Introduction
Breast cancer is a major type of cancer that
threaten the health of women worldwide and has been attracting the
attention of researchers worldwide. Among the 185 countries/regions
in the world, 159 countries have the highest incidence of breast
cancer, with the highest incidence and death rate of female cancer,
with the incidence and death rate of 47.8 and 13.6% per 100,000
people, respectively (1). After
standardized treatment of early breast cancer, the 5-year survival
rate of breast cancer patients can reach 95% (2). The incidence and mortality of cancer
are increasing rapidly worldwide, which is related to the aging of
population and the increase of population. With the improvement of
people's life quality and the improvement of pacing engineering,
the indications for pacemaker implantation are gradually expanding.
At present, the clinical application of pacemaker therapy,
especially ordinary pacemaker, has become very popular. According
to the 2012 ACCF/AHA/HRS Guidelines for Implanted Devices, common
pacemaker implantation indications include 11 aspects (3) (1)
Appropriate indications for permanent pacemaker implantation in
patients with abnormal sinus node function; (2) Indications for permanent pacemaker
implantation in adults with acquired atrioventricular block;
(3) Indications for permanent
pacemaker implantation in patients with chronic double branch
block; (4) Indications of
permanent pacemaker implantation in patients after acute myocardial
infarction; (5) Indications of
permanent pacemaker implantation in patients with hypersensitive
carotid sinus syndrome and cardiogenic neurosyncope; (6) Indications of permanent pacemaker
implantation in patients after heart transplantation; (7) An indication of permanent pacemaker
implantation that automatically detects and terminates tachycardia;
(8) Indications of permanent
pacemaker for tachycardia prevention; (9) Indications of permanent cardiac
pacemaker to prevent atrial fibrillation (AF); (10) Indications of permanent pacemaker
implantation in patients with hypertrophic cardiomyopathy;
(11) Indications for permanent
pacemaker implantation in children and adults with congenital heart
disease (CHD). How to improve the survival rate and quality of life
of breast cancer patients with pacemakers is an issue that we need
to pay common attention to. At present, the treatment of breast
cancer has entered the era of comprehensive therapy, including
surgery, radiation therapy, chemotherapy, targeted therapy,
endocrine therapy and immunotherapy. Therefore, when radiotherapy
is performed on such patients, the path of pacemaker implantation
should be considered for those who need pacemaker implantation, so
as to prepare for safe radiotherapy with pacemaker irradiation dose
control in the later stage. Ionizing radiation generated during
radiotherapy can cause charge accumulation and abnormal current in
the semiconductor components of cardiac pacemaker, which can cause
pacemaker failure or interfere with the function of pacemaker, and
even induce malignant arrhythmia. After implantation of the
pacemaker electrode in the heart cavity, a foreign body rejection
inflammatory reaction occurred at the electrode joint/tissue
interface, resulting in inflammatory edema. Cardiomyocyte damage
and inflammatory edema caused by radiotherapy can inhibit the
conduction of electrical pulse signal of the pacemaker, and even
cause working obstacles for the pacemaker in severe cases.
Therefore, Medtronic recommends that the radiation dose of the
pacemaker should not be greater than or equal to 5 Gy, and that the
pacemaker and pulse generator should be placed in the irradiation
field. When radiotherapists use 3D radiotherapy to delineate the
target area, they should define the pacemaker as the organ at risk
(OAR) and ensure that its maximum tolerance is 2 Gy. Tumor patients
with pacemaker implantation can safely and effectively complete
radiotherapy under certain treatment conditions, which requires us
to take full consideration of the specific situation of patients in
the process of making radiotherapy plan and implementing
radiotherapy, so as to minimize the influence of various factors on
pacemaker.
Case report
In May 2020, the patient, a 64-year-old female,
found a left axillary mass that was not causing any redness,
swelling or pain. In addition, the left axillary mass did not
shrink significantly after oral traditional Chinese medicine (TCM)
(100 ml twice a day, orally in the morning and evening). This
manifestation was not followed by further examination or treatment.
The patient's left axillary mass not only increased in size by
October 2020, but additional masses were also found in the outer
and upper part of the left breast. A breast ultrasound examination
was performed by another hospital (Mianyang Central Hospital of
Sichuan Province, October 2020). In the same month, the patient was
admitted into the Department of Radiology Oncology, West China
Hospital of Sichuan University (Chengdu, China) for further
examination and treatment The patient was diagnosed as left breast
cancer with axillary lymph node metastasis, grade 3, lymphatic
vascular invasion, by biopsy of left breast and left axillary mass.
Following surgery, two cycles of TCbHP (docetaxel, carboplatin,
trastuzumab, pertuzumab) chemotherapy were performed. After
chemotherapy, the renal function of the patient worsened, and the
patient was adjusted to four cycles of THP (docetaxel, trastuzumab,
pertuzumab) chemotherapy. Chemotherapy was followed by endocrine
therapy with trozole, followed by targeted therapy. As the patient
exhibited complications of atrial fibrillation, coronary
atherosclerosis, cardiac insufficiency, hypertension and type 2
diabetes mellitus, antitumor therapy was suspended. The patient was
subsequently admitted into the Department of Cardiology of West
China Hospital of Sichuan University (Chengdu, China) in December
2020. The results of the medical examination are summarized in
Table I.
 | Table IHospital examination results of the
patient. |
Table I
Hospital examination results of the
patient.
| Test | Result |
|---|
| Dynamic
electrocardiogram | Slow ventricular rate
atrial fibrillation, minimum heart rate 29 beats/min, transient
third-degree atrioventricular block, ventricular escape heart rate,
complete right ventricular block and ST-T changes seen during
sleep |
| Echocardiogram | The left ventricle
was enlarged, the interventricular septum was significantly
thickened, the posterior wall of the left ventricle was slightly
thicker and the ascending aorta was widened. Tricuspid
regurgitation (mild) and normal left ventricular systolic diastolic
function were observed |
| Enhanced MRI of
cardiac function | Asymmetrical
thickening of the left ventricular wall. The thickest point was 2.3
cm and myocardial perfusion defect was observed with extensive
delayed enhancement, slightly decreased left ventricular ejection
fraction (45.8%), significantly decreased right ventricular
ejection fraction (31.2%) and enlarged left and right atria |
| Laboratory
examination | Serum creatinine, 151
µmol/l; estimated glomerular filtration rate, 31.46 ml/min/1.73
m2 |
The patient was ultimately diagnosed with the
following: I) Atrial fibrillation with intermittent third-degree
atrioventricular block; ii) cardiac insufficiency with grade III
cardiac function (General physical activity is significantly
limited. No symptoms at rest, less than normal physical activity
can cause fatigue, palpitations, asthma or angina); iii)
hypertrophic cardiomyopathy; iv) coronary atherosclerosis; v)
hypertension; vi) type 2 diabetes mellitus; vii) left breast cancer
with axillary lymph node metastasis; and viii) chronic renal
insufficiency.
