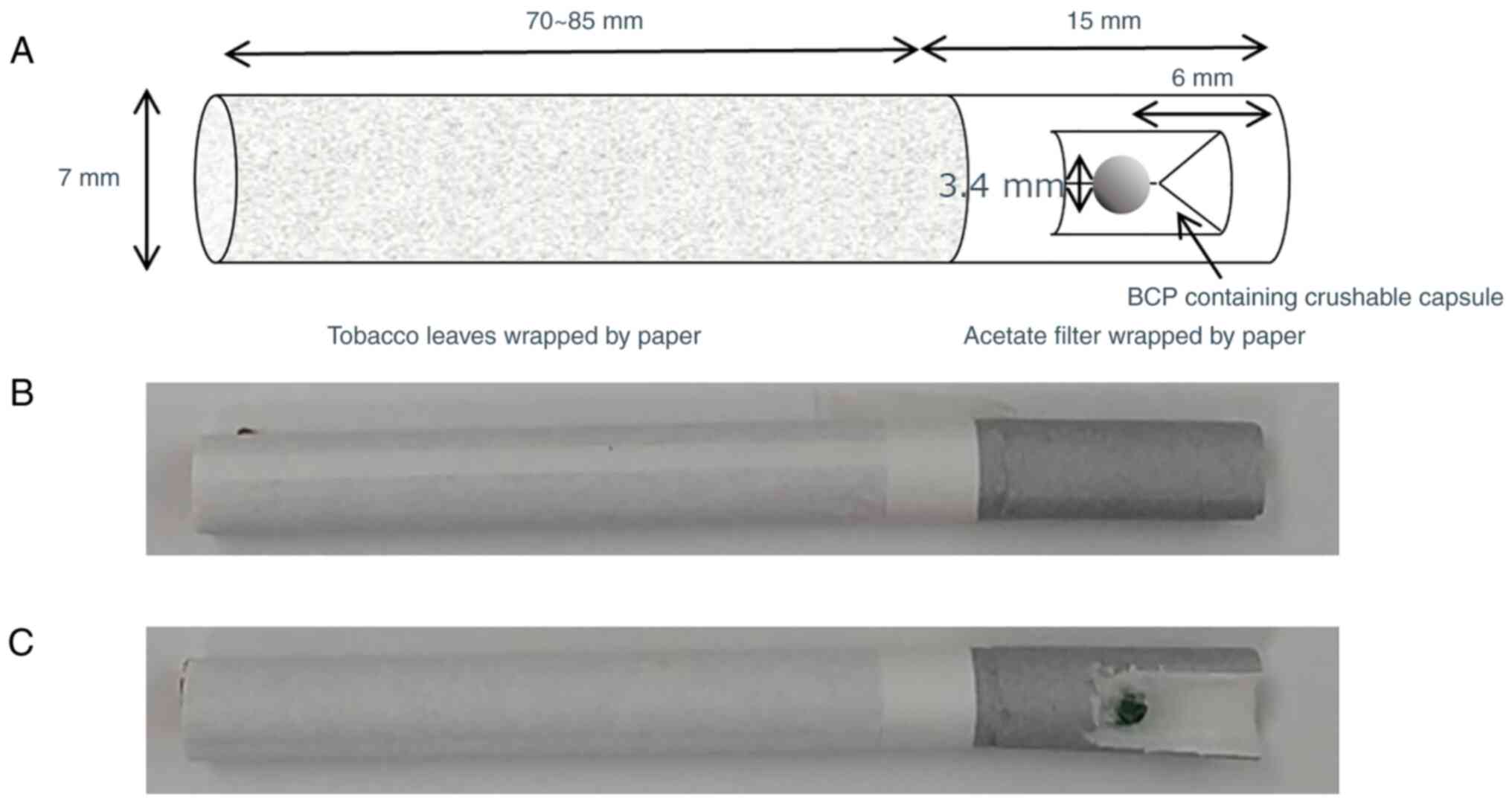
Introduction
Smoking is a worldwide pastime, as shown by the
existence of 1.14 billion current smokers in 2019(1). The report mentioned that both the
ratio and population of smokers were decreasing; for example, the
percentage decreases from 1990 to 2019 were 37.7% for women and
27.5% for men. Although the smoking population is declining, the
number of people who are dying from disease caused by smoking is
increasing. From 2011 to 2019, the number of deaths due to smoking
increased from 5.40 to 7.69 million people worldwide (2). Health hazards from smoking include
malignant tumors, cardiovascular diseases, and respiratory
diseases. In 2010, the number of deaths was reported as 77,400 due
to malignant tumors, 18,100 due to cardiovascular disease, and
33,400 due to respiratory disease (3). These diseases caused by smoking
contribute to rising medical costs worldwide; therefore, the health
of smokers is also important to consider in government health
policies.
Examples of cardiovascular diseases caused by
smoking include ischemic heart disease, stroke, and aortic aneurysm
and dissection. One of the most important risk factors for these
diseases is the hardening of blood vessel walls, which is caused by
nicotine inhalation (4). The pulse
wave velocity (PWV) is one of the indicators of arterial wall
sclerosis (5). There are several
PWV measurement methods; however, in recent years, brachial-ankle
PWV (baPWV), which is easy to measure, has been widely used
(6). Since baPWV shows a
correlation with the Framingham risk score (7,8), it
may be considered an index that reflects the total cardiovascular
risk. For example, a baPWV of 1400 cm/sec corresponds to a moderate
Framingham risk score (8) and
increases the risk of developing hypertension (9). A meta-analysis also showed that baPWV
is an independent predictor of prognosis, with a 12% increase in
cardiovascular disease incidence with an increase of 100 cm/sec
(10).
