Introduction
Endoscopic submucosal dissection (ESD) is a widely
recognized interventional therapy for en bloc resection of
gastrointestinal lesions (1). ESD
is a minimally invasive treatment method. Regardless of the size
and location of the lesion, superficial gastrointestinal tumors can
be completely removed. ESD has higher technical requirements,
especially for larger and more invasive injuries. However, it
carries a high risk of complications, including perforation,
bleeding and post-ESD coagulation syndrome (2,3),
among which hemorrhage is the most serious and common. Yang et
al (4) reported that there has
been no agreement on the definition of bleeding after ESD,
resulting in the reported rates of bleeding after ESD ranging from
1.3 to 13.0%. Post-ESD bleeding can be controlled through
endoscopic intervention or blood transfusion (5), but it cannot be completely prevented
and may lead to life-threatening conditions such as hemorrhagic
shock (6). Acquired hemophilia A
(AHA) is a severe bleeding disorder characterized by an
autoantibody directed against coagulation factor VIII, and these
antibodies arise in individuals with no prior history of AHA; it is
characterized by bleeding in 90% of patients, of which 70% have
severe bleeding (7,8). In total, 50% of AHA cases are
idiopathic, while the remaining 50% are related to pregnancy,
autoimmune diseases, malignant tumors and drugs (9). In clinical practice, multiple
postoperative bleeds combined with AHA is rare. By reviewing both
domestic and foreign literature and combining this with a case
report from Wuhan No. 1 Hospital (Wuhan, China), the clinical
characteristics of rare concurrent AHA after ESD are discussed and
analyzed in the present study. The aim of the present study was to
aid the analysis and diagnosis of clinical ESD postoperative
hemorrhage.
Case report
The patient in the present case report (age, 48
years; sex, male) was admitted to Wuhan No. 1 Hospital (Wuhan,
China) in November 2019 due to irregular stools accompanied by
increased stool frequency for half a year. A colonoscopy was
performed in another hospital due to a change of stool shape, which
suggested rectal polyps. No medical treatment was administered
during this period. The patient had no history of any chronic
disease, such as hypertension, diabetes or heart disease, and
reported no family history of inherited diseases, spontaneous
bleeding or non-continuous bleeding after trauma. After admission,
no obvious abnormalities were found in the blood analysis, liver
function, renal function, carcinoembryonic antigen or coagulation
function [prothrombin time (PT), 10.6 sec (reference value, 9-13
sec); activated partial thromboplastin time (APTT), 28.8 sec
(reference value, 20-40 sec)] tests. Gastroscopy indicated reflux
esophagitis, erosive gastritis and duodenitis. Colonoscopy
performed in Wuhan No. 1 Hospital (Wuhan, China) revealed a
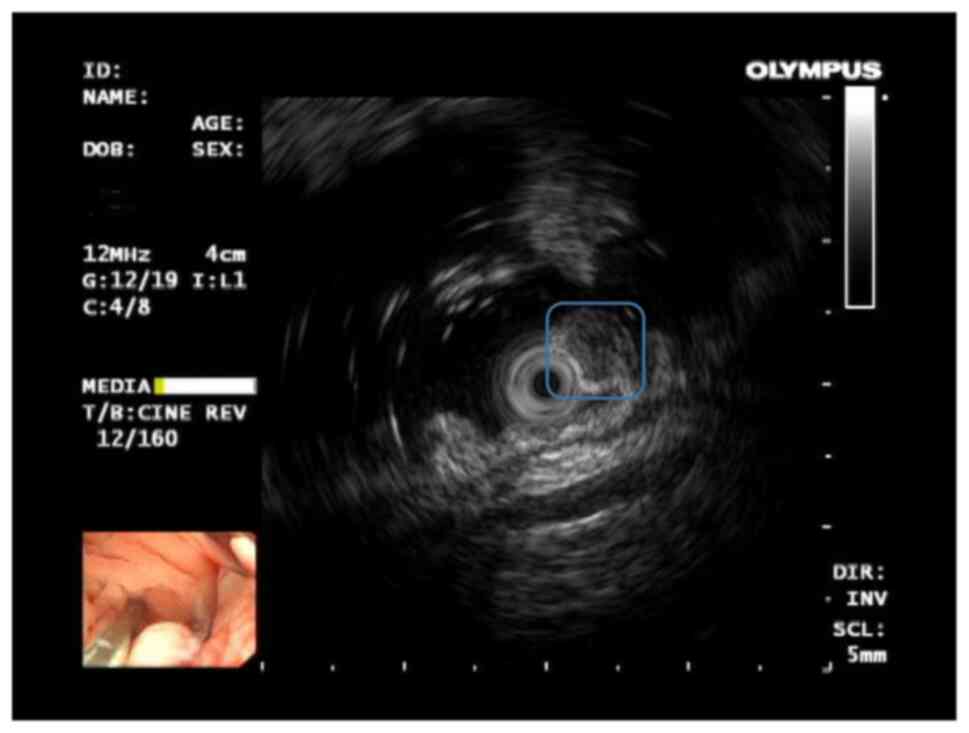
submucosal tumor of the rectum 15 cm from the anus. Ultrasound
colonoscopy (Fig. 1) suggested a
submucosal hypoechoic eminence (considering the possibility of
neuroendocrine neoplasm). Consequently, ESD therapy was performed
on day 4 after admission. ESD involved marking around the lesion ~5
mm from the edge using an Erbe electric knife system (Erbe
Elektromedizin GmbH), submucosal injection to lift the lesion from
the muscularis propria, circumferential incision into the submucosa
outside the marked points and submucosal dissection until the
lesion was removed. The electric cutting mode was ENDO
CUT® Q and FORCED COAG, with effect 3 and power 50.
