Introduction
Stroke was reported to cause 3.94 million (95%
uncertainty interval 3.43-4.58) new cases in China in 2019(1). It has been previously reported that
ischemic stroke constitutes >80% of all cases of stroke
(2). This disease has become a key
threat to public health with high rates of disability and mortality
(3). At present, the primary
therapeutic method for ischemic stroke is to recanalize occluded
arteries through thrombolysis or thrombectomy (4). However, it has been reported that the
restoration and reperfusion of blood flow into previously
blood-deficient area has the potential to aggravate brain tissue
injury and is frequently accompanied by cerebral
ischemia-reperfusion (I/R) injury (CIRI) (5). CIRI is a dynamic and complex
pathophysiological process, which is caused by a range of cellular
and external physiological factors (6). Intracellular Ca2+
overload, overproduction of free radicals, excitatory amino acids,
inflammatory cascade activation, acidosis, increased mitochondrial
permeability and cell apoptosis are reported to be the primary
factors of CIRI (7). Although
thrombolysis and embolectomy restore blood flow to the infarcted
brain tissue, such therapy also results in I/R (8,9).
Therefore, it is important to elucidate the molecular mechanisms
underlying CIR to develop novel strategies with significant
efficacy for treatment of CIR.
TNFα-induced protein 1 (TNFAIP1), which is also
called B12 or BACURD2, has numerous biological functions (10). It has been reported that TNFAIP1 is
a key mediator of inflammation by activating NF-κB activity
(11). Furthermore, TNFAIP1 has
been reported to stimulate DNA polymerase δ activity and interact
with proliferating cell nuclear antigen, which suggests that
TNFAIP1 regulates the inflammatory response, cell proliferation and
cell cycle progression (12).
TNFAIP1 may also promote neurotoxicity (13). Gladwyn-Ng et al (14) previously reported that TNFAIP1
hinders neuronal migratory capabilities in the embryonic cortex and
changes the morphology of the immature neuron (14). Another recent study reported that
TNFAIP1 expression is upregulated following myocardial I/R and that
TNFAIP1 knockdown ameliorates myocardial I/R injury (MIRI) via the
Akt/GSK-3β/nuclear factor erythroid 2-related factor 2 (Nrf2)
pathway (15). However, the role
of TNFAIP1 in CIR and its underlying mechanism remains poorly
understood. Therefore, the present study evaluated the effects of
TNFAIP1 on CIR and investigated how TNFAIP1 may regulate the
pathophysiological process of CIRI.
Materials and methods
Cell culture and treatment
The rat adrenal gland cancer PC12 cell line was
purchased from BioVector NTCC. DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (HyClone; Cytiva) and
1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
were used to cultivate the cells at 37˚C with 5% CO2 for
2 h. To establish the oxygen glucose deprivation and reperfusion
(OGD/R) model in vitro, PC12 cells were cultured in
glucose-free DMEM (Procell Life Science & Technology Co., Ltd.)
in an oxygen-free incubator supplied with 5% CO2 and 95%
N2 at 37˚C for 2 h. Following hypoxia treatment, the
media was replaced with normoxic glucose-containing medium and
cells were transferred to an incubator supplied with 95% air and 5%
CO2 at 37˚C for 24 h. Subsequently, cells were treated
with 5 µM ML385 at room temperature for 6 h or 0.75 µM Erastin at
room temperature for 24 h.
Cell transfection
For knockdown of TNFAIP1 expression, short hairpin
RNAs (shRNAs) targeting TNFAIP1 with a pRNAU6.1 vector backbone
(sh-TNFAIP1#1, 5'-GGAAGTGCTGACCGACAAA-3'; sh-TNFAIP1#2,
5'-GATTGCAGATAGCTAGCTA-3') and appropriate scrambled sequence
negative control (sh-NC, 5'-GGTACGCAATAGGAGTGTGTG-3') were
synthesized by Shanghai GenePharma Co., Ltd. The transfection of
100 nM of recombinants into PC12 cells was performed at 37˚C for 48
h using Lipofectamine® 2000 reagent (Thermo Fisher
Scientific, Inc.). The transfection of cells with sh-TNFAIP1 was
performed 24 h prior to OGD/R treatment. After 48 h, the cells were
collected for subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
Following OGD/R treatment, cells were seeded into
96-well plates at a density of 5x103 cells and
cultivated in DMEM with 10% FBS (HyClone; Cytiva) at room
temperature for 24 h. Each well was treated with 10 µl CCK-8
solution (Sangon Biotech Co., Ltd.) added to each well for further
incubation at 37˚C with 5% CO2 for 4 h. A microplate
reader was used for the assessment of the optical density at 450
nm.
Flow cytometry
A total of 200 µl PC12 cells were rinsed with 1 ml
pre-cold PBS twice and centrifuged. Cells were then resuspended
into 100 µl binding buffer. Following incubation with 5 µl Annexin
V-FITC on ice for 15 min, cells were stained with 10 µl propidium
iodide (10 mg/ml) at 4˚C for 30 min in the dark. A flow cytometer
(BD Biosciences) was used for detection. Flowjo vX.0.7 software
(FlowJo LLC) was used to assess the rates of apoptosis.
Reactive oxygen species (ROS)
detection
ROS Assay kit containing DCFH-DA (MilliporeSigma)
was used to evaluate ROS generation. PC12 cells were probed using
DCFH-DA (10 µM) at 37˚C in the dark for 30 min. Subsequently,
PBS-rinsed cells were imaged using an Axio Observer D1fluorescence
microscope (Carl Zeiss AG; magnification, x200).
Assessment of oxidative stress
markers
PC12 cells were inoculated into six-well plates at a
density of 4x105/well. Following aforementioned
treatment, levels of intracellular glutathione (GSH) and
malondialdehyde (MDA) were assessed using GSH Assay kit (cat. no.
S0073; Beyotime Institute of Biotechnology) and MDA Assay Kit (cat.
no. S0131S; Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. A microplate reader was used for
colorimetric analysis.
ELISA
Following aforementioned treatment, the protein
expression levels of inflammatory factors TNF-α, IL-1β and IL-6 in
cell supernatant were assessed using corresponding ELISA kits (cat.
nos. EK0526, EK0393 and EK0412, respectively; all Wuhan Boster
Biological Technology, Ltd.). A microplate reader was used to
assess the absorbance at 450 nm.
