1. Introduction
Coronaviridae, a family of positive-strand
RNA viruses, are human pathogens that can cause a worldwide
epidemic (1). Several fatal and
novel strains of this family have been spread into the human
population globally over the past decades. The severe acute
respiratory syndrome (SARS)-coronavirus (CoV) was the first lethal
virus that infected 8,096 cases and caused 774 deaths in
2003(2). In 2012, Middle East
respiratory syndrome (MERS)-CoV led to an official 2,442 cases and
led to the death of 842 individuals (3,4).
Finally, SARS-CoV-2 was first reported in China and then caused the
current SARS-CoV-2 pandemic in 2019, which markedly altered human
life (5).
Alphaviruses, transmitted innately by mosquitoes,
are other positive-strand RNA viruses belonging to the family
Togaviridae that induce debilitating symptoms in humans. The
distribution of mosquito-borne alphaviruses is substantially
restricted in areas where vector hosts and reservoirs are present.
However, the 2004-2019 Chikungunya virus (CHIKV) outbreak revealed
the potency of this family to also affect non-endemic regions
(6). The two recent worldwide
outbreaks of CHIKV affected approximately eight million individuals
in almost 50 countries worldwide (7).
While the world is still being affected by the
recent SARS-CoV-2 pandemic, the crucial need for novel antiviral
platform technology research in vaccine development is urgently
required. As regards the unpredictable nature of viral epidemics,
the Coalition for Epidemic Preparedness Innovations accelerates the
expansion of various vaccine platforms against emerging infectious
diseases, such as MERS-CoV and CHIKV before their epidemics appear
(8).
Accordingly, the present comprehensive review aimed
to provide an in-depth insight into the various vaccine
technologies against the most significant zoonotic viral infection
of Coronaviruses and Alphaviruses in different phases of human
clinical trials.
2. Middle East respiratory syndrome
coronavirus
MERS-CoV first emerged in Saudi Arabia in
2012(9). It expanded to 27 other
countries, and according to the World Health Organization, as of
September, 2019, a 34.40% mortality rate was estimated (10). Among the genome encoding four
structural proteins [envelope (E), membrane (M), spike (S),
nucleocapsid (N)], the S protein, as a receptor identification and
viral entrance into host cells, is the primitive target for
effective immune response induction against MERS-CoV infection
(11). During the period of
infection, host furin protease splits the S protein into two
subunits known as the receptor-binding subunit S1 and the
membrane-fusion subunit S2(12).
MERS-CoV differs from SARS-CoV and SARS-CoV-2, as it is in lineage
C, whilst SARS-CoV and SARS-CoV-2 are in lineage B, of β-CoV
(13). MERS-CoV can identify
dipeptidyl peptidase 4 (hDPP4, CD26) as its receptor (14), while SARS-CoV-2 and SARS-CoV enter
host cells through their receptor, angiotensin-converting enzyme 2
(ACE2) (15). Evidence has
indicated that similar to SARS-CoV, MERS-CoV has its origin in
bats, as they are the natural reservoir (16). Moreover, bats and European
hedgehogs are the other natural host (17). Dromedary camels have been
recognized as an intermediate host for MERS (18), so the transmission possibility of
MERS-CoV from camels to humans is well established, as well as
human-to-human transmission (Fig.
1). It leads to clinical symptoms like fever, diarrhea, and
mild to severe respiratory symptoms (Fig. 1). Various analyses have been
conducted based on various vaccine candidates; the present review
provides a summary of current MERS vaccines under preclinical
development (19).
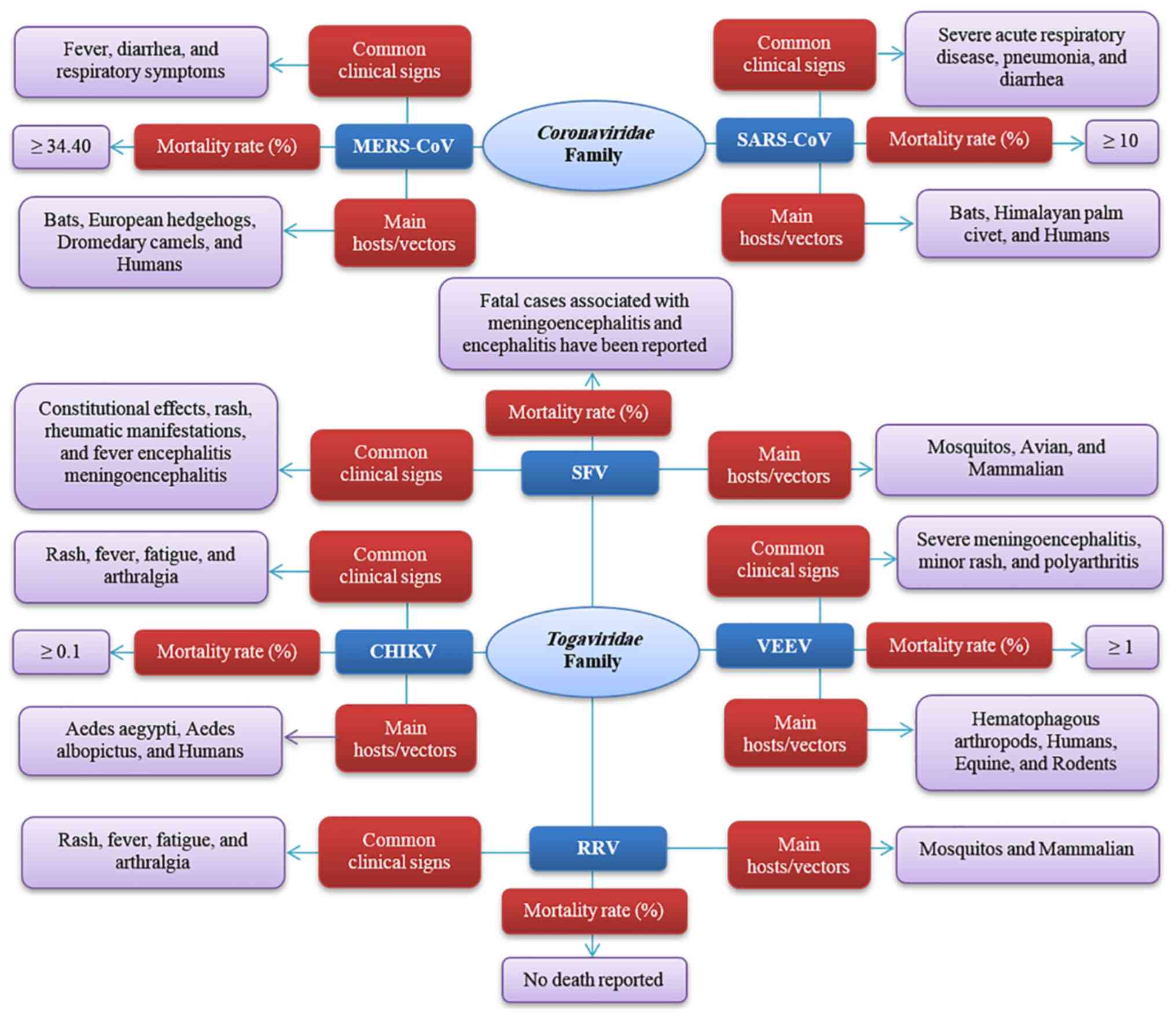 | Figure 1Summary of common clinical signs,
mortality rate, and main hosts/vectors of MERS-CoV, SARS-CoV,
CHIKV, Venezuelan equine encephalitis, RRV and SFV. MERS-CoV,
Middle East respiratory syndrome coronavirus; SARS-CoV, severe
acute respiratory syndrome coronavirus; CHIKV, Chikungunya virus;
RRV, Ross River virus; SFV, Semliki Forest virus; VEEV, Venezuelan
equine encephalitis virus. |
Preclinical MERS-CoV vaccines based on
the viral S structural protein
S-protein-targeted vaccines are under preclinical
development with some studies being performed on different animal
models (11,20).
As regards the generation of MERS-CoV DNA vaccines,
multiple designs of DNA vaccines encoding the MERS-CoV S protein or
its S1 fragment have been tested. GLS-5300, a DNA vaccine
expressing a full-length MERS CoV S-glycoprotein antigen has
revealed a high immunogenic effect in mice, camels and non-human
primates; thus, in 2016, a phase I clinical trial was commenced to
examine the efficacy in humans and accomplished (NCT02670187,
NCT03721718) (Table I) (21,22).
 | Table IMERS, SARS, Chikungunya virus,
Venezuelan equine encephalitis, Ross River virus and Semliki Forest
virus clinically tested vaccine candidates. |
Table I
MERS, SARS, Chikungunya virus,
Venezuelan equine encephalitis, Ross River virus and Semliki Forest
virus clinically tested vaccine candidates.
| Middle East
respiratory syndrome |
|---|
| Author (year) | Vaccine type | Candidate
vaccine | | Clinical trial
phase | Schedule | Protective efficacy
of vaccine | (Refs.) |
|---|
| Modjarrd et
al (2019) | A DNA plasmid
vaccine | GLS-5300 | The vaccine
expressed the MERS CoV spike (S) glycoprotein and consisted of 6
mg/ml of plasmid pGX9101 in sterile water. | Phase I
(completed) | The vaccination
program began with 0.67, 2, or 6 mg of the GLS-5300 at weeks 4 and
12 followed by co-localized intramuscular electroporation. | - | (56) |
| Advantages: No
severe adverse events, rapid manufacture, avoidance of potential
toxicities, immunogenic, induction of seroconversion and T-cell
responses, durable immune responses, polyfunctional CD8+
T-cell response, no laboratory abnormalities of grade 3 or
higher. | Disadvantages:
Solicited symptoms (mild): Injection-site reactions, headache,
malaise or fatigue, administration site pain, and tenderness.
Unsolicited symptoms: Infections. | |
| Koch et al
(2020) | Modified vaccinia
virus Ankara (MVA) | MVA-MERS-S | Modified vaccinia
virus Ankara (MVA) vector that expressed the MERS-CoV spike
glycoprotein (S) | Phase I | Two doses were
administered as follows: 107 or 108
plaque-forming units (PFU) of MVA-MERS-S on days 0 and 28 and a
booster dose of 108 PFU MVA-MERS-S 12 months (±4 months)
after the first immunization. | - | (58) |
| Advantages: No
severe adverse events, favorable safety, persisting T-cell
responses, rapid induction of immunity, induction of both humoral
and cellular immune responses. | Disadvantages:
Solicited local reactions, pain, induration, swelling, headaches,
and fatigue or malaise. | |
| Bosaeed et
al (2022) Folegatti et al (2020) | Simian
adenovirus-vectored vaccine | ChAdOx1 | Contains the MERS
Spike protein antigen. | Phase I, Phase
Ib | The vaccine was
administered to the participants with the following schedule: The
low-dose group (5x109), the intermediate-dose group
(2.5x1010) and the high-dose group (5x1010)
viral particles. | - | (60,61) |
| Advantages: Safe
and well-tolerated, immunogenic, no serious adverse reactions
induction of both humoral and cellular immune responses, inducing
seroconversion and T-cell responses, good durability. | Disadvantages:
Short-lived fever, Injection site pain, fatigue, headache and
malaise. | |
| Severe acute
respiratory syndrome coronavirus |
| Author (year) | | Candidate
vaccine | Components | Clinical trial
phase | Schedule | Protective efficacy
of vaccine | (Refs.) |
| | | Advantages | | | Disadvantages | | |
| Lin et al
(2007) | Inactivated
virus | ISCV (inactivated
SARS-CoV vaccine) | The vaccine was
obtained from a clinical strain (Sino 3) and inactivated by
β-propiolactone. The syringes in which the ISCV was adsorbed to
aluminum hydroxide include sterile saline without
preservatives. | Phase I,
completed | Doses of 16 and 32
SU/ml were administered to participants. | - | (105) |
| Advantages: Safe,
well tolerated, induction of SARS-CoV-specific neutralizing
antibodies. | - | |
| Martin et al
(2008) | DNA vaccine |
VRC-SRSDNA015-00-VP | The vaccine
contains a single closed circular plasmid DNA macromolecule
(VRC-8318). The DNA vaccine was generated at a 4 mg/ml
concentration in phosphate-buffered saline (PBS). The SARS
recombinant plasmid DNA vaccine encodes the SARS spike
glycoprotein. | Phase I,
completed | A 4 mg dose was
administered on days 0, 28 and 56. | - | (104) |
| Advantages: Safe,
well tolerated, immunogenic SARS-CoV-specific antibody,
neutralizing antibody, SARS-CoV-specific CD4+ T-cell responses,
CD8+ T-cell responses, and rapid manufacturing. | Disadvantages: Mild
injection site symptom: Pain/tenderness, swelling and redness. Mild
systemic symptoms: Myalgia, malaise, headache, chills and
fever. | |
| Chikungunya
virus |
| Author (year) | Vaccine type | Candidate
vaccine/manufacturer | Components | Clinical trial
phase | Schedule | Protective efficacy
of vaccine | (Refs.) |
| Harrison et
al (1967) | Live attenuated
virus (LAV) | CHIKV
TSI-GSD-218 | Whole attenuated
virus | Phase II | 1 dose | Forty seroconverted
by day 14 and 98% (57 vaccinees) seroconverted by day 28 | (109) |
| Advantages: Safe
and well-tolerated. | Disadvantages: No
CHIK volunteer developed clinically important reactions to the
vaccine. | (117) |
| Reisinger et
al (2019) | Measles viral
vectored vaccine (VVV) | MV-CHIK | Capsid | Phase II | 2 (28 days) | Results indicated
that the candidate vaccine successfully displayed safety and
tolerability | (120) |
| E3 |
| E2 |
| 6K |
| E1 |
| Advantages: Safe,
well tolerated, and highly immunogenic. | Disadvantages:
Injection site, tenderness injection site pain, and injection site
induration. | |
| Chen et al
(2019) | Virus-like particle
(VLP) |
VRC-CHKVLP059-00-VP | Capsid | Phase II | 3 doses (0, 4, and
20 weeks) | There was no
significant difference between effective concentration (EC50) and
placebo group (still being further assessed) | (138) |
| E3 |
| E2 |
| 6K |
| E1 |
| Advantages: Similar
immune response as native virus, safe, well-tolerated, highly
immunogenic, given without adjuvant, durable vaccine induced
antibodies. | Disadvantages: Mild
transient alanine aminotransferase increases, transient
neutropenia. | (121) |
| Venezuelan equine
encephalitis |
| Author (year) | Vaccine type | Candidate
vaccine/manufacturer | Components | Clinical trial
phase | Schedule | Protective efficacy
of vaccine | (Refs.) |
| Edelman et
al (1979) | Inactivated
vaccine | C-84 |
Formalin-inactivated vaccine for VEE | N/A | 2 Doses | The vaccine
increased preexisting serum neutralizing antibody titers against
the VEE virus in seropositive TC-83 vaccine recipients and elicited
high neutralizing antibody titers in non-immune subjects after a
primary vaccine and two-dose vaccination | (184) |
| Advantages: No
febrile reactions. No wheal and-flare reactions with pseudopod
formation. No systemic anaphylactic reactions. | - | |
| Hannaman et
al (2016) | DNA | - pWRG/VEE/Althea
Technologies, Inc., San Diego, CA | pWRG/VEEV gens | Phase I | 3 Doses | All participants
indicated neutralized ntibody after third dose | (186) |
| Advantages: Robust
antibody response with a high IgG2a/IgG1 rate. | - | |
| Johnson (2020) | Live,
attenuated | Dried TC-83 | Live, attenuated
vaccine for VEE | Phase II | - | In progression | (185) |
| - | - | |
| Ross River
virus |
| Author (year) | Vaccine type | Candidate
vaccine/manufacturer | Components | Clinical trial
phase | Schedule | Protective efficacy
of vaccine | (Refs.) |
| Aichinger et
al (2011) | Inactivated
whole-virus | Ology
Bioservices | Inactivated
whole-virus Vero cell-derived | Phase I and II | 0, 21 Days; 6
months later | Highly immunogenic
in RRV-naïve adults | (193) |
| Advantages: Safe,
well-tolerated, no serious adverse events, no cases of arthritis
associated with RRV, and low rates of fever, no increase in adverse
events after the 2nd or 3rd dose, protects ~99% of individuals who
completed the vaccination. | - | (232) |
| Semliki Forest
virus |
| Author (year) | Vaccine type | Candidate
vaccine/manufacturer | Components | Clinical trial
phase | Schedule | Protective efficacy
of vaccine | (Refs.) |
| Komdeur et
al (2021) | rSFV-based
therapeutic cancer vaccine | Vvax001/University
Medical Center Groningen Groningen, Netherlands Collaborators:
Dutch Cancer Society ViciniVax B. V | The Vvax001 vaccine
consists of a replication-deficient Semliki Forest virus (SFV)
vector which codes HPV derived tumor-antigens | A phase I clinical
trial | Patients received
three sequential doses, with a gap of 3 weeks. | - | (225) |
| Advantages: Safe,
well tolerated, and induced strong HPV16 E6- and E7-specific immune
responses. Capable of inducing HPV16-specific, IFN-g-producing T
cells. No vaccine related grade 3 or 4 adverse events. Elicited
both CD4+ and CD8+ E6- and
E7-specific T-cell responses. | Disadvantages: The
presence of either pre-existing antibodies against the virus or
vaccine-induced responses that may impede booster responses against
the transgenes. | |
In addition, various iral-vectored vaccine
developments have commenced: Various viral vectors have been
formulated dependent on MERS-CoV S and/or its fragments to examine
the immunogenicity against MERS-CoV infection in animal models,
such as mice, camels and non-human primates. The viral-vectored
vaccines include the modified vaccinia virus Ankara (MVA),
adenovirus, vesicular stomatitis virus (VSV) and measles virus (MV)
(23-25).
MVA encodes full-length MERS-CoV S and was tested in dromedary
camels with the result of inducing neutralizing antibody (NAb)
(26). Extensive investigations
have performed based on different recombinant adenovirus
(rAd)-based MERS vaccines expressing full-length S protein, S1,
N-terminal domain (NTD) and the recombinant receptor-binding domain
(RBD) at the preclinical level (27,28).
