Introduction
Smoke inhalation injury (SII) is the most common
cause of mortality in patients with fire burn injuries (1). Most hospitalized patients with burns
usually have accompanying inhalation injury, and the incidence rate
of SII is positively correlated with mortality rates (2). Fires produce a large quantity of
smoke that contains dust particles, poisonous chemical gases and
heat coupled with a near anoxic environment, which can result in
inhalation injuries that are difficult to treat and pose risks to
the preservation of patient life (3). Burns injury patients may undergo
endotracheal intubation and invasive ventilation as the majority of
damage is inflicted to the upper respiratory tract tissue, prior to
the smoke reaching the tracheal carina (4,5).
Supraglottic edema and airway blockage, or lower airway damage and
subsequent respiratory failure can result due to chemical injury
(6).
The chemical poisons within fire smoke are directly
absorbed into the human body through the lung-blood exchange
interface, resulting in systemic damage (7). The combined action of particulate
matter, toxic gases and exposure to anoxic environments often
results in lung injury with rapid deterioration, bringing great
difficulties to timely treatment (8). The clinical signs of SII include
severe airway obstruction and decreased pulmonary gas exchange
function, which further limits treatments in patients with SII
(7). Therefore, the early
detection of high-risk SII sufferers and prevention of injury
progression are of significant importance in SII management and
healthcare.
According to data gathered in the United States
during 2016-2017(6), the incidence
rate of SII is ~10% (9) of burn
injury patients, which positively correlates with the increase of
burns to total body surface area (TBSA) (10). The highest recorded incidence of
SII (14%) was shown in patients with 80-89% TBSA affected by burn
injury (11). The accurate
definition of SII is frequently challenged. In a number of burn
treatment centers, diagnoses rely on evidence consistent with lung
injuries, clinical records and investigations associated with flame
burns and prolonged smoke exposure (12). At the same time, burns to the neck
and face, difficulty in pronunciation/speech, burnt nose hairs,
presence of carbon powder on extension of the tongue, a cough
producing black phlegm, congestion and edema of the pharynx should
also be taken into consideration when diagnosing SII (7,10).
It has been reported that severe SII is an
independent risk factor for burn-related death (13). Airway obstruction and severe
pneumonia are the main causes of mortality associated with SII
(14). Identifying and diagnosing
the degree of SII presents an important clinical problem.
Bronchoscopy can observe the severity of airway injury in the
intensive care management of patients with SII (9). The use of biochemical and
pathological markers, and imaging examination can also be used to
judge the severity and prognosis of SII (15). The relationship between SII and
acute respiratory distress syndrome (ARDS) has not yet been
confirmed, but ARDS caused by burns has been demonstrated to
increase mortality (16). Studies
have shown that there are numerous risk factors for ARDS, including
age, shock, acute physiological response in hospital, inappropriate
mechanical ventilation (MV) methods, acute physiology and chronic
health evaluation (APACHE II) score, lung injury score, serum
fibrinogen and positive end-expiratory pressure (17,18).
Therefore, although challenging, it is practical for clinicians to
identify and diagnose the severity of SII to implement
corresponding treatment methods.
The objective of the present study was to
investigate the characteristics and risk factors for severe SII,
and the relationship with ARDS and mortality in patients with burns
complicated by SII. The current study aimed to assist in the
formulation of improved treatment modalities to promote patient
survival. To achieve this, each indicator was analyzed to observe
whether it could serve as a strong predictor to influence the
prognosis of SII.
Materials and methods
Participants
The selected patients were affected by flame injury
and were from the Department of Burns and Plastic Surgery of
Characteristic Medical Center of Chinese People's Armed Police
Force and 983 Hospital of the Joint Logistics Support Force of the
Chinese People's Liberation Army (Tianjin, China). Patients'
medical records were collected between the dates of January 2016
and December 2021. Before case collection an appropriate
inclusion/exclusion criteria was developed where the exclusion
criteria considered underlying diseases that affected the clinical
data, including: i) Long-term and heavy smoking; ii)
immunosuppressed status (treatment with immune inhibitors,
chemotherapy, radiation therapy, high-dose glucocorticoids) or
diseases affecting immune function (malignant lymphoma, leukemia,
acquired immunodeficiency syndrome, systemic lupus erythematosus,
multiplemyeloma); iii) hematologic disorders (anemia, hemophilia,
myelodysplasticsyndromes, primary thrombocytosis,
erythroblastosis); iv) coronary heart disease, serious arrhythmia
or acute myocardial ischemia; v) single or multiorgan dysfunction
(kidney, liver failure, upper gastrointestinal bleeding, stress
ulcers, cardiac failure, disseminated intravascular coagulation);
vi) pregnancy or nursing; and vii) severe allergy history. The
inclusion criteria was the admission diagnosis was burn injury
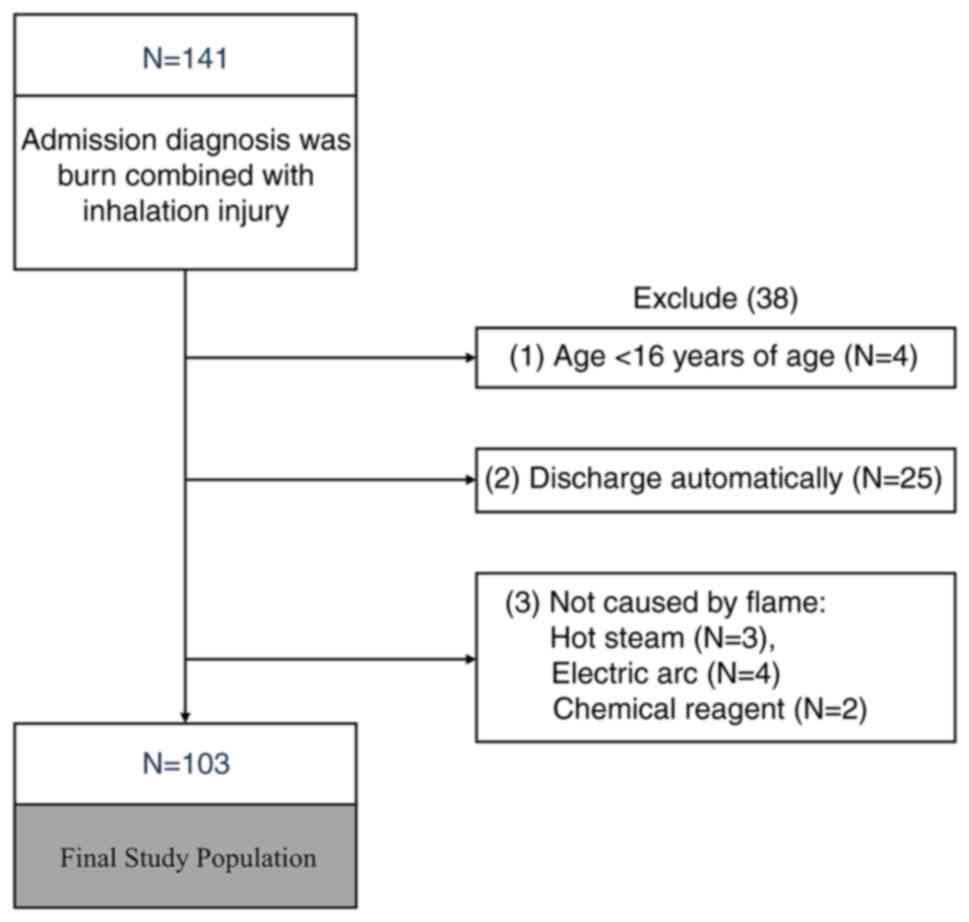
combined with inhalation injury. As shown in Fig. 1, the study collected a total of 141
cases.
Admission diagnosis was burn combined with
inhalation injury, and further exclusion criteria were: i) Age
<16 years old; ii) automatic discharge; and iii) the cause of
injury was not caused by flames (for example hot steam, electric
arc, chemical reagents). A total of 38 cases were excluded, and a
total of 103 cases were included in the final study. The maximum
age was 91 years, the minimum age was 17 years, and the average age
was 46.70±14.21 years. The percentage of female patients was 30.1%.
General data of SII patients' medical records were recorded,
including: Age, sex, weight, time of admission, TBSA afflicted by
burn, II burn area (of total %TBSA) and III burn area (of total
%TBSA).
The extent of SII was divided into mild, moderate
and severe. Determination of the extent of SII included the
combination of hospital records, physical manifestations (burnt
facial hair, carbon deposits in the oropharynx or sputum and facial
burns), blood gas analyses, bronchoscopies and chest radiographies
and pulmonary function (13,19):
i) Mild, dry throat and pain, mild congestion, edema of pharynx,
X-Ray and blood gas analysis without hypoxemia and airway stenosis,
the patients were outside of anoxic environments, and the condition
could be alleviated; (ii) Moderate, airway obstruction, wheezing,
dry rale, throat mucosa, vocal cord mucosa, tracheal mucosa,
congestion, edema and X-Ray analysis revealing tracheal stenosis;
and iii) severe, extensive edema, hemorrhage, ulceration, necrosis
and shedding of trachea and bronchial mucosa, chest imaging
examination showing extensive pulmonary patchy shadows, bullae,
bilateral pleural effusion, progressive aggravation between days
2-15 and blood gas analysis showing mild hypoxemia.