Assessment of Fe2+
levels
Following 48-h transfection, exposure of cells to
OGD/R treatment with or without 5 µM ML385 was performed. Iron
Assay kit (cat. no. ab83366; Abcam) was used to assess levels of
Fe2+ in PC12 cells according to the manufacturer's
protocol. A microplate reader was used to assess the absorbance at
593 nm of each well.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was isolated from cells using RNAiso Plus
(Takara Bio, Inc.) and quantified using QuickDrop (Molecular
Devices LLC) at 260 and 280 nm. cDNA was produced using
PrimeScript™ RT Master Mix (Takara Bio, Inc.) before qPCR using
SYBR® Premix Ex Taq™ II kit (Takara Bio, Inc.) using an
ABI 7500 (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The following thermocycling conditions
were used for qPCR: 95˚C for 10 min; followed by 40 cycles of 95˚C
for 10 sec and 60˚C for 60 sec. The primer sequences used for PCR
were as follows: TNFAIP1 forward (F), 5'-ATCATCATCTTGCCTGGCCC-3'
and reverse (R), 5'-GAACAAAGCTGTTCCCGTGC-3' and GAPDH F,
5'-GTCGTGGAGTCTACTGGCGTCTTCA-3' and R,
5'-TCGTGGTTCACACCCATCACAAACA-3'. GAPDH was used as an internal
reference and the 2-ΔΔCq method
(16) was used for the calculation
of relative mRNA expression levels.
Western blotting
The proteins were quantified using a BCA protein
assay kit (Thermo Fisher Scientific, Inc.) after the extraction of
total proteins from the indicated PC12 cells using RIPA lysis
buffer reagent (Beijing Solarbio Science & Technology Co.,
Ltd.). Following the separation of protein (30 µg/lane) using 10%
SDS-PAGE, the proteins were transferred onto PVDF membranes.
Membranes were blocked using 5% non-fat milk at room temperature
for 2 h. Overnight incubation of membranes was performed at 4˚C
with primary antibodies, supplied by Abcam, as follows: TNFAIP1
(1:1,000; cat. no. ab86934), Bcl-2 (1:1,000; cat. no. ab196495),
Bax (1:1,000; cat. no. ab32503), cleaved caspase 3 (1:5,000; cat.
no. ab214430), Nrf2 (1:1,000; cat. no. ab92946), Lamin B1 (1:1,000;
cat. no. ab16048), heme oxygenase-1 (HO-1; 1:1,000; cat. no.
ab68477), NADPH quinone dehydrogenase 1 (NQO-1; 1:1,000; cat. no.
ab80588), GSH peroxidase 4 (GPX4; 1:1,000; cat. no. ab125066),
ferritin heavy chain (1:1,000; cat. no. ab183781), ferroportin
(FPN; 1:1,000; cat. no. ab239511), transferrin receptor 1 (TFR1;
1:1,000; cat. no. ab84036) and GAPDH (1:1,000; cat. no. ab8245).
The membranes were washed with PBS for three times and then
incubated with the HRP-labeled rabbit anti-mouse secondary
antibodies (1:2,000; cat. no. ab6728; Abcam) at room temperature
for 2 h. The antibody-labeled proteins were visualized using the
ECL Detection Reagent (Shanghai Yeasen Biotechnology Co., Ltd.) and
analyzed using ImageJ (version 1.49; National Institutes of
Health).
Statistical analysis
All the experiments should be repeated at least
three times. Data are presented as the mean ± standard deviation
and were analyzed using SPSS 22.0 software (IBM Corp.). Unpaired
Student's t test or one-way ANOVA followed by Bonferroni's post hoc
test were used for comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
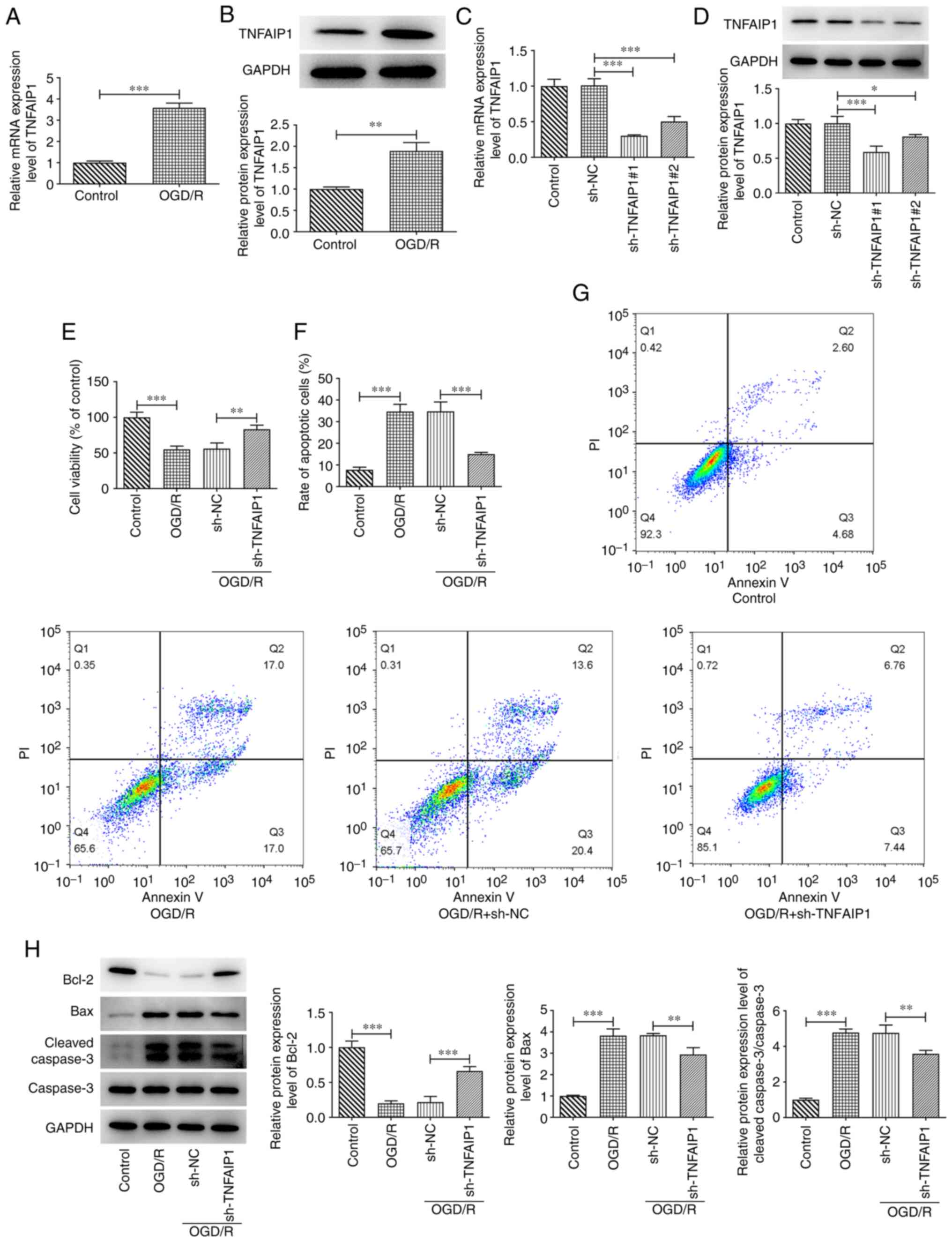
Downregulation of TNFAIP1 decreases
OGD/R-induced PC12 cell injury
To evaluate the role of TNFAIP1 in OGD/R-insulted
PC12 cells, TNFAIP1 mRNA and protein expression levels were
assessed in PC12 cells. The mRNA and protein expression levels of
TNFAIP1 were significantly increased in OGD/R-treated PC12 cells
compared with those in the control group (Fig. 1A and B). TNFAIP1 expression was knocked down in
PC12 cells by transfection with sh-TNFAIP1#1 and #2. RT-qPCR and
western blotting were performed to assess transfection efficiency.