Human adenovirus serotype 5 (Ad5) is the most common Ad vector
applied to develop MERS vaccines among the other types. Adenovirus
type 41 (Ad41) as an enteric pathogen has potential for application
as a vaccine. Both of these vaccines, Ad5-MERS-CoV S and
Ad41-MERS-CoV S, have been investigated in preclinical studies
(23,29). Moreover, chimpanzee adenoviruses
(including AdC68 and ChAdOx1) have been assessed as viral vectors
for MERS vaccines (30).
Another preclinical study, attributed to
VSV-vectored MERS vaccines, which express full-length S protein and
RBD, has also been performed (31). MV-based MERS-CoV vaccines which
express MERS-CoV full-length S protein (MVvac2-MERS-S) and reduced
S protein, a soluble form (MVvac2-MERS-solS), have been established
(32).
Venezuelan equine encephalitis virus (VEEV) replicon
particles (VRPs), a type of alphavirus-based platform as an
encoding MERS-CoV S, can elicit NAb in both young and aged mice
(33-35).
Newcastle disease virus (NDV) has been examined as a
vaccine vector in non-human primates (26). An NDV vector expressing MERS-CoV S
protein was revealed to have a long-lasting induction of NAb titers
in camels (36).
To investigate other platforms, an inactivated dual
rabies/MERS vaccine has been suggested in which the MERS-CoV S1
domain fused to rabies virus G-protein on the rabies virus virion
(34). The inspiration for a
rabies virus vector derived from studies uniting rabies and Ebola
vaccine platforms (37,38).
Protein-based vaccines are capable of promoting
immune reaction. In this group, vaccines based on the RBD (i.e.,
CTD), have comprehensively investigated (39), and those based on S1 and
full-length S proteins are under investigation in animal models
and/or non-human primates (1,40-42).
An RBD fragment is a critical domain that consists of residues
377-588 and RBD proteins, including the mentioned fragment attached
to either the Fc of human IgG (RBD-Fc) or the Foldon (Fd) trimeric
motif (RBD-Fd) attached to the DPP4 receptor; in mice and/or
rabbits, these fusion proteins evoke strong responses against
various strains of MERS-CoV infections (43-45).
Furthermore, a constant CHO-expressed RBD-Fc protein promoted the
survival of hDPP4-Tg mice with MERS-CoV, with no immune toxicity or
eosinophilic immune increment (44). MF59 is an effective adjuvant that
intensely enhances the ability of the RBD protein to evoke potent
reactions (46). Other fragments
of the MERS-CoV S protein, including NTD and S2, are capable of
being elective vaccine goals. The NTD protein can induce defined
humoral and cellular immune responses, which have been analyzed in
Ad5-hDPP4-transduced mice (41).
Investigations on virus-like particle (VLP)- and
bacterium-like particle (BLP)-based vaccine development have also
been performed. There are some MERS vaccines available based on
VLPs and BLPs, some of which express full-length S protein or RBD
and some others are constructed using the S, E and M structural
proteins. Additionally, they have been assessed in animal models
(11,47). A chimeric form of VLP, which was
produced by fusing the MERS-CoV S protein with the matrix protein 1
protein of the H5N1 influenza virus, was shown to elicit particular
antibodies in mice against MERS-CoV (34). The other form of a chimeric,
spherical VLP was established by fusing the canine parvovirus VP2
structural protein gene to the MERS-CoV RBD (11). Moreover, a VLP expressing the
MERS-CoV S, E and M proteins conjugated with an aluminum adjuvant
has been studied in non-human primates (47). In the BLP-based MERS-CoV vaccine
group, RLP3-GEM has been produced as an alternate form to VLP-based
MERS vaccines and the former anchors an RBD linked through three
protein harbors (RLP3) and utilizes Gram-positive booster matrix
(GEM) as a substrate (20). With
the GEL01 adjuvant, the immune induction of this vaccine was tested
in mice (20).
Efforts made with the design of
vaccines based on nanoparticles
Multiple attempts at designing nanoparticle-based
vaccines expressing the MERS-CoV full-length S protein have been
carried out and conducted in insect cells, which were consequently
evaluated for efficacy in mice (48,49).
To achieve nanoparticle vaccines, the nano surfactant treatment and
mechanical disjunction of insect cells, which express S protein was
performed in order to improve nanovesicle formation and
consequently achieve an optimal generation of nanoparticle vaccines
(50). For immune response
improvement, nanoparticle vaccines can be merged into other types
of MERS vaccines, for instance, heterologous priming with rAd5
coding full-length S protein (Ad5/MERS), followed by promoting with
full-length S protein nanoparticles, triggering both Th1 and Th2
immune responses that have a protective effect in mice (51).
Preclinical vaccines based on the
non-S structural proteins of MERS-CoV
As aforementioned, the S protein is the most
substantial one among the other structural proteins in the vaccine
design approach. Apart from the S protein, the N protein may be
another vaccine target; hence, it is conserved among various
strains of the virus. Various N protein-based vaccines have
exhibited potent immunity in immunized mice. Previous studies have
tested MVA or MV vector-based recombinant vaccines presenting the
MERS-CoV N protein (MVA-MERS-N; MVvac2-MERS-N), which causes
MERS-CoV N-specific T-cell induction (including CD8+
T-cells) in mice (24,52).
Preclinical vaccines based on the
inactivated virus
The inactivated MERS-CoV virus, as another vaccine
candidate, has been designed and evaluated at preclinical
assessment. Agrawal et al (53) assessed the immunization of
inactivated MERS-CoV vaccine candidates in mice, which revealed
that it may increase the risk of a hypersensitive-type lung
pathology from MERS-CoV infection (53). Another analysis of inactivated
whole MERS-CoV in mice illustrated enhanced protection (54).
Preclinical vaccines based on
live-attenuated viruses lacking structural, non-structural, or
accessory proteins
MERS-CoV has several protein types; for example, ORF
3-5 as an accessory protein, the E structural protein, and the
nsp16 non-structural protein (nsp), which are ascribed to
pathogenicity. Nevertheless, a recombinant MERS-CoV has been
tested, which lacks ORF 3-5 and was shown to lead to a reduction in
viral titers in cell culture (55). Other research has suggested the
possibility that rapidly generated live-attenuated MERS-CoV
vaccines may have diminished virulence (40).
Clinical trials testing MERS vaccine
platforms
Several clinical trials have been performed testing
MERS vaccine platforms, and the first vaccine candidate was a DNA
plasmid vaccine. Kayvon Modjarrad and colleagues conducted the
first DNA vaccine candidate against the MERS-CoV. That trial is
registered on ClinicalTrials.gov (NCT02670187) and has been
completed (56). The vaccine
candidate GLS-5300, a DNA plasmid vaccine expressing the MERS CoV
spike (S) glycoprotein, was brought out in The Lancet Infectious
Diseases in 2019 (Table I)
(56). To this aim, 75 adults aged
18 to 50 years at the Walter Reed Army Institute for Research
Clinical Trials Center (Silver Spring, MD, USA) participated in the
study and were administered a dose-increment protocol as follows: A
0.67, 2, or 6 mg GLS-5300 intramuscular injection at the starting
point, then immediately at weeks 4 and 12, followed by co-localized
intramuscular electroporation to evaluate the safety of GLS-5300 at
one of these three dose levels. The early results of the study
revealed the safety of the agent. The follow-up after dose 3 was
performed up to the 48th week. To take part in the other groups of
high-dose-administration, a safety monitoring committee should
confirm the vaccination outcome of the first five individuals in
the prior low-dose group. The secondary consequence was
immunogenicity (56). The
ingredient of GLS-5300 is 6 mg/ml plasmid pGX9101 in sterile water
for injection. Plasmid pGX9101 comprises a gene insert planned as
an optimized, full-length, macro consensus of the MERS-CoV
S-glycoprotein raised from publicly present clinical sequences up
to August, 2015 (Table I)
(57). That phase I clinical trial
revealed that the tested vaccine was well-endured, and no critical
adverse events were introduced. The usual adverse effects were
related to the injection site reflexes, which were in line with the
other released clinical trial reports of DNA vaccines or placebo
co-administered through intramuscular injection and electroporation
(56). The effect of GLS-5300
vaccination in cellular stimulation and antibody responses is
similar to MERS in patients who have recovered from CoV infection.
Since DNA vaccines and viral-vectored vaccines use recombinant
technology, they can be included in rapid designing approaches in
the case of emerging infectious diseases. In comparison to living
viral-vectored vaccines, DNA vaccines have this superiority in
rapid production and do not have the possible occurrence of
toxicity (56). Since phase I
clinical trials [(NCT02670187), GLS-5300 (INO-4700)] and phase
I/IIa trials [(NCT03721718), GLS-5300] on MERS-CoV DNA vaccines
have been performed, Inovio Pharmaceuticals generated the GLS-5300
(INO-4700) DNA vaccine.
MVA vector vaccine candidate evokes
humoral and cellular immune responses to MERS-CoV S protein
The MVA vector vaccine candidate was the other
candidate used in clinical trial testing. The open-label, phase I
trial was conducted at the University Medical Center
Hamburg-Eppendorf (Hamburg, Germany) (58). This type of vaccine was based on an
rMVA vector that expresses the full-length MERS-CoV spike
glycoprotein, which relies on the sequence of EMC/2012 (GenBank
accession no. JX869059). The vaccine was constructed by IDT
Biologika GmbH in early chicken embryo fibroblasts. The
participants were healthy adults aged 18 to 55 years. The
participants were injected with doses MVA-MERS-S at
1x107 plaque-forming units (PFU; low-dose group) or
1x108 PFU (high-dose group) intramuscularly at the first
vaccination (Table I). The
amplifier immunization was administrated intramuscularly 28 days
after the first vaccination. The main aims of that study were to
analyze the safety and tolerability of the two dosage plans in
addition to determining the reactogenicity after administration.
The participants in the low-dose or high-dose groups did not
exhibit any severe adverse effects. The comparison of the two-step
vaccination demonstrated that the booster dose elicited humoral and
cellular immune responses to the MERS-CoV spike protein (58). Among various preclinical analyses,
mice vaccinated with MVA-MERS-S produce neutralizing antibodies and
CD8+ T-cells and a protective effect occurs in
Ad-hDPP4-transduced mice infected with MERS-CoV (59). Even though research on animal
models has illustrated the vital role of antibodies in protecting
against MERS-CoV, information obtained on humans has not revealed
the potent connection between the MERS-CoV viral load and
MERS-CoV-specific antibody responses (57). T-cells cause dominant responses in
survivors of MERS-CoV (16). The
importance of T-cell responses has not been proven to be critical
in humans; however, in mouse models, the clearance effect of
T-cells has been revealed (48).
In conclusion, the aforementioned trial (ClinicalTrials.gov, NCT03615911; EudraCT,
2014-003195-23) demonstrates humoral and cellular immunogenicity in
humans. Since vaccination for MVA-MERS-S had no crucial adverse
effects, the vaccine was considered safe. A phase Ib clinical trial
(ClinicalTrials.gov, NCT04119440) on was
conducted in order to scrutinize the safety, tolerability and
immunogenicity of two ascending doses of the candidate vaccine
MVA-MERS-S_DF-1 against MERS (MVA MERS-S). The last update for this
trial was on November 8, 2022.
Adenovirus-vectored vaccine encoding
the full-length spike surface glycoprotein has yielded promising
results
The other phase I clinical trial is based on
assessing the safety and immunogenicity of the candidate simian
adenovirus-vectored vaccine encoding the full-length spike surface
glycoprotein, ChAdOx1 MERS (NCT03399578 and NCT04170829), in humans
was conducted at the Centre for Clinical Vaccinology and Tropical
Medicine (Oxford, UK) (Table I)
(60). A total of 48 healthy
participants aged 18 to 50 years received the ChAdOx1 MERS in a
single injection, intramuscularly. In total, three different doses
were administrated as follows: The low-dose group was administered
5x109 viral particles, the intermediate-dose group with
2.5x1010 viral particles, and the high-dose group with
5x1010 viral particles. ChAdOx1 MERS composition is the
replication-deficient simian adenovirus vector ChAdOx1 expressing a
codon-optimized coding sequence for the full-length spike protein
(S1 and S2 subunits) of the MERS-CoV isolate from a camel in Qatar
(GenBank Accession no. KJ650098.1), containing a 32 amino acid
N-terminal tissue plasminogen activator leader sequence. In that
study, the candidate ChAdOx1 MERS vaccine was evaluated, and the
safety was revealed in all three dose groups, although a higher
reactogenicity profile was considered in the high-dose group. In
addition, no severe adverse reactions were observed (Table I) (60). For the safety evaluation of the
ChAdOx1 MERS vaccine (NCT04170829), a phase Ib trial was conducted
in healthy Middle Eastern adults. The vaccine dosage in this trial
was similar to the clinical trial phase I, and the outcome was
desirable and conformed with the clinical trial phase I (61).
According to preclinical research on the
BVRS-GamVac-Combi vaccine, a heterologous prime-boost immune
vaccine with recombinant adenovirus types 26 and 5, depicted high
titers of specified antigen-neutralizing antibodies in mice
(62); thus, phase I/II clinical
trials of the vaccine (NCT04128059) and BVRS-GamVac (NCT04130594)
are currently underway.
Although various tests have been performed on the
MERS vaccine, no commercial vaccine has been marketed to date, and
all these findings are derived from laboratory-based trials.
3. Severe acute respiratory syndrome
coronavirus
CoVs are included in the family of
Coronaviridae and comprise α-CoV, β-CoV, γ-CoV and δ-CoV
genera (1). The most pathogenic
human CoVs have caused considerable infections, which include
SARS-CoV, MERS-CoV and the newly recognized SARS-CoV-2 [known as
CoV 2019 (COVID-2019)]; all infections are associated with the
genus β-CoV (63). SARS-CoV was
first identified in Guangdong, China, in 2002, resulting in a
worldwide outbreak in 2003, which led to an ~10% fatality (Fig. 1) (64). Several structural proteins,
including nucleocapsid, membrane, envelope and S proteins, which
are expressed by SARS-CoV cause severe infections (65). The target cells to be infected by
this virus are lung epithelial cells and the entry to the host cell
occurs by binding to ACE2(66).
SARS-CoV infection begins with flu-like signs, and subsequently
leads to severe acute respiratory disease, pneumonia, diarrhea and
even death (67). SARS-CoV is
found in bats, which can be transferred into the Himalayan palm
civet as another host that causes the amplification of the virus
(68). There are two probable
mechanisms for the transmission of SARS-CoV as a zoonotic virus,
including animal-to-human and human-to-human (Fig. 1) (67). The evolution of the strategies
regarding the SARS vaccine comprises three generations. Live
attenuated and inactivated vaccines are categorized into the
first-generation group. Related to the natural antigenic substance,
live attenuated vaccines have always yielded significant results
due to their rapid access and potent immunogenic response (69). The successful administration of
these vaccines against variant diseases, such as polio, rubella,
chickenpox, mumps, etc. has been previously reported (69). Based on preclinical research in
which SARS-CoV mutants lacking the E gene were evaluated in
hamsters challenged with SARS-CoV, preventive effects were inferred
(70). Since snp16 can function as
a target for the CoV vaccine, both SARS-CoV and MERS-CoV nsp16
mutant vaccine has evaluation revealed a conservative effect
(71). Another target for live
attenuated CoV vaccine is nsp14, which encodes exoribonuclease
(ExoN). Graham et al assessed the effects of ExoN deletion,
which demonstrated that the ExoN-deleted SARS-CoV vaccine can exert
a protective effect against challenges in these mice (72). Nevertheless, none of the
preclinical analyses of live attenuated vaccines have led to
clinical trials for either SARS-CoV or MERS-CoV (73).
The other form of the vaccine in this category known
as an inactivated vaccine may be achievable by inactivating the
virus, using radiation method (UV-ray, X-ray org-radiation) or
chemicals (such as formalin, methanol, or b-propiolactone), in
which the antigenic feature of the virus remains active, although
it is not able to cause infection (74).
To date, diverse inactivated vaccines are available
against various diseases, such as influenza, polio, hepatitis A,
rabies pathogen, etc. (74).
Various studies have been designed based on first-generation
vaccines against SARS. The assessments have been tested on
different animal models, such as mice (75,76),
hamsters (77), African green
monkey (78) and rhesus monkey
(79), which were revealed to be
safe candidates in animals.
Along with all these data, whole inactivated
vaccines which were tested in both SARS-CoV and MERS-CoV, have
depicted an eosinophil-related lung pathology as a downside
(80). Nonetheless, later research
has manifested that UV-inactivated SARS-CoV with Toll-like receptor
agonists as adjuvants, and formaldehyde-inactivated MERS-CoV with
alum and unmethylated CpG as adjuvants, have the potential of
suppressing or preventing lung injury (81).
As regards second-generation vaccines, protein
subunit vaccines and vector-based vaccines have been assessed. The
first attempts for protein subunit vaccine generation were based on
surrounding full-length S protein-based vaccines; S protein
RBD-based vaccines subsequently attracted increasing attention. The
formulation of a protein subunit vaccine is based on synthetic,
isolated, recombinant, or derived highly antigenic protein base
subunits with the short antigen part proposing a safer strategy in
the vaccine project. Diverse protein subunit vaccines have been
designed successfully against multiple pathogens, such as the
influenza virus, hepatitis B, pneumonia and meningitis, etc.
(82-84).
According to previous studies, the full-length S protein,
extracellular domain of the S protein and trimeric S proteins
(triSpike) have an immunogenic capacity that can exert protective
effects against SARS-CoV infection (84,85).
According to the study by Du et al (86), RBD-based SARS-CoV vaccines have the
potency of evoking RBD-specific IFN-γ producing cellular immune
responses in mice.
Studies using various animal models, such as rabbits
and mice have yielded acceptable outcomes using subunit vaccine
candidates for SARS prevention (87,88).
Moreover, the assessment of the immunogenicity of recombinant
baculovirus-expressed SARS-CoV S protein in a mouse model, yielded
positive results, demonstrating protective effects (85). Other structural proteins, N
protein-based vaccines, have also been tested. Although N
protein-based vaccines cannot impel neutralizing antibodies, they
are more conserved across CoV species than S protein, which renders
them a possible target for a T-cell inducing global CoV vaccine
(89). Testing M protein-based
vaccines has revealed high antibody titers, but no NAb (77). Studies on CoV E protein-based
immunization are limited, and neither neutralizing antibodies nor
protective immunity has been reported (90).
