Introduction
Perfluorooctanoic acid (PFOA) is a newly discovered
organic environmental pollutant distributed in nature, and it has
been widely used in the fields of surfactants, emulsifiers,
textiles and interior decoration in the past few decades (1). Due to the characteristics of
refractory degradation and bioaccumulation, it has been detected in
animals, plants and water, causing serious pollution to the
environment (2,3). Previous studies have demonstrated
that PFOA is harmful to the nervous, immunity and endocrine
systems, and particularly the reproductive system (4-7).
PFOA binds to oestrogen receptors and causes oxidative damage to
the body, thereby impairing male reproductive function (8).
Lipoic acid (LA) with the chemical name
1,2-dithiolane-3-valeric acid is a strong antioxidant, widely
present in living organisms and serves a role in scavenging oxygen
free radicals and chelating metal ions (9). It alleviates the oxidative damage
caused by heavy metals, environmental toxicants or other factors
(10). It also possesses
therapeutic effects in the treatment of diabetes, peripheral
neuropathy, male infertility disease, etc. (11-13).
The present study further explored whether LA could improve the
spermatogenesis disorder induced by PFOA in rats. Male Sprague
Dawley (SD) rats were given 0.01 g/kg PFOA by gavage for 30
consecutive days to establish a PFOA-induced rat model.
Subsequently, rats with reproductive damage were treated with
either a high or low dose of LA. The therapeutic efficiency and
mechanism of action were examined by western blotting, H&E
staining, ELISA and immunofluorescence techniques.
Materials and methods
Materials
A total of 30 specific pathogen-free 6-week-old male
SD rats were purchased from SPF (Beijing) Biotechnology Co., Ltd.
and their weights were ranged between 150 and 170 g. PFOA (96%;
white powder) was purchased from Shanghai Anpu Experimental
Technology Co., Ltd. LA was purchased from Sigma-Aldrich; Merck
KGaA. Anti-rabbit β-actin (cat. no. sc-47778) was purchased from
Santa Cruz Biotechnology, Inc. SDS-PAGE gel, BCA, RIPA lysis
buffer, antifade mounting medium with DAPI (cat. no. P0131), immuno
staining blocking buffer (cat. no. P0102) and H&E staining kits
were all purchased from Beyotime Institute of Biotechnology.
EZ-Link Sulfo-NHS-LC-Biotin (cat. no. 21335) and
streptavidin-fluorescein conjugate (cat. no. S869) were purchased
from Invitrogen; Thermo Fisher Scientific, Inc. The rat
follicle-stimulating hormone (FSH; cat. no. m1059034), rat
testosterone (T; cat. no. ml059506), rat lactate dehydrogenase
(LDH; cat. no. m1059178), rat malondialdehyde (MDA; cat. no.
m1077384) and rat serum succinate dehydrogenase (SDH; cat. no.
ml058919), rat superoxide dismutase (SOD; cat. no. m1059387), rat
glutathione peroxidase (GSH-Px; cat. no. m1097316), rat luteinizing
hormone (LH; cat. no. m1002860) and rat estradiol (E2; cat. no.
m1002871) kits were purchased from Shanghai Enzyme-linked
Biotechnology Co., Ltd. Neutral balsam (cat. no. G8590) was
purchased from Beijing Solarbio Science & Technology Co.,
Ltd.
Animal grouping and treatments
After 1 week of adaptive feeding, the rats were
randomly divided into five groups according to their body weight
based on a random number table, with 6 rats in each group. These
groups were: Control group, PFOA-induced group (PFOA), LA control
group (LA), PFOA-induced low-dose LA medication group [PFOA+LA (L)]
and PFOA-induced high-dose LA medication group [PFOA+LA (H)]. LA
and PFOA were dissolved in corn oil (0.2% of rat body weight), and
all the treatments were administered by gavage at 10 a.m. every
day. The duration of LA treatment was 6 weeks. The control group
was given corn oil continuously for 72 days, the PFOA-induced group
was given 0.01 g/kg PFOA for 30 days and then euthanized after
detection was carried out, the LA control group was given 0.01 g/kg
LA for 42 days and then euthanized after detection was carried out,
the PFOA+LA (L) group was given 0.01 mg/kg PFOA for 30 days to
establish the model and then given the treatment of 0.05 g/kg LA
for 42 days, and the PFOA+LA (H) group was given 0.01 mg/kg PFOA
for 30 days to establish the model and then given the treatment of
0.10 g/kg LA for 42 days. Rats had free access to water and food.
The room temperature and relative humidity of the animal laboratory
were 20-25˚C and 40-60%, respectively, with a 12/12 h day-night
cycle. The body weights of rats were measured and recorded every
weekend, and the weight of rats at the time of euthanasia was ~350
g. After 30 days of gavage of PFOA solution and 42 days of LA
treatment, blood was collected from the abdominal aorta of rats
anesthetized by inhalation of 4% ether and the supernatant was
reserved after centrifugation at 4˚C for 5 min at 600 x g. Rats
were not allowed to live on after blood collection or
biotin-labelled blood-testis barrier (BTB) function analysis.
Euthanasia by cervical dislocation was performed immediately after
the experiment was completed. In addition, the other rats in each
group were sacrificed by cervical dislocation after anaesthesia by
inhalation of 4% ether, their testes and epididymis were quickly
extracted and weighed with a balance. Tissues were homogenized or
sliced after fixation in 4% paraformaldehyde at room temperature
(25˚C) for 15 min. Absence of movement and breath, as well as the
presence of cardiac arrest and pupil dilation for 5 min were used
to confirm death. Animal experiments and treatments were performed
in accordance with the guidelines of the experimental animal
management and ethics committee of Baotou Medical College (Baotou,
China) and humane care was given according to the 3R principle.
