Introduction
Purtscher's retinopathy, also known as ‘angiopathia
retinae traumatica’, first described by Otmar Purtscher in 1910, is
a traumatic angiopathy caused mostly by thoracal or cranial trauma.
The most common retinal symptoms are bilateral ischaemic lesions
(cottonwool spots or Purtscher Flecken) and retinal haemorrhages
(dot-like, preretinal or flame-shaped) (1). Laboratory and clinical observations
have shown that microparticles involved in the pathogenesis of
Purtscher's retinopathy lead to occlusion of small arterioles.
These microparticles may be composed of aggregated leukocytes or
fibrin clots. The phenomenon of intravascular coagulation is well
known after trauma or acute pancreatitis. Purtscher's retinopathy
is associated with both conditions (2). Besides these causes, many other
triggers can cause these changes in the retina (3).
A rare complication of bilateral vision loss has
been described in a young alcoholic with acute pancreatitis and was
verified as Purtscher's retinopathy (4). This finding can develop even several
months after acute pancreatitis (5). We were interested in the case of a
young patient, who suffered from an acute pancreatitis with visual
impairment, which was not caused by Purtscher's retinopathy, but
probably by bilateral ischaemic neuropathy. Therefore, we present
this case report, which has not to date been reported in the
literature.
Case report
A young woman (born 1996), otherwise healthy,
consumed wine daily at a dose of approximately 500 ml per day
(because of personal problems). Due to abdominal pain and collapse,
she was admitted to the intensive care unit of a regional hospital
in Czech Republic, where she was diagnosed with acute pancreatitis.
During the hospitalization, vision was impaired, and she described
‘disappearing around the subject’. On the first ophthalmological
examination, the distance visual acuity was 0.3 and near 0.3
particularly. There was a fragmented foveolar reflex in fundoscopy,
but otherwise the findings were normal. The macular OCT scan was
also normal. Perimetric examination showed concentric constriction
to 40 degrees.
Purtscher's retinopathy was suspected, but the
ocular findings excluded this suspicion. The CT scan of the brain
showed no abnormalities.
On examination at the clinical site in September
2021, visual acuity was 1.0 particularly. The patient reported
subjectively blurred outlines of the letters. The colour vision was
impaired, but after prolonged concentration she was able to read
the Velhagen charts correctly. Ocular findings including
biomicroscopy and fundus fluorescence angiography were in the
physiological range and intraocular pressure was 13/13 mmHg. As
part of this medical follow-up, systemic blood examinations were
performed with the following results: Blood count (CBC), blood
coagulation index, erythrocyte sedimentation rate (ESR), and so on,
would give a better demonstrate of the disease), were normal except
for C-reactive protein (above the upper limit of normal, see
Table I).
 | Table IBlood analysis results. |
Table I
Blood analysis results.
| Blood parameter | Acronym | Results | Reference
interval | Units |
|---|
| Leukocytes | WBC | 5.57 | 4.00-10.00 | 109/l |
| Erythrocytes | RBC | 4.12 | 3.8-5.20 |
1012/l |
| Haemoglobin | HGB | 124 | 120-160 | g/l |
| Haematocrit | HCT | 0.395 | 0.350-0.470 | 1 |
| Thrombocytes | PLT | 298 | 150-400 | 109/l |
| Neutrophils | NE | 59.9 | 45.0-70.0 | % |
| Lymphocytes | LY | 29.3 | 20.0-45.0 | % |
| Monocytes | MO | 9.5 | 2.0-12.0 | % |
| Eosinophils | EO | 0.9 | 0.0-5.0 | % |
| Basophils | BA | 0.4 | 0.0-2.0 | % |
| C-Reactive
proteina | CBC | 5.3 | <5.0 | mg/l |
The patient was recommended treatment with B vitamin
(milgamma N 1 amp. i.m. 3 times per week). Apart from this
recommended medication, the patient was taking velaxin for
depression in the long term.
