Introduction
Periodontitis, an infectious human chronic
inflammatory disease of which the age-standardized prevalence rate
increased by 8.44% (6.62-10.59%) worldwide from 1990 to
2019(1), is caused by accumulation
of subgingival plaque and the action of gram-negative anaerobic
bacteria, such as Porphyromonas gingivalis and Treponema
denticola (2,3). It is characterized by the progressive
destruction of tooth-supporting tissue, eventually leading to tooth
loss (4,5). A number of studies have demonstrated
that periodontitis is associated with the initiation or progression
of various systemic diseases, such as cardiovascular and chronic
kidney disease and diabetes (6-8).
Although progress has been achieved in the treatment of
periodontitis, including skin flap curettage, scaling and root
planting, the effects of the aforementioned therapies only control
the development of this condition (9-11).
Therefore, it is of clinical importance to investigate promising
therapeutic agents for periodontitis to improve oral health and
avert the development of systemic disease.
Periodontal ligament stem cells (PDLSCs),
mesenchymal stem cells located in the periodontal ligament tissue,
harbor osteogenic potential and regenerative capacity to recover
lost/damaged periodontal tissue (12,13).
PDLSCs have been shown to migrate to the site of periodontal
disease and mediate periodontal regeneration (14,15).
PDLSCs are considered an ideal cell source for periodontal tissue
regeneration and repair (16,17).
A body of evidence indicates that endotoxins generated from
periodontal pathogens, notably lipopolysaccharide (LPS) produced by
Porphyromonas gingivalis, disrupt microenvironment
homeostasis and damage periodontal tissue by inhibiting the
viability of PDLSCs and causing disturbance of periodontal ligament
cell differentiation (18,19). LPS is a potent stimulator of
inflammation that produces proinflammatory cytokines. This compound
has been widely used for construction of in vitro
experimental models of periodontitis (20,21).
It is crucial to control the inflammatory damage of PDLSCs for the
restoration of the tissues and to inhibit progression of
periodontitis.
Baricitinib is an oral drug commonly used in
treatment of rheumatoid arthritis (22). A recent clinical study has
suggested that baricitinib improves the periodontal health of
patients with rheumatoid arthritis and decrease the inflammatory
response in periodontal tissue (23). Baricitinib suppresses
osteoclastogenesis by downregulating receptor activator of nuclear
factor-κB ligand (RANKL) expression in osteoblasts (24). In addition, as a Janus kinase
(JAK)1/2 inhibitor, baricitinib increases bone mass in
physiological conditions and ameliorates pathological bone loss by
stimulating osteoblast function (25). Meanwhile, the abnormal expression
of STAT3, an important member of STAT protein family, has been
reported to mediate the over-activation of JAK-STAT3 signaling
pathway, which is related to the process of periodontitis (26). However, whether baricitinib affects
inflammation and osteogenic differentiation of LPS-induced PDLSCs
and its potential regulatory mechanisms remains to be
elucidated.
Materials and methods
Cell culture
Human PDLSCs provided by Shanghai Chuntest
Biotechnology Co., Ltd. were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (FBS; HyClone;
Cytiva) at 37˚C in an incubator with 5% CO2. To induce
osteogenic differentiation, PDLSCs were cultured in osteogenic
differentiation medium, which was composed of α-MEM (Sigma-Aldrich;
Merck KGaA) containing 10 mM β-glycerophosphate, 10% FBS, 50 mM
ascorbic acid and 10 nM dexamethasone for 14 days in a 37˚C
incubator with 5% CO2, as previously described (27).
Treatment protocol
LPS (10 µg/ml; Sigma-Aldrich; Merck KGaA) was used
to induce PDLSCs for 24 h at 37˚C to simulate the inflammatory
microenvironment of periodontitis, as previously described
(21). The biological source of
LPS was Porphyromonas gingivalis. For baricitinib-treated
groups, PDLSCs were treated with baricitinib (1.0, 2.5 and 5.0 µM;
ChemScene) for 24 h at 37˚C in the presence or absence of LPS
(24). In addition, PDLSCs were
pretreated with RO8191 (20 µM; MedChemExpress), an agonist of the
JAK/signal transducer and activator of transcription (STAT)
pathway, for 1 h at 37˚C to investigate the regulatory effect of
baricitinib on this signaling pathway, as previously described
(28).
Cell viability assay
The viability of PDLSCs was evaluated by Cell
Counting Kit-8 (CCK-8) assay (Shanghai Yeasen Biotechnology Co.,
Ltd.) A total of 5x103 PDLSCs were seeded in a 96-well
plate. LPS or baricitinib was employed to treat PDLSCs 24 h prior
to addition of CCK-8 solution (10 µl). Following incubation for
another 4 h, a microplate reader (Molecular Devices, LLC) was
applied to record the optical density values at 450 nm.
Assessment of intracellular reactive
oxygen species (ROS)
The 2,7-dichlorodihydrofluorescein diacetate
(DCFH-DA) probe is a commonly used probe for detecting
intracellular ROS (29). PDLSCs
were seeded in 96-well plates at a density of 2x104
cells/well. Following treatment with LPS, baricitinib or RO8191 and
PDLSCs were incubated with 10 µM DCFH-DA (Beyotime Institute of
Biotechnology) for 20 min at 37˚C. An inverted fluorescence
microscope (Olympus Corporation) (magnification, x400) was used to
measure the fluorescence intensity corresponding to ROS levels from
three random fields.
Determination of superoxide dismutase
(SOD) activity and glutathione (GSH) content
The activity of SOD and content of GSH in the cell
supernatant was evaluated by the SOD assay kit (cat. no. A001-3-2)
and GSH assay kit (cat. no. A006-2-1) supplied by Nanjing Jiancheng
Bioengineering Institute according to the manufacturer's protocol.
The absorbance was detected using a microplate reader (Molecular
Devices, LLC) at a wavelength of 450 and 420 nm.
Detection of inflammatory factors
The protein levels of TNF-α, IL-1β and IL-6 in cell
supernatant was assessed by human TNF-α ELISA kit (cat. no.
F02810), human IL-1β ELISA kit (cat. no. F01220) and human IL-6
ELISA kit (cat. no. F01310) (Shanghai Xitang Biotechnology Co.,
Ltd.) according to the manufacturer's instructions. The optical
density values were determined at 450 nm by a microplate reader
(Molecular Devices, LLC).
Assessment of alkaline phosphatase
(ALP) activity
Following incubation at 37˚C in osteogenic medium
for 14 days, ALP activity of the PDLSCs was assessed by an ALP
assay kit (cat. no. A059-2-1; Nanjing Jiancheng Biotechnology
Institute) according to the manufacturer's instructions. The
optical density was detected at 520 nm using a microplate reader
(Molecular Devices, LLC).
Alizarin red staining
The mineralization potential of PDLSCs was
determined using alizarin red staining after cells were cultured at
37˚C seeded into a 24-well plate at a density of 250 cells/well in
the osteogenic medium with or without LPS, baricitinib or RO8191
for 14 days. PDLSCs were incubated with 4% paraformaldehyde for 30
min at 4˚C, followed by staining with 1% alizarin red solution
(Sigma-Aldrich; Merck KGaA) for 15 min at 37˚C. Following washing
three times with PBS, the images were captured by a light
microscope (magnification, x200; Olympus Corporation) with three
fields for each group. To quantify mineralization, calcium deposits
were desorbed using 10% cetylpyridinium chloride (Sigma-Aldrich;
Merck KGaA) for 30 min at room temperature and the optical density
value was detected using a plate reader at 562 nm (Molecular
Devices, LLC).