BTB function test
A total of 2 rats from each group were anesthetized
by inhalation of 4% ether and their testis tissues were exposed
after disinfection with alcohol. A small opening was created with
surgical scissors to inject a biotin solution (50 µl EZ-Link
Sulfo-NHS-LC-Biotin 10 mg·ml-1 in 1X PBS; cat. no.
21335; Invitrogen; Thermo Fisher Scientific, Inc.) into the testes
of the rat and it was left to disperse for 30 min at room
temperature. Subsequently, the rat testis tissues were rapidly
isolated and placed in liquid nitrogen (-196˚C) for preservation,
and rats were sacrificed by cervical dislocation immediately.
Testis sections (thickness, 8 µm) were obtained from different
levels at -20˚C. Then sections were incubated with
streptavidin-fluorescein conjugate (1:3,000; cat. no. S869;
conjugate, fluorescein; Invitrogen; Thermo Fisher Scientific, Inc.)
for 30 min in the dark after blocking with immuno staining blocking
buffer for 2 h (all at room temperature). The sections were rinsed
with PBS and mounted in a drop of antifade mounting medium with
DAPI at room temperature. An Olympus BX51 fluorescence microscope
(Olympus Corporation) was used for imaging. The Cell sens Dimension
software (V4.1; Olympus Corporation) was used for analysis.
Testicular H&E staining
The testis tissues of rats were quickly extracted
and fixed in 4% paraformaldehyde solution at room temperature for
24 h. Subsequently, the testis tissues were embedded in paraffin
and cut into tissue sections (thickness, 5 µm). The sections were
incubated in xylene for 10 min, absolute ethanol for 5 min, 95%
alcohol for 30 min, 90% alcohol for 30 min, 80% alcohol for 30 min,
70% for alcohol 30 min, and then rinsed in distilled water for 5
min at room temperature for dewaxing. Sections were stained with
hematoxylin for 4 min, rinsed with water for 10 min, differentiated
with hydrochloric acid ethanol for 3 sec, stained with eosin for 1
min and then rinsed with deionized water for 5 min, and finally
sealed with neutral balsam after air-drying naturally (all at room
temperature). An Olympus BX51 fluorescence microscope (Olympus
Corporation) was used for imaging. The Cell sens Dimension software
(V4.1; Olympus Corporation) was used for analysis.
Sperm extraction and counting
The rat bilateral epididymis was stripped into an
Eppendorf tube containing 1.5 ml pre-warmed PBS (37˚C) and
incubated for 20 min on a shaker to completely dissociate the sperm
into the PBS buffer. Subsequently, the semen was filtered with a
70-µm membrane filter and rinsed with 1 ml PBS simultaneously. A
total of 300 µl 5% NaHCO3 was mixed with the
aforementioned semen. The cells in the suspension were counted
using a hemacytometer and Olympus IX51 fluorescence microscope
(Olympus Corporation) after the cell suspension was left to stand
for 1 min to allow the cells to settle on the hemacytometer. Each
sample was counted three times and the mean value was taken.
Detection of SDH, LDH, SOD, MDA and
GSH-Px activity, and T, FSH, LH and E2 levels in testes
The rat testis was dried with filter paper, cut into
small pieces on ice, and then ground into 10% homogenate
mechanically, and the supernatant was collected after
centrifugation at 4˚C for 5 min at 500 x g. The detection operation
was performed using the corresponding ELISA kits according to the
manufacturer's instructions and the absorbance value was detected
using a microplate reader.
Western blotting
Total protein from rat testis tissues was extracted
using RIPA lysis buffer and quantified using a BCA assay. Total
protein (20 µg/lane) was separated by 10% SDS-PAGE and transferred
onto a PVDF membrane that was washed twice with PBS with 0.05%
Tween-20 (PBST) and subsequently blocked at 25˚C for 2 h with 5%
skimmed milk after two washes with PBST. The membrane was
subsequently incubated with primary antibodies against androgen
receptor (AR; 1:2,000; ab133273; Abcam) and β-actin (1:2,000)
overnight at 4˚C. Following incubation with primary antibodies,
membranes were washed three times with PBST and incubated with goat
anti-rabbit IgG (H+L) secondary antibody (conjugate,
DyLight® 488; 1:3,000) for 2 h at 25˚C. The protein
bands were visualized after exposure to enhanced chemiluminescence
solution (SuperSignal™ West Atto; A38555; Thermo Fisher Scientific,
Inc.) on a Tanon-4600 image analysis system (Tanon Science and
Technology Co., Ltd.). The gray value of each band was calculated
and analyzed using ImageJ software (National Institutes of
Health).