It was revealed that the mRNA and protein expressions of TNFAIP1
were significantly reduced compared with those in sh-NC after
transfection with sh-TNFAIP1 (Fig.
1C and D). sh-TNFAIP1#1
exhibited superior transfection efficiency; therefore, sh-TNFAIP1#1
was used for subsequent experiments and was referred to as
sh-TNFAIP1 thereafter. OGD/R treatment significantly suppressed
PC12 cell viability compared with the control, which was
significantly reversed by TNFAIP1 silencing compared with sh-NC
group (Fig. 1E). Furthermore, the
proportion of apoptotic cells was significantly elevated after
OGD/R treatment compared with the control and significantly
reversed by TNFAIP1 knockdown compared with the sh-NC group
(Fig. 1F and G). Consistently, OGD/R significantly
decreased Bcl-2 protein expression levels while significantly
increasing those of Bax and cleaved caspase 3 compared with the
control. However, TNFAIP1 knockdown significantly reversed the
effects of OGD/R on expression levels of these proteins in PC12
cells (Fig. 1H).
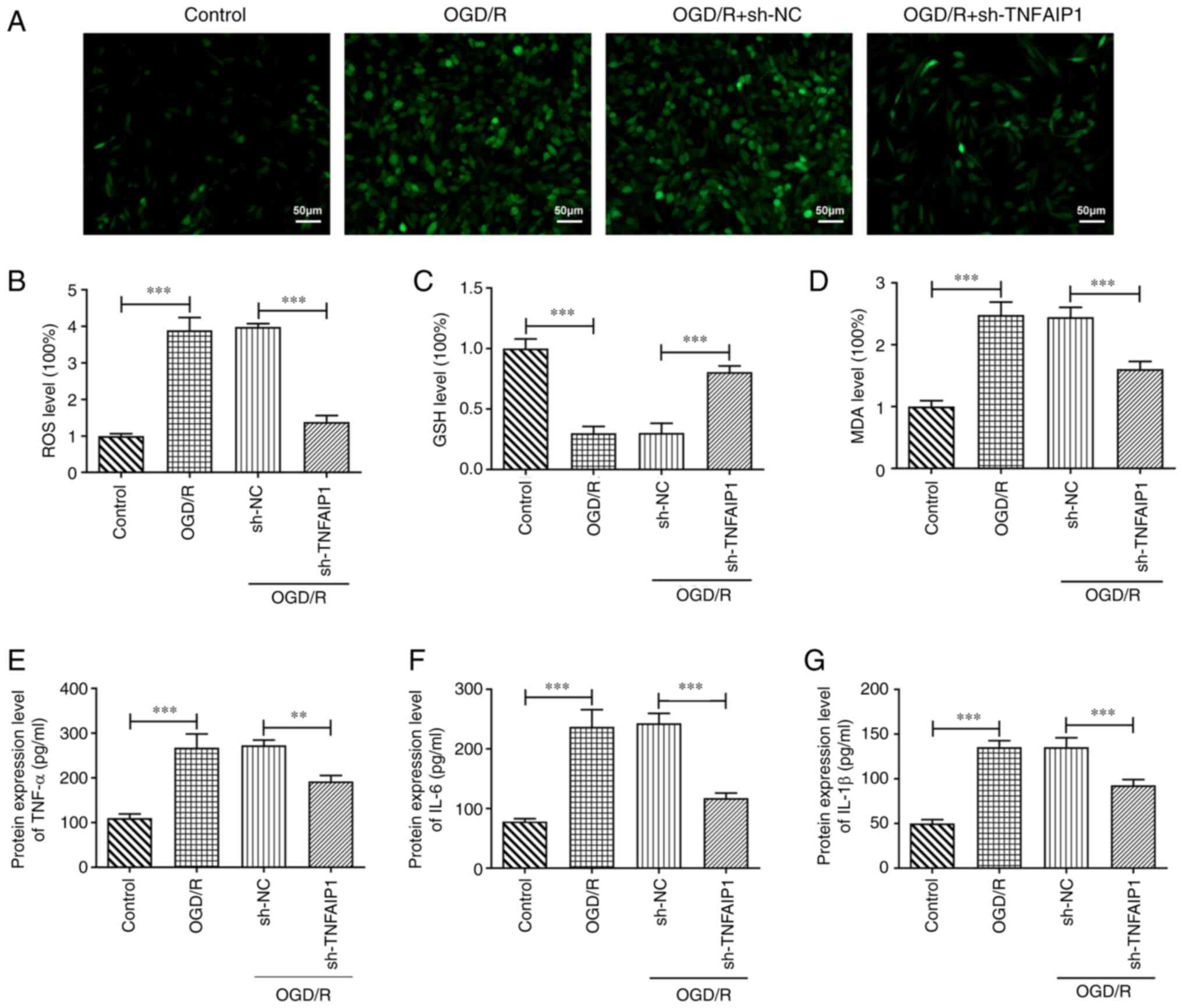
TNFAIP1 silencing alleviates oxidative
stress and the inflammatory response in OGD/R-induced PC12
cells
To assess the impact of TNFAIP1 knockdown in PC12
cells following OGD/R, the levels of oxidative stress and
inflammation were evaluated. OGD/R significantly elevated levels of
ROS compared with the control whereas TNFAIP1 silencing
significantly decreased this enhancement compared with the sh-NC
group (Fig. 2A and B). OGD/R treatment significantly
decreased GSH but significantly increased MDA protein expression
levels in PC12 cells compared with the control; however, knocking
down TNFAIP1 expression significantly reversed OGD/R-induced
oxidative stress compared with the sh-NC group (Fig. 2C and D). Furthermore, protein expression levels
of TNF-α, IL-6 and IL-1β were significantly elevated following
OGD/R compared with the control, which was significantly reversed
after TNFAIP1 knockdown compared with the sh-NC group (Fig. 2E-G).
