Introduction
X-linked hypophosphatemic rickets (XLH) is the most
common hereditary hypophosphatemic disorder. Patients with XLH
suffer from short stature, deformed lower extremities, dental
abnormalities, bone and joint pain, tinnitus, and hearing loss
(1). These manifestations result
primarily from hypophosphatemia caused by renal phosphate wastage.
At the molecular level, XLH is caused by loss-of-function mutations
in the phosphate-regulating endopeptidase homolog of the
X-chromosome (Phex) gene (2). Mutation-driven downregulation of
Phex promotes the expression of fibroblast growth factor 23
(FGF23) (3-5).
FGF23 reduces renal tubule phosphate reabsorption, leading to low
serum inorganic phosphorus. FGF23 also alters the production and
degradation of 1,25-dihydroxyvitamin D by activating intracellular
signaling through binding of fibroblast growth factor receptor 1
(FGFR1) to α-klotho (6-8).
In contrast, excessive FGF23 in a mouse XLH model (Hyp mice) was
indicated to activate ERK phosphorylation in the FGFR3 signaling
pathway in an α-klotho-independent manner (9,10).
Moreover, Kawai et al (11)
demonstrated that activated FGF23 attenuated endochondral
ossification by enhancing FGFR3 signaling.
Activating mutations in the FGFR3 gene cause
achondroplasia, which is the most common form of short-limbed
skeletal dysplasia. We previously described how meclozine, an
antihistamine medicine for motion sickness, attenuated ERK
phosphorylation in chondrocytes with overactive ERK signaling
(12). We subsequently
demonstrated that oral administration of meclozine increased
longitudinal bone growth and suppressed the short-statured
phenotype in a mouse model of achondroplasia in a dose-dependent
manner (13). Thus, the inhibitory
effect of meclozine on FGFR3 was confirmed both in vivo and
in vitro. Komla-Ebri et al (14) developed NVP-BGJ398, an FGFR
tyrosine kinase inhibitor, which improved the skeletal phenotype in
a mouse model of achondroplasia; whereas Wohrle et al
(15) used NVP-BGJ398 to suppress
the hypophosphatemic phenotype of Hyp mice. Hence, inhibition of
FGFR3 signaling could present an alternative treatment for patients
with XLH. Herein, we conducted an in vivo study to examine
the effect of meclozine on bone mineralization associated with
hypophosphatemia in Hyp mice.
Materials and methods
Mice
Hyp mice were obtained from the Jackson Laboratory.
Hyp mice have a large deletion in the 3' untranslated region of the
Phex gene, which results in a loss-of-function mutation.
Female Hyp mice were bred with male wild-type (WT) C57BL/6J mice to
obtain male Hyp mice and their non-Hyp littermates. All mice were
housed in a facility with a 12/12 h light/dark cycle, stable
humidity and temperature, and were fed standard mouse chow
(Oriental Yeast Co.) ad libitum. The animals were genotyped
at 4-6 days by mixing finger tissue with the Viagen DirectPCR Lysis
Reagent (Viagen Biotech, Inc.) and proteinase K solution, followed
by incubation at 55˚C with agitation at 1,000 rpm for 3 h, and 85˚C
without agitation for 45 min (Thermo Mixer C, Eppendorf). The DNA
in the supernatant was collected after centrifugation at 10,000 rpm
for 15 min (Model 5424; Eppendorf). The forward
(5'-CCAAAATTGTTCTTCAGTACACC-3') and reverse
(5'-ATCTGGCAGCACACTGGTATG-3') primers were used for PCR
amplification (16), and the
resulting products were analyzed on a 1% agarose gel containing
ethidium bromide. The presence or absence of a 258-bp band
indicated WT or mutant Phex alleles, respectively. Given
that all parameters were compared within a kinship of male Hyp
mice, but not between kinships, meclozine-administration was
performed only when multiple male Hyp mice were born within
kinship.
Meclozine administration
Hyp mice were divided into meclozine-treated and
untreated (vehicle only) groups; whereas WT mice were treated with
vehicle only. The dose-finding study using a mouse model indicated
that meclozine at 1 and 2 mg/kg/day attenuated FGFR3 signaling in a
dose-dependent manner (13).
Therefore, meclozine was administered at 2 mg/kg/day twice per day
by dissolving it in 0.5% methylcellulose. Body weight was measured
daily before oral administration, which was started at 7 days of
age and continued for 10 consecutive days as described previously
(13).
Serum parameters
After the 10-day-treatment period, mice were
subjected to whole blood collection from the abdominal aorta using
a 26-gauge needle and 1-ml syringe under general anesthesia with
isoflurane. Mice were initially exposed with 3.5 to 4.0% of
isoflurane to fall asleep, then the blood correction was performed
with keeping exposure with 2 to 3% of isoflurane. Mice were
euthanized caused by this exsanguination procedure. Collected blood
samples were left at room temperature for more than 3 h to allow
clotting, and were thereafter centrifuged and the obtained serum
samples were preserved at -20˚C. Serum calcium and phosphate levels
were measured using a DRI-CHEM 7000V biochemical analyzer
(Fujifilm). Serum FGF23 levels were measured using the FGF-23 ELISA
Kit (RRID: AB_2782966; Kainos Laboratories). Serum samples were
excluded from the analysis when the amount of blood drawn was
insufficient for measurement, hemolysis was obvious, the blood
vessel was damaged, or the body fluid in the abdominal cavity was
contaminated.
