Introduction
Nypa fruticans (N. fruticans) Wurmb is
a mangrove plant (Araceae family). It is generally distributed
throughout in the tropical regions of India, the Philippines,
Malaysia, and some parts of Australia (1). Traditionally, the roots, stems, and
leaves of N. fruticans have been used to remedy
tuberculosis, sore throat, and liver diseases (2). N. fruticans contains
polyphenols, flavonoids, protocatechuic acid, chlorogenic acid, and
kaempferol (3,4). N. fruticans has antioxidant,
antidiabetic, and hepatoprotective activities (4-7).
Plants containing phenolic compounds exhibit anti-cancer and
antioxidant activities in humans (8-11).
Inflammatory responses serve in the immune defense system against
stimuli such as infectious agent invasion or endotoxin exposure
resulting in the retrieval of normal cell function and structure
(12). Macrophages are activated
by numerous ligands such as Fzd1, lipopolysaccharide (LPS), and
RANKL (13-15).
Activated macrophages release pro-inflammatory molecules
[cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS),
and cytokines (IL-1β, IL-6, and TNF-α)] (16). LPS propagates signaling cascades by
stimulating major signaling pathways (17). The nuclear factor-kappa B (NF-κB)
is found in numerous cell lines expressing cytokines and growth
factors. NF-κB regulates a variety of target genes involved in cell
survival, cell proliferation, and immune responses (18). Recent studies have shown that
PI3K/Akt plays critical roles in various processes, including cell
survival, cell cycle regulation, and NF-κB activation (19). MAPK, including ERK, JNK, and p38
mediate various cellular and biological processes related to
inflammatory responses. MAPK phosphorylation activates the NF-κB
signaling pathway (20).
Impairment of these pathways could lead to diverse immunological
diseases, including cancer and inflammation. Therefore, the MAPK,
NF-κB, and PI3K/Akt signaling pathways are key targets for
alleviating various inflammatory diseases. Aqueous extracts of N.
fruticans have been previously reported (21). It has also been shown to have an
inflammatory effect on sciatic neuropathy (22). We confirmed the effect of N.
fruticans fractionated with ethyl acetate on the regulation of
inflammatory factors. The present study aimed to verify the
inhibitory effect of alleviating various pro-inflammatory mediators
through regulation of the MAPK and Akt/IκB/NF-κB signaling
pathways, including translocation of NF-κB p65.
Materials and methods
Chemicals and reagents
Dimethyl sulfoxide (DMSO), ethyl acetate, petroleum
ether (PE), ethyl alcohol, and methyl alcohol were purchased from
Merck. All chemical reagents were obtained from Sigma-Aldrich;
Merck KGaA, unless otherwise stated. Antibodies were purchased from
Cell Signaling Technology, Inc., Abcam and Santa Cruz
Biotechnology, Inc.
Experimental materials extraction
Air-dried shoots of N. fruticans were
purchased from Hantteus Yagcho (Seoul, Korea). The shoot extracts
of N. fruticans (484.00 g) were immersed in 80% methanol
(4.20 l) for 7 days using a sonicator (Hwashin Technology Co.,
Ltd.). The obtained extracts were then filtered by filter paper
(Whatman™), evaporated under reduced pressure, and freeze-dried
(N-1110S; Eyela). The powered N. fruticans was sequentially
fractionated by organic solvents of increasing polarity (PE,
chloroform, and ethyl acetate). The ethyl acetate fraction from
N. fruticans (ENF, 0.38 g) was stored at 4˚C. ENF was
diluted in DMSO and further used for experiments.
Analysis of total flavonoid contents
(TFC)
100 µl of ENF (4 mg/ml) was mixed with 900 µl of
distilled water, and followed by 1 ml of 10% AlCl3. The
absorbance of the reaction mixture was recorded at 420 nm using
Xma-3000PC (UV/visible spectrophotometer; Human Corporation) after
30 min. TFC was determined by comparing it with the reference
standard curve for rutin.
Antioxidant capacity evaluation
According to the method of Bondet and Van den Berg,
radical scavenging activity was determined with some modifications
(23,24). The 2,2-diphenyl-1-picryl hydrazyl
(DPPH) solution (300 µM) was dissolved in ethyl alcohol. The ABTS
solution containing 7.4 mM of 2,2'-Azino-bis
(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, and
potassium persulfate (2.6 mM) in distilled water was prepared for
24 h at ambient temperature. The DPPH or ABTS solution was adjusted
to an absorbance value of 1.00 (515 nm or 732 nm, respectively).
The sample (40 µl) was mixed with DPPH or ABTS solution (760 µl)
and reacted for 30 min in the dark at ambient temperature. The data
of radical scavenging activity was expressed as the decrease in the
absorbance of DPPH or ABTS. The rate of decrease in the radicals
was calculated using the formula as follows:
[1-(AbsSample-AbsBlank)/AbsControl]
x100 (%).
Here, AbsSample, AbsBlank and
AbsControl are the absorbance values of the sample,
ethanol or distilled water and radicals, respectively. The standard
compound, L-ascorbic acid, was used at the same concentration.
Then, a curve was constructed, and the equation of the curve was
used to calculate the half-maximal inhibitory concentration
(IC50, µg/ml).
Cell culture
Macrophages (ATCC® CRL-2278™; VA, USA)
were used in this study. Cells were maintained in incubator (5%
CO2, 37˚C), and cultured with DMEM [1%
penicillin/streptomycin and 10% FBS (Biowest)]. Then, 0.2%
prophylactic plasmocin (InvivoGen) was used to suppress the
proliferation of mycoplasma.
Cell viability
To estimate the cell proliferation assay, the
cytotoxic effects of ENF treatment on macrophages were assessed by
the CellTiter 96® AQueous One solution (Promega). The
cells were incubated in each well of 96-well plate for 24 h. The
ENF (25-400 µg/ml) treatment was done at the end of 24 h and the
cells were reacted with the reagent for 2 h in an incubator (37˚C,
5% CO2). The absorbance of supernatant was measured at
490 nm using a microplate reader (ELX808, BioTek).
Nitric oxide (NO) production
The cells (4.0x105 cells/well) were
cultured in 6-well plates, and then treated with the appropriate
concentration of ENF and 1 µg/ml of LPS (L6529, Sigma-Aldrich;
Merck KGaA) for 24 h. Griess A reagent containing 1% sulfanilamide
(w/v) in 5% phosphoric acid (v/v) and Griess B reagent containing
0.1% N-(1-Naphthyl) ethylenediamine dihydrochloride (w/v) in
distilled water were used. The supernatant of the culture medium
was collected, and Griess A/B reagent (1:1) was mixed with it in
equal volumes at room temperature for 10 min. To measure the NO
production, absorbance was measured using a microplate reader at
540 nm.
