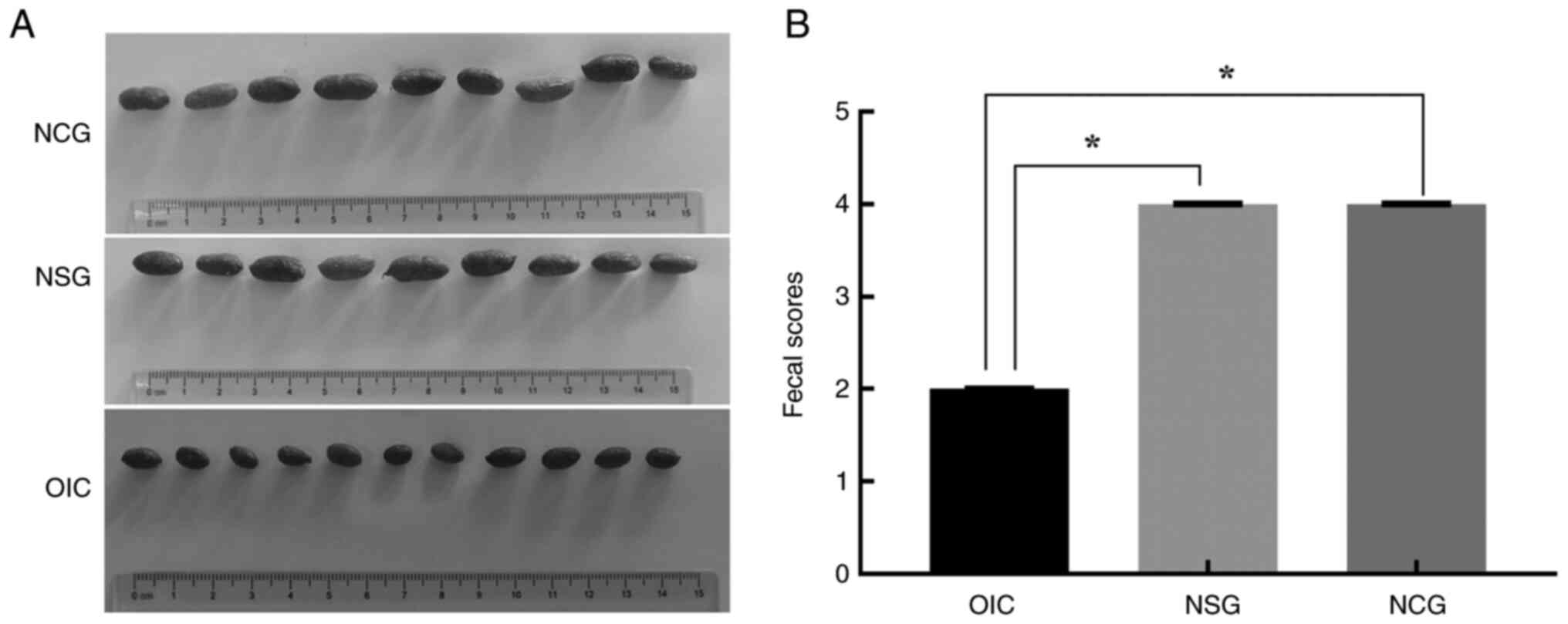
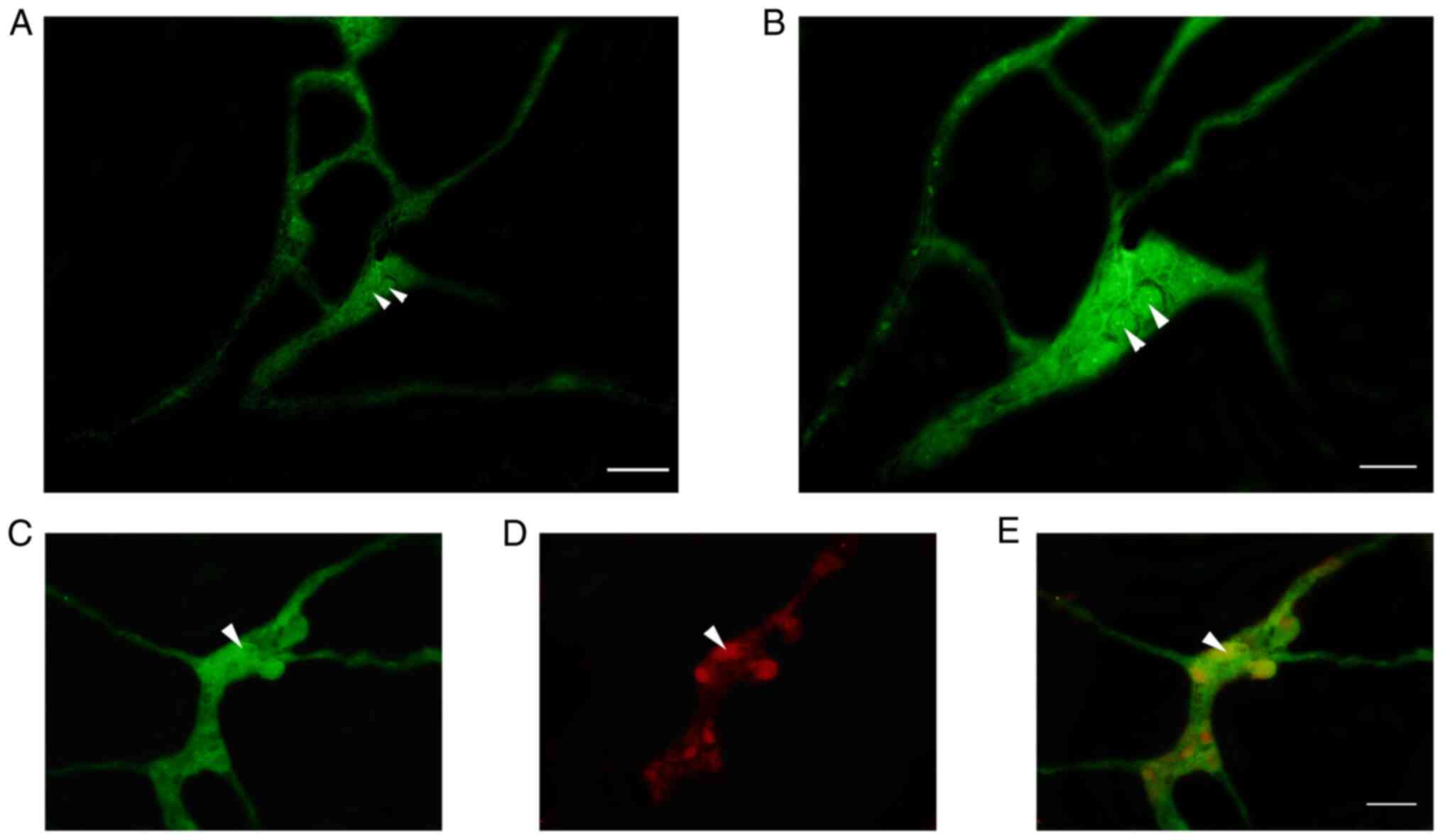
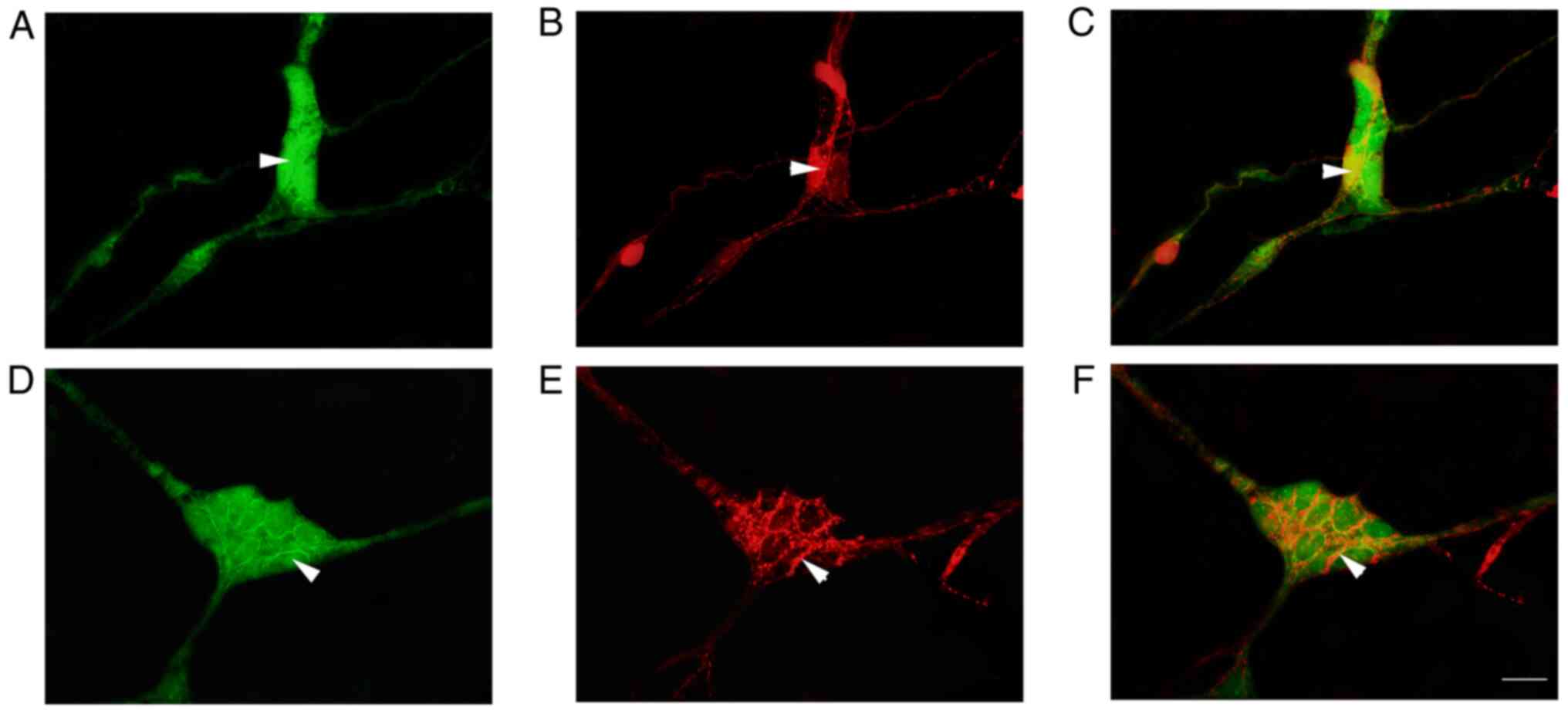
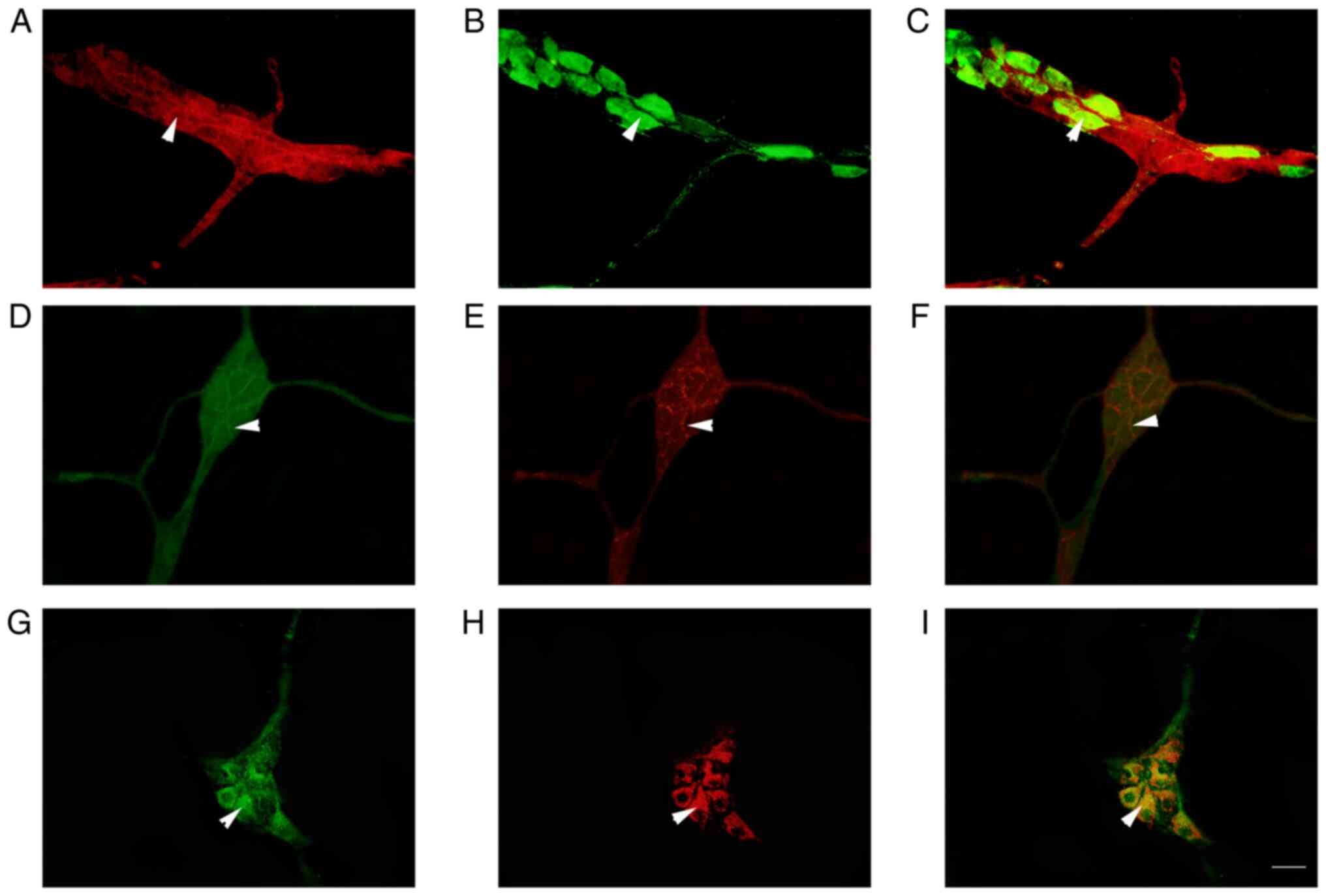
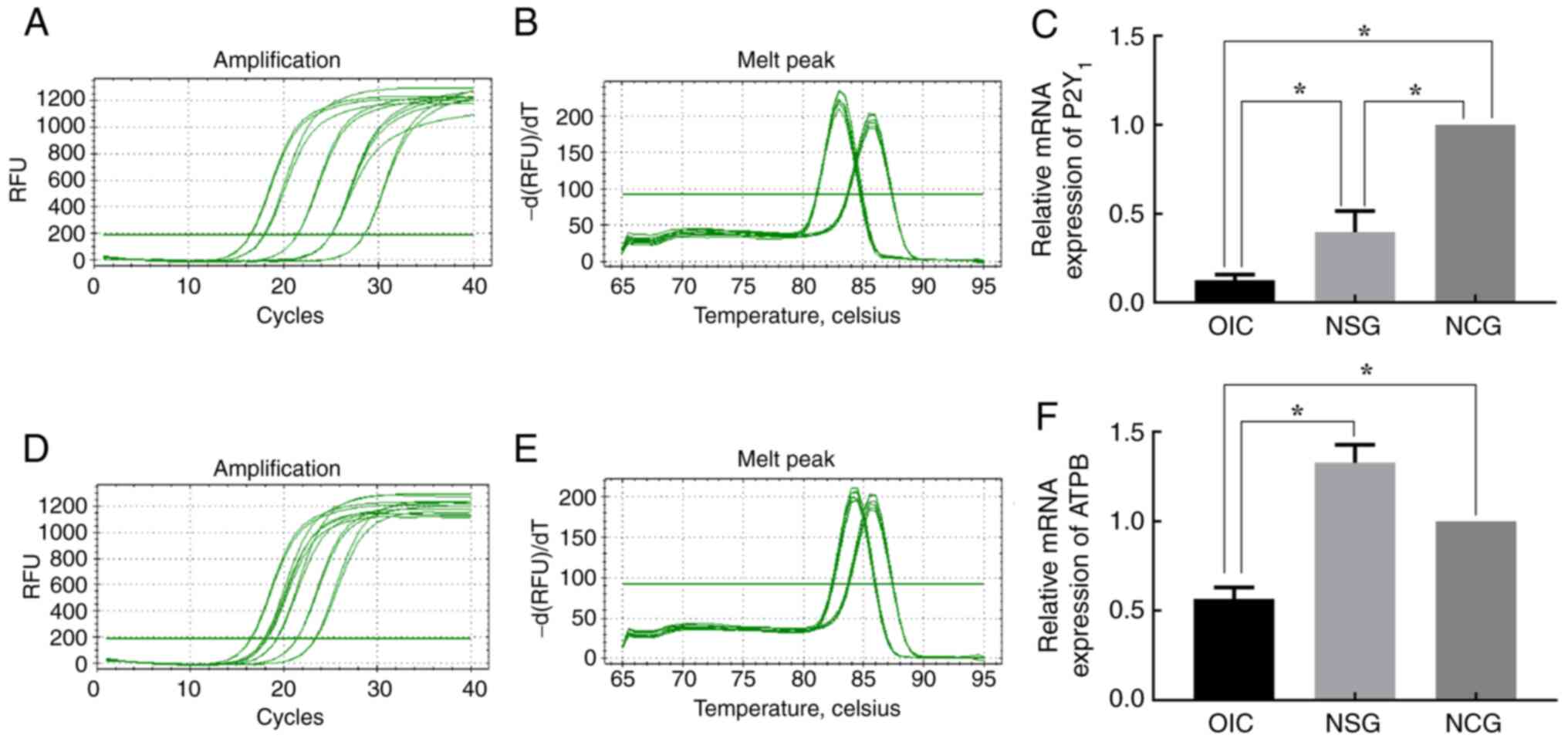
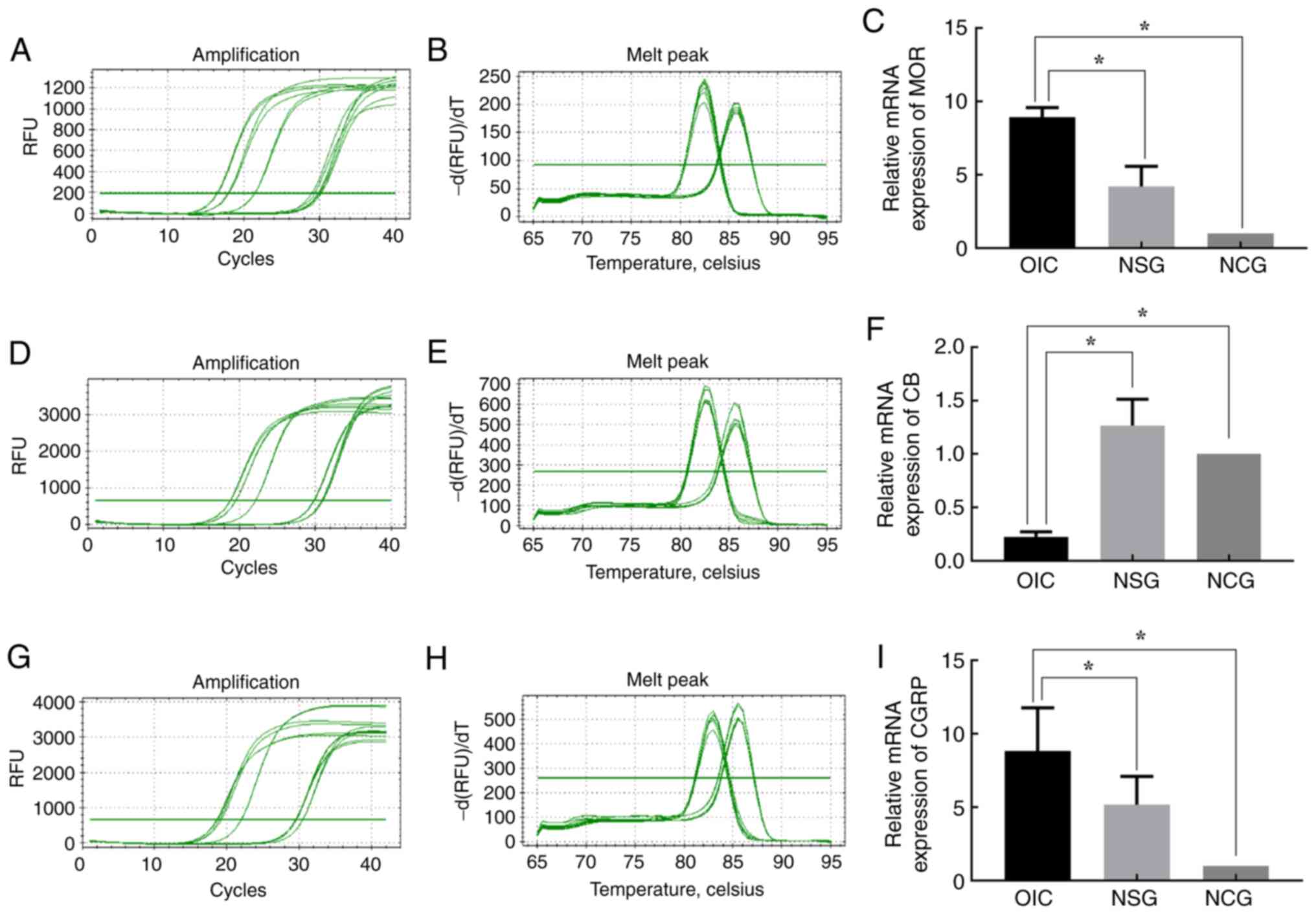
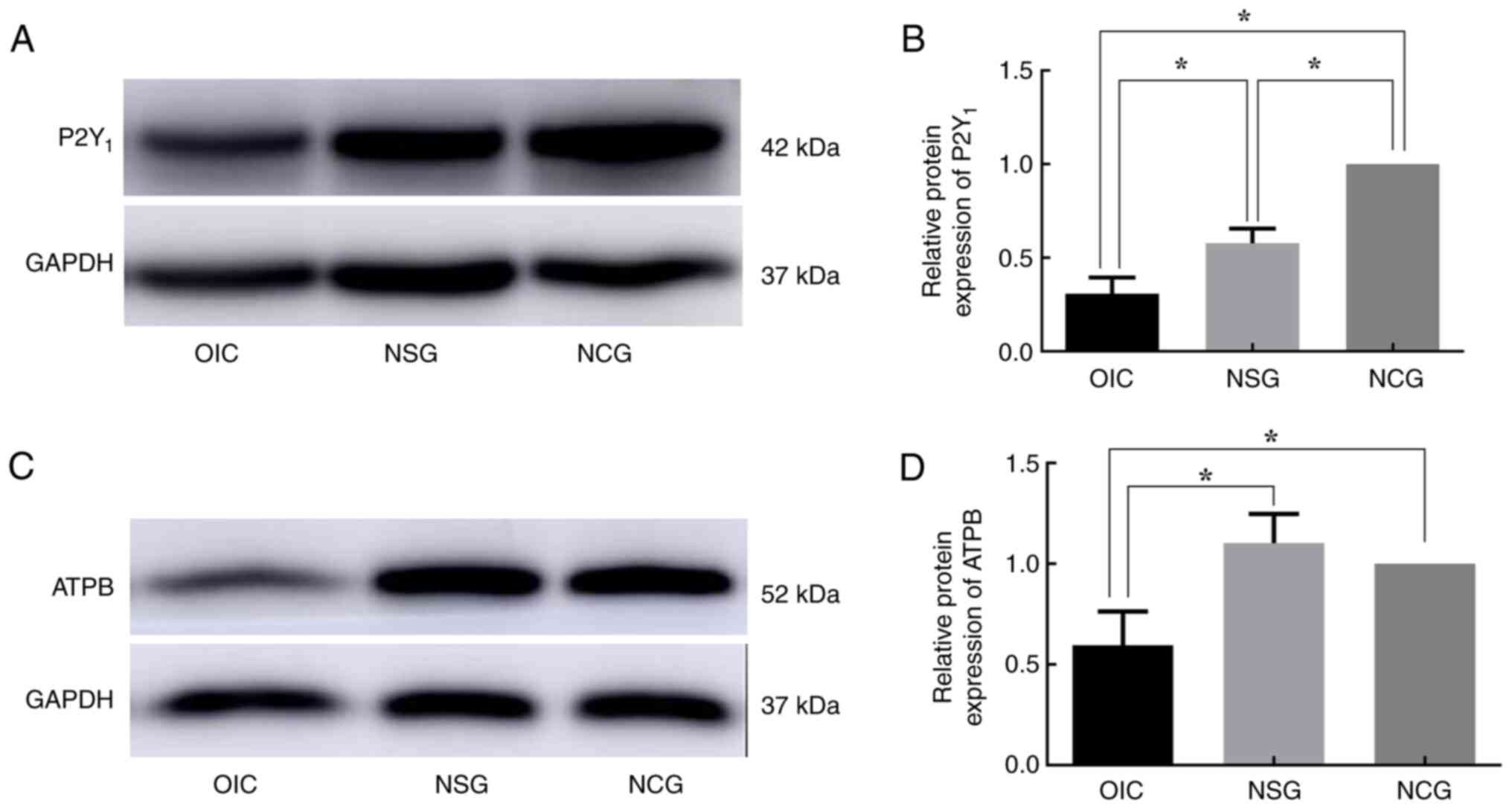
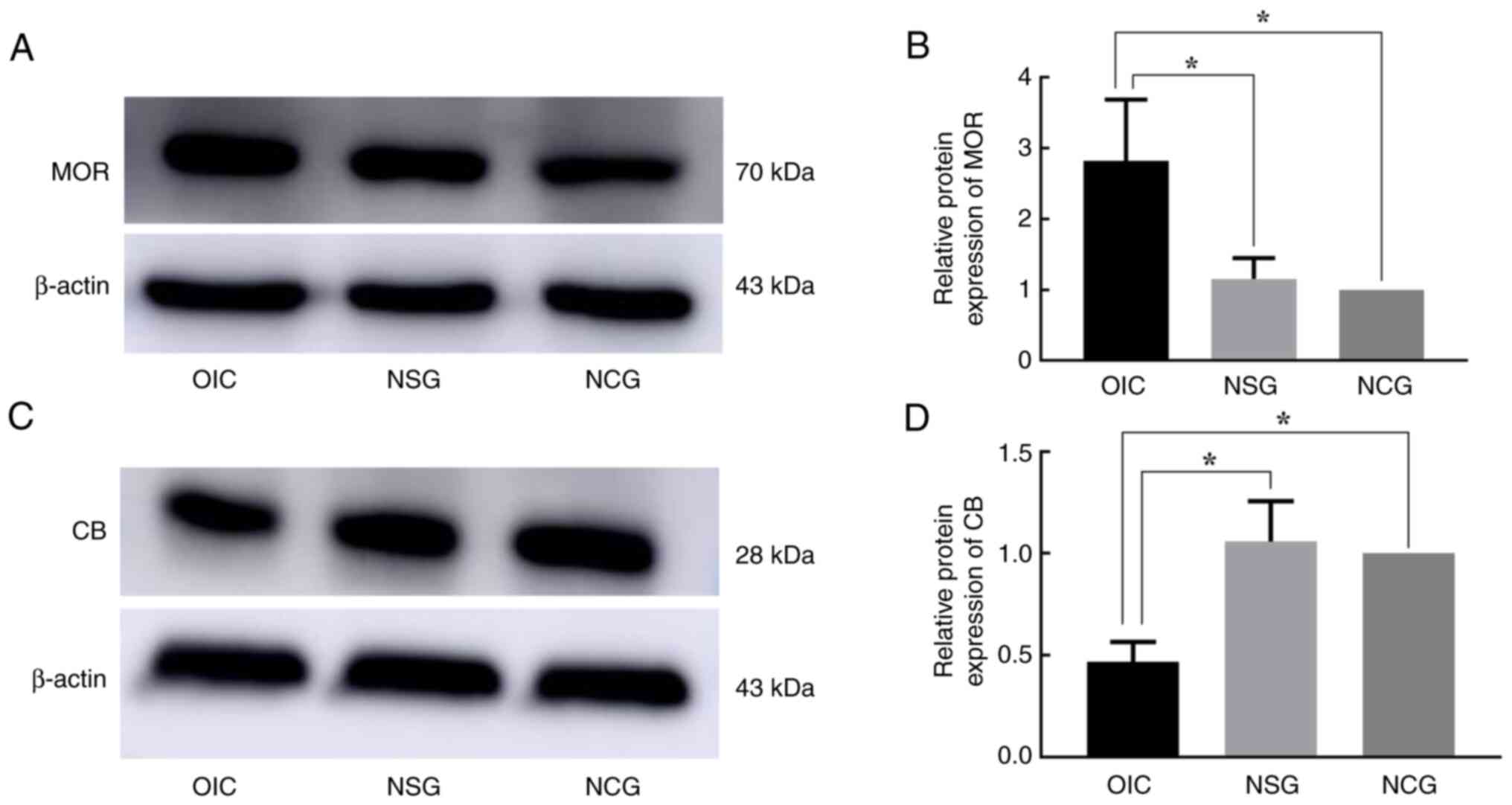
|
1
|
Madariaga-Mazón A, Marmolejo-Valencia AF,
Li Y, Toll L, Houghten RA and Martinez-Mayorga K: Mu-opioid