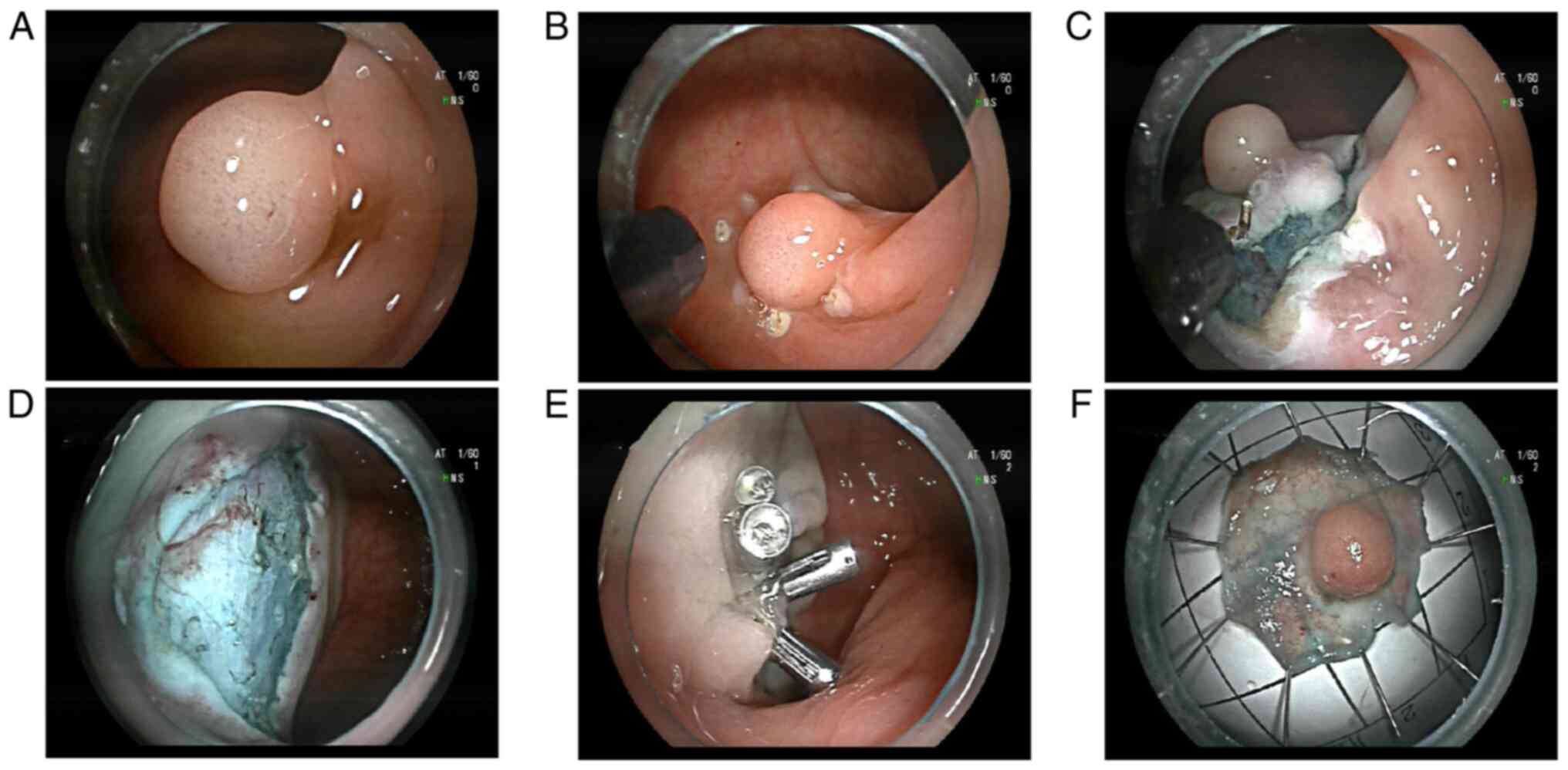
There was ~20 ml of blood loss during the ESD operation, and the
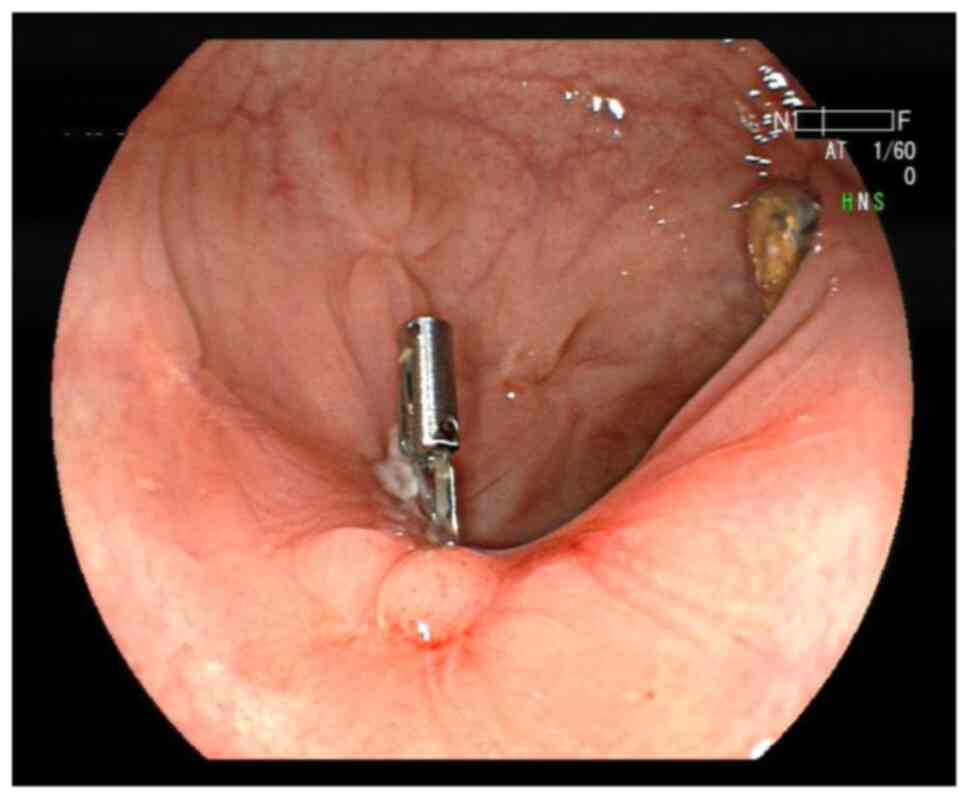
operation went smoothly. Multiple titanium clips completely closed
the wound surface (Fig. 2). The
pathological results indicated a spindle cell tumor in the rectum,
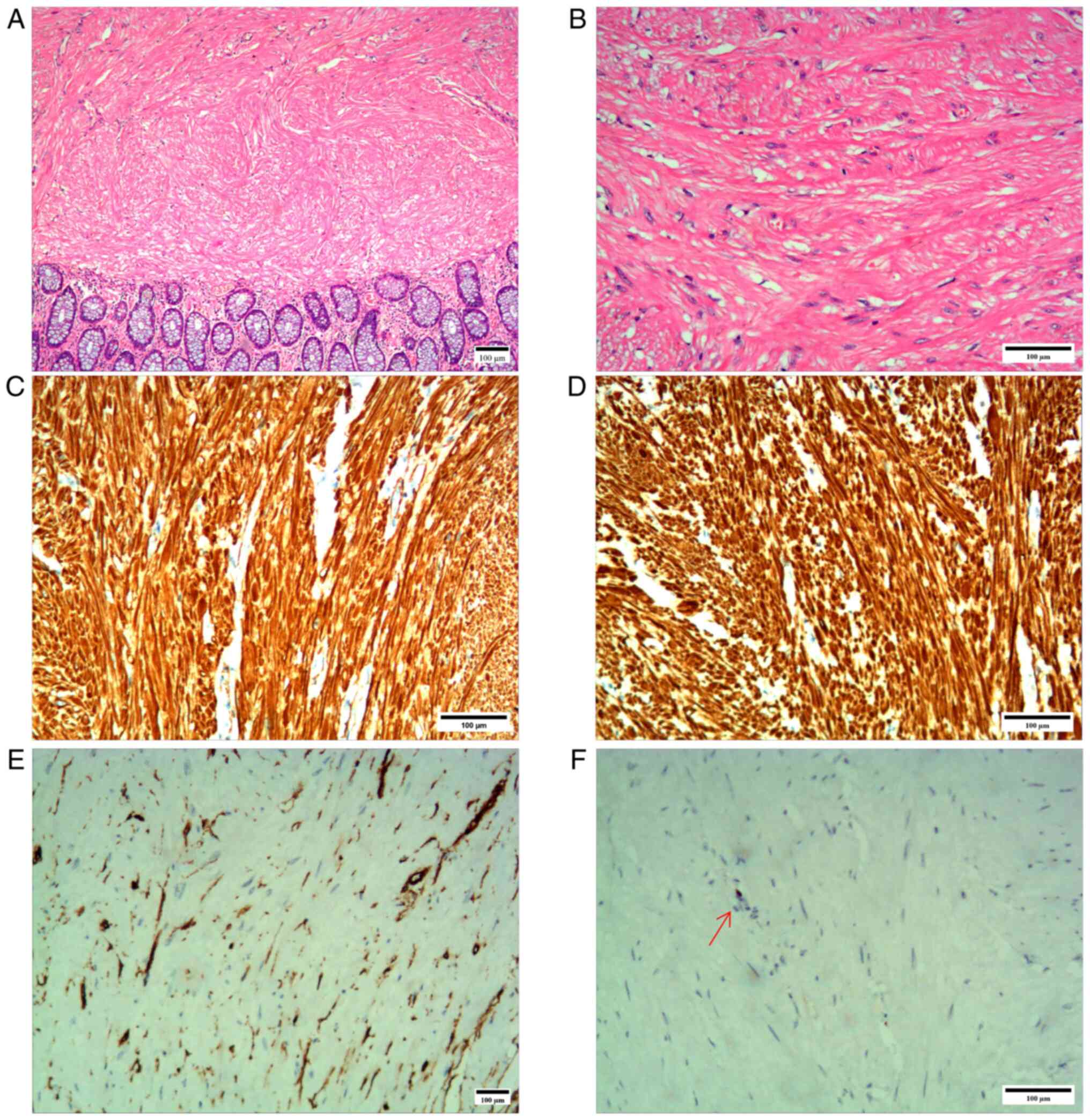
with a diameter of 0.6-0.8 cm. Immunohistochemical results
(Fig. 3) were as follows:
caldesmon(+) (data not shown), CD117(-), CD34 [tumor cell(-);
vascular(+); data not shown], desmin(+), dog-1(-), GFAP(-), Ki-67
(<1%), S-100(-), SMA(+) and Sox10(-). A diagnosis of leiomyoma
was eventually considered (Fig.
3).
The first bleed occurred on day 4 after the
operation, indicated by a small amount of bloody stool. A
colonoscopy was performed and found a little bleeding of the wound,
which a titanium clip was used to stop. On day 7 after the
operation, fresh bloody stool was released again, to an amount of
~800 ml. Under colonoscopy, it was found that part of the titanium
clip closing the ESD wound surface had fallen off, and three small
blood vessels were actively bleeding on the exposed wound surface.
Erbe electric coagulation hemostatic forceps were used to stop the