Western blotting
Cells were seeded in 100 π dishes (4x105
cells/well). After 24 h, the cells were treated with ENF (25, 50,
and 100 µg/ml) for 2 h and then induced with LPS (1 µg/ml) for 30
min or 24 h in macrophages. The cells were washed twice with
ice-cold PBS. The cells were then lysed at 4˚C using a Radio
immunoprecipitation assay (RIPA) buffer [Halt™ protease and
phosphatase inhibitor cocktail, EDTA solution (Thermo Fisher
Scientific, Inc.)]. Following the manufacturer's protocol, a
Bradford assay (Bio-Rad) was employed to determine the protein
concentration. After quantification, the protein was
electrophoresed on a 12% polyacrylamide gel and electro-transferred
to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). A 5%
Bovine serum albumin (BSA, Biosesang) in Tris-buffered saline
containing 0.1% Tween-20 (TBS-T) was used as a blocking agent at
room temperature for 1 h. Subsequently, the membrane was incubated
with primary antibodies (diluted in 3% BSA in TBS-T) at 4˚C for
overnight. The primary antibodies (1:4,000) included an iNOS
(D6B6S) monoclonal antibody (#13120), a phospho-NF-κB p65
monoclonal antibody (p-p65, #3033), an IκB-α (L35A5) monoclonal
antibody (#4814), a phospho-IκB-α (Ser32) (14D4) monoclonal
antibody (p-IκB-α,#2859), a phospho-Akt (Ser473) (D9E) monoclonal
antibody (p-Akt, #4060), a SAPK/JNK polyclonal Antibody (#9252), a
phospho-SAPK/JNK (Thr183/Tyr185) polyclonal antibody (p-JNK#9251),
a p44/42 MAPK (ERK) (137F5) monoclonal antibody (#4695), a
phospho-p44/42 MAPK (ERK) (Thr202/Tyr204) polyclonal antibody
(p-ERK, #9101), a p38 MAPK (D13E1) monoclonal antibody (#8690), and
a phospho-p38 MAPK (Thr180/Tyr182) polyclonal antibody (p-p38,
#9211). Antibodies were purchased from Cell Signaling Technology.
The anti-COX-2 polyclonal antibody (ab15919) and anti-NF-κB p65
polyclonal antibody (ab16502) were purchased from Abcam. The
membrane was then washed thrice with TBS-T for 15 min. After
washing, horse radish peroxidase (HRP)-labeled secondary
antibodies, including anti-mouse IgG-HRP monoclonal antibody
(18-8817-33, Rockland) and anti-rabbit IgG-HRP monoclonal antibody
(18-8816-33) were incubated (1:10,000) for 1 h at room temperature.
Protein bands were detected using an enhanced chemiluminescence
(ECL) substrate solution (Bio-Rad) and visualized using the
ChemiDoc imaging system (Bio-Rad). Western blotting data were
quantified using ImageJ version 1.52a (developed at the National
Institutes of Health).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Macrophages were cultured in Dulbecco's modified
Eagle's medium (DMEM) for 24 h. The following day, the cells were
treated with 25, 50, and 100 µg/ml of ENF for 2 h and then induced
with LPS (1 µg/ml) for 6 h. The cells were washed with PBS and
lysed using 350 µl RLT buffer. Total RNA was extracted from
macrophages using NucleoSpin® RNA Plus (Macherey-Nagel),
as described by the manufacturer and cDNA was synthesized from 1 µg
of extracted RNA using ReverTra Ace-α (Toyobo). The synthesized
cDNA was used as a template, including Quick Taq® HS
DyeMix (Toyobo) and primers (Table
I; COX-2, iNOS, IL-1β, TNF-α, IL-6, and GAPDH). PCR was
performed using a T100 Thermal Cycler (Bio-Rad) and the PCR cycles
were as follows: 25-35 cycles of 30 s at 94˚C (denaturation) and 30
s at each following Tm value of primers
(annealing/extension). The mRNA levels of the target genes were
normalized to the housekeeping gene (GAPDH). The band density was
evaluated using ImageJ software.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| A, RT-PCR |
|---|
| Gene | Primer sequence
(5'-3') | Product size,
bp |
|---|
| iNOS | F:
AATGGCAACATCAGGTCGGCCATCACT | 454 |
| | R:
GCTGTGTGTCACAGAAGTCTCGAACTC | |
| COX-2 | F:
GGAGAGACTATCAAGATAGT | 861 |
| | R:
ATGGTCAGTAGACTTTTACA | |
| GAPDH | F:
AACTTTGGCATTGTGGAAGG | 130 |
| | R:
ATGCAGGGATGATGTTCTGG | |
| TNF-α | F:
CACACTCAGATCATCTTCTC | 198 |
| | R:
TTGAAGAGAACCTGGGAGTA | |
| IL-6 | F:
ATTACACATGTTCTCTGGGA | 312 |
| | R:
TTTTACCTCTTGGTTGAAGA | |
| IL-1β | F:
CAGGATGAGGACATGAGCAC | 329 |
| | R:
CTCTGCAGACTCAAACTCCA | |
| B, RT-qPCR |
| Gene | Primer sequence
(5'-3') | Product size,
bp |
| iNOS | F:
TGGTGGTGACAAGCACATTT | 119 |
| | R:
AAGGCCAAACACAGCATACC | |
| COX-2 | F:
AGAAGGAAATGGCTGCAGAA | 108 |
| | R:
CCCCAAAGATAGCATCTGGA | |
| GAPDH | F:
CCTCCAAGGAGTAAGAAACC | 143 |
| | R:
CTAGGCCCCTCCTGTTATTA | |
Quantitative PCR (qPCR)
For qPCR, the cDNA templates obtained from the
RT-PCR were mixed with the Quanti Tect® SYBR-Green PCR
kit (Qiagen) and all the primers. Primer sequences were designed
using Primer 3 (Table I). All
reactions were performed using Rotor-Gene Q series software
(Qiagen) for qPCR analysis and the reaction conditions were as
follows: 95˚C for 15 min (amplification), 94˚C for 15 sec
(denaturation), 60˚C for 30 sec (annealing), and 72˚C for 30 sec
(extension) with 40 cycles. Housekeeping gene (GAPDH) was used as a
control for relative quantification of mRNA expression. The formula
2-ΔΔCq was used to analyze the
mRNA expression levels, where
ΔΔCq=(Cqtarget-CqGAPDH)sample-(Cqtarget-CqGAPDH)control
(25).
Immunofluorescence
The cells were cultured on glass coverslips for 24 h
and then treated with ENF for 2 h and later with LPS (1 µg/ml) for
30 min. After treatment, cells were fixed with 4% paraformaldehyde
(Biosesang) dissolved in PBS for 15 min at ambient temperature. The
cells were then washed with PBS for 3 min and blocked with 0.1%
Triton X-100 at room temperature. Next, the cells were incubated
with an anti-NF-κB p65 (1:1,000) and an anti-IκB-α (1:1,000) at 4˚C
overnight. Subsequently, we used an anti-rabbit IgG (Alexa
Fluor® 568, ab175471) and an anti-mouse IgG (Alexa
Fluor® 488, ab150113) to stain the cells. The cells were
incubated with DAPI (D1306, Invitrogen; Thermo Fisher Scientific,
Inc.) at room temperature with light blocking. The cells were then
washed at least five times with PBS. Finally, a drop of
fluorescence mounting medium (S3023, Dako) was used prior to
observation under a fluorescence at 400x magnification CKX53
microscope (Olympus) and images were obtained using a digital
single-lens reflex camera (DS126271; Canon).
Statistical analysis
All experiments were repeated at least three times.
Statistical analyses were verified using GraphPad Prism version 5.0
(GraphPad Software, Inc.) and data are presented as mean ± standard
deviation. In the antioxidant capacity analysis of ENF, statistical
comparisons were performed by unpaired t-test. P<0.05 was
considered to indicate a statistically significant difference. In
other experiments, each data point was analyzed using one-way
analysis of variance. The data were analyzed using the Bonferroni
post hoc test.