These diseases caused by smoking have been reduced
mainly through policy changes that induce smoking cessation.
Moreover, some cigarette manufacturers have launched risk-reducing
products such as heat-not-burn tobacco. However, these
correspondences had limitations because there are hundreds of
millions of people who want to quit smoking, but cannot. Therefore,
alternative options are needed to manage their health. Some statins
and food ingredients such as eicosapentaenoic acid (EPA) decrease
baPWV (11-13),
which may be useful for improving smokers' health. We aimed to
develop a method that can be easily ingested with safer food
ingredients instead of pharmaceuticals as an alternative option for
smokers. To overcome these problems, we focused on inhaling
volatile components from cigarette smoke using a flavor capsule
(14). The volatile food additive
that we focused on is β-caryophyllene (BCP), because BCP is a
compound that reduces baPWV and known as an agonist of cannabinoid
receptor 2 (CB2 receptor) (15)
with anti-inflammatory effects. Vascular walls are destroyed and
hardened by matrix metalloproteinase (MMP) production via
inflammation caused by nicotine ingestion (16,17).
Inhaled BCP is transferred into the blood and can prevent vascular
fiber degradation and blood vessel hardening in mice (18,19).
Vascular fibers play a role in maintaining the elasticity and
strength of the vessels; therefore, the prevention of vascular
fiber degradation by BCP is directly linked to reduced baPWV. Based
on these results, we hypothesized that BCP suppresses the hardening
of the blood vessel wall, resulting in a decrease in baPWV.
In terms of palatability, BCP has a weak waxy or
woody odor, so the odor is drowned out by the smell of cigarette
smoke. BCP is present in essential oils at concentrations of 20%
for clove bud oil, 20% for basil oil, 16% for oregano oil, 15% for
hop oil, 11% for cinnamon oil, 8% for rosemary oil, 7% for black
pepper oil, and 5% for lavender oil (20,21).
A clinical study related to BCP has also been reported (22).
Herein, we aimed to examine if inhaling BCP with
cigarette smoke reduced the baPWV of healthy smokers.
Materials and methods
Design of the research
All recruited participants were healthy smokers; the
intervention was inhaling BCP with cigarette smoke, and the
intervention group was compared against the initial status and a
placebo group. The outcomes were changes in blood BCP concentration
over time and the effect of reducing baPWV.
The blood levels of BCP and BCP oxide (BCPO) were
measured in this single-dose study. Vascular function was measured
in a placebo-targeted dose-searching, double-blind, parallel-group
comparative study.
Ethical review
After deliberation and approval by the Institutional
Review Board of Sunsho Pharmaceutical Co., Ltd. (approval number:
21001 for vascular function, and 21002 for BCP concentration in
blood), this study was carried out in accordance with the
Declaration of Helsinki and with ethical considerations. In
addition, prior to the start of the study, informed consent was
requested from each subject individually, and the test was
conducted after explaining that participation in this test was
voluntary and that there was no disadvantage, even if consent was
not given. Written informed consent was obtained from all
participants in this study.
Subjects
A questionnaire was administered at the time of
recruitment, and a screening survey was conducted targeting those
who met the self-report entry criteria and did not violate the
exclusion criteria. Healthy subjects who met the screening criteria
and were judged by us to be appropriate for participation in this
study were selected as the subjects of this study. In the course of
the screening test, if it became clear that the test results were
inconsistent with the self-reported contents, they were excluded
from the analysis, unless there was a specific reason. In addition,
if the instruction in the subject management items was violated
during the research period, or if there was a big problem in the
reliability of the data due to troubles during the research period,
it was considered appropriate to treat them as dropouts or exclude
them from the analysis target.
The eligibility criteria were as follows: healthy
adult men and women and smokers who smoked regular cigarettes. The
exclusion criteria were as follows: non-smokers, smokers who smoked
tobacco other than cigarettes (pipes), smokers who smoked
non-regular type (slim type) cigarettes, those who planned to quit
smoking during the experimental period, those who were being
treated for disease or who were planning to receive treatment,
those who hoped to become pregnant during the experimental period,
those who were pregnant or lactating, those who were taking
medication, or those who consumed health foods (excluding products
whose active ingredients were only vitamins and minerals) on a
daily basis. After the initial survey, those who met the exclusion
criteria were excluded. No other screenings were performed.
Intervention and inspection
methods
BCP (Relaxphytone®) was purchased from
Inabata Koryo Co. Ltd. (Osaka, Japan). Medium-chain triglycerides
(MCT) were purchased from KAO (Tokyo, Japan). The encapsulation of
the BCP/MCT solution was conducted by Sunsho Pharmaceutical Co.,
Ltd. (Shizuoka, Japan) using the dropping method. The diameter of
the capsule was 3.4 mm and the weight of the core was set to 19.30
mg. Three types of capsules were manufactured and their
compositions are described below.
Capsule 1: BCP 5.79 mg, MCT 13.51 mg (BCP 30%)
Capsule 2: BCP 2.90 mg, MCT 16.40 mg (BCP 15%)
Capsule 3: MCT 19.30 mg (placebo)
The cigarettes that the subjects normally smoked
were inquired through the initial questionnaire survey. The
capsules were inserted into the cigarette filters corresponding to
the assigned group. BCP was inhaled via smoking after
insertion.
For the blood levels of BCP and nicotine measured in
the single-dose study, eight volunteer subjects were assigned as
follows: four subjects for capsule 1, three subjects for capsule 2,
and one subject for capsule 3. The allocation method was the same
as that used in the vascular function study.