Knocking down TNFAIP1 expression
suppresses ferroptosis via activation of the Nrf2 signaling pathway
in OGD/R-injured PC12 cells
The potential mechanism by which TNFAIP1 regulated
OGD/R-induced PC12 cells was assessed. Results obtained from
western blotting demonstrated that OGD/R treatment significantly
decreased cytoplasmic (C)-Nrf2 protein expression levels but
significantly elevated those of nuclear (N)-Nrf2 and NQO-1 and
markedly elevated those of HO-1 compared with the control. TNFAIP1
silencing significantly decreased C-Nrf2 protein expression levels
further whereas it significantly increased those of N-Nrf2, HO-1
and NQO-1 compared with sh-NC group (Fig. 3A). To assess the association
between the Nrf2 signaling pathway and ferroptosis downstream of
TNFAIP1 in PC12 cells induced with OGD/R, the Nrf2 inhibitor ML385
was used to treat cells. OGD/R resulted in significantly elevated
Fe2+ levels compared with the control and transfection
with sh-TNFAIP1 significantly reversed the enhancement in
Fe2+ levels that resulted from OGD/R compared with the
sh-NC group. However, ML385 treatment significantly reversed the
inhibitory effects of sh-TNFAIP1 on levels of Fe2+ in
PC12 cells compared with the sh-TNFAIP1 group. It was also
demonstrated that protein expression levels of GPX4, FTH1 and FPN
were significantly decreased whilst those of TFR1 were
significantly increased by OGD/R compared with the control and this
was significantly reversed by TNFAIP1 silencing compared with the
sh-NC group. The effects of TNFAIP1 knockdown on protein expression
levels of GPX4, FTH1 and TFR1 were significantly reversed and the
effect on the protein expression levels of FPN was markedly
reversed by ML385 treatment compared with sh-TNFAIP1 group
(Fig. 3C).
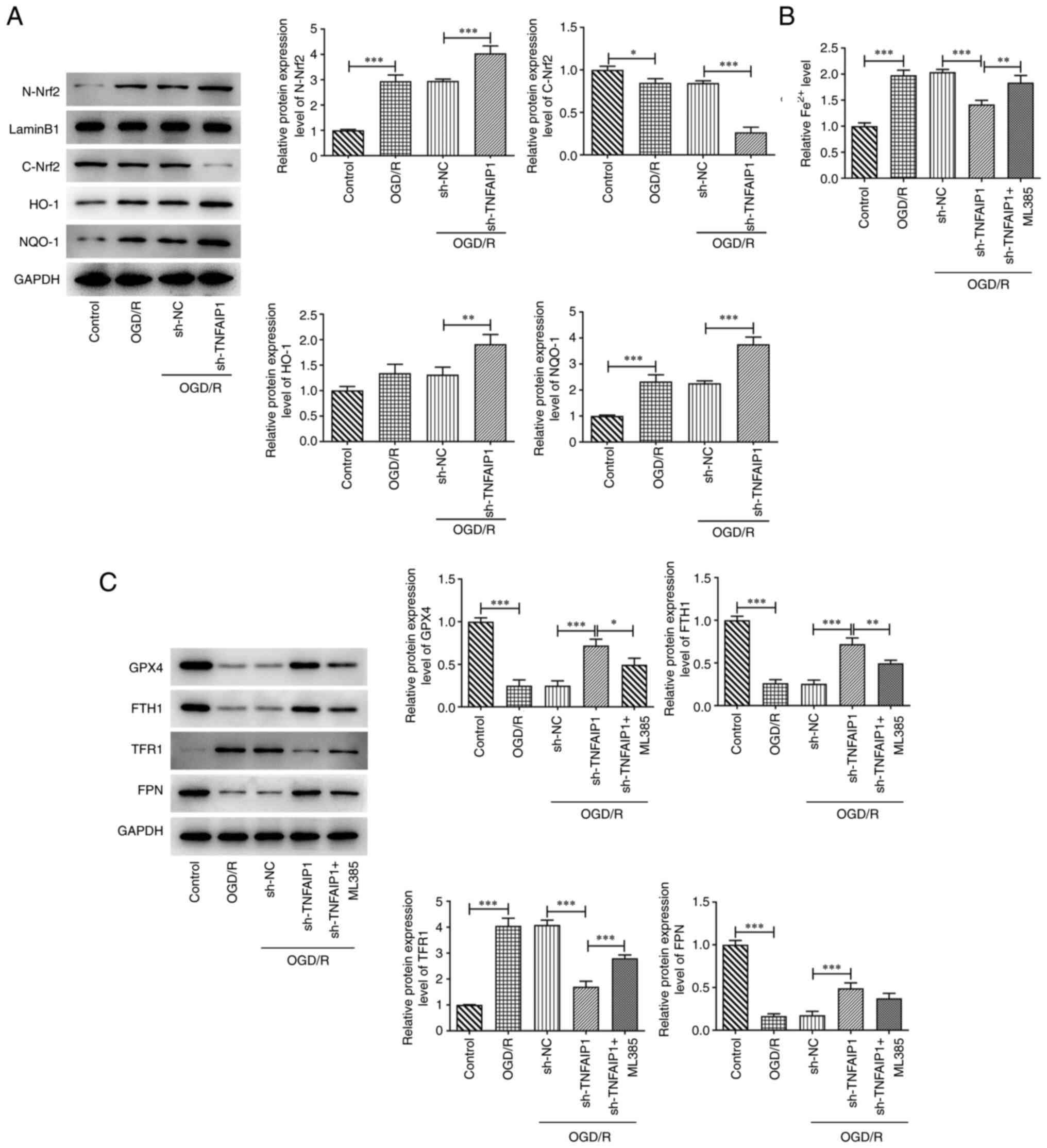 | Figure 3Knockdown of TNFAIP1 inhibits
ferroptosis via activation of the Nrf2 signaling pathway in
OGD/R-induced PC12 cells. (A) Western blotting was performed to
assess protein expression levels of C-Nrf2, N-Nrf2, HO-1 and NQO-1.
