concentration of the
tissue and cells were first detected by using a Bradford Protein
Assay kit (cat. no. P0006; Beyotime Institute of Biotechnology),
then the commercial kit was used to detect the enzyme activity of
the unit volume sample and calculate the enzyme activity under the
same protein concentration. For example, the detection formula of
SOD enzyme activity is as follows: SOD activity (U/mg protein)=[OD
(control group)-OD (test group)/(OD (control group)] ÷ 50% x[(Total
volume)/(Sample volume)] ÷ Protein concentration (mg prot/ml).
Detection of proinflammatory
cytokines
The serum inflammatory cytokines including monocyte
chemoattractant protein-1 (MCP-1), tumor necrosis factor-alpha
(TNF-α), and interleukin-1β (IL-1β) were measured by the Multiskan
FC Microplate Reader (Thermo Fisher Scientific, Inc.). MCP-1 (cat.
no. SEKP-0019), TNF-α (cat. no. SEKM-0034), and IL-1β (cat. no.
SEKM-0002) ELISA kits were obtained all from Beijing Solarbio
Science & Technology Co., Ltd. The manufacturer's instructions
for the corresponding assay kit were followed.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from kidney tissue using
Trizol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's instructions. The concentration
of total RNA was detected using a biological spectrometer (NanoDrop
2000; Thermo Fisher Scientific, Inc.). A total of 1 µg of total RNA
was reverse transcribed into cDNA at 37˚C for 60 min and 4˚C for 5
min using a First-strand cDNA Synthesis kit (cat. no. E6300L; New
England BioLabs, Inc.) according to the manufacturer's protocol.
qPCR assay was performed by using SYBR green dye on Step One
sequence detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The qPCR reaction conditions were subjected to
an initial predenaturation step at 95˚C for 3 min, followed by 39
cycles of 95˚C for 20 sec and 60˚C for 30 sec. Using β-actin as
internal control, the relative abundance of genes was calculated
using the 2-∆∆Cq formula (19). Primer sequences are presented in
Table SI.
Western blot analysis
The total protein was extracted from the kidney
tissues and HK-2 cells. Briefly, 100 mg of kidney tissues or
5x105 HK-2 cells were homogenized in RIPA buffer for 10
min followed by centrifugation at 13,000 x g for 10 min at 4˚C.
Nuclear proteins were extracted by a nuclear extraction kit (cat.
no. ab113474; Abcam). The concentrations were detected by using a
Bradford Protein Assay kit (cat. no. P0006; Beyotime Institute of
Biotechnology). Western blot analyses were performed as previously
described (20). Briefly, sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was
performed by heating the samples for 8 min at 100˚C and loading 10
µg membrane proteins on to a 5-15% linear acrylamide gradient gel
(10 µg protein/lane). After blocked in 5% BSA (cat. no. SW3015;
Beijing Solarbio Science & Technology Co., Ltd.) dissolved in
TBS-20% Tween 20 (TBST) for 2 h at room temperature, PVDF membranes
were then treated overnight at 4˚C with primary antibodies against
Nrf2 (1:3,000; cat. no. 12721), phosphorylated (p)-Akt (1:3,000;
cat. no. 4060), Akt (1:3,000; cat. no. 9272), HO-1 (1:3,000; cat.
no. 43966), NQO1 (1:3,000; cat. no. 62262), GSK-3β (1:3,000; cat.
no. 12456), p-GSK-3β (1:3,000; cat. no. 5558), Fyn (1:3,000; cat.
no. 4023), Histone H3 (1:3,000; cat. no. 4499), Cleaved Caspase-3
(1:3,000; cat. no. 9661) and GAPDH (1:3,000; cat. no. 5174) which
were all obtained from Cell Signaling Technology, Inc. The
membranes were then incubated with a secondary antibody (1:5,000;
anti-rabbit IgG (H + L), cat. no. 14708; Cell Signaling Technology,
Inc.) for 2 h at room temperature, followed by TBST washes. ECL
plus detection reagents (cat. no. P0018FS; Beyotime Institute of
Biotechnology) were used to visualize protein bands. The ImageJ gel
analysis software (version 1.53; National Institutes of Health) was
used for densitometric analysis.
Cell culture and hypoxia/reoxygenation
(H/R) model
The human renal proximal tubular epithelial cell
line (HK-2) was obtained from the Type Culture Collection of the
Chinese Academy of Sciences and cultured at 37˚C in DMEM/F-12 with
1% v/v penicillin/streptomycin and 10% v/v fetal bovine serum (cat.
no. 10100147; Thermo Fisher Scientific, Inc.). A cell H/R model was
established according to the previously described methods (21,22).
Briefly, HK-2 cells were cultured for 12 h under hypoxic conditions
(1% O2, 5% CO2, and 94% N2) in a
medium without nutrients (glucose-free, serum-free) to induce
hypoxic injury. Then, the medium was refreshed again, and the
plates were moved to a normoxic cell incubator (5% CO2
and 95% air) for 2 h. Control cells were incubated in a complete
culture medium in a regular incubator (5% CO2 and 95%
air).
Cells were divided into five groups and were
cultured with corresponding medium supplied with different
reagents. The five groups included: i) Control group; ii) H/R model
group; iii) H/R model + HSYA (5 µg/ml) group, HK-2 cells were
pretreated with 5 µg/ml HSYA for 12 h; iv) H/R model + HSYA (10
µg/ml) group, HK-2 cells were pretreated with 10 µg/ml HSYA for 12
h; 5) H/R model + HSYA (20 µg/ml) group, HK-2 cells were pretreated
with 20 µg/ml HSYA for 12 h; 6) H/R model + HSYA (20 µg/ml) group +
Akt inhibitor LY294002 (30 µM) group, HK-2 cells were pretreated
with 20 µg/ml HSYA and 30 µM LY294002 (cat. no. HY-10108;
MedChemExpress) for 12 h.
Cell viability assay
Cell viability was assayed using CCK-8 (cat. no.
C0037; Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Briefly, HK-2 cells were cultured in
six-well plates at a density of 5x104/well. A total of
10 µl CCK-8 solution/well was added and HK-2 cells were incubated
for 30 min at 37˚C. The amount of formazan dye generated by
cellular dehydrogenase activity was measured by absorbance at 450
nm with a microplate reader.
Statistical analysis
Statistical analyses were performed using SPSS
(version 19.0; IBM Corp.). Three independent experiments are
represented as the mean ± standard deviation. One way analysis of
variance with Tukey's post hoc test and the unpaired Student's
t-test were used for comparisons between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
HSYA alleviates renal function
parameters and histopathology in I/R mice
To determine the effect of HSYA on renal function
after I/R, the concentration of SCr and BUN were evaluated. As
shown in Fig. 1D and E, the concentration of SCr and BUN was
significantly higher in the I/R group compared with the Sham group.
However, compared with the I/R group, SCr and BUN concentrations
were significantly decreased in the HSYA group.
To determine the effect of HSYA on renal injury
after I/R, H&E staining renal histology were evaluated
(Fig. 1F). H&E staining
results revealed renal structural damage, inflammatory cell
infiltration, and extracellular matrix deposition. However,
pretreatment with HSYA reduced the severity of the tubular injury.
Quantitative analysis showed that mice pre-treated with HSYA
exhibited a significantly lower renal tubular damage score
(Fig. 1G). These results indicated
that HSYA ameliorated renal damage in the I/R mice.
HSYA exerts antioxidant effect on the
kidney of I/R mice
To evaluate the antioxidant effect of HSYA on the
kidney of I/R mice, the levels of oxidative stress and antioxidant
biomarkers were analyzed. As revealed in Fig. 2A-C, the activities of T-AOC, SOD,
and CAT significantly decreased in the I/R group compared with the
Sham group. Furthermore, pretreatment with HSYA significantly
increased the activities of T-AOC, SOD, and CAT in comparison with
the I/R group. By contrast, the levels of ROS, MDA, and 8-OH-dG
significantly increased in the I/R group compared with the Sham
group (Fig. 2D-F). Conversely,
HSYA pretreatment significantly decreased the levels of ROS, MDA,
and 8-OH-dG in the kidney of I/R mice. These results indicated that
HSYA treatment alleviates the imbalance of redox metabolic in the
kidney of I/R mice.