The evaluation of the SARS-CoV protein subunit
vaccines in preclinical assessments has revealed promising results,
despite the fact that these have not entered clinical trials
(91). Superior immunogenic
responses are concluded from vector-based vaccines (92). There are disparate viral vectors
that are being used as a transfer instrument in vaccination, such
as the MVA virus, adenovirus, adeno-associated viruses, retrovirus
vector, lentivirus vector, Sendai virus, etc. (88). Adenovirus, as a popular viral
vector vaccine, has been surveyed in order to examine the
effectiveness of the adenovirus-based SARS-CoV vaccine. Based on
research conducted on monkeys and rats, adenoviral vector
representing the S1 fragment has the potency of inducing NAbs
(88,93). A ferret model of SARS-CoV infection
was previously tested; the results revealed that it could prevent
pneumonia (94). Based on research
on a rat model, an adenoviral-based vaccine outlined potent
SARS-CoV-specific humoral immune responses (95).
The other vaccine platform evaluated in the SARS-CoV
challenge was MVA. In a study on mice immunized with attenuated MVA
containing a full-length S gene, a protective outcome was attained
(96). Although NAbs have been
observed in mice, ferrets and monkeys tested with recombinant MVA
expressing SARS-CoV S protein, no protective effects were detected
(97). On the other hand, some
studies have provided conflicting data depicting certain adverse
effects in ferrets, including inflammatory responses and focal
necrosis in the liver while using the MVA vaccine expressing
SARS-CoV S protein (98,99).
Deming et al (100) evaluated the VEEV-based SARS-CoV
vaccine, and concluded that VEE VRPs expressing S protein exerted
protective effects in mouse models. Further investigations have
been conducted using the parainfluenza-based vaccine in hamsters
and monkeys, and attenuated VSV in mice revealed promising results
for SARS-CoV vaccines (78,90,101).
Analyses on viral vector-based vaccines in
comparison to the first-generation vaccines have demonstrated
efficacious results attributed to the presence of the live virus by
recombination of the antigenic protein ingredient of a pathogenic
virus into a non-virulent vector. In due course, the stimulation of
cellular and humoral immunogenicity is obtained. The precise
information about epidemiology, genotoxicity and virology of both
viruses (pathogenic and vector virus) needs to be examined further,
in order to design a proper and effective vaccine (92).
In order for this to be achieved, however, several
obstacles may have to be combatted, such as a risk of mutation and
unanticipated virulence potency, the delay of an actual expected
immune response, and the need for precise information about the
epidemiology, genotoxicity and virology of both viruses (pathogenic
and vector virus), etc. (60,92).
Other vaccine development strategies have been
tested, VLPs, which are self-assembled viral structural proteins
that imitate the compound of native viruses without a viral genome.
Based on a study in which chimeric VLPs composed of SARS-CoV S
protein and mouse hepatitis virus E, M, and N proteins were
analyzed, the induction of NAb responses and the reduction of
SARS-CoV viral titers in mouse lungs were observed (102). Another study that utilized the
same chimeric VLPs as the aforementioned study by Lokugamage et
al (102), revealed the
disadvantage of this vaccine type, which was pulmonary
immunopathology (80).
Conduction of clinical trials
according to the prior favorable consequences obtained from
preclinical studies
DNA vaccines as a rapid and flexible vaccine
development platform consist of genes encoding viral antigenic
components. Among various evaluations including the S, M and N
protein-based vaccines, only the S protein-based DNA vaccine can
exert a conservative effect against SARS-CoV infection. According
to a previous study, DNA encoding full-length S protein can provide
NAbs and exdert protective effects in mice (103). Since the preclinical results were
encouraging, a phase I clinical trial based on SARS-CoV full-length
S protein DNA vaccine was conducted (NCT00099463) (104). In that clinical trial, the
vaccine, VRC-SRSDNA015-00-VP, comprised a single closed circular
plasmid DNA macromolecule (VRC-8318). For this purpose, 10 healthy
adults received a three-dose vaccine schedule and were then tested
in order to evaluate the immunity and safety of the vaccine. The
vaccine administration was based on three doses of 4 mg/ml on days
0, 28 and 56. The results depicted a promising outcome,
demonstrating a safe and well-tolerated vaccine that can elicit
NAbs and exert a protective effect (Table I) (104).
In a study conducted by Sinovac Biotech, the
response to an inactivated vaccine was examined in 36 healthy
adults (SARS-CoV seronegative), aged 21 to 40 years. The clinical
trial was performed in a randomized manner, double-blinded and
placebo-controlled in China (Table
I) (105). The control group
was administered saline with aluminum hydroxide as aluminum
hydroxide absorbed the inactivated vaccine.
The doses were established based on preclinical
assessments in mice, rats and rhesus monkeys, in which the safety
and immunogenicity of the vaccine were proven. The participants
received 16 SU or 32 SU of the vaccine or the placebo, via
intramuscular injection (105).
The results demonstrated a safe and well-tolerated vaccine
candidate, evoking SARS-CoV-specific NAbs (106). Although several clinical trials
have been running for a number of years, thus far, there is no
licensed vaccine available for SARS-CoV.
4. Chikungunya and O'nyong-nyong virus
CHIKV belongs to the Togaviridae family, the
Alphavirus genus. CHIKV is a positive-sense single-stranded RNA
virus, that was first isolated in 1953 in Tanzania. Since then, the
CHIKV epidemic or endemic infections have been reported in 106
countries and territories, including the USA and Europe (107).
CHIKV consists of three genotypes, having a single
serotype behavior (107).
Aedes aegypti and Aedes albopictus mosquitoes are the
vectors of CHIKV (108). Four
nsps, namely nsp1, nsp2, nsp3 and nsp4, and five structural genes
(C-E3-E2-6K-E1) are encoded in most of the CHIKV genome, and are
responsible for viral replication and transcription. CHIKV virions
are spherical, enveloped particles of ~70 nm in diameter. The E1
and E2 glycoproteins form heterodimers and assemble into spikes on
the surface. At the center of the virion, is the nucleocapsid core,
~35 nm in diameter, which is composed of the C protein in a complex
with the viral genome (109).
The clinical symptoms of CHIKV exhibit similarities
with other Alphaviruses, including the O'nyong-nyong virus and the
Ross River virus (RRV) (Fig. 1).
Since there is no effective antiviral treatment for CHIKV
infections, several techniques have been used for the development
of CHIKV vaccines, including non-infectious (110) and infectious DNA vaccines
(111), VLPs and inactivated
virus. Live attenuated vaccines under development include
rationally attenuated Alphavirus chimeras (112) and deletion mutants (113); a vesicular stomatitis-vectored
vaccine (114) and an internal
ribosome entry site-modified CHIKV strain (115). For this purpose, various clinical
human (116-121)
and animal (122) trials have
been performed.
Preclinical studies
The main portion of current available knowledge on
the immune system response to CHIKV has been obtained from animal
models. The first efforts of researchers date back to the attempts
of the Reed Army Institute of Research (WRAIR) in Washington, D.C.,
USA, and are related to the formalin-inactivated virus vaccine
(109). For the preparation of
this candidate vaccine, chick embryo, suckling mouse-brain (SMB)
and green monkey kidney cells (GMKCs) were used. Due to the weak
immune response elicit, chick embryo could not pass the test, and
between SMB and GMKCs, the former was selected for continuation.
However, the study was suspended to limit the risks of inducing
encephalitis in males. Following the success of the CHIKV 168
vaccine in eliciting homologous protection in mice, the African
CHIKV strain 168; the CHIKV strain E.103 isolated from a pool of 78
Aedes africanus mosquitoes (123), the Asian strain BAH-306 isolated
from Thai patients (124), and
the Indian CHIKV strain C-266 isolated from Calcutta by K.V. Shah
were selected to assess heterologous protection in rhesus macaques
(125). CHIKV strain 168 was
administered in 0, 7 and 21 days. After 30 days of homologous and
heterologous challenges, no viremia was observed in all vaccinated
subjects (Table I) (109).
Other candidate vaccines are VLPs which express the
structural protein of viruses that have the ability to infect the
host (original); however, the new structure does not have the
ability of infection, since it is an empty shape (126). Akahata et al (127) at the Vaccine Research Center
(VRC) at the NIH National Institute of Allergy and Infectious
Diseases (NIAID) in Bethesda, MD, USA developed a VLP vaccine. The
vaccine carried structural CHIKV proteins Capsid, E3, E2, 6K and E1
sequences of the CHIKV strain 37997. All macaques (n=6) were
resistant to the CHIKV (strain LR2006 opy-1) challenge and NAbs
were induced (127). Other
CHIK-VLP vaccines were developed based on the expression of
yeast-derived CHIKV-like particles. Saraswat et al (128) used a novel yeast expression
system (Pichia pastoris) and evaluated this as a vaccine
candidate. This elicited neutralizing activity against CHIKV in
BALB/c mice. Various doses of CHIK-VLPs also succeeded in inducing
humoral and cellular immune responses (128).
The MV-CHIKV vaccine is a type of recombinant
live-attenuated vaccine based on inducing high titers of CHIKV
antibodies in mice challenged with the measles virus. It has been
demonstrate that single vaccination with this vaccine candidate
protected all subjects (mice) from a lethal CHIKV challenge
(122). In addition, two chimeric
Alphavirus vaccine candidates for CHIKV have reached the animal
model phase, as demonstrated by Wang et al (129). The first one was designed for
Alphavirus genus members in 2007. Chimeric Alphavirus/CHIKV vaccine
viruses were created employing recombinant DNA methods (129). The Alphavirus backbone includes
Sindbis virus (SINV) strain AR339, the attenuated VEEV vaccine
strain TC-83 and a South American strain of eastern equine
encephalitis virus (EEEV), BeAr436087, which is unable to cause
disease in adult mice. The structural genes and 5'-UTR of the
sub-genomic RNA were obtained from the LR strain of CHIKV. The
results indicated that the CHIKV chimera versions that used the
attenuated VEEV strain TC-83 or naturally attenuated EEEV backbones
were consistently more immunogenic in outbred or inbred mice than
the chimera with a SINV backbone, and also produced robust NAb
responses (129). In the other
study, in order to establish an Alphavirus vaccine, chimeric
genomes encoding VEEV- or EEEV-derived non-structural and
CHIKV-specific structural proteins (VEE/CHIKV and EEE/CHIKV) were
used. To obtain a safer vaccine and to hint replication in mosquito
cells, a novel modification was introduced. Their replication was
also dependent on the function of the encephalomyocarditis virus
internal ribosome entry site. Three different chimeric candidate
vaccines were used, including Sham, VEE/IRES-CHIKV and
VEE/IRES-C/CHIKV. Following a single immunization, mice exhibited a
protective immune response against subsequent CHIKV challenge,
characterized by high titers of NAbs (112).
Another type of candidate vaccine is the MVA. Three
different research groups have investigated new vaccine platforms.
Van Den Doel et al (130),
Weger-Lucarelli et al (131) and García-Arriaza et al
(132) used different candidate
vaccine platforms and demonstrated the efficiency of vaccines
separately. Furthermore, various groups have investigated different
platforms of the CHIKV vaccine, such as VSV (114), adenovirus 5(133), DNA, protein E2(134), E1 and E2(135), live attenuated (115,116,136) and inactivated (FIV) (109).
Clinical trials
Some candidate vaccines could pass through phase I
and II clinical trials. Inactivated (FIV 15562) (109), live attenuated (137), VLP (138), MV-CHIKV (120) and ChAd0x1-CHIKV (NCT03590392)
(6) were the vaccines that proceed
to phase I trials.
On the other hand, some candidate vaccines
proceeded to phase II trials. The MV-CHIKV vaccine is one of the
vaccine candidates against CHIKV. In 2021, this vaccine's safety
and immune response were completed and compared to the commercially
available MMR vaccine, but no publication has been done yet
(ClinicalTrials.gov Identifier:
NCT03101111). In 2000 CHIKV TSI-GSD-218 entered Phase II (117). The platform was based on live
attenuated and the CHIKV strain 15561 (seed) was obtained from an
infected patient during the 1962 outbreak CHIKV in Thailand. The
vaccine was provided by passaging 18 plaque-to-plaque passages in
MRC-5 cultures (116). During the
investigation, 73 healthy volunteers participated in the study. A
total of 59 volunteers received the vaccine once subcutaneously and
14 were immunized with a placebo (tissue culture fluid).
Consequently, the candidate vaccine was highly immunogenic.
According to the results, 69% of the volunteers (n=40)
seroconverted by day 14, and 98% (57 vaccinees) seroconverted by
day 28; in addition, after 12 months NAb was detectable in 85% of
the volunteers (117). The
PXVX0317 [CHIKV-like particle vaccine (CHIKV VLP)], alum adjuvant
vaccine was evaluated in a clinical to determine the safety of the
vaccine in adults, and assess the induction of anti-CHIKV NAb
responses following a single dose of PXVX0317 (40 µg CHIKV VLP,
alum-adjuvanted) as measured 7 days (day 8), 14 days (day 15), and
21 days (day 22) and 56 days (day 57) after vaccination.
Additionally, 25 volunteers participated in the trial
(NCT05065983). A phase III clinical study on PXVX0317 is currently
under research (ClinicalTrials.gov Identifier: NCT05072080).
In 2015, the NIAID conducted a study to investigate
the safety and tolerability of a CHIKV vaccine,
VRC-CHKVLP059-00-VP, in healthy adults. During this project, 400
subjects participated. From the 400 volunteers, 201 received
intramuscular injections 28 days apart (20 µg) and 199 received a
placebo and were followed-up for 72 weeks. After the injection,
severe, and mild-to-moderate unsolicited adverse events were
observed. In addition, 8 weeks after the first administration, the
half maximal effective concentration geometric mean titer in the
vaccine group was 2005 (95% CI, 1680-2392) vs. 43 (95% CI, 32-58;
P<0 .001) in the placebo group. Finally, there was no
significant difference between the treatment and the placebo group.
However, further assessments and phase III trials are warranted
(Table I) (138).
The immunogenicity, safety and tolerability of the
measles-vectored CHIKV vaccine MV-CHIK have been investigated in
double-blind, randomized, placebo-controlled and active-controlled
trial. A total of 263 patients in two different groups received two
different concentrations (5x104 or 5x105 50%
tissue culture infectious dose) intramuscularly, with an interval
of 28 (D0 and D28) or 168 (D28 and D196) days between the prime
vaccine and the booster. At day 56, NAb was detectable. In
addition, the results revealed that a low vaccine dose induced a
PRNT50 titer of 50.16 and 12.87 (short and long intervals,
respectively), while the high dose induced titers of 174.80 and
33.64 (short and long intervals, respectively). The results
indicated that the vaccine candidate succeed to display safety and
tolerability (Table I) (120).
A phase II, multicenter, randomized,
placebo-controlled and double-blind study was conducted to evaluate
the safety and immunogenicity of a two-injection vaccine regimen
(days 0 and 28) with CHIKV virus-like particle vaccine (CHIKV VLP,
VRC-CHKVLP059-00-VP) in healthy adults, ages 18-60 years, that
resided in CHIKV endemic regions (NCT02562482); the results
demonstrated the safety and tolerability of the vaccine; however,
phase III trials are warranted in order to assess the clinical
efficacy (139).
The live recombinant measles-virus-based CHIKV
vaccine exhibited immunogenicity. It was also safe in the presence
of anti-vector immunity and had an acceptable tolerability profile.
This vaccine is the first promising measles-virus-based candidate
vaccine for use in human beings (119).
A phase II study to evaluate the safety and
immunogenicity of the CHIKV vaccine (MV-CHIK-202) was completed in
2021 (NCT02861586). In September 2022, a clinical study was
completed as a randomized double-blind interventional. That study
evaluated the safety and immunogenicity of the investigational V184
live recombinant measles-vectored CHIKV vaccine delivered in two
doses, 28 days apart compared with a saline placebo. After
providing informed consent, individuals were monitored for
eligibility, including verification of previous exposure to the
CHIKV virus. However, the related study has not yet been published
(NCT03807843). In July 2022, a phase III clinical study was
conducted on ~4,060 male and female subjects aged ≥18 years
evaluating the final dose of VLA1553 (NCT04546724). Nevertheless,
the results have not yet been published, at least to the best of
our knowledge.
Although O'nyong-nyong virus infection does not
have any effective antiviral treatment or vaccine, the CHIKV-IRES
(V1/V2) vaccine for CHIKV has been found to elicit a potent
cross-NAb response and to confer protection against O'nyong-nyong
virus challenge in an A129 mouse model (140).
5. Venezuelan equine encephalitis virus
VEEV, of the Alphavirus genus in the
Togaviridae family, is a zoonotic pathogen that is
transmitted via hematophagous arthropods, through mosquitoes. It is
an enveloped virus with a non-segmented, positive-sense RNA genome.
The genus comprises VEEV, EEEV and western equine encephalitis
virus (WEEV) (141). The VEEV
species incorporates six antigenic subtypes, specifically IA/B, IC,
ID and IE (I-VI) (142).
Alphavirus causes acute infections characterized by high-titer
viremia and since vertebrate hosts are infected, induces a variety
of diseases from severe meningoencephalitis to minor rash and
polyarthritis (Fig. 1) (143). The Togaviridae family
induces human disease outbreaks and equine epizootics in the
American continent, including South, Central, and North America,
particularly outbreaks in Texas in 1971(144). Two live-attenuated strains of
VEEV, specifically TC-83 and V3526, can be securely taken care of
at biosafety level 2 control (145). The virus glycopeptide forms the
icosahedral shape with T=4 symmetry. The virus RNA is surrounded by
240 copies of the viral capsid protein-linked N-terminus of the
protein. Moreover, the capsid is bound to the E2 glycoprotein at
the C terminus (146).
