Introduction
Opioids such as morphine are clinically effective in
relieving acute, chronic and intractable pain. However, long-term
use of opioids induces adverse reactions, including respiratory
complications, gastrointestinal (GI) problems and constipation
(1-5).
Opioid-induced constipation (OIC) is characterized by decreased
frequency of bowel movements, changes in stool characteristics and
incomplete excretion. OIC can seriously impact the patient's
quality of life, resulting in discontinuation of the medication and
affecting the patient's pain management (6-8).
Statistically, ~90% of patients experience GI dysfunction, such as
constipation, after taking opioid-containing analgesics (9-12).
Moreover, opioids impact the physiological
functioning of the central nervous system (CNS) as well as the
peripheral nervous system (PNS) (13). The µ-opioid receptors (MOR) are the
primary action sites of opioids in the PNS (13-16).
It has been reported that OIC occurs primarily due to the
activation of the MOR pathway in the GI tract, thereby inhibiting
the release of neurotransmitters in colonic nerve cells and
reducing the sensitivity of sensory neurons of the PNS in
transmitting information across the colon defecation reflex pathway
(13,14). This inhibits the long-distance
transportation mechanism of the colon, causing intestinal contents
to be stuck in colonic cavities for a longer than usual, resulting
in the excessive absorption of water and electrolytes (8,17).
The biological action of purine signaling has been
recognized since 1929(18). The
purinergic receptors are of two subtypes, P1 and P2. The P2-type
receptors can be further sub-classified into two major families:
P2X and P2Y (4,19). The P2Y purinergic receptor family
is subdivided into P2Y purinergic receptor 1 (P2Y1)-like receptor
subtypes (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11) and the P2Y12-like
receptor subtypes (P2Y12-14) (19,20).
Among these, P2Y1 is widely expressed in the enteric nervous system
of the GI tract and is associated with diastolic function of the GI
smooth muscle induced by non-cholinergic and non-adrenergic
neurotransmitters (10,11,21)
such as adenosine triphosphate (ATP), and nitric oxide (NO).
Reportedly these neurotransmitters are the primary regulators of GI
tract cell motility by stimulating hyperpolarization of smooth
muscle cells, leading to the relaxation of the smooth muscle
(14,22).
In vitro experiments have shown that
electrical stimulation of colonic circular muscle cells records two
different functional potentials: Excitatory neuromuscular junction
potential (EJP) and inhibitory neuromuscular junction potential
(IJP) (23). EJP causes colonic
smooth muscle contraction, while IJP is primarily induced by
certain inhibitory neurotransmitters such as ATP and NO (24). Furthermore, IJP comprises fast
inhibitory junction potential (fIJP) and slow-acting neuromuscular
junction potential (sIJP) (11,22,25).
Purine-dependent fIJP induces transient phase relaxation of GI
smooth muscle, while NO-dependent sIJP induces sustained relaxation
of these muscles (9,22). Studies have shown that fIJP is
highly sensitive to MRS2500, a selective antagonist for the P2Y1
receptor, while sIJP is sensitive to the NO synthase inhibitor
NG-nitro-L-arginine methyl ester (10,22).
Endomorphin-2 (EM2; Tyr-Pro-Phe-Phe-Nh2), the
endogenous ligand of MOR, selectively binds to MOR at a high
affinity and regulates visceral information transmission (25). Our previous study has shown that
the endogenous receptor agonist EM2 has no effect on sIJP of
colonic circular muscle cells but it can completely block fIJP,
which is similar to the effect of the P2Y1-selective antagonist
MRS2500 on the colon (9).
The present study was designed to determine the
association between P2Y1- and OIC by examining the distribution of
P2Y1 in the distal colonic submucosal plexus and its expression
changes in the distal colonic submucosa of OIC rats.
Materials and methods
Animals
Sprague-Dawley (SD) male rats (age, 6 weeks; weight,
180-200 g) were selected. All protocols were approved by the
Committee of Animal Use for Research and Education of the Ning Xia
Medical University (Yin Chuan, China; approval nos. 2015-090 and
2018-007). Animals were housed under controlled conditions:
Temperature 22±2˚C, humidity 40-60%, 12/12-h light-dark cycle and
allowed free access to water and food with constant air renewal.
All efforts were made to minimize the number and suffering of
animals.
Immunofluorescence (IF) staining
IF staining was used to observe the possible
morphological association between P2Y1, ATP synthase subunit β
(ATPB) and MOR in the submucosa of the distal colon of normal rats
so only normal SD rats were selected. A total of four untreated
male rats were anesthetized by intraperitoneal (i.p) injection of
10% chloral hydrate solution (300 mg/kg; 3 ml/kg). Once rats were
completely anesthetized after 1 min, an incision was made along the
midline of the abdomen to expose the colon and distal colon tissue
fragments were harvested. There were no peritonitis phenomena such
as muscle tension and intestinal adhesions during sampling. The
left colic flexure was used for the sign to retain the distal
parts. The section was washed with 0.01 mol/l PBS solution and
fixed with paraformaldehyde at room temperature. Then both ends
were ligated and stored at 4˚C for 8 h. Following 4˚C for 24 h in
30% sucrose solution, the preparations were cut along the mesentery
margin and 4˚C stored in 30% sucrose solution. The mucosal and
muscular layers were then removed under a stereo microscope (16X)
while the submucosa was collected in 0.01 mol/l PBS solution.
After rinsing the tissue samples three times with
0.01 mol/l PBS, 1 mol/l hydrochloric acid was added at room
temperature and then rinsed again three times with 0.01 mol/l PBS.
The 1% newborn calf serum (cat. no. 10099141C; Gibco; Thermo Fisher
Scientific, Inc.) was added to the tissue sections to block for 1 h
at room temperature. The tissue samples were divided into six
groups and incubated at room temperature for 1 h and then placed at
4˚C for 48 h with the following pairs of primary antibodies: i)
Anti-P2Y1 (rabbit polyclonal; cat. no. NBP1-30741; 1:200; NOVUS
Biologicals, LLC) and anti-neuronal nuclei antigen (anti-NeuN;
mouse monoclonal; cat. no. 104224; 1:200; Abcam); ii) anti-P2Y1 and
anti-calbindin (anti-CB; mouse monoclonal; cat. no. 11426; 1:300;
Abcam); iii) anti-P2Y1 and anti-calcitonin gene-related peptide
(anti-CGRP; mouse monoclonal; cat. no. 81887; 1:50; Abcam); iv)
anti-MOR (rabbit polyclonal; cat. no. 10275; Abcam, 1:300) and
anti-calbindin (anti-CB; mouse monoclonal; cat. no. 11426; 1:300;
Abcam); v) anti-MOR and anti-CGRP and ⅵ) anti-MOR and anti-ATPB
(mouse monoclonal; cat. no. 14730; 1:200; Abcam). Subsequently,
tissue sections were rinsed with 0.01 mol/l PBS three times and the
corresponding fluorescein-labeled secondary antibodies were added
to the six groups of tissue samples, respectively: i) Alex488
labeled donkey anti-rabbit IgG (cat. no. 6978; 1:500; Abcam) and
Alex594 labeled goat anti-mouse IgG (cat. no. 150116; 1:500;
Abcam); ii) Alex488 labeled donkey anti-rabbit IgG (cat. no. 6978;
1:500; Abcam) and Alex594 labeled goat anti-mouse IgG (cat. no.
150116; 1:500; Abcam); iii) Alex488 labeled donkey anti-rabbit IgG
(cat. no. 6978; 1:500; Abcam) and Alex594 labeled goat anti-mouse
IgG (cat. no. 150116; 1:500; Abcam); iv) Alex594 labeled goat
anti-rabbit IgG (cat. no. 150080; 1:500; Abcam) and Alex488 labeled
donkey anti-mouse IgG (cat. no. 6816; 1:500; Abcam); v) Alex488
labeled donkey anti-rabbit IgG (cat. no. 6978; 1:500; Abcam) and
Alex594 labeled goat anti-mouse IgG (cat. no. 150116; 1:500; Abcam)
and vi) Alex488 labeled donkey anti-rabbit IgG (cat. no. 6978;
1:500; Abcam) and Alex594 labeled goat anti-mouse IgG (cat. no.
150116; 1:500; Abcam) at room temperature. After incubating for 2
h, sections were rinsed again with 0.01 mol/l PBS three times.
Finally, slices were placed on glass slides and covered with
coverslips and fluorescent encapsulated mounting medium (cat no.
ab104139; Abcam). The specimens were imaged using a fluorescent
microscope (40x magnification; SOLYMPUS-BX51; Olympus Corporation).
Distal colonic tissue of the gut was obtained but could not be
effectively repaired after the material was collected. Euthanasia
was performed via cervical dislocation under anesthesia.
