Introduction
Synchronous Mucinous Metaplasia and Neoplasia of the
Female Genital Tract (SMMN-FGT) is a multifocal mucinous lesion
that occurs simultaneously in the female genital tract and was
first described by Mikami et al (1). SMMN-FGT rarely occurs; ~35 cases have
been reported in the literature (1-11).
SMMN-FGT demonstrates a spectrum of morphological features, ranging
from metaplasia without nuclear or architectural abnormalities to
invasive mucinous adenocarcinoma, including minimal deviation
adenocarcinoma (MDA) of the cervix and usually with gastric
differentiation. Whether the disease process is the multifocal
independent occurrence or the widespread dispersal from a single
lesion is still controversial. The present study reported the
clinical data, histological morphology and immunohistochemistry of
this case and is expected to provide a further reference for the
clinicopathological characteristics of the disease and the basis
for its diagnosis and treatment.
Case study
The patient was a 47-year-old married woman who
complained of a pelvic mass during a physical examination a month
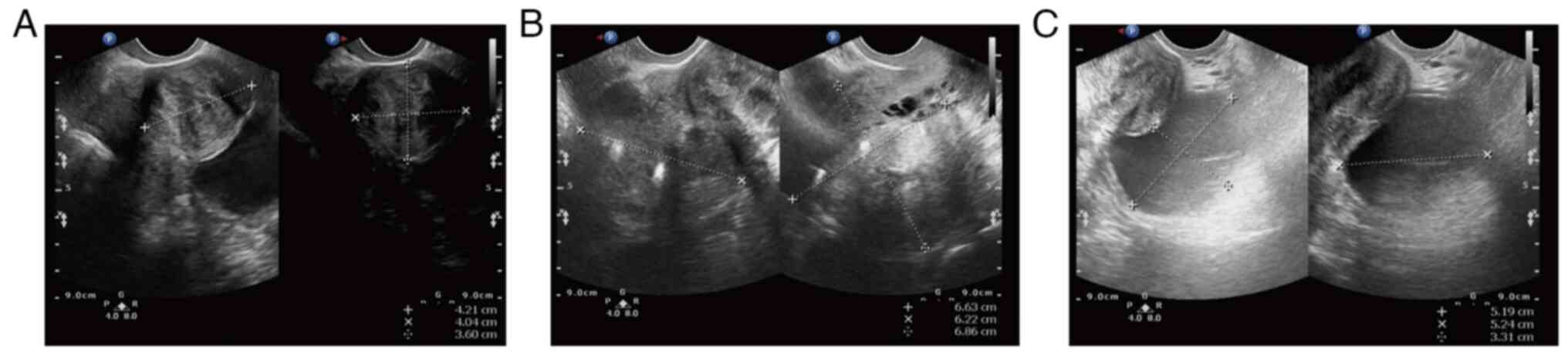
ago (February, 2021). The preoperative transvaginal ultrasound
displayed multiple inhomogeneous hypoechoic echoes without obvious
blood flow signals in the uterine myometrium (the largest one on
the right uterine wall, which occludes the right ovary; Fig. 1A and B). There was a 52x52x33 mm
well-circumscribed anechoic spot in the left ovary without an
obvious blood flow signal (Fig.
1C). Several anechoic spots were detected in the cervix, with a
maximum diameter of 8 mm. The endometrium measured ~5 mm in
thickness. Cervical liquid-based cytology (Thinprep cytologic test,
TCT) was negative for intraepithelial lesions or malignant lesions
and the high-risk HPV test was negative. The patient underwent left
adnexectomy; a frozen section of the left ovarian revealed a
mucinous borderline tumor. The patient underwent a rapid
cytological examination of the peritoneal washing fluid and no
tumor cells were found. Intraoperative examination of the appendix
and other digestive tract organs showed no obvious abnormality.
Total abdominal hysterectomy and bilateral salpingo-oophorectomy
were subsequently performed.
Gross presentation
The removed bilateral ovaries were measured 50x45x25
mm (left) and 32x20x15 mm (right) (grossly view of the specimen) in
size and were multicystic tumors without conspicuous papillae in
the cyst wall. The uterus body measured ~100x45x25 mm in size.
Multiple grey-white nodules were in the intramural and subserosa of
the uterus with a diameter of 2-48 mm. The thickness of the
endometrium was 2-5 mm. The cervical canal was measured ~35 mm in
length and 3 0 mm in diameter, with multiple cervical cystic
lesions scattered in the endocervix. The bilateral fallopian tubes
were grossly unremarkable.