Results
Antioxidant capacity of ENF
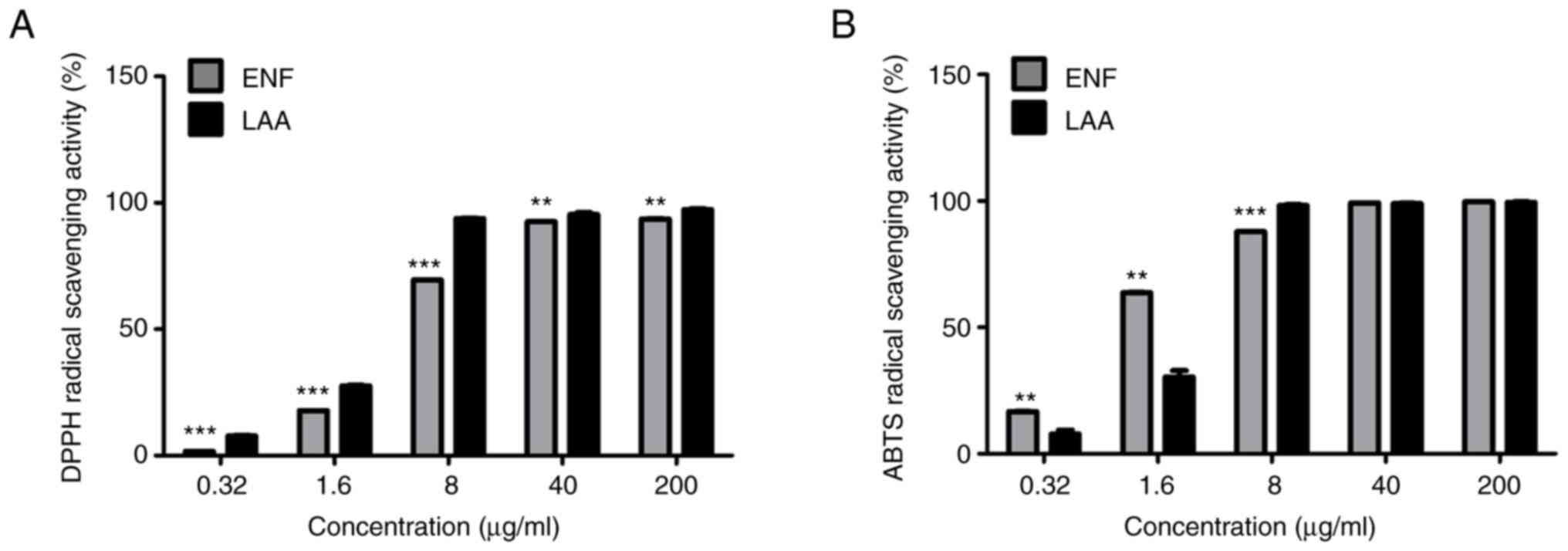
The results of the DPPH and ABTS radical scavenging
are shown in Fig. 1, where it
demonstrated the scavenging ability of ENF against DPPH and ABTS
radicals in a dose-dependent manner. The DPPH radical scavenging
activity of ENF showed maximum inhibition (93.60±0.13% at 200
µg/ml). Meanwhile, the ABTS radical scavenging activity of ENF
showed maximum inhibition (99.67±0.12% at 200 µg/ml). Additionally,
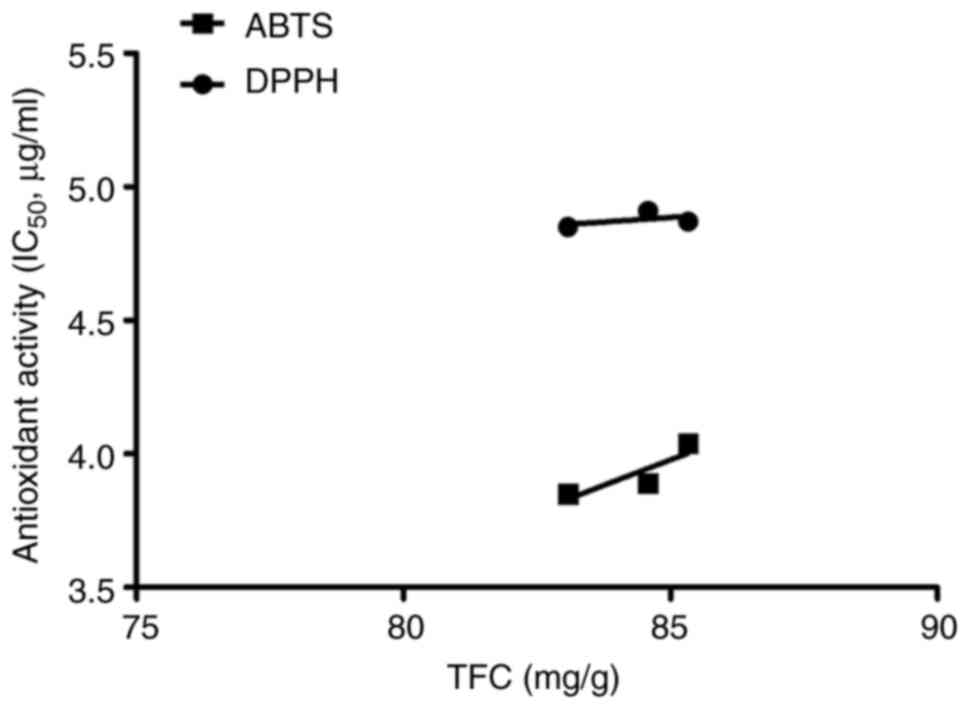
the TFC in ENF was 85.33 mg/g. The reference standard curve of the
rutin was used for normalization (y=0.001x + 0.0433,
R2=0.9974). Pearson correlation coefficient statistics
were analyzed to identify the relationship between total flavonoid
content, ABTS radical scavenging activity, and DPPH radical
scavenging activity (Fig. 2). A
positive moderate correlation was found between the total flavonoid
content and DPPH scavenging activity (r=0.506). There was a
high positive correlation between the total flavonoid content and
ABTS scavenging activity (r=0.865).
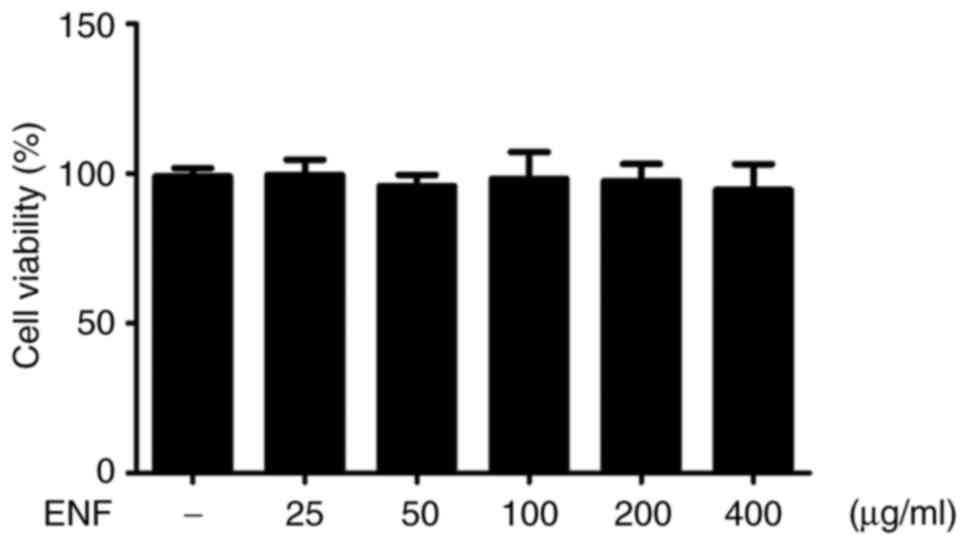
Effects of ENF on cell viability
To estimate the optimal ENF concentration not
affecting the cell viability, macrophages were treated with ENF at
various concentrations (25, 50, 100, 200, and 400 µg/ml). As shown
in Fig. 3, concentrations ranging
from 25-400 µg/ml showed no cytotoxicity as compared to that shown
by the non-treated control (100%). Based on these studies (26,27),
concentrations of 50 (95.83±3.73%) and 100 µg/ml (98.31±8.92%) were
chosen for use in all experiments in this study.
