Introduction
The intestinal mucosal barrier comprises epithelial
cells, mucus, immune cells in the lamina propria, and commensal
microorganisms. Epithelial cells are tightly bound together via
intercellular junctional structures such as tight junctions (TJ).
TJ are composed of transmembrane proteins including claudins and
cytosolic scaffolding proteins including zonula occludens-1 (ZO-1).
Claudin-1, a member of the claudin protein family, is widely
expressed in the intestinal epithelia and regulates the
paracellular permeability (1,2).
ZO-1 interacts with claudins and cytoskeletal actin and plays an
important role in TJ assembly (3).
Additionally, mucus that covers the epithelia contains several
antibacterial peptides and functions as a physical barrier. The
commensal microbiota and host immune cells interact with each
other, which is essential for protection against invading
pathogenic bacteria and toxins (4). Importantly, the function of the
intestinal barrier is strongly influenced by dietary contents
(5). High-fat diet (HFD) increases
the intestinal permeability by inducing the expression of
barrier-disrupting cytokines and reducing mucus secretion (6). Moreover, growing evidence suggests
that HFD is associated with abnormal intestinal flora and gut
dysbiosis (5,7). A high-fat and high-sugar diet alters
the gut microbial structure in only 3.5 days (8). The host mucosal barrier disruption
occurs even more rapidly. The epithelial expression of ZO-1 has
been reported to decrease after 48 h of HFD consumption (9). Moreover, the number of intestinal
immune cells in mice has been observed to have significantly
decreased after 24 h of HFD consumption (10). These findings suggest that
HFD-induced intestinal damage occurs simultaneously with or prior
to the development of obesity-associated metabolic disorders. An
impaired intestinal barrier facilitates the invasion of enteric
microbe-derived antigens into blood vessels, and metabolic
endotoxemia modulates systemic inflammation (11). Therefore, proper functioning of the
gut barrier is critical to prevent the progression of metabolic
inflammation.
The leaves of indigo plants, such as Polygonum
tinctorium contain a wide variety of bioactive molecules.
Tryptanthrin and kaempferol, isolated from P. tinctorium,
possess antibacterial properties (12). Indirubin and its analogues exert
anti-tumor effects by inhibiting cyclin-dependent kinases (13). It was recently reported that P.
tinctorium leaves exerted anti-inflammatory effects by
modulating the interleukin (IL)-10-related pathway in a mouse model
of chemically induced colitis (14). However, the effectiveness of indigo
leaf extract in HFD-induced intestinal damage has not yet been
fully elucidated. Therefore, this study aimed to investigate the
effects of the indigo leaf extract on intestinal damage in HFD-fed
mice.
Materials and methods
Reagents
Rabbit polyclonal anti-ZO-1 (#21773-1-AP) and
anti-Claudin-1 (#28674-1-AP) antibodies were purchased from
Proteintech. The rabbit polyclonal anti-β-actin antibody (#4967)
was purchased from Cell Signaling Technology. Alexa Fluor
488-conjugated anti-rabbit IgG antibody (#A11008) and ProLong Gold
antifade regent with 4',6-diamidino-2-phenylindole (DAPI) were
purchased from Thermo Fisher Scientific. Alcian Blue (AB) solution
(pH2.5) and BCA protein assay kit were purchased from FUJIFILM Wako
Pure Chemical Co. Periodic acid-Schiff (PAS) stain kit and
NucleoSpin RNA kit were obtained from Muto Pure Chemicals Co. and
Macherey-Nagel GmbH and Co. KG, respectively. Moloney murine
leukemia virus (M-MLV) reverse transcriptase and oligo
(dT)18 primers were purchased from Invitrogen; Thermo
Fisher Scientific. Thunderbird™ Next SYBR®
qPCR mix was purchased from Toyobo (Osaka, Japan). Polyvinylidene
fluoride (PVDF) membranes and Luminata Crescendo Western HRP
substrate were purchased from Merck Millipore. Enzyme-linked
immunosorbent assay (ELISA) kit for IL-10 was obtained from
LSBio.
Preparation of indigo Ex
For the purpose of this study, P. tinctorium
was cultivated without pesticides in Aomori. A voucher specimen was
deposited in the herbarium of the Medical Herbal Garden of the
Tohoku Medical and Pharmaceutical University. The indigo Ex was
prepared as previously described (15). Briefly, the air-dried leaves were
powdered and extracted with d-limonene for 48 h. After
filtration, a pale-yellow extract was obtained. In this study, we
used the original indigo Ex AOMORI-BLUE (Japanese Patent no.
6389492). This extract is rich in the principal ingredient,
tryptanthrin (15). The stock
solution, diluted 10,000-fold with water, was provided by the
Aomoriai-Sangyo Co. This solution was diluted 50-fold with
phosphate-buffered saline (PBS) and filter sterilized through a
0.33 µm membrane.
Animal model
Forty-five male C57BL6/J mice (6-7 weeks old) were
purchased from CLEA Japan and maintained on a 12-12 h light/dark
cycle at 22˚C and 50% relative humidity in a specific pathogen-free
environment. After a week of acclimation, the mice were divided
into three groups (n=15 per group). One group was fed a normal chow
(NC) diet and injected intraperitoneally with 10 mg/kg of PBS daily
(NC/PBS group). The others were fed an HFD (High Fat Diet 32: 56.7%
of calories from fat, CLEA Japan) and injected intraperitoneally
with either PBS (HFD/PBS group) or indigo Ex (HFD/indigo Ex group).
Body weights were measured weekly, and after 4 weeks, the mice were
euthanized by cervical dislocation and the tissue samples were
collected. Blood samples obtained by cardiac puncture were
incubated for 30 min at room temperature. The sera were collected
by centrifugation at 3,000 rpm for 10 min at 4˚C, and the serum
levels of total cholesterol (T. Chol), triglycerides (TG), and
glucose were analyzed using a biochemical analyzer (SPOTCHEM EZ
SP-4430; Arkray). All animal experiments were conducted in
accordance with the Guidelines for Animal Experimentation of the
Hirosaki University.
Histological analysis
The colon, epididymal fat, and liver tissues
collected from the mice were fixed in 10% formalin and embedded in
paraffin. Sections (3-5 µm thick) were cut and conventional
hematoxylin and eosin (H&E) staining was performed for
morphological evaluation. Goblet cells were histologically assessed
by AB/PAS staining using a standard protocol.