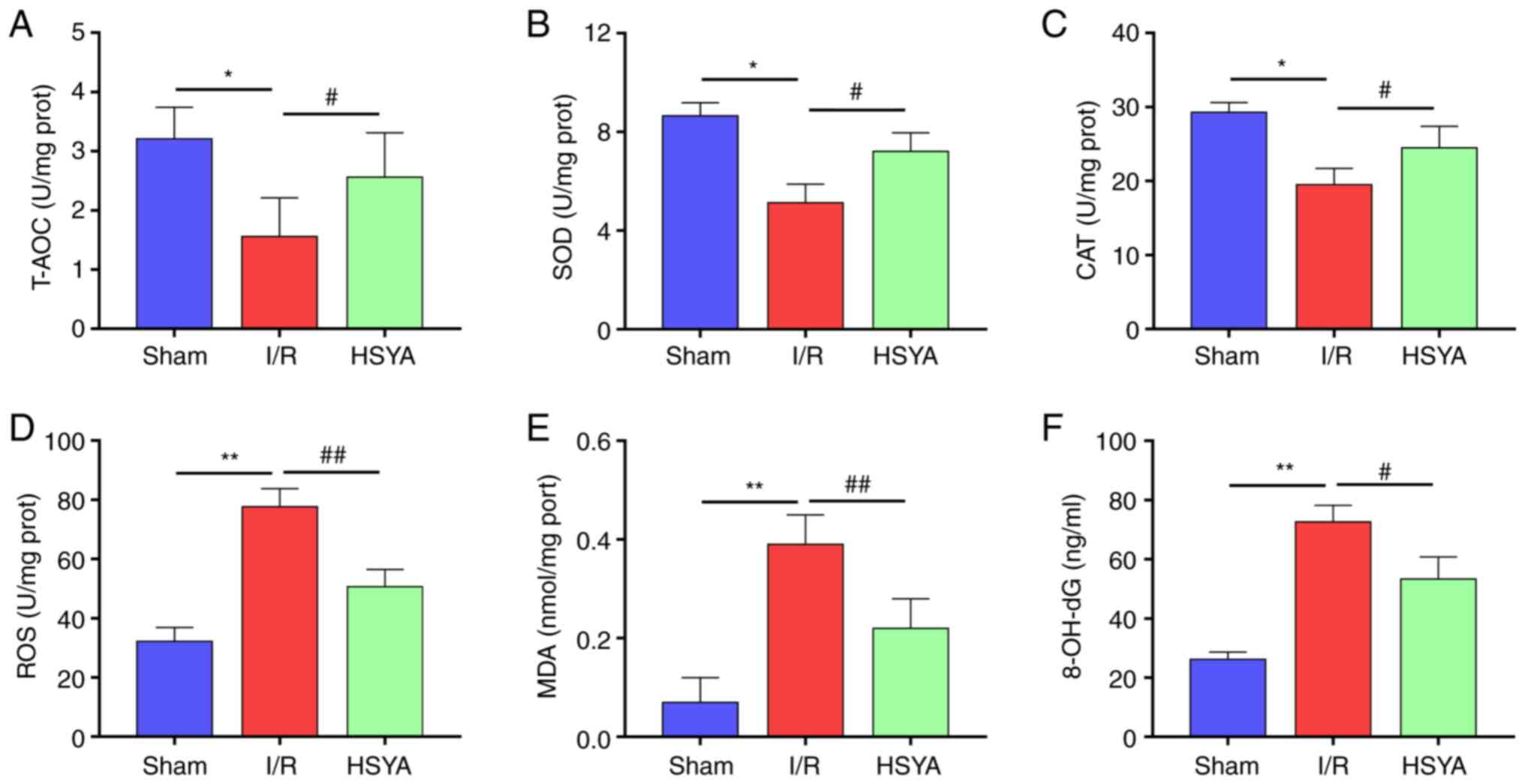 | Figure 2Effect of HSYA on oxidative stress
and antioxidant biomarkers in the kidney of I/R mice. (A-C) The
activities of (A) T-AOC, (B) SOD, and (C) CAT in the kidney tissue.
(D-F) The levels of (D) ROS, (E) MDA, and (F) 8-OH-dG in the kidney
tissue. All data are expressed as the mean ± SD.
*P<0.05 and **P<0.01, Sham group vs.
I/R group; ##P<0.01 and #P<0.05, I/R
group vs. HSYA group. HSYA, Hydroxysafflor yellow A; I/R,
ischemia/reperfusion; SOD, superoxide dismutase; CAT, catalase;
ROS, reactive oxygen species; MDA, malondialdehyde; 8-OH-dG,
8-hydroxyguanosine. |
HSYA ameliorates the expression of
proinflammatory cytokines in I/R mice
The effect of HSYA on the expression of
proinflammatory cytokines in serum and kidney of I/R mice was
further investigated. As revealed in Fig. 3, compared with the Sham group, the
levels of MCP-1, TNF-α and IL-1β significantly increased in the
kidney and serum of I/R mice. Conversely, HSYA pretreatment
significantly decreased the levels of MCP-1, TNF-α, and IL-1β in
the kidney and serum of I/R mice. These results indicated that HSYA
treatment suppresses the inflammatory response in I/R mice.
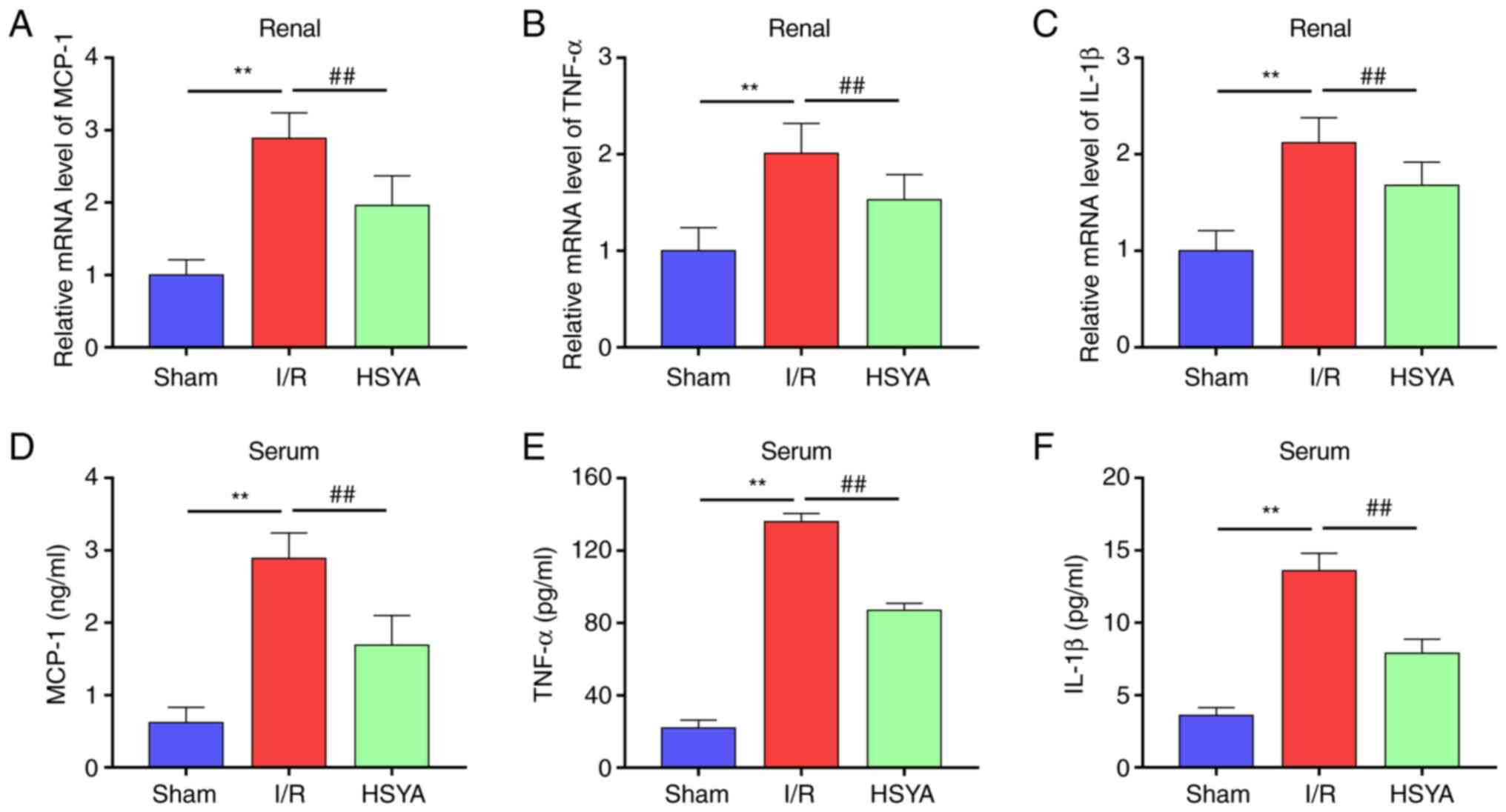 | Figure 3Effect of HSYA on proinflammatory
cytokines in I/R mice. (A-C) The mRNA levels of (A) MCP-1, (B)
TNF-α, and (C) IL-1β in the kidney tissue. (D-F) The levels of (D)
MCP-1, (E) TNF-α, and (F) IL-1β in the serum. All data are
expressed as the mean ± SD. **P<0.01, Sham group vs.
