Introduction
Breast cancer is the most common malignant tumor in
females, accounting for ~25% of all cancer cases (1). The latest statistics show that breast
cancer remains the most common malignant tumor in women, with new
cases accounting for 30% of all cancer cases (2), and its mortality rate is second only
to that of lung cancer, namely ~15% (2). At present, the annual incidence rate
of breast cancer in China has reached 3-4%, and the onset age is
reducing, which seriously affects the health and quality of life of
female patients (3). Tumor
metastasis is an important factor affecting the surgical prognosis
of patients with breast cancer, and certain studies have confirmed
that 90% of patient mortalities can be attributed to breast cancer
metastasis (4,5). Therefore, it is of great importance
to further study the mechanisms of breast cancer metastasis and to
identify effective targets to inhibit breast cancer metastasis.
The mechanism and characteristics of metastasis in
breast cancer have been previously reported. During the occurrence
of tumor metastasis, various components in the tumor
microenvironment interact with each other to directly or indirectly
promote tumor metastasis (6).
Adipocytes are the largest components in breast tissue, and they
release a variety of adipokines, such as leptin, adiponectin,
interleukin-6 (IL-6) and chemokine ligand (CCL)2, which play an
important role in promoting the proliferation, angiogenesis,
diffusion, invasion and metastasis of breast cancer (7). Tumor-related fibroblasts formed after
the activation of normal fibroblasts in breast cancer under the
influence of the tumor play an important role in tumor formation,
development, invasion, metastasis and therapeutic resistance by
secreting various growth factors [such as fibroblast growth factor
(FGF)2 and CCL8] and cytokines (including platelet-derived growth
factor, FGF, IL-6 and hepatocyte growth factor), and remodeling the
extracellular matrix (8-10).
Tumor-associated macrophages (TAMs) can produce chemokines to
promote tumor metastasis, immune escape and lung metastasis in
breast cancer (11,12).
Zinc finger protein A20, also known as tumor
necrosis factor α induced protein 3, is an important endogenous
protective protein for preventing multiple human diseases (13). Abnormal A20 overexpression has been
found in human tumor cell lines and clinical specimens, including
breast cancer resistant strains and inflammatory breast cancer
(14,15). Vendrell et al (14) identified A20 as a new estradiol
regulatory gene, and observed that A20 expression change was
consistent with the expression change of estrogen receptor in
breast cancer cell lines and pathological tissues. The A20 gene was
overexpressed in breast cancer MCF-7 cells with stable A20
metastasis, showing tamoxifen resistance, apoptotic behavior and
increased sensitivity to estradiol. A20 was also highly expressed
in tamoxifen-resistant MVLN and VP (https://www.cellosaurus.org/CVCL_2755) cells, and in
highly aggressive breast cancer tissues. Using its N-terminal
deubiquitination function and C-terminal ubiquitination activity,
A20 can negatively regulate a variety of signaling molecules in the
p38MAPK/NF-κB signaling pathway, reduce the production of cellular
inflammatory factors and inhibit the inflammatory response
(16,17). In recent years, it has been
reported that A20 is involved in the pathological progression of
breast cancer. Sharif-Askari et al (18) found that A20 could serve as a
biomarker for early diagnosis of breast cancer. Song et al
(19) confirmed that A20 was
involved in inflammation-mediated metastatic disease in
triple-negative breast cancer. Therefore, it was hypothesized that
A20 may promote the proliferation and metastasis of breast cancer;
however, the specific mechanism needs to be further verified.
Macrophages can mainly mature and differentiate into
M1 and M2 subgroups. In the tumor microenvironment, macrophages can
be polarized into a particular type of M2 that can promote
tumorigenesis, namely, TAMs (20).
Xiao et al (21) confirmed
that SUMO specific peptidase 3 knockout could promote the
polarization of TAMs to the M2 type and promoted the progression of
breast cancer. Zhao et al (22) found that X-inactive specific
transcript knockdown or microRNA-101 overexpression could induce
macrophages to polarize from the M1 type to the M2 type, thus
promoting the proliferation and migration of breast cancer cells.
Therefore, breast cancer metastasis appeared to be closely
associated with TAMs polarization. Overexpression of A20 promoted
the polarization of pulmonary M1 macrophages to M2(23). Shi et al (24) confirmed that Takeda G
protein-coupled receptor 5 knockout promoted the M1-type
polarization of macrophages by promoting the activation of the
NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3)
inflammasome, while inhibiting the activation of the NLRP3
inflammasome could promote the M2-type polarization of macrophages
(25). In addition, Mouton-Liger
et al (26) confirmed that
A20, as an inhibitor of the NF-κB signaling pathway, could inhibit
NLRP3 inflammasome activity. The role of A20 in regulating TAMs
polarization through the NLRP3 signaling pathway in breast cancer
progression remains unclear.
The present study intended to verify the association
between A20, NLRP3 and TAMs polarization, as well as their effects
on the proliferation and metastasis of breast cancer cells.
Materials and methods
Cell culture
Normal human mammary epithelial cells MCF-10A (cat.
no. MZ-0695), and breast cancer cells MCF-7 (cat. no. MZ-0113),
BT-549 (cat. no. MZ-0031) and MDA-MB-231 (cat. no. 228845) were
obtained from Ningbo Mingzhou Biotechnology Co., Ltd. Human
umbilical vein endothelial cells (HUVECs; cat. no. CL-0122) were
provided by PromoCell GmbH and passaged in endothelial cell growth
medium (PromoCell GmbH). MCF-10A cells were cultured in mammary
epithelium growth medium (Shanghai Yaji Biological Technology Co.,
Ltd.) with 100 ng/ml cholera toxin and 1% Penicillin/Streptomycin.
BT-549 cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) with 0.023 U/ml insulin and 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). MCF-7
and MDA-MB-231 cells were cultured in DMEM with 10% FBS, 50 IU/ml
penicillin and 50 µg/ml streptomycin. Human monocytic leukemia
THP-1 cells were purchased from the Type Culture Collection of the
Chinese Academy of Sciences and cultured in RPMI-1640 medium
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
All cells were maintained at 37˚C in humidified air with 5%
CO2. Two culture wells were employed for each
experiment, including one for the experimental group and the other
one for the control group.