Immunofluorescence (IF) staining
IF staining was performed to examine the epithelial
expression of TJ-associated proteins ZO-1 and Claudin-1. Tissue
sections were immersed in 10 mM citrate buffer (pH 6.0) and
heat-induced antigen retrieval was performed using an autoclave.
Blocking of the endogenous peroxidase of the tissue sections with
3% hydrogen peroxide was followed by that with 5% normal goat serum
for 1 h at room temperature. Thereafter, sections were incubated
with the anti-ZO-1 (1:250) or anti-Claudin-1 (1:250) antibody
overnight at 4˚C. After washing with PBS, the tissue sections were
incubated with Alexa Fluor 488-conjugated anti-rabbit IgG antibody
(1:250) for 1 h at room temperature. The slides were mounted with
ProLong Gold antifade reagent with DAPI, and visualized using a
confocal laser scanning microscope (C1si; Nikon).
Western blotting
Murine colon tissues were lysed with RIPA buffer
containing 0.2% proteinase inhibitor cocktail, and the lysates were
centrifuged at 12,000 rpm for 10 min at 4˚C. The supernatants were
collected, and equal amounts of the protein (15 mg) were loaded
onto a 10-20% sodium dodecyl sulfate (SDS) polyacrylamide gel for
electrophoresis. The separated proteins were transferred onto a
PVDF membrane. After blocking with Tris-buffered saline with Tween
20 (TBS-T, pH 7.4) containing 5% nonfat dry milk, the membranes
were incubated overnight at 4˚C with a primary antibody against
ZO-1 (1:1,000), Claudin-1 (1:1,000), or β-actin (1:2,000).
Membranes were then incubated with an HRP-conjugated secondary
antibody for 1 h at room temperature. Immunodetection was performed
using Luminata Crescendo substrate. The density of each band was
measured using the ImageJ software (16). The levels of ZO-1 and Claudin-1
proteins were normalized to β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the colon tissues using
the NucleoSpin RNA kit according to the manufacturer's
instructions. Single-stranded complementary DNA (cDNA) was
synthesized using oligo (dT)18 primers and M-MLV reverse
transcriptase. RT-qPCR was performed using a Bio-Rad CFX real-time
PCR thermocycler and Thunderbird™ Next SYBR®
qPCR mix. The results were normalized to glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) mRNA levels. Relative gene expression was
calculated using the delta-delta CT method. All assays were
performed in triplicates. The primer sequences used were as
follows: MUC2-F: 5'-CCATTGAGTTTGGGAACATGC-3', R:
5'-TTCGGCTCGGTGTTCAGAG-3', spliced X-box binding protein 1
(sXBP-1)-F: 5'-GAGTCCGCAGCAGGTGC-3', R:
5'-CAAAAGGATATCAGACTCAGAATCTGAA-3', ZO-1-F:
5'-AGGACACCAAAGCATGTGAG-3', R: 5'-GGCATTCCTGCTGGTTACA-3',
Claudin-1-F: 5'-CTGGAAGATGATGAGGTGCAGAAGA-3', R:
5'-CCACTAATGTCGCCAGACCTGAA-3', tumor necrosis factor (TNF)-α-F:
5'-GATCTCAAAGACAACCAACATGTG-3', R: 5'-CTCCAGCTGGAAGACTCCTCCCAG-3',
IL-12p40-F: 5'-GGAAGCACGGCAGCAGAATA-3', R:
5'-AACTTGAGGGAGAAGTAGGAATGG-3', IL-10-F:
5'-TGGCCCAGAAATCAAGGAGC-3', R: 5'-CAGCAGACTCAATACACACT-3', IL-22-F:
5'-GTCAACCGCACCTTTATGCT-3', R: 5'-CATGTAGGGCTGGAACCTGT-3', GAPDH-F:
5'-TGAAGGTCGGTGTGAACGGATTTGG-3', R:
5'-ACGACATACTCAGCACCAGCATCAC-3'.
ELISA
Total protein was extracted from the colon tissues
using RIPA buffer containing 0.2% proteinase inhibitors cocktail as
described above, and the concentration was determined using a BCA
protein assay kit. Colon IL-10 levels were measured using an ELISA
kit, according to the manufacturer's instructions.
Gut microbiota analysis
Gut microbiota analyses was performed at the
Bioengineering Lab. Co., Ltd. (Kanagawa, Japan). Fecal samples were
collected from mice and stored at -20˚C. Total DNA was extracted
using the MPure-12 system and MPure bacterial DNA extraction kit
(MP Bio Japan K.K.). The V3-V4 regions of the 16S ribosomal RNA
(rRNA) genes were amplified using the primers 341F
(5'-ACACTCTTTCCCTACACGACGCTCTTCCGATCT-NNNNN-CCTACGGGNGGCWGCAG-3')
and 805R
(5'-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-NNNNNGACTACHVGGGTATCTAATCC-3').
Amplicons were sequenced using the 2x300-bp paired-end method on a
MiSeq system (Illumina). The microbiome was analyzed using QIIME2
(ver. 2022.2).
Statistical analyses
Data obtained are presented as mean ± standard
deviation (SD). One-way analysis of variance (ANOVA) with post-hoc
Tukey test was performed to analyze the statistical significance
among the three groups, and an unpaired two-tailed t-test
was performed to compare the differences in gut microbiota between
the HFD/PBC and HFD/indigo Ex groups. Statistical significance was
set at P<0.05.
Results
Indigo Ex has no effect on HFD-induced
obesity
First, we investigated the effect of indigo Ex on
diet-induced obesity. The results showed that the body weight of
the HFD-fed mice was significantly higher than that of the NC-fed
mice at 4 weeks. There was no significant difference in the body
weights of the HFD/PBS and HFD/indigo Ex groups (Fig. 1A). Daily food intake in the
HFD/indigo Ex group was similar to that in the HFD/PBS group
(Fig. S1A). Epididymal fat weight
was higher in the HFD/PBC group than in the NC/PBC group, while
indigo Ex had little effect on this increase (Fig. S1B). Histological results confirmed
that HFD induced adipocyte hypertrophy, with indigo Ex having
little effect on them (Fig. S1C).