Considering that the patient was diagnosed with left
breast cancer with axillary lymph node metastasis and required
postoperative radiotherapy, a temporary pacing electrode and
permanent artificial cardiac pacemaker placement' (model no.
A3DR01; Medtronic, Inc.) procedure was performed using the right
axillary vein approach. The electrical pulse generator (model no.
A3DR01; Medtronic, Inc.) was placed in the right subclavian
subcutaneous tissue.
In January 2021, the patient underwent left radical
mastectomy and left axillary lymph node dissection. Postoperative
pathological (HE staining and immunohistochemical staining of
metastatic lymph nodes of breast cancer) assessment showed the
following: i) Invasive ductal carcinoma of the left breast (grade
III; tumor size, 2.0x1.8x1.5 cm); ii) lymphovascular invasion; and
iii) positivity for estrogen receptor (strong-moderate; ~30%
positive cells), progesterone receptor (moderate-strong; ~70%),
human epidermal growth factor receptor 2 (HER2; predisposition to
3+), HER2-fluorescence in situ hybridization and Ki 67
(~30%; data not shown). The ‘left subclavian lymph node’ (1/8,8
were tested, and one was positive) and ‘left axillary level 1 and 2
lymph node’ (13/28,28 axillary lymph nodes were examined, and
cancer metastasis was found in 13 of them) were subsequently
examined for cancer metastasis. This was followed by the
postoperative diagnosis of invasive ductal carcinoma of the left
breast (pT1N3M0 stage III; HER2 overexpression type). After
surgery, the TCbHP chemotherapy regimen (docetaxel 108 mg d1,
carboplatin 500 mg d1, trastuzumab 400 mg d1 and pertuzumab 840 mg
d1) was given for two cycles (3 weeks each). After chemotherapy,
the patient's renal function was aggravated, meaning that the
chemotherapy regimen had to be adjusted to that of the THP regimen
(docetaxel 100 mg d1, trastuzumab 400 mg d1 and pertuzumab 840 mg
d1) for four cycles (3 weeks each). Chemotherapy was followed by
endocrine therapy with Letrozole endocrine therapy (2.5 mg/day for
5 years), thereby targeted therapy was continued. The patient also
attended the department of Radiotherapy, West China Hospital to
receive radiotherapy. The radiotherapy physician fully communicated
with the patient and family members about the procedures of
postoperative radiotherapy, in addition to possible side effects
and impact on the pacemaker (pacemaker dysfunction and permanent
damage), before the patient signed the radiotherapy informed
consent form.
Patient location was captured with Revolution CT ES
(GE USA) and transmitted to the RayStation planning system
(RaySearch Laboratories). The location was secured by the company's
vacuum breast bag. And the CBCT acquisition parameters: scan
sequence: ABC CC, scan Angle from 100˚-260˚, rotation speed
3.18˚/s, FOV diameter 26 cm, length 26 cm, S20 filter plate, the
corresponding pixel size is 0.100 cm spatial resolution. The image
acquisition speed is 5.5 Frames/S, with a total of 400 and 361
frames. Total mAs:36.1 m As, medium resolution reconstruction. The
scanning time of CBCT for each patient was about 1 min, the
scanning volume was 410x263x410 mm, the scanning center was
isocentry, the image resolution was 512x512, and the reconstruction
layer thickness was 3 mm. Radiotherapy physicians delineated the
target area as ‘CTV1’ (left upper and lower clavicle area) and CTV2
(left chest wall). Furthermore, the radiotherapy physicians
delineated the OAR (organs at risk), such as lung, heart, left
anterior descending coronary artery, spinal cord, shoulder joint
and the pacemaker. PCTV1 and PCTV2 are formed by placing 6 mm
outside CTV1 and CTV2, spinal cord was placed 6 mm outside to form
spinal cord PRV, and pacemaker PRV was placed 6 mm outside PM. PRV
means the area of displacement caused by the patient's respiratory
movement due to changes in position during radiotherapy.
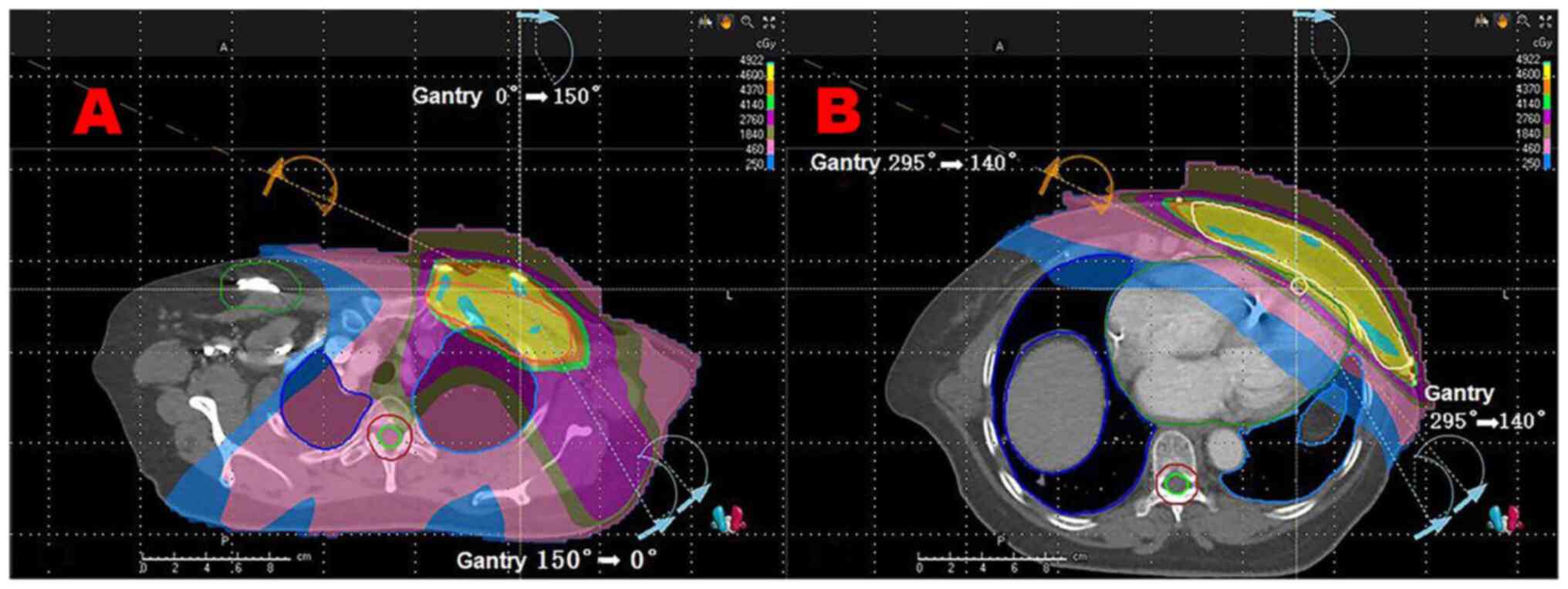
The prescribed dose of PCTV1 and PCTV2 was 46
Gy/23f. Volumetric modulated arc therapy (VMAT) technology, a form
of radiotherapy, was used (4). A
total of four arcs were planned. After designating the pacemaker as
the boundary, the target area was divided into the upper and lower
parts. The upper part had two arcs of 0-150˚, whilst the lower part
had two arcs of 295-140˚. Direct rays passing through the pacemaker
were avoided. The left chest wall was irradiated, whereas the
radiation dose to the heart and lung was controlled and the lead
barrier was locked at 2 cm below the pacemaker. This was set so
that the pacemaker dose is regulated (Fig. 1) and that the dose to the organs at
risk reaches the standard limit dose (Fig. 2).