I/R group; ##P<0.01, I/R group vs. HSYA group. HSYA,
Hydroxysafflor yellow A; I/R, ischemia/reperfusion; MCP-1, monocyte
chemoattractant protein-1; TNF-α, tumor necrosis factor-alpha;
IL-1β, interleukin-1β. |
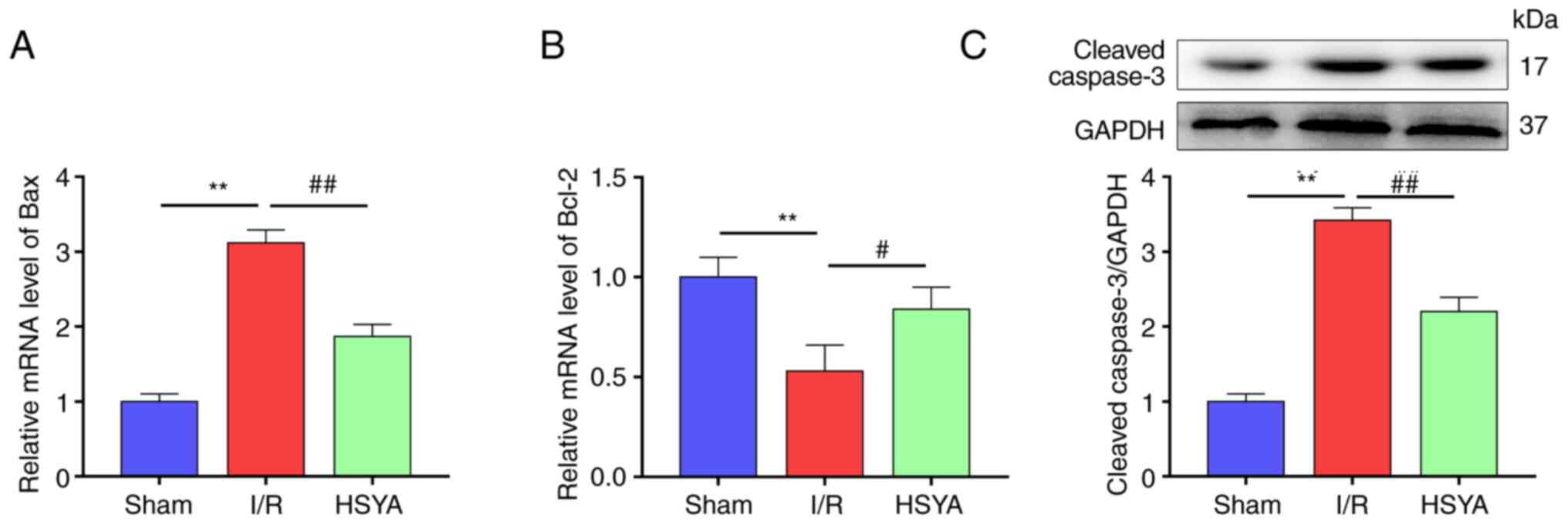
HSYA attenuates apoptosis in the
kidney of I/R mice
To observe the cell apoptosis in the kidney of I/R
mice, the expression of several apoptosis-associated proteins in
the kidney was assessed. As demonstrated in Fig. 4A-C, compared with the Sham group,
the levels of the pro-apoptotic proteins Bax and cleaved caspase-3
were significantly increased and the anti-apoptotic protein Bcl-2
was significantly decreased in the HSYA group. Conversely, HSYA
pretreatment significantly attenuated the decrease of Bcl-2 and the
increase of Bax and caspase-3 in comparison with the I/R group.
HSYA promotes the expression of the
Akt/GSK-3β/Fyn-Nrf2 axis in the kidney of I/R mice
Previous studies demonstrated that Nrf2 plays an
important role in regulating inflammation and redox balance in the
kidney (8-9,23).
In the present study, it was found that the nuclear transfer of
Nrf2 was significantly increased in mice with renal I/R compared
with the Sham group (Fig. 5A and
B). Accordingly, the expression of
Nrf2 target antioxidant genes HO-1 and NQO1 were significantly
higher in the I/R group compared with the Sham group (Fig. 5C-E). In addition, HSYA pretreatment
further increased the nuclear transfer of Nrf2 and significantly
improved the expression of Nrf2 target antioxidant genes HO-1 and
NQO1 in comparison with the I/R group (Fig. 5A-E).
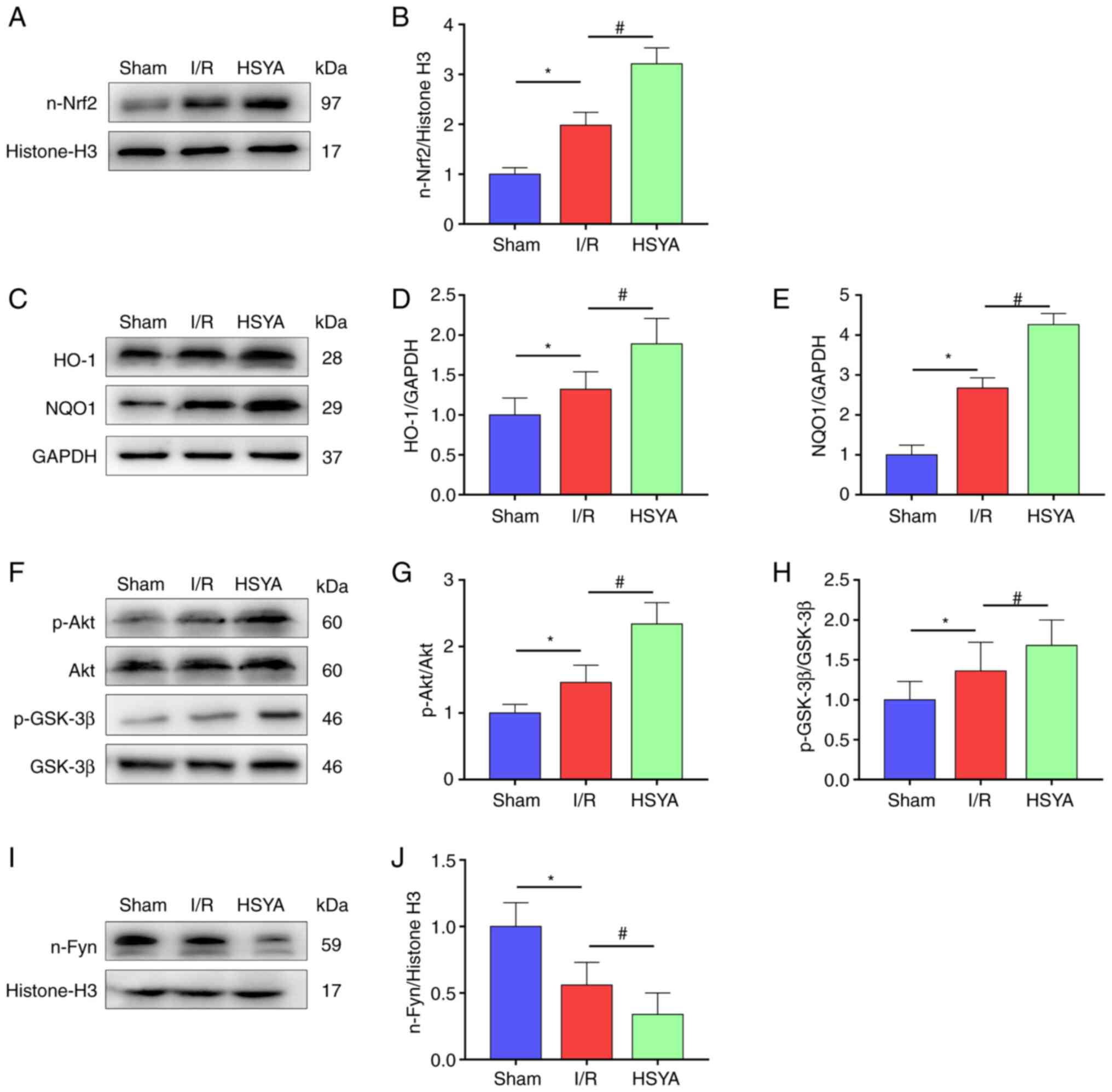 | Figure 5Effect of HSYA on the expression of
the Akt/GSK-3β/Fyn-Nrf2 axis in the kidney of I/R mice. (A and B)
Representative western blot images and summarized data for the
expression of nuclear Nrf2. (C-E) Representative western blot
images and summarized data for the expression of (D) HO-1 and (E)
NQO1. (F-H) Representative western blot images and summarized data
for the ratio of (G) p-Akt/Akt and (H) p-GSK-3β/GSK-3β. (I and J)
Representative western blot images and summarized data for the
expression of nuclear Fyn. The following groups protein bands shown
in this figure originated from the same gel/membrane: 1. HO-1,
NQO1, and GAPDH; 2. n-Nrf2 and Histone-H3; 3. Akt, p-Akt, GSK-3β,
and p-GSK-3β; 4. n-Fyn and Histone-H3. All data are expressed as
the mean ± SD. *P<0.05, Sham group vs. I/R group;
#P<0.05, I/R group vs. HSYA group. HSYA,
Hydroxysafflor yellow A; I/R, ischemia/reperfusion; Nrf2, nuclear
factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; NQO1,
NAD(P)H quinone oxidoreductase 1; p-, phosphorylated. |
Previous studies indicated that the activation of
Nrf2 is regulated by adjusting Fyn-mediated degradation and nuclear
export of Nrf2 (24,25). To investigate the mechanisms by
which HSYA treatment activates Nrf2 transcriptional functions in
the kidney, the total and p-Akt, GSK-3β, and nuclear Fyn levels
were measured. As revealed in Fig.