Cell transfection
Short hairpin (sh)RNA (pGPU6) targeting A20
(sh-A20#1, 5'-GCAACTGGAGTCTCTCAAATC-3' and sh-A20#2,
5'-TTTGAAAGTGGGTGGAATTTA-3') and its corresponding negative control
(sh-NC, 5'-GCAACAAGATGAAGAGCACCAA-3') were designed and provided by
Guangzhou RiboBio Co., Ltd. MDA-MB-231 cells were transfected with
sh-NC or sh-A20#1/2 (1 µg) using Lipofectamine® 2000 for
48 h at 37˚C in 5% CO2.
THP-1 cells were seeded into 6-well plates
(5x105 cells/well) and incubated with 200 ng/ml phorbol
12-myristate 13-acetate (PMA; MedChemExpress) for 24 h. Next, THP-1
cells were respectively cultured in PBS, or medium from MDA-MB-231
cells transfected with sh-NC, or medium from MDA-MB-231 cells
transfected with sh-A20 for 24 h. Finally, THP-1 cells were treated
with the NLRP3 inhibitor MCC950 (1 µM; MedChemExpress) for 1 h at
37˚C.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from MDA-MB-231 cells subjected to
different treatments was extracted with TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). RNA was then reverse
transcribed into cDNA with RevertAid First-Strand cDNA Synthesis
Kit (Takara Bio, Inc.). Sequentially, ~150 ng cDNA was used for
qPCR with SYBR® Premix Ex Taq II RT-PCR Kit (Takara Bio,
Inc.) in a RT-qPCR system. The thermocycling conditions were as
follows: i) 95˚C for 3 min; and ii) 40 cycles of 95˚C for 15 sec,
60˚C for 35 sec and 72˚C for 25 sec. The relative quantification of
A20, CD86, CD80, inducible nitric oxide synthase (iNOS), CD206,
CD163, CD11b, arginase 1 (ARG1) and IL10 was analyzed by the
2-ΔΔCq method (27).
GAPDH was used as an endogenous control. The primer sequences used
in the present study were as follows: A20, forward
5'-TCAAAATGGCTTCCACAGACAC-3' and reverse,
5'-GTCCTTCAGGGTCACCAAGG-3'; CD86, forward
5'-AGCCCACAGGAATGATTCGC-3' and reverse,
5'-TCTGCATAACACCATCATACTCGA-3'; CD80, forward
5'-CGCCTCTCTGAAGATTACCCAAA-3' and reverse,
5'-TAAGACCAGGGCACTTCCCA-3'; iNOS, forward
5'-GATCAAAAACTGGGGCAGCG-3' and reverse, 5'-CTCATCTGGAGGGGTAGGCT-3';
CD206, forward 5'-CATCAGGGTGCAAGGAAGGT-3' and reverse,
5'-TCCATCCGTCCAAAGGAACG-3'; CD163, forward
5'-GAAGACAGAGACAGCGGCTT-3' and reverse,
5'-GGTATCTTAAAGGCTCACTGGGT-3'; CD11b, forward
5'-GCTTTGGTGGCTTCCTTGTG-3' and reverse, 5'-TAGTCGCACTGGTAGAGGCT-3';
ARG1, forward 5'-ACTTAAAGAACAAGAGTGTGATGTG-3' and reverse,
5'-CATGGCCAGAGATGCTTCCA-3'; IL-10, forward
5'-TTGCAAAACCAAACCACAAGACA-3' and reverse,
5'-TCTCGAAGCATGTTAGGCAGG-3'; and GAPDH, forward
5'-AATGGGCAGCCGTTAGGAAA-3' and reverse,
5'-GCGCCCAATACGACCAAATC-3'.
Western blot analysis
MDA-MB-231 cells from different groups were
suspended in PBS and lysed with RIPA buffer (Cell Signaling
Technology, Inc.), followed by centrifugation at 10,000 x g for 10
min at 4˚C to obtain total protein. The protein concentration was
determined using a BCA Protein Assay Kit (Beyotime Institute of
Biotechnology). The protein samples (30 µg per lane) were separated
by SDS-PAGE (10% gels; Bio-Rad Laboratories, Inc.) and then
transferred to PVDF membranes (Bio-Rad Laboratories, Inc.) The
membranes were blocked with 5% non-fat milk for 2 h at room
temperature, and incubated with primary antibodies against A20
(cat. no. ab92324; dilution, 1:1,000; Abcam), VEGFA (cat. no.
ab214424; dilution, 1:1,000; Abcam), NLRP3 (cat. no. ab263899;
dilution, 1:1,000; Abcam), cleaved caspase-1 (cat. no. #4199;
dilution, 1:1,000; Cell Signaling Technology, Inc.), IL-1β (cat.
no. ab254360; dilution, 1:1,000; Abcam), caspase-1 (cat. no. #2225;
dilution, 1:1,000; Cell Signaling Technology, Inc.) and β-actin
(cat. no. ab8227; dilution, 1:1,000; Abcam) overnight at 4˚C. The
following day, the membranes were washed with 0.05% Tween-20/1X
TBST (cat. no. T1085; Beijing Solarbio Science & Technology
Co., Ltd.) three times for 10 min each at room temperature and then
incubated with HRP-conjugated goat anti-rabbit IgG secondary
antibody (cat. no. ab205718; dilution, 1:2,000; Abcam) for 1 h at
room temperature. The protein bands were visualized using enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.) and
quantified by ImageJ 1.8.0 software (National Institutes of
Health).
Cell Counting Kit-8 (CCK-8) assay
MDA-MB-231 cells from different treatment groups
were seeded into 96-well plates (2x103 cells/well), and
incubated at 37˚C with 5% CO2 for 24, 48 and 72 h. CCK-8
reagent (10 µl; MedChemExpress) was added per well after the
indicated time, followed by incubation at 37˚C for another 1 h. The
absorbance values were recorded in a microplate reader (Bio-Rad
Laboratories, Inc.) at 450 nm.
Colony formation assay
MDA-MB-231 cells from different treatment groups
were seeded into 6-well plates (1,000 cells/well) and then cultured
in DMEM at 37˚C for 14 days. Next, the colonies were fixed with 4%
paraformaldehyde at room temperature for 15 min and stained with
0.1% crystal violet at room temperature for 15 min, followed by
observation under an Olympus digital camera (Olympus
Corporation).
Wound healing assay
MDA-MB-231 cells from different treatment groups
were seeded in a 6-well plate (1x105 cells) and cultured
until 80% confluence. The cell layers were scratched with a 100-µl
pipette tip, and the wells were then washed with PBS to remove cell
debris. Next, the cells were incubated with serum-free DMEM for 24
h at 37˚C, and photographed under a light microscope (Olympus
Corporation). The extent of wound healing was calculated as
follows: Wound healing (%)=(wound area at 0 h-wound area at 24
h)/wound area at 0 h.