Microscopic examination
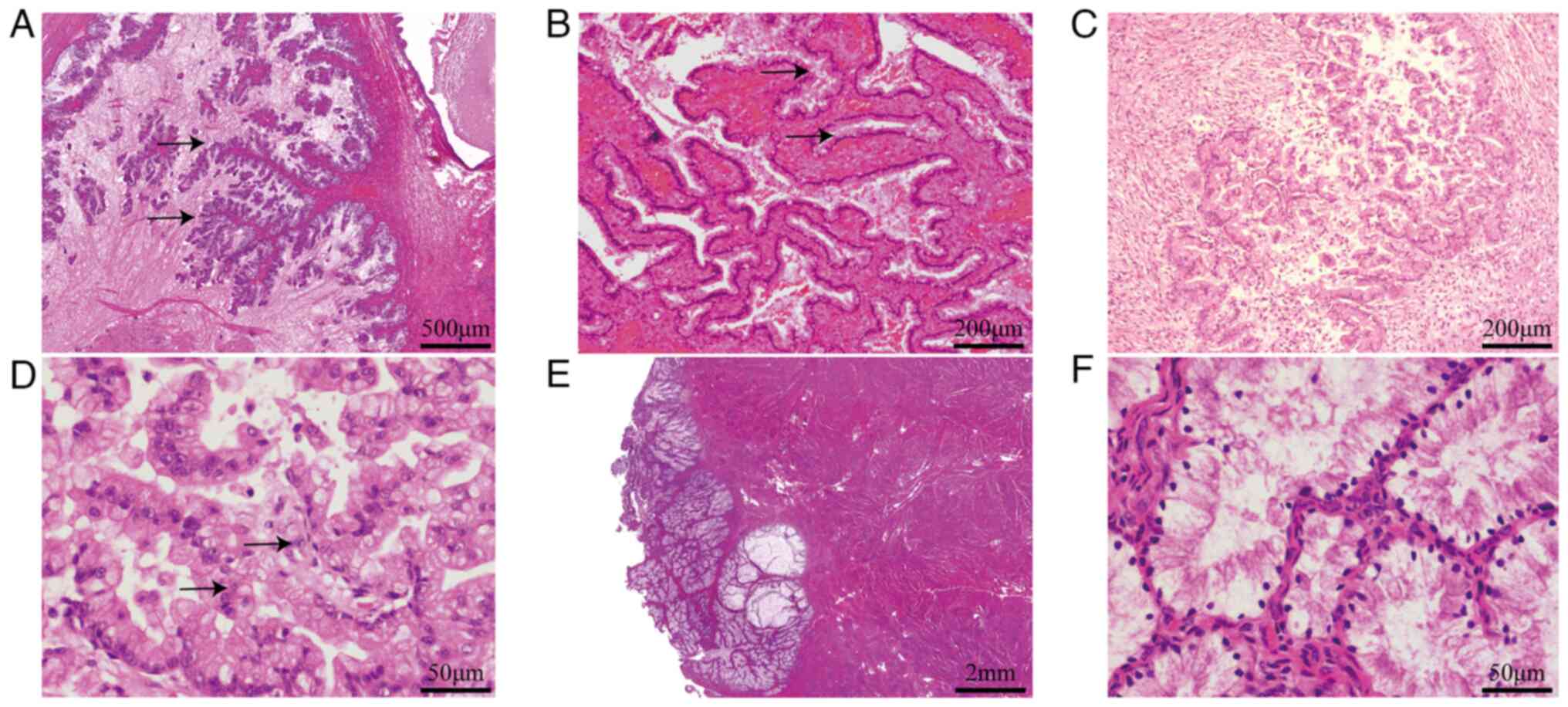
Bilateral ovarian tumors have similar histological
features and contain multiple cysts lined by gastric-type mucinous
epithelium showing variable degrees of stratification, tufting and
villous or slender filiform papillae. Tumor cells are generally
low-grade nuclear atypia. Mitotic activity is predominantly present
in crypts and less prominent on the luminal surface (Fig. 2A). The bilateral fallopian tube
mucosal epithelium showed focal mucinous metaplasia (Fig. 2B).
The cervical lesion in the internal cervical canal
displayed a well-differentiated mucinous adenocarcinoma with focal
superficial invasion and is surrounded by lobular endocervical
glandular hyperplasia (LEGH; Fig.
2C). The tumor was confined to the cervix without vascular
invasion and did not invade beyond the lower uterus. The tumor
cells with abundant clear pale eosinophilic cytoplasm and distinct
cell borders. The cytoplasm contained neutral mucins, which stained
pale pinkish-red. (Fig. 2D).