Histological analysis
Following treatment, mice were subjected to
histological analysis of the first coccygeal vertebra, femur, and
tibia. These samples were collected immediately after euthanasia
and fixed with 4% paraformaldehyde at 4˚C. The samples of the first
coccygeal vertebra were cut in the sagittal plane, while those of
the femur and tibia were cut in the coronal plane; thin sections
were stained with Villanueva Goldner (Kureha Special Laboratory).
The medial mid-shaft cortical bone of the femur, the central part
of the distal metaphyseal cancellous bone (primary spongiosa) of
the femur, and the proximal central part of the first coccygeal
vertebra were identified in low-magnification images. Osteoid
volume (OV) and bone volume (BV) were measured using Image J
software, and the OV/BV served as an index of bone mineralization
(17-19).
The osteoclast number (N.Oc), osteoclast surface (Oc.S), and
osteoclast surface/bone surface (Oc.S/BS) were measured at the
central part of the distal metaphyseal cancellous bone of the femur
with tartrate-resistant acid phosphatase (TRAP) staining to assess
osteoclast activity (19). The
average growth plate thickness, proliferative zone (Pz) thickness,
and hypertrophic zone (Hz) thickness (20) were calculated by measuring the
center, medial quarter, and lateral quarter of the growth plate at
the distal femur stained with hematoxylin and eosin. The percentage
proliferative zone thickness (%Pz) and percentage hypertrophic zone
thickness (%Hz) were calculated relative to the thickness of the
entire growth plate.
Statistical analysis
Histological and serum parameters were normalized to
those of untreated littermate Hyp mice to adjust for environmental
differences such as litter skeletal size among kinship. All data
are expressed as the mean ± SD. Outliers were removed from analysis
using the interquartile range method (21). When equality of variance was
confirmed by Levene's test, statistical differences were determined
by one-way ANOVA with post-hoc Tukey's honest significance test.
When equality of variance was not established by Levene's test,
one-way ANOVA with Welch's correction was performed, and the
Games-Howell method was applied for post-hoc testing. All analyses
were conducted using IBM SPSS Statistics version 27 (IBM).
Statistical significance was set at P<0.05.
Results
Meclozine significantly improves
cortical bone mineralization in Hyp mice
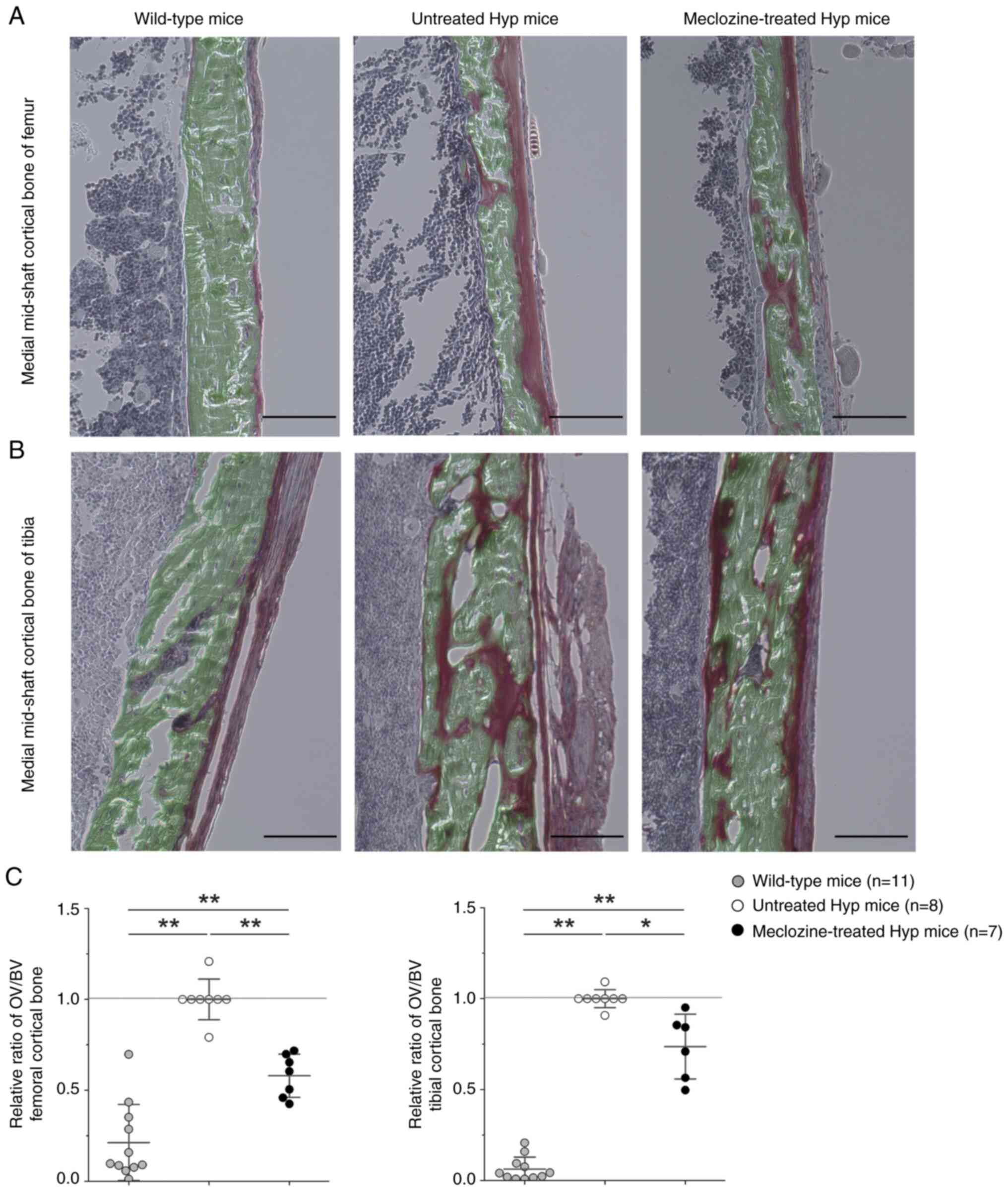
The OV of the femoral and tibial cortical bones,
which was higher in untreated Hyp mice than WT mice, decreased
after treatment with 2 mg/kg/day of meclozine (Fig. 1A and B). Specifically, the OV/BV was 42% lower
in the femur (P<0.01) and 26% lower in the tibia (P<0.01) of
meclozine-treated Hyp mice compared to untreated Hyp mice (Fig. 1C).