Oxygenation index: PaO2/FiO2,
PaO2 is the partial pressure of arterial blood oxygen
and FiO2 is the percentage of inhaled oxygen
concentration (20). The normal
value is 400-500 mmHg, and an oxygenation index of <300 mmHg
indicates pulmonary respiratory dysfunction (16). SII patients with ARDS were defined
according to the Berlin Definition: i) The mean of all
PaO2/FiO2 were measured for each 24 h period
within 1 week by arterial blood gas analysis. If the
PaO2/FiO2 values were <300 mmHg, and
positive end-expiratory pressure or continuous positive airway
pressure were >5 cm H2O; ii) chest imaging, bilateral
opaque infiltrates by radiography of chest; and iii) Origins of
oedema, respiratory failure excluding cardiac failure, or fluid
hyperload as shown by echocardiography (21).
The present study was approved by the Ethics
Committees of Characteristic Medical Center of Chinese People's
Armed Police Force and 983 Hospital of the Joint Logistics Support
Force of the Chinese People's Liberation Army. Written informed
consents were obtained from the patients. This was a retrospective
study and this study did not affect the treatment of patients
received.
Study design
In the present study, the following independent
steps were used for comparative analysis: i) Patients were
classified as three categories (mild, moderate and severe) and
comparisons of demographics, clinical biochemical indices and
prognostic characteristics of the patients with SII were performed
to explore the relationship between the severity of SII, burn area,
mortality, degree of lung injury, biochemical indices and analysis
of risk factors; ii) patients were divided into with or without
ARDS groups and the relevant risk factors of ARDS in SII were
analyzed; and iii) patients were divided into survival and
mortality groups and analysis of related risk factors was performed
in relation to SII-induced death.
Clinical and laboratory measurements
and recordings
From medical records, patients with SII symptoms,
general data, arterial blood gas analyses and blood biochemical
parameters and in-hospital outcomes for patients with SII were
recorded. Body mass index (BMI) is a commonly used measure of body
weight and health and was also collected (22). BMI=weight (kg) divided by height
(m) squared. APACHE II and lung injury prediction score (LIPS) were
used to assess disease severity upon patients' admission. APACHE II
included three portions: Score of acute physiological [temperature,
mean arterial pressure, heart rate, respiratory rate, oxygenation
index, arterial pH, Na, K, HCO3-, blood
creatinine, hematocrit, white blood cells (WBC), Glasgow Coma Score
(23)], age and chronic health. It
was employed to objectively assess the patient severity, formulate
detection and treatment plans, evaluate the treatment effect and
predict the mortality of group and individual patients (24). LIPS was used to identify patients
at risk of developing acute lung injury early in the analysis and
provided an opportunity to implement secondary prevention
strategies. LIPS was composed of 4 formations: i) Predisposing
circumstances, including shock, aspiration, septicemia and
pneumonia; ii) high risk surgical procedures; iii) high risk of
trauma; and iv) risk indicators covering alcohol abuse (BMI >30;
hypoalbuminemia; chemotherapy; FiO2 >35%; respiratory
rate >30 bpm; SpO2 <95%; pH <7.35; and diabetes
mellitus) (25).
Statistical analysis
SPSS 25 (IBM Corp.) software package was used for
data processing and analyses. The measurement data were conformed
to normal distribution [age, BMI, total protein, albumin and uric
acid (UA)], which were expressed as the mean ± standard deviation.
One-way ANOVA and Bonferroni correction was used to determine the
significance of each individual test. For measures with non-normal
distribution [Admission time (h), TBSA, Ⅱ burn area (of total
%TBSA), Ⅲ burn area (of total %TBSA), APACHE II, LIPS, pH,
PaO2/FiO2, PaO2, PaCO2,
lactic acid, WBC, neutrophils, red blood cell (RBC), hemoglobin,
platelet, alanine transaminase (ALT), aspartate aminotransferase
(AST), albumin/globulin (A/G), blood urea nitrogen (BUN), serum
creatinine (SCr)], were expressed as
[M(P25-P75)], and the Kruskal-Wallis H Test
followed by Dunn's post hoc was used for comparison between the
three groups. Counting data (such as percentage of sex, respiratory
infection rate, ARDS rate and mortality rate, the cause of death
rate of ARDS, MODS and shock), were expressed as frequency (n) and
percentage (%) values, and Fisher and chi-square (χ2)
tests were used for comparison among the three groups. Univariate
logistic regression with unadjusted odds ratio (OR) and 95%
confidence interval (CI) was performed to identify potential
parameters for severity, ARDS and mortality of patients with SII.
Subsequently, the significant predictors from the univariate
logistic regression model were verified by multiple logistic
regression, and the independent risk factors were screened
(P<0.05). The severity of SII was analyzed by ordered logistic
regression. Patients with SII that developed ARDS and mortality was
analyzed by binary logistic regression, which was further described
in the receiver-operating characteristic (ROC) curve to calculate
the area under the curve (AUC) and evaluate its accuracy in the
distinction between severe SII, ARDS and mortality. The criteria
for judging the quality of predictive models from AUC was a
follows: i) Perfect, AUC=1; ii) good, 0.85<AUC<1; iii) fair,
0.7<AUC≤0.85; iv) low, 0.5<AUC≤0.7; and v) no predictive
value ≤0.5. The cut-off point was determined as the maximum value
of Youden index (=sensitivity + specificity -1), and the
sensitivity and specificity were calculated respectively. P<0.05
was considered to indicate a statistically significant
difference.
Results
General epidemiological data
From January 2016 to December 2021, a total of 141
patients were admitted with flame injury and were diagnosed with
burn combined with SII. Following the exclusion criteria presented
in Fig. 1, a study population of
103 subjects remained, with a maximum age of 91 and minimum age of
17 years. The average age amongst total SII patients' average age
was 46.70±14.21. The average age of SII patients with mild,
moderate and severe were: 45.08±14.65, 45.07±13.89 and 49.72±14.16,
respectively. There were no significant differences in the age of
patients with SII with mild to moderate and severe SII (F=1.258;
P=0.289). A proportion of 30.1% were female patients. Female
patients showed little difference in mild to moderate and severe
SII, with no significance determined (χ2=2.219;
P=0.315). The average BMI was 22.40±2.39, and there were no
significant differences in BMI between patients with mild, moderate
and severe SII (F=1.003; P=0.371). Time of patients with SII from
burn to admission was 3.00 h (2.00-4.00 h), there was also no
significant difference in the time of previous admission between
patients with mild, moderate and severe SII (χ2=2.073;
P=0.355). Among these patients, 15 died and the fatality rate was
14.6%. Causes of death included: Multiple organ dysfunction
syndrome (MODS) in seven cases, ARDS in six cases and shock in two
cases. The TBSA (%) was 50.00 (29.00-70.00), and the Ⅱ burn area
(of total TBSA %) was 15.00 (8.00-25.00). The Ⅲ burns area (of
total TBSA %) was 30.00 (10.00-50.00). The larger the TBSA and Ⅲ
burns area the more severe the extent of SII (χ2=24.184,
P=6.00x10-6, χ2=23.150,
P=9.00x10-6). With the SII from mild to severe, the
number of patients with respiratory tract infection or that
developed ARDS, patient mortalities and the levels of APACHE II,
LIPS, lactic acid, WBC, ALT, BUN, SCr and UA were all also
significantly increased (P<0.05). Whereas
PaO2/FiO2, PaO2, RBC, hemoglobin,
platelet count, total protein, albumin and A/G were significantly
decreased with the increasing severity of SII (P<0.05) (Table I).
 | Table IBaseline characteristics of the of
the population enrolled in the present study. |
Table I
Baseline characteristics of the of
the population enrolled in the present study.
