Introduction
Sepsis is life-threatening organ dysfunction in
response to infection (1). Due to
the immunosuppressed state, patients with cancer are prone to
infection (2). Cancer accounts for
an estimated 20% of >1 million sepsis hospitalizations (3). Sepsis and septic shock are the most
common causes of death in patients with cancer. A study conducted
by Rudd et al (4) showed
that of the 48.9 million sepsis cases hospitalized in 2017, 22.5%
died. Patients with cancer in combination with sepsis are
associated with higher mortality rates compared to patients with
sepsis (5). Studies have shown
that 28-day mortality rates in patients with sepsis and combined
active cancer ranged from 41.9 to 81.5% (6). A previous study has shown that
mortality from sepsis is associated with anatomical site of
infection (7). Assessing the
strength of the association between the site of infection and
prognosis in patients with cancer with sepsis or septic shock may
help healthcare providers make better clinical decisions (8). However, to the best of our knowledge,
few studies have addressed the impact of specific infection sites
on mortality in patients with cancer with sepsis or sepsis shock.
Hensley et al (3) showed
differences in in-hospital mortality in patients with cancer and
sepsis due to the site of infection. The study by Chebl et
al (2) showed differences in
common sites of infection in patients with hematologic and solid
tumours. However, they did not further analyse the association
between the site of infection and in-hospital mortality in cancer
patients. Therefore, the present study described the distribution
of infection sites in patients with cancer presenting to the
emergency department (ED) with sepsis or septic shock and analysed
the association of specific sites of infection with in-hospital
mortality.
Patients and methods
Data collection
The present study assessed a retrospective
single-centre cohort study by Dagher et al (9) at the American University of Beirut
Medical Center (Beirut, Lebanon) from July 2010 to April 2015. Data
was extracted free of charge from the Dryad Digital Repository
database (doi.org/10.5061/dryad.6qk05). A total of 352
participants were retrospectively reviewed. 176 cancer-free
patients were excluded and 176 patients with active cancer were
selected for analysis. The requirement for ethics approval was
waived. As data was anonymized, the requirement for informed
consent was waived. Because this was a secondary retrospective
chart review study, patients with cancer were not involved in the
design.
Sepsis (9,10) was defined as known or suspected
source of systemic infection plus at least two of the following: i)
Temperature >38 or <36˚C; ii) heart rate >90 beats/min;
iii) respiratory rate >20 breaths/min or arterial carbon dioxide
tension <32 mmHg and iv) white cell count >12,000 or
<4,000/ml.
Septic shock (9,10)
was defined as fulfilling requirements for sepsis plus one of the
following: Systolic blood pressure <90 mmHg, mean arterial
pressure <65 mmHg or lactate >2 mmol/l following initial
fluid replacement.
Bacteraemia was defined as two positive blood
cultures of known skin flora pathogens or one positive blood
culture of non-skin flora pathogens.
Active cancer (9)
was defined as solid or haematological malignancy currently
receiving at least one therapy as follows: i) Chemotherapy; ii)
radiation therapy and iii) surgery.
Statistical analysis
Categorical variables were compared using
χ2 or Fisher's exact probability test. Continuous
variables were compared by Kruskal-Wallis test. Categorical
variables were reported as percentage. Non-normal variables were
reported as the median and interquartile range (IQR). The effect of
the site of infection on mortality was analysed by Cox regression.
Covariates in the multivariate model included age (20-50, 51-70 and
>70 years), sex, hypertension, congestive heart failure,
ejection fraction <40%, tumour type, white blood cell count,
haemoglobin, diagnosis, bacteraemia, chemotherapy, radiation,
surgery, time to antibiotics and steroids. In addition, all-cause
mortality within 60 days was assessed using Kaplan-Meier curves
according to the site of infection. Differences were compared by
the log-rank test. All statistical analyses were performed with
Empower (empowerstats.com; X&Y Solutions,
version 4.1) and R software (R-project.org
version 4.1.3). P<0.05 (two-sided) was considered to indicate a
statistically significant difference.