The mucinous endometrial tumor showed a nodular
expansive growth pattern and invaded the superficial myometrium
(3/15 mm) without vascular invasion (Fig. 2E). It had similar morphological
features to the cervical tumor, such as abundant cytoplasm
containing mucins and atypical mitoses were present but
inconspicuous (Fig. 2F). The
background endometrium displayed hyperplasia.
Immunohistochemical findings
Immunohistochemically, ovarian tumor cells expressed
MUC6, CK7, PAX8 (Fig. 3A-C), a
Ki-67 proliferation index of ~70% (Fig. 3D) and were negative for ER, PR,
CDX-2, CK20 (data not shown). Cervical tumor cells expressed MUC6
(Fig. 3E), were negative for P16
and P53 (Fig. 3F and G) and had a Ki-67 proliferation index of
~50% (Fig. 3H). Endometrial tumor
cells expressed MUC6 (Fig. 3I),
were negative for ER, PR (Fig. 3J
and K), P16 and P53 (data not
shown) and the Ki-67 proliferation index was ~10% (Fig. 3L).
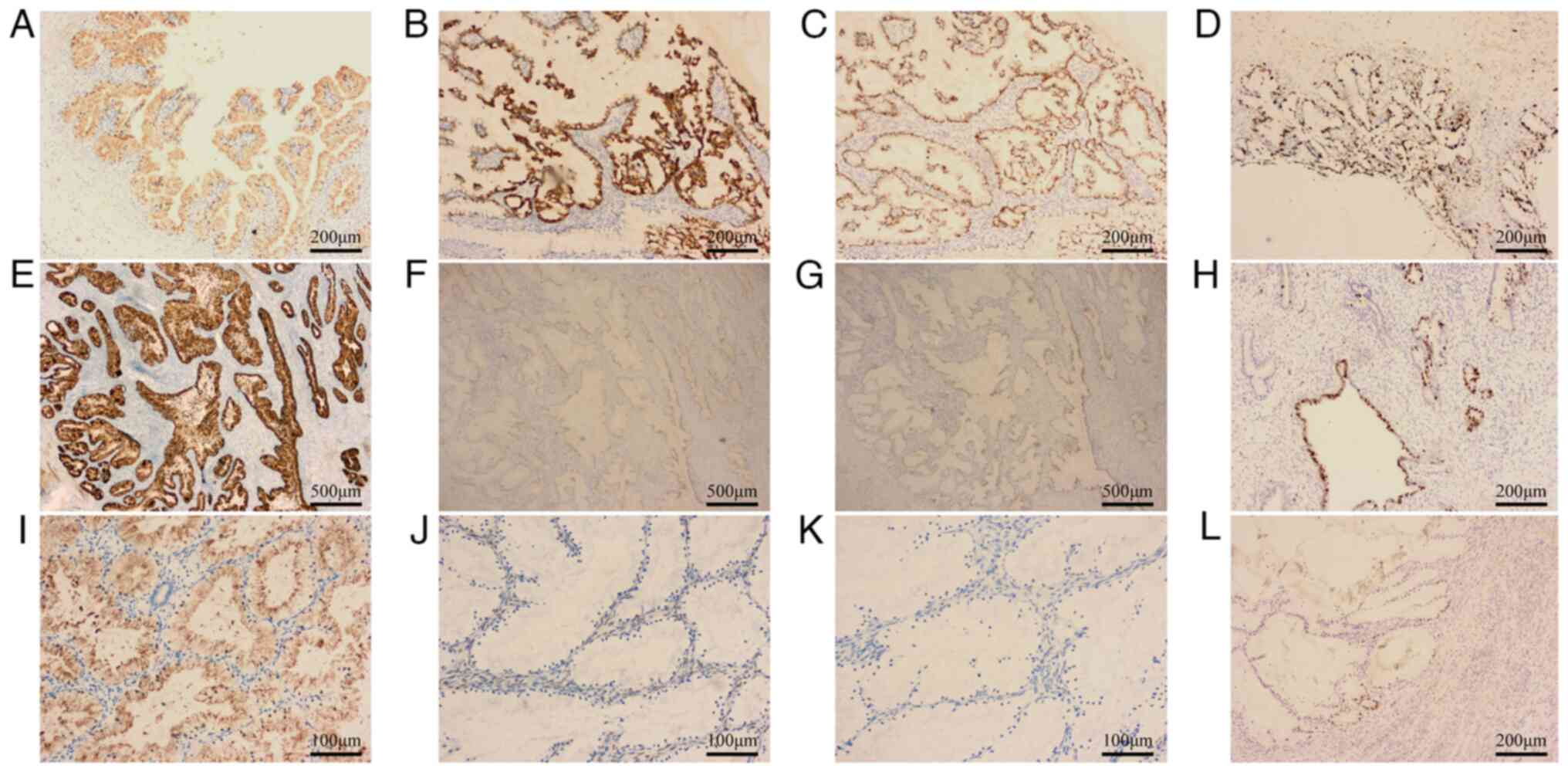 | Figure 3Immunoprofiling of tumor cells.