Model preparation
SD rats were randomly divided into OIC (n=10),
normal saline group (NSG; n=10), and normal control group (NCG;
n=10). For the OIC group, rats were given i.p injection of
loperamide hydrochloride dissolved in 0.9% normal saline (4 mg/kg;
1 ml/100 g) as previously described (26-29)
twice/day for 7 days. For NSG, 0.9% of normal saline (1 ml/100 g)
was i.p injected twice/day for 7 days; rats in the NCG did not
receive any treatment.
Humane endpoints were as follows: i) Persistent
diarrhea; ii) body weight loss >20% of the original weight; iii)
the rat was curled up to the corner of the cage and struggled
excessively during the drug administration and iv) peritonitis,
such as abdominal muscle tension, accompanied by persistent high or
low temperature or intestinal obstruction and intussusception
characterized by increased abdominal circumference. Affected rats
were euthanized. A total of three rats reached humane endpoints due
to the abnormal increase of abdominal circumference.
Tissues used for the IF, WB and RT-PCR experiments
were obtained from the distal colonic area of the gut that could
not be effectively repaired after being collected. Rats were
euthanized via cervical dislocation while still under
anesthesia.
Model evaluation
On the first day of modeling, the traits of rat
feces were observed and recorded. Rat fecal samples were scored
according to the Rome II classification criteria as follows: 1,
Dispersed hard block; 2, small sausage-like pieces; 3, cracks on
the sausage-like surface; 4, sausage-like surface was smooth and
soft; 5, soft lumps but clearly defined; 6, paste-like and unclear
boundary and 7, watery feces (30). Scores 1-2 were considered to
indicate constipation, 3-4 indicated normal stool, whereas 5-7
indicated diarrhea.
After modeling, the fresh feces of each group of
rats were collected to measure the fecal content. The electronic
balance was used to measure the wet weight and check the quality of
each fecal sample.
To determine the GI transit ratio, ~1 ml carmine
suspension (3 g carmine/50 ml hydroxymethyl cellulose) was
intra-gastrically administered. Rats were randomly selected from
each group (n=8/group) 3 h post-treatment. The rats were
anesthetized by i.p injection of 10% chloral hydrate (300 mg/kg; 3
ml/kg) and the pylorus was removed by laparotomy. In the entire
intestinal tract through the end of the rectum, the advancing
distance of the carmine suspension and total length of the
intestinal tract were measured in a tension-free state to determine
the GI transit ratio for each animal. During this experiment, the
sampling of each rat was completed within 5 min and rats were under
anesthesia during euthanasia via cervical dislocation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Rats were anesthetized via i.p injection of 10%
chloral hydrate (300 mg/kg; 3 ml/kg), and the colon was exposed
along the midline of the abdomen. The mucosal layer of the distal
parts (left colic flexure was used for the sector) was retained and
the muscle layer was removed. There were no peritonitis phenomena
such as muscle tension and intestinal adhesion during the whole
process of sampling. Total RNA was extracted using an RNA
extraction kit (cat. no. DP419; TIANGEN) and reverse-transcribed
(incubate for 60 min at 42˚C, then terminate the reaction by
heating at 70˚C for 5 min) into cDNA using the RevertAid kit (cat.
no. K1691; Thermo Fisher Scientific). qPCR was performed using the
SYBR Green Bestar TM qPCR Master Mix kit (DBI Bioscience). The
following thermocycling conditions were used for qPCR: Initial
pre-denaturation at 95˚C for 120 sec; 40 cycles of denaturation at
95˚C for 15 sec, annealing at 58˚C for 30 sec and elongation at
72˚C for 30 sec. Relative gene expression was calculated based on
the 2-ΔΔCq method and normalized to the internal
reference gene β-actin or GAPDH (31). Three biological replicates were
analyzed for each experiment. The following primer pairs were used
for qPCR: MOR forward, 5'-CATGGCCCTTCGGAACCATC-3' and reverse,
5'-TGGCAGACAGCAATGTAGCG-3'; P2Y1 forward,
5'-TTATGTGCAAGCTGCAGAGG-3' and reverse, 5'-CTGCCCAGAGACTTGAGAGG-3';
ATPB forward, 5'-TTGGCAGATGAATGAACCGC-3' and reverse,
5'-GCAGGACATCTTGGCCTTCC-3'; CB forward,
5'-CGACGCTGATGGAAGTGGTTACC-3' and reverse,
5'-GGTGATAGCTCCAATCCAGCCTTC-3'; CGRP forward,
5'-GTGAAGAAGAAGCTCGCCTACTGG-3' and reverse,
5'-CCTCAGCCCCTGTTCCTCCTC-3'; β-actin forward,
5'-TGTCACCAACTGGGACGATA-3' and reverse, 5'-GGGGTGTTGAAGGTCTCAAA-3';
and GAPDH forward, 5'-GACATGCCGCCTGGAGAAAC-3' and reverse,
5'-AGCCCAGGATGCCCTTTAGT-3'. The primers were purchased from Sangon
Biotech Co., Ltd.
Western blotting (WB)
Rats were anesthetized by i.p injection of 10%
chloral hydrate (300 mg/kg; 3 ml/kg) and the colon was exposed
along the midline of the abdomen. There were no peritonitis
phenomena such as muscle tension and intestinal adhesions during
sampling. The distal parts (left colic flexure was used for the
sector) tissues were weighed and proteins were extracted using
lysis buffer (cat. no. KGP-2100; Jiangsu KeyGen Biotech). Total
protein was quantified using a bicinchoninic acid protein assay
(cat. no. KGP-2100; Jiangsu KeyGen Biotech). A total of 40 µg/lane
was pipetted into a well of 10% SDS-PAGE. Proteins were transferred
onto a PVDF membrane at 200 mA constant current flow. PVDF membrane
was incubated with anti-MOR (rabbit polyclonal; cat. no.
NB100-1620; 1:500; NOVUS Biologicals, LLC), anti-P2Y1 (rabbit
polyclonal; cat. no. 85896; Abcam, Cambridge, UK; 1:1,000),
anti-ATPB (mouse monoclonal, cat. no. 14730; 1:1,000; Abcam),
anti-CB (mouse monoclonal; cat. no. 11426; 1:1,000; Abcam),
anti-β-actin (mouse monoclonal; cat. no. TA09; 1:1,000; ZSGB-BIO;
OriGene Technologies, Inc.) and anti-GAPDH (mouse monoclonal; cat.
no. TA08; 1:1,000; ZSGB-BIO; OriGene Technologies, Inc.) antibodies
for 1 h at room temperature and then at 4˚C overnight. Then, the
membrane was incubated with horseradish peroxidase-conjugated goat
anti-mouse IgG (cat. no. ZB-2305; 1:3,000; ZSGB-BIO; OriGene
Technologies, Inc.) or anti-rabbit IgG (cat. no. ZB-2301; 1:3,000;
ZSGB-BIO; OriGene Technologies, Inc.) for 1 h at room temperature
and washed with 1X TBST three times for 10 min each. Ultrasensitive
chemiluminescent reagent (cat. no. BMU102-CN; Abbkine) was used to
visualize protein bands and the images were captured using an
Amersham Imager 600 chemical image system (GE Healthcare
Bio-Sciences AB). The gray value of each protein band was measured
by ImageJ (National Institutes of Health; version 1.53). The ratio
of gray value of the target protein band to the internal reference
protein band was used to determine the relative expression of the
target protein.
In vivo intervention of P2Y1 or MOR
function
After 3 days of adaptation, the i.p injection of
loperamide hydrochloride at a dose of 4 mg/kg twice/day for 7
consecutive days was used to establish the OIC model. On the
following day, OIC rats were sub-divided into the four different
intervention-treated groups: i) Naloxone (4 mg/ml); ii) ATP (4
mg/ml); iii) MRS2179 (0.5 mg/ml) and iv) untreated. All treatments
were administered by i.p injections of 0.5 ml each drug at a
frequency of two injections/day for 5 consecutive days. NSG was
established via i.p injection of the same amount of 0.9% normal
saline, twice/day at 09:00 AM and 06:00 PM consecutively for 12
days. NCG received no treatment. There were six groups with six
rats in each group.