Statistical analysis
All assays were repeated at least three times, and
values are presented as the mean ± standard deviation. Statistical
analysis was performed by one-way analysis of variance followed by
Tukey's test as post hoc tests using SPSS 17.0 statistical software
(SPSS, Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
LA has an ameliorating effect on
physical damage caused by PFOA
Compared with those of the rats in the control and
LA groups, the body and epididymal weights of the rats in the
PFOA-induced group were substantially decreased. The body and
epididymal weights of the rats in PFOA+LA (H) groups were
increased, but there was no change in the rats in the PFOA+LA (L)
group. In addition to body weight, high doses of LA also served a
role in restoring the sex organ coefficients such as the weight
coefficient of testis compared with the PFOA-induced group
(Table I). The treatment with high
dose of LA increased the epididymal mass of PFOA-induced rats to
the normal weights of the rats in the control group.
 | Table ITherapeutic effects of LA on organ
coefficients of rats. |
Table I
Therapeutic effects of LA on organ
coefficients of rats.
| Group | Dose, g/kg | Weight, g | Weight of testis,
g | Weight of epididymis,
g | Weight coefficient of
testis, ‰ | Weight coefficient of
epididymis, ‰ |
|---|
| Control | - | 474.87±38.48 | 3.63±0.10 | 0.84±0.05 | 7.30±0.57 | 1.70±0.10 |
| PFOA | 0.01 |
411.84±33.23a | 3.66±0.20 |
0.77±0.02a |
9.30±0.45a |
1.90±0.18a |
| LA | 0.10 |
469.17±32.24b | 3.68±0.24 |
0.89±0.08b |
8.00±0.63b | 1.80±0.18 |
| PFOA+LA (L) | LA, 0.05; PFOA,
0.01 |
405.99±23.05a,c | 3.72±0.14 |
0.77±0.04a,c |
9.00±0.85a,c | 1.80±0.11 |
| PFOA+LA (H) | LA, 0.10; PFOA,
0.01 |
428.24±23.68a,b,c,d | 3.55±0.23 |
0.83±0.04b,c,d |
8.90±0.66a,b,c |
1.90±0.90a |
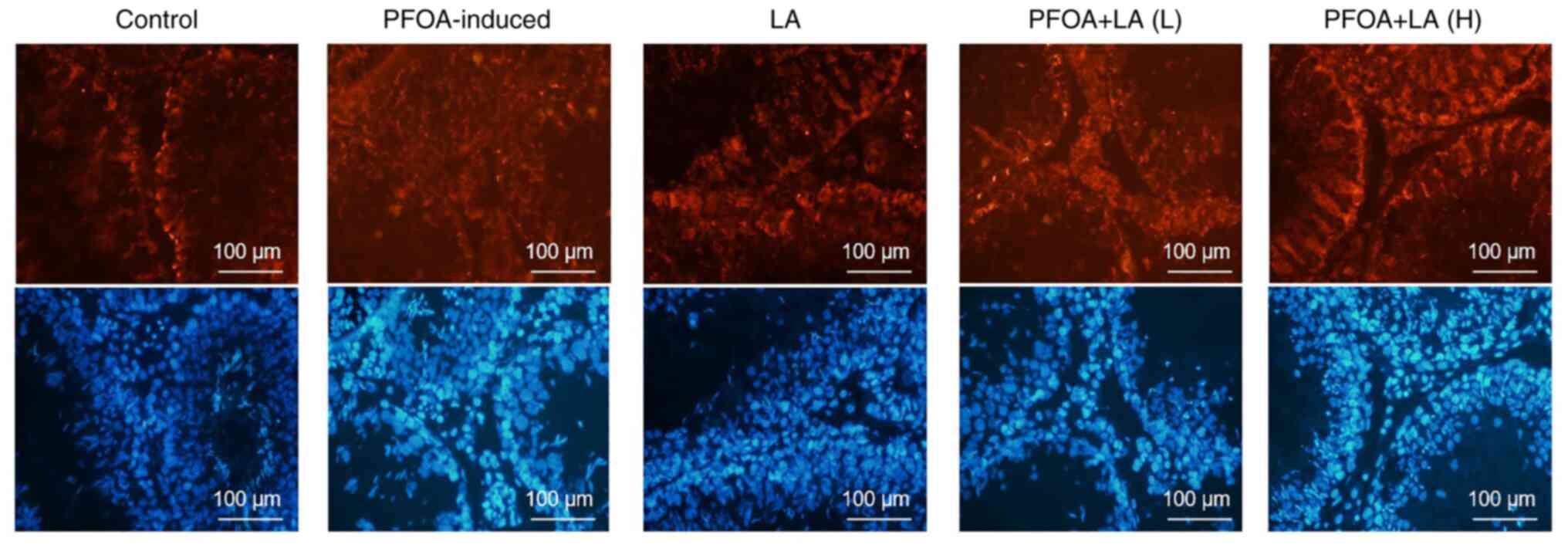
LA has a therapeutic effect on BTB in
PFOA-induced rats
BTB is an immune barrier composed of Sertoli cells
and surrounding structures, which provides a reliable guarantee for
spermatogenesis (14). After being
induced by PFOA, the structure of the seminiferous tubule was
destroyed. Biotin diffused farther, the number of spermatogenic
cells was decreased and the gap between spermatogenic cells was
increased. After treatment with LA, the structural damage of
seminiferous tubules was alleviated. Furthermore, the diffusion
distance of biotin in the seminiferous tubule lumen was reduced,
indicating that the physiological state of the BTB had been
restored (Fig. 1).