Reverse transcription-quantitative
PCR
Total RNA was isolated from PDLSCs with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RT was performed to generate complementary DNA by
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions. The detection of mRNA
expression was performed in the ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using the QuantiNova
SYBR Green PCR kit (Qiagen GmbH). The thermocycling conditions were
as follows: Initial denaturation at 95˚C for 5 min, followed by 40
cycles of denaturation for 15 sec at 95˚C and annealing for 30 sec
at 60˚C. The primer sequences were as follows: TNF-α forward,
5'-CCTCTCTCTAATCAGCCCTCTG-3' and reverse,
5'-GAGGACCTGGGAGTAGATGAG-3'; IL-1β forward,
5'-ATGATGGCTTATTACAGTGGCAA-3' and reverse,
5'-GTCGGAGATTCGTAGCTGGA-3'; IL-6 forward,
5'-ACTCACCTCTTCAGAACGAATTG-3' and reverse,
5'-CCATCTTTGGAAGGTTCAGGTTG-3' and GAPDH forward,
5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse,
5'-GGCTGTTGTCATACTTCTCATGG-3'. The calculation of relative mRNA
expression was performed with the 2-ΔΔCq method
(30). Primers were synthesized by
Shanghai GenePharma Co., Ltd. GAPDH was utilized as an endogenous
control.
Western blot analysis
PDLSCs were lysed in RIPA lysis buffer (Beyotime
Institute of Biotechnology) to extract the total protein, which was
quantified using a bicinchoninic protein assay kit (Beyotime
Institute of Biotechnology). Protein separation was performed using
10% SDS-PAGE (30 µg/lane). The separated proteins were transferred
to PVDF membranes. The blots were blocked with 5% non-fat dry milk
at room temperature for 2 h, followed by incubation with primary
antibodies including p-JAK1 (1:1,000; cat. no. ab138005; Abcam),
JAK1 (1:1,000; cat. no. ab133666; Abcam), p-JAK2 (1:1,000; cat. no.
ab32101; Abcam), JAK2 (1:5,000; cat. no. ab108596; Abcam), p-STAT3
(1:1,000; cat. no. ab68153; Abcam), STAT3 (1:1,000; cat. no.
ab267373; Abcam), OCN (1:1,000; cat. no. ab133612; Abcam), RUNX2
(1:1,000; cat. no. ab236639; Abcam) and GAPDH (1:2,500; cat. no.
ab9485; Abcam) overnight at 4˚C. Subsequently, membranes were
labeled with a horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat. no. ab205718; Abcam) for 1 h at room temperature.
The bands were visualized using an ECL kit (Beyotime Institute of
Biotechnology) and detection system (MilliporeSigma). The band
densities were evaluated using ImageJ software (v1.8.0; National
Institutes of Health).
Statistical analysis
All experiments were repeated independently in
triplicate and data are expressed as the mean ± standard deviation.
GraphPad Prism 6.0 (GraphPad Software, Inc.) was used for data
analysis. The level of significance was analyzed by one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Baricitinib increases viability of
PDLSCs exposed to LPS
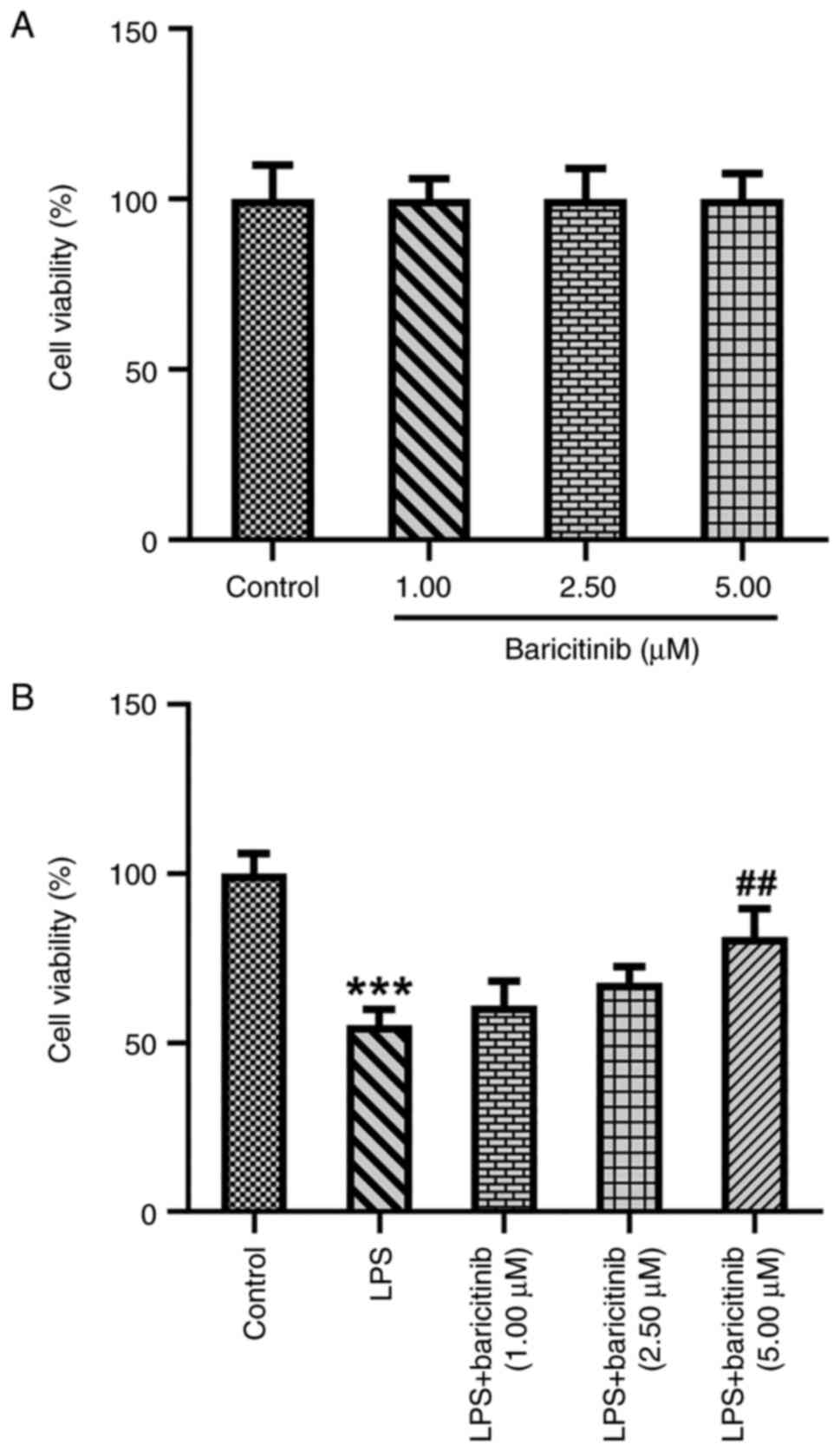
Firstly, cell viability was evaluated by CCK-8 assay
following treatment of PDLSCs with baricitinib (1.0, 2.5 and 5.0
µM). Baricitinib treatment exhibited no significant effect on the
viability of PDLSCs compared with the control group (Fig. 1A). PDLSCs were stimulated by LPS
with or without baricitinib. LPS treatment led to a significant
decrease in PDLSC viability compared with the control group
(Fig. 1B). Baricitinib
dose-dependently increased the viability of PDLSCs following LPS
treatment and PDLSCs viability was significantly exacerbated when
treated by 5 µM baricitinib upon exposure to LPS. These results
demonstrated that baricitinib reversed the LPS-induced decrease in
viability of PDLSCs.