Four weeks of HFD feeding did not lead to apparent steatosis of the
liver, and indigo Ex had no effect on the liver (Fig. S1D). There were no differences in
the serum levels of T. Chol, TG, and glucose between the HFD/PBS
and HFD/indigo Ex groups (Fig.
S1E).
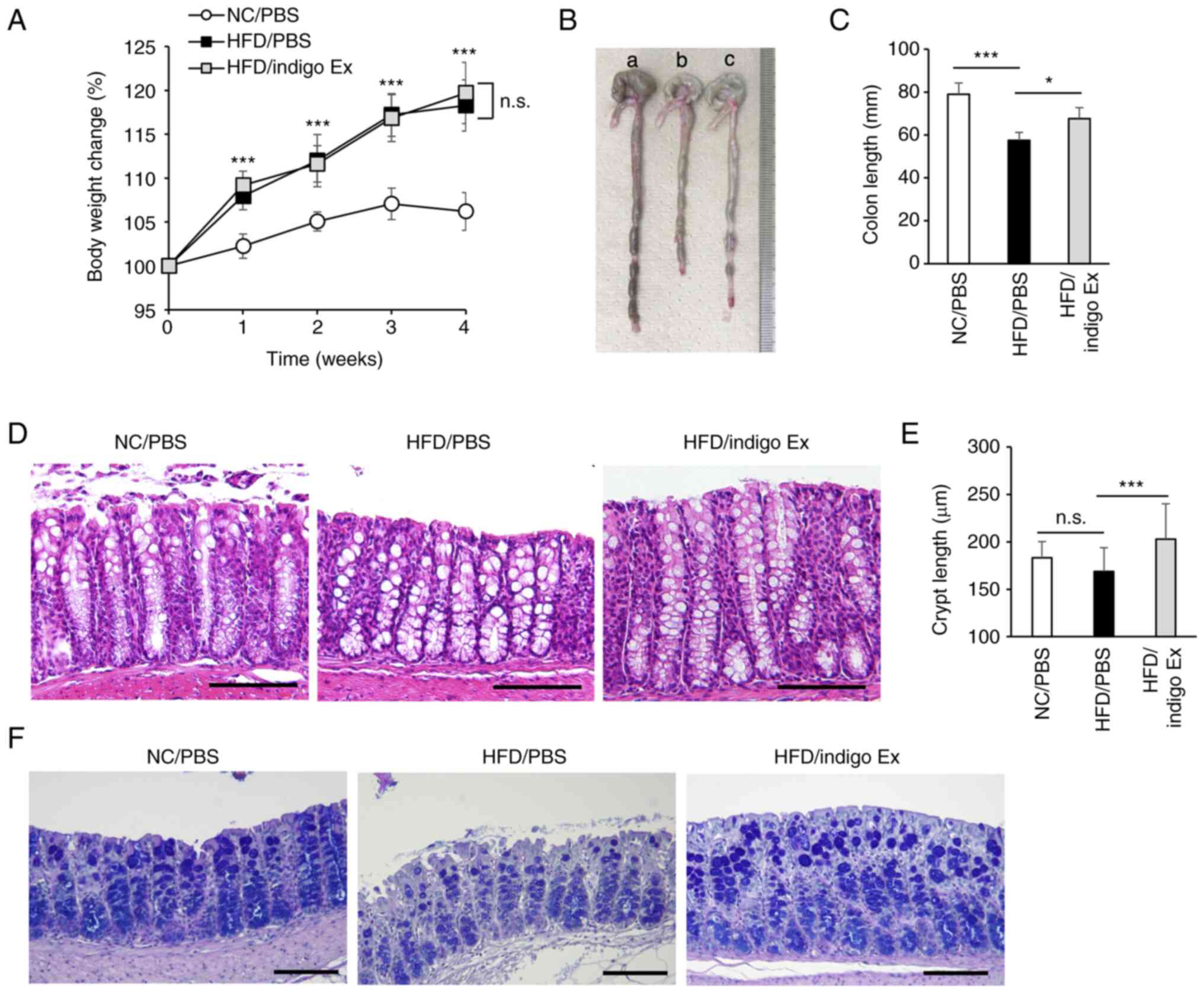 | Figure 1Effects of P. tinctorium leaf
extract (indigo Ex) on HFD-induced intestinal damage in mice. The
mice were divided into three groups. The NC/PBS group was fed an NC
diet and intraperitoneally injected with PBS. The HFD/PBS and
HFD/indigo Ex groups were fed with a HFD and injected with either
PBS or indigo Ex, respectively. (A) Percentage change in the body
weight after 4 weeks. Data are presented as mean ± SD, n=8-9 in
each group. ***P<0.001 vs. NC/PBS; n.s., not
statistically significant. (B) Representative images of the colons
from mice in the NC/PBS (a), HFD/PBS (b), and HFD/indigo Ex (c)
groups. (C) Bar graph depicting the colon length. Data are
presented as mean ± SD, n=8-9 in each group.
***P<0.001, *P<0.05. (D) H&E
staining of the colon sections. Original magnification: 200x. Scale
bar, 100 µm. (E) Bar graph showing the crypt length. Results are
presented as mean ± SD, n=8-9 in each group.
***P<0.001; n.s., not statistically significant. (F)
AB/PAS staining was performed to assess the number of goblet cells.
Scale bar, 100 mm. NC, normal chow; HFD, high-fat diet; PBS,
phosphate-buffered saline; H&E, hematoxylin and eosin; AB/PAS,
Alcian Blue/Periodic acid Schiff; ns, not significant. |
Indigo Ex ameliorates HFD-induced
intestinal epithelial damage
Our results showed that indigo Ex ameliorated the
HFD-induced shortening of the colon in mice (Fig. 1B and C). Moreover, the colon crypt length in
the HFD/indigo Ex group mice was significantly greater than that in
the HFD/PBS group mice (Fig. 1D
and E). There was no difference in
the number of crypts between any of the three groups, and we could
not detect mucosal destruction or increased infiltration of immune
cells (Fig. 1D). To further
evaluate the protective effect of indigo Ex against HFD-induced
intestinal damage, we performed AB/PAS staining to assess the
number of goblet cells. Goblet cells play an essential role in
mucus production, and it has been reported that HFD causes mucin
depletion at a relatively early stage (6,17).