In November 2021, the examination was extended with
electroretinography (pattern electroretinogram), which showed
normal retinal response in both eyes. The visual evoked potential
examination (PVEP) with 1-degree square stimulation showed a small
decrease in both A1 and A2 amplitudes (10 and 9.2 uV), without any
prolonged latency of the P100 ms peak. When smaller squares were
used (15 min), responses were significantly reduced (4.5 and 6 uV).
P100 peak latency was still not prolonged (6). The examination was performed with the
Roland Consult electrophysiological diagnostic system (Germany)
according to the ISCEV methodology. The size of the stimulation
field was 41x31 angular degrees.
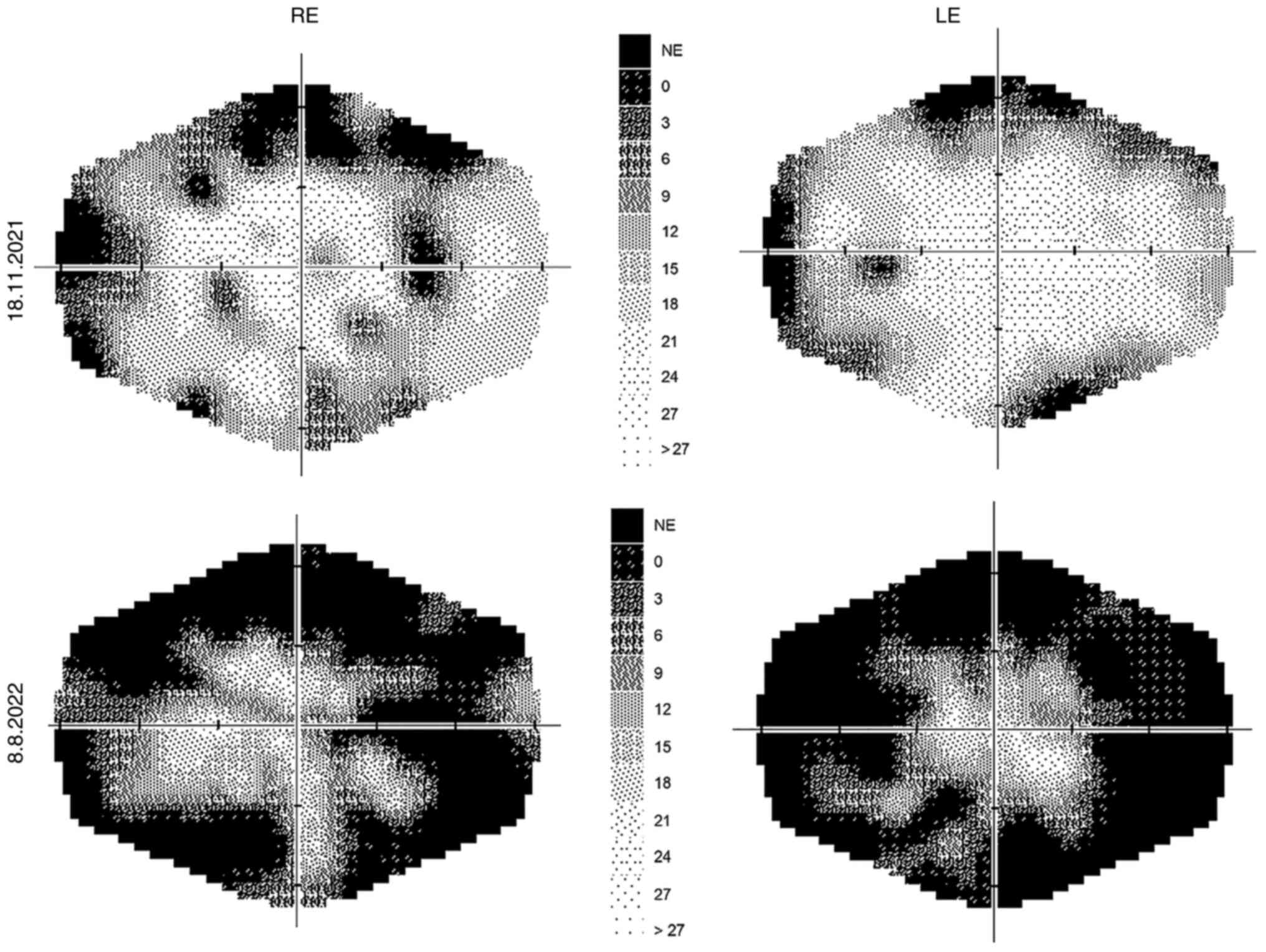
A perimetric examination, which was performed with
the Medmont M700 (Australia), fast threshold program in the range
of 0-30 degrees, also proved a progression of defects (Fig. 1).