(B) Iron Assay kit was used to assess Fe2+ levels. (C)
Western blotting was performed to assess protein expression levels
of GPX4, FTH1, FPN and TFR1. Data are presented as the mean ± SD.
*P<0.05, **P<0.01 and
***P<0.001. TNFAIP1, TNFα-induced protein 1; OGD/R,
oxygen glucose deprivation and reperfusion; Nrf2, nuclear factor
erythroid 2-related factor 2; C, cytoplasmic; N, nuclear; HO-1,
heme oxygenase 1; NQO-1, NADPH quinone dehydrogenase 1; GPX4,
glutathione peroxidase 4; FTH1, ferritin heavy chain; FPN,
ferroportin; TFR1, transferrin receptor 1; sh, short hairpin RNA;
NC, negative control. |
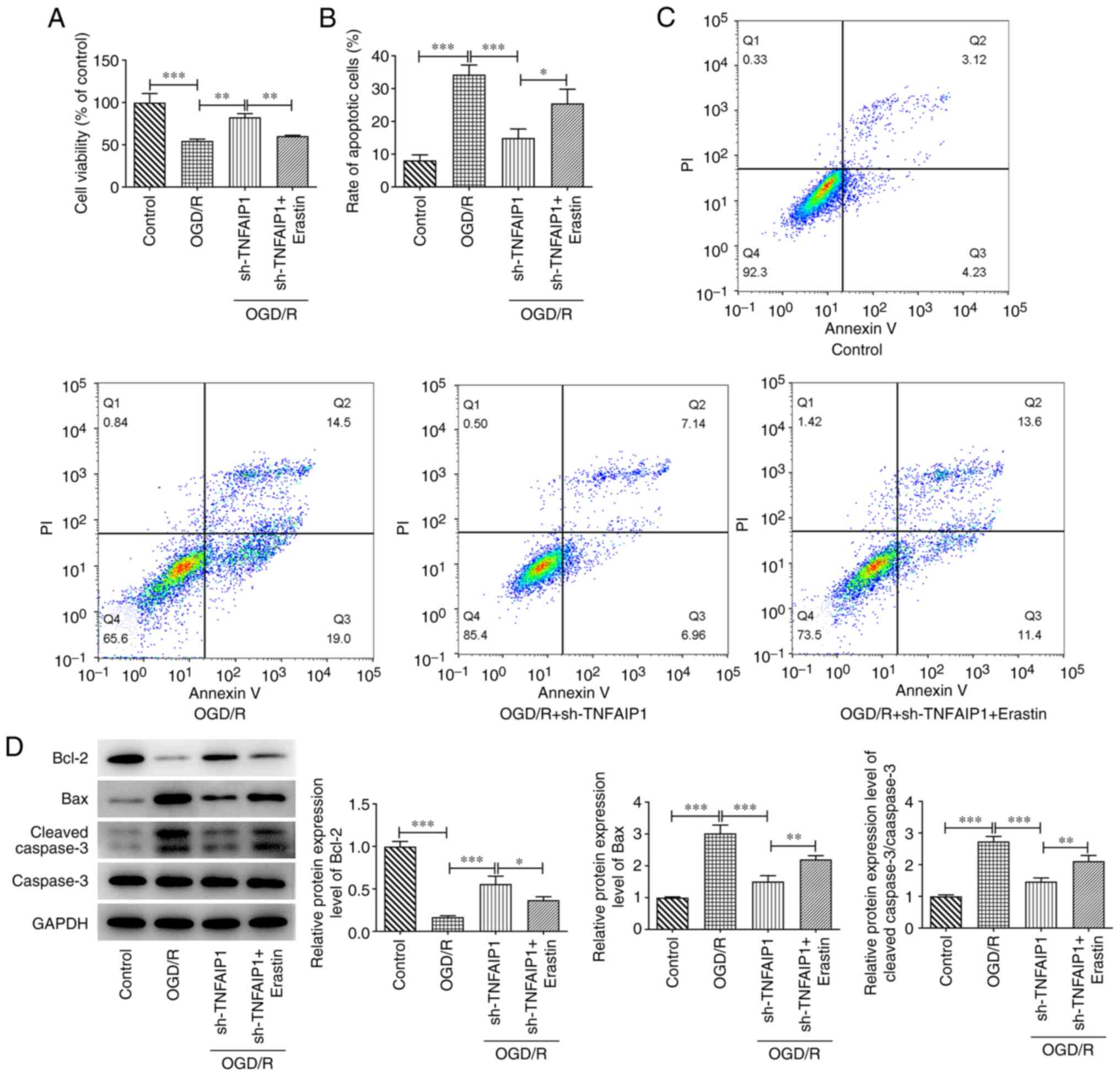
Erastin reverses the impact of TNFAIP1
silencing on OGD/R-induced PC12 cell injury
To assess the role of ferroptosis in TNFAIP-mediated
OGD/R injury, erastin was added to the PC12 cells as a ferroptosis
promoter. Treatment with erastin significantly decreased viability
of OGD/R-induced PC12 cells with TNFAIP1 expression knocked down
compared with sh-TNFAIP1 group (Fig.
4A). Erastin significantly increased cell apoptosis after
treatment with OGD/R and transfection with sh-TNFAIP1 compared with
sh-TNFAIP1 group (Fig. 4B and
C). Significantly decreased Bcl-2
protein expression levels and significantly increased protein
expression levels of Bax and cleaved caspase 3 were observed in
OGD/R-stimulated PC12 cells with TNFAIP1 knockdown after treatment
with erastin compared with the sh-TNFAIP1 group (Fig. 4D).