Baricitinib alleviates LPS-induced
oxidative stress and inflammation in PDLSCs
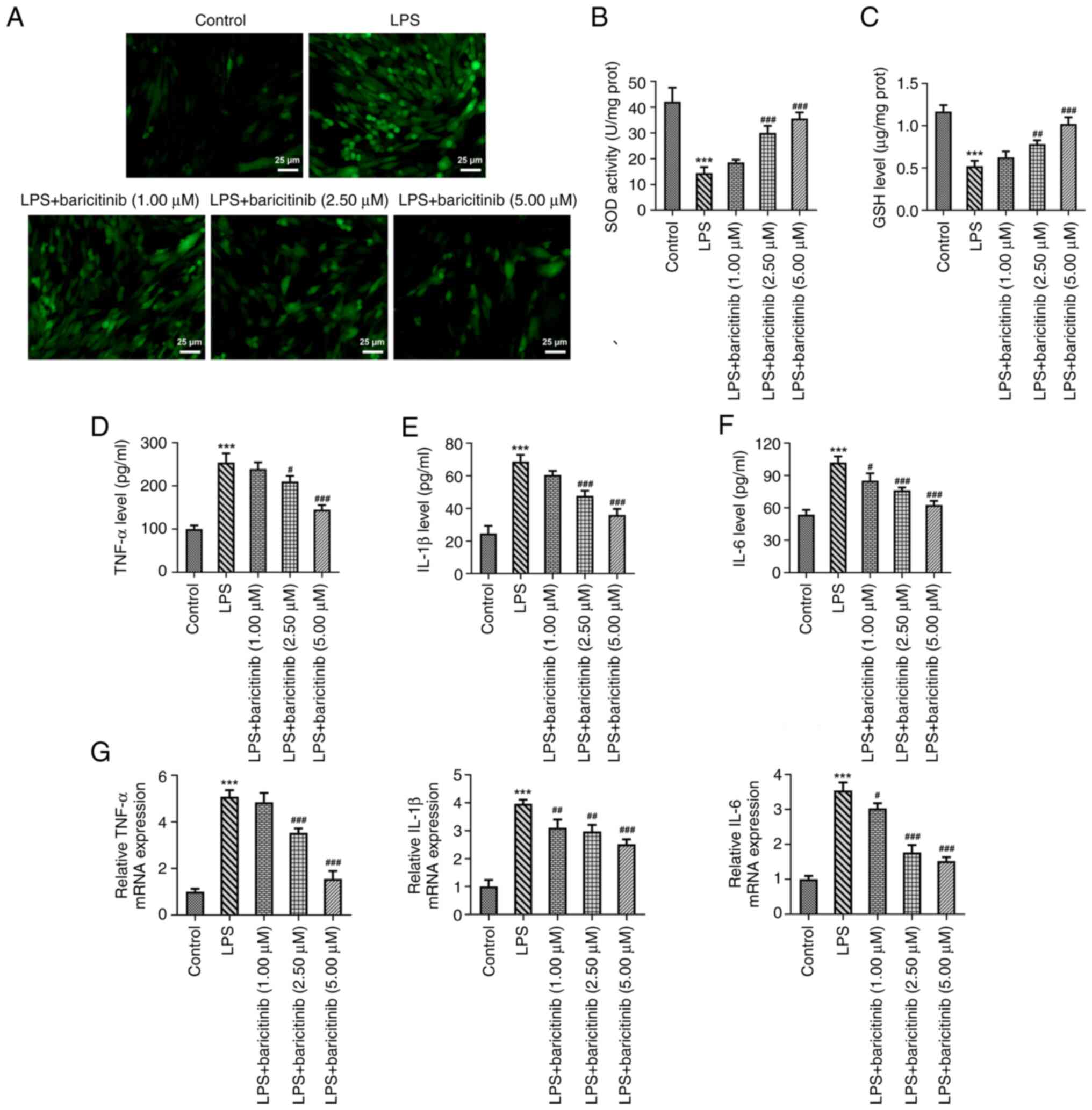
The levels of intracellular ROS were evaluated using
DCFH-DA as a probe; fluorescence intensity reflected the content of
ROS. The fluorescence intensity in the LPS group was markedly
enhanced compared with that of the control group (Fig. 2A). However, baricitinib decreased
the fluorescence intensity induced by LPS in a
concentration-dependent manner. Moreover, decreased SOD activity
and GSH content caused by the LPS challenge gradually recovered
following baricitinib treatment (Fig.
2B and C). In addition, levels
of TNF-α, IL-1β, and IL-6 were elevated following incubation of
PDLSCs with LPS (Fig. 2D-F).
Additional treatment with baricitinib reversed the impact of LPS on
expression levels of the aforementioned inflammatory factors in a
concentration-dependent manner. Consistently, elevated mRNA
expression levels of these inflammatory factors (TNF-α, IL-1β and
IL-6) induced by LPS were also decreased following baricitinib
treatment (Fig. 2G). These data
provide evidence that baricitinib alleviated LPS-induced oxidative
stress and inflammation in PDLSCs.
Baricitinib promotes osteogenic
differentiation in LPS-stimulated PDLSCs
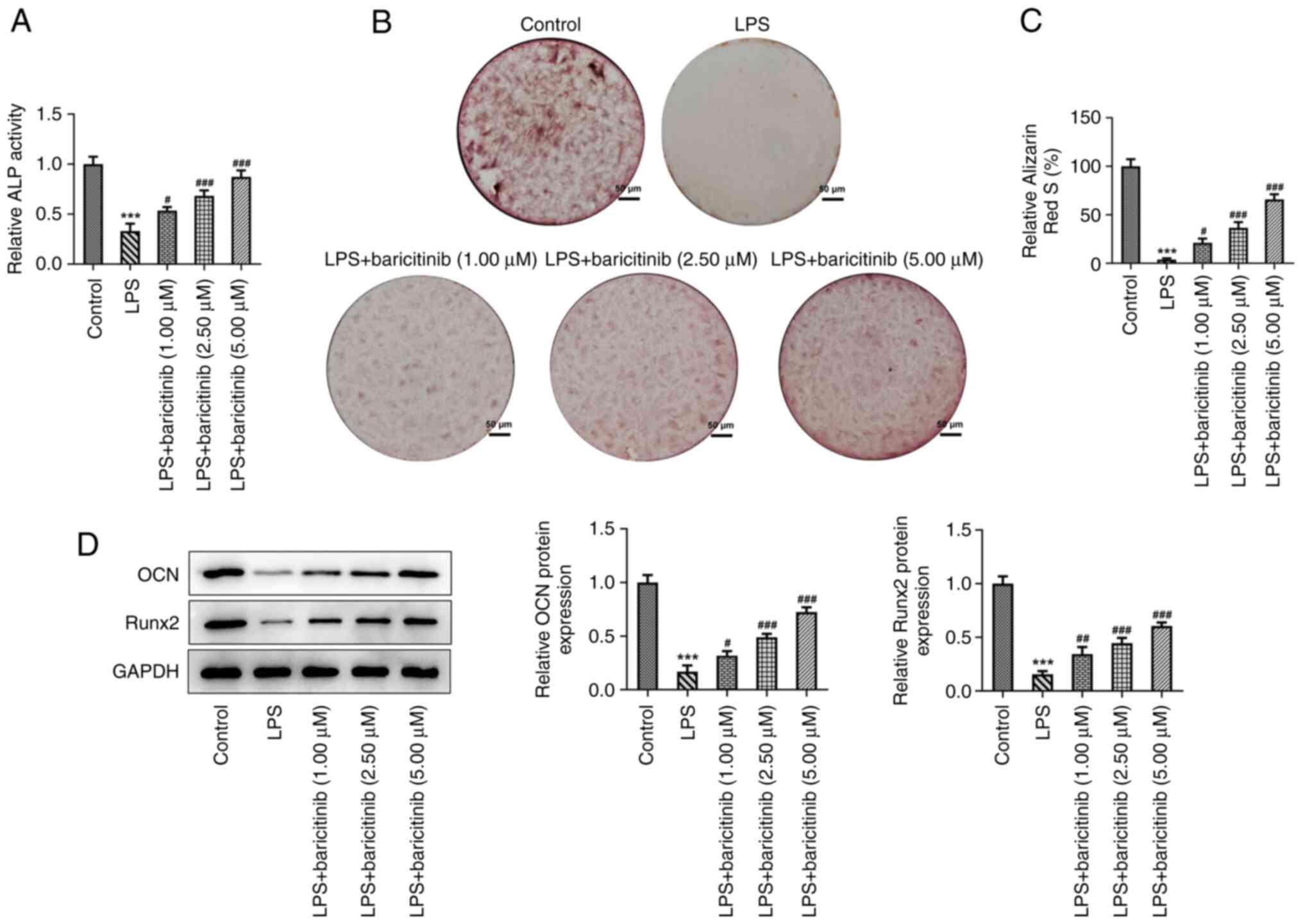
The effect of baricitinib on osteogenic
differentiation of PDLSCs challenged with LPS was investigated. ALP
activity was significantly decreased in LPS group compared with
that of the control group (Fig.