At the follow-up examination 13 months after the
onset of visual impairment, the distance visual acuity (VA) was 0.8
and the near VA was 0.6. Intraocular pressure was 12/12 mmHg.
Colour vision examination was normal again with careful
concentration. Fundoscopy showed slight pigment abnormalities in
the foveal area and mild perifoveal brightening on the left eye.
The findings were otherwise normal. Perimetric examination showed a
progression of changes (Fig.
1).
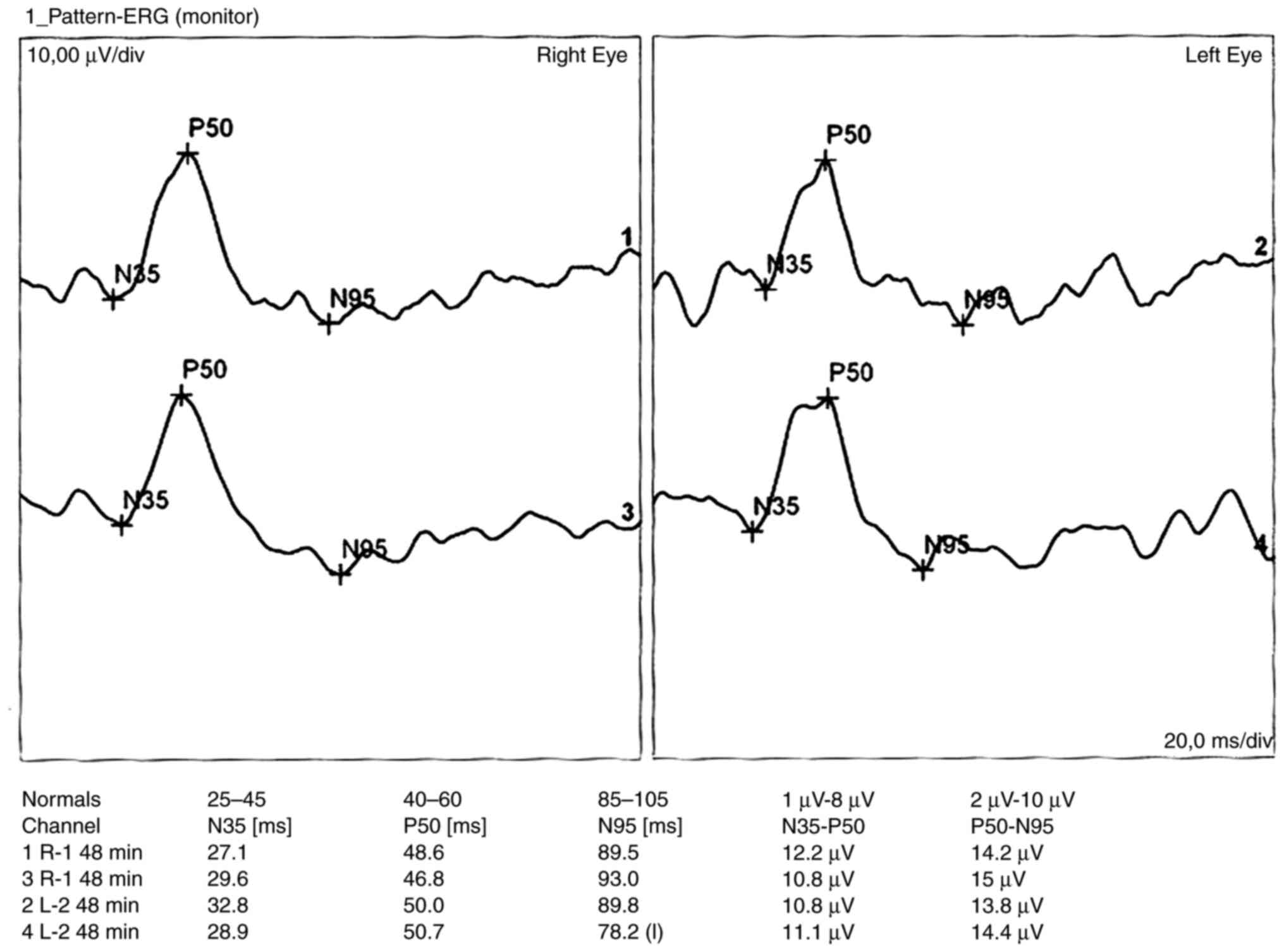
The pattern electroretinogram (PERG)-according to
ISCEV method (using DTC contact electrodes, reference electrode was
placed on the skin near the ipsilateral outer canthus of each eye,
the size of the stimulating area was 15 degrees, the reversal rate