|
Characteristics | Total (n=103) | Mild (n=26) | Moderate
(n=41) | Severe (n=36) | Test value | P-value |
|---|
| Demographics | | | | | | |
|
Age (years,
x̄ ± sd) | 46.70±14.21 | 45.08±14.65 | 45.07±13.89 | 49.72±14.16 | F=1.258 | 0.289 |
|
Female sex,
n (%) | 31 (30.1%) | 10 (38.5%) | 9 (22.0%) | 12 (33.3%) |
X2=2.219 | 0.315 |
|
BMI
(x̄ ± sd) | 22.40±2.39 | 22.23±2.42 | 22.81±2.50 | 22.07±2.22 | F=1.003 | 0.371 |
|
Admission
time [h, M (P25-P75)] | 3.00
(2.00-4.00) | 3.00
(2.00-4.00) | 2.00
(1.00-4.00) | 3.00
(2.00-4.00) |
X2=2.073 | 0.330 |
| Burn area | | | | | | |
|
Total body
surface area [%TBSA, M (P25-P75)] | 50.00
(29.00-70.00) | 26.00
(14.50-46.00) | 55.00
(30.00-60.00)a | 70.00
(46.50-85.00)a |
X2=24.184 |
6.00x10-6 |
|
Ⅱ burn area
[%TBSA, M (P25-P75)] | 15.00
(8.00-25.00) | 10.50
(5.00-25.00) | 15.00
(10.00-25.00) | 17.00
(8.50-24.50) |
X2=0.797 | 0.671 |
|
Ⅲ burns area
[%TBSA, M (P25-P75)] | 30.00
(10.00-50.00) | 10.50
(4.75-22.75) | 30.00
(11.00-47.50)b | 42.50
(30.00-70.00)a |
X2=23.150 |
9.00x10-6 |
| Disease
severity | | | | | | |
|
Respiratory
tract infection, n (%) | 41 (39.81%) | 5 (19.23%) | 15 (36.59%) | 21
(58.33%)a |
X2=18.384 |
4.00x10-6 |
|
ARDS, n
(%) | 20 (19.41%) | 3 (11.53%) | 5 (12.19%) | 12
(33.33%)b,c |
X2=6.950 | 0.014 |
|
APACHE II, M
(P25-P75) | 6.00
(3.00-11.00) | 4.00
(2.00-6.50) | 6.00
(2.00-9.00) | 10.5
(3.25-14.00)a,c |
X2=14.030 | 0.001 |
|
LIPS, M
(P25-P75) | 6.50
(5.50-7.50) | 5.25
(4.00-6.00) | 7.00
(5.50-7.50)a | 7.50
(6.00-8.75)a |
X2=23.559 |
8.00x10-6 |
|
Mortality, n
(%) | 15 (14.6%) | 1 (3.8%) | 3 (7.3%) | 11
(30.6%)a,c |
X2=10.181 | 0.005 |
| Cause of death | | | | | | |
|
ARDS, n
(%) | 6 (5.82%) | - | 1 (2.43%) | 5 (13.89%) | - | - |
|
MODS, n
(%) | 7 (6.80%) | - | 2 (4.88%) | 5 (13.89%) | - | - |
|
Shock, n
(%) | 2 (1.94%) | 1 (3.84%) | - | 1 (2.77%) | - | - |
| Laboratory
analysis | | | | | | |
|
Arterial
blood gas | | | | | | |
|
pH, M
(P25-P75) | 7.38
(7.34-7.42) | 7.39
(7.32-7.43) | 7.36
(7.33-7.41) | 7.39
(7.35-7.42) |
X2=3.927 | 0.140 |
|
PaO2/FiO2
[mmHg, M (P25-P75)] | 293.94
(235.14-402.74) | 320.01
(274.12-413.79) | 300
(247.78-390.86) | 251.57
(215.04-332.67) |
X2=5.147 | 0.076 |
|
PaO2
[mmHg, M (P25-P75)] | 110.00
(86.00-151.00) | 145.50
(95.85-159.75) | 110.00
(94.80-140.00) | 90.50
(80.25-128.25)b |
X2=8.282 | 0.016 |
|
PaCO2
[mmHg, M (P25-P75)] | 37.50
(32.20-41.00) | 36.50
(30.43-40.18) | 37.40
(32.60-39.95) | 38.80
(34.25-42.08) |
X2=1.489 | 0.475 |
|
Lactic acid
[mmol/l, M (P25-P75)] | 4.70
(2.70-8.10) | 4.60
(3.03-6.20) | 3.90
(2.20-5.05) | 8.15
(4.38-10.35)b,d |
X2=18.069 |
1.20x10-4 |
|
Blood cell
analysis | | | | | | |
|
WBC
[109/l, M(P25-P75)] | 18.70
(13.65-27.25) | 13.92
(12.19-18.49) | 16.83
(11.99-24.02) | 26.06
(20.21-30.64)a,d |
X2=22.507 |
1.30x10-5 |
|
Neutrophils
[%, M(P25-P75)] | 87.20
(82.60-90.60) | 86.40
(83.88-90.58) | 86.20
(81.00-89.65) | 88.2
(84.40-91.25) |
X2=1.777 | 0.411 |
|
RBC
[1012/l, M (P25-P75)] | 5.03
(3.52-5.58) | 5.39
(4.96-5.71) | 5.03
(4.32-5.39) | 3.85
(2.23-5.35)a |
X2=13.059 | 0.001 |
|
Hemoglobin
[g/l, M (P25-P75)] | 159.00
(3.52-5.58) | 168.00
(157.75-177.50) | 160.00
(143.00-176.00) | 114.50
(79.75-165.75)a,c |
X2=13.293 | 0.001 |
|
Platelet
[109/l, M(P25-P75)] | 254.00
(195.00-335.00) | 301.00
(227.50-420.25) | 276.00
(206.00-330.00) | 205.5
(129.00-281.50)a,c |
X2=11.059 | 0.004 |
|
Biochemical
analysis | | | | | | |
|
ALT [IU/l,
M(P25-P75)] | 36.00
(24.00-62.00) | 33.00
(19.00-40.75) | 49.00
(24.50-54.50) | 53.00
(27.50-77.50)b |
X2=8.085 | 0.018 |
|
AST [U/l,
M(P25-P75)] | 47.00
(30.00-74.00) | 47.00
(30.00-66.25) | 59.00
(35.00-69.00) | 45.00
(28.00-85.00) |
X2=0.108 | 0.947 |
|
Total
protein (g/l, x̄ ± sd) | 58.12±11.58 | 62.96±10.49 | 57.61±10.71 |
55.22±12.45b | F=3.610 | 0.031 |
|
Albumin
(g/l, x̄ ± sd) | 31.08±10.09 | 36.88±9.71 |
30.63±9.09b |
27.41±9.80a | F=7.577 | 0.001 |
|
A/G
M(P25-P75) | 1.13
(0.90-1.45) | 1.37
(1.10-1.78) | 1.14
(0.94-1.49) | 0.98
(0.83-1.25)a |
X2=13.417 | 0.001 |
|
BUN [mmol/l,
M(P25-P75)] | 6.60
(5.40-8.30) | 6.10
(4.88-7.25) | 6.40
(4.75-8.00) | 8.05
(6.43-9.20)a,c |
X2=12.809 | 0.002 |
|
SCr [µmol/l,
M(P25-P75)] | 87.00
(64.00-134.00) | 71.00
(53.75-98.75) | 67.00
(55.50-123.00) | 134.00
(84.50-167.00)a,d |
X2=19.725 |
5.20x10-5 |
|
UA (mmol/l,
x̄ ± sd) | 401.73±157.09 | 312.57±107.65 | 398.19±144.73 |
470.16±170.18a | F=8.775 |
5.20x10-5 |
Predictors and risk factors for
severity in patients with SII
First, the present study revealed that TBSA, Ⅲ burns
area (of total TBSA %), respiratory tract infection, APACHE II,
LIPS, PaO2/FiO2, PaO2, lactic
acid, WBC, RBC, hemoglobin, platelet count, ALT, total protein,
albumin, A/G, BUN, SCr and UA were associated with severity of SII
(P<0.05), as determined by univariate ordered logistic
regression analysis. There was no association between female sex,
BMI, admission time, Ⅱ burn area (of total TBSA %), pH,
PaO2/FiO2, PaCO2, neutrophils, or
AST with the severity of SII ranging from mild to severe
(P>0.05) (Table II).
Multivariate ordered logistic regression was performed for these
variables with P<0.05 in the univariate ordered logistic
regression analysis. The result (parallelism test P>0.05)
revealed that APACHE II (OR=1.105, 95% CI 1.013-1.206, P=0.025),
LIPS (OR=1.517, 95% CI 1.191-1.931, P=0.021), lactic acid
(OR=1.174, 95% CI 1.052-1.31, P=0.004), WBC (OR=1.120, 95% CI
1.062-1.182, P=5.00x10-5, SCr (OR=1.018, 95% CI
1.007-1.028, P=0.001) and UA (OR=1.005, 95% CI 1.001-1.008,
P=0.012) were independent risk factors for severity of patients
with SII (Table II). The higher
the level of APACHE II, LIPS, Lactic acid, WBC, SCr and UA, the
higher the severity of SII. A clear discrimination of severe SII
from moderate and mild SII groups was demonstrated based on the ROC
curves, which showed that APACHE II, LIPS, lactic acid, WBC, SCr
and UA had sensitivities of 55.6, 38.9, 61.1, 75.0, 72.2 and 58.3%;
specificities of 83.6, 91.0, 80.6, 76.1, 71.6 and 76.4%; and AUC:
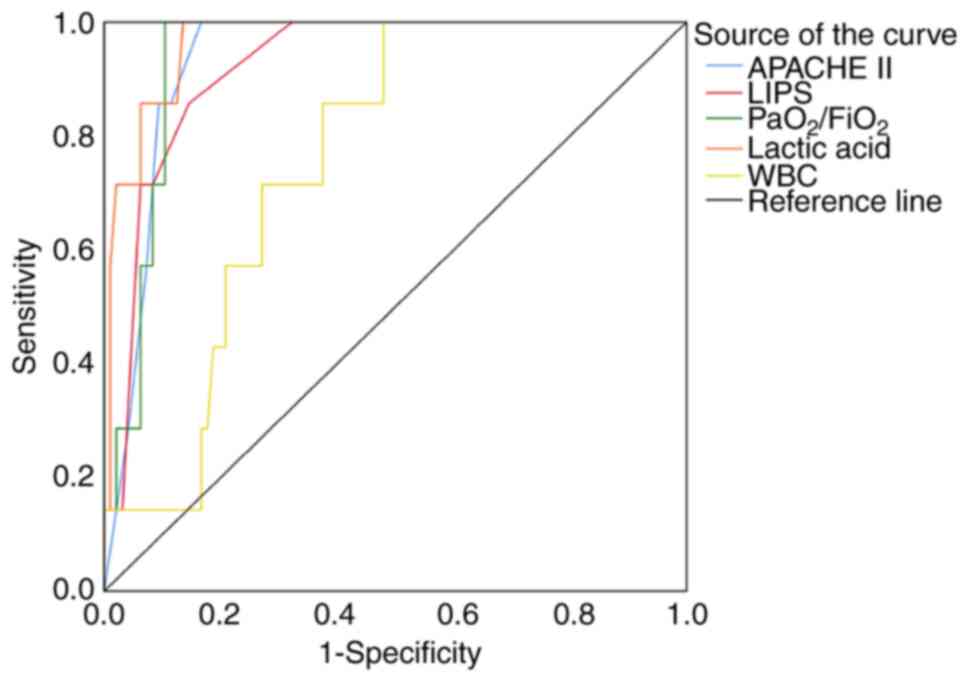
0.710, 0.704, 0.743, 0.774, 0.765 and 0.680 respectively (Table III) (Fig. 2). The results showed that WBC had
the highest AUC (0.774) and sensitivity (75.0%), thus the
reliability of WBC at the cutoff point of 20.91 (109/l)
indicated a strong possibility of severe SII.