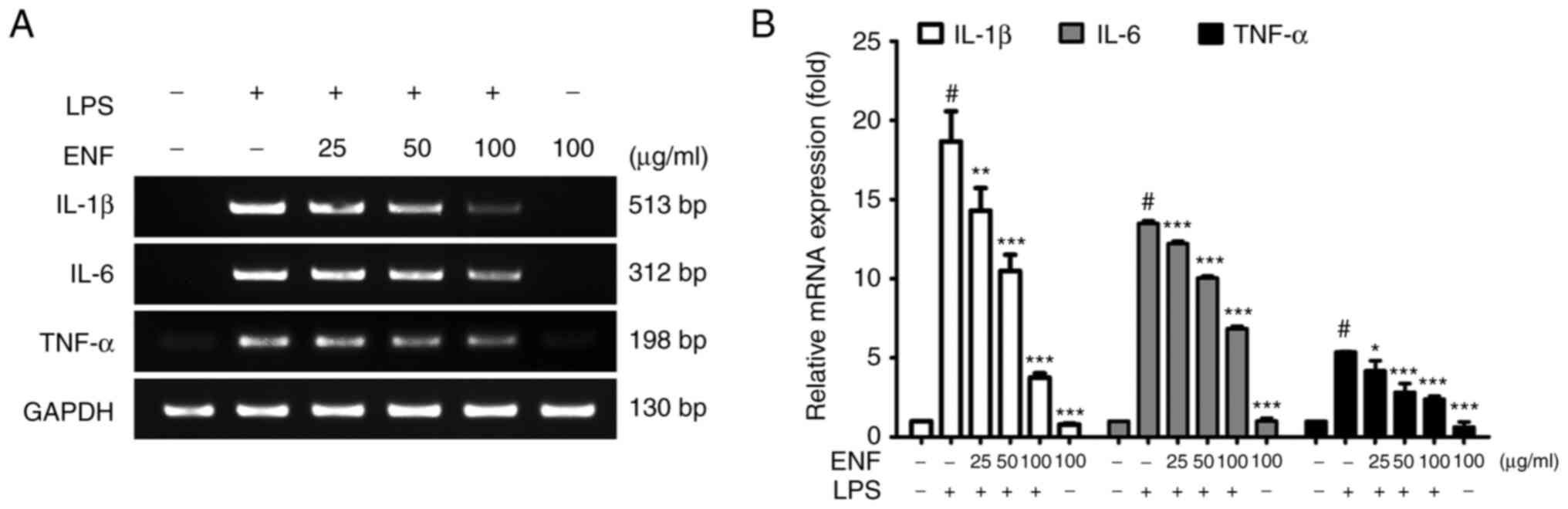
Effects of ENF on cytokines
expression
Cytokines, including TNF-α, IL-6, and IL-1β are
released to amplify the inflammatory response in macrophages
(28). As presented in Fig. 4, the TNF-α mRNA levels were
attenuated by ENF treatment (2.38±0.20-fold at 100 µg/ml) compared
with that in the LPS-induced group (5.37±0.03-fold). The IL-6 mRNA
level was attenuated by ENF treatment (6.85±0.16-fold at 100 µg/ml)
compared with that in the LPS-induced group (13.48±0.18-fold). In
addition, the IL-1β mRNA level was attenuated by ENF treatment
(3.75±0.27-fold at 100 µg/ml) compared with that in the LPS-induced
group (18.66±1.91-fold).
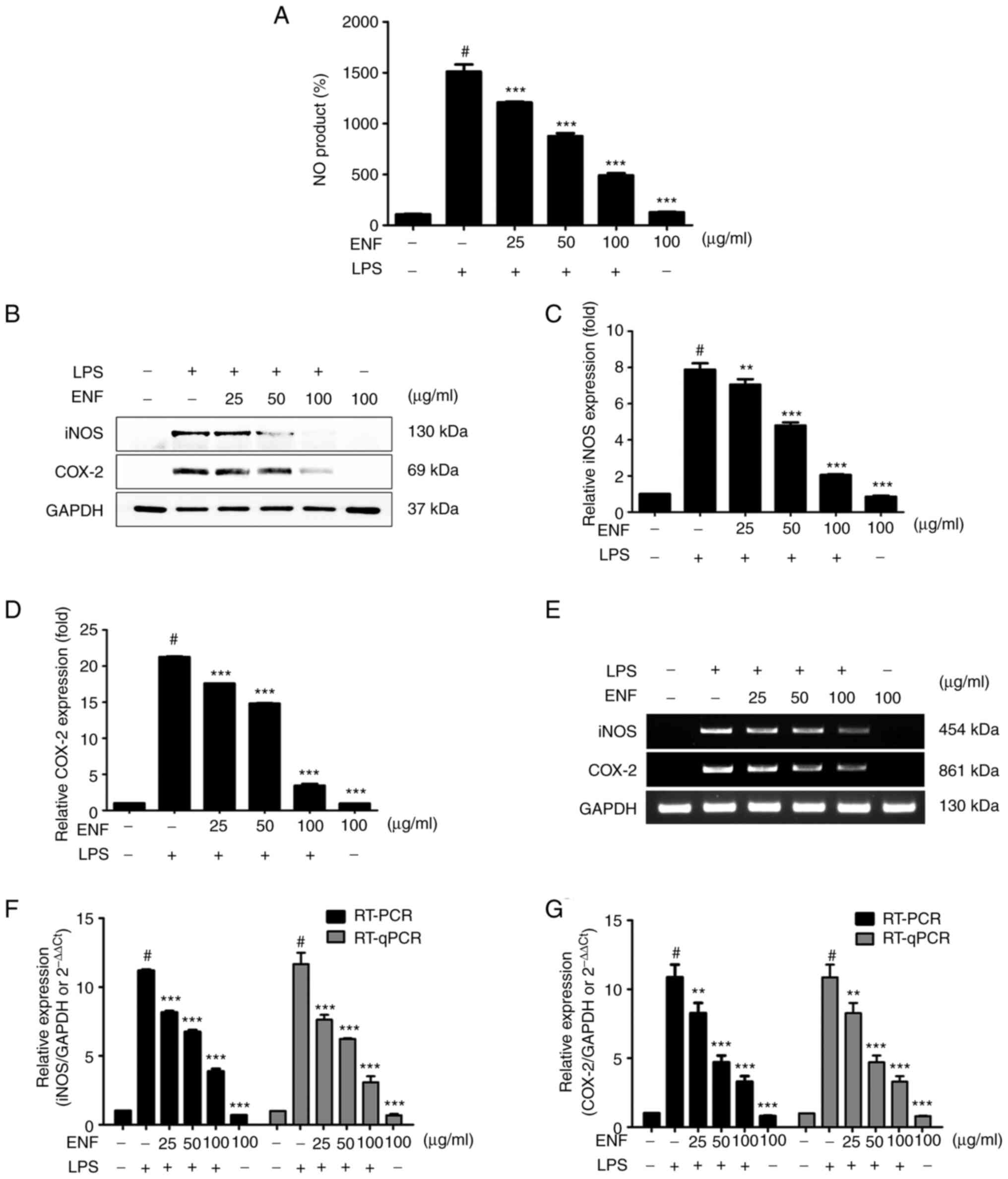
Effects of ENF on NO production,
COX-2, and iNOS
We evaluated the modulatory role of ENF treatment on
NO production in LPS-induced macrophages (Fig. 5A). Nitric oxide production was
significantly increased by LPS (1509.36±71.53%) as compared with
the non-treated control (100%). As presented in Fig. 5A, ENF treatment potently suppressed
NO production at the concentration of 50 (875.44±28.07%) and 100
µg/ml (488.89±21.22%). As shown in Fig. 5C and D, the COX-2 and iNOS expression was
increased by LPS (21.26±0.09-fold and 7.87±0.35-fold, respectively)
at the cellular level. The COX-2 and iNOS expression was
significantly suppressed (3.43±0.22-fold and 2.05±0.04-fold,
respectively) by ENF treatment at 100 µg/ml. The COX-2 and iNOS
mRNA levels correlated with protein expression by RT-PCR analysis
(Fig. 5E). The mRNA level of COX-2
was notably attenuated by ENF treatment at 50 (4.70±0.50-fold) and
100 µg/ml (3.29±0.40-fold) as compared to the LPS-induced group
(10.87±0.92-fold). The mRNA level of iNOS was attenuated through
ENF treatment at 50 (6.74±0.13-fold) and 100 µg/ml (3.88±0.18-fold)
as compared to the LPS-induced group (11.92±0.07-fold).