Meclozine treatment significantly
improves growth plate parameters in Hyp mice
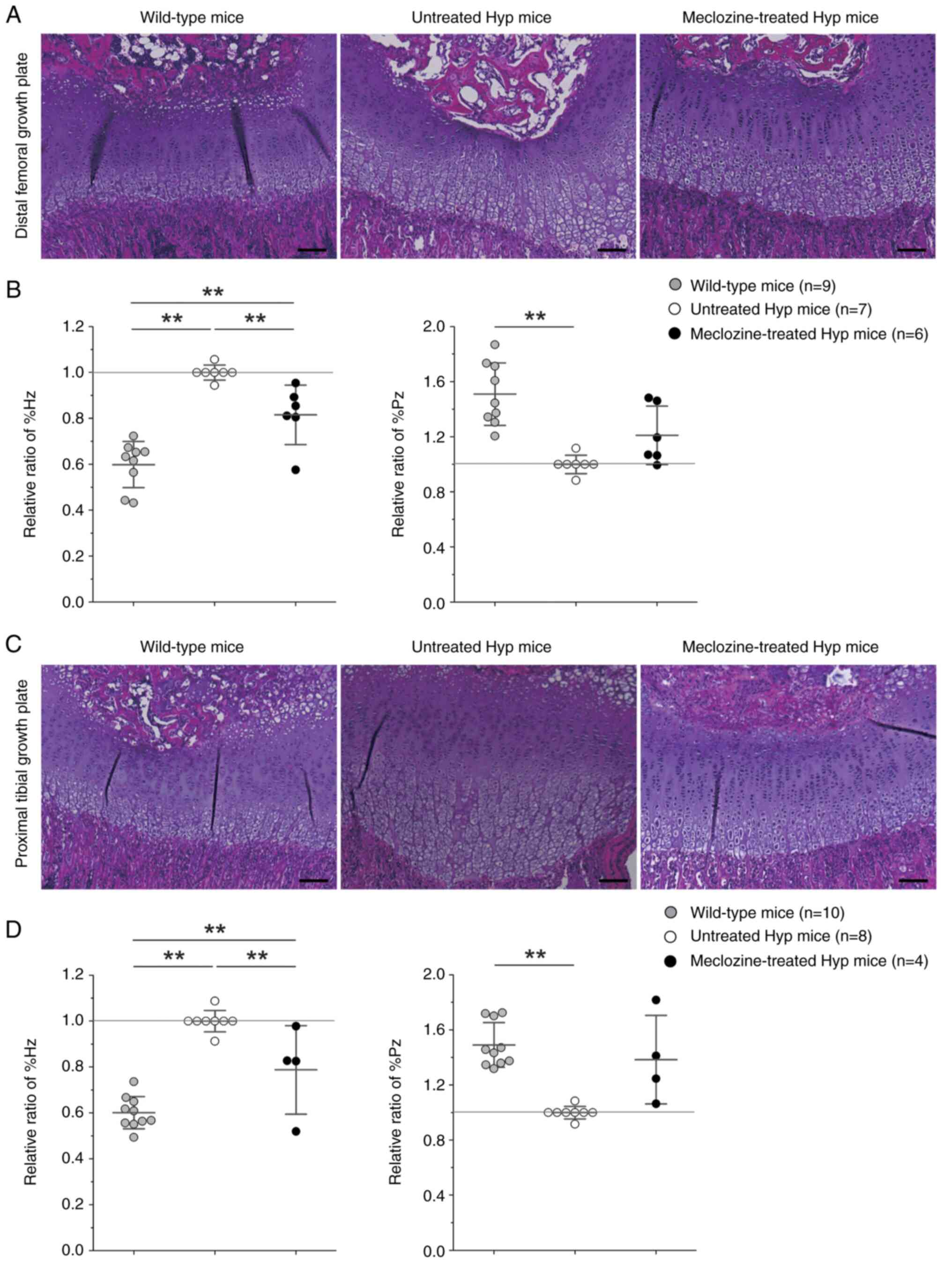
The Hz of the growth plate in the distal femur and
proximal tibia was markedly thickened and presented with an
increased cell size in untreated Hyp mice (Fig. 2A and C). Meclozine treatment decreased the %Hz
in the femur by 18.5% (P<0.01) and in the tibia by 21.2%
(P<0.01) compared to untreated Hyp mice (Fig. 2B and D). Instead, the %Pz of Hyp mice tended to
increase following meclozine treatment.