of the squares equal to 0.8 degrees and the contrast equal to 80%)
was bilaterally normal (Fig. 2).
The PVEP with 1 degree squares stimulation showed borderline
amplitudes (12.5 and 10 uV), with a prolongation of the P100 peak
latency to 121 ms on the right and 129 ms on the left eye. Using
smaller squares (15 min), the responses were borderline on the
right (12.3 uV) and significantly reduced on the left (3.6 uV).
P100 peak latencies were prolonged to 130 ms on the right and 124
ms on the left eye.
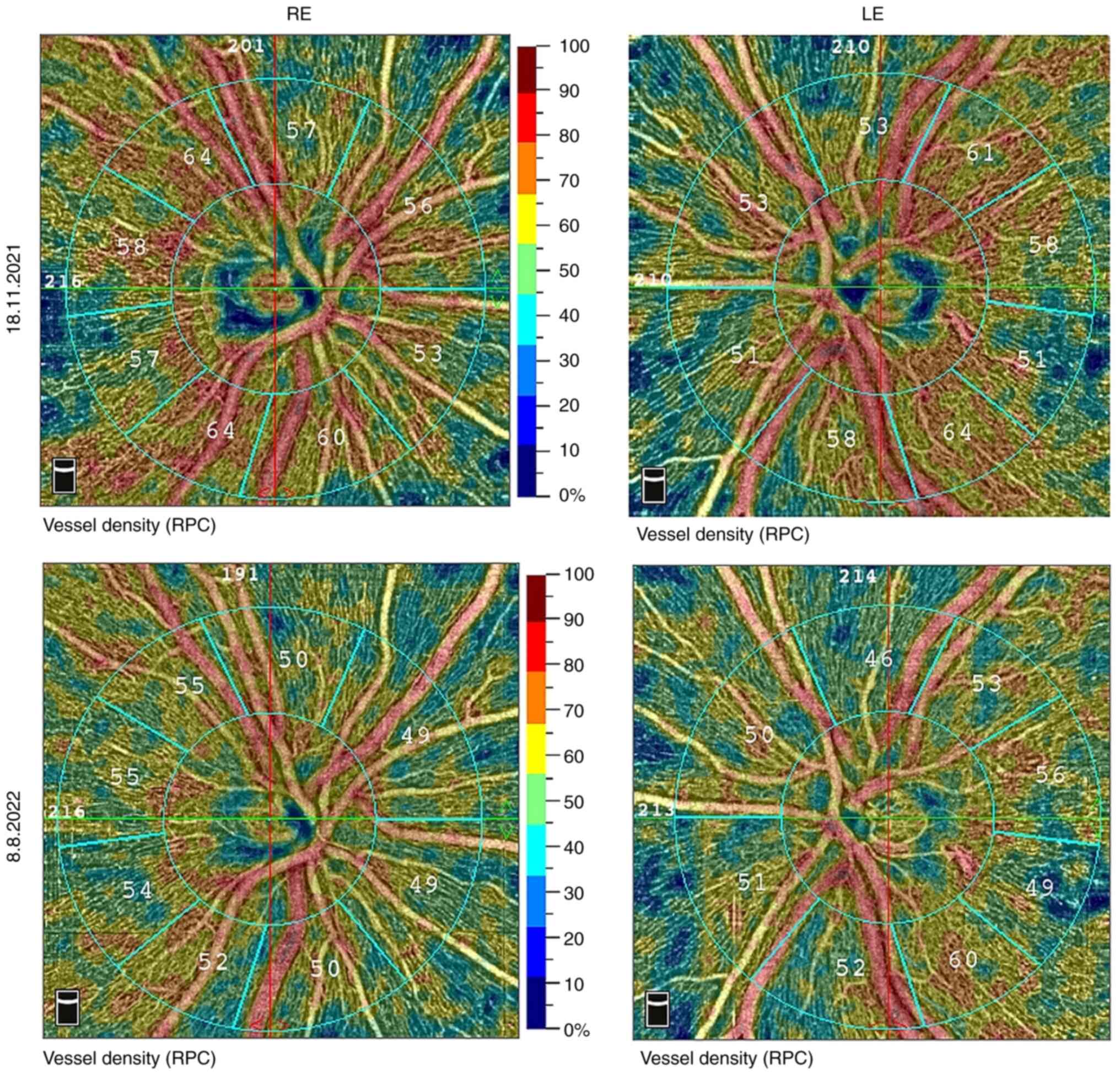
Retinal nerve fibre layer (RNFL) was 131 µm on the
right and 127 µm on the left eye. The vessel density was also
normal. The examination was performed on the Avanti RTVue XR by
Optovue (USA), see results in Fig.
3.