Statistical analysis
The fecal scores were analyzed by the Kruskal-Wallis
H test followed by Dunn's test and the data are presented as the
median ± interquartile range. The GI transit ratio, stool weight
changes, RT-qPCR, WB and intervention of P2Y1 receptor or MOR
function experiments were analyzed by one-way analysis of variance
followed by Tukey's post hoc test; these data are presented as the
mean ± SEM. SPSS 17.0 (SPSS, Inc.) and GraphPad 8.3.0 statistical
software (GraphPad Software, Inc.) were used to perform all
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
The stool score, weight and GI transit
ratio of the OIC model
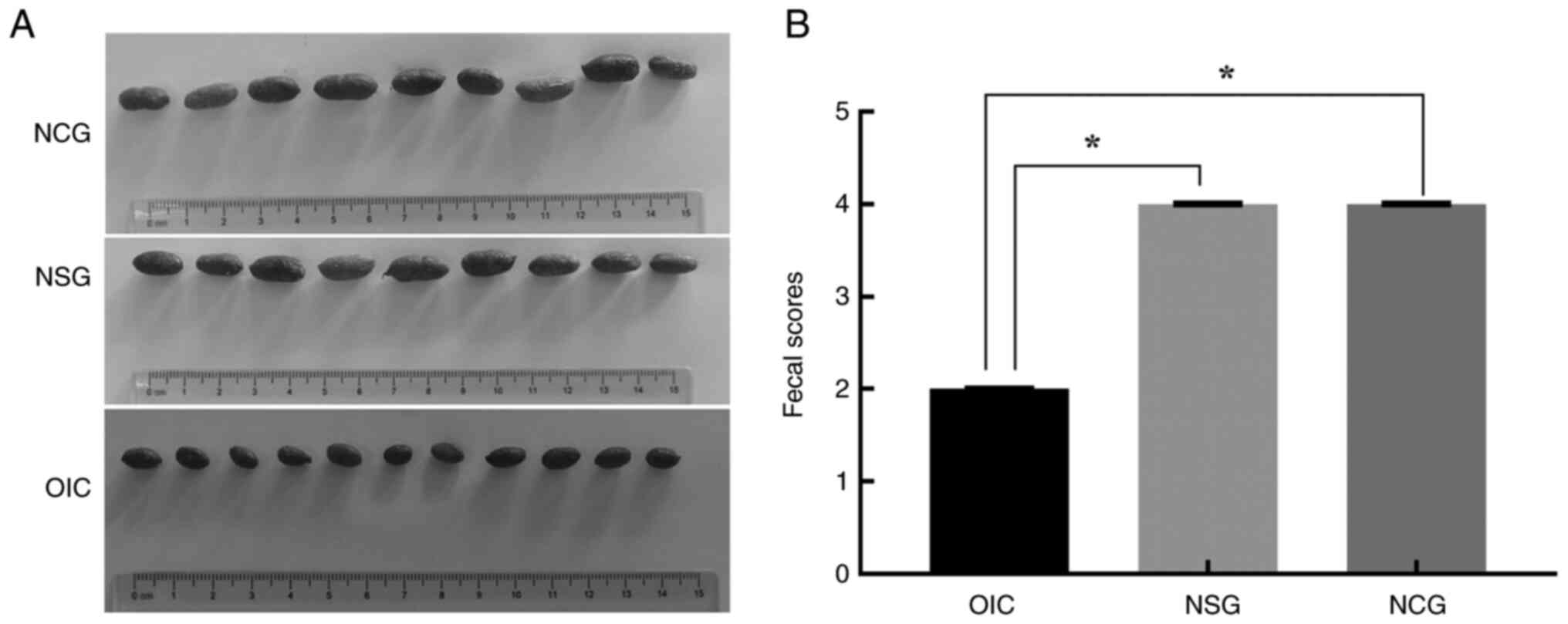
The stool of OIC rats became smaller and harder on
the seventh day of modeling (Fig.
1A), which was close to the small sausage-like pieces with a
score of 2, while the stool morphologies of NCG and NSG rats were
similar, with a sausage-like appearance but a smooth and soft
surface with a score of 4 (Fig.
1B). The stool weight and GI transit ratio of the OIC rats were
significantly lower than NCG and NSG animals on the seventh day of
modeling (Table I).
 | Table IStool weight and gastrointestinal
transit ratio. |
Table I
Stool weight and gastrointestinal
transit ratio.
| Characteristic | OIC | NSG | NCG |
|---|
| Stool weight,
g |
1.50±0.13a,b | 3.10±0.09 | 3.00±0.17 |
| Gastrointestinal
transit ratio, % |
66.80±6.50a,b | 89.80±2.30 | 90.70±1.90 |
Co-expression of P2Y1 with CB and CGRP
in the neurons of submucosal plexus by IF staining
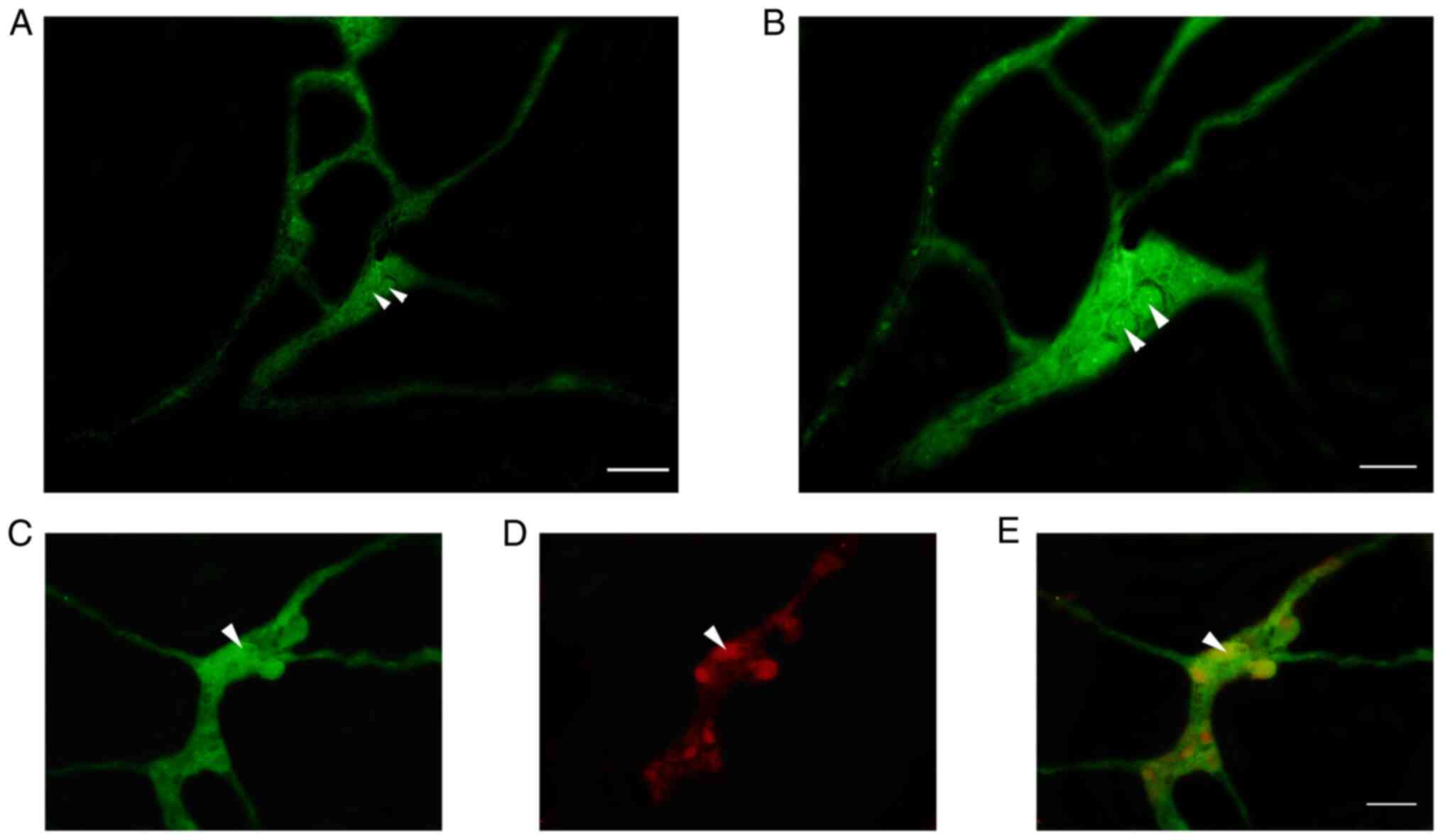
IF analysis revealed that a large number of
P2Y1-positive nerve cells were aggregated in the colonic submucosal
plexus of rats to form ganglia (Fig.
2). In the ganglion, P2Y1 was observed to co-localize with
NeuN-positive enteric neurons in the intestine, suggesting that
these positive cells were neurons (Fig. 2E). The P2Y1-positive nerve cell
body was round or elliptical and the positive marker was primarily
located in the cell bodies and processes, emitting a long
protrusion from the cell body (Figs.