5F-J, significantly increased phosphorylation of GSK-3β and Akt
and decreased nucleus Fyn level were identified in the I/R group
compared with the Sham group. Furthermore, HSYA pretreatment
further increased the phosphorylation of GSK-3β and Akt and
decreased the level of nucleus Fyn in comparison with the I/R group
(Fig. 5F-J). These results
suggested that HSYA attenuates renal damage in I/R mice by
upregulating Akt/GSK-3β/Fyn-Nrf2 axis-mediated antioxidant gene
expression.
HSYA preserves the viability of
H/R-treated HK-2 cells
Since HSYA pretreatment alleviated I/R kidney damage
in vivo, the effect of HSYA on HK-2 cells was next
investigated in vitro (Fig.
6A). As revealed in Fig. 6B,
H/R significantly decreased the viability of HK-2 cells, which was
restored by HSYA treatment in a dose-dependent manner.
Consistently, H/R significantly increased the levels of
pro-apoptotic proteins Bax and cleaved caspase-3 and decreased the
level of anti-apoptotic protein Bcl-2 in HK2 cells (Fig. 6C-E). Meanwhile, HSYA treatment
alleviated the H/R-induced increase in Bax and cleaved caspase-3
expression and the decrease of Bcl-2 expression in HK2 cells
(Fig. 6C-E). These results
suggested that HSYA preserves the viability of H/R-treated HK-2
cells.
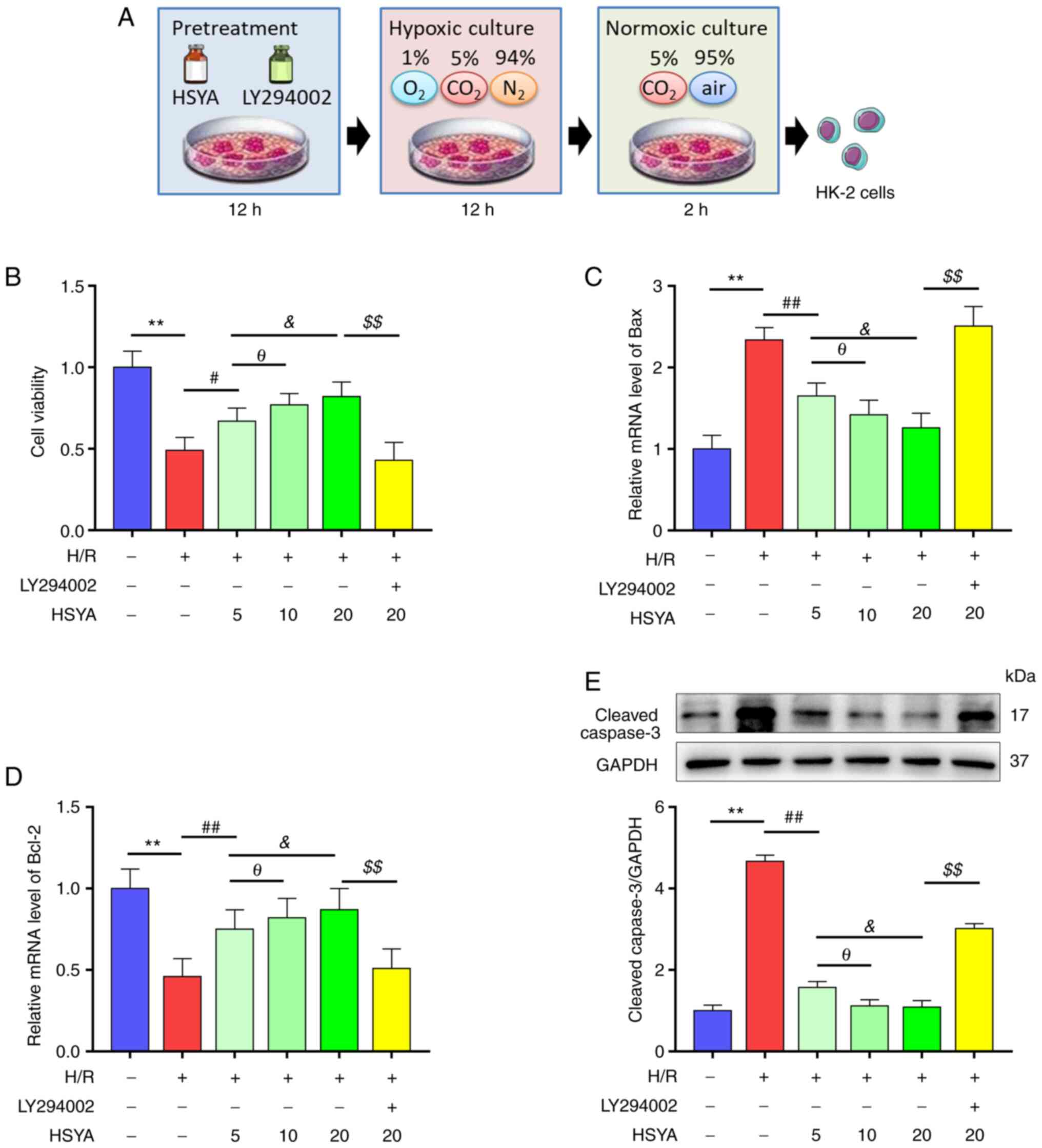 | Figure 6Effect of HSYA on the viability of
H/R-treated HK-2 cells. HK-2 cells were pretreated with or without
Akt inhibitor LY294002 (30 µM) and HSYA at different doses (5, 10,
20 µg/ml) for 12 h, and then were exposed to H/R. (A) The scheme of
the H/R model. (B) Effects of HSYA on the cell viabilities of HK-2
cells. (C and D) Effects of HSYA on the mRNA levels of (C) Bax and
(D) Bcl-2 in HK-2 cells. (E) Effects of HSYA on the expression of
cleaved caspase-3 in HK-2 cells. All data are expressed as the mean
± SD. **P<0.01, control group vs. H/R group;
##P<0.01 and #P<0.05, H/R group vs. 5
µg/ml HSYA group; θP<0.05, 5 µg/ml HSYA group vs. 10
µg/ml HSYA group; &P<0.05, 5 µg/ml HSYA group vs.
20 µg/ml HSYA group; $$P<0.01, 20 µg/ml HSYA group
vs. LY294002 group. This image was produced using images from
Servier Medical Art, licensed under a Creative Common Attribution
3.0 Generic License http://smart.servier.com. HSYA, Hydroxysafflor yellow
A; H/R, hypoxia/reoxygenation. |
HSYA upregulates the antioxidant
capacity and reduces the oxidative stress and inflammation of
H/R-treated HK-2 cells
Furthermore, the effect of HSYA on the antioxidant
capacity, oxidative stress, and inflammation of H/R-treated HK-2
cells was tested. As demonstrated in Fig. 7A-C, H/R significantly decreased the
activities of T-AOC, SOD, and CAT of HK-2 cells. Furthermore,
pretreatment with HSYA significantly increased the activities of
T-AOC, SOD, and CAT of H/R-treated HK-2 cells.