Consistently, we observed that the number of goblet cells was lower
in the HFD/PBS group than in the NC/PBS group, and that indigo Ex
administration prevented this decrease in HFD-fed mice (Fig. 1F). Because MUC2 is the major
component of mucin (18,19), we performed RT-qPCR to analyze the
expression of MUC2 mRNA in the colon tissue of mice from different
groups. The results showed that there was no significant difference
in MUC2 mRNA levels between the HFD/PBS and HFD/indigo Ex groups
(Fig. 2A). Dietary fat-induced
endoplasmic reticulum (ER) stress is associated with impaired
goblet cell function and mucin depletion (17,20).
Therefore, we evaluated the mRNA levels of sXBP-1, an unfolded
protein response (UPR) signaling molecule. Results showed that HFD
and indigo Ex had no effect on sXBP-1 mRNA expression (Fig. 2B).
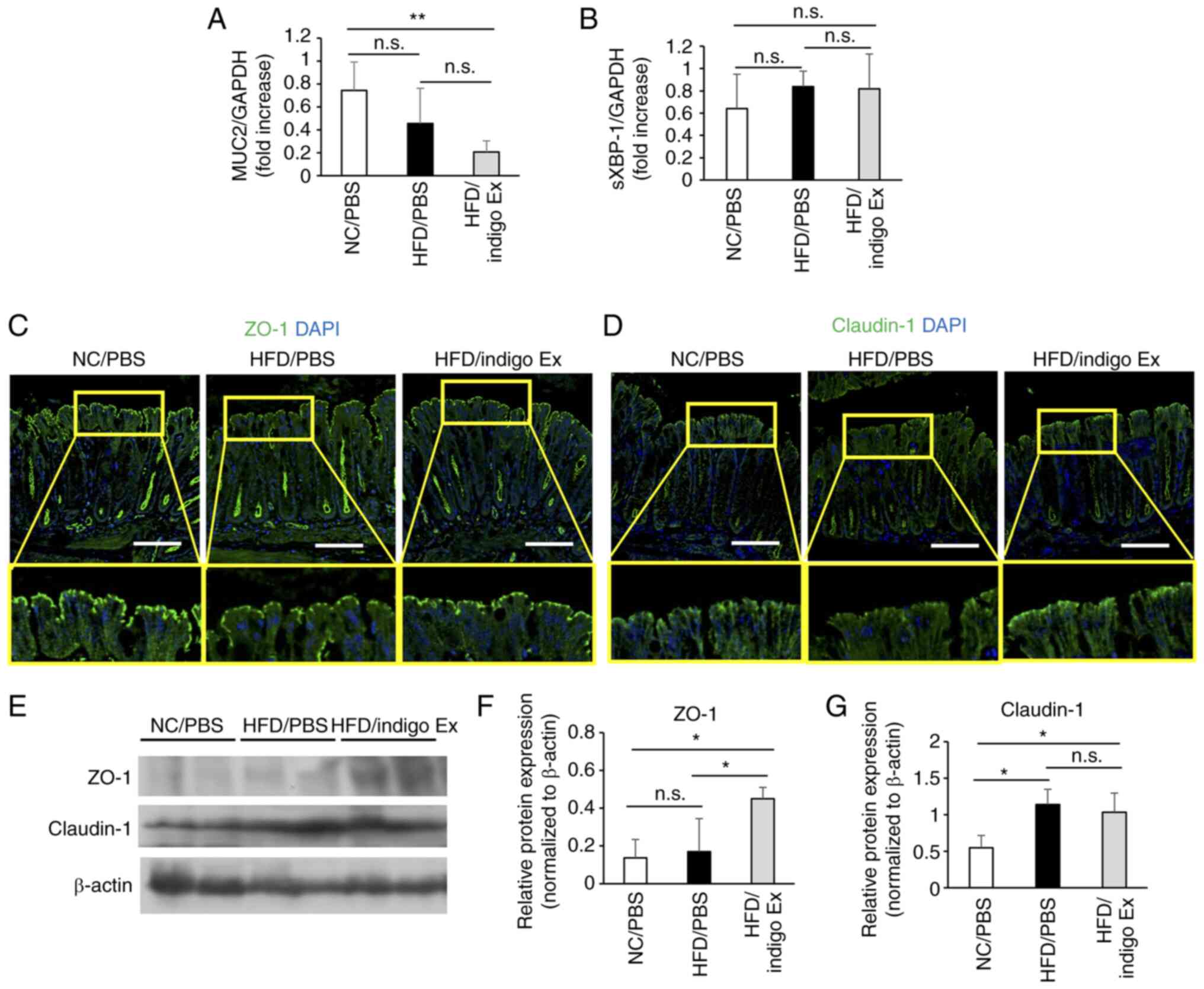 | Figure 2The mRNA levels in the colon of mice
from NC/PBS, HFD/PBS, and HFD/indigo Ex groups. Total RNA was
extracted from the colon tissue and cDNA was synthesized.
Diagrammatic representations of RT-qPCR performed to analyze the
mRNA levels of (A) MUC2 and (B) sXBP-1. Data are presented as mean
± SD, n=3-5 in each group. **P<0.01; n.s., not
statistically significant. (C and D) Diagrammatic representation of
results obtained for immunofluorescence staining performed to
analyze the expression of (C) ZO-1 and (D) Claudin-1 in the colon
mucosa. Both proteins were expressed in the luminal surface of the
epithelial cells (green). Cell nuclei were stained with DAPI
(blue). Representative images for 4-5 mice in each group. Scale
bar, 100 µm. (E) Colon tissue homogenates were prepared, and the
expressions of ZO-1, Claudin-1, and β-actin were analyzed by
western blotting. (F and G) The optical density of each band was
measured using the ImageJ software. Results are presented as mean ±
SD, n=4 in each group. *P<0.05; n.s., not
statistically significant. NC, normal chow; HFD, high-fat diet;
PBS, phosphate-buffered saline; RT-qPCR, reverse
transcription-quantitative PCR; MUC2, mucin-2; sXBP-1, spliced
X-box binding protein-1; ZO-1, zonula occludens-1; DAPI,
4',6-diamidino-2-phenylindole. |
As indigo Ex had a protective effect on goblet
cells, we speculated that the extract may improve HFD-induced
redistribution of TJ-associated proteins. We performed IF staining
to assess the expression of TJ-associated proteins, ZO-1 and
Claudin-1. The results showed that the expression of these proteins
in the mucosal surface layer was markedly decreased in the HFD/PBS
group compared to that in the NC/PBS group, and indigo Ex
administration restored the expression of these proteins (Fig. 2C and D). The results of western blotting showed
that the expression level of ZO-1 protein in the HFD/indigo Ex
group mice was significantly increased compared to that in the
HFD/PBS group mice, whereas there was no difference in the
expression level of Claudin-1 between HFD/PBS group and HFD/indigo
Ex group (Fig. 2E-G). The
expression of ZO-1 and Claudin-1 mRNA was not affected by the
indigo Ex (Fig. 3A and B).