Since a complex VMAT plan with target area segments
was used, the physicist then performed the dose validation of the
VMAT plan using ArcCHECK three-dimensional (3D) array measurements
(Sun Nuclear Corporation) and analyzed the gamma ray pass rate of
the measured data to verify the accuracy of the plan. In addition,
the physician evaluated the planned data from the planning
system.
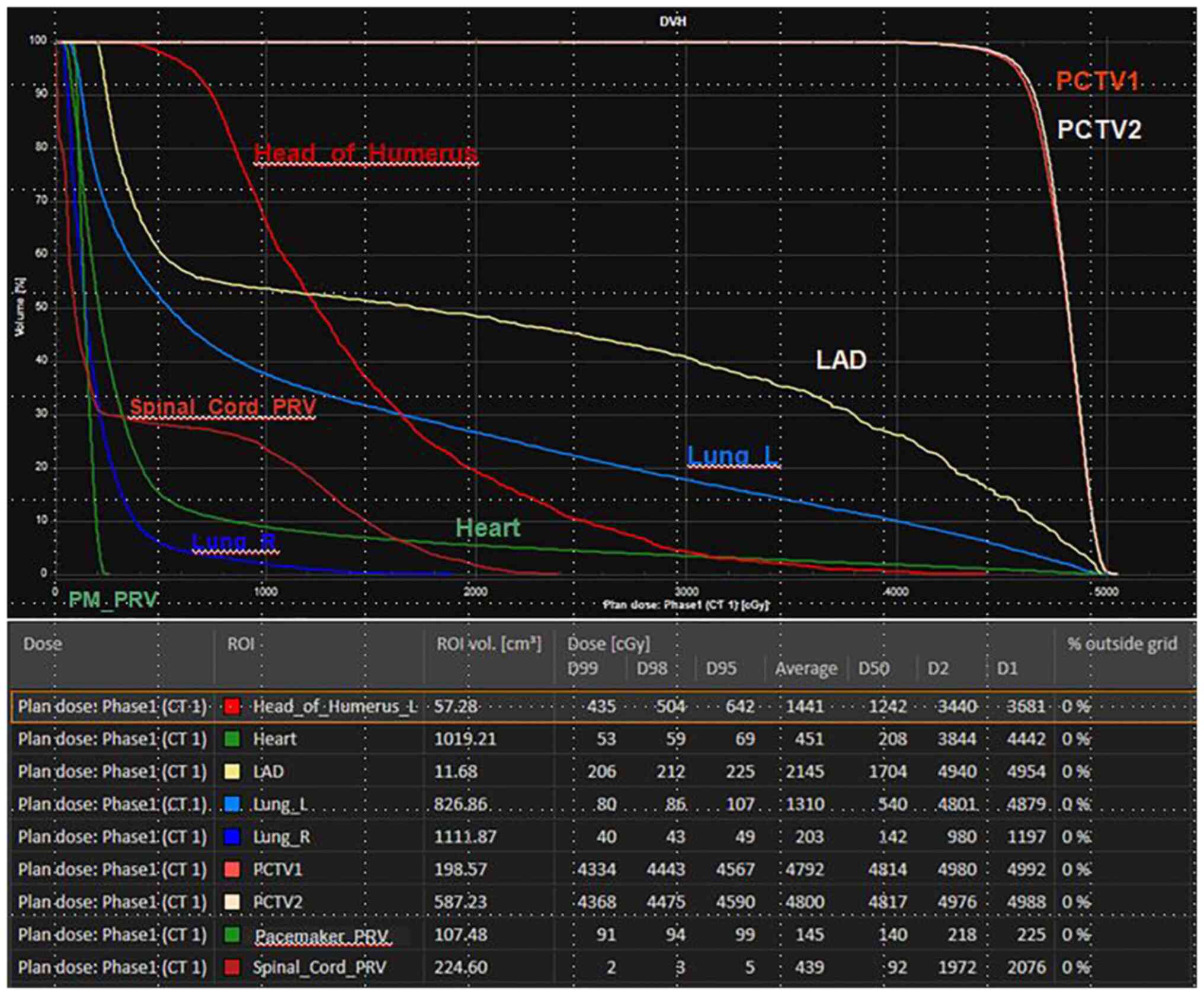
The dose to which the pacemaker was exposed was
measured using a metal oxide semiconductor field effect transistor
during the first treatment (Fig.
3), including the exposure dose during cone beam computerized
tomography (CBCT) and during radiotherapy (inner, central and outer
pacemaker positions; Table II).