bleeding using the electric knife system. After the operation, the
patient temporarily fasted and was treated with octreotide (48 ml
physiological saline + 0.3 mg octreotide via micro pump, 4 ml/h)
for hemostasis, and a blood transfusion was performed. On day 10
after the operation, the stool turned yellow and the patient began
a liquid diet. On day 14 after the operation, fresh bloody stool
was discharged again, with a volume of ~1,000 ml, accompanied by
fatigue. Colonoscopy showed that there was blood spurting from the
exposed ESD wound surface. Consequently, the wound was sealed with
electrocoagulation hemostatic forceps and a nylon rope titanium
clip. On day 18 after the operation, fresh bloody stools appeared
again, and active vascular bleeding was seen under colonoscopy.
Electrocoagulation was applied again to stop the bleeding, and a
nylon rope titanium clip was used to suture the wound. On day 24
after the operation, another bleeding spot was found on the ESD
wound by colonoscopy, and the bleeding was stopped by
electrocoagulation once again. On the same day, a routine blood
examination showed that the white blood cell count was
10.89x109 cells/l (reference range,
3.5-9.5x109 cells/l), the red blood cell count was
3.41x1012 cells/l (reference range,
3.8-5.1x109 cells/l) and the hemoglobin level was 102
g/l (normal range, 130-175 g/l), while coagulation function tests
showed that PT and APTT were normal and the D-dimer level was in
the normal range. On day 27 after the operation, a small amount of
dark red bloody stool was discharged. Endoscopy showed no active
bleeding, and no special treatment was given. Once again, routine
blood examination showed a white blood cell count of
8.81x109 cells/l, a red blood cell count of
2.85x1012 cells/l and a hemoglobin level of 96 g/l.
Coagulation function tests indicated that PT and APTT were still
within the normal range, the fibrinogen level (1.72 g/l; normal
range, 2-4 g/l) was slightly decreased and the level of fibrinogen
degradation products (50.7 mg/l; normal 0.0-5.0 mg/l) was
increased. The activity of plasma antithrombin III was normal.
Fresh bloody stools were once again discharged on day 28 after
operation. One of the ESD wounds was found to be bleeding near the
titanium clip, which was again stopped by electrocoagulation. The
number of bleeding incidents was as high as seven in the month
after the ESD operation, as shown in Fig. 4.