Results
Baseline characteristics and clinical
outcomes
The baseline characteristics of patients with cancer
are shown in Table I. Sites of
infection included lung (n=66; 37.50%), urinary tract (n=47;
26.70%), unknown (n=24; 13.63%), gastrointestinal (n=23; 13.07%)
and other (n=16; 9.10%). Other sites of infection included skin,
oral cavity, catheter, bile and liver (2.84, 0.57, 2.27, 2.84 and
0.57%, respectively). A total of 176 patients with active cancer
were included in the present study, of which 35 patients (19.89%)
had haematological tumours and 141 patients (80.11%) had solid
tumours. In 35 haematological tumours, leukaemia (57.14%) was the
most prevalent type of cancer. In 141 cases of solid tumours, lung
cancer (22.70%) was the most prevalent type of cancer, followed by
breast cancer (14.18%). Overall in-hospital mortality was 47.73%
(n=84), with the lowest mortality in patients with cancer with
urinary tract infection (n=15; 31.91%). Gastrointestinal infection
(n=18; 78.26%) had a higher mortality rate, followed by pneumonia
(n=41; 62.12%).
 | Table IBaseline characteristics of patients
with cancer. |
Table I
Baseline characteristics of patients
with cancer.
| | Infection site | |
|---|
| Variable | Urine tract
(n=47.00) | Unknown
(n=24.00) | Gastrointe stinal
(n=23.00) | Lung (n=66.00) | Other (n=16.00) | P-value |
|---|
| Age, years, n
(%) | | | | | | 0.374 |
|
20-50 | 3.00 (6.38) | 6.00 (25.00) | 2.00 (8.70) | 10.00 (15.15) | 2.00 (12.50) | |
|
51-70 | 19.00 (40.43) | 9.00 (37.50) | 12.00 (52.17) | 32.00 (48.48) | 9.00 (56.25) | |
|
>70 | 25.00 (53.19) | 9.00 (37.50) | 9.00 (39.13) | 24.00 (36.36) | 5.00 (31.25) | |
| Male, n (%) | 34.00 (72.34) | 9.00 (37.50) | 17.00 (73.91) | 43.00 (65.15) | 9.00 (56.25) | 0.039 |
| Hypertension, n
(%) | 30.00 (63.83) | 8.00 (33.33) | 16.00 (69.57) | 34.00 (51.52) | 6.00 (37.50) | 0.039 |
| Congestive heart
failure ejection fraction <40%, n (%) | 8.00 (17.02) | 1.00 (4.17) | 2.00 (8.17) | 13.00 (19.70) | 1.00 (6.25) | 0.255 |
| Tumour type, n
(%) | | | | | | 0.626 |
|
Haematological
malignancy, n (%) | 7.00 (14.89) | 6.00 (25.00) | 3.00 (13.04) | 16.00 (24.24) | 3.00 (18.75) | |
|
Solid
tumour, n (%) | 40.00 (85.11) | 18.00 (75.00) | 20.00 (86.96) | 50.00 (75.76) | 13.00 (81.25) | |
| White blood cell
(1x109/l), n (%) | | | | | | 0.682 |
|
>10 | 27.00 (57.45) | 11.00 (45.83) | 12.00 (52.17) | 35.00 (53.03) | 6.00 (37.50) | |
|
≤10 | 20.00 (42.55) | 13.00 (54.17) | 11.00 (47.83) | 31.00 (46.97) | 10.00 (62.50) | |
| Haemoglobin (g/l), n
(%) | | | | | | 0.600 |
|