 | Figure 2Receiver operating characteristic
curves of APACHE II, LIPS, Lactic acid, WBC, UA, SCr in predicting
for severity of patients with SII. APACHE, acute physiology and
chronic health evaluation; LIPS, lung injury prediction score; WBC,
white blood cells; SCr, serum creatinine; UA, uric acid. |
 | Table IIUnivariate and multivariate logistic
regression analysis of risk factors for severity of SII
patients. |
Table II
Univariate and multivariate logistic
regression analysis of risk factors for severity of SII
patients.
| | Univariate logistic
regression | Multivariate
logistic regression |
|---|
| Parameters | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age
(years)a | 1.019
(0.993-1.045) | 0.160 | - | - |
| Female sex, n
(%)a | 1.094
(0.503-2.382) | 0.821 | - | - |
| BMIa | 0.966
(0.831-1.123) | 0.655 | - | - |
| Admission time
(h)a | 1.009
(0.804-1.267) | 0.939 | - | - |
| Burn area | | | | |
|
Total body
surface area (%TBSA)a,b | 1.041
(1.024-1.058) |
1.00x10-6 | - | - |
|
Ⅱ burn area
(%TBSA)a | 1.012
(0.984-1.041) | 0.421 | - | - |
|
Ⅲ burns area
(%TBSA)a,b | 1.043
(1.024-1.061) | 0.015 | - | - |
| Disease
severity | | | | |
|
Respiratory
tract infection, n (%)a,b | 1.000
(0.917-2.543) |
3.00x10-5 | | |
|
APACHE
IIa,b | 1.702
(1.351-2.144) |
1.22x10-4 | 1.105
(1.013-1.206) | 0.025 |
|
LIPSa,b | 0.758
(0.565-1.018) |
6.00x10-6 | 1.517
(1.191-1.931) | 0.021 |
| Laboratory
analysis | | | | |
|
Arterial
blood gas | | | | |
|
pHa | 49.933
(0.258-9648.611) | 0.145 | - | - |
|
PaO2/FiO2
(mmHg)a | 0.997
(0.993-1.000) | 0.068 | - | - |
|
PaO2
(mmHg)a,b | 0.987
(0.977-0.996) | 0.004 | - | - |
|
PaCO2
(mmHg)a | 0.993
(0.966-1.020) | 0.600 | - | - |
|
Lactic acid
(mmol/l)a,b | 1.194
(1.075-1.328) | 0.001 | 1.174
(1.052-1.311) | 0.004 |
|
Blood cell
analysis | | | | |
|
WBC
(109/l)a,b | 1.118
(1.062-1.177) |
2.10x10-5 | 1.120
(1.062-1.182) |
5.00x10-5 |
|
Neutrophils
(%)a | 1.008
(0.976-1.040) | 0.639 | - | - |
|
RBC
(1012/l)a,b | 0.980
(0.971-0.990) |
6.50x10-5 | - | - |
|
Hemoglobin
(g/l)a,b | 0.997
(.982-1.012) |
5.70x10-5 | - | - |
|
Platelet
(109/l)a,b | 0.995
(0.992-0.998) | 0.003 | - | - |
|
Biochemical
analysis | | | | |
|
ALT
(IU/l)a,b | 1.017
(1.004-1.030) | 0.010 | - | - |
|
AST
(IU/l)a | 1.006
(0.999-1.013) | 0.104 | - | - |
|
Total
protein (g/l)a,b | 0.958
(0.928-0.990) | 0.010 | - | - |
|
Albumin
(g/l)a,b | 0.930
(0.895-0.968) |
3.08x10-4 | - | - |
|
A/Ga,b | 0.461
(0.238-0.895) | 0.022 | - | - |
|
BUN
(mmol/l)a,b | 1.215
(1.060-1.391) | 0.005 | - | - |
|
SCr
(µmol/l)a,b | 1.019
(1.010-1.028) |
3.40x10-5 | 1.018
(1.007-1.028) | 0.001 |
|
UA
(mmol/l)a,b | 1.005
(1.002-1.008) |
1.66x10-4 | 1.005
(1.001-1.008) | 0.012 |
 | Table IIIReceiver operating characteristic
curve analysis of APACHE II, LIPS, lactic acid, WBC, UA and SCr in
predicting severe of SII patients. |
Table III
Receiver operating characteristic
curve analysis of APACHE II, LIPS, lactic acid, WBC, UA and SCr in
predicting severe of SII patients.
| Parameters | AUC | 95% CI | Cut-off | Sensitivity
(%) | Specificity
(%) | Youden index
(%) | P-value |
|---|
| APACHE II | 0.710 | 0.601-0.819 | 9.5 | 55.6 | 83.6 | 39.1 |
4.48x10-4 |
| LIPS | 0.704 | 0.596-0.811 | 7.75 | 38.9 | 91.0 | 29.9 | 0.001 |
| Lactic acid
(mmol/l) | 0.743 | 0.641-0.845 | 5.65 | 61.1 | 80.6 | 41.7 |
5.00x10-5 |
| WBC
(109/l) | 0.774 | 0.677-0.871 | 20.91 | 75.0 | 76.1 | 51.1 |
5.00x10-6 |
| SCr (µmol/l) | 0.765 | 0.664-0.866 | 101.5 | 72.2 | 71.6 | 43.9 |
1.00x10-5 |
| UA (mmol/l) | 0.680 | 0.568-.0791 | 445.0 | 58.3 | 76.4 | 33.0 | 0.003 |
Predictors and risk factors for
patients with SII and ARDS development
The potential factors in relation to patients with
SII and ARDS development as calculated by univariate logistic
regression were TBSA, Ⅲ burns area (of total TBSA %), respiratory
tract infection, APACHE II, LIPS, PaO2/FiO2,
PaO2, lactic acid, WBC, RBC, hemoglobin, platelet count,
ALT, BUN, and UA (P<0.05). There was no relationship between
age, female sex, BMI, admission time, Ⅱ burn area (of total %TBSA),
pH, PaCO2, neutrophils, AST, total protein, albumin, A/G
and SCr associated with patients with SII development of ARDS
(P>0.05) (Table IV).
Multivariate logistic regression revealed that APACHE II (OR=1.881,
95% CI 1.040-3.404, P=0.037), LIPS (OR=2.889, 95% CI 1.025-8.139,
P=0.045), lactic acid (OR=2.095, 95% CI 1.130-3.882, P=0.019) and
WBC (OR=1.281, 95% CI 1.017-1.613, P=0.036) were independent risk
factors for patients with SII with ARDS development. However,
PaO2/FiO2 (OR=0.979, 95% CI 0.966-0.993,
P=0.003) was a protective factor (Table IV). Further ROC curves
demonstrated that APACHE II, LIPS, PaO2/FiO2,
lactic acid and WBC had sensitivities of 100, 87.5, 100, 100 and
100%; specificities of 83.3, 85.4, 89.6, 86.5 and 52.1%; and AUC of
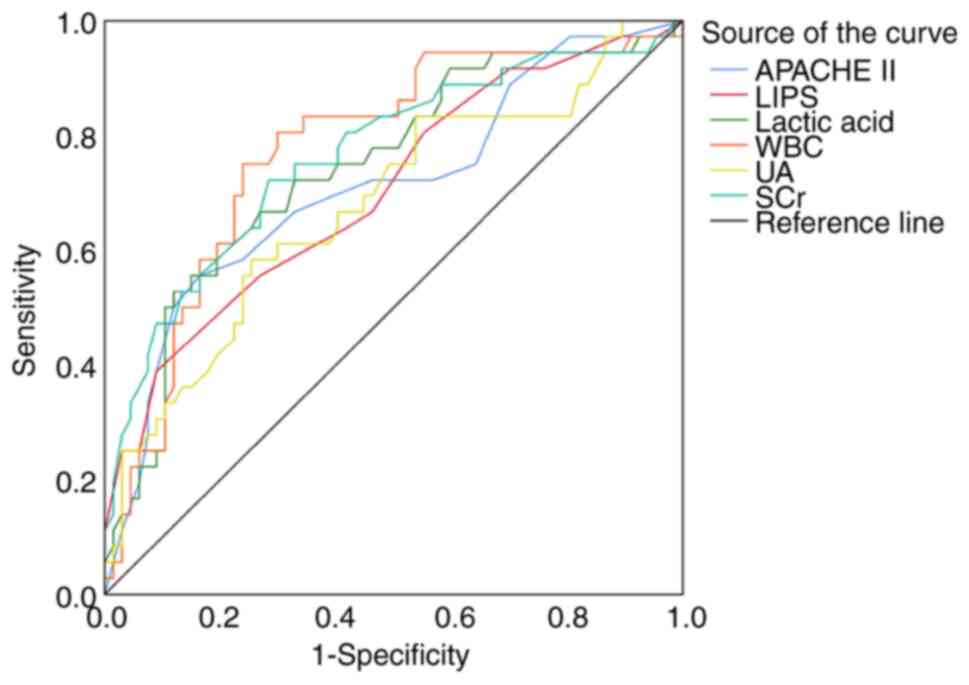
0.934, 0.923, 0.938, 0.966 and 0.760, respectively (Table V) (Fig. 3). The most accurate parameters for
predicting development of ARDS in patients with SII were APACHE II,
LIPS, PaO2/FiO2 and lactic acid
(AUC>0.85). Lactic acid's highest AUC (0.966) and sensitivity
(100%), with the highest reliability of lactic acid having a
cut-off point of 9.6 (mmol/l), suggested that lactic acid was a
strong predictor that patients with SII had developed ARDS.