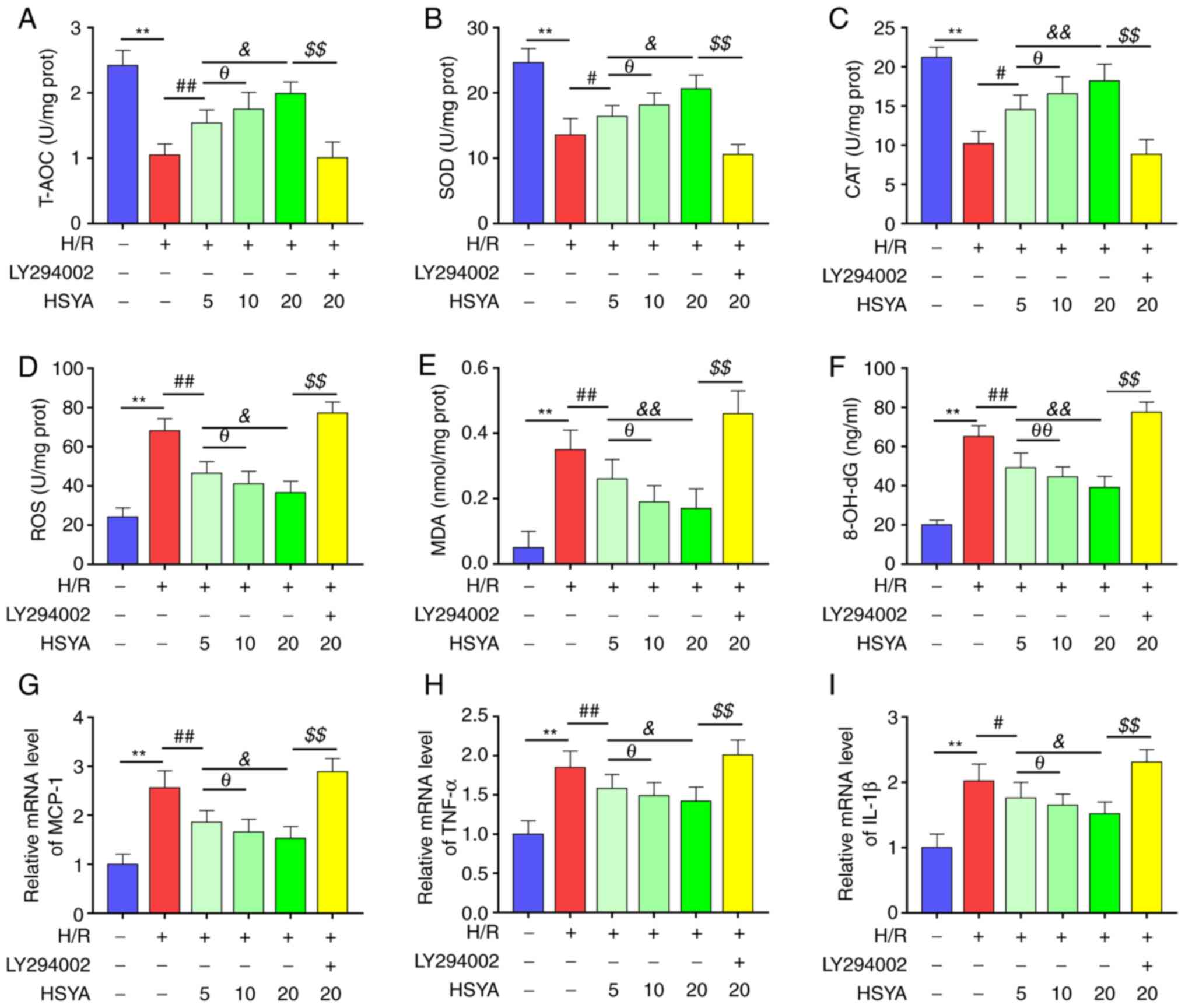 | Figure 7Effect of HSYA on the antioxidant
capacity, oxidative stress, and inflammation of H/R-treated HK-2
cells. The activities of T-AOC (A), SOD (B), and CAT (C) in HK-2
cells. The levels of ROS (D), MDA (E), and 8-OH-dG (F) in HK-2
cells. The mRNA levels of MCP-1 (G), TNF-α (H), and IL-1β (I) in
HK-2 cells. All data are expressed as the mean ± SD.
**P<0.01, control group vs. H/R group;
##P<0.01, #P<0.05, H/R group vs. 5
µg/ml HSYA group; θθP<0.01, θP<0.05, 5
µg/ml HSYA group vs. 10 µg/ml HSYA group;
&&P<0.01, &P<0.05, 5 µg/ml
HSYA group vs. 20 µg/ml HSYA group; $$P<0.01, 20
µg/ml HSYA group vs. LY294002 group. HSYA, Hydroxysafflor yellow A;
H/R, hypoxia/reoxygenation; SOD, superoxide dismutase; CAT,
catalase; ROS, reactive oxygen species; MDA, malondialdehyde;
8-OH-dG, 8-hydroxyguanosine. |
On the other hand, H/R significantly increased the
levels of oxidative stress biomarkers (ROS, MDA, and 8-OH-dG) and
pro-inflammatory cytokines (MCP-1, TNF-α, and IL-1β) of HK-2 cells
(Fig. 7D-I). Conversely, HSYA
pretreatment significantly decreased the levels of oxidative stress
biomarkers and pro-inflammatory cytokines of H/R-treated HK-2
cells. Additionally, the effect of HSYA on the antioxidant
capacity, oxidative stress, and inflammation of H/R-treated HK-2
cells showed a dose-dependent manner.
The Akt/GSK-3β/Fyn-Nrf2 pathway is
involved in the HSYA-mediated protective effect against H/R in HK-2
cells
It was further confirmed whether the
Akt/GSK-3β/Fyn-Nrf2 pathway was critical in the protective effects
observed in H/R-treated HK-2 cells that were pretreated with HSYA.
As shown in Fig. 8A-E, H/R
significantly increased the nuclear transfer of Nrf2 and the
expression of Nrf2 target antioxidant genes HO-1 and NQO1. HSYA
pretreatment further increased the expression of these proteins in
H/R-treated HK-2 cells. Moreover, significantly increased
phosphorylation of Akt and GSK-3β and decreased nucleus Fyn level
was found in HK-2 cells (Fig.
8F-J). HSYA pretreatment significantly increased the
phosphorylation of Akt and GSK-3β and decreased nucleus Fyn level
in H/R-treated HK-2 cells (Fig.
8F-J).
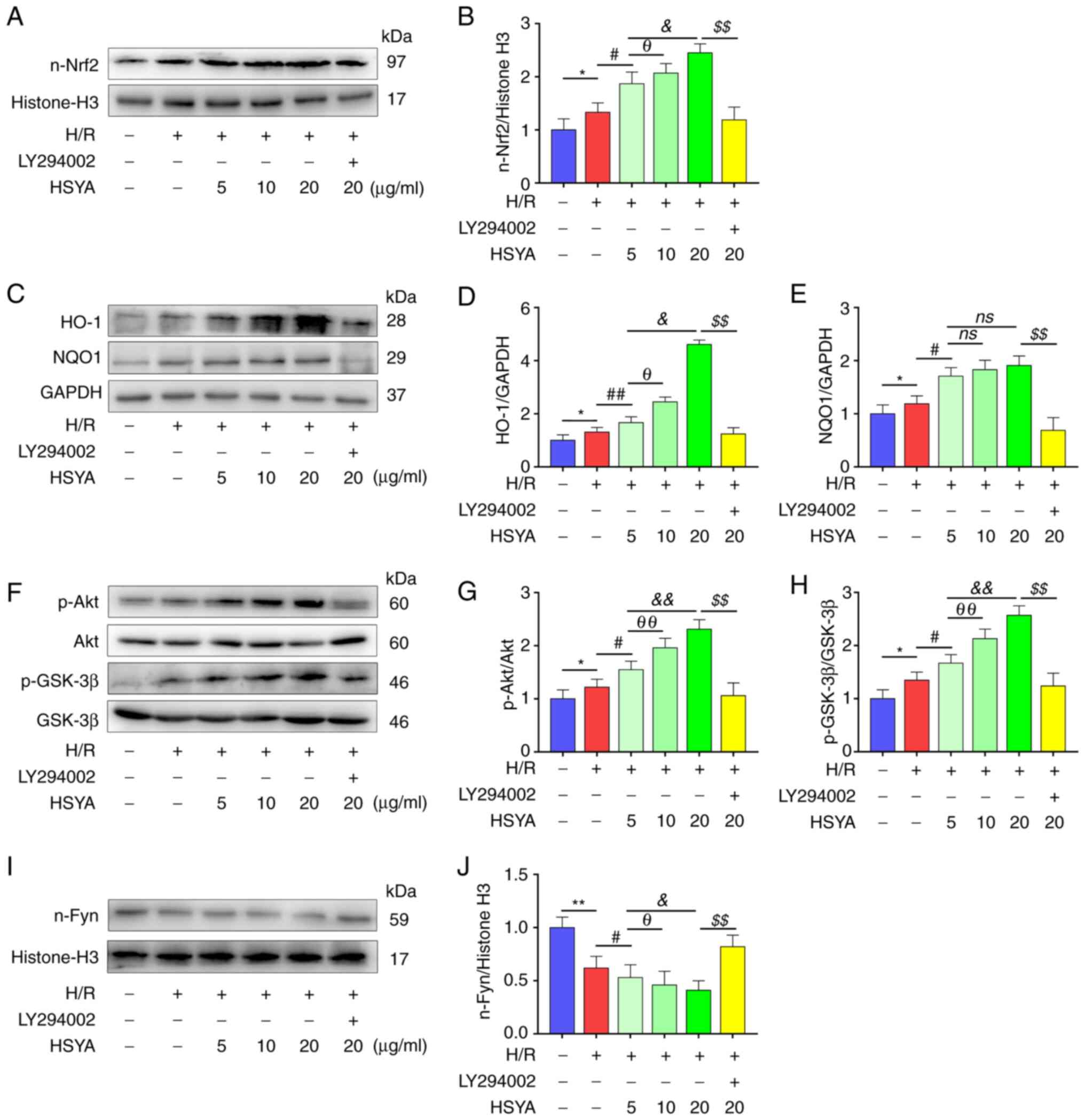 | Figure 8Effect of HSYA on the expression of
the Akt/GSK-3β/Fyn-Nrf2 axis of H/R-treated HK-2 cells. (A and B)
Representative western blot images and summarized data for the
expression of nuclear Nrf2. (C-E) Representative western blot
images and summarized data for the expression of (D) HO-1 and (E)
NQO1. (F-H) Representative western blot images and summarized data
for the ratio of (G) p-Akt/Akt and (H) p-GSK-3β/GSK-3β. (I and J)
Representative western blot images and summarized data for the
expression of nuclear Fyn. The following groups protein bands shown
in this figure originated from the same gel/membrane: 1. HO-1,
NQO1, and GAPDH; 2. n-Nrf2 and Histone-H3; 3. Akt, p-Akt, GSK-3β,
and p-GSK-3β; 4. n-Fyn and Histone-H3. All data are expressed as
the mean ± SD. **P<0.01 and *P<0.05,
control group vs. H/R group; ##P<0.01 and
#P<0.05, H/R group vs. 5 µg/ml HSYA group;
θθP<0.01 and θP<0.05, 5 µg/ml HSYA
group vs. 10 µg/ml HSYA group; &&P<0.01 and
&P<0.05, 5 µg/ml HSYA group vs. 20 µg/ml HSYA
group; $$P<0.01, 20 µg/ml HSYA group vs. LY294002
group. HSYA, Hydroxysafflor yellow A; H/R, hypoxia/reoxygenation;
Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme
oxygenase-1; NQO1, NAD(P)H quinone oxidoreductase 1; p-,
phosphorylated. |
Subsequently, LY294002, an Akt inhibitor, was used
to explore whether HSYA protected HK-2 cells by inhibiting the
Akt/GSK-3β/Fyn-Nrf2 pathway in the present study. As demonstrated
in Fig. 8, significantly reduced
nuclear Nrf2, HO-1, and NQO1 expression was observed in H/R-treated
HK-2 cells after LY294002 supplementation. In addition, as
demonstrated in Figs. 6 and
7, the HSYA-mediated improvement
in antioxidant capacity and reduction in the expression of
oxidative stress, pro-apoptotic, and pro-inflammatory biomarkers in
H/R-treated HK-2 cells were also abrogated by LY294002
supplementation. These data revealed that the protective effects of
HSYA on H/R-treated HK-2 cells were dependent on the
Akt/GSK-3β/Fyn-Nrf2 pathway.