>100 | 22.00 (46.81) | 10.00 (41.67) | 13.00 (56.52) | 32.00 (48.48) | 5.00 (31.25) | |
|
≤100 | 25.00 (53.19) | 14.00 (58.33) | 10.00 (43.48) | 34.00 (51.52) | 11.00 (68.75) | |
| Diagnosis, n (%) | | | | | | 0.193 |
|
Septic
shock | 23.00 (48.94) | 15.00 (62.50) | 17.00 (73.91) | 45.00 (68.18) | 9.00 (56.25) | |
|
Sepsis | 24.00 (51.06) | 9.00 (37.50) | 6.00 (26.09) | 21.00 (31.82) | 7.00 (43.75) | |
| Bacteraemia, n
(%) | 18.00 (38.30) | 13.00 (54.17) | 7.00 (30.43) | 18.00 (27.27) | 10.00 (62.50) | 0.032 |
| Chemotherapy, n
(%) | 42.00 (89.36) | 18.00 (75.00) | 14.00 (60.87) | 58.00 (87.88) | 15.00 (93.75) | 0.011 |
| Radiation therapy, n
(%) | 13.00 (27.66) | 8.00 (33.33) | 3.00 (13.04) | 31.00 (46.97) | 7.00 (43.75) | 0.030 |
| Surgery, n (%) | 26.00 (55.32) | 11.00 (45.83) | 9.00 (39.13) | 22.00 (33.33) | 10.00 (62.50) | 0.093 |
| Time to antibiotics,
h, n (%) | | | | | | 0.832 |
|
0-1 | 17.00 (36.17) | 9.00 (37.50) | 11.00 (50.00) | 25.00 (37.88) | 7.00 (43.75) | |
|
>1 | 30.00 (63.83) | 15.00 (62.50) | 11.00 (50.00) | 41.00 (62.12) | 9.00 (56.25) | |
| Steroids, n
(%) | 13.00 (27.66) | 9.00 (37.50) | 5.00 (21.74) | 27.00 (40.91) | 3.00 (18.75) | 0.239 |
| Hospital days,
median (IQR) | 7.71
(4.44-10.29) | 11.64
(5.10-20.99) | 5.92
(3.50-16.29) | 11.57
(5.05-22.91) | 10.12
(6.30-16.14) | 0.126 |
| In-hospital
mortality, n (%) | 15.00 (31.91) | 14.00 (58.33) | 18.00 (78.26) | 41.00 (62.12) | 7.00 (43.75) | 0.002 |
In the univariable analysis for the entire cohort
(Table II), diagnosis and
chemotherapy were significantly associated with survival. These
variables were included in further analyses.
 | Table IIUnivariate analysis of prognostic
factors in the derivation cohort. |
Table II
Univariate analysis of prognostic
factors in the derivation cohort.
| Variable | Total number of
patients (%) | In-hospital
mortality HR (95% CI) | 28 day mortality HR
(95% CI) | 60 day mortality HR
(95% CI) |
|---|
| Age, years | | | | |
|
20-50 | 23.00 (13.07) | 1.00 | 1.00 | 1.00 |
|
51-70 | 81.00 (46.02) | 0.73
(0.41-1.30) | 0.74
(0.41-1.35) | 0.80
(0.45-1.41) |
|
>70 | 72.00 (40.91) | 0.75
(0.42-1.34) | 0.71
(0.39-1.30) | 0.79
(0.44-1.40) |
| Sex | | | | |
|
Female | 64.00 (36.36) | 1.00 | 1.00 | 1.00 |
|
Male | 112.00 (63.64) | 1.00
(0.66-1.52) | 0.97
(0.63-1.50) | 0.97
(0.65-1.45) |
| Hypertension | | | | |
|
No | 82.00 (46.59) | 1.00 | 1.00 | 1.00 |
|
Yes | 94.00 (53.41) | 1.09
(0.73-1.63) | 1.09
(0.71-1.65) | 1.08
(0.73-1.60) |
| Congestive heart
failure ejection fraction <40% | | | | |
|
No | 151.00 (85.80) | 1.00 | 1.00 | 1.00 |
|