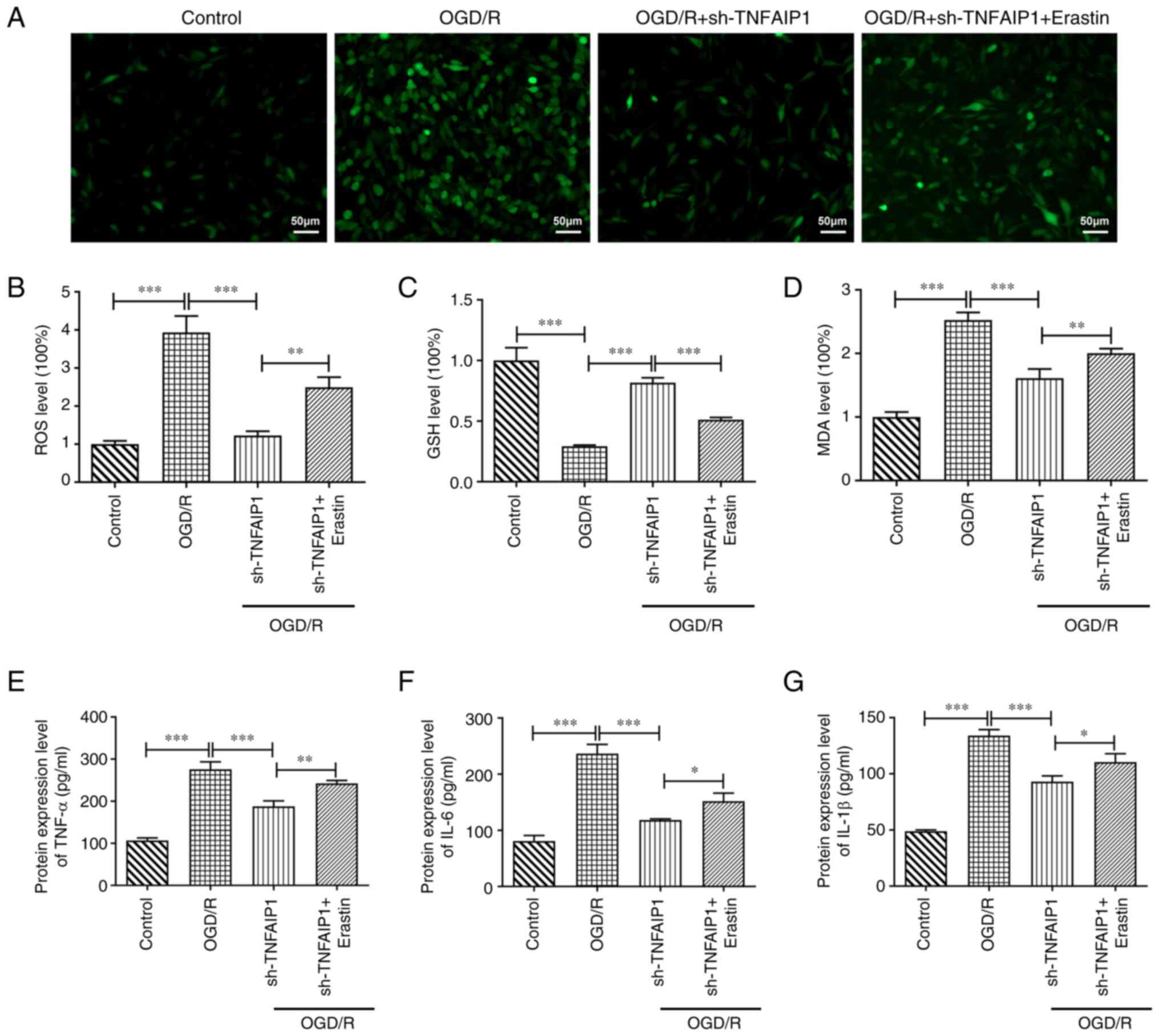
Erastin counteracts the impact of
TNFAIP1 knockdown on oxidative stress and inflammation in PC12
cells following OGD/R injury
Erastin treatment significantly increased the
production of ROS in PC12 cells transfected with sh-TNFAIP1
following OGD/R injury compared with sh-TNFAIP1 group (Fig. 5A and B). Furthermore, co-treatment with erastin
and sh-TNFAIP1 significantly decreased protein expression levels of
GSH and significantly increased MDA protein expression levels
compared with those in the sh-TNFAIP1 group (Fig. 5C and D). Moreover, ELISA demonstrated that
erastin significantly increased the protein expression levels of
TNF-α, IL-6 and IL-1β in OGD/R-stimulated PC12 cells transfected
with sh-TNFAIP1 compared with sh-TNFAIP1 group (Fig. 5E-G).
Discussion
Evidence has demonstrated that the production of
certain cytokines, inflammatory cell infiltration and ROS
production occur in ischemic injury, which can exacerbate the
damage due to cerebral ischemia (17,18).
When reperfusion occurs, recovery of blood supply may result in ROS
production and inflammation, inducing CIRI (19). Therefore, novel effective
therapeutic targets are required for treatment for ischemic stroke.
In the present study, TNFAIP1 knockdown was demonstrated to
increase cell viability and decrease oxidative stress and
inflammation, in addition to suppressing ferroptosis in PC12 cells
insulted with OGD/R, via the Nrf2 signaling pathway. This indicated
the potential to use TNFAIP1 for the attenuation of CIRI.
OGD/R has been frequently used in research for
induction of CIRI and has been reported to stimulate inflammation,
apoptosis, autophagy and endoplasmic reticulum stress in cultured
cortical neurons (20,21). In the present study, the OGD/R
model was established to simulate CIRI in vitro. OGD/R
stimulation was demonstrated to significantly suppress cell
viability, aggravate apoptosis and oxidative stress and induce
inflammatory damage in PC12 cells. TNFAIP1 is an evolutionarily
conserved single-copy gene in humans, mice, rats and nematode worm
that was first reported in umbilical vein endothelial cells
(22). TNFAIP1 has been reported
to serve a key role in neurodevelopment and a number of
neurological diseases (23). For
example, TNFAIP1 is overexpressed in the neurons of the cortex and
hippocampus in the brains of APP/PS1 mice and the upregulation of
TNFAIP1 can induce apoptosis in mice with Alzheimer's disease
(24). A previous study reported
that knocking down TNFAIP1 expression inhibits formaldehyde-induced
neurotoxicity by suppression of cell apoptosis whilst increasing
cell viability and neurite outgrowth by the inhibition of the
AKT/cAMP response element binding protein (CREB) signaling pathway
(25). Qiu et al (26) reported that TNFAIP1 knockdown
elevates cell viability and suppresses apoptosis to prevent
di(2-ethylhexyl) phthalate-induced neurotoxicity by triggering the
CREB signaling pathway. In the present study, TNFAIP1 mRNA and
protein expression levels were significantly upregulated in PC12
cells following OGD/R. TNFAIP1 silencing significantly reversed the
OGD/R-induced decrease in cell viability while reducing cell
apoptosis. Furthermore, oxidative stress and inflammatory response
caused by OGD/R induction were attenuated by TNFAIP1 knockdown,
which supported the protective effects of TNFAIP1 knockdown in PC12
cells following OGD/R induction. TNFAIP1 has been reported as being
induced by TNF-α (27); however,
in the present study it was demonstrated that the inhibition of
TNFAIP1 significantly reduced TNF-α protein expression levels. This
may be linked to previous reports that OGD/R can cause TNFAIP1 to
regulate TNF-α in turn (15,28).