Discussion
It is well accepted that I/R-induced AKI is mainly
characterized by oxidative stress, inflammation, and apoptosis,
which results in higher rates of mortality and morbidity due to the
lack of effective therapies (26).
As the major active component of C. tinctorius L., HSYA has
been studied for the treatment of AKI and presents positive
therapeutic roles (15). The
potential molecular mechanism of HSYA's therapeutic effect was
explored from a new perspective of regulating the Nrf2 antioxidant
signal axis and affecting the redox metabolism of renal tissue. In
the present study, it was demonstrated that pretreatment with HSYA
could significantly attenuate the imbalance of redox metabolism,
inflammatory responses, and apoptosis in renal I/R injury by
targeting the Akt/GSK-3β/Fyn-Nrf2 axis. These findings uncovered
certain novel molecular events in HSYA that protect I/R-induced
AKI.
Accumulating evidence has suggested that oxidative
stress and inflammation play a critical role in the pathology of
I/R injury (27,28). In the present study, significantly
raised oxidative stress biomarkers (ROS, MDA, and 8-OH-dG) and
pro-inflammatory cytokines (MCP-1, TNF-α, and IL-1β), and decreased
antioxidant enzyme activities of T-AOC, SOD, and CAT were also
observed in I/R mice. Meanwhile, I/R resulted in renal dysfunction
and pathological damage. Consistent with previous studies (15,29),
it was found that HSYA treatment ameliorated renal damage and
dysfunction in I/R mice. It has been evidenced that endogenous
inflammatory mediators enhance the renal injury, dysfunction, and
inflammation caused by I/R (30,31).
Previous studies demonstrated that HSYA protected against kidney
injury, which may be related to its anti-inflammatory action
(32-35).
Similarly, it was identified that HSYA treatment significantly
decreased the levels of pro-inflammatory cytokines MCP-1, TNF-α,
and IL-1β in I/R mice and H/R-treated HK-2 cells. Recently, Lee
et al (36) identified that
the activity of SOD was markedly increased and the level of MDA was
markedly decreased in HSYA-treated diabetic nephropathy rats. Wei
et al (37) also found that
HSYA treatment significantly attenuated the elevation of MDA
content, the decrease in SOD activity, and the T-AOC in I/R-induced
brain injury. MDA and 8-OH-dG are end-products of ROS-induced lipid
peroxidation and DNA oxidation that are commonly used as oxidative
stress biomarkers (6). In the
present study, it was reported that HSYA treatment significantly
upregulated the activities of T-AOC, SOD, and CAT, while
significantly downregulated the levels of ROS, MDA, and 8-OH-dG
both in vivo and in vitro. These results indicated
that HSYA treatment alleviates the imbalance of redox metabolic and
inflammatory response induced by kidney I/R injury.
It has been reported that I/R can induce apoptosis
of numerous tissues and organs with the activation of a series of
apoptotic pathways (38,39). Previous studies have demonstrated
that the ratio of pro-apoptotic protein Bax to anti-apoptotic
protein Bcl-2 was an important element in determining the threshold
of apoptosis (40). In the present
study, it was revealed that HSYA treatment markedly restores the
upregulation of Bax and the downregulation of Bcl-2 in kidney cells
induced by in vivo I/R or in vitro H/R. The family of
caspases are crucial executors of programmed apoptosis. Among them,
caspase-3 has been found to be processed into activated fragments
such as cleaved caspase-3 when activated by the apoptotic pathway,
which is considered as an index of apoptosis (41). In the present study, it was
revealed that HSYA treatment significantly reduces the level of
cleaved caspase-3 in kidney cells both in vivo and in
vitro. These data suggested that HSYA intervention can
alleviate I/R-induced renal injury by exerting an anti-apoptotic
effect.
Furthermore, the potential molecular mechanisms
related to the protective roles of HSYA against renal I/R injury
were also examined. The Nrf2 pathway regulates the expression of
several antioxidant and detoxification enzymes including the
catalytic subunits of glutamate cysteine ligase, heme oxygenase-1
(HO-1), and NAD(P)H quinone oxidoreductase 1 (NQO1) by binding to
the antioxidant response element in their promoter regions
(6,42-44).
A previous study suggested that HSYA can improve the antioxidant
capacity of target organs by activating Nrf2 pathway and alleviate
tissue injury induced by I/R (16). Ni et al found that
activation of the AMPK/Nrf-2/HO-1 pathway can inhibit the
inflammatory response of lipopolysaccharide-induced acute lung
injury (45). El-Emam et al
identified that activation of the Nrf-2/HO-1 pathway plays an
anti-apoptotic effect in hepatic I/R injury (46). Previous studies have shown that
although the total Nrf2 expression level (or the Nrf2
nuclear/cytosolic ratio) could change under different physiological
and pathological conditions, the impact on the regulation of
downstream antioxidant gene expression remains highly dependent on
the Nrf2 level that is transported into the nucleus after
activation (24,47,48).
In the present study, it was observed that HSYA treatment
significantly increased the nuclear transfer of Nrf2 and improved
the expression of its downstream target genes HO-1 and NQO1 in
comparison with the I/R group. Both literature studies and the
current research suggested that the activation of Nrf2 was
regulated by Akt/GSK-3β/Fyn-mediated degradation and nuclear export
of Nrf2 (24,43,49).
In the present study, the significantly increased phosphorylation
of GSK-3β and Akt and decreased nucleus Fyn level in the HSYA
treatment group compared with the I/R group were also demonstrated.
In vitro, it was revealed that the intervention of Akt
inhibitor LY294002 significantly reduced nuclear Nrf2, HO-1, and
NQO1 expression in H/R-treated HK-2 cells. In addition,
HSYA-mediated improvement in antioxidant, anti-inflammatory, and
anti-apoptotic effects in H/R-treated HK-2 cells was also abrogated
by LY294002 supplementation. Collectively, these results provided
important insights into the protective effects of HSYA on I/R- or
H/R-induced renal cells injury were dependent on the activation of
Akt/GSK-3β/Fyn-Nrf2 axis.
It should be pointed out that although the in
vitro H/R model is commonly used to simulate I/R injury in
vivo, it is still unable to accurately replicate the complex
internal environment, such as changes in inflammatory conditions
(50,51). In addition, although the present
study showed that HSYA can alleviate renal tubular injury caused by
I/R, it has been claimed that I/R also causes pathological damage
to other components of the kidney. For instance, glomerular
pathological damage that manifests as glomerular hyperplasia,
hypertrophy, mesangial cell hyperplasia, anomalies in the basement
membrane, and inflammatory cell infiltration is also present in I/R
renal injury (52,53). Consequently, the repair effect and
molecular mechanism of HSYA on these I/R injured kidney tissues
need to be further clarified.