3A). By contrast, baricitinib dose-dependently increased ALP
activity relative to the LPS group. The results of alizarin red
staining indicated that the mineralization degree of PDLSCs was
enhanced in the control group; this effect was reduced following
stimulation of the cells with LPS (Fig. 3B and C). Furthermore, in the presence of
baricitinib, the mineralization ability of PDLSCs increased in a
dose-dependent manner. Moreover, the expression levels of proteins
related to osteogenic differentiation, including osteocalcin (OCN)
and Runt-related transcription factor (Runx) 2, were downregulated
following treatment with LPS compared with the control group, as
determined by western blot analysis. This effect was reversed and
the levels of OCN and Runx2 were upregulated following subsequent
treatment of the cells with baricitinib (Fig. 3D). These observations revealed that
baricitinib promoted the osteogenic differentiation of PDLSCs
stimulated by LPS.
Baricitinib inactivates the JAK/STAT
signaling pathway in LPS-stimulated PDLSCs
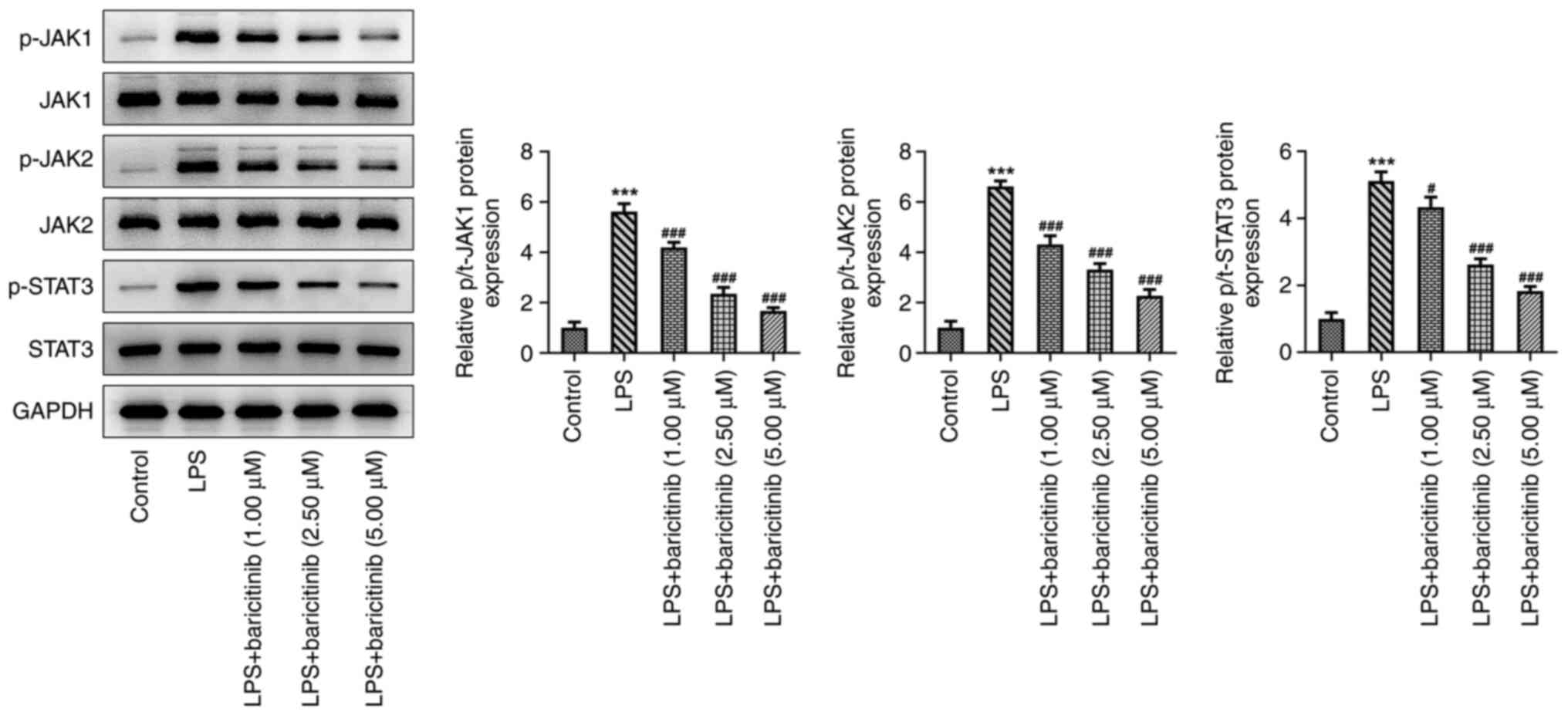
To investigate the underlying mechanism by which
baricitinib regulates the process of PDLSC injury and osteogenic
differentiation triggered by LPS, expression of specific proteins
involved in the JAK/STAT pathway was assessed using western blot
analysis. The expression levels of phosphorylated (p)-JAK1, p-JAK2
and p-STAT3 were significantly increased in the LPS group compared
with the control group (Fig. 4).
By contrast, baricitinib caused a gradual downregulation in the
expression levels of these proteins in a dose-dependent manner; the
highest inhibitory effect was noted in the 5 µM-treated group.
Thereafter, 5 µM baricitinib was applied to the subsequent
experiments. Collectively, these data suggested that baricitinib
suppressed the JAK/STAT signaling pathway in LPS-induced
PDLSCs.