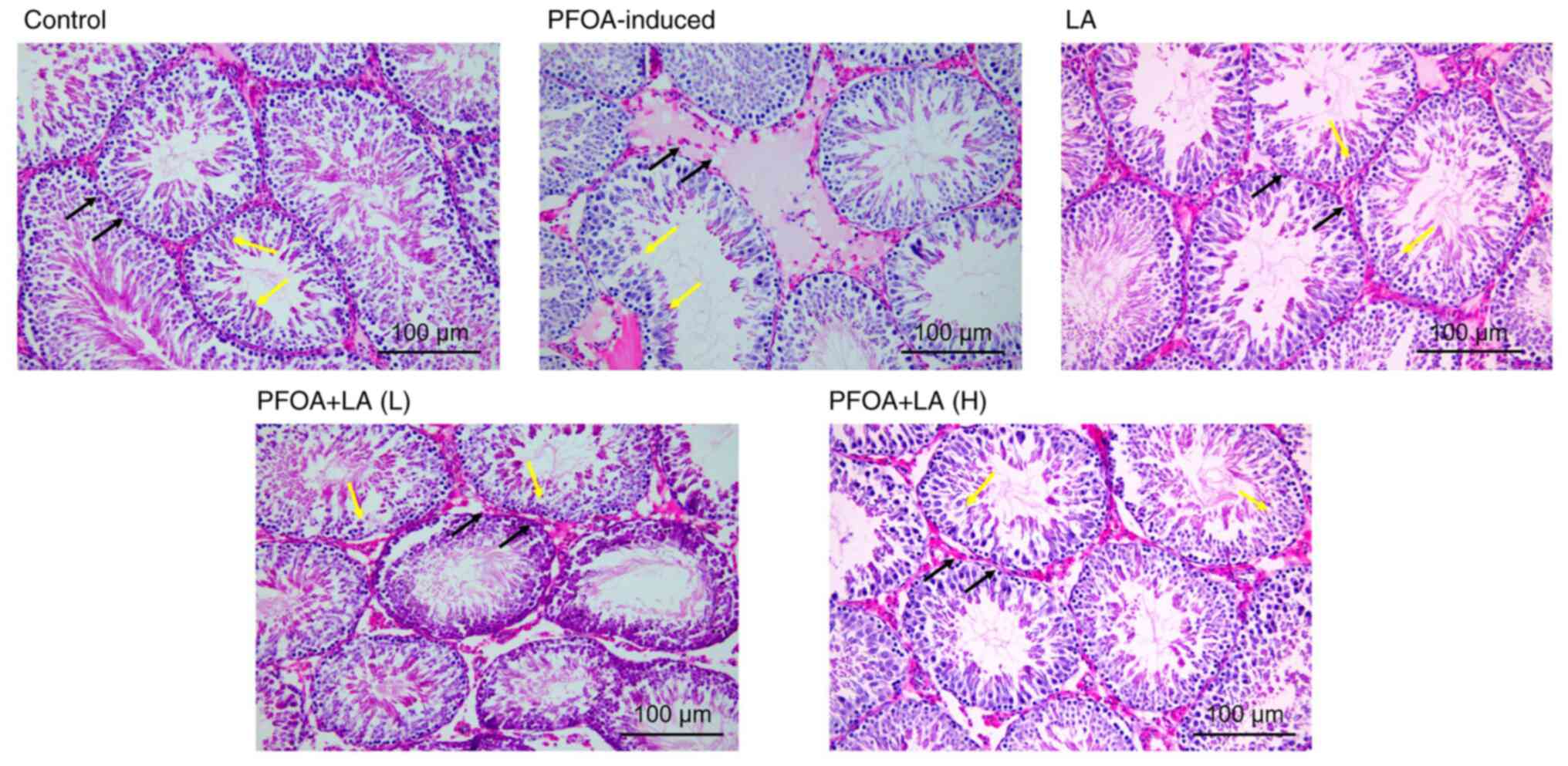
LA has a therapeutic effect on testis
tissues of PFOA-induced rats
H&E staining demonstrated that the seminiferous
tubules in the testis tissue of normal rats were intact and closely
arranged. The spermatogenic cells in the seminiferous tubules were
morphologically regular and distinct, and the formation of sperm
was visible. In contrast to the control group, the seminiferous
tubule lumen in the PFOA-induced rats was narrowed, and the
seminiferous epithelium was thinned. The arrangement of
spermatogenic cells was disordered, and numerous immature
spermatogenic cells fell off into the seminiferous tubule lumen. At
the same time, Sertoli cells and stromal cells were vacuolated. The
number of spermatogenic cells was increased after treatment with
low-dose LA. The histopathology of testes was restored after
treatment with high-dose LA. In addition, the shapes of
seminiferous tubules became more regular, and the spermatogenic
cells were closely arranged with distinct layers (Fig. 2). Staining results indicated that
LA alleviates the PFOA-induced damage to the testes in rats.
LA relieves spermatogenesis disorder
caused by PFOA
Sperm count is an important indicator of
reproductive toxicology, which accurately reflects the degree of
reproductive damage to animals caused by toxicants (15,16).
The sperm counts of rats were reduced after PFOA intoxication
compared with the counts of rats in control and LA groups. After
treatment with LA, the symptoms were relieved to varying degrees,
and the effect was positively related to the dose (Table II).
 | Table IITherapeutic effects of LA on sperm
counts in PFOA-induced rats. |
Table II
Therapeutic effects of LA on sperm
counts in PFOA-induced rats.
| Group | Dose, g/kg | Counts,
x106 |
|---|
| Control | - | 22.50±3.21 |
| PFOA | 0.01 |
11.50±1.52a |
| LA | 0.10 |
19.00±5.10b |
| PFOA+LA (L) | LA, 0.05; PFOA,
0.01 |
15.82±1.72a,b |
| PFOA+LA (H) | LA, 0.10; PFOA,
0.01 |
17.50±1.26b |
LA activates the expression of
testicular marker enzymes
The levels of SDH and LDH in the serum of rats were
decreased after PFOA intoxication. This indicated that PFOA damaged
the production of energy and sperm in the testis tissues. After
treatment with high-dose LA, the levels of SDH and LDH in the serum
of rats in PFOA+LA (H) were increased compared with those in the
PFOA-induced group. The therapeutic effect was positively
associated with the dose of LA administered. Therefore, LA
alleviated serum hormone disorder in rats caused by PFOA (Table III).