The estimated maximum cumulative dose of the pacemaker during
radiotherapy was 225.4 cGy. To reduce the exposed dose of the
pacemaker during CBCT, CBCT verification was performed twice a week
for a total of 10 times, following which the maximum cumulative
dose of the pacemaker was reduced to 70.6 cGy. In the case of
concurrent radiotherapy and CBCT, the maximum involved dose of the
pacemaker was 296.0 cGy (cumulative dose of 23 radiotherapy plus 10
CBCT). CBCT was performed 10 times throughout the course of
treatment, so the remaining 13 times were not validated by CBCT.
They all used the Optical Surface Imaging (OSI) Catalyst~(TM)
system for setup validation. For the remaining three times, optical
surface imaging (OSI) Catalyst™ system (C-Rad) was used to verify
the positioning. The combination of the two methods not only
improved the precision of positioning and guaranteed the accurate
implementation of the complex VMAT plan, but also effectively
controlled the dose to which the pacemaker was exposed.
 | Table IIPacemaker irradiation dose. |
Table II
Pacemaker irradiation dose.
| Pacemaker
position | Cone beam
computerized tomography, cGy | Volumetric modulated
arc therapy, cGy |
|---|
| Inner | 7.06 | 9.80 |
| Central | 3.58 | 9.39 |
| Outer | 3.90 | 6.18 |
An hour before radiotherapy treatment (23 fractions,
5 times a fractions), the patient went to the Cardiology Department
to check the parameters of the pacemaker and confirm that the
instrument is in normal working condition. Before each cycle of
radiotherapy, the patient went to the Cardiology Department to
adjust the pacemaker to the asynchronous pacing mode to prevent
excessive perception. After radiotherapy, the pacemaker was
adjusted back to the original working mode to monitor the status of
the device.
Before and after each cycle of radiotherapy, the
patient was asked if they experienced any discomfort, where her
vital signs were also monitored. During the treatment period, the
patient's state was closely monitored, who was also instructed to
immediately raise her hand to report symptoms, including
palpitation or chest tightness, in which case the therapist would
immediately stop radiotherapy. The treatment room was equipped with
first-aid equipment. Furthermore, if the patient encountered an
emergency (such as Palpitation, chest tightness, tight breath), the
radiotherapy physician, cardiologist and emergency physician would
be immediately contacted for on-site first aid. The patient
successfully completed radiotherapy. During radiotherapy, when the
patient's cardiac pacemaker was adjusted to the asynchronous pacing
mode, light activities (General physical activity, such as climbing
the stairs 2 floors) were causing tiredness and palpitations. After
adjusting back to the original mode, the symptoms were relieved and
there was no cough, tightness of breath or second-degree skin
reaction in the irradiated area.
As of April 2022, the patient has completed
radiotherapy for 5 months and has not complained of any discomfort.
There was no recurrence or metastasis of the tumor in a
comprehensive review. Specific examinations included color
ultrasound of breast chest wall, upper and lower clavicle, axillary
lymph nodes, abdomen, and chest enhanced CT. The cardiac pacemaker
remains in normal working condition.
Discussion
According to the Global Cancer Observatory database,
there were 2,261,419 new cases of breast cancer in 2021, accounting
for 11.7% of the total incidence of cancer (5). In particular, breast cancer has for
the first time surpassed lung cancer as the most common cancer in
women worldwide (5). At present,
the number of patients with cardiovascular diseases in China has
increased to >330 million, where there are ~1 million patients
with bradycardia, with an average annual increase of
300,000-400,000 (6,7). For patients with bradycardia,
implantation of a cardiac pacemaker is the only effective treatment
(6). With the increase in cases of
cardiac pacemaker implantations in China, the number of patients
with heart disease complicated with tumors is likely to
correspondingly increase. The safe implementation of radiotherapy
regimens for such patients is an important obstacle faced by tumor
radiotherapy physicians and therapists.
Cardiac implantable electronic devices (CIEDs)
include cardiac pacemakers and implantable cardiac defibrillators
(ICDs) (8). There are various
factors that affect the use of CIEDs during radiotherapy. Ionizing
radiation and electromagnetic fields generated during radiotherapy
may cause pacemaker dysfunction, interfere with pacing function or
even induce arrhythmias (9).
Device malfunction is reported in 2.5% of patients with pacemakers
and 6.8% of patients with ICD after radiotherapy (10). Regarding the influence of radiation
dose and energy on CIED, the American Association of Physicists in
Medicine (AAPM) recommended in its report no. 34 published in 1994
that in such patients, a cumulative dose received by pacemaker
<2 Gy should be considered when planning radiotherapy (9). In addition, Memorial Sloan Kettering
Cancer Center suggested that CIEDs should be outlined as organs at
risk and optimized to the lowest dose, with a limit of 2-5 Gy for
pacemakers and 0.5-2 Gy for ICDs (11). The device may suffer significant
damage from direct exposure or from the use of energy >6 MV
(10,12). Using energy of ≥10 MV, secondary
neutrons can damage random access memory and additional
semiconductors in modern devices (9,13).
Finally, the distance between the radiation field and the pacemaker
will affect the pacemaker, and the pacemaker will be placed in the
irradiation field, which will cause the pacemaker device working
obstacles. Irradiation field recommendations do not consider
pacemakers (8). The pacemaker
electrical pulse generator is normally implanted subcutaneously in
the left subclavian area above the pectoral muscle through the
subclavian vein. However, due to the left breast cancer radical
surgery, in the present case the cardiologist chose the right
axillary vein approach to implant the pulse generator subcutaneous
device in the right clavicle area in preparation for the subsequent
radiotherapy. In addition, in the present case the manufacturer's
protocols accompanying the pacemaker implanted into the patient
indicated that to prevent excessive perception, the pacemaker can
be programmed to asynchronous pacing mode, where such pacemaker
parameters can be restored after radiotherapy. To prevent device
damage, the pacemaker should be exposed to a dose of <5 Gy based
on manufacturer's guidelines.
Indications for radiotherapy after mastectomy for
patients with breast cancer include T3-4 and T1-2 tumors, ≥4
axillary metastatic lymph nodes and 1-3 positive axillary lymph
node metastases (14). Therefore,
there is a larger degree of heterogeneity in factors determining
the indications for radiotherapy, not only with regards to the
clinical and pathological characteristics of the patient, but also
considering the systemic treatment (15). A previous meta-analysis performed
by the Early Breast Cancer Clinical Trials Collaborative Group
showed that for patients with positive axillary lymph nodes,
post-operative radiotherapy reduced the 10-year overall recurrence
rate and 20-year breast cancer-related mortality rate by 8.8 and
9.3%, respectively, in patients with ≥4 positive axillary lymph
nodes (15). The target areas of
radiotherapy mainly include the chest wall and supraclavicular
lymphatic drainage area. By contrast, the efficacy of internal
breast irradiation is controversial and is recommended in patients
at high risk, such as patients with axillary metastatic lymph nodes
≥4. Modern precision radiotherapy techniques such as stereotactic
radiotherapy, three-dimensional conformal radiotherapy and
intensity modulated radiotherapy are recommended to accurately
assess the dose of radiation delivered to normal tissues such as
the heart. In addition, these techniques are recommended to
adequately balance the benefits and risks of systemic therapy and
radiotherapy in terms of heart-related injury following internal
breast prophylaxis irradiation (15).