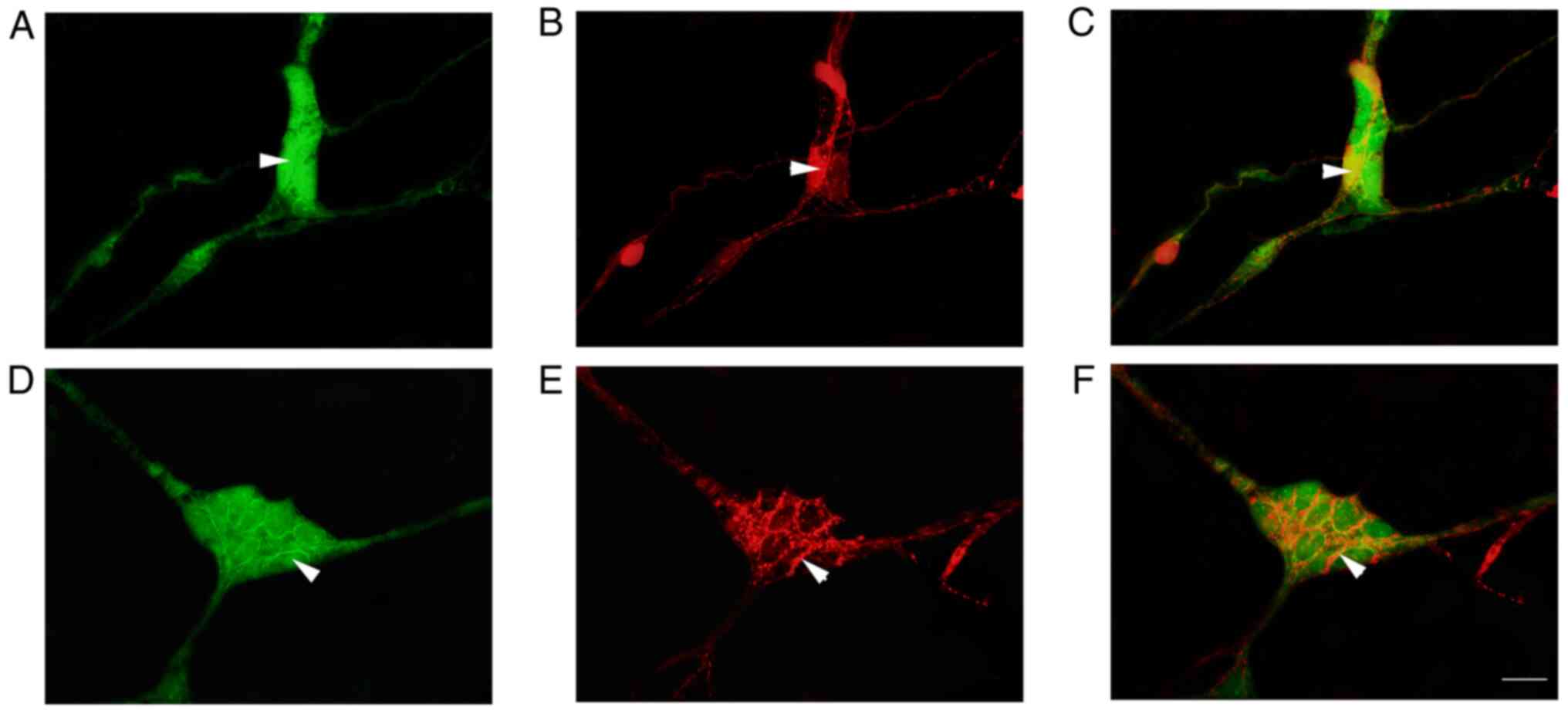
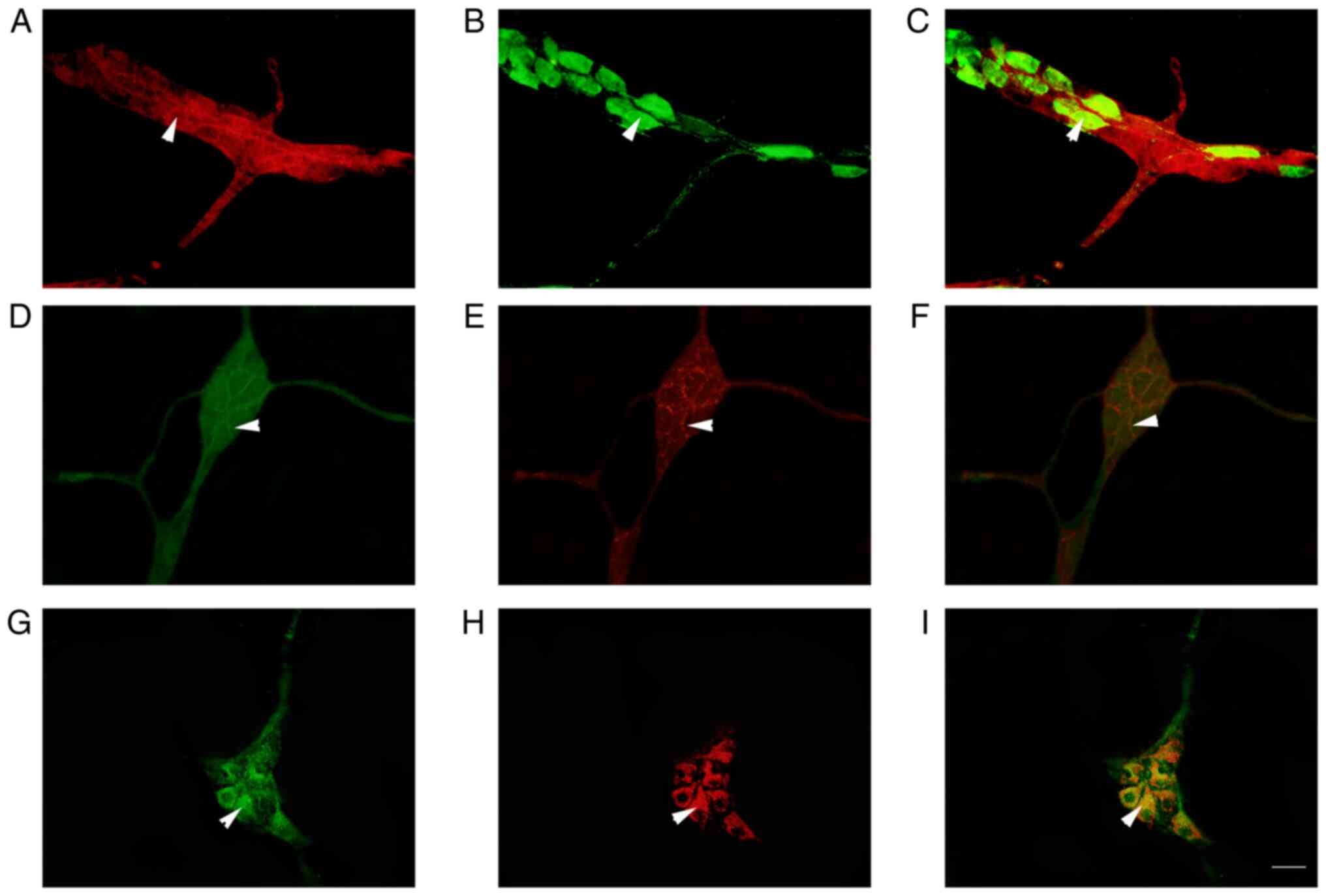
2B and C and 3A and C). CB-positive nerve cell bodies were
round or elliptical, the positive marker was primarily located in
the cytoplasm and several protrusions were identified emitting from
the cell body (Figs. 3B and
4B). In the ganglion, CGRP
protuberances were observed in the submucosal plexus (Figs. 3E and 4E). Moreover, CB and CGRP signals were
detected in the colonic submucosal ganglia and co-localized with
MOR and P2Y1-positive markers, respectively (Figs. 3 and 4). In the ganglion, MOR and ATPB-positive
markers were co-expressed in the intestinal nerve cells and a large
number of MOR-positive nerve fibers surrounded the cell body of
ATPB-positive cells (Fig. 4).