Ovarian tumors positive for (A) MUC6, (B) CK7, (C) PAX-8, (D) Ki-67
proliferation index was ~70% (magnification, x100). Cervical tumors
positive for (E) MUC6, (F) negative for P16 and (G) P53
(magnification, x40), (H) Ki-67 proliferation index was ~50%
(magnification, x100). Endometrial tumors positive for (I) MUC6,
negative for (J) ER and (K) PR (magnification, x200). (L) Ki-67
proliferation index was ~10% (magnification, x100). |
The patient underwent gastroscopy and colonoscopy
following surgery and no gastrointestinal lesions were found.
Combined with the clinical visualization, histological features and
immunohistochemical results, the primary diagnosis was ascertained
as SMMN-FGT by the International Federation of Gynecology and
Obstetrics stage I (12),
including mucinous borderline tumor (MBT) of the bilateral ovaries,
gastric-type cervical adenocarcinoma (GCA), endometrial mucinous
adenocarcinoma and fallopian tubes epithelium mucinous metaplasia.
The secondary diagnosis was multiple uterine leiomyomas. The
patient was been referred to a course of pelvic radiotherapy
followed surgery. The follow-up showed that the patient was alive
and disease-free at 16 months after surgery.
Discussion
Mucinous lesions that occur in two or more sites of
the female genital tract simultaneously, including the cervix,
endometrium, ovary, or fallopian tube, are rare. Occasionally, the
involvement of the urethral orifice and peritoneum has also been
reported (2). These rare mucinous
lesions are termed SMMN-FGT. The distinguishing feature of SMMN-FGT
is that all tumors co-occur and exhibit features of gastric-type
differentiation, such as expression of MUC6 and/or HIK-1083. Most
of the cervix lesions are GCA which is usually called MDA when it
is extremely well-differentiated. The 5th WHO Classification of
female genital tract tumors (13)
classifies it as HPV-independent adenocarcinoma due to its
different pathogenesis from traditional cervical adenocarcinoma.
Some patients are also accompanied by Peutz-Jeghers syndrome
(14). Molecular genetics may be
related to the mutation of STK11(15) and KRAS (16).
To the best of the authors' knowledge, ~35 cases of
SMMN-FGT have been reported in the literature (Table I) (1-11).
The age of the patients ranged from 33-83 years (mean, 51 years
old). The clinical manifestations of SMMN-FGT are not exclusive.
Most patients complained of irregular vaginal bleeding, vaginal
discharge, or abdominal discomfort; some patients exhibit atypical
glandular cells on cervical cytology. The patient in the present
study was a middle-aged woman diagnosed with a pelvic mass by
physical examination but without other symptoms. The negative
cytology result may be that the materials could not be effectively
collected because of the mucinous lesions confined to the internal
cervical canal. In addition, in traditional cervical cancer
screening (cervical cytology combined with HPV test) it is easy to
miss the lesions because it is HPV-independent.
 | Table IClinical and pathological features of
35 patients with SMMN-FGT. |
Table I
Clinical and pathological features of
35 patients with SMMN-FGT.