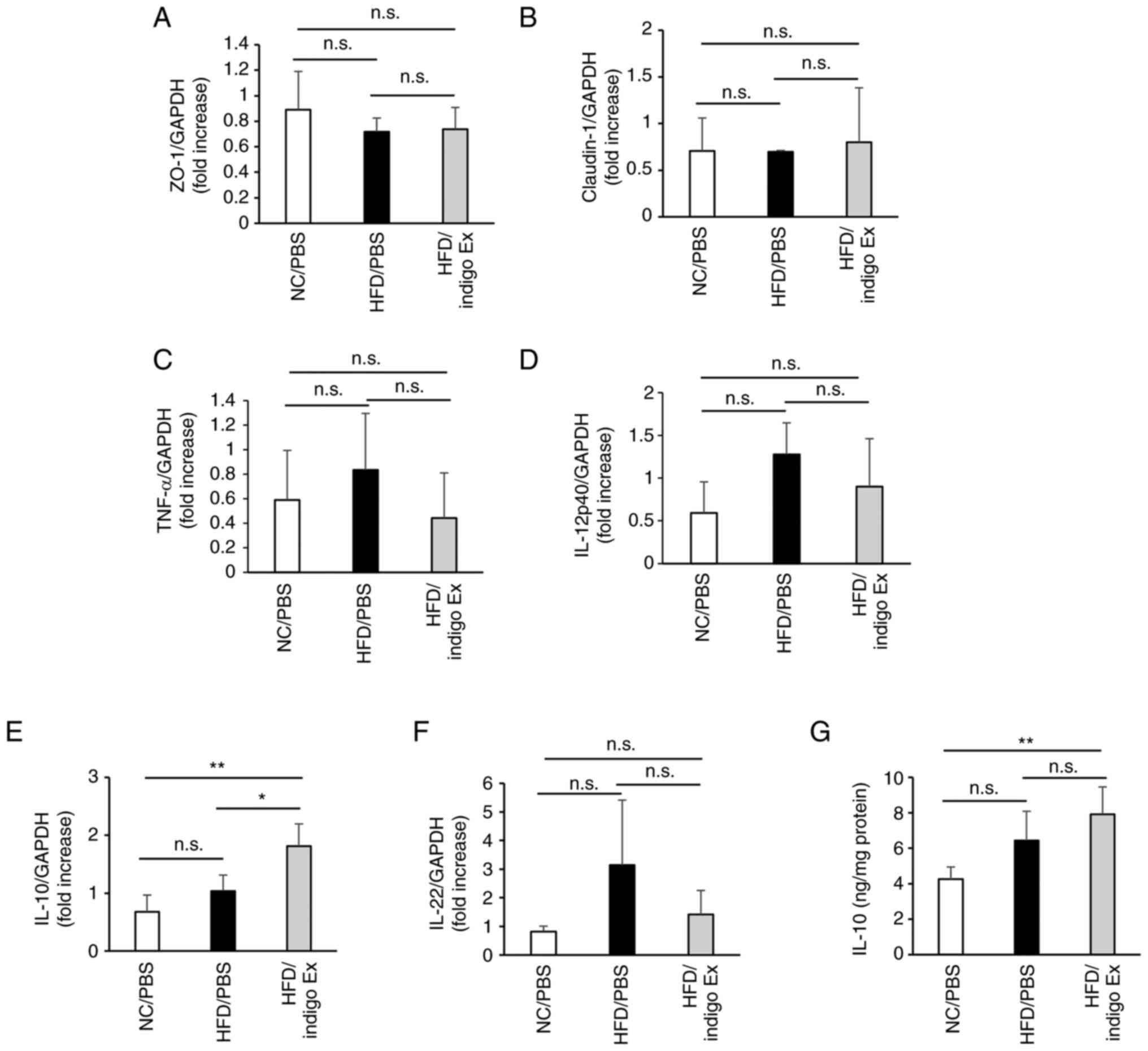 | Figure 3Diagrammatic representations of
RT-qPCR performed to analyze the mRNA levels of (A) ZO-1, (B)
Claudin-1, (C) TNF-α, (D) IL-12p40, (E) IL-10 and (F) IL-22. Data
are presented as mean ± SD, n=3-5 in eac h group.
**P<0.01, *P<0.05; n.s., not
statistically significant. (G) The protein level of IL-10 in murine
colon tissues was determined by ELISA. The results are presented as
mean ± SD, n=5 in each group. **P<0.01; n.s., not
statistically significant. NC, normal chow; HFD, high-fat diet;
PBS, phosphate-buffered saline; ELISA, enzyme-linked immunosorbent
assay; ZO-1, zonula occludens-1; TNF, tumor necrosis factor; IL,
interleukin. |
Next, we examined the mRNA levels of TNF-α,
IL-12p40, IL-10, and IL-22. The results showed no significant
difference in TNF-α and IL-12p40 levels between the NC/PBS,
HFD/PBS, and HFD/indigo Ex groups (Fig. 3C and D). Interestingly, indigo Ex significantly
increased the mRNA levels of IL-10 but not IL-22 (Fig. 3E and F). We performed ELISA for IL-10 using
colon tissue homogenates, and the results indicated that the IL-10
levels were significantly higher in the HFD/indigo Ex group than in
the NC/PBS group (Fig. 3G).