Preclinical studies
To date, to the best of our knowledge, there is no
approved licensed vaccine against VEEV. Reportedly there are
several vaccine candidates against VEEV, which are still in
progress at different stages of development. This system of
classification includes live attenuated viruses, inactivated
viruses, recombinant subunit or chimeric viruses, VLPs, or passive
immunization. The type and features of VEEV candidate vaccines have
been reviewed in detail by Sharma and Knollmann-Ritschel (142). Live attenuated vaccines are
obtained by a mutation in VEEV strains through serial passage in
cell culture or manipulation in the viral genome via mutation.
According to Berge et al (147), initially, TC-83 (a
live-attenuated strain of VEEV) obtained by 83 passages of the
Trinidad donkey (TrD) strain of VEEV in guinea pig heart cells was
used for vaccination in humans. Mexico and Colombia are using live
TC-83 as a vaccine for immunizing equines; however, it is not
currently marketed in the USA (148).
Inactivated candidate vaccines may be suggested as
an alternative candidate, despite the risk of the live virus
escaping. One of the candidates for VEEV vaccines for development
was the formalin-inactivated TrD strain of VEEV (149). Formerly, it was used to vaccinate
equine endemic areas; however, due to the risk of escaped live
viral particles, its administration was restricted. By 1970, the
incomplete inactivated VEEV vaccine was known as the major cause of
outbreaks of VEEV in endemic areas (150). Currently, formalin-inactivated
TC-83 is available for the immunization of horses against VEEV and
WEEV in the USA. During mutations in the 50 non-coding regions,
nsp3, E2, E1 and 30 non-coding regions, and serial passage of the
virulent TrD strain through guinea pig heart cell cultures, TC-83
was developed (147,151). Several side-effects and adverse
effects related to TC-83 have been reported. It can be transmitted
by mosquitos and causes adverse effects in ~20% of recipients; on
the other hand, it has a high rate (almost 18%) of serological
non-response and is likely to cause pancreatic disease (152,153). Another inactivated VEEV was
obtained by using the chemical, 5-iodonaphthyl-1-azide (INA). INA
is a type of hydrophobic alkylating agent, divided into biological
membranes and accumulating hydrophobic domain of the lipid bilayer;
following short-term exposure to UV (long wavelength) it
selectively binds to transmembrane proteins in the viral envelope
and completely inactivates V3000, a full-length infectious clone of
the wild-type TrD strain of VEEV (154,155). INA-inactivated V3526 was shown to
be able to induce immunization in mice against an aerosol challenge
with TrD (156). Another
procedure carried out to inactivate V3526 and generate the VEEV
vaccine was by ionizing gamma radiation; V3526 was exposed to a 50
kGy dose of gamma radiation, which led to a 30-50% loss in epitope
integrity. However, gamma-irradiated V3526 failed to protect mice
against aerosol challenge with virulent TrD and the fatality rate
of underlying mice was almost 60% (157,158). The novel material is known as
manganese-decapeptide-inorganic phosphate complex derived from
gamma radiation-resistant bacteria Deinococcus radiodurans,
was used to protect VEEV epitopes, while the virus genome degraded
completely. Hence, it protected 90% of mice from an aerosol
challenge with TrD (159,160).
Recombinant live attenuated vaccines have been
dedicated to novel techniques to approach vaccines based on an
Alphavirus, which led to a practicable, safe, immunogenic and
effective vaccine against encephalitis Alphaviruses. The genome of
SINV, which is a non-pathogenic member of Alphavirus in humans, is
being used as a vector to design chimeric SIN/VEE virus(es) to
express all the structural proteins of the virulent Alphavirus
(129,161). SIN-83, SAAR/TrD, SIN/TrD and
SIN/ZPC, four different types of SINV vaccines were developed
against VEEV (162). The SIN-83
vaccine-induced immune profile was lower than that of TC-83
vaccination, although it caused negligible disease in mice. SIN-83
vaccination successfully induced immunization against the
intranasal and subcutaneous challenges; however, the result against
intracerebral challenge with heterologous VEEV strain ZPC 738 was
not completely acceptable (161).
In addition, Paessler et al (162) evaluated the immunization
potential of three vaccines in mice and hamsters challenged with
the VEEV strain ZPC 738, which demonstrated 100% protection.
Nevertheless, safety is a priority and live vaccines attract
concern. In a 6-day-old mouse model of VEEV central nervous system
infection, the immune efficacy of three different vaccines, TC-83,
AAR/TRD, SIN/TRD, or SIN/ZPC, and SIN-83 was examined in mice.
Accordingly, the lethal rate was 100% for TC-83; in addition, all
mice vaccinated with SIN-83 survived, which indicated complete
success; the mice administered the other vaccines demonstrated a
moderate rate of survival of 60-80% (162).
Another chimeric vaccine candidate is EILV/TC83, in
which structural genes of EILV (C-E3-E2-6k-E1) were replaced with
those of the TC-83 strain. It induced immunization against the
virulent VEEV 3908 strain in mice (163). MVA was manipulated to express
E3-E2-6k-E1 proteins of the TrD strain of VEEV tested as a vaccine
candidate. MVA-Bavarian Nordic (MVA-BN) was employed as a vector
under the control of a synthetic PrHyb promoter to clone enveloped
proteins of VEEV (164). A single
dose of the MVA-BN-VEEV chimera did not infect mice; however, after
2 weeks, the immune systems of all animals excreted nAb titers
after the booster dose and the animals survived against the
challenge with virulent TrD (164).
Subunit vaccine candidates introduce novel, safe
and high-tech methods for vaccine production. pWRG7077 was utilized
as a vector to express structural genes of the TrD strain of VEEV
(C-E3-E2-6K-E1) in mammals using the gene gun immunization of the
epidermis. In addition, compared with the wild-type plasmid, the
VEEV aerosol challenge protected mice (165) and macaques (166). However, the response of nAbs was
low, and one macaque exhibited low viremia after infection and
exhibited non-sterile immunity (166). DNA vaccination regularly conveys
a DNA plasmid encoding at least one antigen to incite an immune
reaction (167). Another study
demonstrated that a DNA E2 recombinant plasmid vaccine which
contains E2 gene sequences from VEEV IA/B and IE, WEEV and EEEV,
and Mucambo virus and E1 glycoprotein sequences induced a
cross-reactive antibody response against all viruses following
intradermal administration (168).
Another vaccine was developed using novel iDNA
vaccine technology, which is based on infectious DNA. This type of
vaccine possesses the advantages of DNA and live attenuated
vaccines. To develop such a VEEV-DNA vaccine, the full-length
genomic RNA of the TC-83 live attenuated virus was used under the
control of a CMV promoter in a pcDNA3.1-derived plasmid vector.
In vivo developed viral RNA initiated the limited
replication of the vaccine virus. Consequently, to evaluate its
efficacy, a single dose of the pTC-83 iDNA vaccine was administered
to BALB/c mice. Immediately after vaccination, all mice were
seroconverted with no adverse reactions and after 4 weeks, all
animals were challenged with the lethal epidemic strain of VEEV.
All vaccinated animals survived, while the unvaccinated control
groups succumbed to the infection and thus did not survive
(169). Rico et al
(170) designed an alternate
strategy to manage vaccine advancement, which involved using E1
glycoproteins as the antigen for immunization advancement (170). Lipid-antigen-nucleic
acid-complexes containing VEEV E1 and WEEV E1 antigens were formed
through the integration of the purified E1 glycoprotein of the TrD
strain of VEEV and WEEV in cationic liposomes.
Since the major conditions for the replication of
the Alphavirus genome are non-structural proteins and
cis-acting RNA sequences, the genes of structural proteins
can be altered and foreign antigens are highly expressed (171,172). VRPs, which can only replicate
during one cycle due to the lack of structural genes in the virus
offspring, have been shown to exert protective effects against VEEV
infection when administered as early as 6 h prior to viral
infection. The VRP was generated using the V3000 backbone, which
contains a mature VRP263 mRNA transcription initiation site
nucleotide downstream, a V3000 5'UTR, and a genome containing
non-structural genes 1-4. The VRP envelope contains E3 and E1
glycoproteins, derived from V3000(173). This corresponding non-specific
defense mechanism is not yet fully understood; however, the
activation of the innate immune response and the inclusion of the
antiviral state, possibly through the release of endogenous type I
IFN, may mediate defense (174).
Human adenovirus type 5 (Rad/VEEV)-based replication-deficient VEEV
vaccine expressing the E2 glycoprotein VEEV serogroups IA/B has
also been improved. For this, the structural genes of TC-83
(E3-E2-6K) were cloned into PMV100 plasmids and site-directed
mutagenesis was used to convert the TC-2E2 glycoprotein into TDE2
glycoprotein. The modified VEEV structural gene sequence was cloned
into a pMV60 plasmid to create pMV60/VEEV. Subsequently, the
homologous recombination of pMV60/VEEV and pJM17 (containing the
entire genome of Ad5) plasmids in 293 cells was utilized to
generate replication-deficient human adenovirus type 5 (Ad5)
containing VEEV structural proteins (Rad/VEEV) (175). BALB/c mice, 6 to 8 weeks old
immunized intranasally with 107 PFU of recombinant
adenovirus (Rad/VEEV) at various intervals (0 to 21) were protected
from low-to-intermediate doses of infectious VEEV; however, this
immunization was not successful against high doses of infectious
VEEV (175).
nAbs provide a protective shield against peripheral
inoculation or natural Alphavirus infection (175,176). The E2 and E3 glycoproteins of
VEEV can induce monoclonal antibodies which have been shown to
protect mice from challenge with infectious VEEV, whereas E1
glycoproteins provide only weak protection against the infectious
viral challenge (177). In the
study by O'Brien et al (178), they distinguished an extensively
responsive monoclonal immunizer, CUF37-2a, from animals that were
first vaccinated with TC-83 followed by an introduction to six
distinctive serotypes of VEEV (subtypes I, II, III, IV, V and VI).
CUF37-2a was discovered to be explicit to the E2 glycoprotein of
VEEV and recognized all the VEEV subtypes, aside from the subtype
VI, with which it demonstrated a more fragile reactivity. The
antibodies protected the mice from subcutaneous presentation to the
wild-type TrD strain of VEEV (178).
Clinical trials
Currently, multiple groups are performing clinical
trials that aim to study and assess the safety and immunogenicity
of the TC-83 obtained vaccine in adult healthy volunteers. TC-83
has certain disadvantages; thus, it is highly probable that it will
not be affirmed for mass vaccination in the human population. TC-83
is capable of causing an immunological reaction. Vaccines lead to
23-37% spontaneous flu-like symptoms, such as rash, headache,
fever, chills, nausea, diarrhea and myalgia (148,152,179). TC-83 has been isolated from
mosquitoes in the southern states of the USA and poses a
significant environmental risk of spreading following immunization
(180). It has a poor response
rate that is suggested to depend on the HLA typing f the host.
Among responders, during the first year, the antibody titer
decreases following immunization, requiring booster immunizations
to maintain the protective antibody titer. There is also a
possibility of reversion to virulence (152,181). Site-directed mutagenesis has been
employed to generate mutant strains of VEEV that exhibit
differential replication and/or tissue tropism in mice compared to
parent full-length V3000 clones (182,183). Davis et al (183) evaluated V3526, one the
live-attenuated strains of VEEV as a potential vaccine. They
obtained V3526 through the sequence of clonal isolates (J9-1a and
J9-1b) of the mutant V3022 strain. V3526 was produced by
site-specific mutagenesis that removes the furin-like cleavage site
of V3022's E2 (PE2) precursor protein (183). In phase I clinical trials, the
V3526 vaccine demonstrated adverse reactions, such as myalgia,
lymphopenia, pyrexia and tachycardia in the volunteers. Since nasal
and throat samples were positive for V3526 and emerging febrile
reactions co-occurred with this, the development of the vaccine was
terminated. On the other hand, the safety profile provided by this
vaccine was found to be excellent in animal models (141).
Inactivated vaccines for VEEV can
elicit high nAb titers
In the 1970s, a new, formalin-inactivated vaccine
for VEE (C-84) was developed by the US Army based on the TC-83 live
attenuated vaccine. Incidental, mild, local and systemic reactions
were only observed in 28 volunteers; there were no significant
changes in clinical laboratory parameters. The vaccine increased
preexisting serum-neutralizing antibody titers against the VEE
virus in seropositive TC-83 vaccine recipients and elicited high
nAb titers in non-immune subjects after a primary vaccine and
two-dose vaccination (Table I)
(184).
Live attenuated vaccines may be a
potential VEE vaccine
Another research group commenced an investigation
on the VEEV candidate vaccine on February 9, 2017. The study
sponsored by the US Army Medical Research and Development Command
was conducted in phase II and aimed to evaluate the safety and
immunogenicity of the live attenuated vaccine for VEE, dried TC-83,
in 500 healthy volunteers. 18-65 years of age. The volunteers were
administered 0.50 ml of the VEE vaccine subcutaneously in the upper
outer aspect of the triceps region. The estimated study completion
date is on April 1, 2023 (ClinicalTrials.gov Identifier: NCT03051386) (Table I) (185).
DNA vaccines are safer than
inactivated vaccines
On December, 2013, Hannaman et al designed a
study to investigate the DNA vaccine candidate, which expressed the
E3-E2-6K-E1 genes of VEE (pWRG/VEEV) and performed a phase I
clinical study to assess the safety, reactogenicity, tolerability
and immunogenicity of the vaccine. Participants were administered
intramuscular or intradermal electroporation. In the intramuscular
electroporation group, members received 0.50 mg, 2.00 mg of
pWRG/VEE, or the saline placebo in a 1.0 ml injection,
respectively. Participants in the other group (intradermal
electroporation) received 0.08 or 0.30 mg of DNA or saline placebo
in a 0.15 ml injection. The safety of each administration dose was
assessed on days 0, 28 and 56. After two doses, all subjects
exhibited measurable levels of nAbs. In addition, nAbs were
detectable in samples from all subjects after the third vaccination
(Table I) (186).
6. Ross River virus
Ross River virus (RRV) which belongs to the
Togaviridae family of the Alphavirus genus and is a
mosquito-transmitted virus that has a specific molecular
characterization. This zoonotic, positive-strand RNA virus causes a
rash, fever, fatigue and most prominently, arthralgia, which may
persist for months to even years (Fig.
1) (187). This virus causes
the most widespread vector-borne disease in Australia with
>5.000 cases annually (188).
RRV has a complex ecology that has been isolated
from >40 species of mosquitos and also infects/amplifies in at
least 18 animal host species (Fig.
1) (189). There are
currently no approved treatments or vaccines available against the
virus. nAbs may be the only effective path to discovering new
treatments and vaccine designs. There are human monoclonal
antibodies designed for distantly related Alphavirus that binds to
the Mxra8 Alphavirus receptor. With a cryo-electron microscope, the
attachment of these antibodies with RRV reveals a conserved
footprint of neutralizing monoclonal antibody RRV-12 in a region of
the virus surface protein (187).
There are >80 copies of a trimer of
heterodimeric glycoproteins on the surface of the virus. These
heterodimers consist of two glycoproteins (E1 and E2), which are
the target of nAbs (190).
The E2 glycoprotein is exclusively divided into
three domains (A, B and C). The A domain, which is exposed at the
surface, connects domains B and C. The B domain shields the fusion
loop of the E1 protein and the C domain of the E2 glycoprotein.
This B domain of E2 glycoprotein is the target site of monoclonal
nAb in the RRV (191).
RRV-12 monoclonal antibody and its defined epitopes
neutralize and prevent virus entrance to cells in a mouse model.
Accordingly, this monoclonal antibody binds to the B domain of E2
glycoprotein; thus, it is a good candidate for further research on
vaccine developments (187).
CHK-265 cross-reactive murine antibody also inhibits and
neutralizes the RRV, but this inhibition occurs more potently for
CHIK and MAYV viruses (187).
The similarity of both RRV-12 and CHK-265 binding
sites within the B domain reveals the importance of vaccine design
based on epitope for research, particularly when the exact residue
at the binding site is shown. For example, CHK-265 binds to
residues 182-189, 203-206, and 214-218 in the B domain of the E2
glycoprotein (191).
Preclinical trials
In 2011, Holzer et al (192) performed a study to evaluate
inactivated RRV vaccine in active and passive mouse immunization. A
formalin- and UV-inactivated whole virus vaccine was derived from
animal protein-free cell culture. In the first group, the mean
active immunization group, female CD-1 mice received a solution of
500 µl containing vaccine doses of 10, 2.5, 0.625, 0.156, 0.039,
0.01 or 0.0025 µg, on days 0 and 28. After 42 days, the mice were
challenged with the mouse-virulent RRV prototype strain T48 [ATCC
VR-373] in a volume of 100 µl. Another group, the active
immunization group (IFN-α/βR-/-), mice were injected
with a solution of 50 µl containing vaccine doses of 1, 0.25 and
0.063 µg on days 0 and 21. At 42 days after the first shot, the
mice were challenged with 102.5 TCID50 of the
RRV prototype strain T48 in a volume of 10 µl. The passive
immunization of young mice with sera from human vaccines was
carried out using 100 µl complement-inactivated human serum
intraperitoneally. After 24 h, the animals were challenged with
104 TCID50 of infectious RRV T48. In that
study, the vaccine elicited a potent antibody response in both
models (192).
Clinical trials
Another investigation was performed in the same
year with an inactivated whole-virus Vero cell-derived RRV vaccine
in 382 healthy adults. Volunteers received 1.25, 2.5, 5, or 10 µg
aluminum hydroxide-adjuvanted or non-adjuvanted RRV vaccine. After
21 days, the second dose was administered and consequently, booster
doses were injected 6 months later. To evaluate the safety and
immunogenicity of the vaccine, serum IgG and nAb titers were
tested. The results indicated that the optimum concentration of the
vaccine formulation was the adjuvanted 2.5 µg dose. Consequently,
the candidate vaccine, adjuvanted inactivated whole-virus Vero
cell-derived Ross River virus vaccine, was found to be highly
immunogenic in RRV-naïve adults and was well-tolerated at all dose
levels (Table I) (193).
7. Semliki Forest virus
Semliki Forest virus (SFV), as a positive-stranded
RNA enveloped virus, is a member of the Togaviridae family
and the Alphavirus genus along with the Sindbis and VEE virus,
which are arthropod-borne (194).