| Author, year | Case | Age | Symptoms | Cervix | Endometrium | Tube | Ovary | Remarks | FIGO | Treatment | Follow-up | (Refs.) |
|---|
| Giles et al,
1994 | 1 | 48 | Irregular vaginal
bleeding | Mucinous
adenocarcinoma | Papillary mucinous
adenocarcinoma | Mucinous carcinoma
in situ | Invasive mucinous
adenocarcinoma, mucinous cystadenoma | N/A | N/A | TAH + BSO + RT | Disease-free survival
(9 months) | (8) |
| Jackson- York et
al, 1992 | 2 | 48 | Irregular vaginal
bleeding | Papillary mucinous
adenocarcinoma, AIS | Normal | Papillary mucinous
carcinoma (left) | Metastatic
adenocarcinoma (left) | N/A | N/A | TAH + BSO | N/A | (7) |
| Anjarwalla et
al, 2007 | 3 | 65 | Urinary incontinence
18 months | Cervical
agenesis | Mucinous
metaplasia | Pseudopyloric
metaplasia | Atypical mucinous
epithelium (left) | Entire genital
epithelial surface replaced by müllerian epithelial | N/A | TAH + BSO | Disease-free survival
(20 months) | (9) |
| Nagahama et
al, 2013 | 4 | 52 | Increased vaginal
discharge | LEGH | Mucinous
metaplasia | Normal | Normal | Peritoneal cytology
revealed several mucin- containing epithelial clusters | N/A | TAH + BSO,
paclitaxel- carboplatin 5 courses | N/A | (3) |
| Mangili et al,
2004 | 5 | 41 | Left adnexal mass,
abdominal pain | MDA | Simplex hyperplasia
with a component of clear cell metaplasia | Mucinous
metaplasia | MBT (left) | PJS | N/A | TAH + BSO | Disease-free survival
(21 months) | (6) |
| Ikeda et al,
2015 | 6 | 73 | AVD, lower abdominal
pain | Mucinous
adenocarcinoma | Mucinous
adenocarcinoma | Normal | MBT | External urethral
meatus neoplasm | N/A | TAH + BSO, partial
omentectomy, appendectomy and mesenteric and external urethral
meatus neoplasm resection, chemotherapy | Lung metastases were
found after 6 months | (2) |
| Lu et al,
2019 | 7 | 57 | AVD | GCA | Mucinous
metaplasia | MBT (left) | Normal | HPV 16 positive | N/A | TAH + BSO | N/A | (4) |
| Xu et al,
2021 | 8 | 44 | Abdominal mass | Gastric-type mucinous
hyperplasia | Mucinous
metaplasia | Normal | Mucinous
cystadenoma | KRAS G13D
mutation | N/A | TAH + right salpingo-
oophorectomy | N/A | (10) |
| Gu et al,
2018 | 9 | 37-70 Average 54 | AVD, abdominal
mass | Gastric-type mucinous
hyperplasia | LEGH | Mucinous metaplasia
(right) | Mucinous cystadenom a
(left) | N/A | N/A | TAH + BSO | Disease-free survival
(2-34 months) without relapse | (5) |
| | 10 | | AVD | AIS, LEGH, ALEGH | AIS, LEGH, ALEGH | Normal | Mucinous
cystadenoma | N/A | N/A | TAH + BSO | | |
| | 11 | | AVD | GCA | Gastric-type
adenocarcinoma, mucinous metaplasia, MDA | Mucinous metaplasia
(right) | Normal | Normal | N/A | TAH + BSO + PEL, RT,
chemotherapy | | |
| | 12 | | Abdominal mass | GCA, LEGH, ALEGH | LEGH, ALEGH | Mucinous metaplasia
(left) | Mucinous
cystadenoma | N/A | N/A | TAH + BSO,
chemotherapy | | |
| | 13 | | AVD | GCA, MDA | Gastric-type
adenocarcinoma, mucinous metaplasia, MDA | Mucinous metaplasia
(right) | Normal | Tumor invades lymph
nodes and blood vessels | N/A | TAH + BSO + PEL +
PAN, RT, chemotherapy | | |
| | 14 | | AVD | GCA, MDA | Gastric-type
adenocarcinoma, mucinous metaplasia, MDA | AIS (left) | Normal | Vagina
invading | N/A | TAH + BSO + PEL,
RT, chemotherapy | | |
| | 15 | | AVD | AIS, LEGH,
ALEGH | AIS, LEGH,
ALEGH | Mucinous
cystadenoma (right) | Normal | N/A | N/A | TAH + BSO | | |
| Mikami et
al, 2009 | 16 | 47 | AGC on Pap
smears | MDA, AIS, LEGH | Mucinous
adenocarcinoma, mucinous metaplasia | Mucinous
cystadenoma | Normal | Peritoneal washing
positive | IA | TAH + BSO | Disease-freel
surviva (36 months) | (1) |
| | 17 | 60 | Abdominal pain | MDA, AIS, LEGH | Mucinous
metaplasia, LEGH | MBT | MBT | N/A | IA | TAH + BSO | Disease-free
survival (37 months) | |
| | 18 | 65 | AGC on Pap
smears | MDA, AIS, LEGH | Mucinous
metaplasia | Mucinous
metaplasia | Normal | Peritoneal washing
positive | IA | TAH + BSO,
chemotherapy | Disease-free
survival (102 months) | |
| | 19 | 62 | AGC on Pap