Yes | 25.00 (14.20) | 0.77
(0.41-1.44) | 0.67
(0.34-1.34) | 0.87
(0.48-1.55) |
| Tumour type | | | | |
|
Solid
tumour | 141.00 (80.11) | 1.00 | 1.00 | 1.00 |
|
Haematological
malignancy | 35.00 (19.89) | 0.86
(0.51-1.43) | 0.98
(0.58-1.64) | 0.79
(0.48-1.32) |
| White blood cell
(1x109/l), n (%) | | | | |
|
>10 | 91.00 (51.70) | 1.00 | 1.00 | 1.00 |
|
≤10 | 85.00 (48.30) | 0.83
(0.55-1.24) | 0.88
(0.58-1.34) | 0.87
(0.59-1.29) |
| Haemoglobin
categorical, g/l | | | | |
|
>100 | 82.00 (46.59) | 1.00 | 1.00 | 1.00 |
|
≤100 | 94.00 (53.41) | 0.97
(0.65-1.46) | 0.99
(0.65-1.51) | 1.01
(0.68-1.49) |
| Diagnosis | | | | |
|
Septic
shock | 109.00 (61.93) | 1.00 | 1.00 | 1.00 |
|
Sepsis | 67.00 (38.07) | 0.26
(0.16-0.43) | 0.27
(0.16-0.45) | 0.31
(0.19-0.49) |
| Bacteraemia | | | | |
|
No | 110.00 (62.50) | 1.00 | 1.00 | 1.00 |
|
Yes | 66.00 (37.50) | 0.88
(0.57-1.36) | 0.95
(0.61-1.47) | 0.92
(0.61-1.39) |
| Chemotherapy | | | | |
|
No | 29.00 (16.48) | 1.00 | 1.00 | 1.00 |
|
Yes | 147.00 (83.52) | 0.52
(0.32-0.85) | 0.51
(0.31-0.85) | 0.56
(0.35-0.91) |
| Radiation
therapy | | | | |
|
No | 114.00 (64.77) | 1.00 | 1.00 | 1.00 |
|
Yes | 62.00 (35.23) | 0.92
(0.60-1.42) | 0.85
(0.54-1.34) | 1.01
(0.67-1.53) |
| Surgery | | | | |
|
No | 98.00 (55.68) | 1.00 | 1.00 | 1.00 |
|
Yes | 78.00 (44.32) | 0.97
(0.65-1.46) | 1.02
(0.67-1.56) | 1.07
(0.72-1.58) |
| Time to
antibiotics, h | | | | |
|
>1 | 106.00 (60.57) | 1.00 | 1.00 | 1.00 |
|
0-1 | 69.00 (39.43) | 0.71
(0.46-1.09) | 0.67
(0.43-1.05) | 0.70
(0.47-1.07) |
| Steroids | | | | |
|
No | 119.00 (67.61) | 1.00 | 1.00 | 1.00 |
|
Yes | 57.00 (32.39) | 1.19
(0.78-1.83) | 1.21
(0.78-1.88) | 1.25
(0.83-1.88) |
Association between site of infection
and mortality
The urinary tract infection with the lowest
mortality rate (31.91%) was the reference group (Table III). After adjusting for
confounding variables, gastrointestinal infection was associated
with highest in-hospital mortality [hazard ratio (HR), 2.64; 95%
CI, 1.25-5.55), followed by pneumonia (HR, 1.95; 95% CI,
1.03-3.68). The confidence intervals are wide, reflecting the
limited number of patients. The association between site of
infection and 28-day and 60-day mortality was analysed by Cox
regression. Gastrointestinal infection and pulmonary infection had
a higher mortality rate.
 | Table IIICox regression model for
mortality. |
Table III
Cox regression model for
mortality.
| Variable | Not adjusted HR
(95% CI) | P-value | Model
Ia HR (95% CI) | P-value | Model
IIb HR (95% CI) | P-value |
|---|
| In-hospital
mortality | | | | | | |