Quantitative PCR (qPCR) was utilized to normalize mRNA levels and
establish the results of RT-PCR analysis. The qPCR data followed a
pattern similar to that of western blotting and RT-PCR
analysis.
Effects of ENF on Akt/IκB/NF-κB
signaling pathway
NF-κB is a transcription factor complex, that
regulates the expression of proinflammatory mediators (29). As demonstrated in Fig. 6A, ENF treatment suppressed the
phosphorylation of NF-κB p65 (0.99±0.03-fold at 100 µg/ml), without
affecting the total amount of NF-κB p65. The p-IκB-α was increased
by LPS (1.45±0.03-fold) at the cellular level and the p-IκB-α was
significantly suppressed by ENF treatment (0.34±0.02-fold at 100
µg/ml). These results showed that ENF treatment downregulated the
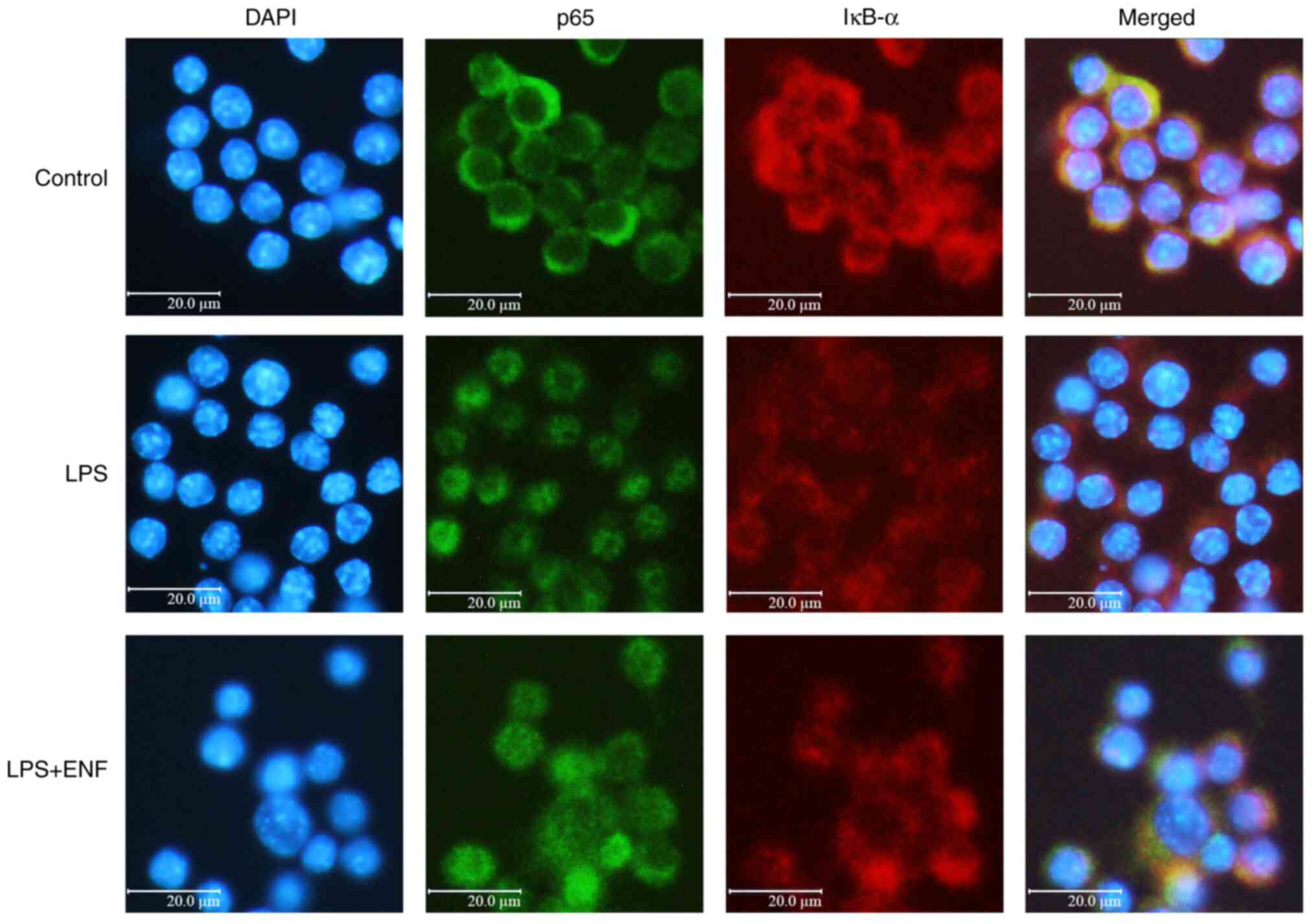
NF-κB signaling by suppressing p-IκB-α. Located in the nucleus,
NF-κB p65 phosphorylation is a major step in the activation of
NF-κB (30). To understand the
molecular mechanism of NF-κB p65 translocation, we investigated the
nuclear fluorescence of IκB-α and NF-κB p65, which are related to
the expression of the NF-κB. As presented in Fig. 7, the NF-κB p65 was translocated
from the cytoplasm to the nucleus as compared with the control
group. Simultaneously, the fluorescence intensity of IκB-α in the
nucleus was increased by ENF treatment of live cells. The increase
in Akt phosphorylation results in the release of proinflammatory
mediators, including cytokines, COX-2, and iNOS (31). As demonstrated in Fig. 6A, phosphorylation of Akt was
increased by LPS (3.27±0.03-fold) at the cellular level. ENF
treatment significantly suppressed the phosphorylation of Akt
(1.39±0.05-fold at 100 µg/ml).