Although comprehensive treatment strategies of
breast cancer has improved the long-term survival rate of patients,
radiotherapy-induced late cardiotoxicity has emerged as a subject
of concern (16). Darby et
al (17) previously reported
that ischemic heart disease after radiotherapy for breast cancer
generally occurs 5 years after radiotherapy. In addition, the
incidence is associated with the mean cardiac radiotherapy dose,
such that for each increment in the mean dose by 1 Gy, the
incidence of cardiac events, such as myocardial infarction,
increases by 7.4%, with no significant threshold which means there
was no significant minimum or maximum dose increase associated with
the incidence of cardiac events (17). The most well-documented
cardiotoxicity reduction study was performed in relation to the
DIBH (Deep Inspiration Breath Holding) technique (18), which is typically used in
combination with 3D conformal radiotherapy planning in tangential
fields. Modern radiotherapy techniques, such as intensity-modulated
radiotherapy (IMRT) and volumetric IMRT, have shown limited and
inconsistent results in reducing cardiac dose. However, the results
have been inconsistent (19). A
previous dosimetry study by Popescu et al (20) found that conventional
intensity-modulated radiotherapy (IMRT) and VMAT improved the dose
distribution in the target region compared with that following 3D
conformal radiotherapy with tangent field. Compared with
conventional IMRT, VMAT reduces the average dose delivered to the
heart and lung, resulting in improved protection of the organs at
risk and shorter irradiation time (21). However, both techniques do increase
the low-dose irradiation of surrounding healthy tissues at the same
time. VMAT is also the treatment that it is commonly adopted by the
authors of the present case report (20).
According to Guidelines for Chinese Physicians
(15) and postoperative staging of
the patient, the target area of radiotherapy should include the
left chest wall and the lymphatic drainage area above and below the
left clavicle. Subject to cardiac dose safety, the internal breast
lymphatic drainage area can also be considered. The patient in the
present case exhibited a combination of hypertrophic
cardiomyopathy, an enlarged heart adjacent to the left chest wall
and pacemaker implantation. Chemotherapy containing paclitaxel and
targeted anti-HER2 therapy are cardiotoxic, meaning that
radiotherapy requires strict control in terms of both cardiac and
pacemaker doses, which poses a great challenge to radiotherapists
and physicians (22). We first
adopted the DIBH-ABC technology, which is the characteristic
technology of our hospital, and adopted the active breathing
control device ABC to achieve deep inspiratory breath holding. ABC
is a respiratory gating device manufactured by Swedish Medical
company. Breathing training was performed for the patient for a
week but discontinued because the patient's breath hold time was
too short to cooperate with the technique. To improve the
conformability of the target area and lower the cardiac dose, the
radiation physicist used both static IMRT and VMAT techniques.
Finally, the VMAT radiotherapy plan for the four arcs of the target
area segment reached the limiting dose for organ and pacemaker
endangerment, after discarding the internal breast target area and
reducing the prescribed dose of radiotherapy to 46 Gy/23f. The
upper part of the two non-tangential field direction arcs with
smaller irradiation angles reduced the dose delivered to the
pacemaker. The lower part of the two tangential field direction
arcs with high conformality of the target area provided an improved
control over the dose delivered to the heart and lung, which locked
the lead gate adjacent to the pacemaker. This reduced the leakage
of radiation from the multi-Leaf Collimator, further controlling
the pacemaker dose. Due to the use of complex VMAT plans in the
target area, the accuracy of the dose was verified by the radiation
physicist before treatment. Chan et al (11) recommended the use of in
vitro dose detection systems, such as optically-stimulated
luminescence dosimeters, thermoluminescent dosimeters and diodes.
The dose delivered to the device is measured on the first day of
treatment to predict the cumulative dose throughout radiotherapy
(11). The dose delivered to the
pacemaker was measured on the first treatment, where the estimated
maximum cumulative dose of the pacemaker throughout radiotherapy
was 225.4 cGy, which was consistent with the radiotherapy plan. The
maximum irradiated dose delivered to the pacemaker at a single CBCT
was also measured to be 7.06 cGy, which provided a basis for
predicting the additional irradiation dose of CBCT delivered to the
pacemaker.
To precisely implement the VMAT radiotherapy plans
in four arcs, image-guided radiotherapy techniques are needed.
Borst et al (23) found
that >50% patients with thoracic tumors who did not receive CBCT
scans to assist with radiotherapy had setup errors of as high as 5
mm off target or higher. A number of studies have previously
demonstrated that CBCT image guidance can reduce the positional
error, decrease the toxic side effects of radiotherapy and help
patients to receive more accurate radiation therapy (24,25).
It has also been shown that the monitoring error of the OSI
Catalyst-(TM) system is 0.24±0.04 mm, with the maximum measurement
error 0.33±0.05 mm (26). Wikström
et al (27) reported that
the pendulum error calculated from the comparison of real-time with
reference images has a repeatability difference of 0.2 mm. Since
OSI is a noninvasive and radiation-free real-time extracorporeal
monitoring system that has high degrees of accuracy for real-time
motion monitoring, it has been proposed for use for the position
verification of patients in a clinical setting (26,28).