 | Table IVUnivariate and multivariate logistic
regression analysis of risk factors for SII patients with acute
respiratory distress syndrome development. |
Table IV
Univariate and multivariate logistic
regression analysis of risk factors for SII patients with acute
respiratory distress syndrome development.
| | Univariate logistic
regression | Multivariate
logistic regression |
|---|
| Parameters | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age,
yearsa | 1.033
(0.981-1.087) | 0.219 | - | - |
| Female sex, n
(%)a | 0.924
(0.169-5.042) | 0.927 | - | - |
| Admission time
(h)a | 1.292
(0.827-2.020) | 0.260 | - | - |
| BMIa | 0.991
(0.716-1.371) | 0.955 | - | - |
| Burn area | | | | |
|
Total body
surface area (%TBSA)a,b | 1.036
(1.000-1.073) | 0.048 | - | - |
|
Ⅱ burn area
(%TBSA)a | 0.999
(0.941-1.061) | 0.978 | - | - |
|
Ⅲ burns area
(%TBSA)a,b | 1.031
(1.001-1.061) | 0.039 | - | - |
|
Respiratory
tract infection, n (%)a,b | 10.457
(1.209-90.441) | 0.033 | - | - |
| Disease
severity | | | | |
|
APACHE
IIa,b | 1.799
(1.186-2.728) | 0.006 | 1.881
(1.040-3.404) | 0.037 |
|
LIPSa,b | 3.844
(1.682-8.784) | 0.001 | 2.889
(1.025-8.139) | 0.045 |
| Laboratory
analysis | | | | |
|
Arterial
blood gas | | | | |
|
pHa | 0.178
(0.000-489.389) | 0.088 | - | - |
|
PaO2/FiO2
(mmHg)a,b | 0.971
(0.953-0.989) | 0.002 | 0.979
(0.966-0.993) | 0.003 |
|
PaO2
(mmHg)a,b | 0.947
(0.909-0.988) | 0.011 | - | - |
|
PaCO2
(mmHg)a,b | 1.015
(0.975-1.056) | 0.477 | - | - |
|
Lactic
acid (mmol/l)a,b | 2.093
(1.301-3.367) | 0.002 | 2.095
(1.130-3.882) | 0.019 |
|
Blood cell
analysis | | | | |
|
WBC
(109/l)a,b | 1.115
(1.023-1.215) | 0.013 | 1.281
(1.017-1.613) | 0.036 |
|
Neutrophils
(%)a | 0.974
(0.928-1.023) | 0.294 | - | - |
|
RBC
(1012/l)a,b | 0.413
(0.229-0.746) | 0.003 | - | - |
|
Hemoglobin
(g/l)a,b | 0.968
(0.948-0.989) | 0.003 | - | - |
|
Platelet
(109/l)a,b | 0.985
(0.974-0.996) | 0.008 | - | - |
|
Biochemical
analysis | | | | |
|
ALT
(IU/l)a,b | 1.023
(1.005-1.040) | 0.011 | - | - |
|
AST
(IU/l)a | 1.003
(0.997-1.009) | 0.365 | - | - |
|
Total
protein (g/l)a | 0.992
(0.929-1.059) | 0.807 | - | - |
|
Albumin
(g/l)a | 0.953
(0.880-1.031) | 0.228 | - | - |
|
A/Ga | 0.196
(0.027-1.432) | 0.108 | | |
|
BUN
(mmol/l)a,b | 1.254
(1.004-1.566) | 0.046 | - | - |
|
SCr
(µmol/l)a | 1.012
(1.000-1.025) | 0.059 | - | - |
|
UA(mmol/l)a,b | 1.010
(1.004-1.015) | 0.001 | - | - |
 | Table VReceiver operating characteristic
curve analysis of APACHE II, LIPS, PaO2/FiO2,
lactic acid and WBC in predicting SII patients with acute
respiratory distress syndrome development. |
Table V
Receiver operating characteristic
curve analysis of APACHE II, LIPS, PaO2/FiO2,
lactic acid and WBC in predicting SII patients with acute
respiratory distress syndrome development.
| Parameters | AUC | 95% CI | Cut-off | Sensitivity
(%) | Specificity
(%) | Youden index
(%) | P-value |
|---|
| APACHE II | 0.934 | 0.883-0.985 | 11.50 | 100 | 83.3 | 83.3 |
1.34x10-4 |
| LIPS | 0.923 | 0.856-0.991 | 7.75 | 0.875 | 85.4 | 71.1 |
1.93x10-4 |
|
PaO2/FiO2 | 0.938 | 0.889-0.986 | 215.07 | 100 | 89.6 | 89.6 |
1.17x10-4 |
| Lactic acid
(mmol/l) | 0.966 | 0.925-1.000 | 9.60 | 100 | 86.5 | 86.5 |
4.10x10-5 |
| WBC
(109/l) | 0.760 | 0.634-0.885 | 18.50 | 100 | 52.1 | 52.1 | 0.022 |
Predictors and risk factors for
mortality of patients with SII
The univariate logistic regression results showed
that TBSA, Ⅱ TBSA (of total TBSA %), Ⅲ TBSA (of total TBSA %),
severity of SII, respiratory tract infection, APACHE II, LIPS,
lactic acid, WBC, PaO2/FiO2, PaO2,
RBC, hemoglobin, platelet count, AST, A/G, BUN and SCr were
associated with mortality of patients with SII (P<0.05). There
was no relationship between female sex, BMI, admission time
moderate of SII, ARDS, pH, neutrophils, ALT, total protein,
albumin, UA and mortality of SII patients (P>0.05) (Table VI). Further multivariate logistic
regression revealed that respiratory tract infection (OR=4.964, 95%
CI 1.179-20.905, P=0.029), lactic acid (OR=1.219, 95% CI
1.044-1.423, P=0.012), WBC (OR=1.157, 95% CI 1.010-1.325, P=0.036)
and SCr (OR=1.023, 95% CI 1.004-1.043, P=0.017) were independent
risk factors for mortalities of patients with SII, whereas
hemoglobin (OR=0.979, 95% CI 0.916-0.983, P=0.003) and A/G
(OR=0.401, 95% CI 0.102-0.931, P=0.020) were protective factors.
ROC curves showed that when lactic acid, WBC, hemoglobin, A/G and
SCr had sensitivities of 60.0, 93.3, 100, 80.0 and 100%;
specificities of 83.0, 67.0, 90.9, 79.5 and 62.5%; and AUC of
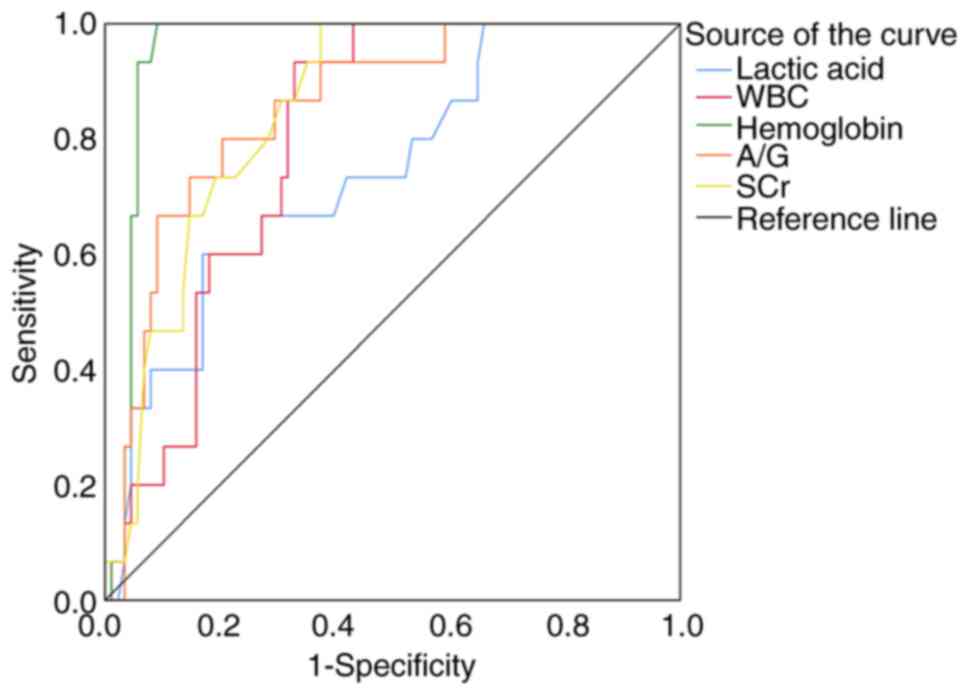
0.741, 0.801, 0.953, 0.854 and 0.852, respectively (Table VII). The results suggested that
hemoglobin had the highest reliability (AUC=0.953) at a cutoff
point of 83.00 (g/l) for predicting mortality in patients with SII
(Fig. 4). A/G and SCr
(AUC>0.85) were also reliable predictors for mortality of
patients with SII.