Blood (5 ml) was collected six times before smoking
and 10, 20, 40, 90 and 180 min after smoking. After blood
collection, plasma was collected by centrifugation. Blood plasma
samples were frozen and stored at -80˚C until analysis.
BCP and BCPO concentrations in the serum were
analyzed using gas chromatography-mass spectrometry (Agilent
7890B-5977B MSD; Agilent Technologies, Santa Clara, CA, USA), a
thermal desorption unit, programmable temperature vaporization
inlet, and multipurpose sampler with dynamic head space option (all
Gerstel GmbH & Co.KG, Mülheim an der Ruhr, Germany) 39. MSD
ChemStation v.F.01.03. 2357 (Agilent), and Mass Hunter
v.B.07.05.2479 (Agilent) were used for data analysis. Tenax TA
cartridges were released from Tenax TA traps using a thermal
desorption cold-trap setup (thermal desorption spectrometer; Markes
International, Ltd., Llantrisant, RCT, UK). An InertCap WAX column
(length: 60 m, outer diameter: 0.25 µm, I.D.: 0.25 mm, GL Sciences,
Inc., Tokyo, Japan) was used for component separation. The oven
temperature was programmed as follows: initial temperature, 40˚C;
ramp rate, 3˚C/min (40 to 145˚C) and 10˚C/min (145 to 240˚C); final
temperature, 240˚C for 5 min. The He inlet pressure was controlled
using an electronic pressure control system to achieve a constant
column flow rate of 1 ml/min. Mass spectrometry analysis was
performed in the ionization mode at a voltage of 70 eV.
For the vascular function measured in the
placebo-targeted study, 19 volunteer subjects were assigned as
follows: seven subjects for capsule 1, seven subjects for capsule
2, and five subjects for capsule 3.
The test period was 12 weeks from September to
December 2021, and the total number of observation days was four:
before, 4 weeks after, 8 weeks after, and 12 weeks after the start
of the test period.
Cigarettes with a capsule in the center of the
filter that the subjects normally smoked were provided. Capsules
were crushed immediately before smoking. The number of cigarettes
smoked per day was counted by collecting cigarette butts. Butts
were also collected for two weeks before the monitoring period.
During the observation period, blood pressure,
baPWV, and ankle brachial index (ABI) were measured using a
BP-203PRE II (Fukuda Colin Co., Ltd., Tokyo, Japan). The % vital
capacity (%VC) and forced expiratory volume in 1 sec
(%FEV1.0) were measured using Chestgragh HI-10 (Chest
Co., Ltd., Tokyo, Japan).
Allocation and blinding method
Subjects were randomly assigned so that sex
differences, age, daily number of cigarettes, smoking history,
amount of nicotine and tar in cigarettes, and care about dietary
balance were as similar as possible among the groups.
The secretariat, who was not in charge of
measurement and analysis, managed and locked the correspondence
table of the subject name, subject number for analysis, and
capsule. The lock was unlocked after all data collection was
completed. The assigned group or measurement results were not
disclosed to the subjects.
Definition of endpoints
The primary endpoint of this study was the reduction
in baPWV by inhaling BCP via cigarette smoke. The secondary
endpoints were investigation of the bioavailability of inhaled BCP
with cigarette smoke; confirmation of the effect of BCP inhalation
on respiratory function such as %VC and %FEV1.0; and
association between respiratory function and blood concentration
and baPWV reduction.
Statistical considerations
All statistical calculations were performed using
EZR (Saitama Medical Center, Jichi Medical University, Saitama,
Japan), a graphical user interface for R (The R Foundation for
Statistical Computing, Vienna, Austria, version 4.0.3). More
precisely, it is a modified version of the R commander (version
2.7-1) that was designed to add statistical functions frequently
used in biostatistics (23). The
Friedman test was used to compare time-series data within the group
for vascular and respiratory function. The Mann-Whitney U test was
used for comparisons between the two groups, and the Steel-Dwass
multiple comparison method following the Kruskal-Wallis test was
used for comparisons among the three groups. The Spearman's rank
correlation test was used to determine the correlation between the
two parameters. P<0.05 was considered to indicate a
statistically significant difference.
Results
Outline of research
An outline of this study is presented in Fig. S1. After enrolment and gathering of
informed consent from 38 people, 19 people were selected based on
the inclusion and exclusion criteria. These participants were
randomly assigned to three groups, five people for placebo group,
seven people for BCP 15% capsule group, and seven people for BCP
30% capsule group. Then, the BCP was placed within their cigarette
capsules (Fig. 1). The
measurements were performed four times and the data were collected
and analyzed. After the end of the intervention, some subjects, one
person for placebo group, three people for BCP 15% capsule group,
and four people for BCP 30% capsule group, underwent a blood
sampling test.
Baseline characteristics
The baseline characteristics of the groups are shown
in Table I. There were no
statistically significant differences in age, cigarettes per day,
nicotine per day, tar per day, pack year, initial baPWV, initial
%VC, and initial %FEV1.0 among the three groups. The
demographic characteristics of the groups, other than the amount of
BCP intake, were comparable.
 | Table IBaseline characteristics of subjects
in each group presented as mean (maximum, and minimum). |
Table I
Baseline characteristics of subjects
in each group presented as mean (maximum, and minimum).