smears | MDA, AIS, LEGH | Mucinous
adenocarcinoma, mucinous metaplasia | MBT | Normal | Vagina
invading | IIA | TAH + BSO + PEL +
PAN, chemotherapy | Died after 62
months | |
| | 20 | 39 | AGC on Pap
smears | AIS, LEGH | Mucinous
adenocarcinoma, mucinous metaplasia | Normal | Normal | N/A | NA | TAH + BSO + PEL,
chemotherapy | Disease-free
survival (13 months) | |
| | 21 | 83 | Ovarian cyst | Normal | Mucinous
metaplasia, LEGH | MBT | MBT | Peritoneal washing
positive | IC | TAH + BSO | Disease-free
survival (25 months) | |
| Chen et al,
2022 | 22 | 56 | AVD | Cervicitis | ALEGH | Mucinous metaplasia
(right) | Mucinous metaplasia
(left) | N/A | N/A | TAH + BSO | Disease-free
survival (80 months) | (11) |
| | 23 | 37 | AVD | ALEGH, GCA in
situ | Mucious
metaplasia | Gastric adenomatous
metaplasia (left) | Mucinous
cystadenoma | N/A | N/A | TAO + BSO | Disease-free
survival (75 months) | |
| | 24 | 41 | AVD, Ovarian
cyst | GCA | Gastric-type
adenocarcinoma | Normal | Mucinous
cystadenoma | N/A | N/A | TAO + BSO | Disease-free
survival (64 months) | |
| | 25 | 36 | AGC on Pap
smears | MDA | MDA | Mucinous carcinoma
(right) | Mucinous carcinoma
(right) | Vaginal wall (+),
Parametrium (+) | N/A | RH + RSO + BPLND +
OMT + AE | Disease-free
survival (60 months) | |
| | 26 | 49 | Ovarian cyst | ALEGH, GCA | Gastric-type
adenocarcinoma | Gastric-type
adenocarcinoma (left) | MBT (left) | N/A | N/A | TAH + LSO | Disease-free
survival (52 months) | |
| | 27 | 54 | AVD | GCA | Gastric-type
adenocarcinoma | Mucinous metaplasia
(Gastric type, right) | Brenner tumor
(right) | N/A | N/A | TAH + BSO + EPH +
BPLND + PALND | Died after 36
months | |
| | 28 | 33 | AVB | LEGH | Mucinous
metaplasia, gastric-type | Mucinous
metaplasia, gastric-type | Reserved, not
checked | N/A | N/A | TAH + BS | Disease-free
survival (43 months) | |
| | 29 | 46 | Ovarian cyst | ALEGH | ALEGH | Normal | Mucinous
cystadenoma (right) | N/A | N/A | TAH + RSO | Disease-free
survival (42 months) | |
| | 30 | 46 | AVD | MDA | MDA | Normal | MBT (left) | N/A | N/A | TAH + BSO | Disease-free
survival (36 months) | |
| | 31 | 39 | AVD | LEGH | Mucinous
metaplasia, Gastric-type | Normal | Reserved, not
checked | N/A | N/A | TAH + BS + BBO | Disease-free
survival (36 months) | |
| | 32 | 51 | Ovarian cyst | LEGH, ALEGH | LEGH | LEGH (right) | Mucinous
cystadenoma (right) | N/A | N/A | TAH + RSO | Disease-free
survival (33 months) | |
| | 33 | 37 | AVB | LEGH | LEGH | Normal | Reserved, not
checked | N/A | N/A | TAH + BS | Disease-free
survival (29 months) | |
| | 34 | 52 | AVD/AVB | GCA | Mucinous
metaplasia | Mucinous metaplasia
(left) | Normal | Vaginal wall
(+) | N/A | RH + BSO +
BPLND | Disease-free
survival (14 months) | |
| Hongliang et
al, 2022 | 35 | 47 | Abdominal mass | GCA | Mucinous
adenocarcinoma | Mucinous
metaplasia | MBT | N/A | IA | TAH + BSO, RT | Disease-free
survival (16 months) | Present case |
Among the described cases, 23 cases showed
gastric-type cervical mucinous adenocarcinoma and three cases
without cervical mucinous lesions. 15 cases of endometrial lesions
manifested as mucinous adenocarcinoma, nine cases were mucinous
metaplasia and eight showed occasional villoglandular growth
associated with LEGH. A total of 12 cases of fallopian tube lesions
were mucinous metaplasia, five cases were mucinous carcinomas and
four cases were mucinous borderline tumors. A total of seven cases
of the ovarian lesions were mucinous borderline tumors, eight were
mucinous cystadenoma and three were ovarian mucinous carcinoma.
Among the 35 cases, 21 cases involved three sites of the female
genital tracts and 10 cases involved four sites. In the patient in
the present study, mucinous lesions involved four sites of the
genital tracts, including the cervix, endometrium, bilateral
ovaries and fallopian tubes. The cervix and endometrium were
gastric-type adenocarcinomas, bilateral ovaries were MBT and
bilateral fallopian tube epithelium were mucinous metaplasia.