receptor biased ligands: A safer and painless discovery of
analgesics? Drug Discov Today. 22:1719–1729. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ford AC, Brenner DM and Schoenfeld PS:
Efficacy of pharmacological therapies for the treatment of
opioid-induced constipation: Systematic review and meta-analysis.
Am J Gastroenterol. 108:1566–1575. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spencer NJ and Hu H: Enteric nervous
system: Sensory transduction, neural circuits and gastrointestinal
motility. Nat Rev Gastroenterol Hepatol. 17:338–351.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Farmer AD, Holt CB, Downes TJ, Ruggeri E,
Del Vecchio S and De Giorgio R: Pathophysiology, diagnosis, and
management of opioid-induced constipation. Lancet Gastroenterol
Hepatol. 3:203–212. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gupta A, Coyne KS, Datto C and Venuti C:
The burden of opioid-induced constipation in younger patients with
chronic noncancer pain. Pain Med. 19:2459–2468. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harada Y, Iizuka S, Saegusa Y, Mogami S,
Fujitsuka N and Hattori T: Mashiningan improves opioid-induced
constipation in rats by activating cystic fibrosis transmembrane
conductance regulator chloride channel. J Pharmacol Exp Ther.
362:78–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Beckett EAH, Staikopoulos V and Hutchinson
MR: Differential effect of morphine on gastrointestinal transit,
colonic contractions and nerve-evoked relaxations in toll-like
receptor deficient mice. Sci Rep. 8(5923)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mozaffari S, Nikfar S and Abdollahi M:
Investigational opioid antagonists for treating opioid-induced
bowel dysfunction. Expert Opin Investig Drugs. 27:235–242.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mañé N, Gil V, Martinez-Cutillas M, Clavé
P, Gallego D and Jiménez M: Differential functional role of
purinergic and nitrergic inhibitory cotransmitters in human colonic
relaxation. Acta Physiol (Oxf). 212:293–305. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gil V, Martinez-Cutillas M, Mañé N, Martin
MT, Jiménez M and Gallego D: P2Y(1) knockout mice lack purinergic
neuromuscular transmission in the antrum and cecum.