Baricitinib improves LPS-induced
oxidative stress and inflammation in PDLSCs by inhibiting JAK/STAT
signaling
To clarify the regulatory effect of baricitinib on
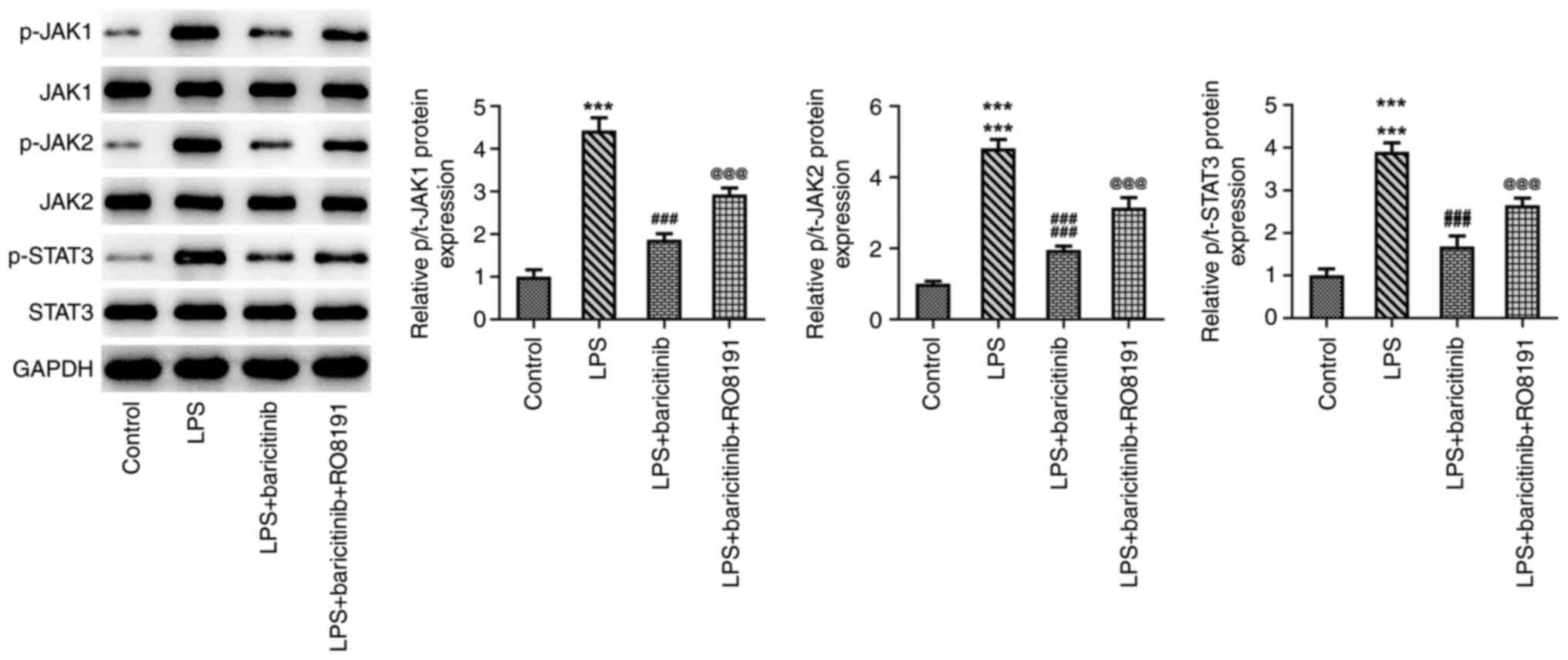
the JAK/STAT signaling pathway in LPS-stimulated PDLSCs, RO8191, an
agonist of the JAK/STAT pathway, was used to treat PDLSCs. RO8191
addition significantly upregulated p-JAK1, p-JAK2 and p-STAT3
expression in PDLSCs treated with LPS and baricitinib compared with
the LPS +Baricitinib group (Fig.
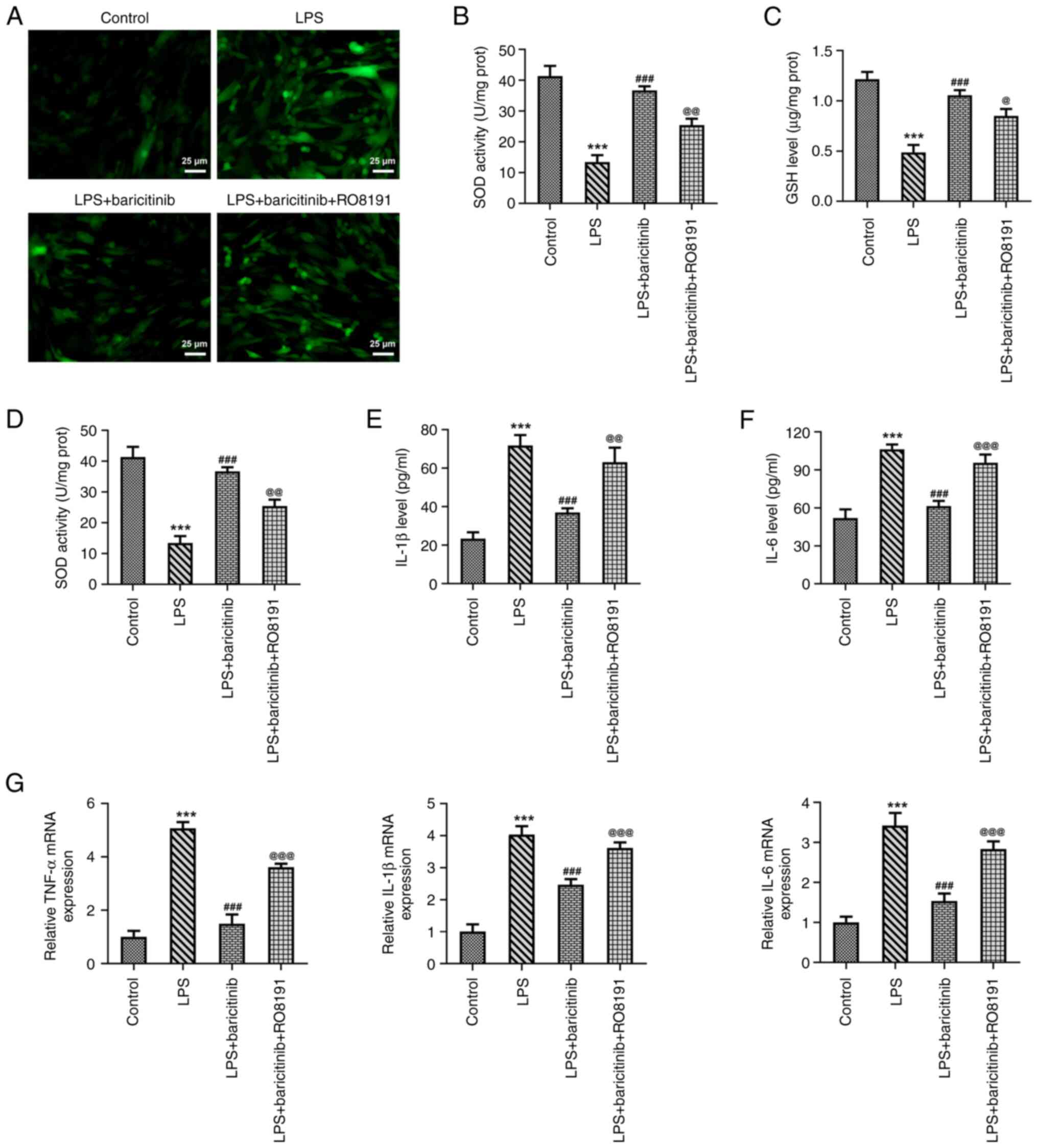
5). Subsequently, levels of oxidative stress and inflammation
were evaluated. RO8191 significantly increased ROS levels compared
with those noted in the LPS + baricitinib group, as determined by
the decreased SOD activity and GSH content (Fig. 6A-C). Moreover, the decline in the
TNF-α, IL-1β and IL-6 levels induced by baricitinib was
significantly reversed following RO8191 treatment (Fig. 6D-G). These findings confirmed that
baricitinib exerted antioxidant and anti-inflammatory effects in
LPS-stimulated PDLSCs by inhibiting JAK/STAT signaling.