The primary differential diagnosis of SMMN-FGT is
metastatic ovarian mucinous adenocarcinoma. Notably, Metastatic
ovarian mucinous adenocarcinoma closely resembles primary or
mucinous borderline tumors and requires close macroscopic and
microscopic observations combined with clinical information for
accurate identification. The features of metastatic ovarian
mucinous adenocarcinoma from cervical cancer were described by
Young and Scully (17) including
i) The ovarian tumor may metastasize from cervical cancer if the
ovarian and cervical cancer occur at a similar time; ii) tumors are
usually present in bilateral ovaries, iii) tumor implants visible
on the surface of the ovary, iv) cervical and ovarian tumors have
similar histological features, v) Cervical tumor usually
infiltrates deep myometrium, vi) cervical tumor widely spread, vii)
tumor invades lymph nodes and blood vessels and viii) deficiency of
mucous metaplasia of the fallopian tubes or endometrial lining.
Moreover, well-differentiated mucinous adenocarcinoma of the
digestive tract, such as well-differentiated mucinous
adenocarcinoma of the gallbladder, metastases to the cervix and
ovary, possesses similar morphological features to cervical MDA and
ovarian mucinous cystadenoma. In the present case, the mucinous
tumors existed on cervical, bilateral ovaries and endometrium with
similar histological characteristics, but the lower uterine
segment, ovarian surface and blood vessels were without tumor
invasion, which demonstrated the ovarian tumor was not metastasized
from the cervical tumor. Moreover, the endometrial and cervical
tumors were limited to the superficial myometrium. Combined with
the clinical features, imaging findings, histology and
immunohistochemistry, the ovarian, endometrial and cervical tumor
occurred simultaneously. Cumulative evidence established SMMN-FGT
as the final histopathological diagnosis.
Treatment of SMMN-FGT is usually based on the stage
of the co-existing adenocarcinoma. Total abdominal hysterectomy
(TAO) and bilateral salpingo-oophorectomy (BSO) are sufficient for
early staging. Most of the 22 reported cases were treated with TAO
and BSO. Some patients received adjuvant radiotherapy and
chemotherapy after surgery. The prognosis of patients is mainly
related to the staging of the most severe lesions (1). One patient died 62 months after
surgery because the lesions invaded the vaginal wall and reoccurred
9 months after surgery. The follow-up of other patients ranged from
2-102 months and they survived disease-free. In the present case,
the patient underwent a rapid cytological examination of the
peritoneal washing fluid during the operation and the results
showed that no tumor cells were found. The appearance of the
resected tumor was limited to the ovary and the surface of the
ovary was smooth and no tumor was observed. Therefore, no
omentectomy was performed during the clinical operation, but this
may also be where treatment is inadequate. Finally, the patient
underwent TAO, BSO and a course of radiotherapy following surgery.
The patient was disease-free 16 months following the completion of
the therapy.
The present study reported a case of SMMN-FGT that
occurred in a 47-year-old woman, including the clinical symptoms,
ultrasound display, gross appearance, microscopic examination and
immunohistochemical findings. However, there are some limitations
to the present study. First, the images of an MRI or CT scan on the
pelvis before surgery were not taken as the preoperative clinical
diagnosis was of a benign ovarian cyst. In addition, the tumors of
the cervix and endometrium were inconspicuous, so the present study
collected a large number of cervical and endometrial tissues for
diagnosis, only to find superficial tumor lesions and that the
gross specimen was damaged, so the present study lacked an image
displaying the gross characteristics of this rare tumor.
The present study presented a case of SMMN-FGT,
which existed in the cervix, endometrium and bilateral annex.
Immunohistochemistry showed that the tumor cells were positive for
gastric-type marker MUC6. Further studies are of great significance
to characterize clinical features of this rare disease for
differential diagnosis and effective therapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Anhui Medical
University Funding Project (grant no. 2020xkj066).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HX contributed to the original conception of the
study, data analyses and wrote the manuscript. YC acquired
hematoxylin and eosin staining, immunohistochemical and ultrasound
images. SZ performed the surgery and contributed to the acquisition
of data. CZ and QW provided pathological diagnosis. MT performed
tissue specimen collection. WZ prepared hematoxylin and eosin and
immunohistochemical sections. HZ performed the analysis of the data
and revised the manuscript. All authors read and approved the final
manuscript. HX and YC confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
Ethics approval was obtained from the Ethics
Committee of Anhui Medical University (approval no. 81220058) and
Anhui Province Maternity and Child Health Hospital (approval no.