To address this issue, a hematologist was invited
for consultation in order to explore the cause of bleeding. Based
on the consultation, further examinations were performed. The
results were as follows: von Willebrand factor antigen, 54.5%
(reference range, 50-150%); coagulation factor VIII activity
(FVIII:C), 22.5% (decreased significantly; reference value,
50-150%); coagulation factor IX, XI and XII activities, 132.3%
(reference range, 50-150%), 85.2% (reference range, 50-150%) and
45.3% (reference range, 50-150%), respectively; PT, 12.7 sec
(reference value range, 9-13 sec); APTT, 43.2 sec (reference range,
23.9-31.9 sec); and international normalized ratio 1.17 (reference
range, 0.7-1.3). It was concluded that the repeated bleeding after
ESD was related to the decrease in FVIII:C, and the treatment plan
was adjusted accordingly. The diet provided to the patient was
mainly liquid, and the patient was given multiple fresh plasma
(containing FVIII, 400 ml, once), cryoprecipitate (containing
FVIII, 400 ml, once) and enteral nutrition (containing amino acids,
fat emulsion, glucose, vitamins, etc.; 1,500 ml, daily for 5 days
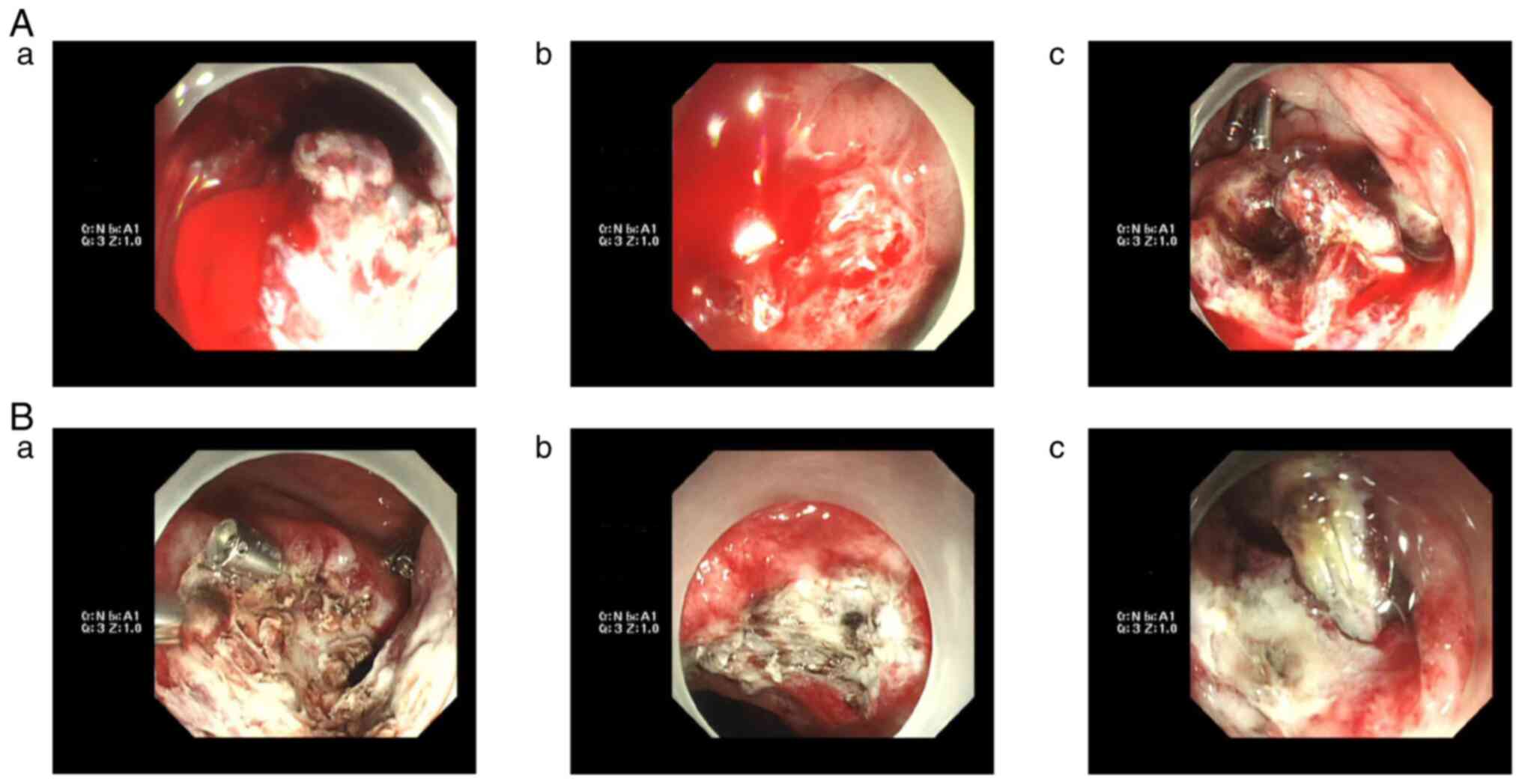
in total). There was no re-bleeding. Colonoscopy showed that the
rectal ESD wound had healed well on the 51st day after the
operation (Fig. 5), showing a
favorable prognosis.