Transwell assay
MDA-MB-231 cells (1x105 cells) from
different treatment groups were seeded in the upper chamber of a
Transwell plate (8.0-µm pore membranes, 24 wells; Corning, Inc.)
that had been coated with Matrigel for 30 min at 37˚C, while
complete medium was added to the lower chamber. After incubation
for 24 h at 37˚C, the cells inside the upper chamber were removed,
and the cells outside the upper chamber were fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet solution for
5 min at room temperature. The invasive cells were observed and
photographed under a light microscope (Olympus Corporation).
Tube formation assay
HUVECs were seeded on a 24-well plate
(7x105 cells/well) coated with Matrigel (BD Biosciences)
and incubated in endothelial cell growth medium from Control, sh-NC
and sh-A20 groups for 48 h at 37˚C. Tube formation was visualized
under a light microscope (Olympus Corporation), and the number of
tubes was calculated using the Image-Pro Plus software (v6.0; Media
Cybernetics, Inc.).
Double staining
immunofluorescence
To identify the polarization states of macrophages
(M1 co-expressing F4/80 + iNOS and M2 co-expressing F4/80 + ARG-1),
double staining immunofluorescence was performed. After blocking
with 3% BSA (Thermo Fisher Scientific, Inc.) at room temperature
for 30 min, THP-1 cells were incubated with anti-F4/80 (cat. no.
sc-377009; dilution, 1:100; Santa Cruz Biotechnology, Inc.),
anti-iNOS (cat. no. sc-7271; dilution, 1:100; Santa Cruz
Biotechnology, Inc.) and anti-ARG-1 (cat. no. #93668; dilution,
1:50; Cell Signaling Technology, Inc.) antibodies. Next, THP-1
cells were washed with PBS and incubated with goat anti-mouse IgG
H&L (Alexa Fluor® 488) secondary antibody (cat. no.
ab150113; dilution, 1:200; Abcam) at room temperature for 1 h in
the dark. Next, the cells were washed with PBS and incubated with
DAPI at room temperature for 10 min in the dark. Finally, THP-1
cells were observed and photographed under a fluorescence
microscope (Olympus Corporation).
Statistical analysis
Data are presented as the mean ± SD from ≥3
experiments and analyzed by GraphPad Prism 1.8.0 program (GraphPad
Software; Dotmatics). One-way ANOVA followed by Tukey's post hoc
test was applied to analyze the differences between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Interference with A20 inhibits the
proliferation of breast cancer cells
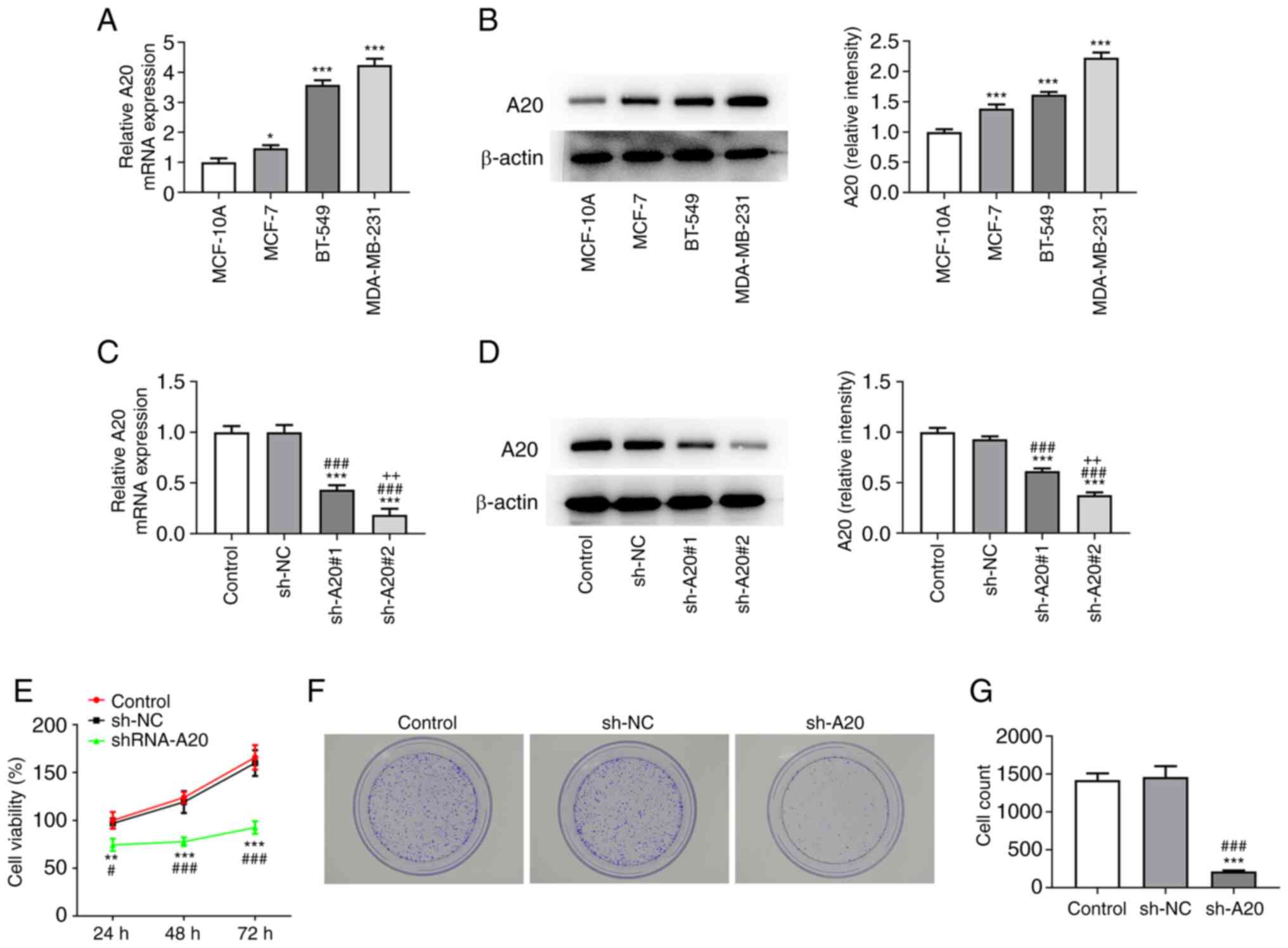
The expression of A20 in breast cancer cells was
increased compared with that in MCF-10A cells, and A20 expression
was the highest in MDA-MB-231 cells; therefore, the MDA-MB-231 cell
line was selected for subsequent experiments (Fig. 1A and B).