Neurogastroenterol Motil. 25:e170–e182. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gallego D, Gil V, Aleu J, Aulí M, Clavé P
and Jiménez M: Purinergic and nitrergic junction potential in the
human colon. Am J Physiol Gastrointest Liver Physiol.
295:G522–G533. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bharucha AE and Wald A: Chronic
constipation. Mayo Clin Proc. 94:2340–2357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Halawi H, Vijayvargiya P, Busciglio I,
Oduyebo I, Khemani D, Ryks M, Rhoten D, Burton D, Szarka LA, Acosta
A and Camilleri M: Effects of naloxegol on whole gut transit in
opioid-naïve healthy subjects receiving codeine: A randomized,
controlled trial. Neurogastroenterol Motil.
30(e13298)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Galligan JJ and Sternini C: Insights into
the role of opioid receptors in the GI tract: Experimental evidence
and therapeutic relevance. Handb Exp Pharmacol. 239:363–378.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Luo M, Li L, Lu CZ and Cao WK: Clinical
efficacy and safety of lactulose for minimal hepatic
encephalopathy: A meta-analysis. Eur J Gastroenterol Hepatol.
23:1250–1257. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Camilleri M, Drossman DA, Becker G,
Webster LR, Davies AN and Mawe GM: Emerging treatments in
neurogastroenterology: A multidisciplinary working group consensus
statement on opioid-induced constipation. Neurogastroenterol Motil.
26:1386–1395. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matsumoto K, Umemoto H, Mori T, Akatsu R,
Saito S, Tashima K, Shibasaki M, Kato S, Suzuki T and Horie S:
Differences in the morphine-induced inhibition of small and large
intestinal transit: Involvement of central and peripheral µ-opioid
receptors in mice. Eur J Pharmacol. 771:220–228. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Beamer E, Conte G and Engel T: ATP release
during seizures - A critical evaluation of the evidence. Brain
Research Bulletin. 151:65–73. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jacobson KA and Müller CE: Medicinal
chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology.
104:31–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kong Q, Quan Y, Tian G, Zhou J and Liu X:
Purinergic P2 receptors: Novel mediators of mechanotransduction.
Front Pharmacol. 12(671809)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu J, Cheng Y, Zhang R, Liu D, Luo YM,
Chen KL, Ren S and Zhang J: P2Y1R is involved in visceral
hypersensitivity in rats with experimental irritable bowel
syndrome. World J Gastroenterol. 23:6339–6349. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
King BF: Burnstock and the legacy of the
inhibitory junction potential and P2Y1 receptors. Purinergic
Signal. 17:25–31. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gil V, Gallego D, Grasa L, Martín MT and
Jiménez M: Purinergic and nitrergic neuromuscular transmission
mediates spontaneous neuronal activity in the rat colon. Am J
Physiol Gastrointest Liver Physiol. 299:G158–G169. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Grasa L, Gil V, Gallego D, Martin MT and
Jiménez M: P2Y(1) receptors mediate inhibitory neuromuscular
transmission in the rat colon. Br J Pharmacol. 158:1641–1652.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li JP, Wang XY, Gao CJ, Liao YH, Qu J, He
ZY, Zhang T, Wang GD and Li YQ: Neurochemical phenotype and
function of endomorphin 2-immunopositive neurons in the myenteric
plexus of the rat colon. Front Neuroanat. 8(149)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shimotoyodome A, Meguro S, Hase T,
Tokimitsu I and Sakata T: Decreased colonic mucus in rats with
loperamide-induced constipation. Comp Biochem Physiol A Mol Integr
Physiol. 126:203–212. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim JE, Kang MJ, Choi JY, Park JJ, Lee MR,
Song BR, Kim HR, Park JW, Choi HJ, Bae SJ and Hwang DY: Regulation
of gastrointestinal hormones during laxative activity of
gallotannin-enriched extract isolated from Galla Rhois in
loperamide-induced constipation of SD rats. Lab Anim Res.
34:223–231. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park SA, Lee GH, Hoang TH, Lee HY, Kang
IY, Chung MJ, Jin JS and Chae HJ: Heat-inactivated lactobacillus
plantarum nF1 promotes intestinal health in loperamide-induced
constipation rats. PLoS One. 16(e0250354)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ma L, Qu Z, Xu L, Han L, Han Q, He J, Luan
X, Wang B, Sun Y and He B: 7,8-dihydroxyflavone enhanced colonic
cholinergic contraction and relieved loperamide-induced
constipation in rats. Dig Dis Sci. 66:4251–4262. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Agrawal A, Houghton LA, Reilly B, Morris J
and Whorwell PJ: Bloating and distension in irritable bowel
syndrome: The role of gastrointestinal transit. Am J Gastroenterol.
104:1998–2004. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Floettmann E, Bui K, Sostek M, Payza K and
Eldon M: Pharmacologic profile of naloxegol, a peripherally acting
µ-opioid receptor antagonist, for the treatment of opioid-induced
constipation. J Pharmacol Exp Ther. 361:280–291. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fan R, Schrott LM, Arnold T, Snelling S,
Rao M, Graham D, Cornelius A and Korneeva NL: Chronic oxycodone
induces axonal degeneration in rat brain. BMC Neurosci.