Discussion
AHA is an autoimmune disease caused by inhibitors of
FVIII, which is rare but life threatening (10). The rarity of AHA means that
clinicians have insufficient treatment experience, which often
leads to delayed diagnosis and treatment. The incidence of AHA
ranges from 0.2 to 1.48 cases per 1 million individuals per year
(11). In the present case report,
the patient had no history of bleeding and no family history of
AHA/bleeding. AHA is usually discovered incidentally when patients
experience bleeding during surgery, trauma or other invasive
examinations, or when APTT is found to be prolonged slightly in a
routine coagulation function test. However, a timely diagnosis
allows for effective hemostasis to be achieved. In clinical
practice, gastrointestinal bleeding is one of the predictable
complications of ESD. According to the literature, the causes of
bleeding after ESD may be related to the following factors: i)
Basic diseases, such as hypertension, liver cirrhosis, coronary
heart disease, old cerebral infarction, renal insufficiency and
atrial fibrillation; ii) drugs used prior to operation, such as
anticoagulant or antiplatelet drugs, antihypertensive drugs (for
example, nifedipine) and heparin replacement therapy; and iii)
lesion factors, including submucosal vascular proliferation,
submucosal fibrosis formation, intraoperative massive bleeding,
arterial bleeding location and quantity, lesion location (for
example, proximal stomach, ileocecal or low rectum), multiple
lesions, larger lesions and longer operation time (12-14).
To further understand the association between
postoperative bleeding and AHA, ‘acquired hemophilia A’,
‘postoperative’ and ‘bleeding’ were used as key words and searched
in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/). A total of six
studies reporting 7 cases were assessed by descriptive analysis
(15-20)
(Table I).
 | Table ICharacteristics of included literature
studies. |
Table I
Characteristics of included literature
studies.
| First author,
year | Age | Sex | Origin | Primary disease | Means of
intervention | Preoperative APTT,
sec | Days of bleeding
after operation | Postoperative APTT,
seca | Factor VIII activity,
%a | FVIII inhibitor,
BU/ml | Treatment for
bleeding | (Refs.) |
|---|
| Hosoya et al,
2013 | 80 | Female | Japan | Superficial
esophageal squamous cell carcinoma | Thoracic
esophagectomy with radical lymph node dissection | 34.9 (normal) | 39 | 140 increased | 1 decreased | 36 | rFVIIa, PSL,
endoscopically with clipping, cyclophosphamide, methylprednisolone
sodium succinate and red cell concentrates | (15) |
| Okamura et al,
2015 | 65 | Male | Japan | Ruptured aneurysmal
subarachnoid hemorrhage, hydrocephalus and neurogenic
dysphagia | Aneurysmal clipping,
external ventricular drainage and percutaneous endoscopic
gastrostomy | 27.2 (normal) | 6 | 79.2 increased | 4 decreased | 10 | PSL | (16) |
| Onishi et al,
2013 | 71 | Male | Japan | Bile duct
obstruction, cholangitis and bile duct cancer | Endoscopic retrograde
biliary drainage, endoscopic nasal biliary drainage tube and
radical pancreaticoduodenectomy | ND | 8 | 83.1 increased | 1 decreased | 8 | Activated prothrombin
complex concentrate, PSL and rituximab | (17) |
| Saito et al,
2018 | 63 | Male | Japan | Gastric cancer | Gastrectomy | ND | 5 | 74.2 increased | 3.18 decreased | 7.59 | Red blood cell
transfusions, PSL and tranexamic acid | (18) |
| Khan et al,
2020 (Case 1) | 12 | Female | China | Congenital
choledochal cyst | Resection of common
bile duct cyst and gallbladder, Roux-en-Y anastomosis of hepatic
duct to jejunum | 45 (normal) | 7 | 150.9 increased | 5 decreased | ND | Blood transfusion and
surgery | (19) |
| Khan et al,
2020 (Case 2) | 18 | Male | China | Large common bile
duct cyst involving left and right hepatic bile ducts | Resection of
choledochal cyst and gallbladder followed by Roux-en-Y
anastomosis | 45 (normal) | 1 | 105.1 increased | 11.2 decreased | 16 | Activated prothrombin
complex concentrates, fresh frozen plasma, blood plasma
cryoprecipitate, intravenous immunoglobulin, methylprednisolone,
plasmapheresis and rFVIIa | (19) |
| Oba et al,
2020 | 46 | Male | Japan | Buccal mucosal
squamous cell carcinoma | Preoperative
chemotherapy and radical oral tumor resection | 31.2 (normal) | 1 | 110.8 increased | 4 decreased | 2 | Red blood cells and
fresh frozen plasma, rFVIIa and prednisolone | (20) |
The patients included in the literature had no
history of chronic disease, hemorrhagic disease or genetic disease.
The age distribution of the patients ranged from 12 to 80 years
old, among which 4 cases were in patients ≥60 years old. The
results of the preoperative examination showed that APTT was normal
in 3 cases and slightly elevated in 2 cases, while in 2 cases, no
relevant data were provided. However, all cases showed
postoperative bleeding with APTT increasing significantly.