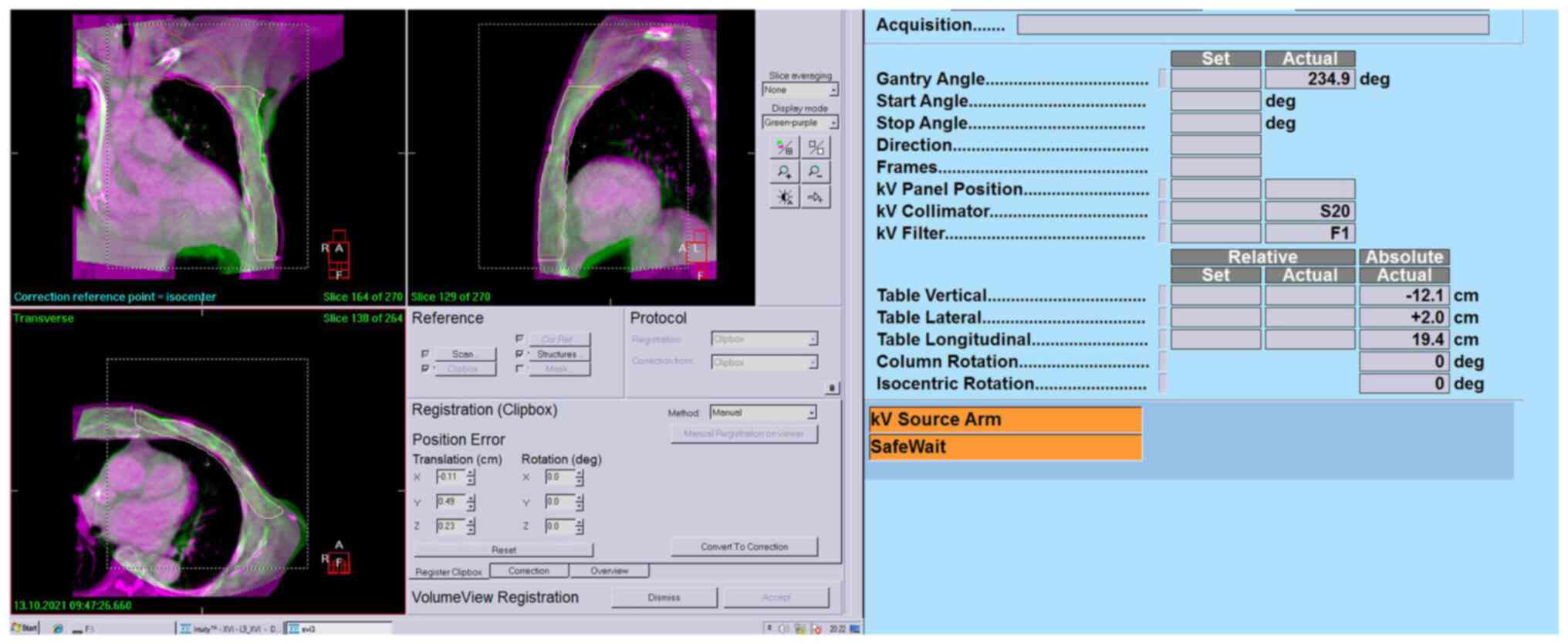
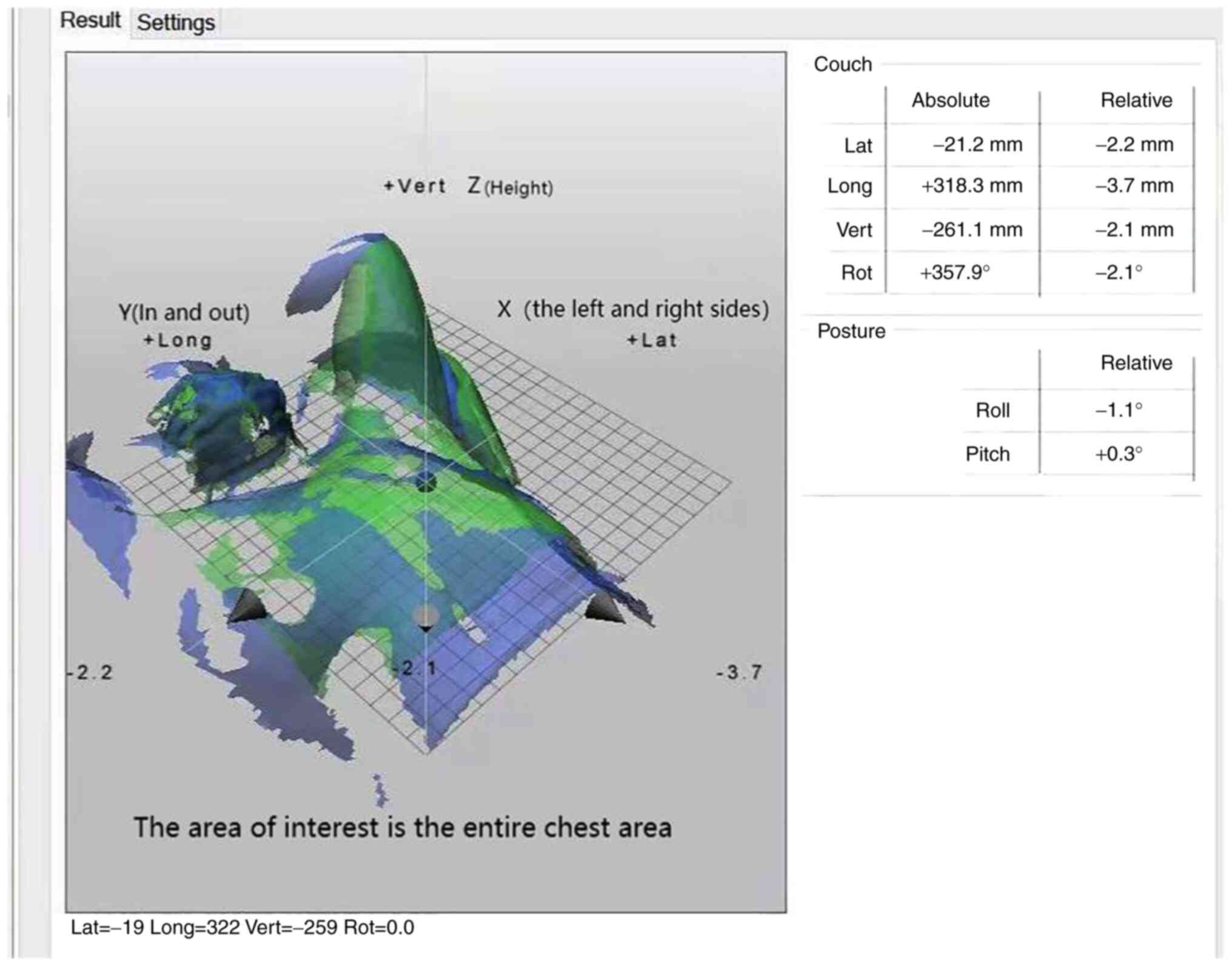
After CBCT scanning, the 3D image of the patient's anatomical
volume reconstructed by the system was registered online with the
imported CT positioning image (Fig.