Baricitinib promotes osteogenic
differentiation of PDLSCs challenged with LPS by inhibiting
JAK/STAT signaling
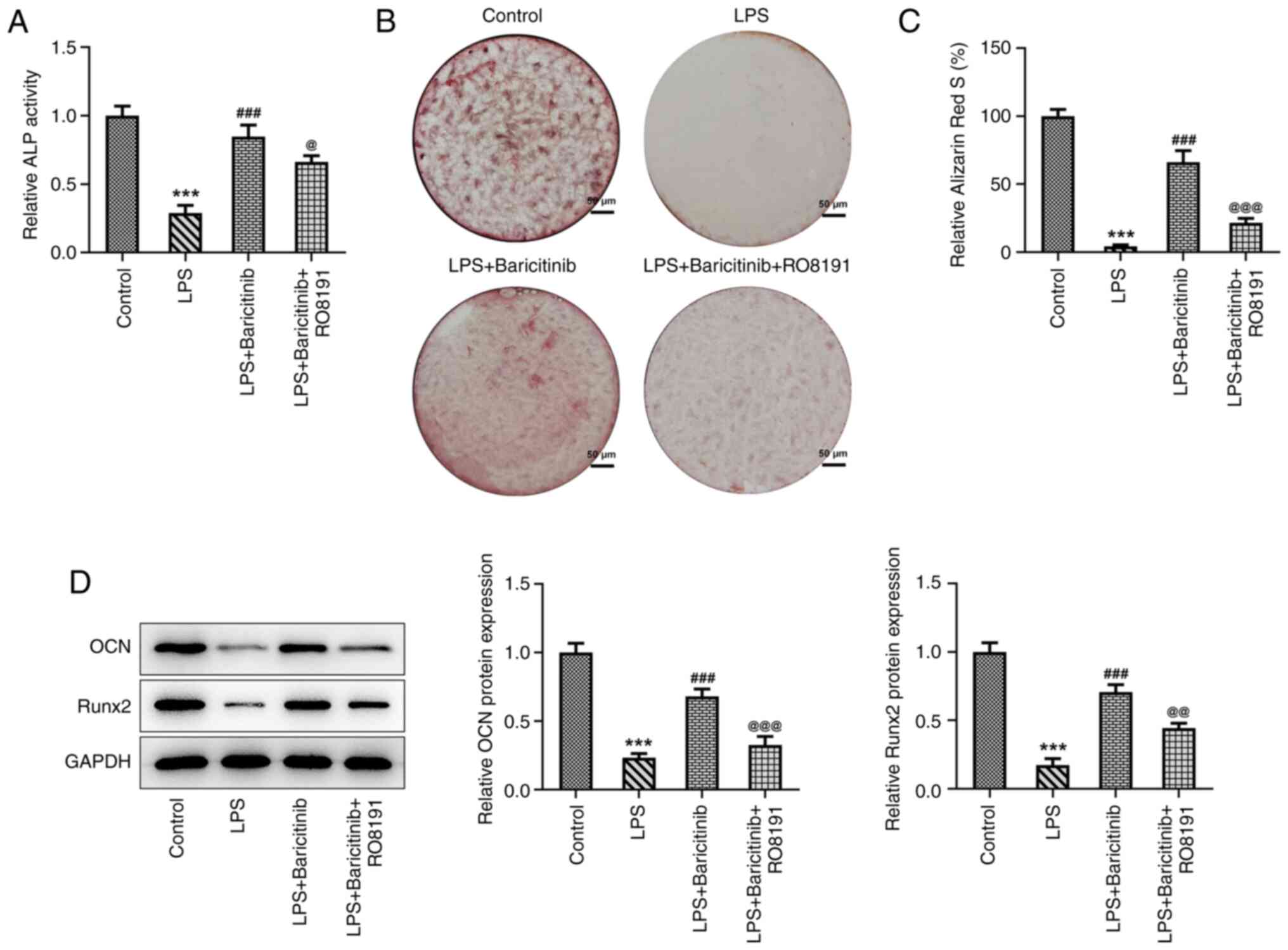
Subsequently, osteogenic differentiation of
LPS-stimulated PDLSCs was assessed with or without baricitinib or
RO8191. The results indicated that addition of RO8191 led to a
significant decrease in ALP activity compared with that in the LPS
+ baricitinib group (Fig. 7A).
Alizarin red staining suggested that the mineralization degree of
PDLSCs was significantly decreased following addition of RO8191 in
PDLSCs co-treated with LPS and baricitinib (Fig. 7B and C). Concomitantly, significant
downregulation of OCN and Runx2 expression was observed in the LPS
+ baricitinib + RO8191 group compared with the LPS + baricitinib
group (Fig. 7D). In summary, these
results revealed that baricitinib promoted osteogenic
differentiation of PDLSCs exposed to LPS by inhibiting JAK/STAT
signaling.
Discussion
Periodontitis is a non-infectious chronic
inflammatory disease that is primarily induced by microbial attack
and affects tooth support structures. This condition is prevalent
worldwide and affects large populations with age-standardized
prevalence rate increased by 8.44% worldwide from 1990 to 2019
(1,31,32).
Due to excellent osteogenic capacity, PDLSCs serve a crucial role
in maintaining periodontal bone mass (33). However, the inflammatory
microenvironment suppresses the osteogenic capacity of PDLSCs,
which is also an important pathogenic feature of periodontitis
(34). Therefore, it is critical
to discover a novel drug that protects PDLSCs from
inflammation-associated injury and improves their functional
characteristics. The present study demonstrated the effects of
baricitinib on LPS-induced damage of PDLSCs and the decrease of
osteogenic differentiation.
Periodontitis is an inflammatory disease mediated by
immunization; LPS produced by dominant bacteria in gingival
crevices serves an indispensable role in the occurrence and
progression of this condition (35). Exposure to LPS leads to increased
levels of certain inflammatory factors, including TNF-α, IL-1β and
IL-6, in PDLSCs (36). Numerous
studies have shown that LPS induces PDLSCs to produce and
accumulate a large amount of ROS, which disrupts the balance
between oxidative and antioxidant systems, resulting in cell and
tissue damage (37,38). SOD and GSH are considered specific
parameters of oxidative stress. A significant increase in the
levels of ROS and expression levels of TNF-α, IL-1β and IL-6, and a
decrease in the levels of SOD and GSH, were noted in PDLSCs
following stimulation with LPS, which was consistent with previous
studies (36-38).
As a U.S Food and Drug Administration-approved oral JAK1/2
inhibitor, baricitinib has been used to treat moderate to extreme
rheumatoid arthritis (39). A
clinical study demonstrated that clinical manifestations and
inflammatory biomarkers were notably improved in patients with
autoinflammatory interferonopathy following baricitinib treatment
(40). Baricitinib is reported to
inhibit tetradecanoylphorbol-13-acetate-induced psoriasis and skin
inflammation in a mouse model (41). Baricitinib improves the periodontal
status of patients with rheumatoid arthritis and decreases the
inflammatory response in the periodontal tissue (23). In the present study, the
antioxidant and anti-inflammatory effects of baricitinib were
verified in LPS-induced PDLSCs, suggesting the potential
application of this compound in the treatment of periodontitis.