Osteoclast activity in Hyp mice tends
to be enhanced by meclozine treatment
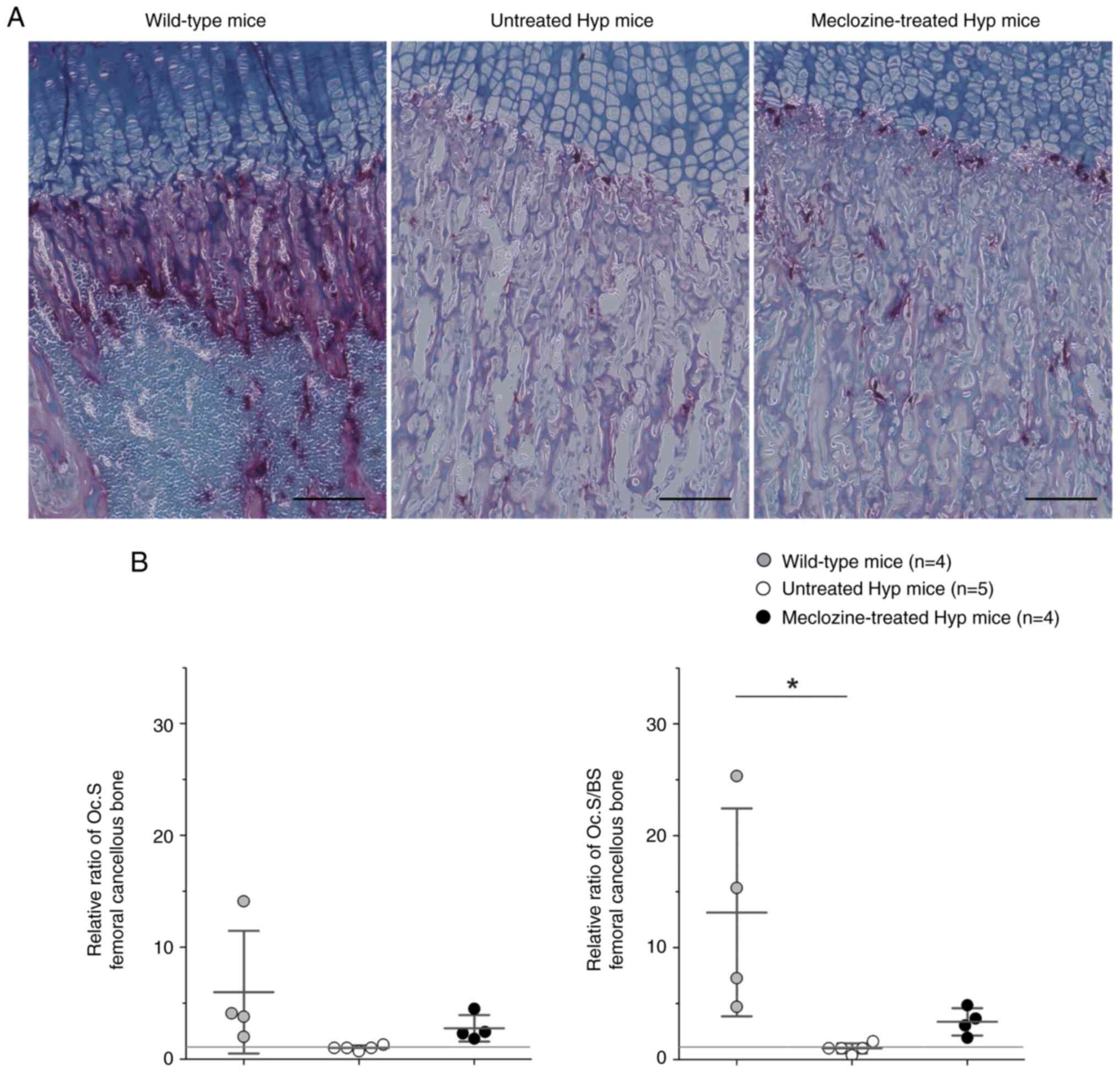
Fig. 3 displays the
histological appearance of the distal femoral growth plate after
TRAP staining. TRAP-positive cells were abundant among hypertrophic
and calcified chondrocytes in WT mice, while they were remarkably
reduced in untreated Hyp mice (Figs.
3A and S1). The Oc.S and
Oc.S/BS were 2.8-fold and 3.4-fold larger in meclozine-treated Hyp
mice than in untreated Hyp mice, respectively, although the
difference was not statistically significant (Fig. 3B).