|
Urinary
tract | 1.00 | | 1.00 | | 1.00 | |
|
Unknown | 1.93
(0.93-3.99) | 0.078 | 1.88
(0.91-3.91) | 0.089 | 1.91
(0.88-4.16) | 0.103 |
|
Gastrointestinal | 3.45
(1.74-6.68) | <0.001 | 2.78
(1.38-5.60) | 0.004 | 2.64
(1.25-5.55) | 0.011 |
|
Lung | 2.19
(1.21-3.96) | 0.009 | 1.91
(1.06-3.46) | 0.032 | 1.95
(1.03-3.68) | 0.039 |
|
Other | 1.30
(0.53-3.19) | 0.566 | 1.35
(0.55-3.31) | 0.519 | 1.31
(0.51-3.37) | 0.577 |
| 28 day
mortality | | | | | | |
|
Urinary
tract | 1.00 | | 1.00 | | 1.00 | |
|
Unknown
site | 1.59
(0.77-3.31) | 0.212 | 1.53
(0.74-3.19) | 0.255 | 1.43
(0.65-3.12) | 0.376 |
|
Gastrointestinal | 2.87
(1.45-5.68) | 0.003 | 2.29
(1.14-4.60) | 0.020 | 2.10
(1.00-4.44) | 0.051 |
|
Lung | 1.75
(0.97-3.16) | 0.062 | 1.52
(0.84-2.74) | 0.165 | 1.55
(0.82-2.94) | 0.175 |
|
Other | 1.02
(0.40-2.60) | 0.971 | 1.02
(0.40-2.63) | 0.962 | 0.92
(0.34-2.46) | 0.870 |
| 60 day
mortality | | | | | | |
|
Urinary
tract | 1.00 | | 1.00 | | 1.00 | |
|
Unknown
site | 1.52
(0.76-3.03) | 0.237 | 1.49
(0.75-2.98) | 0.256 | 1.47
(0.70-3.09) | 0.307 |
|
Gastrointestinal | 2.74
(1.43-5.23) | 0.002 | 2.26
(1.17-4.38) | 0.016 | 2.22
(1.10-4.48) | 0.026 |
|
Lung | 1.78
(1.03-3.05) | 0.038 | 1.56
(0.91-2.69) | 0.108 | 1.58
(0.88-2.84) | 0.124 |
|
Other | 1.17
(0.51-2.68) | 0.708 | 1.20
(0.52-2.76) | 0.664 | 1.09
(0.45-2.62) | 0.847 |
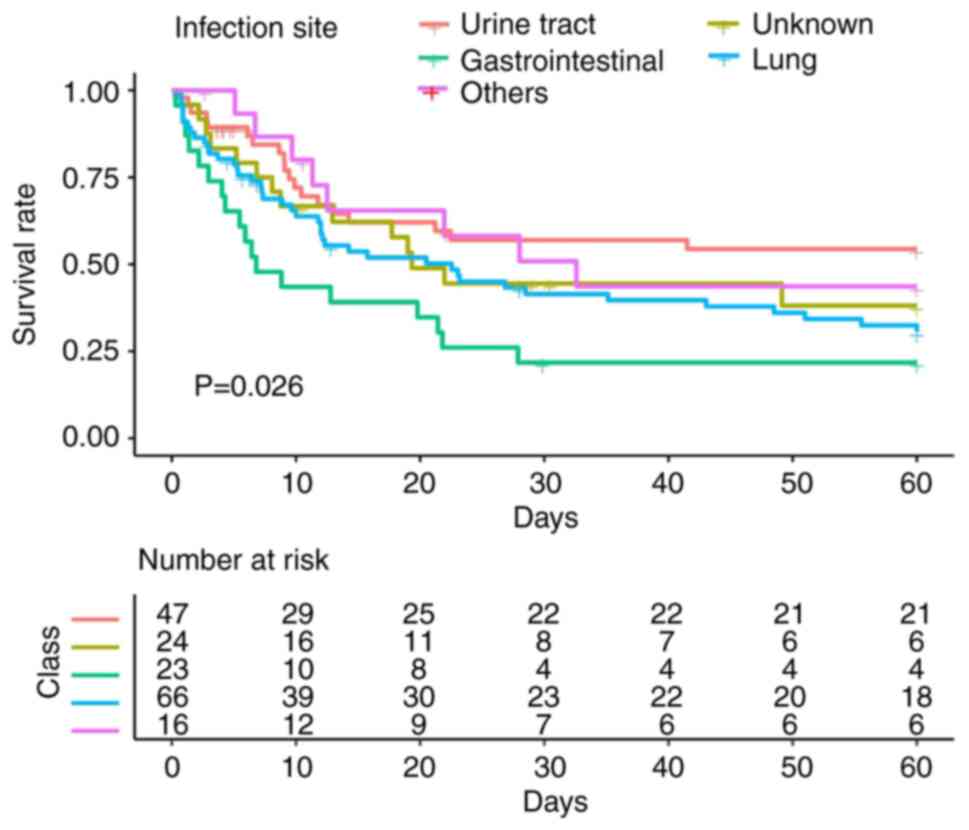
Kaplan-Meier survival curve for each infection site
with 60 day follow-up showed a consistent pattern (Fig. 1) with the highest cumulative
mortality from gastrointestinal infection and pneumonia. Patients
with urinary tract and other site infections had the lowest
cumulative mortality.
To evaluate the relationship between the site of
infection and the mortality of patients with solid or
haematological tumours, a stratified analysis was performed. Among
solid and haematological tumours, gastrointestinal infections were
associated with the highest in-hospital mortality after adjusting
for confounding variables. They were [HR, 2.36; 95% CI, 1.05-5.31],
[HR, 179.91; 95% CI, 5.69-5693.31), respectively (Tables IV and V). The confidence intervals are wide,
reflecting the limited number of patients. Through stratified
analysis to investigate the association between site of infection
and mortality from haematological and solid tumours. We found that
gastrointestinal infection had a higher mortality rate.
 | Table IVCox regression models for mortality
in 141 patients with solid tumours. |
Table IV
Cox regression models for mortality
in 141 patients with solid tumours.