mRNA and protein levels of P2Y1
decreased in OIC rats
ATP is an inhibitory mediators of GI that cause
gastrointestinal dysfunction by activating P2Y1(24). RT-qPCR showed that compared with
the mRNA expression levels in NCG and NSG rats, mRNA levels of the
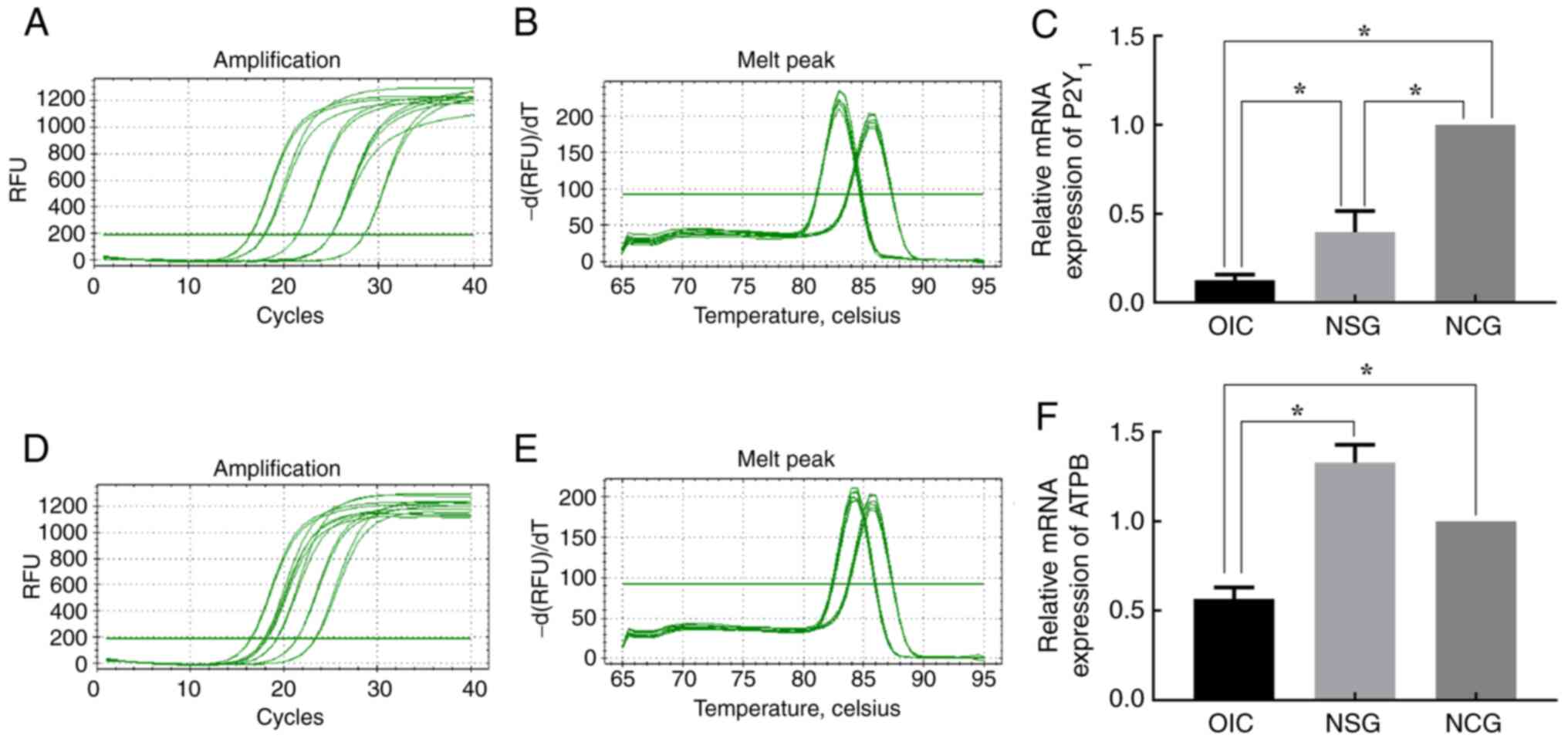
ATPB and P2Y1 were significantly decreased in OIC rats (Fig. 5). RT-qPCR showed that compared with
the mRNA expression levels in NCG and NSG rats, the mRNA levels of
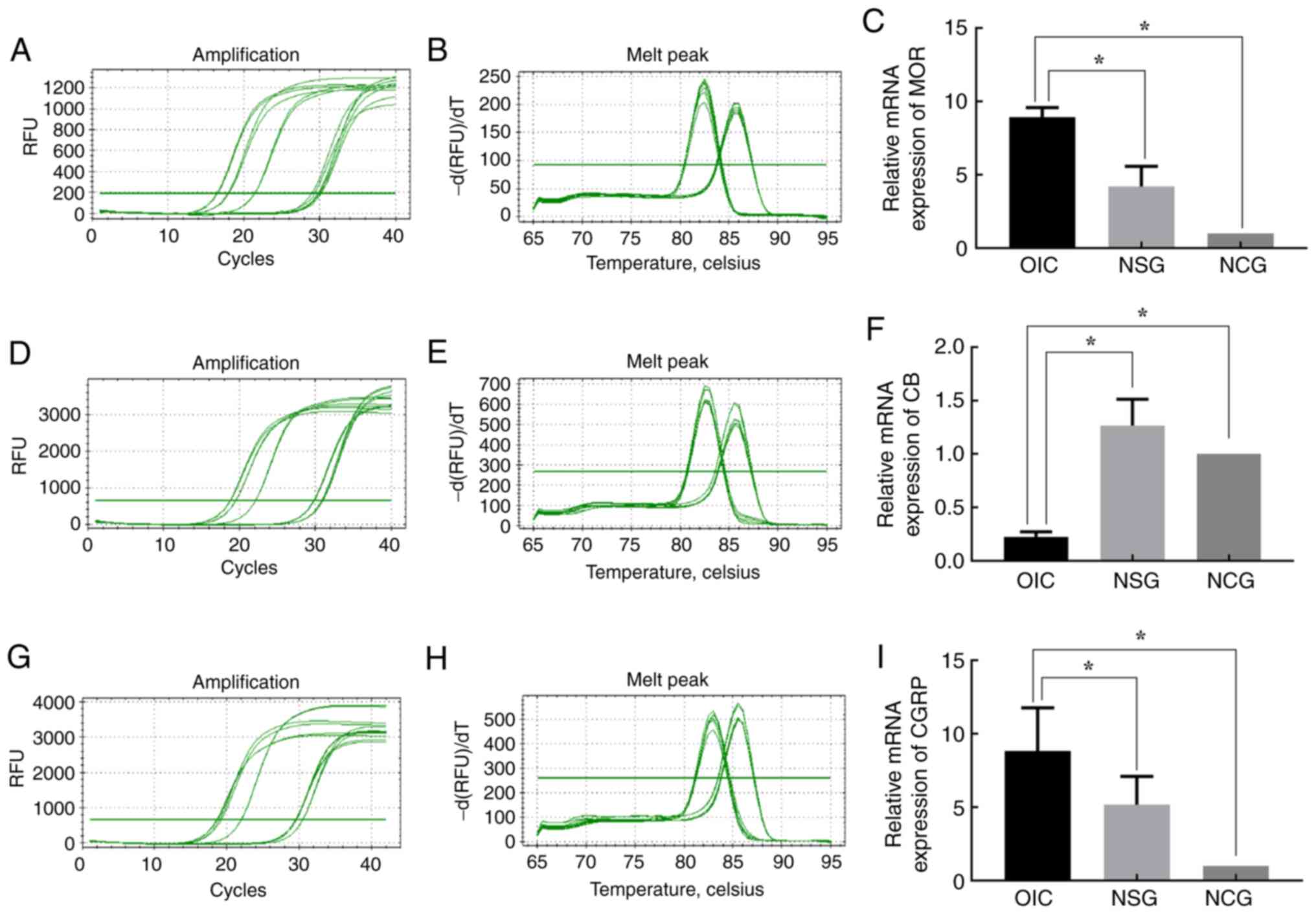
MOR and CGRP in the submucosal layer of OIC rats increased
(Fig. 6A and G and 6C
and I), while the CB mRNA levels
were significantly decreased (Fig.