 | Table VIUnivariate and multivariate logistic
regression analysis of risk factors for mortality of SII
patients. |
Table VI
Univariate and multivariate logistic
regression analysis of risk factors for mortality of SII
patients.
| | Univariate logistic
regression | Multivariate
logistic regression |
|---|
| Parameters | OR (95% CI) |
P-valuea | OR (95% CI) |
P-valueb |
|---|
| Age,
yearsa | 1.015
(1.019-1.110) | 0.437 | - | - |
| Female sex, n
(%)a | 0.313
(0.066-1.480) | 0.143 | - | - |
| BMIa | 0.927
(0.730-1.177) | 0.535 | - | - |
| Admission time
(h) | 1.104
(0.788-1.547) | 0.565 | - | - |
| Burn area | | | | |
|
Total body
surface area (%TBSA)a,b | 1.042
(1.015-1.071) | 0.002 | - | - |
|
Ⅱ burn area
(%TBSA)a,b | 0.935
(0.877-0.997) | 0.040 | - | - |
|
Ⅲ burns area
(%TBSA)a,b | 1.058
(1.029-1.088) |
6.60x10-5 | - | - |
| Degree of lung
injury | | | | |
|
Moderate of
SII, n (%)a | 1.974
(0.194-20.059) | 0.565 | - | - |
|
Severe of
SII, n (%)a,b | 11.000
(1.319-91.720) | 0.027 | - | - |
| Disease
severity | | | | |
|
Respiratory
tract infection, n (%)a,b | 8.138
(2.129-31.109) | 0.002 | 4.964
(1.179-20.905) | 0.029 |
|
ARDS, n
(%)a | 2.554
(0.448-14.564) | 0.291 | - | - |
|
APACHE
IIa,b | 1.170
(1.040-1.317) | 0.009 | - | - |
|
LIPSa,b | 1.583
(1.098-2.282) | 0.014 | - | - |
| Laboratory
analysis | | | | |
|
Arterial
blood gas | | | | |
|
pHa | 0.103
(0.000-234.287) | 0.564 | - | - |
|
PaO2/FiO2
(mmHg)a,b | 0.994
(0.988-1.000) | 0.048 | - | - |
|
PaO2
(mmHg)a,b | 0.981
(0.964-0.999) | 0.036 | - | - |
|
PaCO2
(mmHg)a | 0.978
(0.917-1.043) | 0.502 | - | - |
|
Lactic
acid (mmol/l)a,b | 1.243
(1.083-1.427) | 0.002 | 1.219
(1.044-1.423) | 0.012 |
|
Blood cell
analysis | | | | |
|
WBC
(109/l)a,b | 1.107
(1.033-1.186) | 0.004 | 1.157
(1.010-1.325) | 0.036 |
|
Neutrophils
(%)a | 1.008
(0.956-1.063) | 0.765 | - | - |
|
RBC
(1012/l)a,b | 0.366
(0.230-0.582) |
2.10x10-5 | - | - |
|
Hemoglobin
(g/l)a,b | 0.941
(0.914-0.968) |
3.20x10-5 | 0.949
(0.916-0.983) | 0.003 |
|
Platelet
(109/l)a,b | 0.993
(0.988-0.999) | 0.024 | - | - |
|
Biochemical
analysis | | | | |
|
ALT
(IU/l)a | 1.011
(0.998-1.023) | 0.099 | - | - |
|
AST
(IU/l)a,b | 1.013
(1.003-1.023) | 0.010 | - | - |
|
Total
protein (g/l)a | 0.998
(0.952-1.047) | 0.945 | - | - |
|
Albumin
(g/l)a | 0.949
(0.897-1.005) | 0.075 | - | - |
|
A/Ga,b | 0.029
(0.004-0.209) |
4.34x10-4 | 0.401
(0.102-0.931) | 0.020 |
|
BUN
(mmol/l)a,b | 1.250
(1.053-1.483) | 0.011 | - | - |
|
SCr
(µmol/l)a,b | 1.027
(1.012-1.042) |
3.38x10-4 | 1.023
(1.004-1.043) | 0.017 |
|
UA
(mmol/l)a | 1.003
(1.000-1.007) | 0.067 | - | - |
 | Table VIIReceiver operating characteristic
curve analysis of lactic acid, WBC, hemoglobin, A/G and SCr in
predicting mortality of SII patients. |
Table VII
Receiver operating characteristic
curve analysis of lactic acid, WBC, hemoglobin, A/G and SCr in
predicting mortality of SII patients.
| Parameters | AUC | 95% CI | Cut-off | Sensitivity
(%) | Specificity
(%) | Youden index
(%) | P-value |
|---|
| Lactic acid
(mmol/l) | 0.741 | 0.883-0.985 | 8.70 | 60.0 | 83.0 | 43.0 | 0.003 |
| WBC
(109/l) | 0.801 | 0.856-0.991 | 20.91 | 93.3 | 67.0 | 60.4 |
2.06x10-4 |
| Hemoglobin
(g/l) | 0.953 | 0.889-0.986 | 83.00 | 100 | 90.9 | 90.9 |
2.20x10-8 |
| A/G | 0.854 | 0.925-1.000 | 0.94 | 80.0 | 79.5 | 59.5 |
1.30x10-5 |
| SCr (µmol/l) | 0. 852 | 0.634-0.885 | 95.00 | 100 | 62.5 | 62.5 |
1.40x10-5 |
Discussion
According to the American Burn Association (ABA),
from the total number of patients admitted to 128 burn centers in
the United States in 2016, 3,275 fatalities were associated with
burns from smoke inhalation; a total of 2,745 deaths were from
residential fires, 310 from car accident-related fires and 220 from
other causes (16). Similar to the
present retrospective study, among the 103 patients with burn
combined with inhalation injury, the main causes of injury were
residential-associated (44.8%), with factory accidents (23.0%) and
car accidents (16.1%) accounting for less. The majority of the
fires occurred indoors, causing an abundance of hot air and
pernicious smoke, which is more conducive to SII (26).
In the present study, the average age of injured
adults were 46.70±14.21 years and female patients accounted for
30.1%. In addition, the total SII patients' average BMI was
22.40±2.39, the average BMI of patients with SII in mild to severe
categories were within the normal healthy range. The time interval
from burn to admission was 3.00 h (2.00-4.00 h), there were no
significant difference in sex and the time of admission between
mild, moderate and severe SII. After regression statistical
analysis, sex, BMI and the time of patient admission were not
considered risk factors for patients with severe SII for developing
ARDS or risk of mortality. However, statistical results suggested
that the severity of SII was positively associated with the TBSA.
With increasing SII severity, incidence of respiratory tract
infections, ARDS and mortality also significantly increased.
Accompanying pathologies of burn injuries include MODS, sepsis,
pneumonia and cellulitis. Overall, >60% of deaths from burn
injuries were attributed to MODS (27).
Despite extensive investigations, the etiology of
MODS remains unclear. All cases seem to show uncontrolled systemic
inflammatory response syndrome (SIRS) (28). The causes of post-burn infection
include sepsis, bacteremia after wound treatment, small repetitive
infection and bacterial translocation in the intestines (28). In the present study's results on
mortality of SII, the more serious the inhalation injury was, the
higher the rate of mortality; 15 of the 103 patients died (case
fatality rate of 14.56%). MODS, ARDS and shock are still considered
the main causes of death in burns patients (29). Patients mortalities within 48 h
after SII predominantly result from obstruction and asphyxia
(1). Septic shock caused by
exacerbated inflammatory reaction, rapid and extensive fluid
transfer in burn and non-burn tissues leading to progressive
hypovolemic shock forms an important cause of burn shock-related
mortality (12). In the current
dataset, patients who died within 3-7 days were predominantly
afflicted with ARDS, and those who died after 7 days were
predominantly afflicted with sepsis or wound sepsis complicated
with MODS. The serum marker levels at admission, routine evaluation
and associated parameters (laboratory, clinical examination)
provided important information such as the grade of SII, tendency
to develop into ARDS, mortality and prognosis. Risk stratification
for clinicians will be an extremely important consideration
(30). The present study
demonstrated that APACHE II, LIPS, lactic acid, WBC, ALT, BUN, SCr
and UA were also positively raised with the increasing severity of
SII, whereas PaO2/FiO2, PaO2, RBC,
hemoglobin, platelet count, total protein, albumin and A/G were
decreased with the increasing severity of SII.
The pathophysiology of SII includes direct protein
denaturation and a complex systemic inflammatory response
accompanied by the flow of protein-rich plasma and cellular
contents into the interstitial space, alveoli and bronchial system,
leading to the development of pulmonary oedema, increased airway
resistance and the subsequent formation of fibrin clots and loss of
surface active substances (31,32).