Furthermore, FVIII activities were markedly low and patients were
positive for the FVIII inhibitor. After active treatment of AHA,
bleeding stopped in all the reported cases.
According to the literature, the diagnosis of AHA
mainly depends on the laboratory examination, as well as
determining the related causes and symptoms of bleeding (10,21,22).
When APTT prolongation occurs, the APTT temperature correction test
cannot be performed, which indicates that an endogenous coagulation
factor antibody is present in the plasma. This suggests that FVIII
activity is reduced and an FVIII antibody is present. When the
activity of multiple coagulation factors decreases, it is necessary
to clarify whether to use lupus anticoagulant drugs, so as to
provide a basis for differential diagnosis (10). The key goal of AHA treatment is to
control acute bleeding and eliminate inhibitors (23). Treatment options include the
following: i) Hemostasis treatment, including application of
deaminase-8-d arginine vasopressin, FVIII concentrate, bypass
activation pathway (activated prothrombin complex or recombinant
activated coagulation FVIII), immunoglobulin, plasma exchange and
immunoadsorption; and ii) eradication of inhibitors via the
application of immunosuppressive agents, including glucocorticoids,
cytotoxic drugs (such as, cyclophosphamide and azathioprine), CD20
monoclonal antibody preparation, cetuximab, cyclosporine and
tacrolimus (22,24).
The characteristics and summary of the patient in
the present case report were as follows: i) Middle-aged and male;
ii) no history of hereditary disease or hemorrhagic disease; iii)
diagnosis of rectal submucosal tumor (benign leiomyoma confirmed by
postoperative pathology); iv) ESD treatment was performed; v) APTT
and platelet count were normal before operation; vi) intermittent
and repeated bleeding after ESD; and vii) APTT began to prolong and
FVIII activity decreased after ESD (FVIII inhibitors were not
tested). Hemophilia was not initially considered in this patient
with postoperative bleeding. However, repeated endoscopic
hemostasis was ineffective and APTT began to prolong on day 28
after the operation. Thus, potential AHA should be considered when
repeated bleeding occurs after invasive procedures.
Autoimmune disease refers to the disease caused by
the immune response to autoantibodies, which leads to the damage of
an individual's own tissues. The existence of autoantibodies is not
the same as the presence of autoimmune diseases; autoantibodies may
exist in normal people without autoimmune diseases. A stress state
can lead to the production of autoantibodies, but such antibodies
have no pathogenic effect and belong to a secondary immune response
(25,26). The present patient suffered from
rectal leiomyoma before admission, which was a benign tumor and had
no significant association with AHA. By contrast, the trauma caused
by resection of the rectal leiomyoma may have led to the production
of related antibodies, thus inducing AHA.
In conclusion, when repeated gastrointestinal
bleeding occurs and endoscopic hemostasis is ineffective, even if
there is no history of coagulation disorders or genetic hemorrhage,
the possibility of secondary AHA should not be ignored. The risk of
bleeding caused by a delayed diagnosis of AHA may be one of the
reasons for its high mortality. A timely and accurate diagnosis can
quickly control the bleeding from AHA, which is key to preventing
serious complications.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL and NW contributed to study conception and
design, data collection, the literature search and manuscript
writing. SL and NW contributed equally to this work. ZM and XG
obtained the medical images in the patient treatment and searched
the literature. ZS designed the study and reviewed the manuscript.
All authors have read and approved the final manuscript. SL and ZS
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent to publish this case report
was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Toyonaga T, Man-I M, East JE, Nishino E,
Ono W, Hirooka T, Ueda C, Iwata Y, Sugiyama T, Dozaiku T, et al:
1,635 Endoscopic submucosal dissection cases in the esophagus,
stomach, and colorectum: Complication rates and long-term outcomes.