4). Finally, radiotherapy can be performed after the matching
area and registration mode are determined by radiotherapy
physicians. The OSI system is a stereo imaging system consisting of
three high-definition cameras and led light sources fixed around
the treatment bed at an angle of 120˚. A calibrated CBCT image was
collected as a reference image for OSI when this was first used for
verification. The Light generator was then used to form the image
of the patient's body surface whereas the charge-coupled device
camera was used to obtain the reprojection. The visible light
source calculated the error between the real-time body surface
image and the reference image before directly projecting it onto
the patient's body surface. The therapist calibrated the
positioning error in the three directions before performing the
treatment (Fig. 5).
Chan et al (11) suggested that patients should be
positioned for verification and that the firing field should be
angled to reduce the exposure dose of the device. For the same
consideration, during CBCT imaging before treatment, the collimator
and filter should be reasonably selected to avoid direct radiation
on the pacemaker (11). The
patient received radiotherapy under the medical ELekta Synerg
accelerator. When CBCT was performed, the initial angle of the scan
sequence was pre-set at 120-260˚. The collimator used S20
(collimator with a small-field axial field length of 276.7 mm) and
the F1 filter plate to reduce the radiation dose delivered to the
pacemaker. F1 is a conformal filter, the collimator and filter are
placed in the two slots of the KV source arm. In this manner, the
quality of treatment and the safety of radiotherapy were
optimized.
Various guidelines and reviews have been published
internationally to guide the management of patients with implanted
cardiac electronic devices receiving radiotherapy (11,12,29,30).
Patients are first classified into low-, intermediate- and
high-risk groups by cumulative dose and pacing dependence (Table III), with slight differences
among different guidelines. For example, the risk stratification
reported in the 2019 AAPM TG-203 guidelines (30) (Table
IV) incorporated the presence or absence of neutron irradiation
in addition to proposing stricter limits on the cumulative dose.
Patients are then managed according to the different stages of
radiotherapy with different risk stratifications. Although the
recommendations for patient management vary slightly among
guidelines, all emphasize the importance of close multidisciplinary
collaboration among cardiologists, radiation oncologists,
physicists, therapists and pacemaker specialist technicians.
General management measures during radiotherapy include
reprogramming to asynchronous pacing or placing magnets for
pacemaker-dependent patients, delaying antiarrhythmic therapy and
reprogramming or placing magnets at each radiotherapy session for
patients with ICD (29). Different
management measures are used for patients in different risk strata.
For low-risk patients, the patient symptoms and vital signs are
closely monitored, whilst weekly device checks are required for
those with ICDs only (29). For
medium-risk patients, emergency equipment is required (such as
cardiac monitor and defibrillator), whilst for device-dependent
patients, an external pacemaker should be available and a team of
professionals (cardiologist, pacemaker specialist technician and
resuscitator) should be available to ensure immediate intervention
in case of an emergency, in addition to the device being checked
weekly. For high-risk patients, the benefits of treatment should be
weighed against the risk of inducing damage if device repositioning
is not feasible. Electrocardiogram (ECG) monitoring should be
performed at each radiotherapy session and the device should be
checked within 24 h after each treatment (29). The device should be regulated at 1,
3 and 6 months after the end of radiotherapy (29). In the present case report, the
patient had a minimum cardiac rhythm of 29 beats/min on the 24-h
ambulatory ECG before pacemaker implantation and the predicted
maximum pacemaker involvement dose was 296.0 cGy. According to the
AAPM TG-203 report, since the patient was classified to be a
medium-risk patient, the aforementioned recommended management
measures were followed. The patient successfully completed
radiotherapy with only discomfort caused by changes in pacing
patterns, with no pacemaker damage or functional abnormalities
found upon regular cardiology examinations after radiotherapy was
completed.
 | Table IIIPatient risk category. |
Table III
Patient risk category.
| Patient
classification | <2 Gy | 2-10 Gy | >10 Gy |
|---|
|
Pacing-independenta | Low risk | Intermediate
risk | High risk |
|
Pacing-dependentb | Intermediate
risk | High risk | High risk |
 | Table IVDose region and risk category. |
Table IV
Dose region and risk category.
| | Dose region and risk
category |
|---|
| Patient
classification | <2 Gy | 2-5 Gy | >5 Gy | Neutrons present |
|---|
| Pacing
independent | Low risk | Medium risk | High risk | High risk |
| Pacing dependent | Medium risk | Medium risk | High risk | High risk |
In conclusion, in the era of precision radiotherapy,
the use of modern radiotherapy techniques, including radiotherapy
planning assessment systems, intensity-modulated radiotherapy
techniques, extracorporeal dosimetry and real-time image guidance,
allows for the accurate assessment, limitation and prediction of
the pacemaker dose. In addition, the use of risk-stratified
management measures, with the participation of a multidisciplinary
team, allows for the safe administration of radiotherapy to
patients with pacemaker-implanted tumors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and LX drafted the manuscript. LX performed the
manuscript review and revision. JZ, YH, CL and YS performed patient
planning, radiotherapy and data collection. LZ performed dose
verification and pacemaker dosimetry. YW performed data analysis
and drafted the manuscript. LX interpreted the data. YW and LX
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the present case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao M and Chen W: Interpretation of Global
cancer statistics in GLOBOCAN 2020. Chin J Frontier Medicine
(electronic edition). 13:63–69. 2021.(In Chinese).
|
|
2
|
Coldman AJ and Phillips N: Breast cancer
survival and prognosis by screening history. Br J Cancer.
110:556–559. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hua W and Zhang S: Interpretation of the
2012 ACCF/AHA/HRS Guidelines for the treatment of arrhythmia
devices. Chin J Cardiac Arrhythmias. 2012(467)2012.(In
Chinese).
|
|
4
|
Bradley JA and Mendenhall NP: Novel
Radiotherapy Techniques for Breast Cancer. Annu Rev Med.
69:277–288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Duggan C, Trapani D, Ilbawi AM, Fidarova
E, Laversanne M, Curigliano G, Bray F and Anderson BO: National
health system characteristics, breast cancer stage at diagnosis,
and breast cancer mortality: A population-based analysis. Lancet
Oncol. 22:1632–1642. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
National Center for Cardiovascular
Diseases. Annual report on cardiovascular health and diseases in
China 2020. J Cardiovasc Pulm Dis. 40:1005–1009. 2021.
|
|
7
|
National Cardiovascular Disease Center:
China Cardiovascular Health and Disease Report 2019. Science Press,
2020.
|
|
8
|
Zhang T and Li X: Research progress of
radiotherapy for patients with tumor with implanted cardiac
pacemaker. Chin J Interventional Cardiol. 37:237–240. 2017.(In
Chinese).
|
|
9
|
Marbach JR, Sontag MR, Van Dyk J and
Wolbarst AB: Management of radiation oncology patients with
implanted cardiac pacemakers: Report of AAPM Task Group No. 34.