 | Table IIITherapeutic effects of LA on
testicular marker enzymes. |
Table III
Therapeutic effects of LA on
testicular marker enzymes.
| Group | Dose, g/kg | Serum succinate
dehydrogenase, U/mg | Lactate
dehydrogenase, U/g |
|---|
| Control | - | 1.56±0.31 | 2.70±0.59 |
| PFOA | 0.01 |
1.00±0.41a |
2.10±0.49a |
| LA | 0.10 |
1.73±0.43b |
2.61±0.89b |
| PFOA+LA (L) | LA, 0.05; PFOA,
0.01 |
1.54±0.40b,c |
2.12±0.54a |
| PFOA+LA (H) | LA, 0.10; PFOA,
0.01 |
1.71±0.31a,b,d |
2.42±0.53b |
LA restores the expression of hormones
in PFOA-induced rats
The present study elucidated the mechanism of LA in
treating reproductive injury caused by PFOA by detecting the
changes in FSH, T, E2 and LH levels in rat serum (Table IV). The levels of FSH in rats were
significantly increased after PFOA induction, while the levels of T
and E2 were decreased (P<0.05). After treatment with LA, the
levels of FSH were decreased and those of E2 were increased to
varying degrees compared with the PFOA-induced group, and the
therapeutic effect was positively associated with the dose of LA.
In addition, T levels of rats in the PFOA+LA (H) group returned to
the levels of control group. These results demonstrated that LA
possessed the ability to restore the expression of sex hormones in
PFOA-induced rats.
 | Table IVTherapeutic effects of LA on hormone
levels. |
Table IV
Therapeutic effects of LA on hormone
levels.
| Group | Dose, g/kg |
Follicle-stimulating hormone, pg/ml | Testosterone,
pg/ml | Estradiol,
pg/ml | Luteinizing
hormone, U/ml |
|---|
| Control | - | 0.75±0.02 | 2.82±0.04 | 292.22±71.85 | 10.50±1.03 |
| PFOA | 0.01 |
0.89±0.01a |
2.72±0.03a |
156.15±37.40a |
8.91±1.85a |
| LA | 0.10 |
0.74±0.04b |
2.80±0.03b |
290.00±70.56b | 9.89±1.01 |
| PFOA+LA (L) | LA, 0.05; PFOA,
0.01 |
0.81±0.03a,c |
2.74±0.03a,c |
183.99±25.02a,c |
9.30±1.58a |
| PFOA+LA (H) | LA, 0.10; PFOA,
0.01 |
0.77±0.02b,d |
2.77±0.02b |
246.27±38.92a-d |
9.82±1.90b |
LA attenuates the oxidative stress
injury induced by PFOA in rats
The activities of SOD and GSH-Px in the testis
tissues were decreased after PFOA induction, while the production
of MDA was increased. Treatment with high-dose LA increased the
expression of SOD and GSH-Px and decreased the production of MDA,
but there was no difference in the rats in the PFOA+LA (L) group.
The therapeutic effect was dose-related. These results demonstrated
that LA alleviated the oxidative stress injury caused by PFOA
(Table V).
 | Table VTherapeutic effects of LA on
oxidative injury. |
Table V
Therapeutic effects of LA on
oxidative injury.
| Group | Dose, g/kg | Superoxide
dismutase, U/ml | Glutathione
peroxidase, U/mg | Malondialdehyde,
nmol/mg |
|---|
| Control | - | 18.86±1.37 | 722.88±118.17 | 0.87±0.14 |
| PFOA | 0.01 |
9.60±0.80a |
306.20±77.53a |
1.24±0.35a |
| LA | 0.10 |
18.82±1.34b |
720.78±116.52b |
0.85±0.16b |
| PFOA+LA (L) | LA, 0.05; PFOA,
0.01 |
11.24±1.63a,c |
396.77±101.24a,c | 0.98±0.15 |
| PFOA+LA (H) | LA, 0.10; PFOA,
0.01 |
17.18±1.42b,d |
544.58±89.41a-d |
0.70±0.23b |
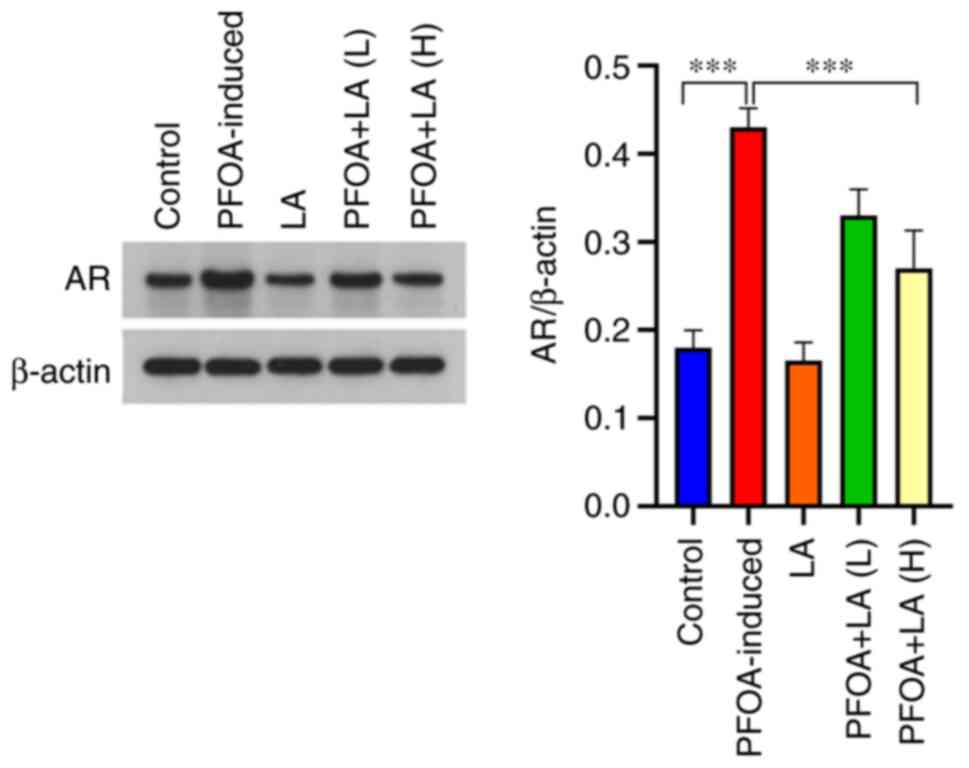
LA reduces AR expression in
PFOA-induced rats
Western blotting results revealed that AR protein
expression in rat testes was increased after PFOA induction
(Fig. 3). The increase in AR
expression has been reported be associated with the destruction of
the hypothalamus-pituitary-testis axis (17). AR expression was decreased after
administration of LA; however, no significant difference was
observed in the rats in the PFOA+LA (L) group, and the therapeutic
effect was positively associated with the dose of LA.
Discussion
The extensive use of PFOA has caused serious
pollution to the environment (3).
PFOA reduces the levels of human serum T and reproductive hormones,
resulting in delayed embryonic development or miscarriage during
pregnancy (18-21).