At the follow-up examination 13 months after the
onset of visual impairment, RNFL was 131 and 124 µm, but there was
a decrease of approximately 10 % in all values in vessel density
(Fig. 3).
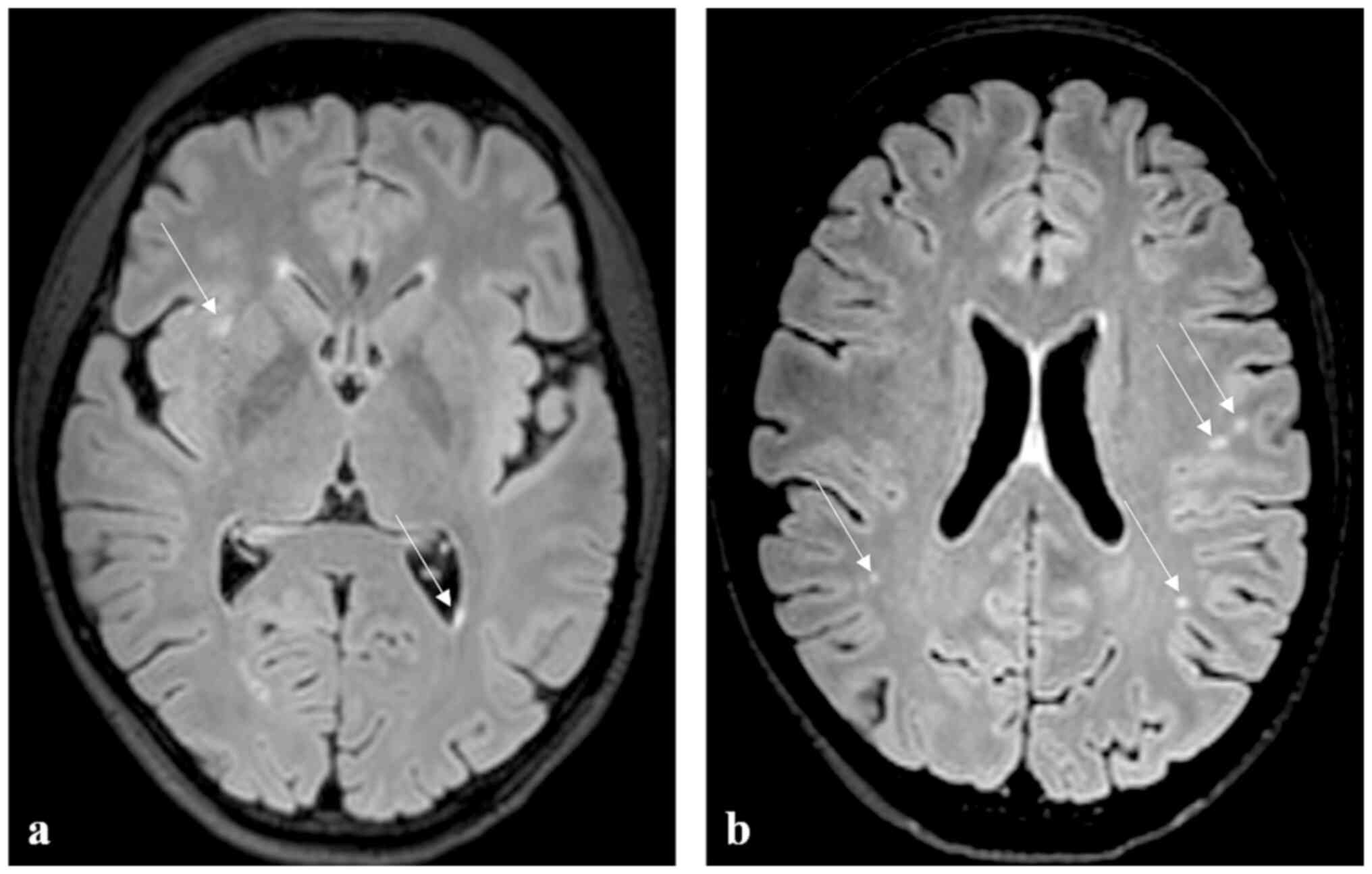
The MRI examination (Fig. 4) was performed in November 2021 on
an Achieva dStream TX SERIES 3T machine (Philips HealthCare, Best,
Nederland) with a 32-channel SENSE RF head coil. The imaging
protocol included T2 mDIXON coronal and axial sequences (TR/TE
3,000/80ms), 3D FLAIR (TR/TE 4,800/269ms), T2 3D DRIVE (TR/TE
2,000/240ms), Turbo Field Echo (TFE) T1 3D sequence (TR/TE 7/3ms),
VenBold (TR/TE 15/21) and DWI (TR/TE 3,616/79ms).
The aetiology seemed to be post-inflammatory,
because of numerous small focuses of non-specific gliosis in the
white matter of both cerebral hemispheres. A follow-up MRI scan 13
months after the onset of the disorder demonstrated also
nonspecific focuses in the white matter of both cerebral
hemispheres subcortically and paraventricularly bilaterally in
stationary number and location as in the November 2021 scan.
Neurological examinations over time (the last one in
August 2022) showed no abnormalities.
We concluded the finding as bilateral optic
neuropathy, probably of ischaemic origin. With the atrophy of the
damaged optic nerve fibres, there was a prolongation of VEP latency
and a decrease in VD over time.
The patient's current status as of October 2022
corresponds to uncorrected distance visual acuity of 0.8 partially,
and 0.6 for uncorrected near visual acuity (in decimal values).