| Characteristic | Placebo | BCP 15% | BCP 30% | P-value |
|---|
| Number | 5 | 7 | 7 | |
| Age, years | 36 (22, 58) | 32 (31, 55) | 35 (20, 49) | 0.782 |
| BCP (mg)/day | 0.0 (0.0, 0.0) | 27.8 (12.2,
46.1) | 44.6 (30.7,
95.5) | 0.002 |
| Cigarettes/day | 8.9 (4.9,
28.7) | 9.60 (4.2,
15.9) | 7.7 (5.3,
16.5) | 0.915 |
| Nicotine
(mg)/day | 11.1 (0.5,
20.7) | 4.8 (0.7,
20.1) | 3.3 (1.0,
25.2) | 0.782 |
| Tar (mg)/day | 129.0 (5.0,
236.1) | 57.6 (7.3,
234.3) | 40.8 (10.2,
201.8) | 0.687 |
| Pack year | 9.50 (0.49,
26.10) | 8.03 (2.52,
27.82) | 5.00 (0.00,
14.03) | 0.51 |
| Initial PWV
(cm/sec) | 1,368.5 (1,118.0,
1,546.0) | 1,472.50 (1,166.0,
1,632.0) | 1,507.50 (1,032.5,
1,602.0) | 0.617 |
| Initial %VC | 118 (99, 149) | 102 (79, 129) | 99 (76, 113) | 0.156 |
| Initial
%FEV1.0 | 83.0 (76.5,
91.4) | 83.7 (57.4,
89.7) | 75.4 (19.0,
100.0) | 0.788 |
BCP and BCPO concentration in
blood
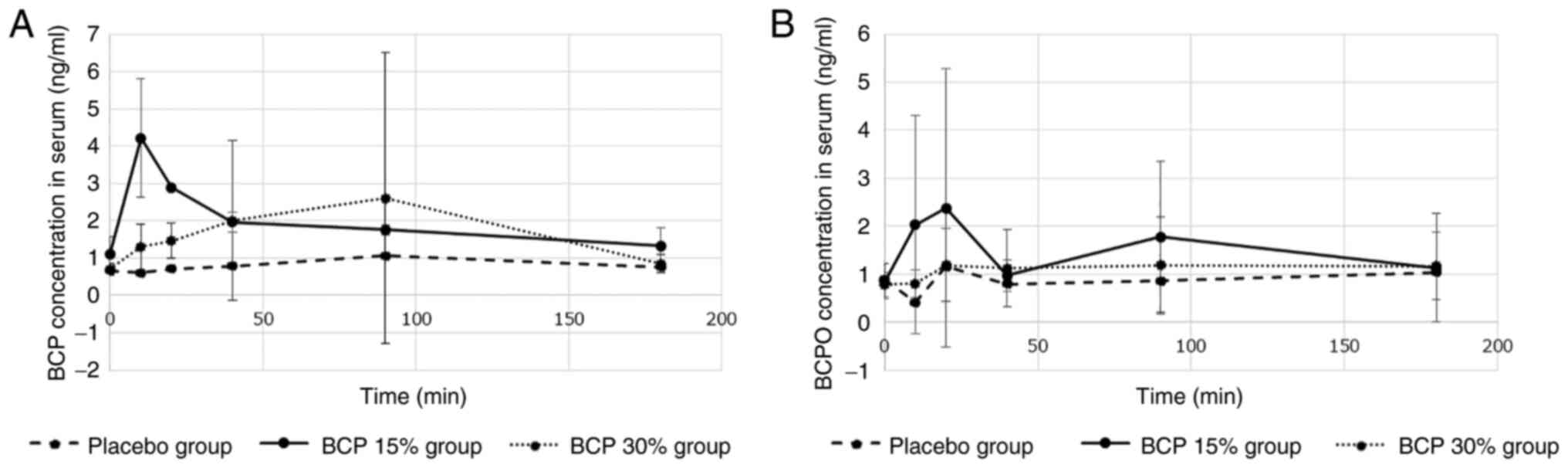
The results are summarized in Fig. 2. The maximum concentration of BCP
reached 4.2 ng/ml 10 min after smoking in the BCP 15% group.
Subsequently, the concentration decreased exponentially. In
contrast, the maximum concentration reached 2.6 ng/ml 90 min after
smoking in the BCP 30% group. The BCP concentration in the placebo
group was constant at 1 ng/ml. The concentration of BCPO was
similar to that of BCP.
Vascular function
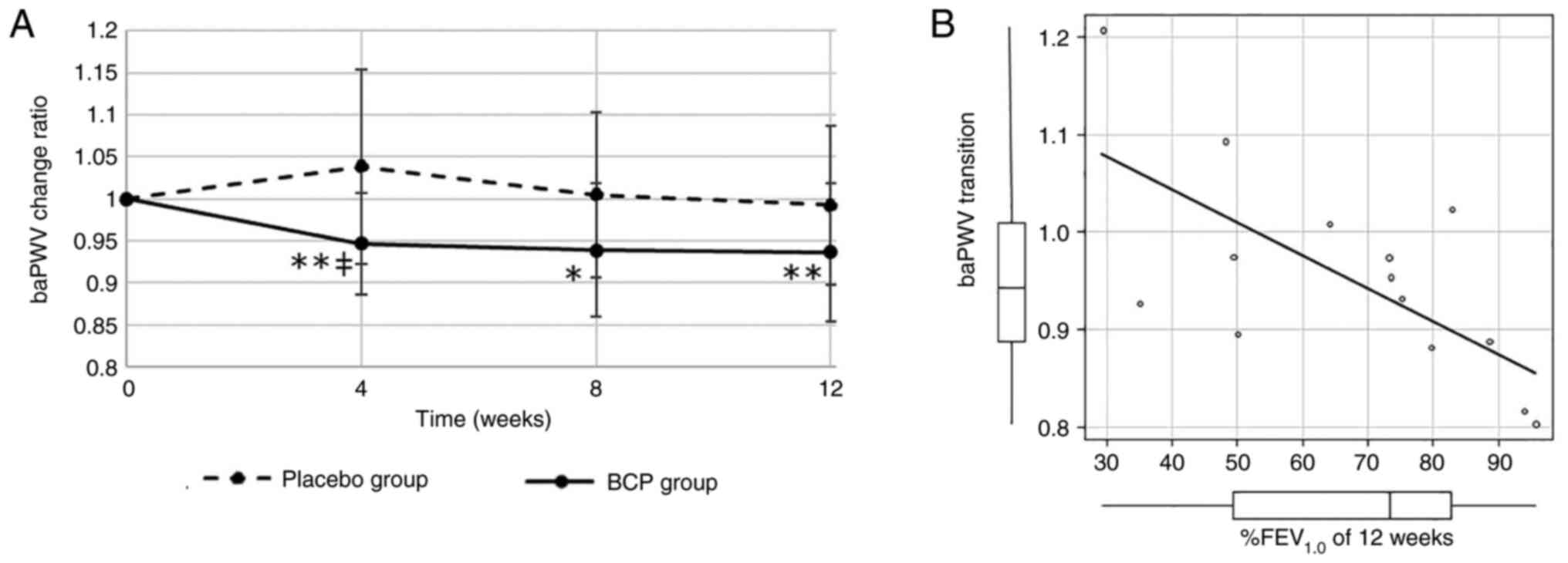
The baPWV transition of the BCP and placebo groups
is shown in Fig. 3A for subjects
whose initial baPWV was >1,300 cm/sec. In the BCP group, baPWV
was reduced at 4, 8 and 12 weeks after the intervention started,
and the difference from the initial value was statistically
significant (P=0.03, 0.09 and 0.03). In contrast, the baPWV did not
change significantly in the placebo group. The difference in baPWV
between the BCP and placebo groups for four weeks was statistically
significant (P=0.08). In all cases, there was a small difference
between the BCP and placebo groups, but this was not statistically
significant (Fig. S2A).
There was little difference among the BCP 30%, BCP
15%, and placebo groups for all cases. However, the baPWV
transitions of the two BCP intervention groups were less than those
of the placebo group at 4, 8 and 12 weeks after intervention for
the sub-analysis of subjects whose initial baPWV was larger than
1300 cm/sec (Fig. S2B and
C).
Except for baPWV, vascular function data such as
diastolic and systolic blood pressure (mean of left and right
arms), pulse pressure, heart rate, and ABI were not significantly
changed during the 12 week study (Fig. S3A).
Respiratory function
The transition of %VC and %FEV1.0 for
each group is shown in Fig. S4.
Both %VC and %FEV1.0 did not change significantly in any
of the groups.
Correlation analysis
The correlation between %FEV1.0 and baPWV
transition for 12 weeks was strong and statistically significant
(Fig. 3B). The correlations
between %FEV1.0 and the area under the curve of blood
concentration (AUC) of BCP and between the AUC of BCP and baPWV
transition at 12 weeks are shown in Fig. S5. These correlations were moderate
but not statistically significant.
Number of cigarettes smoked
The number of cigarettes smoked by each participant
was monitored during the study and is plotted in Fig. S6. There was no correlation between