When MDA-MB-231 cells were transfected with sh-A20#1
and sh-A20#2, the expression of A20 was decreased, and was the
lowest in sh-A20#2 group, which was therefore selected for the
following experiments (Fig. 1C and
D). MDA-MB-231 cells transfected
with sh-A20 showed decreased viability (Fig. 1E). The proliferation of MDA-MB-231
cells was suppressed by interference with A20 (Fig. 1F and G).
Interference with A20 inhibits the
invasion, migration and angiogenesis of breast cancer cells
The invasion and migration of MDA-MB-231 cells were
inhibited by interference with A20 (Fig. 2A-D). The tube formation of HUVECs
was suppressed in the culture medium from sh-A20-transfected
MDA-MB-231 cells (Fig. 2E). The
expression of VEGFA in HUVECs cultured in culture medium from
sh-A20-transfected MDA-MB-231 cells was decreased (Fig. 2F).
Interference with A20 inhibits M2-like
polarization of macrophages
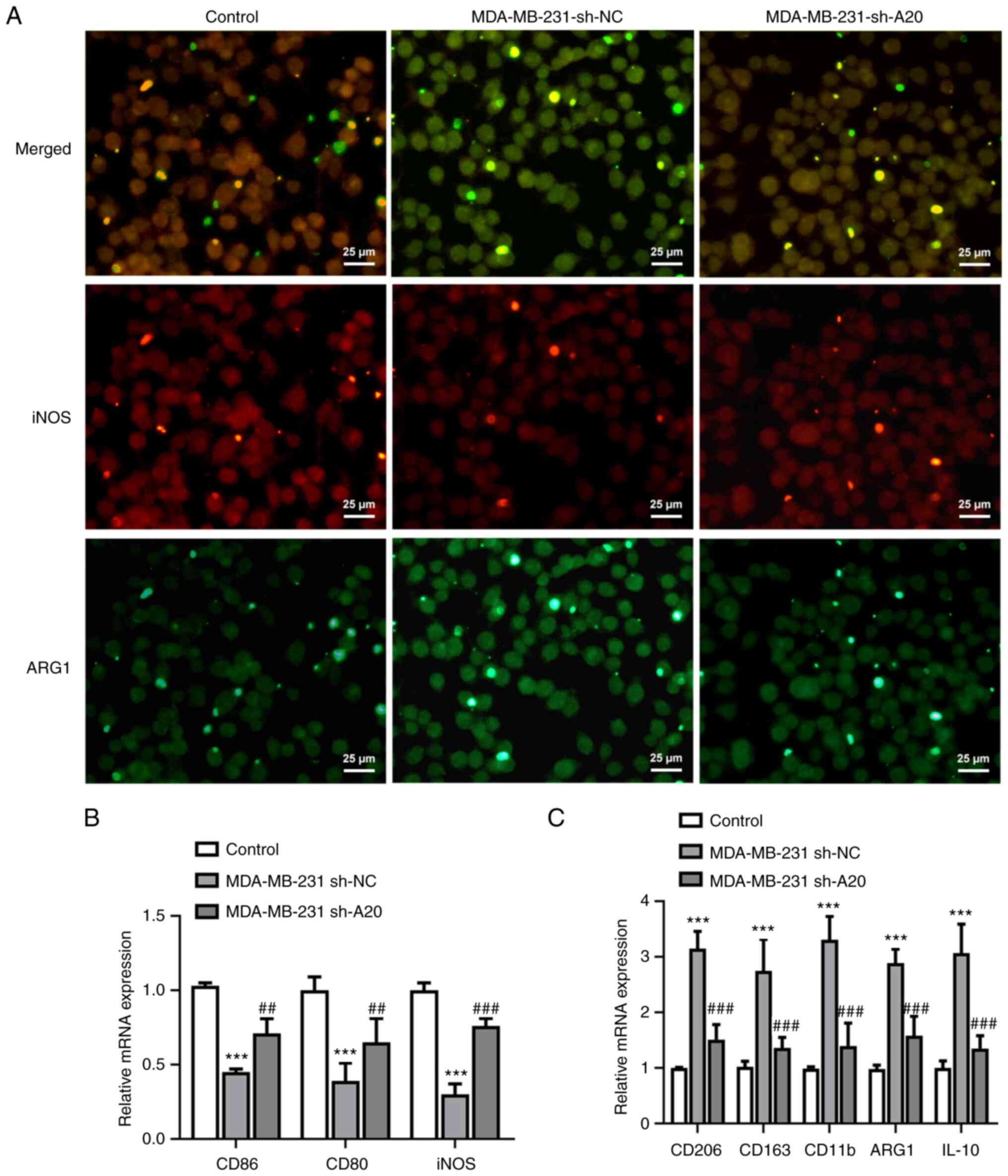
After treating THP-1 cells with PMA, THP-1 cells
cultured in PBS showed a high percentage of M1-like polarization of
macrophages. When PMA-treated THP-1 cells were cultured in culture
medium from sh-NC-transfected MDA-MB-231 cells, M1-like
polarization of macrophage marker iNOS expression was decreased,
while M2-like polarization of macrophage marker ARG1 expression was
increased. When PMA-treated THP-1 cells were cultured in culture
medium from sh-A20-transfected MDA-MB-231 cells, NOS expression was
increased, while ARG1 expression was declined (Fig. 3A). Correspondingly, the levels of
CD86, CD80 and iNOS, which are related to M1-like polarization of
macrophages, were lower in the in the MDA-MB-231 sh-NC group
compared with the control group, while the levels of CD206, CD163,
CD11b, ARG1 and IL10, which are related to M2-like polarization of
macrophages, were increased in the MDA-MB-231 sh-NC group. The
levels of CD86, CD80 and iNOS (related to M1-like polarization of
macrophages) were upregulated, while the levels of CD206, CD163,
CD11b, ARG1 and IL10 (related to M2-like polarization of
macrophages) were downregulated in the MDA-MB-231 sh-A20 group
(Fig. 3B and C).