Indigo Ex does not alter the gut
microbial composition in HFD-fed mice
We performed 16S rRNA gene sequencing to clarify
whether the effects of the indigo Ex were accompanied by changes in
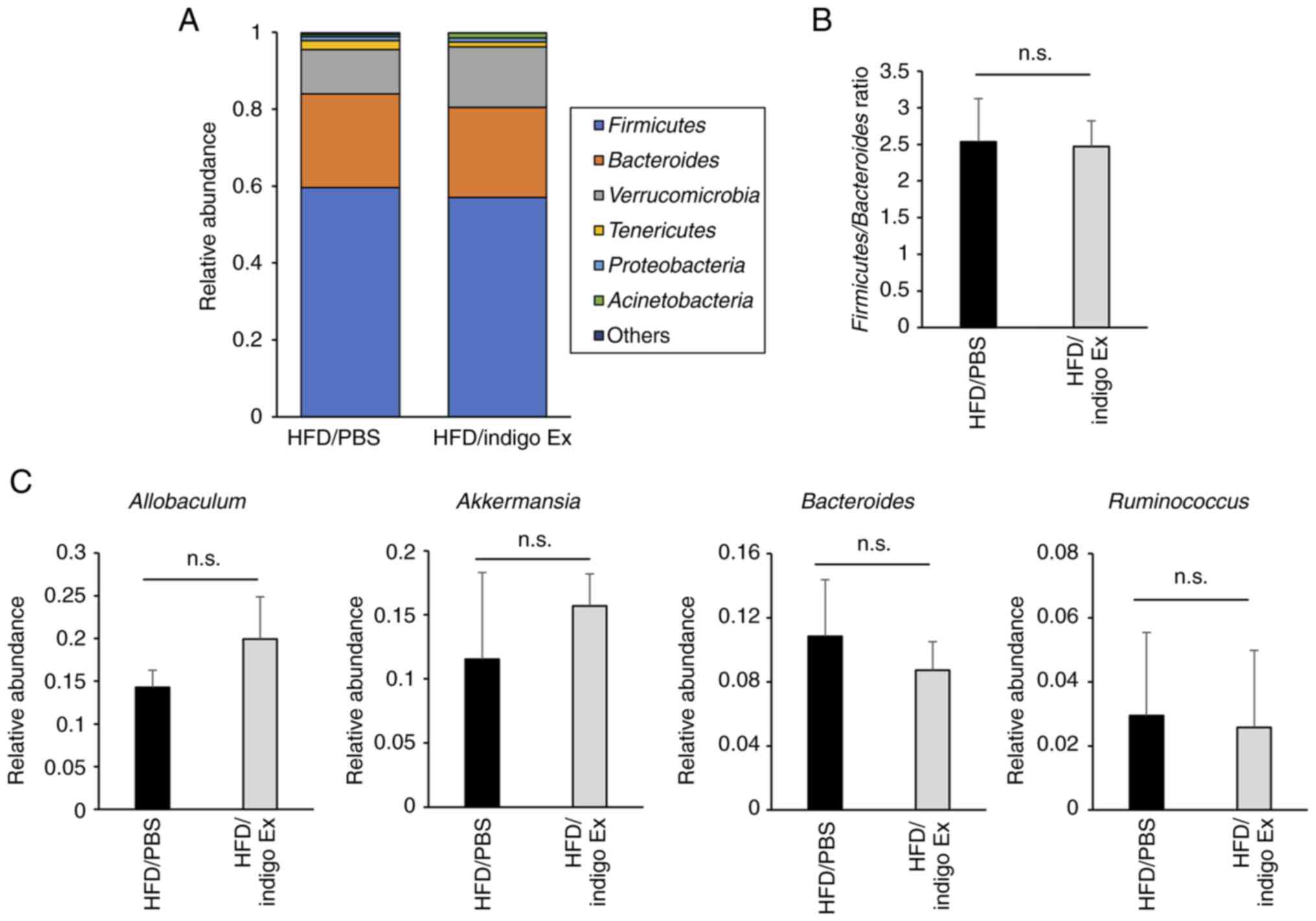
the gut microbial structure. As shown in Fig. 4A, there were no significant
differences in the microbial composition between the HFD/PBS and
HFD/indigo Ex groups at the phylum level. Similarly, there was no
significant difference in the Firmicutes/Bacteroides
ratio between the two groups (Fig.
4B). At the genus level, indigo Ex had no effect on the
relative abundance of Allobaculum, Akkermansia,
Bacteroides, and Ruminococcus (Fig. 4C).
Discussion
The molecular mechanisms underlying HFD-induced
impairment of the intestinal mucosal barrier are complex and not
fully understood. Recent studies have demonstrated that long-term
HFD consumption negatively affects TJ integrity and enhances the
intestinal permeability (21,22).
Moreover, increased expression of proinflammatory cytokines and
intestinal inflammation reduce the expression of TJ-associated
proteins (6,23). A previous study showed that
saturated fatty acids induced ER stress in epithelial cells
(24). Elevated ER stress in
goblet cells reduces mucin secretion, leading to intestinal barrier
disruption (17). Furthermore,
alterations in the structure of the gut microbiota affect the
function of both epithelial and immune cells. In this study, we
demonstrated that indigo Ex ameliorates the decrease in the number
of goblet cells in the colon of HFD-fed mice. Additionally, indigo
Ex restored the expression of ZO-1 and Claudin-1 proteins, whereas
that of mRNA was not affected, suggesting that the extract
modulated the expression of these proteins in a
post-transcriptional manner. These results indicated that indigo Ex
could protect against HFD-induced intestinal epithelial damage. It
has been reported that the TJ-associated proteins are regulated
post-translationally by phosphorylation, palmitoylation, and
glycosylation in response to various stimuli (25,26).