the number of cigarettes smoked and the amount of BCP in
capsules.
Discussion
In this study, we determined the bioavailability of
inhaled BCP and its effects on vascular stiffness by inhaling BCP
via smoking. As a result of the bioavailability, inhaled BCP was
transferred to the blood and reached 4 ng/ml in serum. The baPWV
decreased by 10% in subjects whose initial baPWV was >1,300
cm/sec, but BCP did not have any effect on respiratory function
such as %VC and %FEV1.0.
It has been reported that the bioavailability and
absorption rate of inhaled compounds are higher than those of oral
compounds (24). In the case of
cigarette smoking, it was reported that the maximum blood
concentration of nicotine was 10-20 ng/ml, and the time for maximum
blood concentration was approximately 10 min (25-27).
For BCP, the results of this study were consistent with these
reports regarding the maximum blood concentration and the
transition of blood concentration. BCP was detected in the blood of
the placebo group. The reason for this detection was considered to
be BCP ingested from food. It is known that 0.4-0.5 mg per day per
person of BCP is ingested from food in Europe and the United States
(28).
In this study, baPWV was reduced by 10% in the BCP
group when the initial baPWV was larger than 13,00 cm/sec. The
range of initial baPWV was 1300-1600 cm/sec; hence, 10% of baPWV
means 130-160 cm/sec. In previous reports, the one-year
intervention of EPA (1,800 mg/day) reduced baPWV, and the changes
in baPWV of the EPA and placebo groups were -10 and +40,
respectively (13). In the case of
medicines for treating dyslipidemia, ezetimibe and simvastatin
reduced the aortic PWV by 10% for six weeks (11). Other reports showed that a one-year
intervention with fluvastatin also reduced baPWV from 1,800 to
1,650 cm/sec (12). Compared to
these functional foods and medicines, BCP was considered to have
the same or greater effect in reducing baPWV. It is known that the
baPWV is influenced by blood pressure. However, in this study,
baPWV was reduced, but blood pressure did not change. This result
suggests that blood vessel flexibility is restored without lowering
the blood pressure.
There was no difference in %VC and
%FEV1.0 during the 12 week monitoring period comparing
the BCP capsule and placebo groups. We concluded that respiratory
function did not change significantly during the study; therefore,
the BCP did not adversely affect respiratory function. Participants
with low %FEV1.0 could not capture BCP efficiently in
the blood through the lungs. People with a low %FEV1.0
are those suffering from chronic obstructive pulmonary disease
(COPD), and it is thought that their airway is narrow and gas
cannot be inhaled well because the air in the alveoli does not
change. In fact, it was reported that patients with COPD and low
%FEV1.0 had high blood PaCO2 and low
PaO2 (29). People with
a low %FEV1.0 are considered to have difficulty in
taking BCP, as well as oxygen and carbon dioxide. In this study,
people with a low %FEV1.0 had low blood concentrations
of BCP and a low baPWV reducing transition. Individual differences
of %FEV1.0 and/or absorption efficiency of BCP may have
affected the blood concentration of BCP and vascular function.