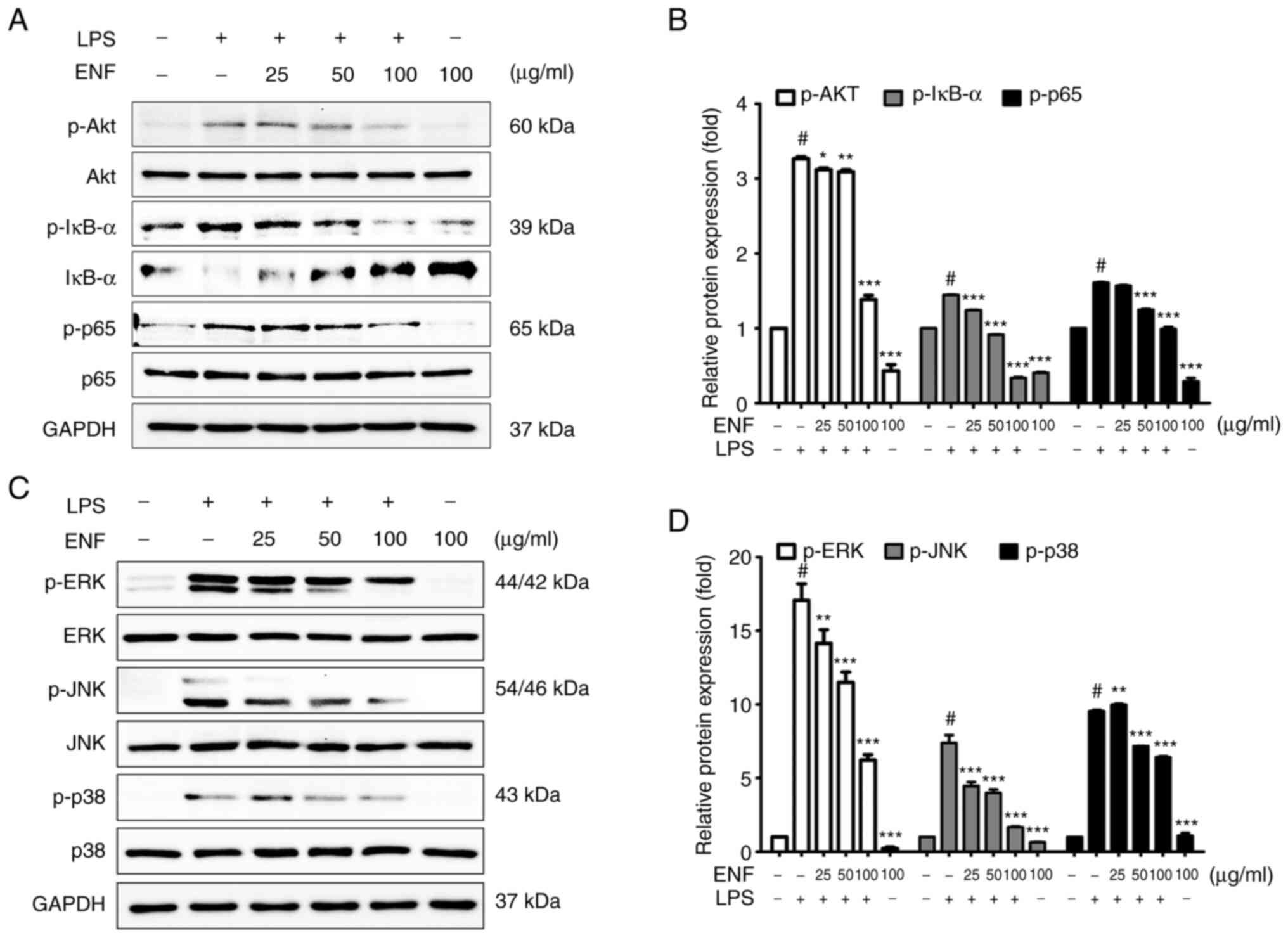 | Figure 6Inhibitory effect of ENF on
Akt/IκB/NF-κB and MAPK signaling protein expression. (A) Western
blotting results of protein phosphorylation levels of Akt, IκB-α,
and NF-κB p65. (B) Phosphorylation levels of Akt, IκB-α, and NF-κB
p65. (C) Western blotting results of protein phosphorylation levels
of ERK, JNK, and p38. (D) Phosphorylation levels of ERK, JNK, and
p38. Values are presented as the mean ± SD. #P<0.001
vs. untreated group; *P<0.05, **P<0.01,
and ***P<0.001 vs. LPS-induced group. ENF, ethyl
acetate fraction from Nypa fruticans Wurmb; LPS,
lipopolysaccharide; p-, phosphorylated. |
Effects of ENF on MAPK signaling
pathway
MAPK (JNK, ERK, and p38) signaling pathway
stimulates the NF-κB signaling pathway (32). To evaluate whether ENF exerts the
modulatory role on phosphorylation of the MAPK, the JNK, ERK, and
p38 expression was investigated by western blotting. As
demonstrated in Fig. 6D, LPS
enhanced the phosphorylation of JNK (7.38±0.54-fold), ERK
(17.07±1.11-fold), and p38 (9.55±0.08-fold) at the cellular level.
ENF treatment significantly suppressed p-JNK (1.67±0.05-fold),
p-ERK (6.22±0.37-fold), and p-p38 (6.42±0.06-fold at 100 µg/ml)
without affecting the total amount of JNK, ERK, and p38. A previous
study reported that blocking the phosphorylation of MAPK pathways
is associated with the regulation of proinflammatory mediators
(33).
Discussion
Phytochemicals (alkaloids, terpenes, saponins,
phenolics, and flavonoids) are naturally occurring compounds found
in plants (34,35). They exert in the protection of
human health, preventing various (inflammation, colorectal cancer,
liver cancer, and DNA damage) diseases (36-39).
Other plants belonging to the Araceae family contain phytochemicals
such as sterols, saponins, phenols, and alkaloids (34,40,41).
N. fruticans contains phytochemicals, such as chlorogenic
acid, kaempferol, and protocatechuic acid (4,21,42),
which have antioxidant, anticancer, and anti-inflammatory effects
(43-45).
There are studies related to the various bioactivities of N.
fruticans such as antidiabetic, antioxidant, and
anti-inflammatory effects (21,46,47).
ENF showed a similar level of DPPH radical scavenging activity
(IC50, 3.93±0.10 µg/ml) compared to L-ascorbic acid
(IC50, 2.65±0.02 µg/ml). ENF scavenged ABTS radical
(IC50, 4.88±0.03 µg/ml) similar as compared to
L-ascorbic acid (IC50, 2.36±0.10 µg/ml). IC50
values for DPPH and ABTS radicals of ENF were not statistically
significant when compared with L-ascorbic acid (P<0.001). These
results can be inferred that the total flavonoid content in ENF
contributes to its antioxidant capacity. Based on research on the
relationship between flavonoid content and effects of
anti-inflammatory (48) and
capacity of free radicals in the inflammatory processes (49), it was confirmed that the
anti-inflammatory effect of ENF was related to its antioxidant
capacity. LPS-induced macrophages secrete cytokines, which are
pro-inflammatory mediators that trigger inflammatory response
(50). The transport of cytokines,
including IL-6, IL-1β, and TNF-α was reduced by ENF treatment,
which could lead to regulation of the cascade. Apparently,
pro-inflammatory mediators are expressed in response to
inflammation via cytokines (51,52).
Since excessive NO production can lead to cytotoxicity (53), cancer (54), and autoimmune diseases (55), it is supported that blocking of
iNOS expression and NO production can prevent inflammatory
diseases. This study showed that ENF treatment suppressed NO
production in LPS-induced macrophages. The suppression of COX-2 and
iNOS expression by ENF showed a similar pattern to the inhibition
of NO production, demonstrating that a decrease in the expression
of iNOS by ENF suppresses NO production. At the basal level, NF-κB
binds to IκB-α and is localized in the cytoplasm. An external
stimulus, such as LPS induces phosphorylation of IκB-α, causing IκB
degradation via ubiquitination of the NF-κB dimer. The released
NF-ĸB p65 translocated into the nucleus and promotes the
transcription of inflammatory mediators. We investigated the
molecular mechanisms of IκB-α and NF-κB p65 in macrophages
following ENF treatment. We confirmed that ENF treatment inhibited
IκB-α phosphorylation, thereby attenuating the NF-κB p65
translocation to the nucleus. Immunofluorescence analysis showed
that ENF treatment increased the fluorescence intensity of IκB-α,
thereby increasing residual NF-κB p65 in the cytoplasm. The
inhibition of IκB-α phosphorylation causes NF-κB p65 to remain in
the cytoplasm, and it is thought that ENF treatment regulates
proinflammatory mediators by reducing the NF-κB signaling pathway.