We speculated that indigo Ex might affect the post-translational
modulation of TJ proteins. Alternatively, the indigo Ex might
indirectly attenuate the epithelial cell damage by increasing mucus
production. The results of IF staining showed that the epithelial
expression of ZO-1 and Claudin-1 was markedly decreased in the
HFD-fed mice, while the protein level of Claudin-1 in colon tissue
homogenates was increased, as shown by western blotting. We
speculate that this discrepancy may be ascribed to the results
obtained using whole tissue homogenates, while failing to reflect
the changes in the expression pattern of the TJ proteins in the
mucosal surface layer. Histologically, our study neither detected
mucosal destruction nor immune cell infiltration; moreover, the
mRNA levels of the proinflammatory cytokines were not increased in
the colons of HFD-fed mice. In addition, the level of sXBP-1 mRNA
in HFD-fed mice was similar to that in the NC-fed mice. According
to previous reports, ER stress occurs at least 11 weeks after HFD
consumption (17,20). Our results indicated that indigo Ex
exerts a protective effect independently of intestinal inflammation
or ER stress. Further studies are however required to determine the
precise mechanisms through which indigo Ex exerts a protective
effect on epithelial cells.
Indigo Ex increased the mRNA levels of IL-10, but
not IL-22. Using chemical compounds, indigo is known to act as an
aryl hydrocarbon receptor (AhR) agonist and induce IL-22
expression, leading to the improvement of intestinal barrier
function under HFD-fed conditions (27). Our results showed that indigo Ex
did not induce IL-22 mRNA expression, suggesting that the
protective effects of indigo Ex were not mediated by IL-22. Some
beneficial effects of indigo leaves may be unrelated to AhR
signaling activation. Previously, we reported that P.
tinctorium leaves improved murine colitis independently of AhR
(14).
3,5,4'-trihydroxy-6,7-methylenedioxyflavone-O-glycosides and
their aglycones, which are present in P. tinctorium, have
been reported to suppress experimental colitis by inducing
IL-10(28). IL-10 plays a key role
in decreasing epithelial stress and enhancing TJ integrity
(23,29). Our results revealed that the colon
IL-10 level was enhanced in the HFD/indigo Ex group mice, while
there was no significant difference between HFD/PBS and HFD/indigo
Ex groups. We speculate that the induction of IL-10 is modestly
associated with the effects of indigo Ex in HFD-fed mice. In the
present study, we were unable to identify the cellular source of
IL-10 and the extract components responsible for the IL-10
increase. These important issues need to be addressed in future
research.
A growing body of evidence suggests that commensal
microbes influence the epithelial expression of TJ-associated
proteins. Dietary fat-induced alterations in the gut microbiota
structure lead to epithelial cell dysfunction. Specifically, HFD
decreases the abundance of barrier-promoting microbes, such as
Lactobacillus spp., Bifidobacterium spp., and
Akkermansia muciniphila (6). In addition, it is well-known that HFD
leads to an increase in the Firmicutes/Bacteroidetes
ratio (30,31). Our findings demonstrated that
indigo Ex had little effect on the gut microbial composition at 4
weeks after HFD consumption, suggesting that the protective effects
of indigo Ex are not mediated by alterations in the gut microbiota
structure.
Moreover, we could not confirm the prophylactic
effects of indigo Ex on obesity itself. Presumably, the 4-week
treatment period was too short to evaluate the effects of indigo Ex
on obesity-associated metabolic disorders, and long-term treatment
may be required. Nevertheless, indigo Ex may prevent the
exacerbation of metabolic inflammation, along with exerting
favorable effects on the metabolic profile.
In conclusion, P. tinctorium leaf extract
increased the number of goblet cells and ameliorated the
redistribution of TJ proteins in the colon mucosa of HFD-fed mice,
thereby preventing the HFD-induced intestinal damage. P.
tinctorium leaf extract may contain natural compounds for
treating obesity-associated intestinal damage, and could be
developed as a potential therapeutic agent for intestinal barrier
dysfunction and metabolic inflammation.
Supplementary Material
(A) Dairy food intake in the three
groups. ***P<0.001; n.s., not statistically
significant. (B) Weight of the epididymal fat.
*P<0.05; n.s., not statistically significant.
Hematoxylin and eosin staining of the (C) adipose tissue and (D)
liver. Original magnification: 200x. Scale bar, 100 μm. (E)
Serum levels of T. Chol, TG and glucose. ***P<0.001,
*P<0.05; n.s., not statistically significant. T.
Chol, total cholesterol; TG, triglyceride.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Interdisciplinary
Collaborative Research Grant for Young Scientists, Hirosaki
University and JSPS KAKENHI (grant no. 20K08346).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The metagenome data are available in the DDBJ Sequenced
Read Archive using the accession numbers DRR433110-DRR433117
(https://ddbj.nig.ac.jp/resource/bioproject/PRJDB15098).
Authors' contributions
SK, HS, HK, KM and KSa conceived and designed the
study. SK and YH performed the experiments and wrote the original
manuscript. JD, YT, KSe, TM, HH, SF and TI performed the data
analyses and revised the manuscript. HS and HK confirm the
authenticity of the raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
This study design was approved by the Animal
Research Committee of Hirosaki University (approval no.
M20016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Garcia-Hernandez V, Quiros M and Nusrat A:
Intestinal epithelial claudins: Expression and regulation in
homeostasis and inflammation. Ann NY Acad Sci. 1397:66–79.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chekakkot C, Ghim J and Ryu SH: Mechanisms
regulating intestinal barrier integrity and its pathological
implications. Exp Mol Med. 50:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Suzuki T: Regulation of intestinal
epithelial permeability by tight junctions. Cell Mol Life Sci.
70:631–659. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Massier L, Blüher M, Kovacs P and
Chakaroun RM: Impaired intestinal barrier and tissue bacteria:
Pathomechanisms for metabolic diseases. Front Endocrinol
(Lausanne). 12(616506)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Forgie AJ, Fouhse JM and Willing BP:
Diet-microbe-host interactions that affect gut mucosal integrity
and infection resistance. Front Immunol. 10(1802)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rohr MW, Narasimhulu CA, Rudeski-Rohr TA
and Parthasarathy S: Negative effects of a high-fat diet on
intestinal permeability: A review. Adv Nutr. 11:77–91.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Martinez KB, Leone V and Chang EB: Western
diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut
Microbes. 8:130–142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Carmody RN, Gerber GK, Luevano JM Jr,
Gatti DM, Somes L, Svenson KL and Turnbaugh PJ: Diet dominates host
genotype in shaping the murine gut microbiota. Cell Host Microbe.