LA is an important cofactor in mitochondrial metabolism, and it can
scavenge a variety of oxygen free radicals (22,23).
Therefore, the present study aimed to explore whether LA has a
therapeutic effect on PFOA-induced reproductive damage.
The integrity of the BTB is an important condition
to ensure the progress of spermatogenesis (14). In addition, the body weights and
sperm counts are indicators of animal reproductive toxicity
(24). After being induced by
PFOA, the rats exhibited destroyed seminiferous tubule structure,
decreased sperm counts, decreased weights of testes and epididymis,
irregular shape of seminiferous tubules, disordered arrangement of
spermatogenic cells, and decreased numbers of spermatogenic cells.
These symptoms were alleviated after the intervention of LA, and
the therapeutic effect was positively associated with the dose.
LDH and SDH serve an important role in the process
of spermatogenesis and can be used as indicators to evaluate
spermatogenesis (25,26). SDH exists in seminiferous tubules
and mitochondria of spermatogenic cells, and it can convert
sorbitol to fructose (27).
Fructose generates energy through the glycolysis pathway in sperm
and participates in the energy metabolism of sperm (28). LDH is the key enzyme of sperm
glycolysis, and it participates in the development of spermatogenic
cells and the energy metabolism of sperm (27,29).
After treatment with high-dose LA, the activities of SDH and LDH
returned to normal levels, which alleviated the testicular energy
metabolism disorder caused by PFOA in rats.
When the testis is stimulated by external stimuli or
harmful substances, it will generate a defensive response, and the
testis will produce excess reactive oxygen species (ROS) (30). The excessive ROS produced exceed
the antioxidant scavenging capacity of the testis, causing
peroxidative damage to the testis (31). MDA is a product of lipid
peroxidation and its amount can be used as a marker to assess
oxidative damage (32). In
addition, SOD and GSH-Px are indispensable antioxidant defence
enzymes in the testes (33). The
present study revealed that the production of MDA in the testis
tissues of PFOA-induced rats was increased, while the activities of
SOD and GSH-Px were decreased. After LA treatment, the production
of MDA was decreased, and the activities of SOD and GSH-Px were
increased, indicating that LA had a therapeutic effect on
PFOA-induced testicular tissue peroxidation.
Furthermore, the present study explored the
mechanism of LA in treating reproductive injury induced by PFOA at
the hormone level. The hypothalamus-pituitary-testis axis is
closely related to spermatogenesis (34). The hypothalamus secretes a
gonadotropin-releasing hormone that acts on the pituitary,
prompting the pituitary to secrete FSH (35,36).
FSH not only binds to receptors on Sertoli cells to regulate
spermatogenesis and differentiation of spermatogenic cells but also
promotes Leydig cells to synthesize T (17). AR mainly exists in the Sertoli
cells of the testes and is a necessary intermediate substance for
androgen to serve the role of spermatogenic regulation (18,37).
T serves a role in male reproduction by affecting the synthesis of
E2(38). The levels of FSH were
increased, and the levels of T and E2 were decreased in the
PFOA-induced rats. However, the levels of LH in the plasma did not
change after induction of PFOA, which is consistent with the
results of another study (39).
This result may be caused by PFOA disrupting the rat
hypothalamic-pituitary-testis axis to initiate the feedback
regulation of FSH. Western blotting results demonstrated that AR
expression was increased after exposure to PFOA. The levels of
serum hormone and AR expression in rats returned to normal after LA
treatment, indicating that LA exerted therapeutic effects via the
hypothalamic-pituitary-testis axis.