Akt is widely known as a downstream target of several cellular
processes (56). The
phosphorylation of Akt was inhibited by ENF treatment. Since Akt
activates NF-κB by phosphorylating IκB kinase (IKK), inhibition of
Akt phosphorylation through ENF treatment induces downstream NF-κB
signaling pathway. Ligands activating cellular inflammatory
responses induce phosphorylation of factors (JNK, ERK, and p38) in
the MAPK signaling pathway in macrophages (57). ENF treatment suppressed the
inflammatory cascade by alleviating the MAPK signaling pathway,
which participate in NF-κB activation. In summary, ENF exhibits
anti-inflammatory effects by modulating the nuclear translocation
of NF-κB p65, which regulates the MAPK and Akt/IκB signaling
pathways. The ethyl acetate fraction of N. fruticans can
therefore be used as a functional material derived from natural
products with the potential to regulate inflammatory responses.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HJP wrote the manuscript, performed the experiments
and analyzed the data. HJP and SYH performed the experiments and
interpreted the data. SOO and JBL participated in drafting the
article. SSO, JBL and SMM were involved in interpretation of data.
TWJ and JHP confirm the authenticity of all the raw data. JHP
designed the experiments, wrote and edited the manuscript. HJP and
TWJ made substantial contributions to the conception and
acquisition of data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tamunaidu P and Saka S: Chemical
characterization of various parts of nipa palm (Nypa
fruticans). Ind Crops Prod. 34:1423–1428. 2011.
|
|
2
|
Bandaranayake W: Traditional and medicinal
uses of mangroves. Mangroves and Salt Marshes. 2:133–148. 1998.
|
|
3
|
Hossain MF and Islam MA: Utilization of
mangrove forest plant: Nipa palm (Nypa fruticans Wurmb.). Am
J Agric Fores. 3:156–160. 2015.
|
|
4
|
Prasad N, Yang B, Kong KW, Khoo HE, Sun J,
Azlan A, Ismail A and Romli ZB: Phytochemicals and antioxidant
capacity from Nypa fruticans Wurmb. fruit. Evid Based
Complement Alternat Med. 2013(154606)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yusoff NA, Yam MF, Beh HK, Abdul Razak KN,
Widyawati T, Mahmud R, Ahmad M and Asmawi MZ: Antidiabetic and
antioxidant activities of Nypa fruticans Wurmb. vinegar
sample from Malaysia. Asian Pac J Trop Med. 8:595–605.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yusoff NA, Lim V, Al-Hindi B, Abdul Razak
KN, Widyawati T, Anggraini DR, Ahmad M and Asmawi MZ: Nypa
fruticans Wurmb. Vinegar's aqueous extract stimulates insulin
secretion and exerts hepatoprotective effect on STZ-induced
diabetic rats. Nutrients. 9(925)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsai SJ and Yin MC: Anti-glycative and
anti-inflammatory effects of protocatechuic acid in brain of mice
treated by D-galactose. Food Chem Toxicol. 50:3198–3205.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ahn SK, Goo YM, Ko KH, Lee SJ, Moon YH and
Lee SS, Kim JW and Lee SS: Study on the evaluation of nutritional
values and antioxidant activities for herbal medicine by-products.
J Agric Life Sci. 48:101–110. 2014.
|
|
9
|
Kim EY, Baik IH, Kim JH, Kim SR and Rhyu
MR: Screening of the antioxidant activity of some medicinal plants.
KOREAN J FOOD SCI. TECHNOL. 36:333–338. 2004.
|
|
10
|
Nirmala MJ, Samundeeswari A and Sankar PD:
Natural plant resources in anti-cancer therapy-A review. Res Plant
Biol. 1:01–14. 2011.
|
|
11
|
Wang Q, Kuang H, Su Y, Sun Y, Feng J, Guo
R and Chan K: Naturally derived anti-inflammatory compounds from
Chinese medicinal plants. J Ethnopharmacol. 146:9–39.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lawrence T, Willoughby DA and Gilroy DW:
Anti-inflammatory lipid mediators and insights into the resolution
of inflammation. Nat Rev Immunol. 2:787–795. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Neumann J, Schaale K, Farhat K, Endermann
T, Ulmer AJ, Ehlers S and Reiling N: Frizzled1 is a marker of
inflammatory macrophages, and its ligand Wnt3a is involved in
reprogramming Mycobacterium tuberculosis-infected macrophages.
FASEB J. 24:4599–4612. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sweet MJ and Hume DA: Endotoxin signal
transduction in macrophages. J Leukoc Biol. 60:8–26.
1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang R, Wang X, Zhou Y and Xiao Y:
RANKL-induced M1 macrophages are involved in bone formation. Bone
Res. 5(17019)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
So MS, Lee JS and Yi SY: Induction of
nitric oxide and cytokines in macrophages by Codonopsis lanceolata.
Korean J Food Sci Technol. 36:986–990. 2004.
|
|
17
|
Bjorkbacka H, Fitzgerald KA, Huet F, Li X,
Gregory JA, Lee MA, Ordija CM, Dowley NE, Golenbock DT and Freeman
MW: The induction of macrophage gene expression by LPS
predominantly utilizes Myd88-independent signaling cascades.
Physiol Genomics. 19:319–330. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kanarek N, London N, Schueler-Furman O and
Ben-Neriah Y: Ubiquitination and degradation of the inhibitors of
NF-kappaB. Cold Spring Harb Perspect Biol.
2(a000166)2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rajaram MV, Ganesan LP, Parsa KV, Butchar
JP, Gunn JS and Tridandapani S: Akt/Protein kinase B modulates
macrophage inflammatory response to Francisella infection and
confers a survival advantage in mice. J Immunol. 177:6317–6324.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Haque MA, Jantan I and Harikrishnan H:
Zerumbone suppresses the activation of inflammatory mediators in
LPS-stimulated U937 macrophages through MyD88-dependent
NF-κB/MAPK/PI3K-Akt signaling pathways. Int Immunopharmacol.
55:312–322. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bae GS and Park SJ: The anti-inflammatory
effect of Nypa fruticans Wurmb fruit on
lipopolysaccharide-induced inflammatory response on RAW 264.7
cells. Korea J Herbol. 31:79–84. 2016.
|
|
22
|
Kang MS and Hyun KY: Antinociceptive and
anti-inflammatory effects of Nypa fruticans wurmb by
suppressing TRPV1 in the sciatic neuropathies. Nutrients.
12(135)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bondet V, Brand-Williams W and Berset C:
Kinetics and mechanisms of antioxidant activity using the DPPH.
free radical method. LWT-Food Sci Technol. 30:609–615. 1997.
|
|
24
|
van den Berg R, Haenen GRMM, van den Berg
H and Bast A: Applicability of an improved Trolox equivalent
antioxidant capacity (TEAC) assay for evaluation of antioxidant
capacity measurements of mixtures. Food Chem. 66:511–517. 1999.