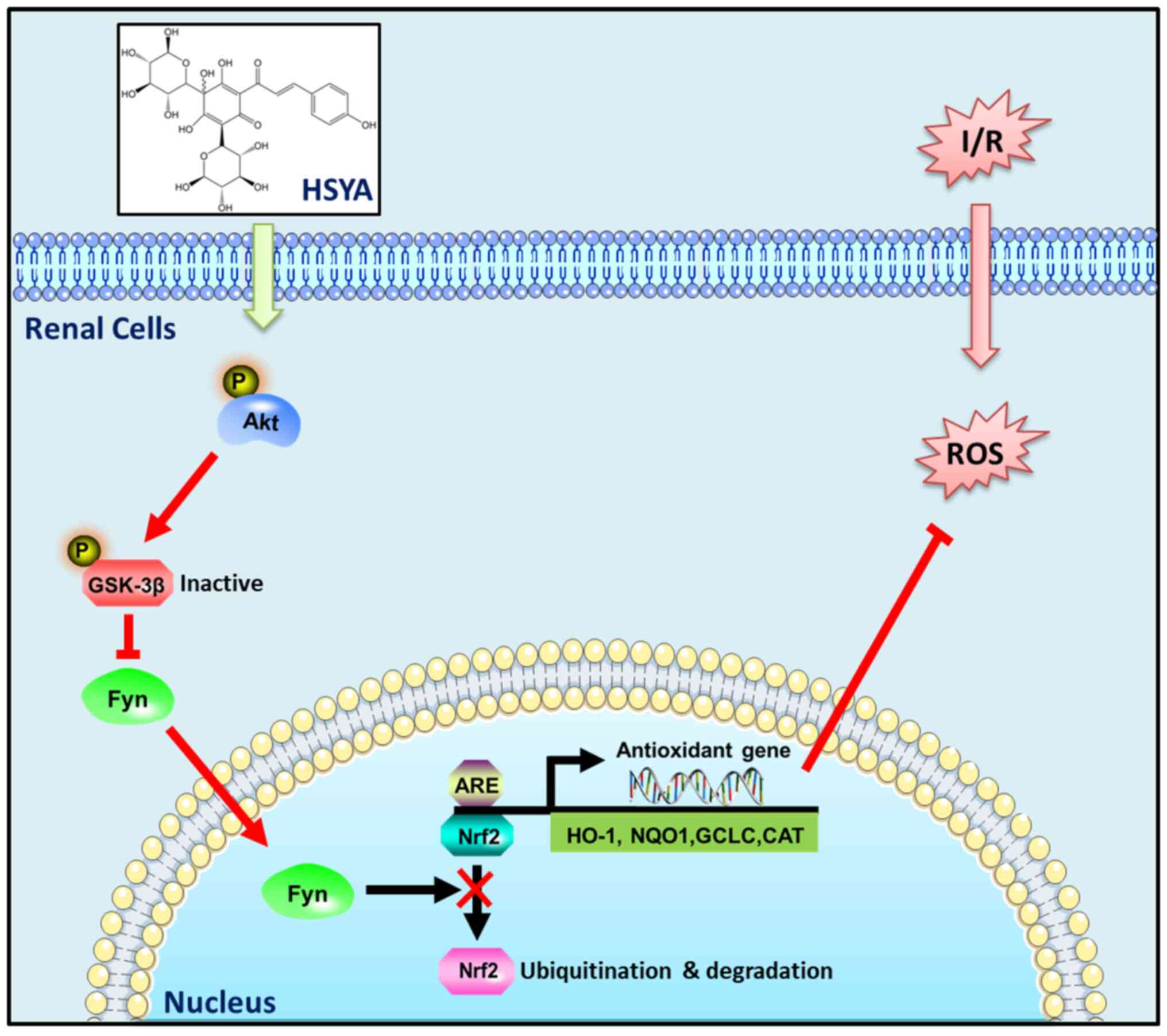
In summary, the results of the present study
revealed that HSYA exerts antioxidant, anti-inflammatory, and
anti-apoptotic effects against AKI induced by I/R, at least in
part, via targeting the Akt/GSK-3β/Fyn-Nrf2 axis (Fig. 9). Our studies present a promising
use for HSYA in the treatment of renal I/R injury.
Supplementary Material
Primer sequences used for reverse
transcription quantitative PCR.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 32200731 and 32070781), the
Shandong Provincial Natural Science Foundation (grant nos.
ZR2021MC064 and ZR2020MH166) and the Shandong Province Traditional
Chinese Medicine Science and Technology Project (grant no.
2020Q061).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, YX and XL conceived and designed the
experiments. ZG, JK, KH, ZL and XT performed the experiments. CW,
HZ and YZ analyzed the data. YX wrote the paper. YW and YX confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study protocol was reviewed and approved
(approval no. 2021-11) by the Ethics Committee of Binzhou Medical
University (Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bao N and Dai D: Dexmedetomidine protects
against ischemia and reperfusion-induced kidney injury in rats.
Mediators Inflamm. 2020(2120971)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Malek M and Nematbakhsh M: Renal
ischemia/reperfusion injury; from pathophysiology to treatment. J
Renal Inj Prev. 4:20–27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Diao C, Chen Z, Qiu T, Liu H, Yang Y, Liu
X, Wu J and Wang L: Inhibition of PRMT5 attenuates oxidative
stress-induced pyroptosis via activation of the Nrf2/HO-1 signal
pathway in a mouse model of renal ischemia-reperfusion injury. Oxid
Med Cell Longev. 2019(2345658)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu H, Wang L, Weng X, Chen H, Du Y, Diao
C, Chen Z and Liu X: Inhibition of Brd4 alleviates renal
ischemia/reperfusion injury-induced apoptosis and endoplasmic
reticulum stress by blocking FoxO4-mediated oxidative stress. Redox
Biol. 24(101195)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tang C, Han H, Liu Z, Liu Y, Yin L, Cai J,
He L, Liu Y, Chen G, Zhang Z, et al: Activation of BNIP3-mediated
mitophagy protects against renal ischemia-reperfusion injury. Cell
Death Dis. 10(677)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Y and Xiong Y, Zhang A, Zhao N, Zhang
J, Zhao D, Yu Z, Xu N, Yin Y, Luan X and Xiong Y: Oligosaccharide
attenuates aging-related liver dysfunction by activating Nrf2
antioxidant signaling. Food Sci Nutr. 8:3872–3881. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jiang GP, Liao YJ, Huang LL, Zeng XJ and
Liao XH: Effects and molecular mechanism of pachymic acid on
ferroptosis in renal ischemia reperfusion injury. Mol Med Rep.
23(63)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun Q, Zeng C, Du L and Dong C: Mechanism
of circadian regulation of the NRF2/ARE pathway in renal
ischemia-reperfusion. Exp Ther Med. 21(190)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pei J, Cai S, Song S, Xu Y, Feng M, Luo G,
Wang Y, Sun F, Shi H and Xu S: Normobaric hyperoxia plays a
protective role against renal ischemia-reperfusion injury by
activating the Nrf2/HO-1 signaling pathway. Biochem Biophys Res
Commun. 532:151–158. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hu ZC, Xie ZJ, Tang Q, Li XB, Fu X, Feng
ZH, Xuan JW, Ni WF and Wu AM: Hydroxysafflor yellow A (HSYA)
targets the NF-κB and MAPK pathways and ameliorates the development
of osteoarthritis. Food Funct. 9:4443–4456. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen L, Xiang Y, Kong L, Zhang X, Sun B,
Wei X and Liu H: Hydroxysafflor yellow A protects against cerebral
ischemia-reperfusion injury by anti-apoptotic effect through
PI3K/Akt/GSK3β pathway in rat. Neurochem Res. 38:2268–2275.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou D, Ding T, Ni B, Jing Y and Liu S:
Hydroxysafflor Yellow A mitigated myocardial ischemia/reperfusion
injury by inhibiting the activation of the JAK2/STAT1 pathway. Int
J Mol Med. 44:405–416. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ye J, Lu S, Wang M, Ge W, Liu H, Qi Y, Fu
J, Zhang Q, Zhang B, Sun G and Sun X: Hydroxysafflor yellow a
protects against myocardial ischemia/reperfusion injury via
suppressing NLRP3 inflammasome and activating autophagy. Front
Pharmacol. 11(1170)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang S, Shi Z, Li C, Ma C, Bai X and Wang
C: Hydroxysafflor yellow A attenuates ischemia/reperfusion-induced
liver injury by suppressing macrophage activation. Int J Clin Exp
Pathol. 7:2595–2608. 2014.PubMed/NCBI
|
|
15
|
Bai J, Zhao J, Cui D, Wang F, Song Y,
Cheng L, Gao K, Wang J, Li L, Li S, et al: Protective effect of
hydroxysafflor yellow A against acute kidney injury via the
TLR4/NF-κB signaling pathway. Sci Rep. 8(9173)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hu T, Wei G, Xi M, Yan J, Wu X, Wang Y,
Zhu Y, Wang C and Wen A: Synergistic cardioprotective effects of
Danshensu and hydroxysafflor yellow A against myocardial
ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1
pathway. Int J Mol Med. 38:83–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yakulov TA, Todkar AP, Slanchev K, Wiegel
J, Bona A, Groß M, Scholz A, Hess I, Wurditsch A, Grahammer F, et
al: CXCL12 and MYC control energy metabolism to support adaptive
responses after kidney injury. Nat Commun. 9(3660)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brooks C, Wei Q, Cho SG and Dong Z:
Regulation of mitochondrial dynamics in acute kidney injury in cell
culture and rodent models. J Clin Invest. 119:1275–1285.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Zhao N and Xiong Y, Zhang J, Zhao
D, Yin Y, Song L, Yin Y, Wang J, Luan X and Xiong Y: Downregulated
recycling process but not de novo synthesis of glutathione limits
antioxidant capacity of erythrocytes in hypoxia. Oxid Med Cell
Longev. 2020(7834252)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yu W, Sheng M, Xu R, Yu J, Cui K, Tong J,
Shi L, Ren H and Du H: Berberine protects human renal proximal
tubular cells from hypoxia/reoxygenation injury via inhibiting
endoplasmic reticulum and mitochondrial stress pathways. J Transl
Med. 11(24)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu C, Chen K, Wang H, Zhang Y, Duan X,
Xue Y, He H, Huang Y, Chen Z, Ren H, et al: Gastrin attenuates
renal ischemia/reperfusion injury by a PI3K/Akt/bad-mediated
anti-apoptosis signaling. Front Pharmacol.