The genome of wild-type SFV expresses non-structural proteins that
are in control of the transcription and replication of viral RNA
and structural proteins, such as the capsid protein and envelope
glycoproteins. The membrane envelope possesses the E1, E2, and E3
glycoproteins, which are involved in receptor distinction and
fusion (195). Animal hosts for
SFV differ from mosquitos to avian and mammalian species (Fig. 1) (196). Some vaccine strategies are based
on peptides or protein subunits (197,198), recombinant vaccinia
virus-vectored vaccines (199,200), adenovirus, or baculovirus-derived
vectors (201,202) and naked DNA-based vaccines
(203) are followed (204).
In the process of vaccine development, the SFV
system was applied first as a DNA or an RNA-derived vaccine, in
which the structural genes were replaced with an external gene
(203,205). The recombinant SFV (rSFV)
particles, which have the ability to infect host cells without
replication, have been studied in mice in different virus models,
such as the influenza virus (206), louping ill virus (207), human immunodeficiency virus
(208), and human papillomavirus
(209).
The inactivation of the SFV can be achieved using
different processes, such as formalin, β-propiolactone,
hydroxylamine and 2-ethyl ethylenimine; formalin inactivation
appears to be the optimal process. These types of vaccines have
been evaluated in white mice (210).
Various analyses have been conducted to assess the
efficacy of different vaccine platforms, such as testing the
recombinant SFV particles expressing the hepatitis C virus
non-structural protein 3 (NS3) (211), and constructing and testing the
infectious but non-replicative SFV particles encoding Brucella
abortus Cu, Zn superoxide dismutase (SOD) (212-218).
Vectors based on the SFV have been broadly tested in vitro
and in vivo to express heterologous genes in animal cells
(219).
Some clinical trials were conducted an RNA replicon
vaccine based on the SFV, in which these replication-defective SFV
replicon particles demonstrated potent immune responses in animal
models against a variety of viral and tumor antigens (172,206,220-222).
These preclinical studies have promised to certify other clinical
studies of this vector platform (223).
Preclinical and clinical trials
To achieve the rSFV vectors, the structural genes
were deleted from the SFV genome and replaced by the targeted gene.
This genome was packaged in a viral particle containing a
nucleocapsid and a membrane envelope (195). In order to develop a therapeutic
vaccine against HPV-induced cancers, an SFV-based vector platform
was utilized (224). An
rSFV-based therapeutic vaccine, Vvax001, encoding a fusion protein
of HPV16 E6 and E7, which is the first-in-human clinical, was
developed by Komdeur et al (225). The results of the phase I
revealed the safety of the Vvax001, which can elicit potent immune
responses from participants (Table
I) (225). Several studies
have been conducted based on using rSFV-based RNA for vaccination
against a range of pathogens, as for example in a study in which
mice were immunized with rSFV RNA encoding HIV-1 HXB2 gp160 protein
which led to an antigen-specific humoral immune response (108,226,227,228). In another study, the intradermal
electroporation with the SFV-based RNA, expressing luciferase and
galactosidase proteins, exerted potent cellular and humoral
responses in mice (229). These
vectors have also been used as antitumor vaccines (200). In another assessment, the potency
of the SFV-based self-amplifying RNA as a recombinant vaccine
against HIV-1C in mice was evaluated, as it is considered an
encouraging approach for preventing the transmission and
eradication of HIV/AIDS. Consequently, the extraction of clear
cell-mediated and humoral immune responses in mice by rSFV2 gen RNA
encoding HIV-1C antigens illustrated the potency of self-amplifying
rSFV2gen RNA as a promising candidate for anti-HIV vaccine
development (208). In the
process of searching for new respiratory syncytial virus (RSV)
vaccine candidates, a strategy based on an Alphavirus vector and
SFV particles, which are self-abortive vector particles expressing
RSV F and G proteins from an RNA replicon, was designed (206).
The analysis of recombinant RNA based on the SFV
replicon expressing the nucleoprotein of the influenza virus was
tested in mice, which demonstrated that the self-replicative
recombinant SFV RNA may be useful as a nucleic acid vaccine
(228).
The SFV expression system was estimated as a basis
for avian vaccine development due to preliminary studies revealing
that 1-day-old specific pathogen-free (SPF) chicks were sensitive
to infection with a strain of SFV, generating SFV-specific
antibodies, but exhibiting no clinical disease. Intramuscular
immunization with recombinant replication-defective SFV particles,
which express the Escherichia coli lacZ reporter gene, led
to high titers of β-gal-specific antibodies at 4 weeks p.i. after
two inoculations. However, significantly lower antibody levels were
observed in chicks immunized with a recombinant SFV-based DNA
construct or a conventional CMV promoter-based DNA plasmid. rSFV
particles which encode the protective VP2 protein or the
VP2/VP4/VP3 polyprotein of infectious bursal disease virus (IBDV)
were produced and the antigens that were expressed were
characterized in cell culture. Proteins were generated and found to
respond against a range of IBDV-specific monoclonal antibodies. The
outcome of the immunization of 1-day-old SPF chicks with rSFV
particles expressing the IBDV proteins was specific antibodies
evoked in all birds and the production of nAbs in some, but not all
birds (230).
8. Conclusions and future perspectives
Having noted the absence of effective therapeutic
drugs and the rapid worldwide spread of viral infections, the
development of novel vaccine technologies, such as recombinant
subunit and DNA vaccines, should not be neglected. It is a fact
that finding any single nucleotide polymorphisms in the genome of
viruses may reduce the cross-protective immune responses against
these (231). Therefore, finding
much more effective and functional antiviral vaccines needs to be
prioritized before the emergence of new viral pandemics. The
present review aimed to provide sufficient data on various vaccine
platforms in different stages of human clinical trials against the
most significant zoonotic viruses of Coronavirus and Alphavirus.
Over the past decades, the massive outbreaks of these viral
families, such as MERS-CoV, SARS-CoV-2 and CHIKV, illustrate the
essential need for stimulating research activities with large-scale
investment to design and formulate new vaccine candidates in the
future. As there are other viruses belonging to these families that
have the propensity to ‘spill over’ around the globe, further
investigations that aim to contribute toward the development of new
vaccine generations are warranted. Consequently, these approaches
may be employed when society requires to confront the next
unavoidable contagious infection outbreak.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AT, SSB and DM designed the study. ES, DAS and DM
were involved in the screening of the literature for inclusion in
the review. AT was involved in quality assessment. SSB, HZ, ES,
NSG, MK, ST, DAS and HS were involved in the writing and revision
of the manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The authors declare that they have no competing interests.
References
|
1
|
Du L, Tai W, Zhou Y and Jiang S: Vaccines
for the prevention against the threat of MERS-CoV. Expert Rev
Vaccines. 15:1123–1134. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hui DSC and Zumla A: Severe acute
respiratory syndrome: Historical, epidemiologic, and clinical
features. Infect Dis Clin North Am. 33:869–889. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramadan N and Shaib H: Middle East
respiratory syndrome coronavirus (MERS-CoV): A review. Germs.
9:35–42. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Donnelly CA, Malik MR, Elkholy A,
Cauchemez S and Van Kerkhove MD: Worldwide reduction in MERS cases
and deaths since 2016. Emerg Infect Dis. 25:1758–1760.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Folegatti PM, Harrison K, Preciado-Llanes
L, Lopez FR, Bittaye M, Kim YC, Flaxman A, Bellamy D, Makinson R,
Sheridan J, et al: A single dose of ChAdOx1 Chik vaccine induces
neutralizing antibodies against four chikungunya virus lineages in
a phase 1 clinical trial. Nat Commun. 12(4636)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zeller H, Van Bortel W and Sudre B:
Chikungunya: Its history in Africa and Asia and its spread to new
regions in 2013-2014. J Infect Dis. 214 (Suppl 5):S436–S440.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bernasconi V, Kristiansen PA, Whelan M,
Román RG, Bettis A, Yimer SA, Gurry C, Andersen SR, Yeskey D, Mandi
H, et al: Developing vaccines against epidemic-prone emerging
infectious diseases. Bundesgesundheitsblatt Gesundheitsforschung
Gesundheitsschutz. 63:65–73. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zaki AM, Van Boheemen S, Bestebroer TM,
Osterhaus AD and Fouchier RA: Isolation of a novel coronavirus from
a man with pneumonia in Saudi Arabia. N Engl J Med. 367:1814–1820.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
World Health Organization (WHO): ‘MERS
situation update, September 2019’. WHO, Geneva, 2019. https://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-september-2019.html.
|
|
11
|
Wang C, Zheng X, Gai W, Wong G, Wang H,
Jin H, Feng N, Zhao Y, Zhang W, Li N, et al: Novel chimeric
virus-like particles vaccine displaying MERS-CoV receptor-binding
domain induce specific humoral and cellular immune response in
mice. Antiviral Res. 140:55–61. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu X, Zhang S, Jiang L, Cui Y, Li D, Wang
D, Wang N, Fu L, Shi X, Li Z, et al: Structural basis for the
neutralization of MERS-CoV by a human monoclonal antibody MERS-27.
Sci Rep. 5(13133)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Letko M, Marzi A and Munster V: Functional
assessment of cell entry and receptor usage for SARS-CoV-2 and
other lineage B betacoronaviruses. Nat Microbiol. 5:562–569.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li F and Du L: MERS coronavirus: An
emerging zoonotic virus. Viruses. 11(663)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara
H, Geng Q, Auerbach A and Li F: Structural basis of receptor
recognition by SARS-CoV-2. Nature. 581:221–224. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Woo PCY, Lau SKP, Chen Y, Wong EYM, Chan
KH, Chen H, Zhang L, Xia N and Yuen KY: Rapid detection of MERS
coronavirus-like viruses in bats: Pote1ntial for tracking MERS
coronavirus transmission and animal origin. Emerg Microbes Infect.
7(18)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cotten M, Watson SJ, Zumla AI, Makhdoom
HQ, Palser AL, Ong SH, Al Rabeeah AA, Alhakeem RF, Assiri A,
Al-Tawfiq JA, et al: Spread, circulation, and evolution of the
Middle East respiratory syndrome coronavirus. mBio. 5:e01062–13.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Azhar EI, El-Kafrawy SA, Farraj SA, Hassan
AM, Al-Saeed MS, Hashem AM and Madani TA: Evidence for
camel-to-human transmission of MERS coronavirus. N Engl J Med.
370:2499–2505. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Alshukairi AN, Zheng J, Zhao J, Nehdi A,
Baharoon SA, Layqah L, Bokhari A, Al Johani SM, Samman N, Boudjelal
M, et al: High prevalence of MERS-CoV infection in camel workers in
Saudi Arabia. mBio. 9:e01985–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li E, Chi H, Huang P, Yan F, Zhang Y, Liu
C, Wang Z, Li G, Zhang S, Mo R, et al: A novel bacterium-like
particle vaccine displaying the MERS-CoV receptor-binding domain
induces specific mucosal and systemic immune responses in mice.
Viruses. 11(799)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chi H, Zheng X, Wang X, Wang C, Wang H,
Gai W, Perlman S, Yang S, Zhao J and Xia X: DNA vaccine encoding
Middle East respiratory syndrome coronavirus S1 protein induces
protective immune responses in mice. Vaccine. 35:2069–2075.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Al-Amri SS, Abbas AT, Siddiq LA, Alghamdi
A, Sanki MA, Al-Muhanna MK, Alhabbab RY, Azhar EI, Li X and Hashem
AM: Immunogenicity of candidate MERS-CoV DNA vaccines based on the
spike protein. Sci Rep. 7(44875)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ababneh M, Alrwashdeh M and Khalifeh M:
Recombinant adenoviral vaccine encoding the spike 1 subunit of the
Middle East respiratory syndrome coronavirus elicits strong humoral
and cellular immune responses in mice. Vet World. 12:1554–1562.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bodmer BS, Fiedler AH, Hanauer JRH, Prüfer
S and Mühlebach MD: Live-attenuated bivalent measles virus-derived
vaccines targeting Middle East respiratory syndrome coronavirus
induce robust and multifunctional T cell responses against both
viruses in an appropriate mouse model. Virology. 521:99–107.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Langenmayer MC, Lülf-Averhoff AT,
Adam-Neumair S, Fux R, Sutter G and Volz A: Distribution and
absence of generalized lesions in mice following single dose
intramuscular inoculation of the vaccine candidate MVA-MERS-S.
Biologicals. 54:58–62. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Adney DR, Wang L, Van Doremalen N, Shi W,
Zhang Y, Kong WP, Miller MR, Bushmaker T, Scott D, de Wit E, et al:
Efficacy of an adjuvanted Middle East respiratory syndrome
coronavirus spike protein vaccine in dromedary camels and alpacas.
Viruses. 11(212)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Alharbi NK, Qasim I, Almasoud A, Aljami
HA, Alenazi MW, Alhafufi A, Aldibasi OS, Hashem AM, Kasem S,
Albrahim R, et al: Humoral immunogenicity and efficacy of a single
dose of ChAdOx1 MERS vaccine candidate in dromedary camels. Sci
Rep. 9(16292)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hashem AM, Algaissi A, Agrawal AS, Al-Amri
SS, Alhabbab RY, Sohrab SS, S Almasoud A, Alharbi NK, Peng BH,
Russell M, et al: A highly immunogenic, protective, and safe
adenovirus-based vaccine expressing Middle East respiratory
syndrome coronavirus S1-CD40L fusion protein in a transgenic human
dipeptidyl peptidase 4 mouse model. J Infect Dis. 220:1558–1567.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lemiale F, Haddada H, Nabel GJ, Brough DE,
King CR and Gall JG: Novel adenovirus vaccine vectors based on the
enteric-tropic serotype 41. Vaccine. 25:2074–2084. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alharbi NK, Padron-Regalado E, Thompson
CP, Kupke A, Wells D, Sloan MA, Grehan K, Temperton N, Lambe T,
Warimwe G, et al: ChAdOx1 and MVA based vaccine candidates against
MERS-CoV elicit neutralising antibodies and cellular immune
responses in mice. Vaccine. 35:3780–3788. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu R, Wang J, Shao Y, Wang X, Zhang H,
Shuai L, Ge J, Wen Z and Bu Z: A recombinant VSV-vectored MERS-CoV
vaccine induces neutralizing antibody and T cell responses in
rhesus monkeys after single dose immunization. Antiviral Res.
150:30–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhou Y, Yang Y, Huang J, Jiang S and Du L:
Advances in MERS-CoV vaccines and therapeutics based on the
receptor-binding domain. Viruses. 11(60)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Agnihothram S, Menachery VD, Yount BL Jr,
Lindesmith LC, Scobey T, Whitmore A, Schäfer A, Heise MT and Baric
RS: Development of a broadly accessible Venezuelan equine
encephalitis virus replicon particle vaccine platform. J Virol.
92:e00027–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lan J, Yao Y, Deng Y, Chen H, Lu G, Wang
W, Bao L, Deng W, Wei Q, Gao GF, et al: Recombinant receptor
binding domain protein induces partial protective immunity in
rhesus macaques against Middle East respiratory syndrome
coronavirus challenge. EEBioMedicine. 2:1438–1446. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Q, Qi J, Yuan Y, Xuan Y, Han P, Wan
Y, Ji W, Li Y, Wu Y, Wang J, et al: Bat origins of MERS-CoV
supported by bat coronavirus HKU4 usage of human receptor CD26.
Cell Host Microbe. 16:328–337. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu RQ, Ge JY, Wang JL, Yu S, Zhang HL,
Wang JL, Wen ZY and Bu ZG: Newcastle disease virus-based MERS-CoV
candidate vaccine elicits high-level and lasting neutralizing
antibodies in Bactrian camels. J Integr Agric. 16:2264–2273.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pallesen J, Wang N, Corbett KS, Wrapp D,
Kirchdoerfer RN, Turner HL, Cottrell CA, Becker MM, Wang L, Shi W,
et al: Immunogenicity and structures of a rationally designed
prefusion MERS-CoV spike antigen. Proc Natl Acad Sci USA.
114:E7348–E7357. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wirblich C, Coleman CM, Kurup D, Abraham
TS, Bernbaum JG, Jahrling PB, Hensley LE, Johnson RF, Frieman MB
and Schnell MJ: One-health: A safe, efficient, dual-use vaccine for
humans and animals against Middle East respiratory syndrome
coronavirus and rabies virus. J Virol. 91:e02040–16.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tai W, Wang Y, Fett CA, Zhao G, Li F,
Perlman S, Jiang S, Zhou Y and Du L: Recombinant receptor-binding
domains of multiple Middle East respiratory syndrome coronaviruses
(MERS-CoVs) induce cross-neutralizing antibodies against divergent
human and camel MERS-CoVs and antibody escape mutants. J Virol.
91:e01651–16. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Papageorgiou N, Lichière J, Baklouti A,
Ferron F, Sévajol M, Canard B and Coutard B: Structural
characterization of the N-terminal part of the MERS-CoV
nucleocapsid by X-ray diffraction and small-angle X-ray scattering.
Acta Crystallogr D Struct Biol. 72:192–202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang L, Li L, Yan L, Ming Z, Jia Z, Lou Z
and Rao Z: Structural and biochemical characterization of
endoribonuclease Nsp15 encoded by Middle East respiratory syndrome
coronavirus. J Virol. 92:e00893–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tang J, Zhang N, Tao X, Zhao G, Guo Y,
Tseng CTK, Jiang S, Du L and Zhou Y: Optimization of antigen dose
for a receptor-binding domain-based subunit vaccine against MERS
coronavirus. Hum Vaccin Immunother. 11:1244–1250. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Du L, Zhao G, Kou Z, Ma C, Sun S, Poon VK,
Lu L, Wang L, Debnath AK, Zheng BJ, et al: Identification of a
receptor-binding domain in the S protein of the novel human
coronavirus Middle East respiratory syndrome coronavirus as an
essential target for vaccine development. J Virol. 87:9939–9942.
2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nyon MP, Du L, Tseng CTK, Seid CA, Pollet
J, Naceanceno KS, Agrawal A, Algaissi A, Peng BH, Tai W, et al:
Engineering a stable CHO cell line for the expression of a
MERS-coronavirus vaccine antigen. Vaccine. 36:1853–1862.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tao X, Garron T, Agrawal AS, Algaissi A,
Peng BH, Wakamiya M, Chan TS, Lu L, Du L, Jiang S, et al:
Characterization and demonstration of the value of a lethal mouse
model of Middle East respiratory syndrome coronavirus infection and
disease. J Virol. 90:57–67. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hao W, Wojdyla JA, Zhao R, Han R, Das R,
Zlatev I, Manoharan M, Wang M and Cui S: Crystal structure of
Middle East respiratory syndrome coronavirus helicase. PLoS Pathog.