Interference with A20 promotes the
activation of the NLRP3 inflammasome
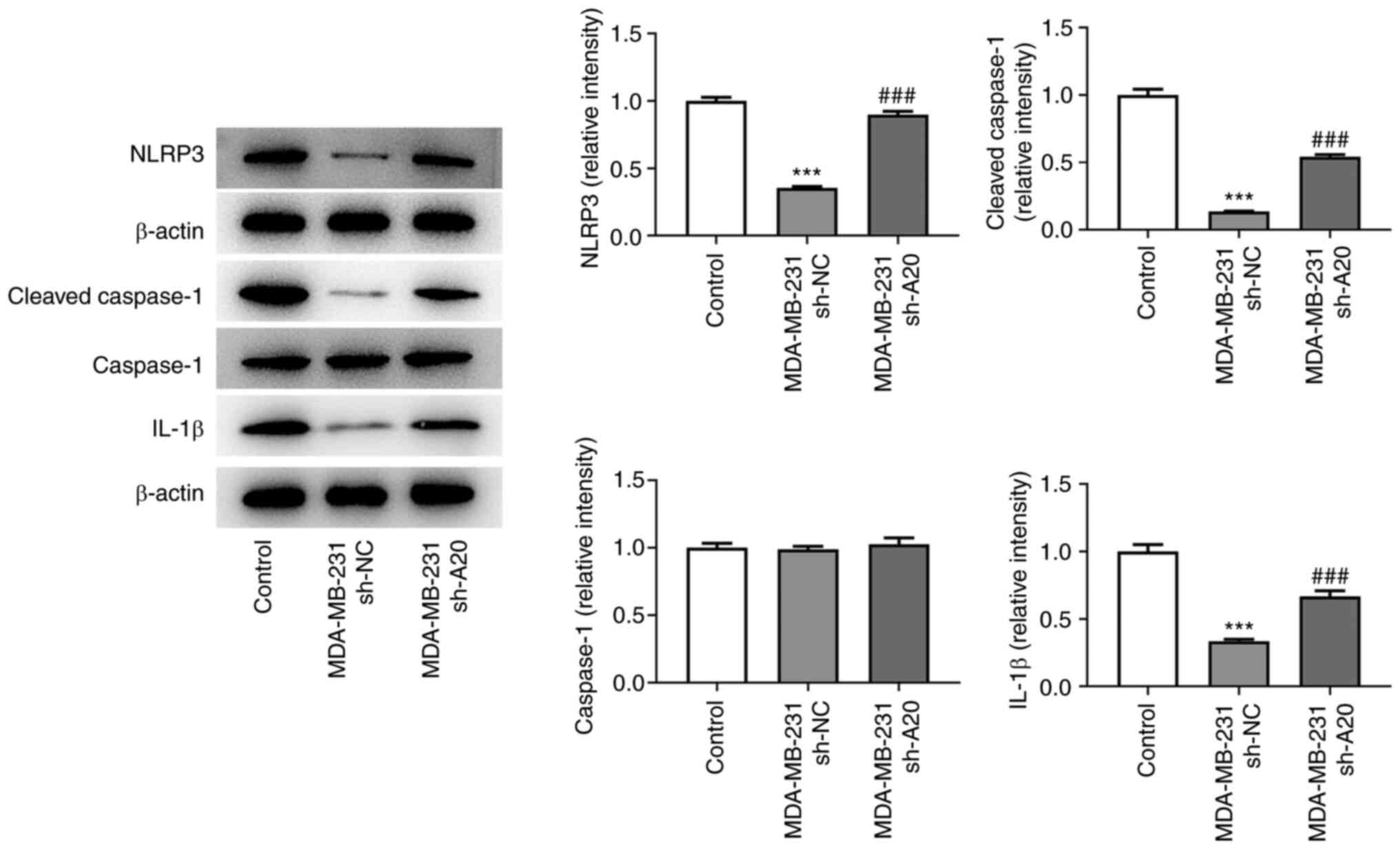
The expression of NLRP3, cleaved caspase-1 and IL-1β
in THP-1 cells cultured in culture medium from sh-NC-transfected
MDA-MB-231 cells was decreased. However, the culture medium from
sh-A20-transfected MDA-MB-231 cells increased the levels of NLRP3,
cleaved caspase-1 and IL-1β in THP-1 cells (Fig. 4).
Interference with A20 inhibits
macrophage proliferation and recruitment, and M2-like polarization
through the NLRP3 inflammasome pathway
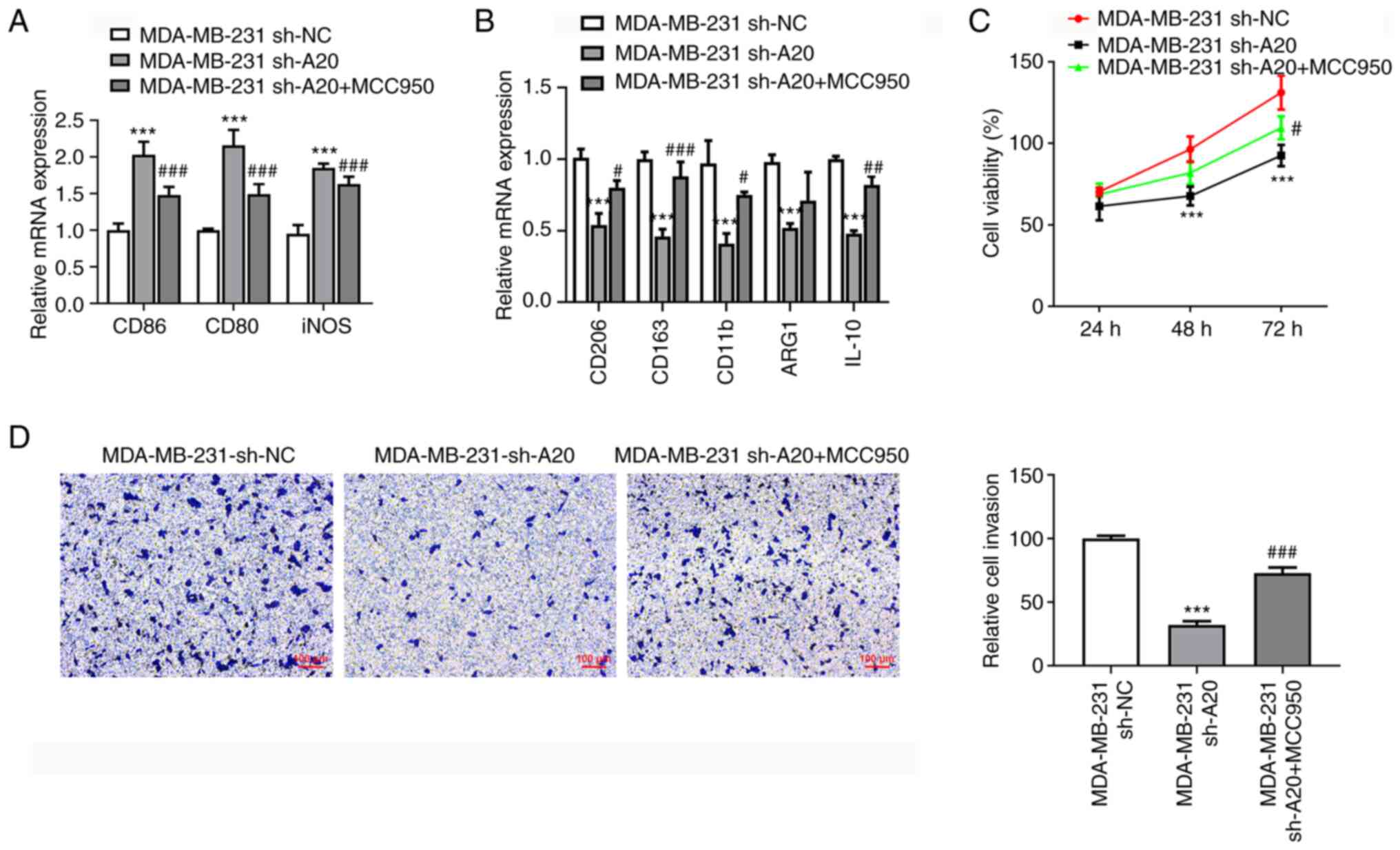
MCC950 suppressed the levels of CD86, CD80 and iNOS
(related to M1-like polarization of macrophages), and enhanced the
levels of CD206, CD163, CD11b and IL10 (related to M2-like
polarization of macrophages) (Fig.
5A and B). The culture medium
from sh-A20-transfected MDA-MB-231 cells suppressed the
proliferation of THP-1 cells, which was reversed by MCC950
(Fig. 5C). The invasion ability of
THP-1 cells cultured in culture medium from sh-A20-transfected
MDA-MB-231 cells was inhibited, and MCC950 restored the invasion
ability of THP-1 cells cultured in culture medium from
sh-A20-transfected MDA-MB-231 cells (Fig. 5D).
Discussion
A20 has been reported to be abnormally highly
expressed in tumor tissues. Guo et al (28) found that increased expression of
A20 in glioma could inhibit caspase-8 cleavage, as well as inhibit
the tumor necrosis factor-related apoptosis-inducing ligand-induced
apoptosis of tumor cells through the action of ubiquitin ligase.
Wang et al (29) found that
the expression of A20 was increased in 86 cases of
cholangiocarcinoma, and that A20 affected the time of tumor lymph
node metastasis, which was considered a novel prognostic factor for
cholangiocarcinoma. Jin et al (30) found that, in non-small cell lung
cancer cells, high expression of A20 inhibited the caspase-8
precursor, which was activated by the E3 ubiquitination enzyme
Cullin3 through its deubiquitination effect, and inhibited the
apoptosis signaling pathway through polyubiquitination. Lerebours
et al (15) used RT-qPCR to
compare and analyze NF-κB-related genes in 60 pathological tissues
from 35 cases of inflammatory breast cancer and 22 cases of
non-inflammatory breast cancer, and found that the expression
levels of 35 genes, including A20, were significantly upregulated
in inflammatory breast cancer tissues. The present study
demonstrated that interference with A20 inhibited the
proliferation, invasion and migration of breast cancer cells, as
well as HUVECs tube formation.
The inflammasome is a multiprotein complex composed