17:72–84. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mouries J, Brescia P, Silvestri A, Spadoni
I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini
L, et al: Microbiota-driven gut vascular barrier disruption is a
prerequisite for non-alcoholic steatohepatitis development. J
Hepatol. 71:1216–1228. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tanaka S, Nemoto Y, Takei Y, Morikawa R,
Oshima S, Nagaishi T, Okamoto R, Tsuchiya K, Nakamura T, Stutte S
and Watanabe M: High-fat diet-derived free fatty acids impair the
intestinal immune system and increase sensitivity to intestinal
epithelial damage. Biochem Biophys Res Commun. 522:971–977.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cani PD, Bibiloni R, Knauf C, Waget A,
Neurinck AM, Delzenne NM and Burcelin R: Changes in gut microbiota
control metabolic endotoxemia-induced inflammation in high-fat
diet-induced obesity and diabetes in mice. Diabetes. 57:1470–1480.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kataoka M, Hirata K, Kunikata T, Ushio S,
Iwaki K, Ohashi K, Ikeda M and Kurimoto M: Antibacterial action of
tryptanthrin and kaempferol, isolated from the indigo plant
(Polygonum tinctorium Lour.), against Helicobacter
pylori-infected Mongolian gerbils. J Gastroenterol. 36:5–9.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hoessel R, Leclerc S, Endicott JA, Nobel
ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, et
al: Indirubin, the active constituent of a Chinese antileukaemia
medicine, inhibits cyclin-dependent kinases. Nat Cell Biol.
1:60–67. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Asari T, Kikuchi H, Kawaguchi S, Sakuraba
H, Yoshida S, Akemoto Y, Maeda T, Shinji O, Murai Y, Higuchi N, et
al: Polygonum tinctorium leaves suppress sodium dextran
sulfate-induced colitis through interleukin-10-related pathway.
Biochem Biophys Rep. 30(101272)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hagiyama M, Takeuchi F, Sugano A,
Yoneshige A, Inoue T, Wada A, Kajiyama H, Takaoka Y, Sasaki K and
Ito A: Indigo plant leaf extract inhibits the binding of SARS-CoV-2
spike protein to angiotensin-converting enzyme 2. Exp Ther Med.
23(274)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to ImageJ: 25 Years of image analysis. Nat Methods.
9:671–675. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gulhane M, Murray L, Lourie R, Tong H,
Sheng YH, Wang R, Kang A, Schreiber V, Wong KY, Magor G, et al:
High fat diets induce colonic epithelial cell stress and
inflammation that is reversed by IL-22. Sci Rep.
6(28990)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Heazlewood CK, Cook MC, Eri R, Price GR,
Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ,
et al: Aberrant mucin assembly in mice causes endoplasmic reticulum
stress and spontaneous inflammation resembling ulcerative colitis.
PLoS Med. 5(e54)2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Birchenough GM, Johansson ME, Gustafsson
JK, Bergström JH and Hannson GC: New developments in goblet cell
mucus secretion and function. Mucosal Immunol. 8:712–719.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dai YJ, Liu WB, Abasubong KP, Zhang DD, Li
XF, Xiao K, Wang X and Jiang GZ: The mechanism of
lipopolysaccharide escaping the intestinal barrier in Megalobrama
amblycephala fed a high-fat diet. Front sNutr.
9(853409)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ghezzal S, Postal BG, Quevrain E, Brot L,
Seksik P, Leturque A, Thenet S and Carrière V: Palmitic acid
damages gut epithelium integrity and initiates inflammatory
cytokine production. Biochim Biophys Acta Mol Cell Biol Lipids.
1865(158530)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nascimento JC, Matheus VA, Oliveira RB,
Tada SFS and Collares-Buzato CB: High-fat diet induces disruption
of the tight junction-mediated paracellular barrier in the proximal
small intestine before the onset of type 2 diabetes and
endotoxemia. Dig Did Sci. 66:3359–3374. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Capaldo CT and Nusrat A: Cytokine
regulation of tight junctions. Biochim Biophys Acta. 1788:864–871.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Escoula Q, Bellenger S, Narce M and
Bellenger J: Docosahexaenoic and eicosapentaenoic acids prevent
altered-Muc2 secretion induced by palmitic acid by alleviating
endoplasmic reticulum stress in LS174T goblet cells. Nutrients.
11(2179)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Reiche J and Huber O: Post-translational
modifications of tight junction transmembrane proteins and their
direct effect on barrier function. Biochim Biophys Acta Biomembr.
1862(183330)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Butt AM, Khan IB, Hussain M, Idress M, Lu
J and Tong Y: Role of post translational modifications and novel
crosstalk between phosphorylation and O-beta-GlcNAc modifications
in human claudin-1, -3 and -4. Mol Biol Rep. 39:1359–1369.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lin YH, Luck H, Khan S, Schneeberger PHH,
Tsai S, Clemente-Casares X, Lei H, Leu YL, Chan YT, Chen HY, et al:
Aryl hydrocarbon receptor agonist indigo protects against
obesity-related insulin resistance through modulation of intestinal
and metabolic tissue immunity. Int J Obes (Lond). 43:2407–2421.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kimura H, Tokuyama-Nakai S, Hirabayashi Y,
Ishihara T, Jisaka M and Yokota K: Anti-inflammatory and
bioavailability studies on dietary
3,5,4'-trihydroxy-6,7-methylenedioxyflavone-O-glycosides and their
aglycone from indigo leaves in a murine model of inflammatory bowel
disease. J Pharm Biomed Anal. 193(113716)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hasnain SZ, Tauro S, Das I, Tong H, Chen
AC, Jeffery PL, McDonald V, Florin TH and McGuckin MA: IL-10
promotes production of intestinal mucus by suppressing protein
misfolding and endoplasmic reticulum stress in goblet cells.
Gastroenterol. 144:357–368.e9. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hildebrandt MA, Hoffman C, Sherrill-Mix
SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F
and Wu GD: High-fat diet determines the composition of the murine
gut microbiome independently of obesity. Gastroenterol.
137:1716–1724.e1-e2. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Murphy EA, Velazquez KT and Herbert KM:
Influence of high-fat diet on gut microbiota: A driving force for
chronic disease risk. Curr Opin Clin Nutr Metab Care. 18:515–520.
2015.PubMed/NCBI View Article : Google Scholar
|