13(e1006474)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang C, Zheng X, Gai W, Zhao Y, Wang H,
Wang H, Feng N, Chi H, Qiu B, Li N, et al: MERS-CoV virus-like
particles produced in insect cells induce specific humoural and
cellular imminity in rhesus macaques. Oncotarget. 8:12686–12694.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Coleman CM, Venkataraman T, Liu YV, Glenn
GM, Smith GE, Flyer DC and Frieman MB: MERS-CoV spike nanoparticles
protect mice from MERS-CoV infection. Vaccine. 35:1586–1589.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Coleman CM, Liu YV, Mu H, Taylor JK,
Massare M, Flyer DC, Glenn GM, Smith GE and Frieman MB: Purified
coronavirus spike protein nanoparticles induce coronavirus
neutralizing antibodies in mice. Vaccine. 32:3169–3174.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kato T, Takami Y, Kumar Deo V and Park EY:
Preparation of virus-like particle mimetic nanovesicles displaying
the S protein of Middle East respiratory syndrome coronavirus using
insect cells. J Biotechnol. 306:177–184. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jung SY, Kang KW, Lee EY, Seo DW, Kim HL,
Kim H, Kwon T, Park HL, Kim H, Lee SM and Nam JH: Heterologous
prime-boost vaccination with adenoviral vector and protein
nanoparticles induces both Th1 and Th2 responses against Middle
East respiratory syndrome coronavirus. Vaccine. 36:3468–3476.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Veit S, Jany S, Fux R, Sutter G and Volz
A: CD8+ T cells responding to the Middle East respiratory syndrome
coronavirus nucleocapsid protein delivered by vaccinia virus MVA in
mice. Viruses. 10(718)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Agrawal AS, Tao X, Algaissi A, Garron T,
Narayanan K, Peng BH, Couch RB and Tseng CTK: Immunization with
inactivated Middle East respiratory syndrome coronavirus vaccine
leads to lung immunopathology on challenge with live virus. Hum
Vaccin Immunother. 12:2351–2356. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Deng Y, Lan J, Bao L, Huang B, Ye F, Chen
Y, Yao Y, Wang W, Qin C and Tan W: Enhanced protection in mice
induced by immunization with inactivated whole viruses compare to
spike protein of middle east respiratory syndrome coronavirus.
Emerg Microbes Infect. 7(60)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Scobey T, Yount BL, Sims AC, Donaldson EF,
Agnihothram SS, Menachery VD, Graham RL, Swanstrom J, Bove PF, Kim
JD, et al: Reverse genetics with a full-length infectious cDNA of
the Middle East respiratory syndrome coronavirus. Proc Natl Acad
Sci USA. 110:16157–16162. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Modjarrad K, Roberts CC, Mills KT,
Castellano AR, Paolino K, Muthumani K, Reuschel EL, Robb ML, Racine
T, Oh MD, et al: Safety and immunogenicity of an anti-Middle East
respiratory syndrome coronavirus DNA vaccine: A phase 1,
open-label, single-arm, dose-escalation trial. Lancet Infect Dis.
19:1013–1022. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Muthumani K, Falzarano D, Reuschel EL,
Tingey C, Flingai S, Villarreal DO, Wise M, Patel A, Izmirly A,
Aljuaid A, et al: A synthetic consensus anti-spike protein DNA
vaccine induces protective immunity against Middle East respiratory
syndrome coronavirus in nonhuman primates. Sci Transl Med.
7(301ra132)2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Koch T, Dahlke C, Fathi A, Kupke A,
Krähling V, Okba NMA, Halwe S, Rohde C, Eickmann M, Volz A, et al:
Safety and immunogenicity of a modified vaccinia virus Ankara
vector vaccine candidate for Middle East respiratory syndrome: An
open-label, phase 1 trial. Lancet Infect Dis. 20:827–838.
2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhao J, Li K, Wohlford-Lenane C,
Agnihothram SS, Fett C, Zhao J, Gale MJ Jr, Baric RS, Enjuanes L,
Gallagher T, et al: Rapid generation of a mouse model for Middle
East respiratory syndrome. Proc Natl Acad Sci USA. 111:4970–4975.
2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Folegatti PM, Bittaye M, Flaxman A, Lopez
FR, Bellamy D, Kupke A, Mair C, Makinson R, Sheridan J, Rohde C, et
al: Safety and immunogenicity of a candidate Middle East
respiratory syndrome coronavirus viral-vectored vaccine: A
dose-escalation, open-label, non-randomised, uncontrolled, phase 1
trial. Lancet Infect Dis. 20:816–826. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bosaeed M, Balkhy HH, Almaziad S, Aljami
HA, Alhatmi H, Alanazi H, Alahmadi M, Jawhary A, Alenazi MW,
Almasoud A, et al: Safety and immunogenicity of ChAdOx1 MERS
vaccine candidate in healthy Middle Eastern adults (MERS002): an
open-label, non-randomised, dose-escalation, phase 1b trial. Lancet
Microbe. 3:e11–e20. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Dolzhikova IV, Grousova DM, Zubkova OV,
Tukhvatulin AI, Kovyrshina AV, Lubenets NL, Ozharovskaia TA, Popova
O, Esmagambetov IB, Shcheblyakov DV, et al: Preclinical studies of
immunogenity, protectivity, and safety of the combined vector
vaccine for prevention of the Middle East respiratory syndrome.
Acta Naturae. 12:114–123. 2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chan JFW, Lau SKP and Woo PCY: The
emerging novel Middle East respiratory syndrome coronavirus: the
‘knowns’ and ‘unknowns’. J Formos Med Assoc. 112:372–381.
2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Du L, He Y, Zhou Y, Liu S, Zheng BJ and
Jiang S: The spike protein of SARS-CoV-a target for vaccine and
therapeutic development. Nat Rev Microbiol. 7:226–236.
2009.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Peiris JS, Guan Y and Yuen KY: Severe
acute respiratory syndrome. Nat Med. 10 (12 Suppl):S88–S97.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
66
|
Ge XY, Li JL, Yang XL, Chmura AA, Zhu G,
Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, et al: Isolation and
characterization of a bat SARS-like coronavirus that uses the ACE2
receptor. Nature. 503:535–538. 2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Enjuanes L, Dediego ML, Alvarez E, Deming
D, Sheahan T and Baric R: Vaccines to prevent severe acute
respiratory syndrome coronavirus-induced disease. Virus Res.
133:45–62. 2008.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ithete NL, Stoffberg S, Corman VM,
Cottontail VM, Richards LR, Schoeman MC, Drosten C, Drexler JF and
Preiser W: Close relative of human Middle East respiratory syndrome
coronavirus in bat, South Africa. Emerg Infect Dis. 19:1697–1699.
2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Minor PD: Live attenuated vaccines:
Historical successes and current challenges. Virology. 479:379–392.
2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lamirande EW, DeDiego ML, Roberts A,
Jackson JP, Alvarez E, Sheahan T, Shieh WJ, Zaki SR, Baric R,
Enjuanes L and Subbarao K: A live attenuated severe acute
respiratory syndrome coronavirus is immunogenic and efficacious in
golden Syrian hamsters. J Virol. 82:7721–7724. 2008.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Robson F, Khan KS, Le TK, Paris C,
Demirbag S, Barfuss P, Rocchi P and Ng WL: Coronavirus RNA
proofreading: molecular basis and therapeutic targeting. Mol Cell.
79:710–727. 2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Graham RL, Becker MM, Eckerle LD, Bolles
M, Denison MR and Baric RS: A live, impaired-fidelity coronavirus
vaccine protects in an aged, immunocompromised mouse model of
lethal disease. Nat Med. 18:1820–1826. 2012.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Menachery VD, Gralinski LE, Mitchell HD,
Dinnon KH III, Leist SR, Yount BL Jr, Graham RL, McAnarney ET,
Stratton KG, Cockrell AS, et al: Middle East respiratory syndrome
coronavirus nonstructural protein 16 is necessary for interferon
resistance and viral pathogenesis. mSphere. 2:e00346–17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Stauffer F, El-Bacha T and Da Poian AT:
Advances in the development of inactivated virus vaccines. Recent
Pat Antiinfect Drug Discov. 1:291–296. 2006.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Escriou N, Callendret B, Lorin V,
Combredet C, Marianneau P, Février M and Tangy F: Protection from
SARS coronavirus conferred by live measles vaccine expressing the
spike glycoprotein. Virology. 452:32–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zuñiga S, Pascual-Iglesias A, Sanchez CM,
Sola I and Enjuanes L: Virulence factors in porcine coronaviruses
and vaccine design. Virus Res. 226:142–151. 2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
DeDiego ML, Alvarez E, Almazán F, Rejas
MT, Lamirande E, Roberts A, Shieh WJ, Zaki SR, Subbarao K and
Enjuanes L: A severe acute respiratory syndrome coronavirus that
lacks the E gene is attenuated in vitro and in vivo. J Virol.
81:1701–1713. 2007.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Bukreyev A, Lamirande EW, Buchholz UJ,
Vogel LN, Elkins WR, St Claire M, Murphy BR, Subbarao K and Collins
PL: Mucosal immunisation of African green monkeys (Cercopithecus
aethiops) with an attenuated parainfluenza virus expressing the
SARS coronavirus spike protein for the prevention of SARS. Lancet.
363:2122–2127. 2004.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Qin E, Shi H, Tang L, Wang C, Chang G,
Ding Z, Zhao K, Wang J, Chen Z, Yu M, et al: Immunogenicity and
protective efficacy in monkeys of purified inactivated Vero-cell
SARS vaccine. Vaccine. 24:1028–1034. 2006.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Tseng CT, Sbrana E, Iwata-Yoshikawa N,
Newman PC, Garron T, Atmar RL, Peters CJ and Couch RB: Immunization
with SARS coronavirus vaccines leads to pulmonary immunopathology
on challenge with the SARS virus. PLoS One.
7(e35421)2012.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Iwata-Yoshikawa N, Uda A, Suzuki T,
Tsunetsugu-Yokota Y, Sato Y, Morikawa S, Tashiro M, Sata T,
Hasegawa H and Nagata N: Effects of Toll-like receptor stimulation
on eosinophilic infiltration in lungs of BALB/c mice immunized with
UV-inactivated severe acute respiratory syndrome-related
coronavirus vaccine. J Virol. 88:8597–8614. 2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Chang CY, Hong WWL, Chong P and Wu SC:
Influence of intron and exon splicing enhancers on mammalian cell
expression of a truncated spike protein of SARS-CoV and its
implication for subunit vaccine development. Vaccine. 24:1132–1141.
2006.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Feng Y, Wan M, Wang XJ, Zhang PY, Yu YL
and Wang LY: Expression of predicted B cell epitope peptide in S2
subunit of SARS coronavirus spike protein in E. coli and
identification of its mimic antigenicity. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 23:113–116. 2007.PubMed/NCBI(In Chinese).
|
|
84
|
Li J, Ulitzky L, Silberstein E, Taylor DR
and Viscidi R: Immunogenicity and protection efficacy of monomeric
and trimeric recombinant SARS coronavirus spike protein subunit
vaccine candidates. Viral Immunol. 26:126–132. 2013.PubMed/NCBI View Article : Google Scholar
|
|
85
|
He Y, Li J, Heck S, Lustigman S and Jiang
S: Antigenic and immunogenic characterization of recombinant
baculovirus-expressed severe acute respiratory syndrome coronavirus
spike protein: Implication for vaccine design. J Virol.
80:5757–5767. 2006.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Du L, Zhao G, Chan CC, Li L, He Y, Zhou Y,
Zheng BJ and Jiang S: A 219-mer CHO-expressing receptor-binding
domain of SARS-CoV S protein induces potent immune responses and
protective immunity. Viral Immunol. 23:211–219. 2010.PubMed/NCBI View Article : Google Scholar
|
|
87
|
He Y, Zhou Y, Liu S, Kou Z, Li W, Farzan M
and Jiang S: Receptor-binding domain of SARS-CoV spike protein
induces highly potent neutralizing antibodies: Implication for
developing subunit vaccine. Biochem Biophys Res Commun.
324:773–781. 2004.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Liu RY, Wu LZ, Huang BJ, Huang JL, Zhang
YL, Ke ML, Wang JM, Tan WP, Zhang RH, Chen HK, et al: Adenoviral
expression of a truncated S1 subunit of SARS-CoV spike protein
results in specific humoral immune responses against SARS-CoV in
rats. Virus Res. 112:24–31. 2005.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Wang N, Shang J, Jiang S and Du L: Subunit
vaccines against emerging pathogenic human coronaviruses. Front
Microbiol. 11(298)2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Buchholz UJ, Bukreyev A, Yang L, Lamirande
EW, Murphy BR, Subbarao K and Collins PL: Contributions of the
structural proteins of severe acute respiratory syndrome
coronavirus to protective immunity. Proc Natl Acad Sci USA.
101:9804–9809. 2004.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Li YD, Chi WY, Su JH, Ferrall L, Hung CF
and Wu TC: Coronavirus vaccine development: From SARS and MERS to
COVID-19. J Biomed Sci. 27(104)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Bouard D, Alazard-Dany N and Cosset FL:
Viral vectors: From virology to transgene expression. Br J
Pharmacol. 157:153–165. 2009.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Gao W, Tamin A, Soloff A, D'Aiuto L,
Nwanegbo E, Robbins PD, Bellini WJ, Barratt-Boyes S and Gambotto A:
Effects of a SARS-associated coronavirus vaccine in monkeys.
Lancet. 362:1895–1896. 2003.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Kobinger GP, Figueredo JM, Rowe T, Zhi Y,
Gao G, Sanmiguel JC, Bell P, Wivel NA, Zitzow LA, Flieder DB, et
al: Adenovirus-based vaccine prevents pneumonia in ferrets
challenged with the SARS coronavirus and stimulates robust immune
responses in macaques. Vaccine. 25:5220–5231. 2007.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zhang CH, Lu JH, Wang YF, Zheng HY, Xiong
S, Zhang MY, Liu XJ, Li JX, Wan ZY, Yan XG, et al: Immune responses
in Balb/c mice induced by a candidate SARS-CoV inactivated vaccine
prepared from F69 strain. Vaccine. 23:3196–3201. 2005.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Bisht H, Roberts A, Vogel L, Bukreyev A,
Collins PL, Murphy BR, Subbarao K and Moss B: Severe acute
respiratory syndrome coronavirus spike protein expressed by
attenuated vaccinia virus protectively immunizes mice. Proc Natl
Acad Sci USA. 101:6641–6646. 2004.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Chen Z, Zhang L, Qin C, Ba L, Yi CE, Zhang
F, Wei Q, He T, Yu W, Yu J, et al: Recombinant modified vaccinia
virus Ankara expressing the spike glycoprotein of severe acute
respiratory syndrome coronavirus induces protective neutralizing
antibodies primarily targeting the receptor binding region. J
Virol. 79:2678–2688. 2005.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Weingartl H, Czub M, Czub S, Neufeld J,
Marszal P, Gren J, Smith G, Jones S, Proulx R, Deschambault Y, et
al: Immunization with modified vaccinia virus Ankara-based
recombinant vaccine against severe acute respiratory syndrome is
associated with enhanced hepatitis in ferrets. J Virol.
78:12672–12676. 2004.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Czub M, Weingartl H, Czub S, He R and Cao
J: Evaluation of modified vaccinia virus Ankara based recombinant
SARS vaccine in ferrets. Vaccine. 23:2273–2279. 2005.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Deming D, Sheahan T, Heise M, Yount B,
Davis N, Sims A, Suthar M, Harkema J, Whitmore A, Pickles R, et al:
Vaccine efficacy in senescent mice challenged with recombinant
SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med.
3(e525)2006.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Kapadia SU, Rose JK, Lamirande E, Vogel L,
Subbarao K and Roberts A: Long-term protection from SARS
coronavirus infection conferred by a single immunization with an
attenuated VSV-based vaccine. Virology. 340:174–182.
2005.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Lokugamage KG, Yoshikawa-Iwata N, Ito N,
Watts DM, Wyde PR, Wang N, Newman P, Kent Tseng CT, Peters C and
Makino S: Chimeric coronavirus-like particles carrying severe acute
respiratory syndrome coronavirus (SCoV) S protein protect mice
against challenge with SCoV. Vaccine. 26:797–808. 2008.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Yang ZY, Kong WP, Huang Y, Roberts A,
Murphy BR, Subbarao K and Nabel GJ: A DNA vaccine induces SARS
coronavirus neutralization and protective immunity in mice. Nature.
428:561–564. 2004.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Martin JE, Louder MK, Holman LA, Gordon
IJ, Enama ME, Larkin BD, Andrews CA, Vogel L, Koup RA, Roederer M,
et al: A SARS DNA vaccine induces neutralizing antibody and
cellular immune responses in healthy adults in a phase I clinical
trial. Vaccine. 26:6338–6343. 2008.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Lin J, Zhang JS, Su N, Xu JG, Wang N, Chen
JT, Chen X, Liu YX, Gao H, Jia YP, et al: Safety and immunogenicity
from a phase I trial of inactivated severe acute respiratory
syndrome coronavirus vaccine. Antivir Ther. 12:1107–1113.
2007.PubMed/NCBI
|
|
106
|
Tang L, Zhu Q, Qin E, Yu M, Ding Z, Shi H,
Cheng X, Wang C, Chang G, Zhu Q, et al: Inactivated SARS-CoV
vaccine prepared from whole virus induces a high level of
neutralizing antibodies in BALB/c mice. DNA Cell Biol. 23:391–394.
2004.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Leta S, Beyene TJ, De Clercq EM, Amenu K,
Kraemer MUG and Revie CW: Global risk mapping for major diseases
transmitted by Aedes aegypti and Aedes albopictus.