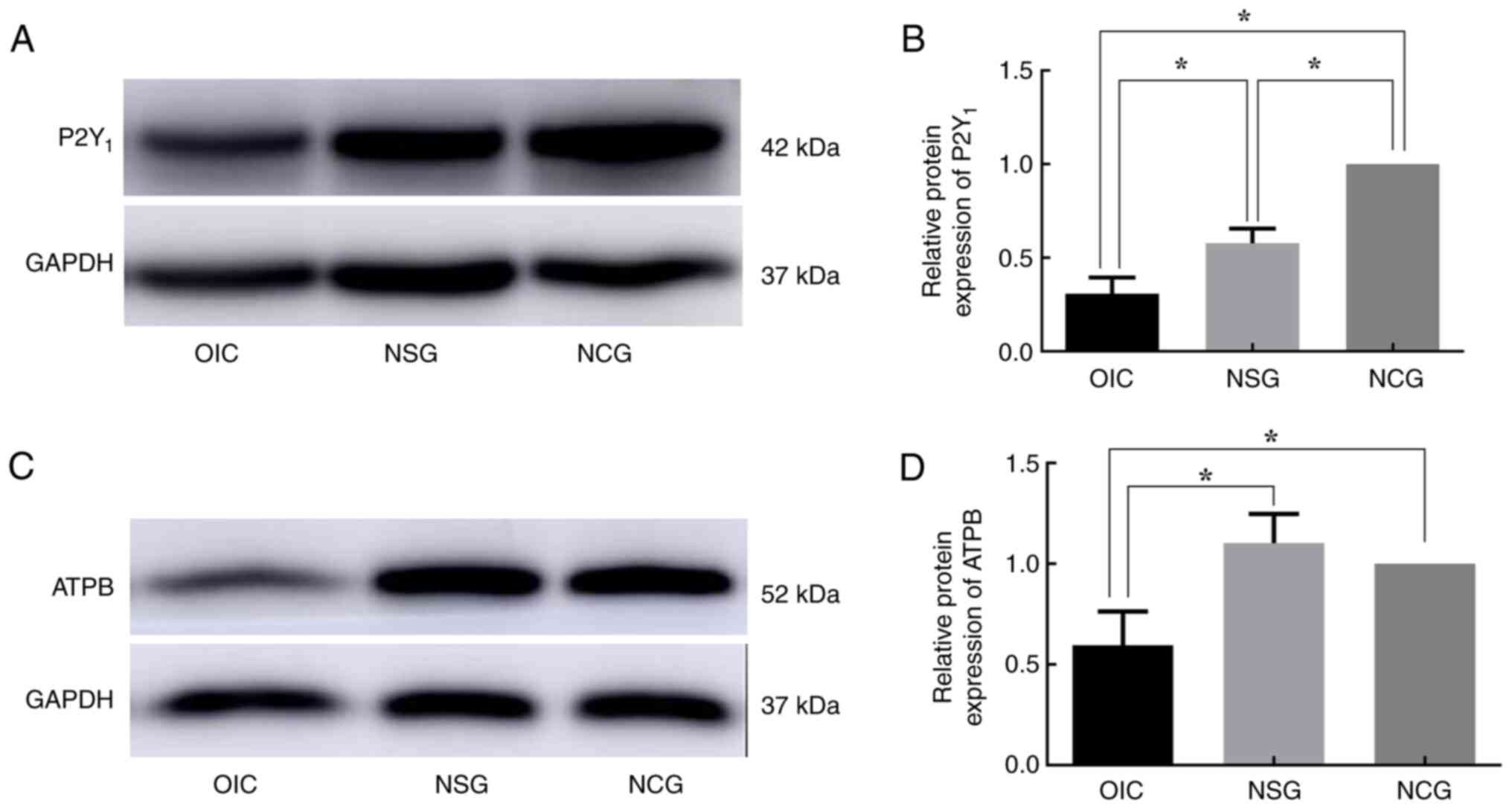
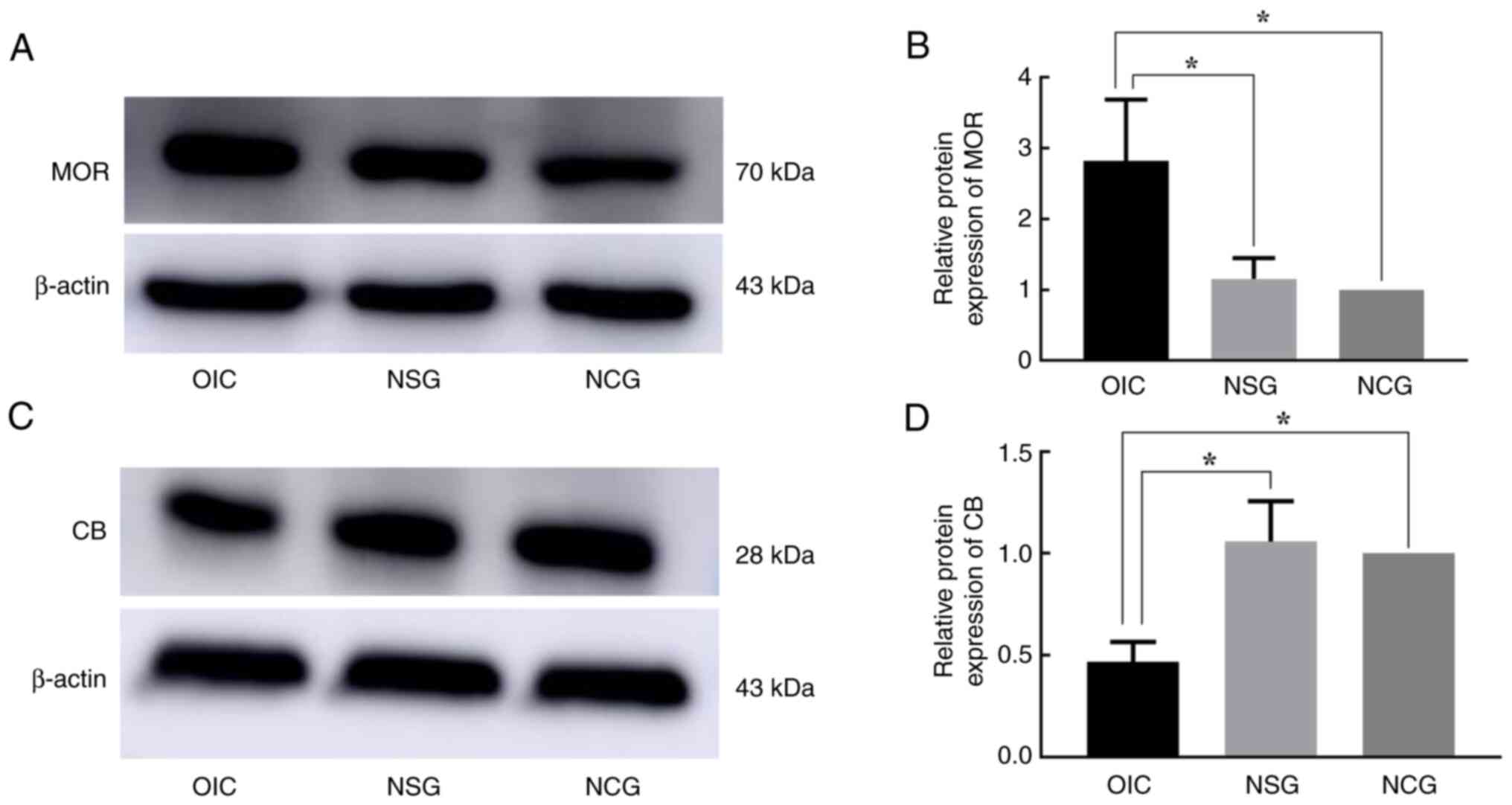
6D-F). Consistent with RT-qPCR results, WB analysis revealed
that MOR protein levels in the submucosal layer of OIC rats were
significantly increased, while those of ATPB, P2Y1, and CB proteins
were significantly decreased compared with those of the NCG and NSG
rats. At the same time, compared with NCG, the expression of P2Y1
protein was significantly decreased in NSG rats (Figs. 7 and 8).
ATP relieves OIC of rats
Compared with that in the NSG and NCG, the naloxone
and ATP treatment groups had no significant change in their rate of
GI transit. Compared with that in the NCG, NSG, OIC,
naloxone-treated and ATP-treated groups, the MRS2179 group
exhibited significantly decreased GI transport function (Table II).
 | Table IIGastrointestinal transit ratio
changes in rats. |
Table II
Gastrointestinal transit ratio
changes in rats.
| Group | Advanced length,
cm | Intestinal length,
cm | Gastrointestinal
transit ratio, % |
|---|
| OIC |
105.7±14.6a,b,c,d | 134.5±11.8 |
78.4±6.4a,b,c,d |
| Naloxone | 127.0±6.7 | 137.0±5.4 | 92.7±2.1 |
| ATP | 126.0±5.1 | 137.0±2.4 | 92.0±4.0 |
| MRS2179 |
91.5±13.6a,b,c,d | 130.0±12.8 |
70.4±5.5a,b,c,d |
| NSG | 119.5±9.7 | 132.0±11.2 | 90.6±2.1 |
| NCG | 120.8±7.4 | 131.8±8.2 | 91.7±2.1 |
GI transit ratio analysis suggested that the P2Y1
agonist ATP significantly relieved constipation symptoms in rats,
which is similar to the effect of MOR antagonist naloxone, whereas
the P2Y1 inhibitor MRS2179 aggravated the constipation symptoms in
this OIC rat model.
Discussion
Effective analgesic sites for opioids such as
morphine are primarily located in the CNS, and the main cause of
constipation is activation of the peripheral MOR signaling pathway,
leading to GI dysfunction (13,15,32-36).
Loperamide hydrochloride is an opioid receptor agonist that
decreases water and electrolyte secretion from the mucosal layers
of the intestine by binding to MORs in the GI tract. This
ligand-receptor interaction inhibits the long-distance transport of
intestinal contents via the GI tract, causing accumulation of toxic
waste materials in the intestinal lumen (37). In the present study, an OIC rat
model was evaluated according to the Rome II stool typing criteria
(30,38-41).
After 7 days of modeling, the stool samples of OIC rats became
smaller and harder, while GI transit ratio and stool weight of
these OIC rats significantly decreased. This was consistent with
the characteristics of OIC stools in previous reports, indicating
that the OIC rat model was successfully established (28,29).
The functional activity of the GI tract is primarily
regulated by the enteric nervous system (ENS), which mainly
includes the submucosal plexus between the mucosa and the submucosa
and the myenteric plexus located between the circular and
longitudinal muscle (14,41-44).
Enteric nerve cells are divided into intrinsic primary afferent or
sensory neurons, intermediate nerve cells and motor nerve cells.