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jang TW and Park JH: Anti-Inflammatory
Effects of Abeliophyllum distichum Nakai (Cultivar Okhwang 1)
Callus through Inhibition of PI3K/Akt, NF-κB, and MAPK Signaling
Pathways in Lipopolysaccharide-Induced Macrophages. Processes.
9(1071)2021.
|
|
27
|
Kim EA, Kim SY, Ye BR, Kim J, Ko SC, Lee
WW, Kim KN, Choi IW, Jung WK and Heo SJ: Anti-inflammatory effect
of Apo-9'-fucoxanthinone via inhibition of MAPKs and NF-kB
signaling pathway in LPS-stimulated RAW 264.7 macrophages and
zebrafish model. Int Immunopharmacol. 59:339–346. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Adcock IM, Ford PA, Bhavsar P, Ahmad T and
Chung KF: Steroid resistance in asthma: Mechanisms and treatment
options. Curr Allergy Asthma Rep. 8:171–178. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Caban M, Chojnacka K, Owczarek K,
Laskowska J, Fichna J, Podsedek A, Sosnowska D and Lewandowska U:
Spent hops (Humulus lupulus L.) extract as modulator of the
inflammatory response in lipopolysaccharide stimulated raw 264.7
macrophages. J Physiol Pharmacol: Apr 27, 2020 (Epub ahead of
print).
|
|
30
|
Biswas SK and Lewis CE: NF-κB as a central
regulator of macrophage function in tumors. J Leukoc Biol.
88:877–884. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ngabire D, Seong Y, Patil MP, Niyonizigiye
I, Seo YB and Kim GD: Anti-inflammatory effects of aster incisus
through the inhibition of NF-κB, MAPK, and akt pathways in
LPS-stimulated RAW 264.7 macrophages. Mediators Inflamm.
2018(4675204)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Schulze-Osthoff K, Ferrari D, Riehemann K
and Wesselborg S: Regulation of NF-kappaB activation by MAP kinase
cascades. Immunobiology. 198:35–49. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim NY, Cheong SH, Lee KJ, Sok DE and Kim
MR: Anti-inflammatory effects of Ribes diacanthum Pall mediated via
regulation of Nrf2/HO-1 and NF-κB signaling pathways in
LPS-stimulated RAW 264.7 macrophages and a TPA-induced dermatitis
animal model. Antioxidants (Basel). 9(622)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
El-Desouky SK, Kim KH, Ryu SY, Eweas AF,
Gamal-Eldeen AM and Kim YK: A new pyrrole alkaloid isolated
fromArum palaestinum Boiss. and its biological activities. Arch
Pharm Res. 30:927–931. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Koes RE, Quattrocchio F and Mol JNM: The
flavonoid biosynthetic pathway in plants: Function and evolution.
BioEssays. 16:123–132. 1994.
|
|
36
|
Leiherer A, Mündlein A and Drexel H:
Phytochemicals and their impact on adipose tissue inflammation and
diabetes. Vascul Pharmacol. 58:3–20. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Johnson IT: Phytochemicals and cancer.
Proc Nutr Soc. 66:207–215. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rajendran P, Ho E, Williams DE and
Dashwood RH: Dietary phytochemicals, HDAC inhibition, and DNA
damage/repair defects in cancer cells. Clin Epigenetics.
3(4)2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nishino H, Satomi Y, Tokuda H and Masuda
M: Cancer control by phytochemicals. Curr Pharm Des. 13:3394–3399.
2007.PubMed/NCBI
|
|
40
|
Roshan R, Ahmed S and Hasan MM: Arisaema
jacquemontii Blume (Araceae): A review of medicinal uses,
phytochemistry and pharmacology. J Pharmacogn Phytochem. 6:429–432.
2017.
|
|
41
|
Dong H, Geng Y, Wang X, Song X, Wang X and
Yu J: Chemical constituents from scindapsus officinalis (Roxb.)
Schott. and their anti-inflammatory activities. Molecules.
23(2577)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yusoff NA, Ahmad M, Al Hindi B, Widyawati
T, Yam MF, Mahmud R, Razak KN and Asmawi MZ: Aqueous extract of
Nypa fruticans Wurmb. vinegar alleviates postprandial
hyperglycemia in normoglycemic rats. Nutrients. 7:7012–7026.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sahoo S, Ghosh G, Das D and Nayak S:
Phytochemical investigation and in vitro antioxidant activity of an
indigenous medicinal plant Alpinia nigra BL Burtt. Asian Pac J Trop
Biomed. 3:871–876. 2013.
|
|
44
|
Xu XH, Li T, Fong CM, Chen X, Chen XJ,
Wang YT, Huang MQ and Lu JJ: Saponins from Chinese medicines as
anticancer agents. Molecules. 21(1326)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Souto AL, Tavares JF, Da Silva MS, Diniz
Mde F, de Athayde-Filho PF and Barbosa Filho JM: Anti-inflammatory
activity of alkaloids: An update from 2000 to 2010. Molecules.
16:8515–8534. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Choi JH, Hwang JW, Lee SG, Heo SH and Kang
H: Antioxidant effect of hot water extracts from 3 types Indonesia
plants (Hibiscus petals, Moringa Oleifera gymnosperm, and Nipa
Fruticans Wurmb). Journal of Naturopathy. 10:42–47. 2021.
|
|
47
|
Reza H, Haq WM, Das AK, Rahman S, Jahan R
and Rahmatullah M: Anti-hyperglycemic and antinociceptive activity
of methanol leaf and stem extract of Nypa fruticans Wurmb.
Pak J Pharm Sci. 24:485–488. 2011.PubMed/NCBI
|
|
48
|
Maleki SJ, Crespo JF and Cabanillas B:
Anti-inflammatory effects of flavonoids. Food Chem.
299(125124)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Forman HJ and Zhang H: Targeting oxidative
stress in disease: Promise and limitations of antioxidant therapy.
Nat Rev Drug Discov. 20:689–709. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dang Y, Mu Y, Wang K, Xu K, Yang J, Zhu Y
and Luo B: Papaverine inhibits lipopolysaccharide-induced
microglial activation by suppressing NF-κB signaling pathway. Drug
Des Devel Ther. 10:851–859. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Samad TA, Moore KA, Sapirstein A, Billet
S, Allchorne A, Poole S, Bonventre JV and Woolf CJ:
Interleukin-1beta-mediated induction of Cox-2 in the CNS
contributes to inflammatory pain hypersensitivity. Nature.
410:471–475. 2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zeilhofer HU and Brune K: Analgesic
strategies beyond the inhibition of cyclooxygenases. Trends
Pharmacol Sci. 27:467–474. 2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kröncke KD, Fehsel K and Kolb-Bachofen V:
Nitric oxide: Cytotoxicity versus cytoprotection-how, why, when,
and where? Nitric Oxide. 1:107–120. 1997.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hofseth LJ, Hussain SP, Wogan GN and
Harris CC: Nitric oxide in cancer and chemoprevention. Free Radic
Biol Med. 34:955–968. 2003.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liew FY: Regulation of nitric oxide
synthesis in infectious and autoimmune diseases. Immunol Lett.
43:95–98. 1994.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Grandage VL, Gale RE, Linch DC and Khwaja
A: PI3-kinase/Akt is constitutively active in primary acute myeloid
leukaemia cells and regulates survival and chemoresistance via
NF-kB, MAPkinase and p53 pathways. Leukemia. 19:586–594.
2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bode JG, Ehlting C and Häussinger D: The
macrophage response towards LPS and its control through the
p38(MAPK)-STAT3 axis. Cell Signal. 24:1185–1194. 2012.PubMed/NCBI View Article : Google Scholar
|