Meclozine has a marginal effect on
metaphyseal bone mineralization in Hyp mice
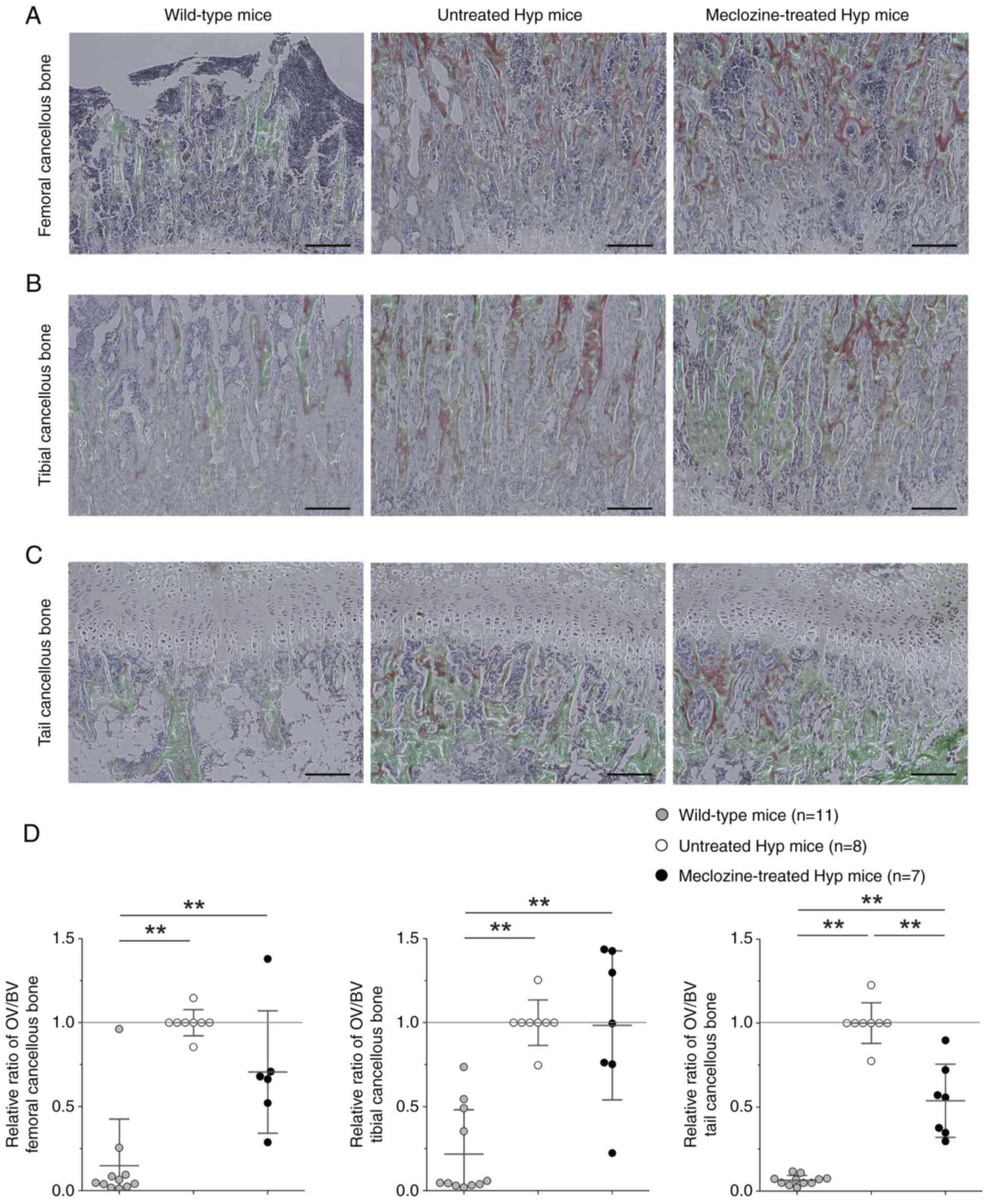
In the trabecular bone, untreated Hyp mice presented
larger OV and smaller BV than WT mice in the distal femur (Fig. 4A), proximal tibia (Fig. 4B), and first coccygeal vertebra
(Fig. 4C). The OV/BV was reduced
by 46% (P<0.01) in the coccygeal vertebra of meclozine-treated
Hyp mice compared to untreated Hyp mice; whereas no statistical
difference was detected in the distal femur and proximal tibia
(Fig. 4D).
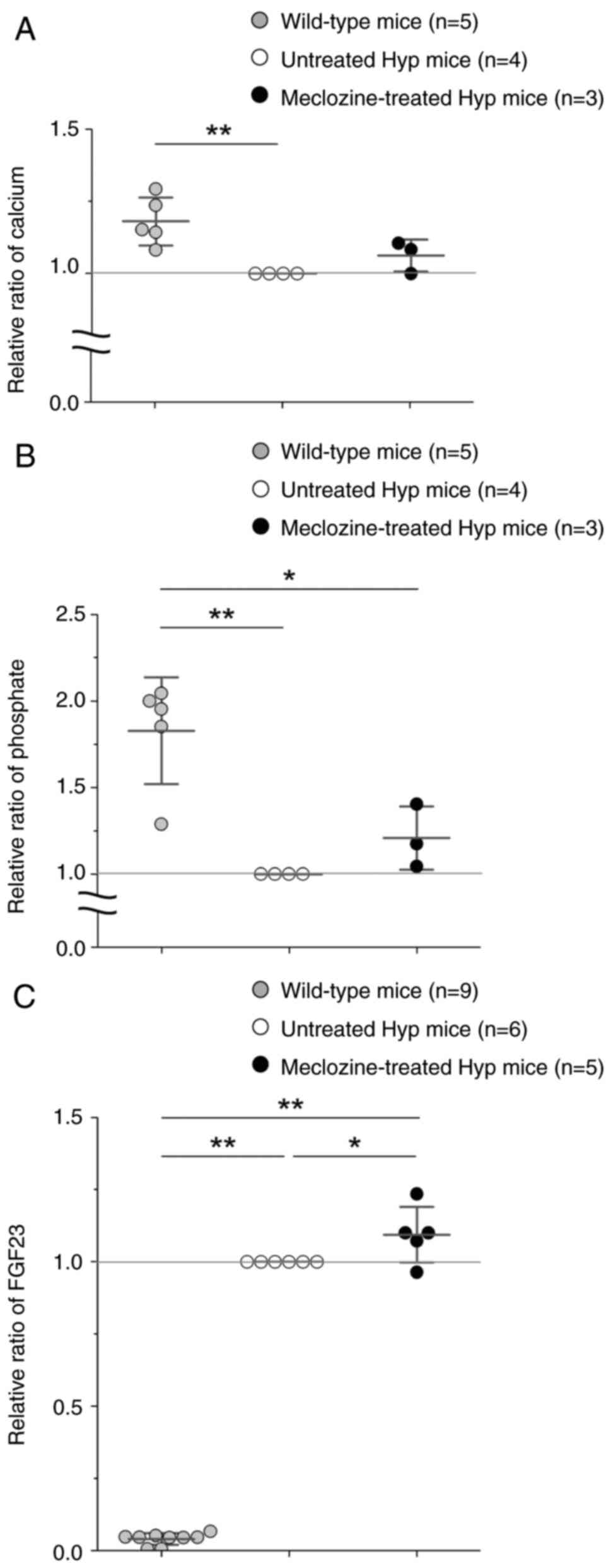
Meclozine slightly ameliorates serum
calcium and phosphate levels, while boosting FGF23 secretion in Hyp
mice
Serum calcium and phosphate levels were
significantly higher in WT mice than in untreated Hyp mice
(Fig. 5A and B). The 10-day-treatment with meclozine
slightly increased serum calcium and phosphate levels, but had an
even greater effect on serum FGF23 levels in Hyp mice (Fig. 5C).