Among these, the intrinsic primary afferent nerve cells are the
first neurons in the reflex pathways activated by mucosal
stimulation. These neurons transmit mechanical and chemical
stimulatory information to the motor nerve cells in the intestinal
plexus, thereby regulating the GI tract smooth muscle movement
(14,42,45,46).
The MOR is a type of opioid receptor and the primary site action of
morphine and other opioid preparations acting on the PNS. Compared
with other opioid receptors, MOR is more densely distributed in the
colon than in the stomach and small intestine (14,16).
In the present study, MOR was co-expressed with CB and CGRP in the
colonic nerve cells. Studies have shown that CB is primarily
expressed in the primary sensory afferent neurons and participates
in the sensory information transmission along the GI tract
(43,46-48).
CGRP is released by the submucosal plexus cells that transmit
mechanical and chemical irritative sensory information along the GI
tract (46,49,50).
It is hypothesized that MOR may be closely related to the
transmission of sensory information in the rat colon.
The movement of GI smooth muscle is primarily
dominated by motor nerve cells, including excitatory and inhibitory
nerve cells, of which inhibitory motor neurons are predominantly
non-cholinergic or non-adrenergic inhibitory nerve cells (51). ATP is an important extracellular
secondary messenger in the colon that is released by both
non-cholinergic and non-adrenergic neurons in the ENS. ATP
regulates the functional activity of the colon by activating the
receptors such as P2Y1 (51,52).
Previous studies have shown that P2Y1s are abundantly expressed in
the GI smooth muscle cells and are involved in regulating
transmission of neural information (24,52-54).
The current study showed that P2Y1s are expressed in the submucosal
plexus and co-expressed with CB and CGRP in the nerve cells of rat
colonic tissues. P2Y1s are highly sensitive to stimulatory sensory
signals, such as mechanical stimuli during GI disorder (52). The present study revealed that the
P2Y1s are not only distributed in the distal colonic submucosal
plexus cells but are also involved in transmission of neural
information in the rat colon.
In the present study, the mRNA expression levels of
MOR and CGRP were significantly increased in the distal submucosa
of OIC rats, while levels of P2Y1, ATPB and CB were significantly
decreased. It was also shown that protein expression levels of MOR,
P2Y1, ATPB and CB were similar to their respective mRNA levels. GI
dysfunction-induced constipation is associated with abnormal
colorectal nerve signaling (55).
Long-term use of opioid preparations may cause irreversible
perturbations to the MOR signaling axis in the colorectal regions,
leading to chronic constipation (13,14,32).
In the colonic submucosal plexus, MORs are distributed on the
surface of nerve cells to regulate colonic smooth muscle motility
through the release of enteric neurotransmitters (14). IF showed that both MOR and ATPB
were co-expressed in the distal colonic submucosal plexus of rats.
P2Y1 is the dominant purine receptor in the colon and is associated
with transmission of sensory information in the colonic submucosa
(32,52). A decrease in P2Y1 expression was
also observed in the NSG group. Here, NSG can be used as the
solvent control group, and this change of P2Y1 can be understood as
the gastrointestinal changes induced by 0.9% saline solvent.
However, the expression of P2Y1 in OIC rat colon was lower than
that in NSG in our study. Based on the above results, it is
speculated that MOR, ATP and P2Y1s have similar morphological basis
to form a complete neural regulatory pathway in the colonic nervous
system of rats.
Moreover, in vivo experiments showed that the
P2Y1 agonist ATP could significantly relieve the symptoms of
constipation in rats, which was similar to the effect of the MOR
antagonist naloxone, while MRS2179 aggravated the symptoms of
constipation. ATP is an endogenous agonist with a strong affinity
for P2Y1s that activates P2Y1 and causes relaxation of colonic
smooth muscle, thereby relieving OIC (52,56).
MRS2179 is a selective antagonist for P2Y1s (56). In vivo peristalsis
experiments in the distal colon of guinea pigs have revealed that
the propulsion kinetics of epoxy-coated artificial pellets
attenuate the effect of MRS2179 (56,57).
In the present study, the changes of P2Y1 in the
submucosa and its possible association with OIC were mainly
discussed. However, regarding the part of the intestinal muscular
plexus, future research is needed to explore the distribution
characteristic of P2Y1 in the myenteric plexus.
Taken together, the results of the present study
suggested that P2Y1s may serve an important role in regulating the
OIC pathway and interference with the function of P2Y1s relieved
symptoms of constipation. Moreover, the study demonstrated that the
P2Y1 expression was associated with the occurrence of OIC in this
rat model and co-expression of MOR and P2Y1s may be associated with
the development of OIC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 31560280 and 31860275).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and XR confirm the authenticity of all the raw
data. XJ and DW designed the methodology. YZ, FL and XR performed
experiments and analyzed the data. YZ and BJ contributed to the
acquisition and interpretation of data. YZ wrote the manuscript.
JL, HJ and LW contributed to the conception of the study, obtained
funding, designed the project and gave final approval of the
version to be published. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The experimental procedures were performed with the
approval of the Ethics Committee of Ningxia Medical University
(Yinchuan, China; approval nos. 2015-090 and 2018-007).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Madariaga-Mazón A, Marmolejo-Valencia AF,
Li Y, Toll L, Houghten RA and Martinez-Mayorga K: Mu-opioid
receptor biased ligands: A safer and painless discovery of
analgesics? Drug Discov Today. 22:1719–1729. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ford AC, Brenner DM and Schoenfeld PS:
Efficacy of pharmacological therapies for the treatment of
opioid-induced constipation: Systematic review and meta-analysis.
Am J Gastroenterol. 108:1566–1575. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spencer NJ and Hu H: Enteric nervous
system: Sensory transduction, neural circuits and gastrointestinal
motility. Nat Rev Gastroenterol Hepatol. 17:338–351.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Farmer AD, Holt CB, Downes TJ, Ruggeri E,
Del Vecchio S and De Giorgio R: Pathophysiology, diagnosis, and
management of opioid-induced constipation. Lancet Gastroenterol
Hepatol. 3:203–212. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gupta A, Coyne KS, Datto C and Venuti C:
The burden of opioid-induced constipation in younger patients with
chronic noncancer pain. Pain Med. 19:2459–2468. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harada Y, Iizuka S, Saegusa Y, Mogami S,
Fujitsuka N and Hattori T: Mashiningan improves opioid-induced
constipation in rats by activating cystic fibrosis transmembrane
conductance regulator chloride channel. J Pharmacol Exp Ther.
362:78–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Beckett EAH, Staikopoulos V and Hutchinson
MR: Differential effect of morphine on gastrointestinal transit,
colonic contractions and nerve-evoked relaxations in toll-like
receptor deficient mice. Sci Rep. 8(5923)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mozaffari S, Nikfar S and Abdollahi M:
Investigational opioid antagonists for treating opioid-induced
bowel dysfunction. Expert Opin Investig Drugs. 27:235–242.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mañé N, Gil V, Martinez-Cutillas M, Clavé
P, Gallego D and Jiménez M: Differential functional role of
purinergic and nitrergic inhibitory cotransmitters in human colonic
relaxation. Acta Physiol (Oxf). 212:293–305. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gil V, Martinez-Cutillas M, Mañé N, Martin
MT, Jiménez M and Gallego D: P2Y(1) knockout mice lack purinergic
neuromuscular transmission in the antrum and cecum.
Neurogastroenterol Motil. 25:e170–e182. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gallego D, Gil V, Aleu J, Aulí M, Clavé P
and Jiménez M: Purinergic and nitrergic junction potential in the
human colon. Am J Physiol Gastrointest Liver Physiol.
295:G522–G533. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bharucha AE and Wald A: Chronic
constipation. Mayo Clin Proc. 94:2340–2357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Halawi H, Vijayvargiya P, Busciglio I,
Oduyebo I, Khemani D, Ryks M, Rhoten D, Burton D, Szarka LA, Acosta
A and Camilleri M: Effects of naloxegol on whole gut transit in
opioid-naïve healthy subjects receiving codeine: A randomized,
controlled trial. Neurogastroenterol Motil.
30(e13298)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Galligan JJ and Sternini C: Insights into
the role of opioid receptors in the GI tract: Experimental evidence
and therapeutic relevance. Handb Exp Pharmacol. 239:363–378.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Luo M, Li L, Lu CZ and Cao WK: Clinical
efficacy and safety of lactulose for minimal hepatic
encephalopathy: A meta-analysis. Eur J Gastroenterol Hepatol.
23:1250–1257. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Camilleri M, Drossman DA, Becker G,
Webster LR, Davies AN and Mawe GM: Emerging treatments in
neurogastroenterology: A multidisciplinary working group consensus
statement on opioid-induced constipation. Neurogastroenterol Motil.