Int J Infect Dis. 67:25–35. 2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Vega-Rúa A, Zouache K, Girod R, Failloux
AB and Lourenço-de-Oliveira R: High level of vector competence of
Aedes aegypti and Aedes albopictus from ten American
countries as a crucial factor in the spread of chikungunya virus. J
Virol. 88:6294–6306. 2014.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Harrison VR, Binn LN and Randall R:
Comparative immunogenicities of chikungunya vaccines prepared in
avian and mammalian tissues. Am J Trop Med Hyg. 16:786–791.
1967.PubMed/NCBI
|
|
110
|
Mallilankaraman K, Shedlock DJ, Bao H,
Kawalekar OU, Fagone P, Ramanathan AA, Ferraro B, Stabenow J,
Vijayachari P, Sundaram SG, et al: A DNA vaccine against
chikungunya virus is protective in mice and induces neutralizing
antibodies in mice and nonhuman primates. PLoS Negl Trop Dis.
5(e928)2011.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Tretyakova I, Hearn J, Wang E, Weaver S
and Pushko P: DNA vaccine initiates replication of live attenuated
chikungunya virus in vitro and elicits protective immune response
in mice. J Infect Dis. 209:1882–1890. 2014.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Wang E, Volkova E, Adams AP, Forrester N,
Xiao SY, Frolov I and Weaver SC: Chimeric alphavirus vaccine
candidates for chikungunya. Vaccine. 26:5030–5039. 2008.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Hallengärd D, Kakoulidou M, Lulla A,
Kümmerer BM, Johansson DX, Mutso M, Lulla V, Fazakerley JK, Roques
P, Le Grand R, et al: Novel attenuated chikungunya vaccine
candidates elicit protective immunity in C57BL/6 mice. J Virol.
88:2858–2866. 2014.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Chattopadhyay A, Wang E, Seymour R, Weaver
SC and Rose JK: A chimeric vesiculo/alphavirus is an effective
alphavirus vaccine. J Virol. 87:395–402. 2013.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Plante K, Wang E, Partidos CD, Weger J,
Gorchakov R, Tsetsarkin K, Borland EM, Powers AM, Seymour R,
Stinchcomb DT, et al: Novel chikungunya vaccine candidate with an
IRES-based attenuation and host range alteration mechanism. PLoS
Pathog. 7(e1002142)2011.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Levitt NH, Ramsburg HH, Hasty SE, Repik
PM, Cole FE Jr and Lupton HW: Development of an attenuated strain
of chikungunya virus for use in vaccine production. Vaccine.
4:157–162. 1986.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Edelman R, Tacket CO, Wasserman SS,
Bodison SA, Perry JG and Mangiafico JA: Phase II safety and
immunogenicity study of live chikungunya virus vaccine TSI-GSD-218.
Am J Trop Med Hyg. 62:681–685. 2000.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Kushnir N, Streatfield SJ and Yusibov V:
Virus-like particles as a highly efficient vaccine platform:
Diversity of targets and production systems and advances in
clinical development. Vaccine. 31:58–83. 2012.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Ramsauer K, Schwameis M, Firbas C, Müllner
M, Putnak RJ, Thomas SJ, Desprès P, Tauber E, Jilma B and Tangy F:
Immunogenicity, safety, and tolerability of a recombinant
measles-virus-based chikungunya vaccine: A randomised,
double-blind, placebo-controlled, active-comparator, first-in-man
trial. Lancet Infect Dis. 15:519–527. 2015.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Reisinger EC, Tschismarov R, Beubler E,
Wiedermann U, Firbas C, Loebermann M, Pfeiffer A, Muellner M,
Tauber E and Ramsauer K: Immunogenicity, safety, and tolerability
of the measles-vectored chikungunya virus vaccine MV-CHIK: A
double-blind, randomised, placebo-controlled and active-controlled
phase 2 trial. Lancet. 392:2718–2727. 2019.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Chang LJ, Dowd KA, Mendoza FH, Saunders
JG, Sitar S, Plummer SH, Yamshchikov G, Sarwar UN, Hu Z, Enama ME,
et al: Safety and tolerability of chikungunya virus-like particle
vaccine in healthy adults: A phase 1 dose-escalation trial. Lancet.
384:2046–2052. 2014.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Brandler S, Ruffié C, Combredet C, Brault
JB, Najburg V, Prevost MC, Habel A, Tauber E, Desprès P and Tangy
F: A recombinant measles vaccine expressing chikungunya virus-like
particles is strongly immunogenic and protects mice from lethal
challenge with chikungunya virus. Vaccine. 31:3718–3725.
2013.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Weinbren M, Haddow AJ and Williams M: The
occurrence of chikungunya virus in Uganda. I. Isolation from
mosquitoes. Trans R Soc Trop Med Hyg. 52:253–262. 1958.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Halstead SB and Buescher EL: Hemorrhagic
disease in rodents infected with virus associated with Thai
hemorrhagic fever. Science. 134:475–476. 1961.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Shah KV, Gibbs CJ and Banerjee G:
Virological investigation of the epidemic of haemorrhagic fever in
Calcutta: Isolation of three strains of chikungunya virus. Indian J
Med Res. 52:676–683. 1964.PubMed/NCBI
|
|
126
|
Liu H, Fang G, Wu H, Li Z and Ye Q:
L-cysteine production in Escherichia coli based on rational
metabolic engineering and modular strategy. Biotechnol J.
13(1700695)2018.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Akahata W, Yang ZY, Andersen H, Sun S,
Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG and Rao S: A
virus-like particle vaccine for epidemic chikungunya virus protects
nonhuman primates against infection. Nat Med. 16:334–338.
2010.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Saraswat S, Athmaram TN, Parida M, Agarwal
A, Saha A and Dash PK: Expression and characterization of yeast
derived chikungunya virus like particles (CHIK-VLPs) and its
evaluation as a potential vaccine candidate. PLoS Negl Trop Dis.
10(e0004782)2016.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Wang E, Petrakova O, Adams AP, Aguilar PV,
Kang W, Paessler S, Volk SM, Frolov I and Weaver SC: Chimeric
Sindbis/eastern equine encephalitis vaccine candidates are highly
attenuated and immunogenic in mice. Vaccine. 25:7573–7581.
2007.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Van Den Doel P, Volz A, Roose JM,
Sewbalaksing VD, Pijlman GP, van Middelkoop I, Duiverman V, van de
Wetering E, Sutter G, Osterhaus AD and Martina BE: Recombinant
modified vaccinia virus Ankara expressing glycoprotein E2 of
chikungunya virus protects AG129 mice against lethal challenge.
PLoS Negl Trop Dis. 8(e3101)2014.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Weger-Lucarelli J, Chu H, Aliota MT,
Partidos CD and Osorio JE: A novel MVA vectored chikungunya virus
vaccine elicits protective immunity in mice. PLoS Negl Trop Dis.
8(e2970)2014.PubMed/NCBI View Article : Google Scholar
|
|
132
|
García-Arriaza J, Cepeda V, Hallengärd D,
Sorzano CÓS, Kümmerer BM, Liljeström P and Esteban M: A novel
poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and
protects mice against chikungunya infection. J Virol. 88:3527–3547.
2014.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Wang D, Suhrbier A, Penn-Nicholson A,
Woraratanadharm J, Gardner J, Luo M, Le TT, Anraku I, Sakalian M,
Einfeld D and Dong JY: A complex adenovirus vaccine against
chikungunya virus provides complete protection against viraemia and
arthritis. Vaccine. 29:2803–2809. 2011.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Kumar M, Sudeep AB and Arankalle VA:
Evaluation of recombinant E2 protein-based and whole-virus
inactivated candidate vaccines against chikungunya virus. Vaccine.
30:6142–6149. 2012.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Khan M, Dhanwani R, Rao PVL and Parida M:
Subunit vaccine formulations based on recombinant envelope proteins
of chikungunya virus elicit balanced Th1/Th2 response and
virus-neutralizing antibodies in mice. Virus Res. 167:236–246.
2012.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Chu H, Das SC, Fuchs JF, Suresh M, Weaver
SC, Stinchcomb DT, Partidos CD and Osorio JE: Deciphering the
protective role of adaptive immunity to CHIKV/IRES a novel
candidate vaccine against Chikungunya in the A129 mouse model.
Vaccine. 31:3353–3360. 2013.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Malinoski FJ, Ksiazek T, Ramsburg H,
Lupton HW and Meadors GF (eds): Safety and immunogenicity of a new
chikungunya virus vaccine: double-blind, placebo controlled human
trial. 38th Annual Meeting of the Amer Soc Trop Med Hyg, Section
8.2, 1989.
|
|
138
|
Chen P, Demirji J, Ivleva VB, Horwitz J,
Schwartz R and Arnold F: The transient expression of CHIKV VLP in
large stirred tank bioreactors. Cytotechnology. 71:1079–1093.
2019.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Chen GL, Coates EE, Plummer SH, Carter CA,
Berkowitz N, Conan-Cibotti M, Cox JH, Beck A, O'Callahan M, Andrews
C, et al: Effect of a chikungunya virus-like particle vaccine on
safety and tolerability outcomes: A randomized clinical trial.
JAMA. 323:1369–1377. 2020.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Partidos CD, Paykel J, Weger J, Borland
EM, Powers AM, Seymour R, Weaver SC, Stinchcomb DT and Osorio JE:
Cross-protective immunity against o'nyong-nyong virus afforded by a
novel recombinant chikungunya vaccine. Vaccine. 30:4638–4643.
2012.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Fine DL, Roberts BA, Teehee ML, Terpening
SJ, Kelly CL, Raetz JL, Baker DC, Powers AM and Bowen RA:
Venezuelan equine encephalitis virus vaccine candidate (V3526)
safety, immunogenicity and efficacy in horses. Vaccine.
25:1868–1876. 2007.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Sharma A and Knollmann-Ritschel B: Current
understanding of the molecular basis of Venezuelan equine
encephalitis virus pathogenesis and vaccine development. Viruses.
11(164)2019.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Howley PM, Knipe DM and Whelan S: Fields
virology: Emerging viruses. Lippincott Williams & Wilkins,
2020.
|
|
144
|
Weaver SC, Pfeffer M, Marriott K, Kang W
and Kinney RM: Genetic evidence for the origins of Venezuelan
equine encephalitis virus subtype IAB outbreaks. Am J Trop Med Hyg.
60:441–448. 1999.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Chosewood LC and Wilson DE: Centers for
disease control and prevention: Biosafety in microbiological and
biomedical laboratories, US Department of Health and Human
Services. Public Health Service, Centers for Disease Control and
Prevention, National Institutes of Health, Washington, DC,
2009.
|
|
146
|
Lundberg L, Carey B and Kehn-Hall K:
Venezuelan equine encephalitis virus capsid-the clever caper.
Viruses. 9(279)2017.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Berge TO, Banks IS and Tigertt WD:
Attenua-lion of Venezuelan equine encephalomyelitis virus by ire
vitro cultivation in guinea-pig heart cells. Am J Hyg. 73:209–218.
1961.
|
|
148
|
Paessler S and Weaver SC: Vaccines for
Venezuelan equine encephalitis. Vaccine. 27 (Suppl 4):D80–D85.
2009.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Cole FE Jr, May SW and Robinson DM:
Formalin-inactivated Venezuelan equine encephalomyelitis (Trinidad
strain) vaccine produced in rolling-bottle cultures of chicken
embryo cells. Appl Microbiol. 25:262–265. 1973.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Kinney RM, Tsuchiya KR, Sneider JM and
Trent DW: Molecular evidence for the origin of the widespread
Venezuelan equine encephalitis epizootic of 1969 to 1972. J Gen
Virol. 73:3301–3305. 1992.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Kinney RM, Johnson BJ, Welch JB, Tsuchiya
KR and Trent DW: The full-length nucleotide sequences of the
virulent Trinidad donkey strain of Venezuelan equine encephalitis
virus and its attenuated vaccine derivative, strain TC-83.
Virology. 170:19–30. 1989.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Pittman PR, Makuch RS, Mangiafico JA,
Cannon TL, Gibbs PH and Peters CJ: Long-term duration of detectable
neutralizing antibodies after administration of live-attenuated VEE
vaccine and following booster vaccination with inactivated VEE
vaccine. Vaccine. 14:337–343. 1996.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Rayfield E, Gorelkin L, Cumow R and
Jahrling P: Virus-induced pancreatid disease induced by Venezuelan
equine encephalitis virus. Alterations in glucose tolerance and
insulin release. J Am Diab Assoc. 25:621–623. 1976.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Sharma A, Raviv Y, Puri A, Viard M,
Blumenthal R and Maheshwari RK: Complete inactivation of Venezuelan
equine encephalitis virus by 1,5-iodonaphthylazide. Biochem Biophys
Res Commun. 358:392–398. 2007.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Sharma A, Gupta P, Glass PJ, Parker MD and
Maheshwari RK: Safety and protective efficacy of INA-inactivated
Venezuelan equine encephalitis virus: Implication in vaccine
development. Vaccine. 29:953–959. 2011.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Gupta P, Sharma A, Spurgers KB, Bakken RR,
Eccleston LT, Cohen JW, Honnold SP, Glass PJ and Maheshwari RK:
1,5-Iodonaphthyl azide-inactivated V3526 protects against aerosol
challenge with virulent venezuelan equine encephalitis virus.
Vaccine. 34:2762–2765. 2016.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Martin SS, Bakken RR, Lind CM, Garcia P,
Jenkins E, Glass PJ, Parker MD, Hart MK and Fine DL: Comparison of
the immunological responses and efficacy of gamma-irradiated V3526
vaccine formulations against subcutaneous and aerosol challenge
with Venezuelan equine encephalitis virus subtype IAB. Vaccine.
28:1031–1040. 2010.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Fine DL, Jenkins E, Martin SS, Glass P,
Parker MD and Grimm B: A multisystem approach for development and
evaluation of inactivated vaccines for Venezuelan equine
encephalitis virus (VEEV). J Virol Methods. 163:424–432.
2010.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Gaidamakova EK, Myles IA, McDaniel DP,
Fowler CJ, Valdez PA, Naik S, Gayen M, Gupta P, Sharma A, Glass PJ,
et al: Preserving immunogenicity of lethally irradiated viral and
bacterial vaccine epitopes using a radio-protective Mn2+-peptide
complex from Deinococcus. Cell Host Microbe. 12:117–124.
2012.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Gayen M, Gupta P, Morazzani EM,
Gaidamakova EK, Knollmann-Ritschel B, Daly MJ, Glass PJ and
Maheshwari RK: Deinococcus Mn2+-peptide complex: A novel
approach to alphavirus vaccine development. Vaccine. 35:3672–3681.
2017.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Paessler S, Fayzulin RZ, Anishchenko M,
Greene IP, Weaver SC and Frolov I: Recombinant sindbis/Venezuelan
equine encephalitis virus is highly attenuated and immunogenic. J
Virol. 77:9278–9286. 2003.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Paessler S, Ni H, Petrakova O, Fayzulin
RZ, Yun N, Anishchenko M, Weaver SC and Frolov I: Replication and
clearance of Venezuelan equine encephalitis virus from the brains
of animals vaccinated with chimeric SIN/VEE viruses. J Virol.
80:2784–2796. 2006.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Erasmus JH, Seymour RL, Kaelber JT, Kim
DY, Leal G, Sherman MB, Frolov I, Chiu W, Weaver SC and Nasar F:
Novel insect-specific eilat virus-based chimeric vaccine candidates
provide durable, mono-and multivalent, single-dose protection
against lethal alphavirus challenge. J Virol. 92:e01274–17.
2018.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Hu WG, Steigerwald R, Kalla M, Volkmann A,
Noll D and Nagata LP: Protective efficacy of monovalent and
trivalent recombinant MVA-based vaccines against three encephalitic
alphaviruses. Vaccine. 36:5194–5203. 2018.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Kafai NM, Williamson LE, Binshtein E,
Sukupolvi-Petty S, Gardner CL, Liu J, Mackin S, Kim AS, Kose N,
Carnahan RH, et al: Neutralizing antibodies protect mice against
Venezuelan equine encephalitis virus aerosol challenge. J Exp Med.
219(e20212532)2022.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Dupuy LC, Richards MJ, Reed DS and
Schmaljohn CS: Immunogenicity and protective efficacy of a DNA
vaccine against Venezuelan equine encephalitis virus aerosol
challenge in nonhuman primates. Vaccine. 28:7345–7350.
2010.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Lee J, Arun Kumar S, Jhan YY and Bishop
CJ: Engineering DNA vaccines against infectious diseases. Acta
Biomater. 80:31–47. 2018.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Dupuy LC, Locher CP, Paidhungat M,
Richards MJ, Lind CM, Bakken R, Parker MD, Whalen RG and Schmaljohn
CS: Directed molecular evolution improves the immunogenicity and
protective efficacy of a Venezuelan equine encephalitis virus DNA
vaccine. Vaccine. 27:4152–4160. 2009.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Tretyakova I, Lukashevich IS, Glass P,
Wang E, Weaver S and Pushko P: Novel vaccine against Venezuelan
equine encephalitis combines advantages of DNA immunization and a
live attenuated vaccine. Vaccine. 31:1019–1025. 2013.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Rico AB, Phillips AT, Schountz T, Jarvis
DL, Tjalkens RB, Powers AM and Olson KE: Venezuelan and western
equine encephalitis virus E1 liposome antigen nucleic acid
complexes protect mice from lethal challenge with multiple
alphaviruses. Virology. 499:30–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Ni H, Yun NE, Zacks MA, Weaver SC, Tesh
RB, Da Rosa APT, Powers AM, Frolov I and Paessler S: Recombinant
alphaviruses are safe and useful serological diagnostic tools. Am J
Trop Med Hyg. 76:774–781. 2007.PubMed/NCBI
|
|
172
|
Liljeström P and Garoff H: A new
generation of animal cell expression vectors based on the Semliki
Forest virus replicon. Biotechnology (N Y). 9:1356–1361.
1991.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Konopka JL, Thompson JM, Whitmore AC, Webb
DL and Johnston RE: Acute infection with Venezuelan equine
encephalitis virus replicon particles catalyzes a systemic
antiviral state and protects from lethal virus challenge. J Virol.
83:12432–12442. 2009.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Grieder FB, Davis BK, Zhou XD, Chen SJ,
Finkelman FD and Gause WC: Kinetics of cytokine expression and
regulation of host protection following infection with molecularly
cloned Venezuelan equine encephalitis virus. Virology. 233:302–312.