Discussion
Coagulation abnormalities can present with a
spectrum of findings, from isolated intravascular thrombosis to
severe disseminated intravascular coagulation. Purtscher's
retinopathy, caused by microembolisation in choroidal and retinal
arterioles, should be included among the various systemic
manifestations of acute pancreatitis. Such visual disturbance may
be a rare systemic manifestation of acute pancreatitis that has not
been associated with a severe or complicated clinical course. There
is no targeted treatment of these ocular complications, so the
outcome therefore depends on the resolution of the primary
pancreatic disease (7).
The course of the optic nerve can be divided into
intraorbital, intracanalicular and intracranial sections. The blood
supply to these different parts of the nerve is provided by many
arterial branches, therefore an ischaemia of the posterior optic
nerve may not be caused by occlusion of a single artery. The
intraorbital segment is supplied by both the peripheral centripetal
vascular system from the pial plexus and the axial centrifugal
vascular system composed of branches of the central retinal artery
(8).
Manifestation of non-arteritic posterior ischaemic
neuropathy (PION) is rare and usually arises from a small vessel
disease. It is associated with multifactorial systemic diseases
such as diabetes mellitus, hypertension, atherosclerosis or other
causes (carotid artery dissection, carotid cavernous fistula)
(9), migraine (8,10,11),
associated with haemodialysis (8,12),
or head injury (8). Perioperative
conditions can also be the cause (13-18).
However, histopathological findings of PION may vary
between patients, and the lack of accurate data is also due to only
sporadic case reports in the available literature. It has been
reported that the optic nerve can be spared in either the
peripheral or central segment, but there are also cases of complete
loss of the axons with total optic nerve infarction. This
variability is due to the different routes of vascular supply from
the central retinal artery, as mentioned above. Specifically,
ischaemia involving centrifugal vascular systems spares the central
portion of the nerve, whereas ischaemia involving centripetal
vascular supply systems spares the peripheral portion of the nerve.
The latter case of ischaemia is more common in both arteritic and
non-arteritic PION (8).
In non-arteritic PION, visual acuity ranges from
20/25 or better in 17 % of patients, 20/40 or better in 20 % and
20/200 or worse in 69 % 18. Central visual field defects are common
in both arteritic and non-arteritic PION (7).
In cases of acute ischaemic injury to the posterior
part of the optic nerve, cytotoxic oedema occurs and results in
water molecules accumulating intracellularly from the extracellular
space, restricting diffusion across the cell membrane. Thus,
ischaemia of the pial branches supplying the periphery, or of the
central retinal artery supplying the centre, could both be
theoretically determined using DWI, but only a few cases have been
reported diagnosing PION successfully in this manner (19).
Our patient, therefore, could have suffered an
ischaemia of the intracranial part of the peripheral and possibly
central part of the visual pathway. This ischaemia could also be
induced by inflammatory products in pancreatitis. That is what the
brain MRI results showed.
The fundoscopy findings showing normal RNFL and VD
values, and pathological VEP support this hypothesis. Subsequent
follow-up examination showed prolonged P100 peak latency in VEP and
decreased VD values. These findings may be indicative of ongoing
atrophy in the visual pathway.
In conclusion, posterior ischaemic neuropathy is a
relatively rare disease. Its association with acute pancreatitis
has not yet been described in the literature. Therefore, it is
important to consider this neuro-ophthalmological abnormality in
patients with acute pancreatitis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC conceptualized and designed the study, and is
lead author of the manuscript. ZD, MK, MF and JL implemented
clinical investigations and outcome assessment, and share
co-authorship of the manuscript. All authors have read and approved
the final manuscript. HC and JL confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The present case report was performed according to
the Declaration of Helsinki and was approved by the Internal Ethics
Committee of the Ophthalmology Clinic JL (Prague, Czech Republic).
All data used were anonymized.