Discussion
Burosumab, a recombinant human monoclonal antibody
that targets FGF23, remains very expensive to administer to all
patients with XLH. Additionally, some children fear the burosumab
administration via injection, while oral administration of sodium
phosphate does not always achieve the correct dosage due to
increased renal phosphate wasting (22). We previously demonstrated that
meclozine suppressed the short-statured phenotype of achondroplasia
mice by attenuating FGFR3 signaling (12,13,23),
and clinical trials of meclozine in children with achondroplasia
are under way (24). To the best
of our knowledge, current study demonstrated the first evidence of
the effect of meclozine on improving bone mineralization in a mouse
model of XLH. Oral administration of meclozine, which can be a
cost-reduced treatment, would be reasonable considering long-term
administration to patients with XLH from children to adults. The
present study provides evidence that meclozine promotes bone
mineralization, improves growth plate structure, and marginally
increases serum calcium and phosphorus levels in Hyp mice. The
limited effect of meclozine on calcium and phosphorus may be
explained by the partial contribution of FGFR3 hyperactivation to
the etiology of hypophosphatemic rickets.
Histological analyses demonstrated that meclozine
decreased OV in both cortical and cancellous bones, and realigned
the growth plate structure of Hyp mice. Similar findings have
previously been reported after treatment of Hyp mice with other
FGFR3 inhibitors, such as NVP-BGJ398 and MAPK inhibitors (15,25).
Downregulation of indian hedgehog, which is in the downstream of
FGFR3, has also been shown involved in FGF23/α-klotho signaling
(11). These results indicate that
hyperactivation of FGFR3 signaling could be related to abnormal
bone metabolism and disturbed growth plate structure including Pz
and Hz through the MAPK pathway in hypophosphatemic rickets. The
role of osteoclasts in the pathogenic skeletogenesis of Hyp mice is
unclear. The number of osteoclasts was reported to be lower in
4-week-old Hyp mice than in WT mice (26). Administration of a MAPK inhibitor
during lactation was demonstrated to inhibit osteoclastogenesis in
Hyp mice (25). We confirmed that
osteoclast activity was reduced in 17-day-old Hyp mice but could be
slightly rescued in the distal femoral growth plate upon meclozine
administration. Endochondral ossification requires precise
orchestration of vascularization in hypertrophic chondrocytes,
extracellular matrix remodeling, and recruitment of osteoclasts and
osteoblasts. Osteoclast deficiency causes impaired growth plate
ossification, resulting in the accumulation of hypertrophic
chondrocytes and inhibition of vascularization. Increased
osteoclast activity at the growth plate could be related to the
reduced width of hypertrophic chondrocytes and accelerated
calcification after treatment of Hyp mice with meclozine.
One-week treatment with a MAPK inhibitor was
depicted to reduce serum FGF23 levels in Hyp mice (25). However, abnormally elevated serum
FGF23 levels in Hyp mice were upregulated by 8-week administration
of NVP-BGJ398 and 4-week administration of a MAPK inhibitor
(15,27). Here, we indicate that repeated
administration of meclozine slightly augmented serum FGF23 levels
in Hyp mice. These conflicting outcomes could result from the
complicated feedback loop controlling FGF23 metabolism. Meclozine,
as well as other FGFR inhibitors, have been presented to elevate
FGF23 and serum phosphorus levels in Hyp mice (15,27).
Downstream signaling of highly activated FGF23 in Hyp mice may be
disturbed by these FGFR inhibitors, leading to the suppression of
renal phosphate excretion and consequent increase in serum
phosphorus levels, although the exact mechanism of phosphate
homeostasis has not been fully elucidated (27). In theory, elevated serum phosphorus
levels stimulate the phosphorus-regulating hormone FGF23.
The current study has several limitations. First, we
administered 2 mg/kg/day of meclozine to 7-day-old Hyp mice for 10
days, thus mimicking the protocol used for achondroplasia mice
(13). The peak drug concentration
of meclozine at 2 mg/kg/day in mice (13) was lower than that of meclozine 25
mg/body in adult humans (28).