26:1386–1395. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matsumoto K, Umemoto H, Mori T, Akatsu R,
Saito S, Tashima K, Shibasaki M, Kato S, Suzuki T and Horie S:
Differences in the morphine-induced inhibition of small and large
intestinal transit: Involvement of central and peripheral µ-opioid
receptors in mice. Eur J Pharmacol. 771:220–228. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Beamer E, Conte G and Engel T: ATP release
during seizures - A critical evaluation of the evidence. Brain
Research Bulletin. 151:65–73. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jacobson KA and Müller CE: Medicinal
chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology.
104:31–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kong Q, Quan Y, Tian G, Zhou J and Liu X:
Purinergic P2 receptors: Novel mediators of mechanotransduction.
Front Pharmacol. 12(671809)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu J, Cheng Y, Zhang R, Liu D, Luo YM,
Chen KL, Ren S and Zhang J: P2Y1R is involved in visceral
hypersensitivity in rats with experimental irritable bowel
syndrome. World J Gastroenterol. 23:6339–6349. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
King BF: Burnstock and the legacy of the
inhibitory junction potential and P2Y1 receptors. Purinergic
Signal. 17:25–31. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gil V, Gallego D, Grasa L, Martín MT and
Jiménez M: Purinergic and nitrergic neuromuscular transmission
mediates spontaneous neuronal activity in the rat colon. Am J
Physiol Gastrointest Liver Physiol. 299:G158–G169. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Grasa L, Gil V, Gallego D, Martin MT and
Jiménez M: P2Y(1) receptors mediate inhibitory neuromuscular
transmission in the rat colon. Br J Pharmacol. 158:1641–1652.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li JP, Wang XY, Gao CJ, Liao YH, Qu J, He
ZY, Zhang T, Wang GD and Li YQ: Neurochemical phenotype and
function of endomorphin 2-immunopositive neurons in the myenteric
plexus of the rat colon. Front Neuroanat. 8(149)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shimotoyodome A, Meguro S, Hase T,
Tokimitsu I and Sakata T: Decreased colonic mucus in rats with
loperamide-induced constipation. Comp Biochem Physiol A Mol Integr
Physiol. 126:203–212. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim JE, Kang MJ, Choi JY, Park JJ, Lee MR,
Song BR, Kim HR, Park JW, Choi HJ, Bae SJ and Hwang DY: Regulation
of gastrointestinal hormones during laxative activity of
gallotannin-enriched extract isolated from Galla Rhois in
loperamide-induced constipation of SD rats. Lab Anim Res.
34:223–231. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park SA, Lee GH, Hoang TH, Lee HY, Kang
IY, Chung MJ, Jin JS and Chae HJ: Heat-inactivated lactobacillus
plantarum nF1 promotes intestinal health in loperamide-induced
constipation rats. PLoS One. 16(e0250354)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ma L, Qu Z, Xu L, Han L, Han Q, He J, Luan
X, Wang B, Sun Y and He B: 7,8-dihydroxyflavone enhanced colonic
cholinergic contraction and relieved loperamide-induced
constipation in rats. Dig Dis Sci. 66:4251–4262. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Agrawal A, Houghton LA, Reilly B, Morris J
and Whorwell PJ: Bloating and distension in irritable bowel
syndrome: The role of gastrointestinal transit. Am J Gastroenterol.
104:1998–2004. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Floettmann E, Bui K, Sostek M, Payza K and
Eldon M: Pharmacologic profile of naloxegol, a peripherally acting
µ-opioid receptor antagonist, for the treatment of opioid-induced
constipation. J Pharmacol Exp Ther. 361:280–291. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fan R, Schrott LM, Arnold T, Snelling S,
Rao M, Graham D, Cornelius A and Korneeva NL: Chronic oxycodone
induces axonal degeneration in rat brain. BMC Neurosci.
19(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mañé N, Jiménez-Sábado V and Jiménez M:
BPTU, an allosteric antagonist of P2Y1 receptor, blocks nerve
mediated inhibitory neuromuscular responses in the gastrointestinal
tract of rodents. Neuropharmacology. 110:376–385. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Coyne KS, LoCasale RJ, Datto CJ, Sexton
CC, Yeomans K and Tack J: Opioid-induced constipation in patients
with chronic noncancer pain in the USA, Canada, Germany, and the
UK: Descriptive analysis of baseline patient-reported outcomes and
retrospective chart review. Clinicoecon Outcomes Res. 6:269–281.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Takai N, Miyajima N, Tonomura M and Abe K:
Relationship between receptor occupancy and the antinociceptive
effect of mu opioid receptor agonists in male rats. Brain Res.
1680:105–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Siemens W and Becker G: Methylnaltrexone
for opioid-induced constipation: Review and meta-analyses for
objective plus subjective efficacy and safety outcomes. Ther Clin
Risk Manag. 12:401–412. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bharucha AE, Locke GR, Zinsmeister AR,
Seide BM, McKeon K, Schleck CD and Melton LJ III: Differences
between painless and painful constipation among community women. Am
J Gastroenterol. 101:604–612. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Young A, Viswanath A, Kalladka M, Khan J,
Eliav E and Diehl SR: Mouse model demonstrates strain differences
in susceptibility to opioid side effects. Neurosci Lett.
675:110–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen W, Chung HH and Cheng JT:
Opiate-induced constipation related to activation of small
intestine opioid µ2-receptors. World J Gastroenterol. 18:1391–1396.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Grundy D and Schemann M: Enteric nervous
system. Curr Opin Gastroenterol. 21:176–182. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gulati R, Komuravelly A, Leb S, Mhanna MJ,
Ghori A, Leon J and Needlman R: Usefulness of assessment of stool
form by the modified bristol stool form scale in primary care
pediatrics. Pediatr Gastroenterol Hepatol Nutr. 21:93–100.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Palus K, Bulc M, Czajkowska M, Miciński B
and Całka J: Neurochemical characteristics of calbindin-like
immunoreactive coeliac-cranial mesenteric ganglion complex (CCMG)
neurons supplying the pre-pyloric region of the porcine stomach.
Tissue Cell. 50:8–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Verheijden S and Boeckxstaens GE:
Neuroimmune interaction and the regulation of intestinal immune
homeostasis. Am J Physiol Gastrointest Liver Physiol. 314:G75–G80.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Galligan JJ and Akbarali HI: Molecular
physiology of enteric opioid receptors. Am J Gastroenterol Suppl.
2:17–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Palus K, Bulc M and Całka J: Changes in
VIP-, SP- and CGRP-like immunoreactivity in intramural neurons
within the pig stomach following supplementation with low and high
doses of acrylamide. Neurotoxicology. 69:47–59. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
da Silva MV, Marosti AR, Mendes CE,
Palombit K and Castelucci P: Submucosal neurons and enteric glial
cells expressing the P2X7 receptor in rat experimental colitis.
Acta Histochem. 119:481–494. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Olsson C: Calbindin immunoreactivity in
the enteric nervous system of larval and adult zebrafish (Danio
rerio). Cell Tissue Res. 344:31–40. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nishimura E, Buchan AM and McIntosh CH:
Autoradiographic localization of mu- and delta-type opioid
receptors in the gastrointestinal tract of the rat and guinea pig.
Gastroenterology. 91:1084–1094. 1986.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kaur R, O'Shaughnessy CT, Jarvie EM,
Winchester WJ and McLean PG: Characterization of a calcitonin
gene-related peptide release assay in rat isolated distal colon.
Arch Pharm Res. 32:1775–1781. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mitsui R, Miyamoto S, Takano H and
Hashitani H: Properties of submucosal venules in the rat distal
colon. Br J Pharmacol. 170:968–977. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Van Crombruggen K, Van Nassauw L,
Timmermans JP and Lefebvre RA: Inhibitory purinergic P2 receptor
characterisation in rat distal colon. Neuropharmacology.
53:257–271. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kotova PD, Bystrova MF, Rogachevskaja OA,
Khokhlov AA, Sysoeva VY, Tkachuk VA and Kolesnikov SS: Coupling of
P2Y receptors to Ca2+ mobilization in mesenchymal
stromal cells from the human adipose tissue. Cell Calcium. 71:1–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Barańska J, Czajkowski R and Pomorski P:
P2Y1 receptors-properties and functional activities. Adv
Exp Med Biol. 1051:71–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Buvinic S, Bravo-Zehnder M, Boyer JL,
Huidobro-Toro JP and González A: Nucleotide P2Y1 receptor regulates
EGF receptor mitogenic signaling and expression in epithelial
cells. J Cell Sci. 120:4289–4301. 2007.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Burnstock G: Purine and pyrimidine
receptors. Cell Mol Life Sci. 64:1471–1483. 2007.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Strong DS, Cornbrooks CF, Roberts JA,
Hoffman JM, Sharkey KA and Mawe GM: Purinergic neuromuscular
transmission is selectively attenuated in ulcerated regions of
inflamed guinea pig distal colon. J Physiol. 588:847–859.
2010.PubMed/NCBI View Article : Google Scholar
|