Furthermore, Yi et al (25)
reported that clearance of ROS suppresses formaldehyde-mediated
upregulation of TNFAIP1 expression; however in the present study,
it was demonstrated that TNFAIP1-silencing inhibited production of
ROS in OGD/R-treated PC12 cells.
It has been previously reported that the Nrf2
signaling pathway is associated with I/R process in numerous types
of tissue (29,30). Zhao et al (31) reported that sulforaphane attenuates
liver injury from intestinal I/R via the Nrf2/antioxidant response
element pathway. Wei et al (32) reported that Nrf2 protects against
neuronal and capillary degeneration following retinal I/R injury.
Furthermore, another study reported that TNFAIP1 expression is
increased following I/R injury and TNFAIP1 knockdown alleviates
MIRI via the AKT/GSK3β/Nrf2 signaling pathway (15). Therefore, it was hypothesized that
TNFAIP1 is involved in neuron injury from CIRI. In the present
study, C-Nrf2 protein expression levels were significantly
decreased and N-Nrf2 protein expression levels were significantly
enhanced following OGD/R treatment, whereas transfection with
sh-TNFAIP significantly decreased C-Nrf2 protein expression levels
and significantly increased protein expression levels of N-Nrf2
further. It can be hypothesized that excessive oxidative stress due
to OGD/R promoted separation of Kelch-like ECH-associated protein 1
from Nrf-2 to activate Nrf2 and that silencing of TNFAIP1
expression may prolong activation of the Nrf2 signaling pathway
following OGD/R. The Nrf2 signaling pathway is a key pathway that
leads to ferroptosis (33). Yuan
et al (34) reported that
kaempferol inhibits OGD/R-induced ferroptosis in neurons via the
AKT/Nrf2/GPX4 signaling pathway. The present study demonstrated
that OGD/R significantly enhanced levels of Fe2+ and
TFR1 protein expression levels but significantly diminished protein
expression levels of GPX4, FTH1 and FPN. TNFAIP1 silencing
significantly reversed this trend and ML385 treatment prevented
this reversal. Erastin was added to the PC12 cells to promote
ferroptosis, which demonstrated that erastin reversed the
beneficial effects of TNFAIP1 on viability, oxidative stress and
inflammatory damage in OGD/R-induced PC12 cells. These results
suggested that downregulation of TNFAIP1 expression may alleviate
OGD/R-induced neuronal cell damage by inhibition of ferroptosis via
regulation of the Nrf2 signaling pathway.
In summary, the present study demonstrated the
potential protective role of TNFAIP1 silencing in OGD/R-injured
neurocytes and the key role of Nrf2-mediated ferroptosis in the
viability, oxidative stress, apoptosis and inflammatory damage of
PC12 cells following OGD/R stimulation, which suggested that
TNFAIP1 may be a promising therapeutic target for CIRI. However,
there are also some limitations of the present study. First, the
function of TNFAIP1 in CIRI in a clinical setting was not explored.
Second, an in vivo CIRI model was not established to
investigate the role of TNFAIP1 in CIRI.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Basic Public
Welfare Research Program of Zhejiang Province, China (grant no.
LGF20H270003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX and KL designed the present study and drafted and
revised the manuscript. LX, JZ, HS, GZ and XJ performed the
experiments. ML and PZ reviewed the literature and analyzed the
data. LX and KL confirmed the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma Q, Li R, Wang L, Yin P, Wang Y, Yan C,
Ren Y, Qian Z, Vaughn MG, McMillin SE, et al: Temporal trend and
attributable risk factors of stroke burden in China, 1990-2019: An
analysis for the global burden of disease study 2019. Lancet Public
Health. 6:e897–e906. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Grysiewicz RA, Thomas K and Pandey DK:
Epidemiology of ischemic and hemorrhagic stroke: incidence,
prevalence, mortality, and risk factors. Neurol Clin. 26:871–895.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Owens B: Stroke. Nature.
510(S1)2014.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Pan J, Konstas AA, Bateman B, Ortolano GA
and Pile-Spellman J: Reperfusion injury following cerebral
ischemia: Pathophysiology, MR imaging, and potential therapies.
Neuroradiology. 49:93–102. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang L, Chen C, Zhang X, Li X, Chen Z,
Yang C, Liang X, Zhu G and Xu Z: Neuroprotective effect of curcumin
against cerebral ischemia-reperfusion via mediating autophagy and
inflammation. J Mol Neurosci. 64:129–139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang H, Chen S, Zhang Y, Xu H and Sun H:
Electroacupuncture ameliorates neuronal injury by
Pink1/Parkin-mediated mitophagy clearance in cerebral
ischemia-reperfusion. Nitric Oxide. 91:23–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang J, Chen M, Cao RY, Li Q and Zhu F:
The role of circular RNAs in cerebral ischemic diseases: Ischemic
stroke and cerebral ischemia/reperfusion injury. Adv Exp Med Biol.