| Variable | Not adjusted HR
(95% CI) | P-value | Model
Ia HR (95% CI) | P-value | Model
IIb HR (95%
CI) | P-value |
|---|
| In-hospital
mortality | | | | | | |
|
Urinary
tract | 1.0 | | 1.0 | | 1.0 | |
|
Unknown | 2.21
(0.99-4.95) | 0.053 | 2.25
(1.00-5.05) | 0.049 | 2.33
(0.97-5.63) | 0.060 |
|
Gastrointestinal | 2.93
(1.39-6.16) | 0.005 | 2.22
(1.04-4.75) | 0.039 | 2.36
(1.05-5.31) | 0.039 |
|
Lung | 2.32
(1.22-4.42) | 0.010 | 2.07
(1.08-3.95) | 0.028 | 2.19
(1.10-4.35) | 0.026 |
|
Other | 1.05
(0.37-2.94) | 0.929 | 1.14
(0.40-3.22) | 0.807 | 1.14
(0.37-3.50) | 0.816 |
| 28 day
mortality | | | | | | |
|
Urinary
tract | 1.0 | | 1.0 | | 1.0 | |
|
Unknown | 1.75
(0.78-3.93) | 0.179 | 1.74
(0.77-3.93) | 0.181 | 1.65
(0.68-4.01) | 0.269 |
|
Gastrointestinal | 2.37
(1.13-4.97) | 0.023 | 1.81
(0.85-3.85) | 0.124 | 1.70
(0.75-3.85) | 0.201 |
|
Lung | 1.76
(0.93-3.34) | 0.085 | 1.56
(0.82-2.99) | 0.176 | 1.65
(0.83-3.31) | 0.156 |
|
Other | 0.76
(0.25-2.31) | 0.626 | 0.80
(0.26-2.44) | 0.689 | 0.69
(0.21-2.27) | 0.539 |
| 60 day
mortality | | | | | | |
|
Urinary
tract | 1.0 | | 1.0 | | 1.0 | |
|
Unknown | 1.69
(0.79-3.62) | 0.175 | 1.72
(0.80-3.69) | 0.161 | 1.71
(0.74-3.95) | 0.207 |
|
Gastrointestinal | 2.24
(1.12-4.49) | 0.023 | 1.77
(0.87-3.60) | 0.115 | 1.95
(0.92-4.17) | 0.084 |
|
Lung | 1.83
(1.02-3.28) | 0.042 | 1.64
(0.91-2.95) | 0.099 | 1.72
(0.92-3.21) | 0.092 |
|
Other | 0.96
(0.38-2.43) | 0.925 | 1.02
(0.40-2.61) | 0.966 | 0.95
(0.34-2.63) | 0.920 |
 | Table VCox regression models for mortality
in 35 patients with haematological tumours. |
Table V
Cox regression models for mortality
in 35 patients with haematological tumours.
| Variable | Not adjusted HR
(95% CI) | P-value | Model
Ia HR (95% CI) | P-value | Model
IIb HR (95%
CI) | P-value |
|---|
| In-hospital
mortality | | | | | | |
|
Urinary
tract | 1.0 | | 1.0 | | 1.0 | |
|
Unknown
site | 1.45
(0.24-8.71) | 0.683 | 1.68
(0.28-10.20) | 0.571 | 76.67
(1.84-3202.38) | 0.022 |
|
Gastrointestinal | 13.99
(2.04-95.89) | 0.007 | 30.51
(3.71-251.17) | 0.002 | 179.91
(5.69-5693.31) | 0.003 |
|
Lung | 2.05
(0.43-9.67) | 0.366 | 2.59
(0.54-12.43) | 0.234 | 16.96
(0.86-334.76) | 0.062 |
|
Other | 3.16
(0.44-22.66) | 0.253 | 4.95
(0.66-37.40) | 0.121 | 66.81
(1.10-4062.06) | 0.045 |
| 28 day
mortality | | | | | | |
|
Urinary
tract | 1.0 | | 1.0 | | 1.0 | |
|
Unknown
site | 1.45
(0.24-8.71) | 0.683 | 1.68
(0.28-10.20) | 0.571 | 76.67
(1.84-3202.38) | 0.023 |
|
Gastrointestinal | 13.99
(2.04-95.89) | 0.007 | 30.51
(3.71-251.17) | 0.002 | 179.91
(5.69-5683.31) | 0.003 |
|
Lung | 2.05
(0.43-9.67) | 0.366 | 2.59
(0.54-12.43) | 0.234 | 16.96
(0.86-334.76) | 0.063 |
|
Other | 3.16
(0.44-22.66) | 0.253 | 4.95
(0.66-37.40) | 0.121 | 66.81
(1.10-4062.06) | 0.045 |
| 60 day
mortality | | | | | | |
|
Urinary
tract | 1.0 | | 1.0 | | 1.0 | |
|
Unknown
site | 1.45
(0.24-8.71) | 0.683 | 1.68
(0.28-10.20) | 0.571 | 76.67
(1.84-3202.38) | 0.023 |
|
Gastrointestinal | 13.99
(2.04-95.89) | 0.007 | 30.51
(3.71-251.17) | 0.002 | 179.91
(5.69-5693.31) | 0.003 |
|
Lung | 2.05
(0.43-9.67) | 0.366 | 2.59
(0.54-12.43) | 0.234 | 16.96
(0.86-334.76) | 0.063 |
|
Other | 3.16
(0.44-22.66) | 0.253 | 4.95
(0.66-37.40) | 0.121 | 66.81
(1.10-4062.06) | 0.045 |
Discussion
Although infection is the most common cause of
in-hospital mortality in patients with cancer (11), to the best of our knowledge, there
are no previous studies examining the influence of infection sites
on patients with cancer.
In the present single cohort study of patients with
cancer admitted to the ED due to sepsis or septic shock, it was