19(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mañé N, Jiménez-Sábado V and Jiménez M:
BPTU, an allosteric antagonist of P2Y1 receptor, blocks nerve
mediated inhibitory neuromuscular responses in the gastrointestinal
tract of rodents. Neuropharmacology. 110:376–385. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Coyne KS, LoCasale RJ, Datto CJ, Sexton
CC, Yeomans K and Tack J: Opioid-induced constipation in patients
with chronic noncancer pain in the USA, Canada, Germany, and the
UK: Descriptive analysis of baseline patient-reported outcomes and
retrospective chart review. Clinicoecon Outcomes Res. 6:269–281.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Takai N, Miyajima N, Tonomura M and Abe K:
Relationship between receptor occupancy and the antinociceptive
effect of mu opioid receptor agonists in male rats. Brain Res.
1680:105–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Siemens W and Becker G: Methylnaltrexone
for opioid-induced constipation: Review and meta-analyses for
objective plus subjective efficacy and safety outcomes. Ther Clin
Risk Manag. 12:401–412. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bharucha AE, Locke GR, Zinsmeister AR,
Seide BM, McKeon K, Schleck CD and Melton LJ III: Differences
between painless and painful constipation among community women. Am
J Gastroenterol. 101:604–612. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Young A, Viswanath A, Kalladka M, Khan J,
Eliav E and Diehl SR: Mouse model demonstrates strain differences
in susceptibility to opioid side effects. Neurosci Lett.
675:110–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen W, Chung HH and Cheng JT:
Opiate-induced constipation related to activation of small
intestine opioid µ2-receptors. World J Gastroenterol. 18:1391–1396.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Grundy D and Schemann M: Enteric nervous
system. Curr Opin Gastroenterol. 21:176–182. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gulati R, Komuravelly A, Leb S, Mhanna MJ,
Ghori A, Leon J and Needlman R: Usefulness of assessment of stool
form by the modified bristol stool form scale in primary care
pediatrics. Pediatr Gastroenterol Hepatol Nutr. 21:93–100.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Palus K, Bulc M, Czajkowska M, Miciński B
and Całka J: Neurochemical characteristics of calbindin-like
immunoreactive coeliac-cranial mesenteric ganglion complex (CCMG)
neurons supplying the pre-pyloric region of the porcine stomach.
Tissue Cell. 50:8–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Verheijden S and Boeckxstaens GE:
Neuroimmune interaction and the regulation of intestinal immune
homeostasis. Am J Physiol Gastrointest Liver Physiol. 314:G75–G80.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Galligan JJ and Akbarali HI: Molecular
physiology of enteric opioid receptors. Am J Gastroenterol Suppl.
2:17–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Palus K, Bulc M and Całka J: Changes in
VIP-, SP- and CGRP-like immunoreactivity in intramural neurons
within the pig stomach following supplementation with low and high
doses of acrylamide. Neurotoxicology. 69:47–59. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
da Silva MV, Marosti AR, Mendes CE,
Palombit K and Castelucci P: Submucosal neurons and enteric glial
cells expressing the P2X7 receptor in rat experimental colitis.
Acta Histochem. 119:481–494. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Olsson C: Calbindin immunoreactivity in
the enteric nervous system of larval and adult zebrafish (Danio
rerio). Cell Tissue Res. 344:31–40. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nishimura E, Buchan AM and McIntosh CH:
Autoradiographic localization of mu- and delta-type opioid
receptors in the gastrointestinal tract of the rat and guinea pig.
Gastroenterology. 91:1084–1094. 1986.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kaur R, O'Shaughnessy CT, Jarvie EM,
Winchester WJ and McLean PG: Characterization of a calcitonin
gene-related peptide release assay in rat isolated distal colon.
Arch Pharm Res. 32:1775–1781. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mitsui R, Miyamoto S, Takano H and
Hashitani H: Properties of submucosal venules in the rat distal
colon. Br J Pharmacol. 170:968–977. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Van Crombruggen K, Van Nassauw L,
Timmermans JP and Lefebvre RA: Inhibitory purinergic P2 receptor
characterisation in rat distal colon. Neuropharmacology.
53:257–271. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kotova PD, Bystrova MF, Rogachevskaja OA,
Khokhlov AA, Sysoeva VY, Tkachuk VA and Kolesnikov SS: Coupling of
P2Y receptors to Ca2+ mobilization in mesenchymal
stromal cells from the human adipose tissue. Cell Calcium. 71:1–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Barańska J, Czajkowski R and Pomorski P:
P2Y1 receptors-properties and functional activities. Adv
Exp Med Biol. 1051:71–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Buvinic S, Bravo-Zehnder M, Boyer JL,
Huidobro-Toro JP and González A: Nucleotide P2Y1 receptor regulates
EGF receptor mitogenic signaling and expression in epithelial
cells. J Cell Sci. 120:4289–4301. 2007.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Burnstock G: Purine and pyrimidine
receptors. Cell Mol Life Sci. 64:1471–1483. 2007.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Strong DS, Cornbrooks CF, Roberts JA,
Hoffman JM, Sharkey KA and Mawe GM: Purinergic neuromuscular
transmission is selectively attenuated in ulcerated regions of
inflamed guinea pig distal colon. J Physiol. 588:847–859.
2010.PubMed/NCBI View Article : Google Scholar
|