Administration of meclozine at 2 mg/kg/day to mice is likely to be
clinically relevant, although different doses of meclozine, age of
Hyp mice, and treatment protocols would likely lead to different
results. Further studies such as a long-term toxicity tests using
several kinds of animals are required to apply meclozine treatment
to the human clinical setting. Second, the detailed biological
molecular mechanism of action of meclozine remains unknown,
although we previously demonstrated that meclozine attenuates ERK
phosphorylation downstream of FGFR3 signaling in chondrocytes
(12). Meclozine was found to
inhibit both ERK and p38 phosphorylation downstream of receptor
activator of nuclear factor-κB ligand signaling in an OVX mouse
model of postmenopausal osteoporosis (29). Moreover, we recently suggested that
meclozine attenuated ERK and p38 pathways in FGF2-treated murine
chondrocytes (30).
In conclusion, repeated administration of meclozine
for 10 days partially ameliorated bone quality and growth plate
structure in Hyp mice, probably due to inhibition of FGFR3
signaling. Thus, this mechanism successfully suppressed
pathological phenotypes in mouse models of both achondroplasia and
hypophosphatemic rickets.
Supplementary Material
N.Oc. Dots indicate the relative N.Oc,
and bars indicate the mean ± SD. Statistical significance was
analyzed by one-way ANOVA with post-hoc Tukey's honest significance
test. *P<0.05. Hyp mice, X-linked hypophosphatemic
mice; N.Oc, number of osteoclasts.
Acknowledgements
The authors would like to thank Mr. Ryusaku Esaki
(Department of Orthopaedic Surgery, Nagoya University Graduate
School of Medicine, Nagoya, Aichi, Japan) for technical assistance
for animal studies and Professor Tamio Ohno (Division of
Experimental Animals, Nagoya University Graduate School of
Medicine, Nagoya, Aichi, Japan) for technical support.
Funding
Funding: This work was supported by the Japan Agency for Medical
Research and Development (grant nos. JP20ek0109414 and
JP22ek0109513), Grants-in-Aid For Scientific Research from the
Japan Society for the Promotion of Science (grant no. JP19K09646)
and Takeda Science Foundation (grant no. 2018041703).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YK, MM and HK designed the study. YK and KM acquired
the data. YK, BO, TM, SI and KO analyzed and interpreted the data.
YK and MM drafted the paper, while TM, SI, KO and HK revised it
critically. YK and MM confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal care and experiments conformed to the
institutional guidelines of Nagoya University, and all experimental
protocols were approved by the Institutional Animal Care and Use
Committee of Nagoya University (approval no. M210231; Nagoya,
Aichi, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Skrinar A, Dvorak-Ewell M, Evins A, Macica
C, Linglart A, Imel EA, Theodore-Oklota C and San Martin J: The
lifelong impact of X-Linked hypophosphatemia: Results from a burden
of disease survey. J Endocr Soc. 3:1321–1334. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alizadeh Naderi AS and Reilly RF:
Hereditary disorders of renal phosphate wasting. Nat Rev Nephrol.
6:657–665. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carpenter TO, Shaw NJ, Portale AA, Ward
LM, Abrams SA and Pettifor JM: Rickets. Nat Rev Dis Primers.
3(17101)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Michigami T and Ozono K: Roles of
phosphate in skeleton. Front Endocrinol (Lausanne).
10(180)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Michigami T: Skeletal mineralization:
Mechanisms and diseases. Ann Pediatr Endocrinol Metab. 24:213–219.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Andrukhova O, Zeitz U, Goetz R, Mohammadi
M, Lanske B and Erben RG: FGF23 acts directly on renal proximal
tubules to induce phosphaturia through activation of the
ERK1/2-SGK1 signaling pathway. Bone. 51:621–628. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shimada T, Hasegawa H, Yamazaki Y, Muto T,
Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S and Yamashita
T: FGF-23 is a potent regulator of vitamin D metabolism and
phosphate homeostasis. J Bone Miner Res. 19:429–435.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen G, Liu Y, Goetz R, Fu L, Jayaraman S,
Hu MC, Moe OW, Liang G, Li X and Mohammadi M: α-Klotho is a
non-enzymatic molecular scaffold for FGF23 hormone signalling.
Nature. 553:461–466. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Murali SK, Andrukhova O, Clinkenbeard EL,
White KE and Erben RG: Excessive osteocytic Fgf23 secretion
contributes to pyrophosphate accumulation and mineralization defect
in hyp mice. PLoS Biol. 14(e1002427)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Murali SK, Roschger P, Zeitz U, Klaushofer
K, Andrukhova O and Erben RG: FGF23 regulates bone mineralization
in a 1,25(OH)2 D3 and klotho-independent manner. J Bone Miner Res.
31:129–142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kawai M, Kinoshita S, Kimoto A, Hasegawa
Y, Miyagawa K, Yamazaki M, Ohata Y, Ozono K and Michigami T: FGF23
suppresses chondrocyte proliferation in the presence of soluble
α-Klotho both in vitro and in vivo. J Biol Chem. 288:2414–2427.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Matsushita M, Kitoh H, Ohkawara B, Mishima
K, Kaneko H, Ito M, Masuda A, Ishiguro N and Ohno K: Meclozine
facilitates proliferation and differentiation of chondrocytes by
attenuating abnormally activated FGFR3 signaling in achondroplasia.
PLoS One. 8(e81569)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Matsushita M, Esaki R, Mishima K, Ishiguro
N, Ohno K and Kitoh H: Clinical dosage of meclozine promotes
longitudinal bone growth, bone volume, and trabecular bone quality
in transgenic mice with achondroplasia. Sci Rep.