of pattern recognition receptors, the adaptor protein
apoptosis-associated speck-like protein containing a CARD and
pro-caspase-1, and the NLRP3 inflammasome is the most extensively
studied (26). Previous studies
have shown that the initiation and activation of NLRP3 is widely
involved in the occurrence and development of a variety of
diseases, and also plays an important role in the metastasis of
breast cancer. Wang et al (31) indicated that NLRP3 promoted the
migration and invasion of breast cancer cells, and NLRP3 induced
IL-1β secretion through a caspase-1-dependent pathway to promote
the EMT of breast cancer cells. Ershaid et al (32) confirmed that NLRP3 signals derived
from cancer-related fibroblasts could promote the growth and
metastasis of breast cancer. Yao et al (33) found that berberine could inhibit
the proliferation and migration of triple-negative breast cancer
cells by inhibiting the NLRP3 pathway. A20 could regulate the
activity of microglia, and microglia A20 deficiency amplified
lipopolysaccharide (LPS)-induced cell damage and NLRP3 inflammasome
activation, thus aggravating the neuroinflammatory response
(34). Deletion of A20 induced
NF-κB expression in macrophages, which was dependent on NLRP3
inflammasome activation and neuroinflammation (35). A20 overexpression protected against
pristine-induced lupus nephritis by suppressing NF-κB and NLRP3
inflammasome activation in macrophages of mice (36). A20-deficient macrophages exhibited
spontaneous NLRP3 inflammasome activity to LPS alone (37). The present study showed that
interference with A20 activated the NLRP3 inflammasome in THP-1
cells.
TAMs are known to interfere with a variety of cancer
treatments, such as chemotherapy, radiation and immunotherapy.
Often, depleting M2 TAMs or reprogramming them to the M1 phenotype
enhances the efficacy of these therapies (38,39).
Silencing of the monocarboxylate transporter 1 (MCT-1) gene
suppressed the EMT and the activation of matrix metalloproteinases
in invasive triple-negative breast cancer cells, and M2 macrophages
were decreased, while tumor-suppressive M1 macrophages were
increased via MCT-1 knockdown (40). Downregulation of the long
non-coding RNA small nucleolar RNA host gene 1 attenuated M2
macrophage polarization to inhibit the metastasis of tumor cells
and the angiogenesis of breast cancer cells (41). Lactate could induce macrophage
M2-polarization to promote breast cancer proliferation, migration
and angiogenesis (42). Ginkgolide
B enhanced the expression of M2 microglial markers, and suppressed
the expression of M1 microglial markers by inhibiting NLRP3
inflammasome activation (43).