Subsequently, the effects of baricitinib on
osteogenic differentiation of PDLSCs incubated with LPS were
analyzed by evaluating the expression of several osteogenic
differentiation markers and mineralization in PDLSCs. ALP is an
early marker of osteogenic differentiation and a key hydrolase in
cellular osteogenic differentiation (42). The increase in the activity of ALP
reflects maturation of osteogenic differentiation (43). Alizarin red staining forms
mineralized nodules through the specific binding of alizarin red S
to calcium ions, which indicates mineralization capacity of cells
(44). OCN, a non-collagen protein
abundant in the bone tissue, is a specific indicator of bone
metabolism and osteocyte activity; its expression levels reflect
the rate of bone formation (45).
Runx2 is a key gene of bone formation and an important indicator of
osteogenic differentiation of mesenchymal stem cells (46). Baricitinib can increase bone mass
in steady-state conditions and ameliorate pathological bone loss by
stimulating osteoblast function (25). Murakami et al (24) demonstrated that baricitinib
inhibits osteoclast formation in vitro by suppressing the
expression of RANKL. The present results indicated that under the
treatment of baricitinib, levels of ALP, Runx2 and OCN and
mineralization degree were elevated, which confirmed that
baricitinib promoted osteogenic differentiation of PDLSCs,
highlighting its potential to treat periodontitis.
To investigate the underlying mechanism of
baricitinib on the impact of LPS-stimulated PDLSCs, expression
levels of proteins involved in the JAK/STAT pathway were assessed.
A previous study revealed that Porphyromonas gingivalis
activates the JAK/STAT signaling pathway and enhances ROS
accumulation, thereby promoting the occurrence and development of
periodontitis (47).
Downregulation of Toll-like receptor 4 expression and the
JAK1/STAT3 signaling pathway can improve diabetic periodontitis in
a mouse model (48). Zheng et
al (26) revealed that
inhibition of JAK/STAT signaling suppresses progression of chronic
periodontitis. Here, upregulation of p-JAK1, JAK2 and STAT3
expression in PDLSCs induced by LPS was inhibited by baricitinib.
Subsequent addition of RO8191, an agonist of the JAK/STAT pathway,
abolished the protective effects of baricitinib on LPS-triggered
oxidative stress, inflammation and loss of osteogenic
differentiation, suggesting that this compound alleviated
LPS-induced PDLSC injury and promoted osteogenic differentiation by
inhibiting JAK/STAT signaling.
The present study demonstrated that baricitinib,
with its antioxidant, anti-inflammatory and pro-osteogenic
differentiation effects, may be a promising agent in protecting
PDLSCs from periodontitis-induced injury. The present study may
provide insight into the use of baricitinib for the treatment of
periodontitis. Further in vivo animal studies will be
performed in the future to confirm the effects of baricitinib on
periodontitis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PY and FS designed the study. PY, FS and YZ
performed the experiments and analyzed the data. PY drafted the
manuscript and interpreted the data. YZ revised the manuscript for
important intellectual content. All authors have read and approved
the final manuscript. PY and YZ confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen MX, Zhong YJ, Dong QQ, Wong HM and
Wen YF: Global, regional, and national burden of severe
periodontitis, 1990-2019: An analysis of the global burden of
disease study 2019. J Clin Periodontol. 48:1165–1188.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Socransky SS, Haffajee AD, Cugini MA,
Smith C and Kent RL Jr: Microbial complexes in subgingival plaque.
J Clin Periodontol. 25:134–144. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Byrne SJ, Dashper SG, Darby IB, Adams GG,
Hoffmann B and Reynolds EC: Progression of chronic periodontitis
can be predicted by the levels of Porphyromonas gingivalis and
Treponema denticola in subgingival plaque. Oral Microbiol Immunol.
24:469–477. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Papapanou PN, Sanz M, Buduneli N, Dietrich
T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani
F, et al: Periodontitis: Consensus report of workgroup 2 of the
2017 world workshop on the classification of periodontal and
peri-implant diseases and conditions. J Periodontol. 89 (Suppl
1):S173–S182. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hajishengallis G: Periodontitis: From
microbial immune subversion to systemic inflammation. Nat Rev
Immunol. 15:30–44. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Polak D and Shapira L: An update on the
evidence for pathogenic mechanisms that may link periodontitis and
diabetes. J Clin Periodontol. 45:150–166. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wallace K, Shafique S and Piamjariyakul U:
The relationship between oral health and hemodialysis treatment
among adults with chronic kidney disease: A systematic review.
Nephrol Nurs J. 46:375–394. 2019.PubMed/NCBI
|
|
8
|
Khumaedi AI, Purnamasari D, Wijaya IP and
Soeroso Y: The relationship of diabetes, periodontitis and
cardiovascular disease. Diabetes Metab Syndr. 13:1675–1678.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Trikka D and Vassilopoulos S: Periodontal
regeneration with enamel matrix derivative in the management of
generalized aggressive periodontitis: A case report with 11-year
follow-up and literature review. J Int Soc Prev Community Dent.
9:13–20. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bella P and Istvan G: The comprehensive
periodontal, resorative end prosthodontic therapy of chronic
periodontitis case presentation. Fogorv Sz. 109:125–135.
2016.PubMed/NCBI(In English, Hungarian).
|
|
11
|
Ashouri Moghaddam A, Radafshar G,
Jahandideh Y and Kakaei N: Clinical evaluation of effects of local
application of aloe vera gel as an adjunct to scaling and root
planning in patients with chronic periodontitis. J Dent (Shiraz).
18:165–172. 2017.PubMed/NCBI
|
|
12
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aydin S and Şahin F: Stem cells derived
from dental tissues. Adv Exp Med Biol. 1144:123–132.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Zheng Y, Ding G, Fang D, Zhang C,
Bartold PM, Gronthos S, Shi S and Wang S: Periodontal ligament stem
cell-mediated treatment for periodontitis in miniature swine. Stem
Cells. 26:1065–1073. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nagata M, Iwasaki K, Akazawa K, Komaki M,
Yokoyama N, Izumi Y and Morita I: Conditioned medium from
periodontal ligament stem cells enhances periodontal regeneration.
Tissue Eng Part A. 23:367–377. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Diomede F, D'Aurora M, Gugliandolo A,
Merciaro I, Ettorre V, Bramanti A, Piattelli A, Gatta V, Mazzon E,
Fontana A and Trubiani O: A novel role in skeletal segment
regeneration of extracellular vesicles released from
periodontal-ligament stem cells. Int J Nanomed. 13:3805–3825.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Trubiani O, Marconi GD, Pierdomenico SD,
Piattelli A, Diomede F and Pizzicannella J: Human oral stem cells,
biomaterials and extracellular vesicles: A promising tool in bone
tissue repair. Int J Mol Sci. 20(4987)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramenzoni LL, Russo G, Moccia MD, Attin T
and Schmidlin PR: Periodontal bacterial supernatants modify
differentiation, migration and inflammatory cytokine expression in
human periodontal ligament stem cells. PLoS One.