The number of cigarettes smoked was not affected by
BCP, suggesting that the improvement effect of BCP is not because
of the reduction of nicotine intake but the activity of BCP itself.
BCP is reported to be an agonist of the CB2 receptor (15). Compared to the cannabinoid type 1
receptor, which is expressed in central nervous cells and is
related to mental effects, the CB2 receptor is expressed mainly in
immune cells and is related to inflammation. BCP has also been
reported to interact with peroxisome proliferator-activated
receptors (PPARs), in particular PPAR α and γ (30-32).
PPARs are involved in both metabolic and inflammatory responses.
According to some reports, BCP shows anti-inflammatory and
anti-oxidative stress (33-37).
Previous studies have reported that nicotine induces aortic
stiffness with degeneration of the aorta in mice (4,16,38).
We recently reported that inhalation of BCP attenuates
nicotine-induced murine aortic stiffness via the CB2 dependent
pathway (19). These studies
suggest that BCP can reduce inflammation in the human body caused
by hazardous substances such as aldehydes in the mainstream smoke
of cigarettes.
A limitation of this study was the sample size and
monitoring period. In this study, only a few statistically
significant differences in baPWV reduction were observed owing to
the small number of subjects. To confirm the preventive effect of
BCP, it is necessary to have deteriorated vascular and respiratory
functions in the placebo group, which would take at least one year.
Moreover, this study contains content that makes use of patents
that are owned by Sunsho Pharmaceutical Co., Ltd., INABATA KORYO,
Co., Ltd., and Kindai University.
In conclusion, BCP with cigarette smoke can be
inhaled into the blood, thereby reducing the baPWV in humans. BCP
is, to the best of our knowledge, the first compound that can
comprehensively contribute to the health of smokers who cannot quit
smoking and can be easily ingested. BCP microcapsules can be placed
in cigarette wrapping paper to reduce the risk of sidestream smoke
and contribute to improved public health.
Supplementary Material
A systematic illustration of the study
design criteria. BCP, β-caryophyllene.
(A) The baPWV transition in the BCP
and placebo groups during this study for all case. Solid lines
represent the BCP group, and dashed lines indicate the placebo
group. baPWV transition in the BCP 30%, BCP 15%, and placebo groups
during this study for (B) all cases and (C) for subjects whose
initial baPWV was >1,300 cm/sec. Solid lines represent the BCP
30% group, dotted lines indicate the BCP 15% group, and dashed
lines represent the placebo group. *P<0.1 vs. the
initial baPWV. BCP, β-caryophyllene; baPWV, brachial-ankle pulse
wave velocity; %FEV1.0, forced expiratory volume in 1
sec.
Transitions of (A) diastolic and
systolic blood pressure, (B) pulse pressure, (C) heart rate and (D)
ABI. Data are the mean of left and right arms. ABI, ankle brachial
index.
Transitions of (A) %VC and (B)
%FEV1.0 in the BCP 30%, BCP 15%, and placebo groups
during this study. Solid lines represent the BCP 30% group, dotted
lines indicate the BCP 15% group, and dashed lines represent the
placebo group. BCP, β-caryophyllene; %FEV1.0, forced
expiratory volume in 1 sec; %VC, % vital capacity.
(A) Correlation between
%FEV1.0 in 12 weeks and the AUC of BCP. Spearman's rank
correlation coefficient is 0.548, and the P-value is 0.171. (B)
Correlation between the AUC of BCP and baPWV transition. Spearman's
rank correlation coefficient is -0.714, and the P-value is 0.0576.
%FEV1.0, forced expiratory volume in 1 sec; BCP,
β-caryophyllene; baPWV, brachial-ankle pulse wave velocity; AUC,
area under the curve of blood concentration.
Transition of the number of smoked
cigarettes per day during the study. Average and standard deviation
per week are plotted. Solid lines represent the BCP 30% group,
dotted lines indicate the BCP 15% group, and dashed lines represent
the placebo group. BCP, β-caryophyllene.
Acknowledgements
The authors deeply thank Dr. Kei Takagi (Takaoka
Hospital) for providing health care to the subjects. The authors
appreciate Ms. Yoko Yamamoto (Takaoka Hospital) for the blood
sampling. The authors would also like to thank Mr. Kazuhiko Tadano,
Mr. Tetsuya Katsumata, Ms. Motoyo Koyama, Mr. Takanori Kobayashi,
Mr. Mitsuhiro Fukuda, Mr. Tomoya Suzuki, and Dr. Hirofumi Watanabe
(Sunsho Pharmaceutical Co., Ltd.) for their support in this
project.
Funding
Funding: This work was financially supported by Sunsho
Pharmaceutical Co., Ltd. for data collection, statistical analyses,
and writing of the manuscript and INABATA KORYO, Co., Ltd. for raw
material offering and gas chromatography-mass spectrometry
analyses.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KY conceptualized and designed the details of this
research, collected clinical data and was a major contributor in
writing the manuscript. KT and YT analyzed data statistically,
collected clinical data, and reviewed and edited the manuscript. YM
designed the details of this research, and reviewed and edited the
manuscript. SM and YY analyzed serum samples by gas
chromatography-mass spectrometry, provided raw materials, and
reviewed and edited the manuscript. NZ and NU conceptualized and
designed the details of this research, and reviewed and edited the
manuscript. KY, SM, YY, NZ and NU confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Institutional
Review Board of Sunsho Pharmaceutical Co., Ltd. (approval no. 21001
for vascular function, and 21002 for BCP concentration in blood).