In summary, LA was effective in treating
PFOA-induced reproductive damage by regulating the oxidative stress
pathway in the testes and the hypothalamic-pituitary-testis axis.
LA may be applied to humans to protect the reproductive system
against poisons in the future.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Inner Mongolia
Autonomous Region-level College Students Innovation and
Entrepreneurship Training Program (grant no. 201910127005), the
Baotou Medical College Scientific Research Fund Project (grant no.
BYJJ-YF-2018021) and the Baotou Medical College Postgraduate
Research and Innovation Funding Project (grant no.
bycx2019003).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JB, ZZ and RW participated in the conception, design
and data acquisition of the article. WZ and NG participated in the
analysis and interpretation of data, drafting the manuscript and
made critical revisions for important intellectual content. JB
ensured that questions related to the integrity of any part of the
work were appropriately investigated and resolved. ZZ, RW, WZ, NG
and JB confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Baotou Medical College, Inner Mongolia University of
Science and Technology (Baotou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zareitalabad P, Siemens J, Hamer M and
Amelung W: Perfluorooctanoic acid (PFOA) and
perfluorooctanesulfonic acid (PFOS) in surface waters, sediments,
soils and wastewater-a review on concentrations and distribution
coefficients. Chemosphere. 91:725–732. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zheng Z, Yu H, Geng WC, Hu XY, Wang YY, Li
Z, Wang Y and Guo DS: Guanidinocalix[5]arene for sensitive
fluorescence detection and magnetic removal of perfluorinated
pollutants. Nat Commun. 10(5762)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xiao L, Ling Y, Alsbaiee A, Li C, Helbling
DE and Dichtel WR: β-Cyclodextrin polymer network sequesters
perfluorooctanoic acid at environmentally relevant concentrations.
J Am Chem Soc. 139:7689–7692. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Johansson N, Eriksson P and Viberg H:
Neonatal exposure to PFOS and PFOA in mice results in changes in
proteins which are important for neuronal growth and synaptogenesis
in the developing brain. Toxicol Sci. 108:412–418. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chang ET, Adami HO, Boffetta P, Wedner HJ
and Mandel JS: A critical review of perfluorooctanoate and
perfluorooctanesulfonate exposure and immunological health
conditions in humans. Crit Rev Toxicol. 46:279–331. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Croce L, Coperchini F, Tonacchera M,
Imbriani M, Rotondi M and Chiovato L: Effect of long- and
short-chain perfluorinated compounds on cultured thyroid cells
viability and response to TSH. J Endocrinol Invest. 42:1329–1335.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wan HT, Lai KP and Wong CKC: Comparative
analysis of PFOS and PFOA toxicity on sertoli cells. Environ Sci
Technol. 54:3465–3475. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu W, Yang B, Wu L, Zou W, Pan X, Zou T,
Liu F, Xia L, Wang X and Zhang D: Involvement of NRF2 in
perfluorooctanoic acid-induced testicular damage in male mice. Biol
Reprod. 93(41)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park S, Karunakaran U, Jeoung NH, Jeon JH
and Lee IK: Physiological effect and therapeutic application of
alpha lipoic acid. Curr Med Chem. 21:3636–3645. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG,
Yang YY, Guo SQ, Wang S, Guo T, Wang ZY and Guo C: α-Lipoic acid
improves abnormal behavior by mitigation of oxidative stress,
inflammation, ferroptosis, and tauopathy in P301S Tau transgenic
mice. Redox Biol. 14:535–548. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Corrêa LBNS, da Costa CAS, Ribas JAS,
Boaventura GT and Chagas MA: Antioxidant action of alpha lipoic
acid on the testis and epididymis of diabetic rats: Morphological,
sperm and immunohistochemical evaluation. Int Braz J Urol.
45:815–824. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chukanova EI and Chukanova AS:
Alpha-lipoic acid in the treatment of diabetic polyneuropathy. Zh
Nevrol Psikhiatr Im S S Korsakova. 118:103–109. 2018.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
13
|
Morini M, Roccatagliata L, Dell'Eva R,
Pedemonte E, Furlan R, Minghelli S, Giunti D, Pfeffer U, Marchese M
and Noonan D: Alpha-lipoic acid is effective in prevention and
treatment of experimental autoimmune encephalomyelitis. J
Neuroimmunol. 148:146–153. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cheng CY and Mruk DD: The blood-testis
barrier and its implications for male contraception. Pharmacol Rev.
64:16–64. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xiao Y, Xu B, Bordiga M, Li H, Travaglia
F, Bai S, Chen J and Bai W: Cyanidin-3-O-glucoside supplement
improves sperm quality and spermatogenesis in a mice model of
ulcerative colitis. Nutrients. 14(984)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gautam R, Priyadarshini E, Nirala JP,
Meena R and Rajamani P: Modulatory effects of Punica granatum L
juice against 2115 MHz (3G) radiation-induced reproductive toxicity
in male Wistar rat. Environ Sci Pollut Res Int. 28:54756–54765.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gill-Sharma MK: Testosterone retention
mechanism in sertoli cells: A biochemical perspective. Open Biochem
J. 12:103–112. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eggert A, Cisneros-Montalvo S, Anandan S,
Musilli S, Stukenborg JB, Adamsson A, Nurmio M and Toppari J: The
effects of perfluorooctanoic acid (PFOA) on fetal and adult rat
testis. Reprod Toxicol. 90:68–76. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lu H, Zhang H, Gao J, Li Z, Bao S, Chen X,
Wang Y, Ge R and Ye L: Effects of perfluorooctanoic acid on stem
Leydig cell functions in the rat. Environ Pollut. 250:206–215.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li D, Song P, Liu L and Wang X:
Perfluorooctanoic acid exposure during pregnancy alters the
apoptosis of uterine cells in pregnant mice. Int J Clin Exp Pathol.