7(7371)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Komla-Ebri D, Dambroise E, Kramer I,
Benoist-Lasselin C, Kaci N, Le Gall C, Martin L, Busca P, Barbault
F, Graus-Porta D, et al: Tyrosine kinase inhibitor NVP-BGJ398
functionally improves FGFR3-related dwarfism in mouse model. J Clin
Invest. 126:1871–1884. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Wohrle S, Henninger C, Bonny O, Thuery A,
Beluch N, Hynes NE, Guagnano V, Sellers WR, Hofmann F, Kneissel M,
et al: Pharmacological inhibition of fibroblast growth factor (FGF)
receptor signaling ameliorates FGF23-mediated hypophosphatemic
rickets. J Bone Miner Res. 28:899–911. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Miyagawa K, Yamazaki M, Kawai M, Nishino
J, Koshimizu T, Ohata Y, Tachikawa K, Mikuni-Takagaki Y, Kogo M,
Ozono K and Michigami T: Dysregulated gene expression in the
primary osteoblasts and osteocytes isolated from hypophosphatemic
Hyp mice. PLoS One. 9(e93840)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Akagi H, Ochi H, Soeta S, Kanno N,
Yoshihara M, Okazaki K, Yogo T, Harada Y, Amasaki H and Hara Y: A
comparison of the process of remodeling of
hydroxyapatite/Poly-D/L-Lactide and beta-tricalcium phosphate in a
loading site. Biomed Res Int. 2015(730105)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mishima K, Kitoh H, Ohkawara B, Okuno T,
Ito M, Masuda A, Ishiguro N and Ohno K: Lansoprazole upregulates
polyubiquitination of the TNF receptor-associated factor 6 and
facilitates Runx2-mediated osteoblastogenesis. EBioMedicine.
2:2046–2061. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dempster DW, Compston JE, Drezner MK,
Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR
and Parfitt AM: Standardized nomenclature, symbols, and units for
bone histomorphometry: A 2012 update of the report of the ASBMR
Histomorphometry Nomenclature Committee. J Bone Miner Res. 28:2–17.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fuente R, Gil-Pena H, Claramunt-Taberner
D, Hernández-Frías O, Fernández-Iglesias Á, Hermida-Prado F,
Anes-González G, Rubio-Aliaga I, Lopez JM and Santos F: Marked
alterations in the structure, dynamics and maturation of growth
plate likely explain growth retardation and bone deformities of
young Hyp mice. Bone. 116:187–195. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Anagnostou E, Dimopoulou P, Sklavos S,
Zouvelou V and Zambelis T: Identifying jitter outliers in single
fiber electromyography: Comparison of four methods. Muscle Nerve.
63:217–224. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Imel EA: Burosumab for pediatric X-linked
hypophosphatemia. Curr Osteoporos Rep. 19:271–277. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Matsushita M, Hasegawa S, Kitoh H, Mori K,
Ohkawara B, Yasoda A, Masuda A, Ishiguro N and Ohno K: Meclozine
promotes longitudinal skeletal growth in transgenic mice with
achondroplasia carrying a gain-of-function mutation in the FGFR3
gene. Endocrinology. 156:548–554. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kitoh H, Matsushita M, Mishima K, Nagata
T, Kamiya Y, Ueda K, Kuwatsuka Y, Morikawa H, Nakai Y and Ishiguro
N: Pharmacokinetics and safety after once and twice a day doses of
meclizine hydrochloride administered to children with
achondroplasia. PLoS One. 15(e0229639)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fuente R, Gil-Pena H, Claramunt-Taberner
D, Hernández-Frías O, Fernández-Iglesias Á, Alonso-Durán L,
Rodríguez-Rubio E, Hermida-Prado F, Anes-González G, Rubio-Aliaga
I, et al: MAPK inhibition and growth hormone: A promising therapy
in XLH. FASEB J. 33:8349–8362. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hayashibara T, Hiraga T, Sugita A, Wang L,
Hata K, Ooshima T and Yoneda T: Regulation of osteoclast
differentiation and function by phosphate: Potential role of
osteoclasts in the skeletal abnormalities in hypophosphatemic
conditions. J Bone Miner Res. 22:1743–1751. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang MY, Ranch D, Pereira RC, Armbrecht
HJ, Portale AA and Perwad F: Chronic inhibition of ERK1/2 signaling
improves disordered bone and mineral metabolism in hypophosphatemic
(Hyp) mice. Endocrinology. 153:1806–1816. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Z, Lee B, Pearce D, Qian S, Wang Y,
Zhang Q and Chow MS: Meclizine metabolism and pharmacokinetics:
Formulation on its absorption. J Clin Pharmacol. 52:1343–1349.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guo J, Li W, Wu Y, Jing X, Huang J, Zhang
J, Xiang W, Ren R, Lv Z, Xiao J and Guo F: Meclizine prevents
ovariectomy-induced bone loss and inhibits osteoclastogenesis
partially by upregulating PXR. Front Pharmacol.
8(693)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Takemoto G, Matsushita M, Okamoto T, Ito
T, Matsuura Y, Takashima C, Chen-Yoshikawa TF, Ebi H, Imagama S,
Kitoh H, et al: Meclozine attenuates the MARK pathway in mammalian
chondrocytes and ameliorates FGF2-Induced bone hyperossification in
larval zebrafish. Front Cell Dev Biol. 9(694018)2022.PubMed/NCBI View Article : Google Scholar
|