NLRP3 inflammasome was decreased by glycyrrhizin treatment, which
upregulated the protein expression levels of M2 microglia-related
markers but downregulated those of M1 microglia-related markers in
injured spinal cord (44). A20
overexpression inhibited the NLRP3 inflammasome, and MCC950
suppressed the activation of M1 macrophage polarization through A20
silencing in P. gingivalis (Pg). LPS and IFN-γ stimulated THP-1
cells (45). The present study
also indicated that interference with A20 increased the expression
of M1 microglial markers, and decreased the expression of M2
microglia-related markers by activating the NLRP3 inflammasome to
suppress the proliferation, invasion and migration of breast cancer
cells as well as HUVEC angiogenesis through the NLRP3 inflammasome
pathway. MCC950 weakened the inhibitory effect of sh-A20 on
macrophage proliferation and M2-like polarization.
In conclusion, A20 expression was increased in
breast cancer cells, and interference with A20 suppressed the
proliferation, invasion and migration of breast cancer cells, and
the angiogenesis of HUVECs. In addition, interference with A20
inhibited macrophage proliferation and M2-like polarization through
the activation of the NLRP3 inflammasome pathway. The current
findings revealed for the first time the regulatory role of A20 in
the invasion and migration of breast cancer cells through the NLRP3
inflammasome pathway, which was mediated by TAMs polarization.
Additional cell lines and animal models will be explored in future
studies. Furthermore, the potential clinical application of A20
needs to be evaluated in future clinical studies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Longyan City Science
and Technology Plan Project (grant no. 2021LYF17043).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW conceived and designed the current study. YaZ
conducted the experiments and analyzed the data with the help of
SW, YuZ and CH. YaZ wrote the manuscript, while XW revised it. XW
and YaZ confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gendoo DMA, Zon M, Sandhu V, Manem VSK,
Ratanasirigulchai N, Chen GM, Waldron L and Haibe-Kains B:
MetaGxData: Clinically annotated breast, ovarian and pancreatic
cancer datasets and their use in generating a multi-cancer gene
signature. Sci Rep. 9(8770)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li X, Xin P, Wang C, Wang Z, Wang Q and
Kuang H: Mechanisms of traditional chinese medicine in the
treatment of mammary gland hyperplasia. Am J Chin Med. 45:443–458.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Soni A, Ren Z, Hameed O, Chanda D, Morgan
CJ, Siegal GP and Wei S: Breast cancer subtypes predispose the site
of distant metastases. Am J Clin Pathol. 143:471–478.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Park M, Kim D, Ko S, Kim A, Mo K and Yoon
H: Breast cancer metastasis: Mechanisms and therapeutic
implications. Int J Mol Sci. 23(6806)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Picon-Ruiz M, Morata-Tarifa C,
Valle-Goffin JJ, Friedman ER and Slingerland JM: Obesity and
adverse breast cancer risk and outcome: Mechanistic insights and
strategies for intervention. CA Cancer J Clin. 67:378–397.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhao C, Wu M, Zeng N, Xiong M, Hu W, Lv W,
Yi Y, Zhang Q and Wu Y: Cancer-associated adipocytes: Emerging
supporters in breast cancer. J Exp Clin Cancer Res.
39(156)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Katoh M: FGFR inhibitors: Effects on
cancer cells, tumor microenvironment and whole-body homeostasis
(Review). Int J Mol Med. 38:3–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Farmaki E, Chatzistamou I, Kaza V and
Kiaris H: A CCL8 gradient drives breast cancer cell dissemination.
Oncogene. 35:6309–6318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun X, Fan W and Wu D: Research progress
of actions of cancer-associated fibroblasts in invasion, metastasis
and drug resistance in breast cancer. Chinese J Gen Surg.
29:618–624. 2020.
|
|
11
|
Li J, Wang S, Wang N, Zheng Y, Yang B,
Wang X, Zhang J, Pan BZ and Wang Z: Aiduqing formula inhibits
breast cancer metastasis by suppressing TAM/CXCL1-induced Treg
differentiation and infiltration. Cell Commun Signal.
19(89)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nie G, Cao X, Mao Y, Lv Z, Lv M, Wang Y,
Wang H and Liu C: Tumor-associated macrophages-mediated CXCL8
infiltration enhances breast cancer metastasis: Suppression by
Danirixin. Int Immunopharmacol. 95(107153)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Malynn BA and Ma A: A20: A multifunctional
tool for regulating immunity and preventing disease. Cell Immunol.
340(103914)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vendrell JA, Ghayad S, Ben-Larbi S,
Dumontet C, Mechti N and Cohen PA: A20/TNFAIP3, a new
estrogen-regulated gene that confers tamoxifen resistance in breast
cancer cells. Oncogene. 26:4656–4667. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lerebours F, Vacher S, Andrieu C, Espie M,
Marty M, Lidereau R and Bieche I: NF-kappa B genes have a major
role in inflammatory breast cancer. BMC Cancer.
8(41)2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Priem D, Devos M, Druwé S, Martens A,
Slowicka K, Ting AT, Pasparakis M, Declercq W, Vandenabeele P, van
Loo G and Bertrand MJM: A20 protects cells from TNF-induced
apoptosis through linear ubiquitin-dependent and -independent
mechanisms. Cell Death Dis. 10(692)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li L, Huang B, Song S, Sohun H, Rao Z, Tao
L, Jin Q, Zeng J, Wu R, Ji K, et al: A20 functions as mediator in
TNFα-induced injury of human umbilical vein endothelial cells
through TAK1-dependent MAPK/eNOS pathway. Oncotarget.
8:65230–65239. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sharif-Askari FS, Al-Khayyal N, Talaat I,
Sharif-Askari NS, Rawat S, Jundi M, SyrjÄnen K, Hamoudi R and
Bendardaf R: Immunohistochemical assessment of TNFAIP3/A20
expression correlates with early tumorigenesis in breast cancer.