Surg Endosc. 27:1000–1008. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nishizawa T and Yahagi N: Endoscopic
mucosal resection and endoscopic submucosal dissection: Technique
and new directions. Curr Opin Gastroenterol. 33:315–319.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Arimoto J, Higurashi T, Kato S, Fuyuki A,
Ohkubo H, Nonaka T, Yamaguchi Y, Ashikari K, Chiba H, Goto S, et
al: Risk factors for post-colorectal endoscopic submucosal
dissection (ESD) coagulation syndrome: A multicenter, prospective,
observational study. Endosc Int Open. 6:E342–349. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang CH, Qiu Y, Li X and Shi RH: Bleeding
after endoscopic submucosal dissection of gastric lesions. J Dig
Dis. 21:139–146. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Patel N, Patel K, Ashrafian H, Athanasiou
T, Darzi A and Teare J: Colorectal endoscopic submucosal
dissection: Systematic review of mid-term clinical outcomes. Dig
Endosc. 28:405–416. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kataoka Y, Tsuji Y, Sakaguchi Y, Minatsuki
C, Asada-Hirayama I, Niimi K, Ono S, Kodashima S, Yamamichi N,
Fujishiro M and Koike K: Bleeding after endoscopic submucosal
dissection: Risk factors and preventive methods. World J
Gastroenterol. 22:5927–5935. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Knoebl P, Marco P, Baudo F, Collins P,
Huth-Kühne A, Nemes L, Pellegrini F, Tengborn L and Lévesque H:
EACH2 Registry Contributors. Demographic and clinical data in
acquired hemophilia A: Results from the European Acquired
Haemophilia Registry (EACH2). J Thromb Haemost. 10:622–631.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baudo F, Collins P, Huth-Kühne A, Lévesque
H, Marco P, Nemes L, Pellegrini F, Tengborn L and Knoebl P: EACH2
registry contributors. Management of bleeding in acquired
hemophilia A: Results from the European Acquired Haemophilia
(EACH2) Registry. Blood. 120:39–46. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Franchini M and Lippi G: Acquired
hemophilia A. Adv Clin Chem. 54:71–80. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kruse-Jarres R, Kempton CL, Baudo F,
Collins PW, Knoebl P, Leissinger CA, Tiede A and Kessler CM:
Acquired hemophilia A: Updated review of evidence and treatment
guidance. Am J Hematol. 92:695–705. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Webert KE: Acquired hemophilia A. Semin
Thromb Hemost. 38:735–741. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Terasaki K, Dohi O, Naito Y, Azuma Y,
Ishida T, Kitae H, Matsumura S, Ogita K, Takayama S, Mizuno N, et
al: Effects of Guidelines for gastroenterological endoscopy in
patients undergoing antithrombotic treatment on postoperative
bleeding after endoscopic submucosal dissection for early gastric
cancer:A propensity score-matching analysis. Digestion.
102:256–264. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Suzuki S, Chino A, Kishihara T, Uragami N,
Tamegai Y, Suganuma T, Fujisaki J, Matsuura M, Itoi T, Gotoda T, et
al: Risk factors for bleeding after endoscopic submucosal
dissection of colorectal neoplasms. World J Gastroenterol.
20:1839–1845. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ogasawara N, Yoshimine T, Noda H, Kondo Y,
Izawa S, Shinmura T, Ebi M, Funaki Y, Sasaki M and Kasugai K:
Clinical risk factors for delayed bleeding after endoscopic
submucosal dissection for colorectal tumors in Japanese patients.
Eur J Gastroenterol Hepatol. 28:1407–1414. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hosoya Y, Matsumura M, Madoiwa S, Zuiki T,
Matsumoto S, Nunomiya S, Lefor A, Sata N and Yasuda Y: Acquired
hemophilia A caused by factor VIII inhibitors: Report of a case.
Surg Today. 43:670–674. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Okamura T, Komatsu M, Ito A, Ito T, Suga
T, Arakura N, Sakai H and Tanaka E: A case of acquired hemophilia A
diagnosed after percutaneous endoscopic gastrostomy. Clin J
Gastroenterol. 8:290–293. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Onishi I, Kayahara M, Munemoto M, Sakai S,
Makino I, Hayashi H, Nakagawara H, Tajima H, Takamura H, Kitagawa
H, et al: Management of postoperative hemorrhage associated with
factor VIII inhibitor: Report of a case. Surg Today. 43:1058–1061.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Saito M, Ogasawara R, Izumiyama K, Mori A,
Kondo T, Tanaka M, Morioka M and Ieko M: Acquired hemophilia A in
solid cancer: Two case reports and review of the literature. World
J Clin Cases. 6:781–785. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khan UZ, Yang X, Masroor M, Aziz A, Yi H
and Liu H: Surgery-associated acquired hemophilia A: A report of 2
cases and review of literature. BMC Surg. 20(213)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oba S, Nakahira M, Kogashiwa Y, Ebihara Y
and Sugasawa M: Acquired hemophilia A presenting as massive
postoperative bleeding in a patient with oral squamous cell
carcinoma. Case Rep Otolaryngol. 2020(8961785)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Collins PW: Management of acquired
haemophilia A. J Thromb Haemost. 9 (Suppl 1):S226–S235.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Coulson S, Mohanaruban A, Shlebak A and
Sergot A: An unusual cause of bleeding in an elderly patient. Clin
Med (Lond). 12:150–152. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Charlebois J, Rivard GÉ and St-Louis J:
Management of acquired hemophilia A: review of current evidence.
Transfus Apher Sci. 57:717–720. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Constantinescu C, Jitaru C, Pasca S, Dima
D, Dirzu N, Coriu D, Zdziarska J, Ghiaur G, Mahlangu J and
Tomuleasa C: Unexplained hemorrhagic syndrome? Consider acquired
hemophilia A or B. Blood Rev. 53(100907)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pandey Y, Atwal D, Konda M, Roy A and
Sasapu A: Acquired hemophilia A. Proc (Bayl Univ Med Cent).
33:71–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Eggenhuizen PJ, Ng BH and Ooi JD: Treg
enhancing therapies to treat autoimmune diseases. Int J Mol Sci.
21(7015)2020.PubMed/NCBI View Article : Google Scholar
|