Combined, these events further limit the air flow to the alveoli,
which increases the probability of respiratory infection (15,33).
In the present patient dataset, the number of respiratory
infections was directly proportional to the severity of SII,
patients SII and ARDS and mortality. APACHE II is a reliable,
convenient and commonly utilized scoring system for clinicians to
evaluate disease severity in critically ill patients (24). The higher the score, the worse the
pathological condition and prognosis (18). As a novel system for predicting
lung injury, a higher LIPS score is associated with more serious
lung injury (34). In the present
study data, the increase of the severity of SII was associated with
increases in APACHE II and LIPS, which could be performed as
independent risk factors for severity of SII and ARDS development
of patients with SII. Comparisons of the AUC of the two groups
demonstrated that APACHE II and LIPS had relatively higher AUC
(AUC>0.9) in patients with SII and ARDS group. APACHE II
>11.5 and LIPS >7.75 indicated the possibility that patients
with SII had ARDS.
Fires rapidly consume oxygen, resulting in low
oxygen content in the environment (anoxic). Moreover, the extreme
heat of the smoke passing through the respiratory tract results in
edema and mucosal detachment, which are prone to drive upper
respiratory tract obstructions, severe hypoxemia and decreased
PaO2/FiO2 in patients with inhalation injury
(16). The normal range of
PaO2/FiO2 is 400-500 (mmHg) (21). The average
PaO2/FiO2 of patients with mild and moderate
SII was shown to be between 300-400 (mmHg), but the average
PaO2/FiO2 of patients with severe SII was
lower than 300 (mmHg), indicating that patients with severe SII
were likely to have respiratory disorders. In the present study,
PaO2/FiO2 was a protective factor in
determining ARDS development of SII patients. With the AUC=0.938,
PaO2/FiO2<215.07 (mmHg) was accurate to
indicate possibility that patients with SII developed ARDS.
In the stress state of large-area burns,
pathophysiological changes such as microcirculation disturbance,
tissue ischemia and hypoxia lead to insufficient oxygenated blood
perfusion to important organs, and subsequent increases in level of
lactic acid (35). It has been
demonstrated that base deficit and serum lactic acid have
well-known associations with mortality in burn patients (36). The present study showed that the
average value of lactic acid in patients with SII was higher
compared with the normal range (0.5-2.2 mmol/l), especially in
patients with moderate and severe SII. Lactic acid is an
intermediate product of anaerobic metabolism of glucose, which can
be excreted through normal metabolic pathways. In the present
study, while lactic acid was suggested to be an independent risk
factor for determining the severity of SII, patients with ARDS and
SII and SII mortality. Compared with other groups, lactic acid had
the highest AUC (0.966) in ARDS group. When lactic acid >9.60
(mmol/l), it indicated the relative possibility that patients with
SII developed ARDS. The present data also showed that there were no
significant difference in pH measurements between the mild,
moderate and severe patient groups. Therefore, after regression
statistical analysis confirmed the finding, pH was not considered a
risk factor for patients with severe SII for developing ARDS or
risk of mortality.
Infections are the most common complications in
hospitalized patients with severe burns (37). WBC is an effective predictor for
early blood stream infection in burn patients (38). The average increase of WBC at
admission may be due to the initialization of systemic inflammation
causing WBC mobilization (39). In
the present study, WBC average values in mild, moderate and severe
SII were significantly higher compared with normal patients. Yet
the normal range of WBC was 4-10 (109/l). The results
suggested that SII caused a large leukocyte recruitment response.
WBC was raised with the increased severity of SII. Moreover, in the
current research, WBC was suggested to form an independent risk
factor for determining the severity of SII, ARDS development in
patients with SII and SII mortality. Consistent with our previous
study that the severity of thermal burn injury is associated with
WBC activation, WBC and neutrophil counts were significantly
increased on admission day (40).
Compared with the other indicators in severity of SII group, the
highest AUC and sensitivity were 0.774 and 75.0%, respectively.
Thus, WBC had reliable prediction at a cut-off point of 20.91
(109/l) for indicating the possibility of severe
SII.
In trauma bleeding after burns, hemolysis of red
blood cells in the burn area occurs under the direct influence of
heat (41,42). A variety of injury mechanisms cause
red blood cell rupture, resulting in a reduction in the amount of
red blood cells and a decrease in hemoglobin values (43). Early thrombocytopenia in burns is
caused by the destruction of platelets or their accumulation in the
skin near the burn scabs (44).
The present study showed that RBC, hemoglobin and platelet count
decreased with increasing severity of SII. Furthermore, hemoglobin
was a protective factor for mortality of patients with SII, and the
normal hemoglobin range was 110-150 (g/l). The ROC curve showed
that the AUC and sensitivity of hemoglobin were 0.953 and 100%,
respectively. These data indicated that hemoglobin, at a cut-off of
83.00 (g/l), was a highly reliable predictor for mortality of
patients with SII.
Total protein, albumin and A/G also decreased with
increasing severity of SII. Due to severe massive burns, plasma
proteins can be lost through traumatic massive protein leakage and
tissue breakdown, with a decrease in albumin and more loss of
albumin than globulin, reversing the A/G ratio (normal A/G range is
1.5-2.5) (45). The present study
revealed A/G as a protective factor for SII mortality and that A/G
had great accuracy in predicting mortality (AUC=0.854). Conversely
ALT, BUN, SCr and UA were positively raised with increasing
severity of SII. Elevated ALT in the early phase of injury can be
due to shock or hypovolemia, resulting in ischemia and hypoxia in
the liver, and leading to liver damage (46). Reduced effective circulating blood
volume due to various causes after burns leads to reduced renal
blood flow and decreased glomerular filtration rate, resulting in
increased BUN, SCr and UA stasis (47). In the present study, SCr was an
independent risk factor for predicting the severity of SII and SII
mortality. The ROC curve results showed that SCr had accuracy in
predicting mortality (AUC=0.852). UA was also shown to be an
independent risk factors for the severity of SII, but the AUC was
0.680, which was relatively low compared with the other indicators
within the same group.
In conclusion, SII remains a major cause of
morbidity and mortality in burn patients worldwide. The current
study concluded that combined serum, blood gas markers and clinical
indicators could predict severity SII, the probability to develop
ARDS combined with SII and the mortality of SII. The present
findings suggested that APACHE II, LIPS, lactic acid, WBC, UA and
SCr were risk factors for severity of SII in patients, and WBC
>20.91 (109/l) could be a reliable indicator for
severe SII. APACHE II, LIPS, lactic acid and WBC were risk factors
for patients with SII to develop ARDS, whereas
PaO2/FiO2 was protective factor against
patients with SII developing ARDS. Lactic acid >9.60 (mmol/l)
had the greatest accuracy in predicting patients with SII
developing ARDS. Lactic acid, WBC and SCr were risk factors for
mortality, whereas hemoglobin and A/G were protective factors
against mortality. Hemoglobin <83.00 (g/l) had the greatest
accuracy in predicting mortality. These patients had no indications
of previous medical histories. The present study proposed that
these indices could be convenient assessment parameters to devise
better treatment plans to preempt worsening conditions. However,
the small number of cases in the present study may have affected
the results of the statistical analyses. In addition, the present
study did not evaluate long-term results, thus it is necessary to
analyze the risk factors in larger patient numbers and in long-term
accumulation of datasets.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by funding from National
Natural Science Foundation of China, (grant no. 81971878), Opening
Project of Military Logistics (grant no. BLB19J006), Tianjin
Natural Science Foundation (grant no. 20JCQNJC01260), Tianjin
University Independent Innovation Fund (grant no. 2020XRG-002) and
Tianjin University Independent Innovation Fund (grant no.
2021XZS-0025).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH, HF and QL contributed to the study design and
provided the funding acquisition. ZN, ZD, XG, YC and LY contributed
to data collection and wrote the manuscript. ZN, ZD, WZ, HW, JS and
YC provided resources and participated in the data analysis. ZN and
ZD performed data validation, and retouched the manuscript. ZN, ZD
and GX confirmed the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committees of
Characteristic Medical Center of Chinese People's Armed Police
Force and 983 Hospital of the Joint Logistics Support Force of the
Chinese People's Liberation Army. Written informed consents were
obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mercel A, Tsihlis ND, Maile R and Kibbe
MR: Emerging therapies for smoke inhalation injury: A review. J
Transl Med. 18(141)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guo B, Bai Y, Ma Y, Liu C, Wang S, Zhao R,
Dong J and Ji HL: Preclinical and clinical studies of
smoke-inhalation-induced acute lung injury: Update on both
pathogenesis and innovative therapy. Ther Adv Respir Dis.
13(1753466619847901)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Walker PF, Buehner MF, Wood LA, Boyer NL,
Driscoll IR, Lundy JB, Cancio LC and Chung KK: Diagnosis and
management of inhalation injury: An updated review. Crit Care.