Written informed consent was given by each subject
individually.
Patient consent for publication
Written informed consent was obtained from the
participants in this study for publication of this paper.
Competing interests
KY, KT, YT, and YM are employees of Sunsho
Pharmaceutical Co., Ltd., and SM and YY are employees of INABATA
KORYO, Co., Ltd. Kindai University, INABATA KORYO, Co., Ltd., and
Sunsho Pharmaceutical Co., Ltd. applied patents related to this
research (PCT/JP2021/040234, JP/2021/149802, JP/2021/149803,
JP/2022/074711). BCP used in this research was purchased from
INABATA KORYO, Co., Ltd. The BCP-containing capsules and placebo
capsules were manufactured by Sunsho Pharmaceutical Co., Ltd.
References
|
1
|
GBD, Tobacco collaborators. Spatial,
temporal, and demographic patterns in prevalence of smoking tobacco
use and attributable disease burden in 204 countries and
territories, 1990-2019: A systematic analysis from the Global
burden of Disease Study 2019. Lancet. 2021:2337–2360.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Murakami Y, Miura K, Okamura T and Ueshima
H: EPOCH-JAPAN Research Group. Population attributable numbers and
fractions of deaths due to smoking: A pooled analysis of 180,000
Japanese. Prev Med. 52:60–65. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ikeda N, Inoue M, Iso H, Ikeda S, Satoh T,
Noda M, Mizoue T, Imano H, Saito E, Katanoda K, et al: Adult
mortality attributable to preventable risk factors for
non-communicable diseases and injuries in Japan: A comparative risk
assessment. PLoS Med. 9(e1001160)2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang S, Zhang C, Zhang M, Liang B, Zhu H,
Lee J, Viollet B, Xia L, Zhang Y and Zou MH: Activation of
AMP-activated protein kinase α2 by nicotine instigates formation of
abdominal aortic aneurysms in mice in vivo. Nat Med. 18:902–910.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Bramwell JC and Hill AV: Velocity of
transmission of the pulse-wave. Lancet. 199:891–892. 1922.
|
|
6
|
Yamashina A, Tomiyama H, Takeda K, Tsuda
H, Arai T, Hirose K, Koji Y, Hori S and Yamamoto Y: Validity,
reproducibility, and clinical significance of noninvasive
brachial-ankle pulse wave velocity measurement. Hypertens Res.
25:359–364. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tanaka H, Munakata M, Kawano Y, Ohishi M,
Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T
and Ozawa T: Comparison between carotid-femoral and brachial-ankle
pulse wave velocity as measures of arterial stiffness. J Hypertens.
27:2022–2027. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamashina A, Tomiyama H, Arai T, Hirose K,
Koji Y, Hirayama Y, Yamamoto Y and Hori S: Brachial-ankle pulse
wave velocity as a marker of atherosclerotic vascular damage and
cardiovascular risk. Hypertens Res. 26:615–622. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tomiyama H, Matsumoto C, Yamada J, Yoshida
M, Odaira M, Shiina K, Nagata M and Yamashina A: Predictors of
progression from prehypertension to hypertension in Japanese men.
Am J Hypertens. 22:630–636. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vlachopoulos C, Aznaouridis K,
Terentes-Printzios D, Ioakeimidis N and Stefanadis C: Prediction of
cardiovascular events and all-cause mortality with brachial-ankle
elasticity index: A systematic review and meta-analysis.
Hypertension. 60:556–562. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mäki-Petäjä KM, Booth AD, Hall FC, Wallace
SM, Brown J, McEniery CM and Wilkinson IB: Ezetimibe and
Simvastatin reduce inflammation, disease activity, and aortic
stiffness and improve endothelial function in rheumatoid arthritis.
J Am Coll Cardiol. 50:852–858. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ichihara A, Hayashi M, Koura Y, Tada Y,
Kaneshiro Y and Saruta T: Long-term effects of statins on arterial
pressure and stiffness of hypertensives. J Hum Hypertens.
19:103–109. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tomiyama H, Takazawa K, Osa S, Hirose K,
Hirai A, Iketani T, Monden M, Sanoyama K and Yamashina A: Do
eicosapentaenoic acid supplements attenuate age-related increases
in arterial stiffness in patients with dyslipidemia?: A preliminary
study. Hypertens Res. 28:651–655. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kyriakos CN, Zatoński MZ and Filippidis
FT: Flavour capsule cigarette use and perceptions: a systematic
review. Tob Control: Oct 4, 2021 (Epub ahead of print).
|
|
15
|
Gertsch J, Leonti M, Raduner S, Racz I,
Chen JZ, Xie XQ, Altmann KH, Karsak M and Zimmer A:
Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci
USA. 105:9099–9104. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kugo H, Zaima N, Tanaka H, Urano T, Unno N
and Moriyama T: The effects of nicotine administration on the
pathophysiology of rat aortic wall. Biotech Histochem. 92:141–148.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kugo H, Zaima N, Onozato M, Miyamoto C,
Hashimoto K, Yanagimoto K and Moriyama T: Suppressive effects of
dietary EPA-rich fish oil on the degradation of elastin fibers in
the aortic wall in nicotine-administered mice. Food Funct.