found that the site of infection was associated with in-hospital
mortality when the urinary tract infection group was used as a
reference group. The present data showed that the lung, urinary
system, gastrointestinal tract, and unknown site are common sites
of infection, which is consistent with previous studies (3,12).
The highest mortality was associated with
gastrointestinal infection. Chemotherapy and radiation therapy have
been widely used in patients with cancer in recent years, as well
as repeated antibiotics to prevent infections (13). The treatment of cancer and
antibiotics may promote intestinal mucosal destruction, placing
patients at a significantly higher risk of developing refractory
enteritides, such as clostridium difficile-associated colitis and
neutropenic enterocolitis (14).
The treatment of gastrointestinal infection is becoming clinically
challenging due to long-term chemotherapy and depressed immune
systems (15). Despite
improvements in management of pneumonia, this remains a common
cause of morbidity and mortality in patients with cancer (16).
Unknown site infections may lead to poor outcomes
due to the inability to obtain culture samples of unknown site
infections in a short time and the inability to select appropriate
treatment regimens based on pathogenetic and drug distribution
characteristics. Unknown site infection is associated with fatal
outcomes in patients without cancer (8). However, infection of unknown site was
not considered statistically significant in the present study after
adjusting for comorbidities and severity markers. Given the small
sample size of the present study, the association between infection
of the unknown site and in-hospital mortality needs to be confirmed
in other studies.
The association between infection site and mortality
may differ due to differences in underlying immune mechanisms
between detailed cancer types and cancer sites (2). It has been reported that the risk of
death associated with sepsis varies by tumour location and
treatment (17). Through
stratified analysis, the present study found that the infection
site had a consistent effect on the mortality of patients with
solid and haematological tumours. However, due to the limitation of
the sample size, it is not possible to analyse the effect of the
infection site on the mortality of solid tumours such as lung
cancer or other types of tumour. Therefore, the association between
infection sites and mortality in different types of cancer needs
further study.
The present study has certain limitations. First,
the site of infection may be misclassified because there may be not
enough time in a busy ED to estimate the exact site of infection.
Second, this was a single-centre retrospective study to evaluate
the association between the site of infection and mortality in
patients with solid or haematological tumours admitted to the ED
with sepsis or septic shock. Thus, the findings may not be
generalizable to the general population. Third, the patient stage
of disease may be a factor in mortality. However, the present study
was a secondary analysis and cannot further assess the stage of
patients with cancer. Therefore, in-hospital mortality may be
overestimated because the population analysed in the current study
included patients diagnosed with terminal cancer (9).
In conclusion, the site of infection was associated
with in-hospital mortality in patients with cancer diagnosed with
sepsis or septic shock.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analysed during the
current study are available in the Dryad Digital Repository
database (doi.org/10.5061/dryad.6qk05).