19(351)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cancio LC: Airway management and smoke
inhalation injury in the burn patient. Clin Plast Surg. 36:555–567.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Otterness K and Ahn C: Emergency
department management of smoke inhalation injury in adults. Emerg
Med Pract. 20:1–24. 2018.PubMed/NCBI
|
|
6
|
Jones SW, Williams FN, Cairns BA and
Cartotto R: Inhalation injury: Pathophysiology, diagnosis, and
treatment. Clin Plast Surg. 44:505–511. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Deutsch CJ, Tan A, Smailes S and
Dziewulski P: The diagnosis and management of inhalation injury: An
evidence based approach. Burns. 44:1040–1051. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reid A and Ha JF: Inhalational injury and
the larynx: A review. Burns. 45:1266–1274. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kahn SA, Bader B, Flamm T and Woods J:
Revisions in the national burn repository improve the rate of
firefighter injury data capture. J Burn Care Res. 40:412–415.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ching JA, Shah JL, Doran CJ, Chen H, Payne
WG and Smith DJ Jr: The evaluation of physical exam findings in
patients assessed for suspected burn inhalation injury. J Burn Care
Res. 36:197–202. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Veeravagu A, Yoon BC, Jiang B, Carvalho
CM, Rincon F, Maltenfort M, Jallo J and Ratliff JK: National trends
in burn and inhalation injury in burn patients: Results of analysis
of the nationwide inpatient sample database. J Burn Care Res.
36:258–265. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Foncerrada G, Culnan DM, Capek KD,
González-Trejo S, Cambiaso-Daniel J, Woodson LC, Herndon DN,
Finnerty CC and Lee JO: Inhalation injury in the burned patient.
Ann Plast Surg. 80:S98–S105. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Palmieri TL: Inhalation injury: Research
progress and needs. J Burn Care Res. 28:549–554. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Carr JA, Phillips BD and Bowling WM: The
utility of bronchoscopy after inhalation injury complicated by
pneumonia in burn patients: Results from the national burn
repository. J Burn Care Res. 30:967–974. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Murtaza B, Sharif MA, Tamimy MS, Dar MF,
Aslam A, Kazmi STM and Ishaque M: Clinico-pathological profile and
outcome of inhalational burns. J Coll Physicians Surg Pak.
19:609–613. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Legrand M, Dépret F and Mallet V:
Management of Burns. N Engl J Med. 381:1188–1189. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dessap AM, Boissier F, Charron C, Bégot E,
Repessé X, Legras A, Brun-Buisson C, Vignon P and Vieillard-Baron
A: Acute cor pulmonale during protective ventilation for acute
respiratory distress syndrome: Prevalence, predictors, and clinical
impact. Intensive Care Med. 42:862–870. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Luo J, Yu H, Hu YH, Liu D, Wang YW, Wang
MY, Liang BM and Liang ZA: Early identification of patients at risk
for acute respiratory distress syndrome among severe pneumonia: A
retrospective cohort study. J Thorac Dis. 9:3979–3995.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Albright JM, Davis CS, Bird MD, Ramirez L,
Kim H, Burnham EL, Gamelli RL and Kovacs EJ: The acute pulmonary
inflammatory response to the graded severity of smoke inhalation
injury. Crit Care Med. 40:1113–1121. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Toffaletti JG and Rackley CR: Monitoring
oxygen status. Adv Clin Chem. 77:103–124. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ranieri VM, Rubenfeld GD, Thompson BT,
Ferguson ND, Caldwell E, Fan E, Camporota L and Slutsky AS: Acute
respiratory distress syndrome: The berlin definition. JAMA.
307:2526–2533. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Swift JA, Langley-Evans SC, Pearce J,
Jethwa PH, Taylor MA, Avery A, Ellis S, McMullen S and Elliott-Sale
KJ: Antenatal weight management: Diet, physical activity, and
gestational weight gain in early pregnancy. Midwifery. 49:40–46.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wijdicks EF, Bamlet WR, Maramattom BV,
Manno EM and McClelland RL: Validation of a new coma scale: The
FOUR score. Ann Neurol. 58:585–593. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Knaus WA, Draper EA, Wagner DP and
Zimmerman JE: APACHE II: A severity of disease classification
system. Crit Care Med. 13:818–829. 1985.PubMed/NCBI
|
|
25
|
Gajic O, Dabbagh O, Park PK, Adesanya A,
Chang SY, Hou P, Anderson H III, Hoth JJ, Mikkelsen ME, Gentile NT,
et al: Early identification of patients at risk of acute lung
injury: Evaluation of lung injury prediction score in a multicenter
cohort study. Am J Respir Crit Care Med. 183:462–470.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Grabowska T, Skowronek R, Nowicka J and
Sybirska H: Prevalence of hydrogen cyanide and carboxyhaemoglobin
in victims of smoke inhalation during enclosed-space fires: A
combined toxicological risk. Clin Toxicol (Phila). 50:759–763.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bloemsma GC, Dokter J, Boxma H and Oen IM:
Mortality and causes of death in a burn centre. Burns.
34:1103–1107. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jeschke MG, van Baar ME, Choudhry MA,
Chung KK, Gibran NS and Logsetty S: Burn injury. Nat Rev Dis
Primers. 6(11)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Greenhalgh DG, Saffle JR, Holmes JH IV,
Gamelli RL, Palmieri TL, Horton JW, Tompkins RG, Traber DL, Mozingo
DW, Deitch EA, et al: American Burn Association consensus
conference to define sepsis and infection in burns. J Burn Care
Res. 28:776–790. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Koniman R, Kaushik M, Teo SH, Tan CW, Li
HH, Foo WYM, Tan BK, Chong SJ and Tan HK: Renal outcomes of
intensive care burn patients in an Asian tertiary centre. Burns.
46:400–406. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chao KY, Lin YW, Chiang CE and Tseng CW:
Respiratory management in smoke inhalation injury. J Burn Care Res.
40:507–512. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dries DJ and Endorf FW: Inhalation injury:
Epidemiology, pathology, treatment strategies. Scand J Trauma
Resusc Emerg Med. 21(31)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bergquist M, Hästbacka J, Glaumann C,
Freden F, Huss F and Lipcsey M: The time-course of the inflammatory
response to major burn injury and its relation to organ failure and
outcome. Burns. 45:354–363. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Murray JF, Matthay MA, Luce JM and Flick
MR: An expanded definition of the adult respiratory distress
syndrome. Am Rev Respir Dis. 138:720–723. 1988.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jones AE, Shapiro NI, Trzeciak S, Arnold
RC, Claremont HA and Kline JA: Lactate clearance vs central venous
oxygen saturation as goals of early sepsis therapy: A randomized
clinical trial. JAMA. 303:739–746. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chotalia M, Pirrone C, Ali M, Mullhi R,
Torlinska B, Mangham T, England K and Torlinski T: The utility of
arterial blood gas parameters and chest radiography in predicting
appropriate intubations in burn patients with suspected inhalation
injury-A retrospective cohort study. Burns. 47:1793–1801.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lachiewicz AM, Hauck CG, Weber DJ, Cairns
BA and van Duin D: Bacterial infections after burn injuries: Impact
of multidrug resistance. Clin Infect Dis. 65:2130–2136.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liao PH, Kao CC, How CK, Yang YS, Chen MC,
Hung-Tsang DY and Lee YT: Initial white blood cell count and
revised Baux score predict subsequent bloodstream infection in burn
patients: A retrospective analysis of severe burn patients from the
Formosa color dust explosion of 2015. J Formos Med Assoc.
120:1719–1728. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schmidt K, Zivkovic AR, Thiele M, Horter
J, Brenner T, Weigand MA, Kleinschmidt S and Hofer S: Point-of-care
measured serum cholinesterase activity predicts patient outcome
following severe burns. Burns. 47:863–872. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Laggner M, Lingitz MT, Copic D, Direder M,
Klas K, Bormann D, Gugerell A, Moser B, Radtke C, Hacker S, et al:
Severity of thermal burn injury is associated with systemic
neutrophil activation. Sci Rep. 12(1654)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Schaden E, Kimberger O, Kraincuk P, Baron
DM, Metnitz PG and Kozek-Langenecker S: Perioperative treatment
algorithm for bleeding burn patients reduces allogeneic blood
product requirements. Br J Anaesth. 109:376–381. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Welling H, Ostrowski SR, Stensballe J,
Vestergaard MR, Partoft S, White J and Johansson PI: Management of
bleeding in major burn surgery. Burns. 45:755–762. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rizzo JA, Ross E, Ostrowski ML, Gomez BG,
Aden JK and Cap AP: Intraoperative blood transfusions in burn
patients. Transfusion. 61 (Suppl 1):S183–S187. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu G, Zhuang M, Fan X, Hong X, Wang K,
Wang H, Chen Z, Sun Y and Xia Z: Blood transfusions in severe burn
patients: Epidemiology and predictive factors. Burns. 42:1721–1727.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Eljaiek R, Heylbroeck C and Dubois MJ:
Albumin administration for fluid resuscitation in burn patients: A
systematic review and meta-analysis. Burns. 43:17–24.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mert S, Bulutoglu B, Chu C, Dylewski M,
Lin FM, Yu YM, Yarmush ML, Sheridan RL and Uygun K: Multiorgan
metabolomics and lipidomics provide new insights into fat
infiltration in the liver, muscle wasting, and liver-muscle
crosstalk following burn injury. J Burn Care Res. 42:269–287.
2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Folkestad T, Brurberg KG, Nordhuus KM,
Tveiten CK, Guttormsen AB, Os I and Beitland S: Acute kidney injury
in burn patients admitted to the intensive care unit: A systematic
review and meta-analysis. Crit Care. 24(2)2020.PubMed/NCBI View Article : Google Scholar
|