1997.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Phillpotts RJ, O'brien L, Appleton RE,
Carr S and Bennett A: Intranasal immunisation with defective
adenovirus serotype 5 expressing the Venezuelan equine encephalitis
virus E2 glycoprotein protects against airborne challenge with
virulent virus. Vaccine. 23:1615–1623. 2005.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Phillpotts RJ, Jones LD and Howard SC:
Monoclonal antibody protects mice against infection and disease
when given either before or up to 24 h after airborne challenge
with virulent Venezuelan equine encephalitis virus. Vaccine.
20:1497–1504. 2002.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Parker MD, Buckley MJ, Melanson VR, Glass
PJ, Norwood D and Hart MK: Antibody to the E3 glycoprotein protects
mice against lethal Venezuelan equine encephalitis virus infection.
J Virol. 84:12683–12690. 2010.PubMed/NCBI View Article : Google Scholar
|
|
178
|
O'Brien LM, Underwood-Fowler CD, Goodchild
SA, Phelps AL and Phillpotts RJ: Development of a novel monoclonal
antibody with reactivity to a wide range of Venezuelan equine
encephalitis virus strains. Virol J. 6(206)2009.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Alevizatos AC, McKinney RW and Feigin RD:
Live, attenuated Venezuelan equine encephalomyelitis virus vaccine.
I. Clinical effects in man. Am J Trop Med Hyg. 16:762–768.
1967.PubMed/NCBI
|
|
180
|
Pedersen CE Jr, Robinson DM and Cole FE
Jr: Isolation of the vaccine strain of Venezuelan equine
encephalomyelitis virus from mosquitoes in Louisiana. Am J
Epidemiol. 95:490–496. 1972.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Erwin-Cohen R, Porter A, Pittman P, Rossi
C and Dasilva L: Host responses to live-attenuated Venezuelan
equine encephalitis virus (TC-83): Comparison of naïve, vaccine
responder and nonresponder to TC-83 challenge in human peripheral
blood mononuclear cells. Hum Vaccin Immunother. 8:1053–1065.
2012.PubMed/NCBI View Article : Google Scholar
|
|
182
|
Grieder FB, Davis NL, Aronson JF, Charles
PC, Sellon DC, Suzuki K and Johnston RE: Specific restrictions in
the progression of Venezuelan equine encephalitis virus-induced
disease resulting from single amino acid changes in the
glycoproteins. Virology. 206:994–1006. 1995.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Davis NL, Powell N, Greenwald GF, Willis
LV, Johnson BJ, Smith JF and Johnston RE: Attenuating mutations in
the E2 glycoprotein gene of Venezuelan equine encephalitis virus:
Construction of single and multiple mutants in a full-length cDNA
clone. Virology. 183:20–31. 1991.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Edelman R, Ascher MS, Oster CN, Ramsburg
HH, Cole FE and Eddy GA: Evaluation in humans of a new, inactivated
vaccine for Venezuelan equine encephalitis virus (C-84). J Infect
Dis. 140:708–715. 1979.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Johnson DM: Development of rationally
designed live attenuated vaccines for Lassa fever and Venezuelan
equine encephalitis. Electron Theses Diss. (3393)2020.
|
|
186
|
Hannaman D, Dupuy LC, Ellefsen B and
Schmaljohn CS: A phase 1 clinical trial of a DNA vaccine for
Venezuelan equine encephalitis delivered by intramuscular or
intradermal electroporation. Vaccine. 34:3607–3612. 2016.PubMed/NCBI View Article : Google Scholar
|
|
187
|
Powell LA, Miller A, Fox JM, Kose N, Klose
T, Kim AS, Bombardi R, Tennekoon RN, Dharshan de Silva A, Carnahan
RH, et al: Human mAbs broadly protect against arthritogenic
alphaviruses by recognizing conserved elements of the Mxra8
receptor-binding site. Cell Host Microbe. 28:699–711.e7.
2020.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Tupanceska D, Zaid A, Rulli NE, Thomas S,
Lidbury B, Matthaei K, Ramirez R and Mahalingam S: Ross River
virus: An arthritogenic alphavirus of significant importance in the
asia pacific. In: Lal SK (ed). Emerging viral diseases of Southeast
Asia. Vol. 4. Issues Infect Dis. Basel, Karger, pp94-111, 2007.
|
|
189
|
Claflin SB and Webb CE: Ross River virus:
Many vectors and unusual hosts make for an unpredictable pathogen.
PLoS Pathog. 11(e1005070)2015.PubMed/NCBI View Article : Google Scholar
|
|
190
|
Earnest JT, Basore K, Roy V, Bailey AL,
Wang D, Alter G, Fremont DH and Diamond MS: Neutralizing antibodies
against Mayaro virus require Fc effector functions for protective
activity. J Exp Med. 216:2282–2301. 2019.PubMed/NCBI View Article : Google Scholar
|
|
191
|
Fox JM, Long F, Edeling MA, Lin H, van
Duijl-Richter MKS, Fong RH, Kahle KM, Smit JM, Jin J, Simmons G, et
al: Broadly neutralizing alphavirus antibodies bind an epitope on
E2 and inhibit entry and egress. Cell. 163:1095–1107.
2015.PubMed/NCBI View Article : Google Scholar
|
|
192
|
Holzer GW, Coulibaly S, Aichinger G,
Savidis-Dacho H, Mayrhofer J, Brunner S, Schmid K, Kistner O,
Aaskov JG, Falkner FG, et al: Evaluation of an inactivated Ross
River virus vaccine in active and passive mouse immunization models
and establishment of a correlate of protection. Vaccine.
29:4132–4141. 2011.PubMed/NCBI View Article : Google Scholar
|
|
193
|
Aichinger G, Ehrlich HJ, Aaskov JG,
Fritsch S, Thomasser C, Draxler W, Wolzt M, Müller M, Pinl F, Van
Damme P, et al: Safety and immunogenicity of an inactivated whole
virus Vero cell-derived Ross River virus vaccine: A randomized
trial. Vaccine. 29:9376–9384. 2011.PubMed/NCBI View Article : Google Scholar
|
|
194
|
Strauss JH and Strauss EG: The
alphaviruses: Gene expression, replication, and evolution.
Microbiol Rev. 58:491–562. 1994.PubMed/NCBI View Article : Google Scholar
|
|
195
|
Ljungberg K and Liljeström P:
Self-replicating alphavirus RNA vaccines. Expert Rev Vaccines.
14:177–194. 2015.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Frolov I, Hoffman TA, Prágai BM, Dryga SA,
Huang HV, Schlesinger S and Rice CM: Alphavirus-based expression
vectors: Strategies and applications. Proc Natl Acad Sci USA.
93:11371–11377. 1996.PubMed/NCBI View Article : Google Scholar
|
|
197
|
Choo QL, Kuo G, Ralston R, Weiner A, Chien
D, Van Nest G, Han J, Berger K, Thudium K, Kuo C, et al:
Vaccination of chimpanzees against infection by the hepatitis C
virus. Vaccination of chimpanzees against infection by the
hepatitis C virus USA. 91:1294–1298. 1994.PubMed/NCBI View Article : Google Scholar
|
|
198
|
Lópex-Días de Cerio AL, Casares N, Lasarte
JJ, Sarobe P, Pérez-Mediavilla LA, Ruiz M, Prieto J and
Borrás-Cuesta F: T(h)1 but not T(h)0 cell help is efficient to
induce cytotoxic T lymphocytes by immunization with short synthetic
peptides. Int Immunol. 11:2025–2034. 1999.PubMed/NCBI View Article : Google Scholar
|
|
199
|
Shirai M, Akatsuka T, Pendleton CD,
Houghten R, Wychowski C, Mihalik K, Feinstone S and Berzofsky JA:
Induction of cytotoxic T cells to a cross-reactive epitope in the
hepatitis C virus nonstructural RNA polymerase-like protein. J
Virol. 66:4098–4106. 1992.PubMed/NCBI View Article : Google Scholar
|
|
200
|
Leitner WW, Ying H and Restifo NP: DNA and
RNA-based vaccines: Principles, progress and prospects. Vaccine.
18:765–777. 1999.PubMed/NCBI View Article : Google Scholar
|
|
201
|
Baumert TF, Vergalla J, Satoi J, Thomson
M, Lechmann M, Herion D, Greenberg HB, Ito S and Liang TJ:
Hepatitis C virus-like particles synthesized in insect cells as a
potential vaccine candidate. Gastroenterology. 117:1397–1407.
1999.PubMed/NCBI View Article : Google Scholar
|
|
202
|
Makimura M, Miyake S, Akino N, Takamori K,
Matsuura Y, Miyamura T and Saito I: Induction of antibodies against
structural proteins of hepatitis C virus in mice using recombinant
adenovirus. Vaccine. 14:28–36. 1996.PubMed/NCBI View Article : Google Scholar
|
|
203
|
Brinster C and Inchauspé G: DNA vaccines
for hepatitis C virus. Intervirology. 44:143–153. 2001.PubMed/NCBI View Article : Google Scholar
|
|
204
|
Amara RR, Villinger F, Altman JD, Lydy SL,
O'Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS,
et al: Control of a mucosal challenge and prevention of AIDS by a
multiprotein DNA/MVA vaccine. Science. 292:69–74. 2001.PubMed/NCBI View Article : Google Scholar
|
|
205
|
Ying H, Zaks TZ, Wang RF, Irvine KR,
Kammula US, Marincola FM, Leitner WW and Restifo NP: Cancer therapy
using a self-replicating RNA vaccine. Nat Med. 5:823–827.
1999.PubMed/NCBI View
Article : Google Scholar
|
|
206
|
Berglund P, Fleeton MN, Smerdou C and
Liljeström P: Immunization with recombinant Semliki Forest virus
induces protection against influenza challenge in mice. Vaccine.
17:497–507. 1999.PubMed/NCBI View Article : Google Scholar
|
|
207
|
Fleeton MN, Liljeström P, Sheahan BJ and
Atkins GJ: Recombinant Semliki Forest virus particles expressing
louping ill virus antigens induce a better protective response than
plasmid-based DNA vaccines or an inactivated whole particle
vaccine. J Gen Virol. 81:749–758. 2000.PubMed/NCBI View Article : Google Scholar
|
|
208
|
Ajbani SP, Velhal SM, Kadam RB, Patel VV
and Bandivdekar AH: Immunogenicity of Semliki Forest virus based
self-amplifying RNA expressing Indian HIV-1C genes in mice. Int J
Biol Macromol. 81:794–802. 2015.PubMed/NCBI View Article : Google Scholar
|
|
209
|
Van De Wall S, Ljungberg K, IP PP, Boerma
A, Nijman HW, Liljeström P and Daemen T: Development of a next
generation Semliki Forest virus-based DNA vaccine against cervical
cancer. Hum Gene Ther. 25(A30)2014.
|
|
210
|
Mussgay M and Weiland E: Preparation of
inactivated vaccines against alphaviruses using Semliki Forest
virus-white mouse as a model. I. Inactivation experiments and
evaluation of double inactivated subunit vaccines. Intervirology.
1:259–268. 1973.PubMed/NCBI View Article : Google Scholar
|
|
211
|
Brinster C, Chen M, Boucreux D,
Paranhos-Baccala G, Liljeström P, Lemmonier F and Inchauspé G:
Hepatitis C virus non-structural protein 3-specific cellular immune
responses following single or combined immunization with DNA or
recombinant Semliki Forest virus particles. J Gen Virol.
83:369–381. 2002.PubMed/NCBI View Article : Google Scholar
|
|
212
|
Oñate AA, Donoso G, Moraga-Cid G, Folch H,
Céspedes S and Andrews E: An RNA vaccine based on recombinant
Semliki Forest virus particles expressing the Cu,Zn superoxide
dismutase protein of Brucella abortus induces protective
immunity in BALB/c mice. Infect Immun. 73:3294–3300.
2005.PubMed/NCBI View Article : Google Scholar
|
|
213
|
Jiang X and Baldwin CL: Effects of
cytokines on intracellular growth of Brucella abortus.
Infect Immun. 61:124–134. 1993.PubMed/NCBI View Article : Google Scholar
|
|
214
|
Jones SM and Winter AJ: Survival of
virulent and attenuated strains of Brucella abortus in
normal and gamma interferon-activated murine peritoneal
macrophages. Infect Immun. 60:3011–3014. 1992.PubMed/NCBI View Article : Google Scholar
|
|
215
|
Stevens MG, Pugh GW Jr and Tabatabai LB:
Effects of gamma interferon and indomethacin in preventing
Brucella abortus infections in mice. Infect Immun.
60:4407–4409. 1992.PubMed/NCBI View Article : Google Scholar
|
|
216
|
Zhan Y and Cheers C: Endogenous gamma
interferon mediates resistance to Brucella abortus
infection. Infect Immun. 61:4899–4901. 1993.PubMed/NCBI View Article : Google Scholar
|
|
217
|
Ko J and Splitter GA: Molecular
host-pathogen interaction in brucellosis: Current understanding and
future approaches to vaccine development for mice and humans. Clin
Microbiol Rev. 16:65–78. 2003.PubMed/NCBI View Article : Google Scholar
|
|
218
|
Muñoz-Montesino C, Andrews E, Rivers R,
González-Smith A, Moraga-Cid G, Folch H, Céspedes S and Oñate AA:
Intraspleen delivery of a DNA vaccine coding for superoxide
dismutase (SOD) of Brucella abortus induces SOD-specific
CD4+ and CD8+ T cells. Infect Immun. 72:2081–2087. 2004.PubMed/NCBI View Article : Google Scholar
|
|
219
|
Colmenero P, Berglund P, Kambayashi T,
Biberfeld P, Liljeström P and Jondal M: Recombinant Semliki Forest
virus vaccine vectors: The route of injection determines the
localization of vector RNA and subsequent T cell response. Gene
Ther. 8:1307–1314. 2001.PubMed/NCBI View Article : Google Scholar
|
|
220
|
Smerdou C and Liljestrom P: Two-helper RNA
system for production of recombinant Semliki forest virus
particles. J Virol. 73:1092–1098. 1999.PubMed/NCBI View Article : Google Scholar
|
|
221
|
Berglund P, Quesada-Rolander M, Putkonen
P, Biberfeld G, Thorstensson R and Liljeström P: Outcome of
immunization of cynomolgus monkeys with recombinant Semliki Forest
virus encoding human immunodeficiency virus type 1 envelope protein
and challenge with a high dose of SHIV-4 virus. AIDS Res Hum
Retroviruses. 13:1487–1495. 1997.PubMed/NCBI View Article : Google Scholar
|
|
222
|
Chen M, Hu KF, Rozell B, Örvell C, Morein
B and Liljeström P: Vaccination with recombinant alphavirus or
immune-stimulating complex antigen against respiratory syncytial
virus. J Immunol. 169:3208–3216. 2002.PubMed/NCBI View Article : Google Scholar
|
|
223
|
Singh A, Koutsoumpli G, van de Wall S and
Daemen T: An alphavirus-based therapeutic cancer vaccine: From
design to clinical trial. Cancer Immunol Immunother. 68:849–859.
2019.PubMed/NCBI View Article : Google Scholar
|
|
224
|
Daemen T, Riezebos-Brilman A, Regts J,
Dontje B, van der Zee A and Wilschut J: Superior therapeutic
efficacy of alphavirus-mediated immunization against human
papilloma virus type 16 antigens in a murine tumour model: Effects
of the route of immunization. Antivir Ther. 9:733–742.
2004.PubMed/NCBI
|
|
225
|
Komdeur FL, Singh A, van de Wall S,
Meulenberg JJM, Boerma A, Hoogeboom BN, Paijens ST, Oyarce C, de
Bruyn M, Schuuring E, et al: First-in-human phase I clinical trial
of an SFV-based RNA replicon cancer vaccine against HPV-induced
cancers. Mol Ther. 29:611–625. 2021.PubMed/NCBI View Article : Google Scholar
|
|
226
|
Vignuzzi M, Gerbaud S, van der Werf S and
Escriou N: Naked RNA immunization with replicons derived from
poliovirus and Semliki Forest virus genomes for the generation of a
cytotoxic T cell response against the influenza A virus
nucleoprotein. J Gen Virol. 82:1737–1747. 2001.PubMed/NCBI View Article : Google Scholar
|
|
227
|
Brand D, Lemiale F, Turbica I, Buzelay L,
Brunet S and Barin F: Comparative analysis of humoral immune
responses to HIV type 1 envelope glycoproteins in mice immunized
with a DNA vaccine, recombinant Semliki Forest virus RNA, or
recombinant Semliki Forest virus particles. AIDS Res Hum
Retroviruses. 14:1369–1377. 1998.PubMed/NCBI View Article : Google Scholar
|
|
228
|
Zhou X, Berglund P, Rhodes G, Parker SE,
Jondal M and Liljeström P: Self-replicating Semliki Forest virus
RNA as recombinant vaccine. Vaccine. 12:1510–1514. 1994.PubMed/NCBI View Article : Google Scholar
|
|
229
|
Johansson DX, Ljungberg K, Kakoulidou M
and Liljeström P: Intradermal electroporation of naked replicon RNA
elicits strong immune responses. PLoS One. 7(e29732)2012.PubMed/NCBI View Article : Google Scholar
|
|
230
|
Phenix KV, Wark K, Luke CJ, Skinner MA,
Smyth JA, Mawhinney KA and Todd D: Recombinant Semliki Forest virus
vector exhibits potential for avian virus vaccine development.
Vaccine. 19:3116–3123. 2001.PubMed/NCBI View Article : Google Scholar
|
|
231
|
Goo J, Jeong Y, Park YS, Yang E, Jung DI,
Rho S, Park U, Sung H, Park PG, Choi JA, et al: Characterization of
novel monoclonal antibodies against MERS-coronavirus spike protein.
Virus Res. 278(197863)2020.PubMed/NCBI View Article : Google Scholar
|
|
232
|
Heinz FX, Holzmann H, Essl A and Kundi M:
Field effectiveness of vaccination against tick-borne encephalitis.
Vaccine. 25:7559–7567. 2007.PubMed/NCBI View Article : Google Scholar
|