8:2829–2835. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Takemoto Y, Kishi C, Sugiura Y, Yoshioka
Y, Matsumura S, Moriyama T and Zaima N: Distribution of inhaled
volatile β-caryophyllene and dynamic change of liver metabolites in
mice. Sci Rep. 11(1728)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kishi C, Higashihara M, Takemoto Y, Kamei
M, Yoshioka Y, Matsumura S, Yamada K, Kobayashi T, Matahira Y,
Moriyama T and Zaima N: Inhaled volatile β-caryophyllene is
incorporated into the aortic wall and attenuates nicotine-induced
aorta degeneration via a CB2 receptor-dependent pathway. Biomed
Pharmacother. 153(113423)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He Y, Galaj E, Bi GH, Wang XF, Gardner E
and Xi ZX: β-caryophyllene, a dietary terpenoid, inhibits nicotine
taking and nicotine seeking in rodents. Br J Pharmacol.
177:2058–2072. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Varga ZV, Matyas C, Erdelyi K, Cinar R,
Nieri D, Chicca A, Nemeth BT, Paloczi J, Lajtos T, Corey L, et al:
β-caryophyllene protects against alcoholic steatohepatitis by
attenuating inflammation and metabolic dysregulation in mice. Br J
Pharmacol. 175:320–334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Amalraj A, Jacob J, Varma K and Gopi S:
Preparation and characterization of liposomal β-caryophyllene
(Rephyll) by nanofiber weaving technology and its effects on
delayed onset muscle soreness (DOMS) in humans: A randomized,
double-blinded, crossover-designed, and placebo-controlled study.
ACS Omega. 5:24045–24056. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Patton JS, Fishburn CS and Weers JG: The
lungs as a portal of entry for systemic drug delivery. Proc Am
Thorac Soc. 1:338–344. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Henningfield JE: Nicotine medications for
smoking cessation. N Engl J Med. 333:1196–1203. 1995.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Benowitz NL, Hukkanen J and Jacob P: III:
Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp
Pharmacol. 192:29–60. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
D'Ruiz CD, Graff DW and Yan XS: Nicotine
delivery, tolerability and reduction of smoking urge in smokers
following short-term use of one brand of electronic cigarettes. BMC
Public Health. 15(991)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Adams TB, Gavin CL, McGowen MM, Waddell
WJ, Cohen SM, Feron VJ, Marnett LJ, Munro IC, Portoghese PS,
Rietjens IM and Smith RL: The FEMA GRAS assessment of aliphatic and
aromatic terpene hydrocarbons used as flavor ingredients. Food Chem
Toxicol. 49:2471–2494. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fard MR and Zarezadeh N: Relationship
between FEV1 and PaO2, PaCO2 in patients with
chronic bronchitis. Tanaffos. 3:41–46. 2004.
|
|
30
|
Youssef DA, El-Fayoumi HM and Mahmoud MF:
Beta-caryophyllene protects against diet-induced dyslipidemia and
vascular inflammation in rats: Involvement of CB2 and PPAR-γ
receptors. Chem Biol Interact. 297:16–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Irrera N, D'Ascola A, Pallio G, Bitto A,
Mazzon E, Mannino F, Squadrito V, Arcoraci V, Minutoli L, Campo GM,
et al: β-caryophyllene mitigates collagen antibody induced
arthritis (CAIA) in mice through a cross-talk between CB2 and
PPAR-γ receptors. Biomolecules. 9(326)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu C, Jia Y, Lee JH, Jun HJ, Lee HS, Hwang
KY and Lee SJ: Trans-caryophyllene is a natural agonistic ligand
for peroxisome proliferator-activated receptor-α. Bioorg Med Chem
Lett. 24:3168–3174. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ames-Sibin AP, Barizão CL, Castro-Ghizoni
CV, Silva FMS, Sá-Nakanishi AB, Bracht L, Bersani-Amado CA,
Marçal-Natali MR, Bracht A and Comar JF: β-caryophyllene, the major
constituent of copaiba oil, reduces systemic inflammation and
oxidative stress in arthritic rats. J Cell Biochem.
119:10262–10277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Al-Taee H, Azimullah S, Meeran MFN, Alaraj
Almheiri MK, Al Jasmi RAA, Tariq S, Ab Khan M, Adeghate E and Ojha
S: β-caryophyllene, a dietary phytocannabinoid attenuates oxidative
stress, inflammation, apoptosis and prevents structural alterations
of the myocardium against doxorubicin-induced acute cardiotoxicity
in rats: An in vitro and in vivo study. Eur J Pharmacol.
858(172467)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Brito LF, Oliveira HBM, das Neves Selis N,
E Souza CLSE, Júnior MNS, de Souza EP, Silva LSCD, de Souza
Nascimento F, Amorim AT, Campos GB, et al: Anti-inflammatory
activity of β-caryophyllene combined with docosahexaenoic acid in a
model of sepsis induced by Staphylococcus aureus in mice. J Sci
Food Agric. 99:5870–5880. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fontes LBA, Dias DDS, Aarestrup BJV,
Aarestrup FM, Da Silva Filho AA and Corrêa JODA: β-caryophyllene
ameliorates the development of experimental autoimmune
encephalomyelitis in C57BL/6 mice. Biomed Pharmacother. 91:257–264.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Basha RH and Sankaranarayanan C:
β-caryophyllene, a natural sesquiterpene lactone attenuates
hyperglycemia mediated oxidative and inflammatory stress in
experimental diabetic rats. Chem Biol Interact. 245:50–58.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wagenhäuser MU, Schellinger IN, Yoshino T,
Toyama K, Kayama Y, Deng A, Guenther SP, Petzold A, Mulorz J,
Mulorz P, et al: Chronic nicotine exposure induces murine aortic
remodeling and stiffness segmentation-implications for abdominal
aortic aneurysm susceptibility. Front Physiol.
9(1459)2018.PubMed/NCBI View Article : Google Scholar
|