American Association of Physicists in Medicine. Med Phys. 21:85–90.
1994.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Zaremba T, Jakobsen AR, Søgaard M,
Thøgersen AM, Johansen MB, Madsen LB and Riahi S: Risk of device
malfunction in cancer patients with implantable cardiac device
undergoing radiotherapy: A population-based cohort study. Pacing
Clin Electrophysiol. 38:343–356. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chan MF, Young C, Gelblum D, Shi C, Rincon
C, Hipp E, Li J and Wang D: A Review and Analysis of Managing
Commonly Seen Implanted Devices for Patients Undergoing Radiation
Therapy. Adv Radiat Oncol. 6(100732)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gauter-Fleckenstein B, Israel CW,
Dorenkamp M, Dunst J, Roser M, Schimpf R, Steil V, Schäfer J,
Höller U and Wenz F: DEGRO/DGK. DEGRO/DGK guideline for
radiotherapy in patients with cardiac implantable electronic
devices. Strahlenther Onkol. 191:393–404. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Soejima T, Yoden E, Nishimura Y, Ono S,
Yoshida A, Fukuda H, Fukuhara N, Sasaki R, Tsujino K and Norihisa
Y: Radiation therapy in patients with implanted cardiac pacemakers
and implantable cardioverter defibrillators: A prospective survey
in Japan. J Radiat Res. 52:516–521. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wei J, Guo Y, Wang Y, Wu Z, Bo J, Zhang B,
Zhu J and Han W: Clinical development of CAR T cell therapy in
China: 2020 update. Cell Mol Immunol. 18:792–804. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chinese Physicians Association, Radiation
Oncology Therapy Physicians Branch. Radiation Therapy Guidelines
for Breast Cancer (CMA 2020 Edition). Chin J Radiat Oncol.
30:321–342. 2021.(In Chinese).
|
|
16
|
Wang S: Radiotherapy for Left-sided Breast
Cancer: Focusing on Heart Dosimetry (unpublished PhD thesis).
Zhejiang University, 2018.
|
|
17
|
Darby SC, Ewertz M, McGale P, Bennet AM,
Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante
B, et al: Risk of ischemic heart disease in women after
radiotherapy for breast cancer. N Engl J Med. 368:987–998.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shengye W: Cardiac dosimetry of
radiotherapy for left breast cancer (unpublished PhD thesis).
Zhejiang University, 2018.
|
|
19
|
Duma MN, Baumann R, Budach W, Dunst J,
Feyer P, Fietkau R, Haase W, Harms W, Hehr T, Krug D, et al:
Heart-sparing radiotherapy techniques in breast cancer patients: A
recommendation of the breast cancer expert panel of the German
society of radiation oncology (DEGRO). Strahlenther Onkol.
195:861–871. 2019.PubMed/NCBI View Article : Google Scholar : (In English).
|
|
20
|
Popescu CC, Olivotto IA, Beckham WA,
Ansbacher W, Zavgorodni S, Shaffer R, Wai ES and Otto K: Volumetric
modulated arc therapy improves dosimetry and reduces treatment time
compared to conventional intensity-modulated radiotherapy for
locoregional radiotherapy of left-sided breast cancer and internal
mammary nodes. Int J Radiat Oncol Biol Phys. 76:287–295.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ahmad SS, Duke S, Jena R, Williams MV and
Burnet NG: Advances in radiotherapy. BMJ. 345(e7765)2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Han Y, Chen K, Li Q and Chen J: Study on
the application of A-DIBH method to reduce the cardiac dose in
patients undergoing radiotherapy after breast-conserving surgery
for left-sided breast cancer. Chin Modern Doctor. 60:84–87+197.
2022.(In Chinese).
|
|
23
|
Borst GR, Sonke JJ, Betgen A, Remeijer P,
van Herk M and Lebesque JV: Kilo-voltage cone-beam computed
tomography setup measurements for lung cancer patients; first
clinical results and comparison with electronic portal-imaging
device. Int J Radiat Oncol Biol Phys. 68:555–561. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hansen EK, Larson DA, Aubin M, Chen J,
Descovich M, Gillis AM, Morin O, Xia P and Pouliot J: Image-guided
radiotherapy using megavoltage cone-beam computed tomography for
treatment of paraspinous tumors in the presence of orthopedic
hardware. Int J Radiat Oncol Biol Phys. 66:323–326. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu Y, Zhong R, Liu Z, Ye C, Chen L, He Z
and Bai S: Management of cone beam CT technique for patient
positioning in radiotherapy. J Cancer Control Treatment.
23:156–158. 2010.(In Chinese).
|
|
26
|
Chen L, Bai L, Li G, Quan H and Bai S:
Real-time motion tracking accuracy of optical surface imaging
system. Chin J Med Physics. 38:1053–1056. 2021.(In Chinese).
|
|
27
|
Wikström K, Nilsson K, Isacsson U and
Ahnesjö A: A comparison of patient position displacements from body
surface laser scanning and cone beam CT bone registrations for
radiotherapy of pelvic targets. Acta Oncol. 53:268–277.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gierga DP, Riboldi M, Turcotte JC, Sharp
GC, Jiang SB, Taghian AG and Chen GT: Comparison of target
registration errors for multiple image-guided techniques in
accelerated partial breast irradiation. Int J Radiat Oncol Biol
Phys. 70:1239–1246. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Salerno F, Gomellini S, Caruso C, Barbara
R, Musio D, Coppi T, Cardinale M, Tombolini V and de Paula U:
Management of radiation therapy patients with cardiac defibrillator
or pacemaker. Radiol Med. 121:515–520. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Miften M, Mihailidis D, Kry SF, Reft C,
Esquivel C, Farr J, Followill D, Hurkmans C, Liu A, Gayou O, et al:
Management of radiotherapy patients with implanted cardiac
pacemakers and defibrillators: A Report of the AAPM TG-203†. Med
Phys. 46:e757–e788. 2019.PubMed/NCBI View
Article : Google Scholar
|