11(540479)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nezu M and Suzuki N: Roles of Nrf2 in
protecting the kidney from oxidative damage. Int J Mol Sci.
21(2951)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dai X, Yan X, Zeng J, Chen J, Wang Y, Chen
J, Li Y, Barati MT, Wintergerst KA, Pan K, et al: Elevating CXCR7
improves angiogenic function of EPCs via Akt/GSK-3β/Fyn-mediated
Nrf2 activation in diabetic limb ischemia. Circ Res. 120:e7–e23.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rong H, Liang Y and Niu Y: Rosmarinic acid
attenuates β-amyloid-induced oxidative stress via
Akt/GSK-3β/Fyn-mediated Nrf2 activation in PC12 cells. Free Radic
Biol Med. 120:114–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Borthwick E and Ferguson A: Perioperative
acute kidney injury: Risk factors, recognition, management, and
outcomes. BMJ. 341(c3365)2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu MY, Yiang GT, Liao WT, Tsai A, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Amirzargar MA, Yaghubi F, Hosseinipanah M,
Jafari M, Pourjafar M, Rezaeepoor M, Rezaei H, Roshanaei G,
Hajilooi M and Solgi G: Anti-inflammatory effects of valproic acid
in a rat model of renal ischemia/reperfusion injury: Alteration in
cytokine profile. Inflammation. 40:1310–1318. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Min J and Wei C: Hydroxysafflor yellow A
cardioprotection in ischemia-reperfusion (I/R) injury mainly via
Akt/hexokinase II independent of ERK/GSK-3β pathway. Biomed
Pharmacother. 87:419–426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ramesh G and Reeves WB: TNF-alpha mediates
chemokine and cytokine expression and renal injury in cisplatin
nephrotoxicity. J Clin Invest. 110:835–842. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Patel NS, Chatterjee PK, Di Paola R,
Mazzon E, Britti D, De Sarro A, Cuzzocrea S and Thiemermann C:
Endogenous interleukin-6 enhances the renal injury, dysfunction,
and inflammation caused by ischemia/reperfusion. J Pharmacol Exp
Ther. 312:1170–1178. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ye J, Lu S, Wang M, Ge W, Liu H, Qi Y, Fu
J, Zhang Q, Zhang B, Sun G and Sun X: Corrigendum: Hydroxysafflor
yellow a protects against myocardial ischemia/reperfusion injury
via suppressing NLRP3 inflammasome and activating autophagy. Front
Pharmacol. 12(671318)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hu N, Duan J, Li H, Wang Y, Wang F, Chu J,
Sun J, Liu M, Wang C, Lu C and Wen A: Hydroxysafflor yellow a
ameliorates renal fibrosis by suppressing TGF-β1-induced
epithelial-to-mesenchymal transition. PLoS One.
11(e0153409)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li J, Zhang S, Lu M, Chen Z, Chen C, Han
L, Zhang M and Xu Y: Hydroxysafflor yellow A suppresses
inflammatory responses of BV2 microglia after oxygen-glucose
deprivation. Neurosci Lett. 535:51–56. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu S and Wang Y, Wen H, Sun X and Wang Y:
Hydroxysafflor Yellow A inhibits TNF-α-induced inflammation
of human fetal lung fibroblasts via NF-κB signaling pathway.
Evid Based Complement Alternat Med. 2019(4050327)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lee M, Zhao H, Liu X, Liu D, Chen J, Li Z,
Chu S, Kou X, Liao S, Deng Y, et al: Protective effect of
hydroxysafflor yellow a on nephropathy by attenuating oxidative
stress and inhibiting apoptosis in induced type 2 diabetes in rat.
Oxid Med Cell Longev. 2020(7805393)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wei X, Liu H, Sun X, Fu F, Zhang X, Wang
J, An J and Ding H: Hydroxysafflor yellow A protects rat brains
against ischemia-reperfusion injury by antioxidant action. Neurosci
Lett. 386:58–62. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yao L, Chen H, Wu Q and Xie K:
Hydrogen-rich saline alleviates inflammation and apoptosis in
myocardial I/R injury via PINK-mediated autophagy. Int J Mol Med.
44:1048–1062. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ye L, He S, Mao X, Zhang Y, Cai Y and Li
S: Effect of hepatic macrophage polarization and apoptosis on liver
ischemia and reperfusion injury during liver transplantation. Front
Immunol. 11(1193)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yang E, Zha J, Jockel J, Boise LH,
Thompson CB and Korsmeyer SJ: Bad, a heterodimeric partner for
Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell.
80:285–291. 1995.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liang Y, Yan C and Schor NF: Apoptosis in
the absence of caspase 3. Oncogene. 20:6570–6578. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kubben N, Zhang W, Wang L, Voss TC, Yang
J, Qu J, Liu GH and Misteli T: Repression of the antioxidant NRF2
pathway in premature aging. Cell. 165:1361–1374. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xiong Y, Wang Y, Zhang J, Zhao N, Zhang H,
Zhang A, Zhao D, Yu Z, Yin Y, Song L, et al: hPMSCs protects
against D-galactose-induced oxidative damage of CD4(+) T cells
through activating Akt-mediated Nrf2 antioxidant signaling. Stem
Cell Res Ther. 11(468)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xiong Y, Xiong Y, Zhang H, Zhao Y, Han K,
Zhang J, Zhao D, Yu Z, Geng Z, Wang L, et al: hPMSCs-derived
exosomal miRNA-21 protects against aging-related oxidative damage
of CD4(+) T cells by targeting the PTEN/PI3K-Nrf2 axis. Front
Immunol. 12(780897)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ni YL, Shen HT, Su CH, Chen WY, Huang-Liu
R, Chen CJ, Chen SP and Kuan YH: Nerolidol suppresses the
inflammatory response during lipopolysaccharide-induced acute lung
injury via the modulation of antioxidant enzymes and the
AMPK/Nrf-2/HO-1 pathway. Oxid Med Cell Longev.
2019(9605980)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
El-Emam SZ, Soubh AA, Al-Mokaddem AK and
Abo El-Ella DM: Geraniol activates Nrf-2/HO-1 signaling pathway
mediating protection against oxidative stress-induced apoptosis in
hepatic ischemia-reperfusion injury. Naunyn Schmiedebergs Arch
Pharmacol. 393:1849–1858. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jain AK and Jaiswal AK: GSK-3beta acts
upstream of Fyn kinase in regulation of nuclear export and
degradation of NF-E2 related factor 2. J Biol Chem.
282:16502–16510. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kaspar JW and Jaiswal AK: Tyrosine
phosphorylation controls nuclear export of Fyn, allowing Nrf2
activation of cytoprotective gene expression. FASEB J.
25:1076–1087. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhao Y, Song W, Wang Z, Wang Z, Jin X, Xu
J, Bai L, Li Y, Cui J and Cai L: Resveratrol attenuates testicular
apoptosis in type 1 diabetic mice: Role of Akt-mediated Nrf2
activation and p62-dependent Keap1 degradation. Redox Biol.
14:609–617. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ding C, Ding X, Zheng J, Wang B, Li Y,
Xiang H, Dou M, Qiao Y, Tian P and Xue W: miR-182-5p and
miR-378a-3p regulate ferroptosis in I/R-induced renal injury. Cell
Death Dis. 11(929)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dong Y, Yin J, Chen T, Wen J, Zhang Q, Li
X, Lin W, Liu F, Fan Y and Wang N: Dl-3-n-butylphthalide
pretreatment attenuates renal ischemia/reperfusion injury. Biochem
Biophys Res Commun. 557:166–173. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Golmohammadi MG, Banaei S, Nejati K and
Chinifroush-Asl MM: Vitamin D3 and erythropoietin protect against
renal ischemia-reperfusion injury via heat shock protein 70 and
microRNA-21 expression. Sci Rep. 10(20906)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen Y, Lin L, Tao X, Song Y, Cui J and
Wan J: The role of podocyte damage in the etiology of
ischemia-reperfusion acute kidney injury and post-injury fibrosis.
BMC Nephrol. 20(106)2019.PubMed/NCBI View Article : Google Scholar
|