1087:309–325. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moussaddy A, Demchuk AM and Hill MD:
Thrombolytic therapies for ischemic stroke: Triumphs and future
challenges. Neuropharmacology. 134:272–279. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gobin YP, Starkman S, Duckwiler GR,
Grobelny T, Kidwell CS, Jahan R, Pile-Spellman J, Segal A, Vinuela
F and Saver JL: MERCI 1: A phase 1 study of mechanical embolus
removal in cerebral ischemia. Stroke. 35:2848–2854. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang CL, Wang C, Yan WJ, Gao R, Li YH and
Zhou XH: Knockdown of TNFAIP1 inhibits growth and induces apoptosis
in osteosarcoma cells through inhibition of the nuclear factor-κB
pathway. Oncol Rep. 32:1149–1155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao Y, Li S, Xia N, Shi Y and Zhao CM:
Effects of XIST/miR-137 axis on neuropathic pain by targeting
TNFAIP1 in a rat model. J Cell Physiol. 233:4307–4316.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang L, Liu N, Hu X, Zhang W, Wang T, Li
H, Zhang B, Xiang S, Zhou J and Zhang J: CK2 phosphorylates TNFAIP1
to affect its subcellular localization and interaction with PCNA.
Mol Biol Rep. 37:2967–2973. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu N, Yu Z, Xun Y, Li M, Peng X, Xiao Y,
Hu X, Sun Y, Yang M, Gan S, et al: TNFAIP1 contributes to the
neurotoxicity induced by Aβ25-35 in Neuro2a cells. BMC Neurosci.
17(51)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gladwyn-Ng IE, Li SS, Qu Z, Davis JM, Ngo
L, Haas M, Singer J and Heng JI: Bacurd2 is a novel interacting
partner to Rnd2 which controls radial migration within the
developing mammalian cerebral cortex. Neural Dev.
10(9)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wen L, Yang QH, Ma XL, Li T, Xiao S and
Sun CF: Inhibition of TNFAIP1 ameliorates the oxidative stress and
inflammatory injury in myocardial ischemia/reperfusion injury
through modulation of Akt/GSK-3β/Nrf2 pathway. Int Immunopharmacol.
99(107993)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao J, Wang H, Dong L, Sun S and Li L:
miRNA-20b inhibits cerebral ischemia-induced inflammation through
targeting NLRP3. Int J Mol Med. 43:1167–1178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pei H, Song X, Peng C, Tan Y, Li Y, Li X,
Ma S, Wang Q, Huang R, Yang D, et al: TNF-α inhibitor protects
against myocardial ischemia/reperfusion injury via Notch1-mediated
suppression of oxidative/nitrative stress. Free Radic Biol Med.
82:114–121. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hao MQ, Xie LJ, Leng W and Xue RW: Trim47
is a critical regulator of cerebral ischemia-reperfusion injury
through regulating apoptosis and inflammation. Biochem Biophys Res
Commun. 515:651–657. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qin H, Tan W, Zhang Z, Bao L, Shen H, Wang
F, Xu F and Wang Z: 15d-prostaglandin J2 protects cortical neurons
against oxygen-glucose deprivation/reoxygenation injury:
Involvement of inhibiting autophagy through upregulation of Bcl-2.
Cell Mol Neurobiol. 35:303–312. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu Y, Qu X, Yan M, Li D and Zou R: Tricin
attenuates cerebral ischemia/reperfusion injury through inhibiting
nerve cell autophagy, apoptosis and inflammation by regulating the
PI3K/Akt pathway. Hum Exp Toxicol.
41(9603271221125928)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wolf FW, Marks RM, Sarma V, Byers MG, Katz
RW, Shows TB and Dixit VM: Characterization of a novel tumor
necrosis factor-alpha-induced endothelial primary response gene. J
Biol Chem. 267:1317–1326. 1992.PubMed/NCBI
|
|
23
|
Liu Y, Sun H and Sun Y: LncRNA p21,
downregulating miR-181b, aggravates neuropathic pain by
upregulating Tnfaip1 and inhibit the AKT/CREB axis. Brain Res Bull.
171:150–161. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xiao Y, Li Y, Zhang H, Yang L, Jiang Y,
Wei C, Feng X, Xun Y, Yuan S, Xiang S and Liu N: TNFAIP1 is
upregulated in APP/PS1 mice and promotes apoptosis in SH-SY5Y cells
by binding to RhoB. J Mol Neurosci. 71:1221–1233. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yi J, Zhu M, Qiu F, Zhou Y, Shu P, Liu N,
Wei C and Xiang S: TNFAIP1 mediates formaldehyde-induced
neurotoxicity by inhibiting the Akt/CREB pathway in N2a cells.
Neurotox Res. 38:184–198. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qiu F, Zhou Y, Deng Y, Yi J, Gong M, Liu
N, Wei C and Xiang S: Knockdown of TNFAIP1 prevents
di-(2-ethylhexyl) phthalate-induced neurotoxicity by activating
CREB pathway. Chemosphere. 241(125114)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tang X, Tangkham T, Aljahdali B, Lee S, Su
M and Dibart S: The role of TNF-α induced protein 1 in the
activation of pro-apoptotic proteins. Hum Cell. 34:1123–1129.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aljahdali BH: Regulation of TNF-α gene
expression by TNFAIP1 as an activator or suppressor in response to
lipopolysaccharide (unpublished PhD thesis). Boston University,
2019.
|
|
29
|
Xiao C, Xia ML, Wang J, Zhou XR, Lou YY,
Tang LH, Zhang FJ, Yang JT and Qian LB: Luteolin attenuates cardiac
ischemia/reperfusion injury in diabetic rats by modulating Nrf2
antioxidative function. Oxid Med Cell Longev.
2019(2719252)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
He M, Pan H, Chang RC, So KF, Brecha NC
and Pu M: Activation of the Nrf2/HO-1 antioxidant pathway
contributes to the protective effects of Lycium barbarum
polysaccharides in the rodent retina after
ischemia-reperfusion-induced damage. PLoS One.
9(e84800)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao HD, Zhang F, Shen G, Li YB, Li YH,
Jing HR, Ma LF, Yao JH and Tian XF: Sulforaphane protects liver
injury induced by intestinal ischemia reperfusion through Nrf2-ARE
pathway. World J Gastroenterol. 16:3002–3010. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wei Y, Gong J, Yoshida T, Eberhart CG, Xu
Z, Kombairaju P, Sporn MB, Handa JT and Duh EJ: Nrf2 has a
protective role against neuronal and capillary degeneration in
retinal ischemia-reperfusion injury. Free Radic Biol Med.
51:216–224. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23(101107)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yuan Y, Zhai Y, Chen J, Xu X and Wang H:
Kaempferol ameliorates oxygen-glucose
deprivation/reoxygenation-induced neuronal ferroptosis by
activating Nrf2/SLC7A11/GPX4 axis. Biomolecules.
11(923)2021.PubMed/NCBI View Article : Google Scholar
|