14(e0219181)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cheng M and Zhou Q: Targeting EZH2
ameliorates the LPS-inhibited PDLSC osteogenesis via Wnt/β-catenin
pathway. Cells Tissues Organs. 209:227–235. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang W, Yuan C, Geng T, Liu Y, Zhu S,
Zhang C, Liu Z and Wang P: Lipopolysaccharide inhibits osteogenic
differentiation of periodontal ligament stem cells partially
through toll-like receptor 4-mediated ephrinB2 downregulation. Clin
Oral Investig. 24:3407–3416. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fu Z, Wang X, Li B and Tang Y:
Fraxinellone alleviates inflammation and promotes osteogenic
differentiation in lipopolysaccharide-stimulated periodontal
ligament stem cells by regulating the bone morphogenetic protein
2/Smad pathway. Arch Oral Biol. 121(104927)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Honda S and Harigai M: The safety of
baricitinib in patients with rheumatoid arthritis. Expert Opin Drug
Saf. 19:545–551. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ancuța C, Pomîrleanu C, Mihailov C,
Chirieac R, Ancuța E, Iordache C, Bran C and Țănculescu O: Efficacy
of baricitinib on periodontal inflammation in patients with
rheumatoid arthritis. Joint Bone Spine. 87:235–239. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Murakami K, Kobayashi Y, Uehara S, Suzuki
T, Koide M, Yamashita T, Nakamura M, Takahashi N, Kato H, Udagawa N
and Nakamura Y: A Jak1/2 inhibitor, baricitinib, inhibits
osteoclastogenesis by suppressing RANKL expression in osteoblasts
in vitro. PLoS One. 12(e0181126)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Adam S, Simon N, Steffen U, Andes FT,
Scholtysek C, Müller DIH, Weidner D, Andreev D, Kleyer A, Culemann
S, et al: JAK inhibition increases bone mass in steady-state
conditions and ameliorates pathological bone loss by stimulating
osteoblast function. Sci Transl Med. 12(eaay4447)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zheng XY, Mao CY, Qiao H, Zhang X, Yu L,
Wang TY and Lu EY: Plumbagin suppresses chronic periodontitis in
rats via down-regulation of TNF-α, IL-1β and IL-6 expression. Acta
Pharmacol Sin. 38:1150–1160. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shao Q, Liu S, Zou C and Ai Y: Effect of
LSD1 on osteogenic differentiation of human periodontal ligament
stem cells in periodontitis. Oral Dis: Nov 5, 2021 (Epub ahead of
print).
|
|
28
|
Zheng X, Zhu Y, Wang X, Hou Y and Fang Y:
Silencing of ITGB6 inhibits the progression of cervical carcinoma
via regulating JAK/STAT3 signaling pathway. Ann Transl Med.
9(803)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Eruslanov E and Kusmartsev S:
Identification of ROS using oxidized DCFDA and flow-cytometry.
Methods Mol Biol. 594:57–72. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sanz M, Marco Del Castillo A, Jepsen S,
Gonzalez-Juanatey JR, D'Aiuto F, Bouchard P, Chapple I, Dietrich T,
Gotsman I, Graziani F, et al: Periodontitis and cardiovascular
diseases: Consensus report. J Clin Periodontol. 47:268–288.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lundmark A, Hu YOO, Huss M, Johannsen G,
Andersson AF and Yucel-Lindberg T: Identification of salivary
microbiota and its association with host inflammatory mediators in
periodontitis. Front Cell Infect Microbiol. 9(216)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Menicanin D, Mrozik KM, Wada N, Marino V,
Shi S, Bartold PM and Gronthos S: Periodontal-ligament-derived stem
cells exhibit the capacity for long-term survival, self-renewal,
and regeneration of multiple tissue types in vivo. Stem Cells Dev.
23:1001–1011. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li L, Liu W, Wang H, Yang Q, Zhang L, Jin
F and Jin Y: Mutual inhibition between HDAC9 and miR-17 regulates
osteogenesis of human periodontal ligament stem cells in
inflammatory conditions. Cell Death Dis. 9(480)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shirasugi M, Nakagawa M, Nishioka K,
Yamamoto T, Nakaya T and Kanamura N: Relationship between
periodontal disease and butyric acid produced by periodontopathic
bacteria. Inflamm Regen. 38(23)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hoare A, Soto C, Rojas-Celis V and Bravo
D: Chronic inflammation as a link between periodontitis and
carcinogenesis. Mediators Inflamm. 2019(1029857)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhao B, Zhang W, Xiong Y, Zhang Y, Zhang D
and Xu X: Effects of rutin on the oxidative stress, proliferation
and osteogenic differentiation of periodontal ligament stem cells
in LPS-induced inflammatory environment and the underlying
mechanism. J Mol Histol. 51:161–171. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Duan Y, An W, Wu H and Wu Y: Salvianolic
acid C attenuates LPS-induced inflammation and apoptosis in human
periodontal ligament stem cells via toll-like receptors 4
(TLR4)/nuclear factor kappa B (NF-κB) pathway. Med Sci Monit.
25:9499–9508. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Smolen JS, Genovese MC, Takeuchi T, Hyslop
DL, Macias WL, Rooney T, Chen L, Dickson CL, Riddle Camp J,
Cardillo TE, et al: Safety profile of baricitinib in patients with
active rheumatoid arthritis with over 2 years median time in
treatment. J Rheumatol. 46:7–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sanchez GAM, Reinhardt A, Ramsey S,
Wittkowski H, Hashkes PJ, Berkun Y, Schalm S, Murias S, Dare JA,
Brown D, et al: JAK1/2 inhibition with baricitinib in the treatment
of autoinflammatory interferonopathies. J Clin Invest.
128:3041–3052. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bhaskarmurthy DH and Prince SE: Effect of
baricitinib on TPA-induced psoriasis like skin inflammation. Life
Sci. 279(119655)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Douglas TEL, Vandrovcová M, Kročilová N,
Keppler JK, Zárubová J, Skirtach AG and Bačáková L: Application of
whey protein isolate in bone regeneration: Effects on growth and
osteogenic differentiation of bone-forming cells. J Dairy Sci.
101:28–36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jiang B, Xu J, Zhou Y, Mao J, Guan G, Xu X
and Mei L: Estrogen enhances osteogenic differentiation of human
periodontal ligament stem cells by activating the Wnt/β-catenin
signaling pathway. J Craniofac Surg. 31:583–587. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yu M, Wang L, Ba P, Li L, Sun L, Duan X,
Yang P, Yang C and Sun Q: Osteoblast progenitors enhance osteogenic
differentiation of periodontal ligament stem cells. J Periodontol.
88:e159–e168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sun H, Feng K, Hu J, Soker S, Atala A and
Ma PX: Osteogenic differentiation of human amniotic fluid-derived
stem cells induced by bone morphogenetic protein-7 and enhanced by
nanofibrous scaffolds. Biomaterials. 31:1133–1139. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Deng Y, Wu S, Zhou H, Bi X, Wang Y, Hu Y,
Gu P and Fan X: Effects of a miR-31, Runx2, and Satb2 regulatory
loop on the osteogenic differentiation of bone mesenchymal stem
cells. Stem Cells Dev. 22:2278–2286. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li J, Li L, Wang X and Xiao L:
Porphyromonas gingivalis inhibition of MicroRNA-205-5p expression
modulates proinflammatory cytokines in gingival epithelial cells.
Biochem Genet. 58:566–579. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang Q, Li H, Xie H, Fu M, Guo B, Ding Y,
Li W and Yu H: 25-Hydroxyvitamin D3 attenuates experimental
periodontitis through downregulation of TLR4 and JAK1/STAT3
signaling in diabetic mice. J Steroid Biochem Mol Biol. 135:43–50.
2013.PubMed/NCBI View Article : Google Scholar
|