Authors' contributions
YC, JH, JX, RQ and TL made substantial contributions
to conception and design, acquisition of data and analysis and
interpretation of data. YC and TL were involved in drafting the
manuscript and revising it critically for important intellectual
content. TL gave final approval of the version to be published. All
authors have participated sufficiently in the work to take
responsibility for appropriate portions of the content. YC and TL
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. YC was
responsible for the drafting of the manuscript. YC and JH confirm
the authenticity of all the raw data. YC, JH, JX and RQ conceived
and designed the study. YC and TL performed the literature
research. YC, JH and RQ performed the data analysis. YC and TL
edited the manuscript. TL reviewed the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The requirement for ethics approval and informed
consent was waived as the data were anonymized.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
López R, Pérez-Araos R, Baus F, Moscoso C,
Salazar Á, Graf J, Montes JM and Samtani S: Outcomes of sepsis and
septic shock in cancer patients: Focus on lactate. Front Med
(Lausanne). 8(603275)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chebl RB, Safa R, Sabra M, Chami A,
Berbari I, Jamali S, Makki M, Tamim H and Dagher GA: Sepsis in
patients with haematological versus solid cancer: A retrospective
cohort study. BMJ Open. 11(e038349)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hensley MK, Donnelly JP, Carlton EF and
Prescott HC: Epidemiology and outcomes of cancer-related versus
non-cancer-related sepsis hospitalizations. Crit Care Med.
47:1310–1316. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990-2017: Analysis for the global burden of disease
study. Lancet. 395:200–211. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tripathi H, Mukhopadhyay S and Mohapatra
SK: Sepsis-associated pathways segregate cancer groups. BMC Cancer.
20(309)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang YG, Zhou JC and Wu KS: High 28-day
mortality in critically ill patients with sepsis and concomitant
active cancer. J Int Med Res. 46:5030–5039. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abe T, Tokuda Y, Shiraishi A, Fujishima S,
Mayumi T, Sugiyama T, Deshpande GA, Shiino Y, Hifumi T, Otomo Y, et
al: In-hospital mortality associated with the misdiagnosis or
unidentified site of infection at admission. Crit Care.
23(202)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Caraballo C, Ascuntar J, Hincapié C,
Restrepo C, Bernal E and Jaimes F: Association between site of
infection and in-hospital mortality in patients with sepsis
admitted to emergency departments of tertiary hospitals in
Medellin, Colombia. Rev Bras Ter Intensiva. 31:47–56.
2019.PubMed/NCBI View Article : Google Scholar : (In Spanish,
English).
|
|
9
|
Dagher GA, El Khuri C, Chehadeh AA, Chami
A, Bachir R and Zebian D: Are patients with cancer with sepsis and
bacteraemia at a higher risk of mortality? A retrospective chart
review of patients presenting to a tertiary care centre in Lebanon.
BMJ Open. 7(e013502)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nazer L, Lopez-Olivo MA, Cuenca JA, Awad
W, Brown AR, Abusara A, Sirimaturos M, Hicklen RS and Nates JL:
All-cause mortality in cancer patients treated for sepsis in
intensive care units: A systematic review and meta-analysis.
Support Care Cancer. 2022:1–11. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bhat S, Muthunatarajan S, Mulki SS, Bhat
KA and Kotian KH: Bacterial infection among cancer patients:
Analysis of isolates and antibiotic sensitivity pattern. Int J
Microbiol. 2021(8883700)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jordan KR, Loman BR, Bailey MT and Pyter
LM: Gut microbiota-immune-brain interactions in
chemotherapy-associated behavioral comorbidities. Cancer.
124:3990–3999. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Itani M, Menias CO, Mellnick VM, El Zakhem
A, Elsayes K, Katabathina V and Revzin MV: Imaging of abdominal and
pelvic infections in the cancer patient. Abdom Radiol (NY).
46:2920–2941. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Su Z, Lu L, Chen F, Chen J and Chen X: Gut
microbiota and sunitinib-induced diarrhea in metastatic renal cell
carcinoma: A pilot study. Cancer Manag Res. 13:8663–8672.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Anderson EJ: Respiratory infections.
Cancer Treat Res. 161:203–236. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shvetsov YB, Ogino MH, Glibetic N, Asato
CB, Wilkens LR, Le Marchand L and Matter ML: Association of sepsis
mortality with specific cancer sites and treatment type: The
multiethnic cohort study. J Pers Med. 11(146)2021.PubMed/NCBI View Article : Google Scholar
|