Anticancer Res. 41:739–745. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Song C, Kendi AT, Lowe VJ and Lee S: The
A20/TNFAIP3-CDC20-CASP1 axis promotes inflammation-mediated
metastatic disease in triple-negative breast cancer. Anticancer
Res. 42:681–695. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiao M, Bian Q, Lao Y, Yi J, Sun X, Sun X
and Yang J: SENP3 loss promotes M2 macrophage polarization and
breast cancer progression. Mol Oncol. 16:1026–1044. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao Y, Yu Z, Ma R, Zhang Y, Zhao L, Yan
Y, Lv X, Zhang L, Su P, Bi J, et al:
lncRNA-Xist/miR-101-3p/KLF6/C/EBPα axis promotes TAM polarization
to regulate cancer cell proliferation and migration. Mol Ther
Nucleic Acids. 23:536–551. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Y, Song Z, Bi J, Liu J, Tong L, Song
Y, Bai C and Zhu X: A20 protein regulates
lipopolysaccharide-induced acute lung injury by downregulation of
NF-κB and macrophage polarization in rats. Mol Med Rep.
16:4964–4972. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shi Y, Su W, Zhang L, Shi C, Zhou J, Wang
P, Wang H, Shi X, Wei S, Wang Q, et al: TGR5 regulates macrophage
inflammation in nonalcoholic steatohepatitis by modulating NLRP3
inflammasome activation. Front Immunol. 11(609060)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu T, Wang L, Liang P, Wang X, Liu Y, Cai
J, Chen Y, Wang L, Gao G and Tian Y: USP19 suppresses inflammation
and promotes M2-like macrophage polarization by manipulating NLRP3
function via autophagy. Cell Mol Immunol. 18:2431–2442.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mouton-Liger F, Rosazza T, Sepulveda-Diaz
J, Ieang A, Hassoun SM, Claire E, Mangone G, Brice A, Michel PP,
Corvol JC and Corti O: Parkin deficiency modulates NLRP3
inflammasome activation by attenuating an A20-dependent negative
feedback loop. Glia. 66:1736–1751. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guo Q, Dong H, Liu X, Wang C, Liu N, Zhang
J, Li B, Cao W, Ding T, Yang Z and Zhang X: A20 is overexpressed in
glioma cells and may serve as a potential therapeutic target.
Expert Opin Ther Targets. 13:733–741. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Y, Wan M, Zhou Q, Wang H, Wang Z,
Zhong X, Zhang L, Tai S and Cui Y: The prognostic role of SOCS3 and
A20 in human cholangiocarcinoma. PLoS One.
10(e0141165)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jin Z, Li Y, Pitti R, Lawrence D, Pham VC,
Lill JR and Ashkenazi A: Cullin3-based polyubiquitination and
p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis
signaling. Cell. 137:721–735. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang Y, Zhang H, Xu Y, Peng T, Meng X and
Zou F: NLRP3 induces the autocrine secretion of IL-1β to promote
epithelial-mesenchymal transition and metastasis in breast cancer.
Biochem Biophys Res Commun. 560:72–79. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ershaid N, Sharon Y, Doron H, Raz Y, Shani
O, Cohen N, Monteran L, Leider-Trejo L, Ben-Shmuel A, Yassin M, et
al: NLRP3 inflammasome in fibroblasts links tissue damage with
inflammation in breast cancer progression and metastasis. Nat
Commun. 10(4375)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yao M, Fan X, Yuan B, Takagi N, Liu S, Han
X, Ren J and Liu J: Berberine inhibits NLRP3 Inflammasome pathway
in human triple-negative breast cancer MDA-MB-231 cell. BMC
Complement Altern Med. 19(216)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Voet S, Mc Guire C, Hagemeyer N, Martens
A, Schroeder A, Wieghofer P, Daems C, Staszewski O, Vande Walle L,
Jordao MJC, et al: A20 critically controls microglia activation and
inhibits inflammasome-dependent neuroinflammation. Nat Commun.
9(2036)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vande Walle L, Van Opdenbosch N, Jacques
P, Fossoul A, Verheugen E, Vogel P, Beyaert R, Elewaut D,
Kanneganti TD, van Loo G and Lamkanfi M: Negative regulation of the
NLRP3 inflammasome by A20 protects against arthritis. Nature.
512:69–73. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li M, Shi X, Qian T, Li J, Tian Z, Ni B
and Hao F: A20 overexpression alleviates pristine-induced lupus
nephritis by inhibiting the NF-κB and NLRP3 inflammasome activation
in macrophages of mice. Int J Clin Exp Med. 8:17430–17440.
2015.PubMed/NCBI
|
|
37
|
Duong BH, Onizawa M, Oses-Prieto JA,
Advincula R, Burlingame A, Malynn BA and Ma A: A20 restricts
ubiquitination of pro-interleukin-1β protein complexes and
suppresses NLRP3 inflammasome activity. Immunity. 42:55–67.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Germano G, Frapolli R, Belgiovine C,
Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M,
Pasqualini F, et al: Role of macrophage targeting in the antitumor
activity of trabectedin. Cancer Cell. 23:249–262. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
De Palma M and Lewis CE: Macrophage
regulation of tumor responses to anticancer therapies. Cancer Cell.
23:277–286. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Weng YS, Tseng HY, Chen YA, Shen PC, Al
Haq AT, Chen LM, Tung YC and Hsu HL: MCT-1/miR-34a/IL-6/IL-6R
signaling axis promotes EMT progression, cancer stemness and M2
macrophage polarization in triple-negative breast cancer. Mol
Cancer. 18(42)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zong S, Dai W, Guo X and Wang K:
LncRNA-SNHG1 promotes macrophage M2-like polarization and
contributes to breast cancer growth and metastasis. Aging (Albany
NY). 13:23169–23181. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B,
Yang J, Pan J, Hu S, Zhang C, et al: Tumor-derived lactate induces
M2 macrophage polarization via the activation of the ERK/STAT3
signaling pathway in breast cancer. Cell Cycle. 17:428–438.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang Y, Zhao Y, Zhang J, Gao Y, Li S,
Chang C, Yu D and Yang G: Ginkgolide B inhibits NLRP3 inflammasome
activation and promotes microglial M2 polarization in
Aβ1-42-induced microglia cells. Neurosci Lett.
764(136206)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Su XQ, Wang XY, Gong FT, Feng M, Bai JJ,
Zhang RR and Dang XQ: Oral treatment with glycyrrhizin inhibits
NLRP3 inflammasome activation and promotes microglial M2
polarization after traumatic spinal cord injury. Brain Res Bull.
158:1–8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hou L, Ye Y, Gou H, Tang H, Zhou Y, Xu X
and Xu Y: A20 inhibits periodontal bone resorption and
NLRP3-mediated M1 macrophage polarization. Exp Cell Res.
418(113264)2022.PubMed/NCBI View Article : Google Scholar
|