YYLL2022-2020xkj066-11-1.0).
Patient consent for publication
Written informed consent for publication was
obtained from patient, including the patient's data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mikami Y, Kiyokawa T, Sasajima Y, Teramoto
N, Wakasa T, Wakasa K and Hata S: Reappraisal of synchronous and
multifocal mucinous lesions of the female genital tract: A close
association with gastric metaplasia. Histopathology. 54:184–191.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ikeda Y, Yasuda M, Kato T, Yano Y,
Kurosaki A and Hasegawa K: Synchronous mucinous metaplasia and
neoplasia of the female genital tract with external urethral meatus
neoplasm: A case report. Gynecol Oncol Rep. 12:27–30.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nagahama K, Yamanaka S, Nakayama T,
Tokinaga A, Asai-Sato M, Miyagi E, Tanaka R and Furuya M: A case of
synchronous mucinous metaplasia and neoplasia of the female genital
tract without an STK11 or KRAS mutation. Gynecol Oncol Case Rep.
5:4–5. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lu J, Gao L, Yu H, Xia L, Guo X and Zheng
F: A patient from Zhejiang, China, with synchronous mucinous
metaplasia and neoplasms of the female genital tract: A case
report. J Obstet Gynaecol Res. 45:1382–1385. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gu WY, Tao X, Zhang LL, Wang L, Zhou XR
and Ning Y: Synchronous mucinous metaplasia and neoplasia of the
female genital tract. Zhonghua Bing Li Xue Za Zhi. 47:845–850.
2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
6
|
Mangili G, Taccagni G, Garavaglia E,
Carnelli M and Montoli S: An unusual admixture of neoplastic and
metaplastic lesions of the female genital tract in the
Peutz-Jeghers Syndrome. Gynecol Oncol. 92:337–342. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jackson-York GL and Ramzy I: Synchronous
papillary mucinous adenocarcinoma of the endocervix and fallopian
tubes. Int J Gynecol Pathol. 11:63–67. 1992.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Giles A, Yoon J and Lindley R: Multifocal
mucinous neoplasia of the female genital system. Histopathology.
25:281–283. 1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Anjarwalla S, Rollason TP, Rooney N and
Hirschowitz L: Atypical mucinous metaplasia and intraepithelial
neoplasia of the female genital tract-a case report and review of
the literature. Int J Gynecol Cancer. 17:1147–1150. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu ZY, Wu J, Song XX, Meng G and Gui ZH: A
case of Synchronous Mucinous Metaplasia and Neoplasia of the Female
Genital Tract with KRAS gene mutation. Chin J Clin and Experiment
Pathol. 37:1147–1148. 2021.(In Chinese).
|
|
11
|
Chen M, Li J-J, Tao X, Zhu C-Q, Dong X-H,
Yao L-Q and Li Q: Clinical analysis of 14 cases of synchronous
mucinous metaplasia and neoplasia of the female genital tract.
Fudan Univ J Med Sci. 49:402–410. 2022.
|
|
12
|
Bhatla N and Denny L: FIGO cancer report
2018. Int J Gynaecol Obstet. 143 (Suppl 2):S2–S3. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lokuhetty D, White VA and Watanabe R:
Female genital Tumours. In: WHO Classification of Tumours. Vol 4.
5th Edition. Internal Agency for Research on Cancer (IARC), Lyon,
2020.
|
|
14
|
Banno K, Kisu I, Yanokura M, Masuda K,
Ueki A, Kobayashi Y, Hirasawa A and Aoki D: Hereditary
gynecological tumors associated with Peutz-Jeghers syndrome
(Review). Oncol Lett. 6:1184–1188. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kuragaki C, Enomoto T, Ueno Y, Sun H,
Fujita M, Nakashima R, Ueda Y, Wada H, Murata Y, Toki T, et al:
Mutations in the STK11 gene characterize minimal deviation
adenocarcinoma of the uterine cervix. Lab Invest. 83:35–45.
2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yoo SH, Park BH, Choi J, Yoo J, Lee SW,
Kim YM and Kim KR: Papillary mucinous metaplasia of the endometrium
as a possible precursor of endometrial mucinous adenocarcinoma. Mod
Pathol. 25:1496–1507. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Young RH and Scully RE: Mucinous ovarian
tumors associated with mucinous adenocarcinomas of the cervix. A
clinicopathological analysis of 16 cases. Int J Gynecol Pathol.
7:99–111. 1988.PubMed/NCBI View Article : Google Scholar
|