Patient consent for publication
All details, medical records, figures, medical
history or test results were used with the written informed consent
for publication from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Purtscher O: Angiopathia retinae
traumatica. Lymphorrhagien des Augengrundes. Albrecht von Graefe
Arch Ophth. 82:347–371. 1912.
|
|
2
|
Behrens-Baumann W, Scheurer G and Schroer
H: Pathogenesis of Purtscher's retinopathy. An experimental study.
Graefes Arch Clin Exp Ophthalmol. 230:286–291. 1992.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tripathy K and Patel BC: Purtscher
retinopathy. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2022.
|
|
4
|
Suárez Crespo JF, de Teresa Galván FJ,
Sánchez Rico C, González Galilea A, Pinel Julián LM, Espinosa
Aguilar MD and López de Hierro Ruiz M: Purtscher's retinopathy and
acute alcoholic pancreatitis. Rev Esp Enferm Dig. 885:513–515.
1996.PubMed/NCBI(In Spanish).
|
|
5
|
Sharma AG, Kazim NA, Eliott D, Houghton O
and Abrams GW: Purtscher's retinopathy that occurred 6 months
before acute pancreatitis. Am J Ophthalmol. 141:205–207.
2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lešták J, Nutterová E, Pitrová Š, Krejčová
H, Bartošová L and Forgáčová V: High tension versus normal tension
glaucoma. A comparison of structural and functional examinations. J
Clinic Exp Ophthalmol S. (5)2012.
|
|
7
|
Campo SM, Gasparri V, Catarinelli G and
Sepe M: Acute pancreatitis with Purtscher's retinopathy: Case
report and review of the literature. Dig Liver Dis. 32:729–732.
2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hayreh SS: Posterior ischaemic optic
neuropathy: Clinical features, pathogenesis, and management. Eye
(Lond). 18:1188–1206. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oh DJ, Chhadva P, Kanu LN, Liu CY and
Macintosh PW: Sudden-onset blindness from a spontaneous
carotid-cavernous fistula with secondary central retinal artery
occlusion and posterior ischemic optic neuropathy.
Neuroophthalmology. 43:107–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee AG, Brazis PW and Miller NR: Posterior
ischemic optic neuropathy associated with migraine. Headache.
36:506–510. 1996.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Foroozan R, Marx DP and Evans RW:
Posterior ischemic optic neuropathy associated with migraine.
Headache. 48:1135–1139. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Buono LM, Foroozan R, Savino PJ,
Danesh-Meyer HV and Stanescu D: Posterior ischemic optic neuropathy
after hemodialysis. Ophthalmology. 110:1216–1218. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Buono LM and Foroozan R: Perioperative
posterior ischemic optic neuropathy: Review of the literature. Surv
Ophthalmol. 50:15–26. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Weber ED, Colyer MH, Lesser RL and
Subramanian PS: Posterior ischemic optic neuropathy after minimally
invasive prostatectomy. J Neuroophthalmol. 27:285–287.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eli IM, Kim RB, Kilburg C, Pecha TJ,
Couldwell WT and Menacho ST: Postoperative posterior ischemic optic
neuropathy after left far-lateral craniectomy for resection of
craniocervical meningioma. World Neurosurg. 114:339–343.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Levinson B and Reddy S: Posterior ischemic
optic neuropathy after extensive spine surgery: A case report and
review of the literature. AANA J. 87:37–42. 2019.PubMed/NCBI
|
|
17
|
Oliver JD, Kobets AJ, Judy BF and Cohen
AR: Posterior ischemic optic neuropathy following supine craniotomy
for epidural abscess in a child. Childs Nerv Syst. 37:2657–2660.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hayreh SS: Ischemic optic neuropathy. Prog
Retin Eye Res. 28:34–62. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Maramattom BV, Sundar S, Thomas D and
Panikar D: Postoperative posterior ischemic optic neuropathy (PION)
following right pterional meningioma surgery. Ann Indian Acad
Neurol. 19:374–376. 2016.PubMed/NCBI View Article : Google Scholar
|