11:5602–5611. 2018.PubMed/NCBI
|
|
21
|
Tsai MS, Lin CY, Lin CC, Chen MH, Hsu SH,
Chien KL, Sung FC, Chen PC and Su TC: Association between
perfluoroalkyl substances and reproductive hormones in adolescents
and young adults. Int J Hyg Environ Health. 218:437–443.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Solmonson A and DeBerardinis RJ: Lipoic
acid metabolism and mitochondrial redox regulation. J Biol Chem.
293:7522–7530. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tibullo D, Li Volti G, Giallongo C, Grasso
S, Tomassoni D, Anfuso CD, Lupo G, Amenta F, Avola R and Bramanti
V: Biochemical and clinical relevance of alpha lipoic acid:
Antioxidant and anti-inflammatory activity, molecular pathways and
therapeutic potential. Inflamm Res. 66:947–959. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Marty MS, Erraguntla N, North C, Barranco
WT, Kirman CR, Cagen S, Rushton EK, Shen H, Koehler MW and Budinsky
R: A reproductive and developmental toxicity screening study of
1,3-butadiene in Sprague-Dawley rats. Regul Toxicol Pharmacol.
127(105066)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang GL, Yu F, Dai DZ, Cheng YS, Zhang C
and Dai Y: CPU86017-RS attenuate hypoxia-induced testicular
dysfunction in mice by normalizing androgen biosynthesis genes and
pro-inflammatory cytokines. Acta Pharmacol Sin. 33:470–478.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu YZ, Sun H, Fu Y, Wang J, Song M, Li M,
Li YF and Miao LG: Effects of sub-chronic aluminum chloride on
spermatogenesis and testicular enzymatic activity in male rats.
Life Sci. 102:36–40. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cao W, Aghajanian HK, Haig-Ladewig LA and
Gerton GL: Sorbitol can fuel mouse sperm motility and protein
tyrosine phosphorylation via sorbitol dehydrogenase. Biol Reprod.
80:124–133. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Calvert SJ, Reynolds S, Paley MN, Walters
SJ and Pacey AA: Probing human sperm metabolism using 13C-magnetic
resonance spectroscopy. Mol Hum Reprod. 25:30–41. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xie J, Yu J, Zhang Z, Liu D, Fan Y, Wu Y,
Ma H, Wang C and Hong Z: AMPK pathway is implicated in low level
lead-induced pubertal testicular damage via disordered glycolysis.
Chemosphere. 291(132819)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Teves ME and Roldan ERS: Sperm bauplan and
function and underlying processes of sperm formation and selection.
Physiol Rev. 102:7–60. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang K, Gao Y, Wang C, Liang M, Liao Y and
Hu K: Role of oxidative stress in varicocele. Front Genet.
13(850114)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Meng L, Liu J, Wang C, Ouyang Z, Kuang J,
Pang Q and Fan R: Sex-specific oxidative damage effects induced by
BPA and its analogs on primary hippocampal neurons attenuated by
EGCG. Chemosphere. 264(128450)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jia ZQ, Liu D, Sheng CW, Casida JE, Wang
C, Song PP, Chen YM, Han ZJ and Zhao CQ: Acute toxicity,
bioconcentration, elimination and antioxidant effects of fluralaner
in zebrafish, Danio rerio. Environ Pollut. 232:183–190.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chimento A, Sirianni R, Casaburi I and
Pezzi V: Role of estrogen receptors and g protein-coupled estrogen
receptor in regulation of hypothalamus-pituitary-testis axis and
spermatogenesis. Front Endocrinol (Lausanne). 5(1)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim K, Lee BJ, Cho BN, Kang SS, Choi WS,
Park SD, Lee CC, Cho WK and Wuttke W: Blockade of noradrenergic
neurotransmission with diethyldithiocarbamic acid decreases the
mRNA level of gonadotropin-releasing hormone in the hypothalamus of
ovariectomized, steroid-treated prepubertal rats.
Neuroendocrinology. 59:539–544. 1994.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim K, Lim IS, Cho BN, Kang SS, Lee BJ,
Choi KH, Chung CH, Lee CC, Cho WK and Wuttke W: A partial blockade
of catecholaminergic neurotransmission with 6-hydroxydopamine
decreases mRNA level of gonadotropin releasing hormone in the male
rat hypothalamus. Neuroendocrinology. 58:146–152. 1993.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wu RC, Jiang M, Beaudet AL and Wu MY:
ARID4A and ARID4B regulate male fertility, a functional link to the
AR and RB pathways. Proc Natl Acad Sci USA. 110:4616–4621.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Alemany M: The roles of androgens in
humans: Biology, metabolic regulation and health. Int J Mol Sci.
23(11952)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bookstaff RC, Kamel F, Moore RW, Bjerke DL
and Peterson RE: Altered regulation of pituitary
gonadotropin-releasing hormone (GnRH) receptor number and pituitary
responsiveness to GnRH in
2,3,7,8-tetrachlorodibenzo-p-dioxin-treated male rats. Toxicol Appl
Pharmacol. 105:78–92. 1990.PubMed/NCBI View Article : Google Scholar
|